Abstract
Hippo pathway components are structurally and functionally conserved and are notable for their role in controlling organ size. More diverse functions of the Hippo pathway have been recognized, including development, tissue homeostasis, wound healing and regeneration, immunity, and tumorigenesis. During embryogenesis, different signaling pathways are repeatedly and cooperatively activated, leading to differential gene expression in specific developmental contexts. In this article, we present an overview on the regulation and function of the Hippo pathway in mammalian early development. We introduce the Hippo pathway components and major upstream signals that act through this pathway to influence embryogenesis. We also discuss the roles of Hippo pathway in tissue specification and organ development during organogenesis.
Keywords: HIPPO, YAP/TAZ, stem cells, embryogenesis, early development
Hippo pathway and its role in organ size control
The Hippo pathway is the most recently discovered, major signaling pathway identified to play a role in limiting tissue and organ growth [1, 2]. Subsequent studies demonstrate that Hippo is highly conserved in mammals and controls development and tissue/organ homeostasis whereas dysregulation of the Hippo pathway contributes to human diseases, such as cancer [3, 4]. Core components of the Hippo pathway include a kinase cascade and a transcription module [5]. Genetic and biochemical studies have established that the Hippo kinase cascade functions to inhibit the transcription module that represents the major functional output of the Hippo pathway, thereby inhibiting cell and tissue growth. Unlike many developmental pathways that are defined by specific morphogens/hormones and their corresponding receptors, the Hippo pathway responds to diverse signals, such as hormones, cell-cell or cell-matrix contact, mechanic cues, cellular energy status, and various stress, to control cell growth, differentiation, tissue/organ development and homeostasis. As such, the Hippo pathway can receive and integrate a wide range of environmental and physiological signals to modulate transcription and cellular activity.
In addition to a key role in tissue and organ homeostasis in adults, a large number of recent studies have established the fundamental function of this pathway in mammalian embryonic development. Although many reviews on the Hippo pathway have been published, most focus on the signaling mechanisms, cancer implication, or a specific tissue type [5–7]. This review aims to provide the current landscape of the Hippo pathway in early development with an emphasis of mouse genetic studies. Here, we summarize the Hippo pathway components, mechanisms of regulation, and upstream regulatory signals, particularly those important in development. This review mainly focuses on the functions of the Hippo pathway in early developmental stages in a chronological order from zygotes to organogenesis. We discuss the functional cross talk between Hippo and other developmental signals as well as therapeutic potential of manipulating the Hippo pathway in regenerative medicine. We aim to provide readers with a comprehensive view of the Hippo pathway in early development and key open questions in the field.
Core components of Hippo pathway
A large number of signals act through various mechanisms to control the Hippo pathway activity. The core components of the Hippo pathway are highly conserved and consist of a kinase cascade and downstream transcription effectors (Fig. 1). In mammals, the kinase cascade includes MST1/2 (Mammalian STE20 like Kinase 1/2), MAP4K (Mitogen-Activated Protein Kinase Kinase Kinase Kinase) family, and LATS1/2 (Large Tumor Suppressor Kinase 1/2), while the downstream effectors are further grouped into YAP1 (Yes Associated Protein 1, also known as YAP) / WWTR1 (WW Domain Containing Transcription Regulator 1, also known as TAZ) transcription co-activators and DNA-binding protein TEAD 1/2/3/4 (TEA Domain Transcription Factor 1/2/3/4) .
Figure 1.
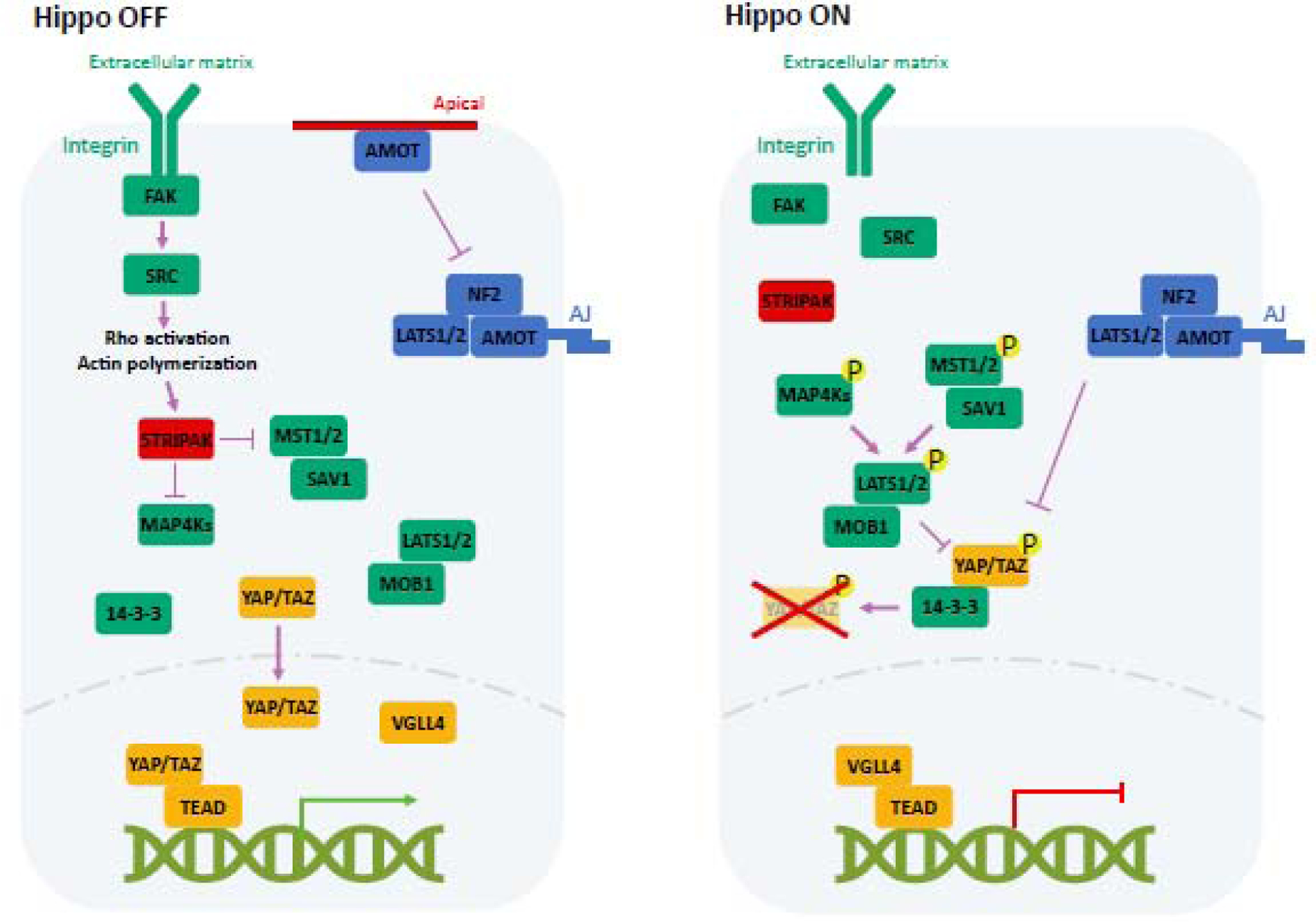
The mammalian Hippo pathway and its regulation by cell-cell and cell-matrix contact. MST1/2, MAP4Ks and LATS1/2 kinases are activated by phosphorylation. YAP/TAZ are inhibited by LATS dependent phosphorylation, which promotes YAP/TAZ cytoplasmic localization and degradation. STRIPAK inhibits MST and MAP4Ks by dephosphorylation. ECM acts via integrin to modulate the Hippo pathway. Cell-cell contact signal is detected by adherens junction and tight junction and mediated by AMOT family protein to stimulate Hippo signaling. In the absence of nuclear YAP/TAZ, VGLL4 binds TEAD to repress transcription.
At the top of the kinase cascade, protein phosphatase PP2A in the STRIPAK (striatin-interacting phosphatase and kinase) complexes (see Glossary) limits the activity of MST1/2 or MAP4Ks by direct dephosphorylation [8] (Fig. 1). When stimulated by upstream signals, the STRIPAK-mediated dephosphorylation is relieved, thereby MST1/2 or MAP4Ks are phosphorylated and activated, possibly due to autophosphorylation or by the upstream TAO kinase [9, 10]. SAV1 (Salvador Family WW Domain Containing Protein 1) and MOB1A/B (MOB Kinase Activator 1A/B), which are regulatory subunits of MST and LATS, respectively, are then phosphorylated by the active MST1/2 and recruit LATS1/2 for activation by MST [11–13]. Besides MST1/2, MAP4K proteins can also phosphorylate and activate LATS1/2 [14, 15]. NF2 (Neurofibromin 2), a FERM domain protein, is a critical upstream regulator that promotes the activation of Hippo kinase cascade by interacting with upstream signals and Hippo pathway components [16, 17]. YAP/TAZ is then phosphorylated by LATS1/2, resulting in their binding with 14-3-3 and cytoplasmic sequestration [18]. The LATS-phosphorylated YAP/TAZ can undergo further phosphorylation by casein kinase 1δ/ε, and this phosphorylation recruits SCF E3 ligase, leading to protein ubiquitination and degradation [19, 20]. YAP/TAZ can also be phosphorylated by other kinases, however, YAP/TAZ inhibition by LATS dependent phosphorylation represents the major and most widely used mechanism in Hippo signaling.
YAP/TAZ don’t have any DNA binding domain and act as a co-activator. The output of the Hippo pathway is mainly dependent on their binding to TEAD family members, which share a highly conserved DNA binding domain and a YAP/TAZ binding domain [21]. One unique feature of TEAD is that it can be auto-palmitoylated and this palmitoylation is critical for TEAD function [22]. In the absence of YAP/TAZ, TEAD interacts with VGLL4 (Vestigial Like Family Member 4), a protein containing Vg motif with transcriptional repression activity, to form a default repression complex [23]. Once shuttled into the nucleus, YAP/TAZ binds TEAD to displace VGLL4, thus forming a transcriptional activation complex, which is responsible for the induction of the majority of Hippo target genes.
Upstream regulators of Hippo pathway in embryogenesis
Unlike classical signaling pathways that are defined by specific ligands and receptors, the Hippo pathway works as an integrator of signals from the biochemical, physical, and architectural environment. As this review focuses on embryogenesis, we will only discuss the Hippo pathway’s regulatory factors relevant in early development. Besides Hippo, the embryonic development is also regulated by various other signaling pathways, the crosstalk between Hippo and these signaling pathways should not be overlooked:
Cell polarity and adhesion
Cell polarity, the asymmetric distribution of constituents in a cell, is an essential feature of life. The molecular basis of cell polarity is a set of evolutionarily conserved proteins called the PAR-aPKC system [24]. Researchers have revealed how cell polarity cooperates with junction-associated scaffolding angiomotin (AMOT) family proteins to regulate Hippo pathway (Fig. 1). When cells are non-polar, AMOT is phosphorylated on serine 176 (S176) and distributed in adherens junctions (AJs) [25], and interacts with NF2 and LATS kinases to facilitate the phosphorylation of YAP/TAZ [26, 27]. When cells exhibit polarity, on the other hand, AMOT is sequestered from basolateral AJs to apical domains by cell polarity regulator Par-aPKC system [25], where AMOT interacts with F-actin and suppresses YAP/TAZ activity [28].
Mechanical force
During embryogenesis, Hippo pathway is constantly regulated by mechanical force from the extracellular matrix (ECM) and neighboring cells (Fig. 1). Specifically, high stiffness of ECM induces nuclear localization of YAP/TAZ, whereas low ECM stiffness promotes YAP/TAZ cytoplasmic localization [29, 30]. Most studies have indicated that integrin and its downstream SRC/FAK kinases are involved in the initial events in response to matrix stiffness [31]. Rho family of GTPases and the cytoskeleton are key mediators from mechanical cues to the Hippo pathway [29, 32, 33]. However, the detailed mechanism of how cytoskeleton controls the activity of YAP/TAZ is still unknown.
GPCR
Strict spatial and temporal control of soluble factors is the key feature of embryonic development. G protein–coupled receptors (GPCRs) have been linked to Hippo regulation [34–38]. Based on the types of coupled G proteins, GPCR signaling can either activate or inhibit the Hippo pathway [34, 39] (Fig. 2). Mechanistically, Rho-GTPases and the F-actin cytoskeleton relay GPCR signaling to Hippo [34]. Rho also acts in part via STRIPAK to inhibit the Hippo kinase cascade [8].
Figure 2.
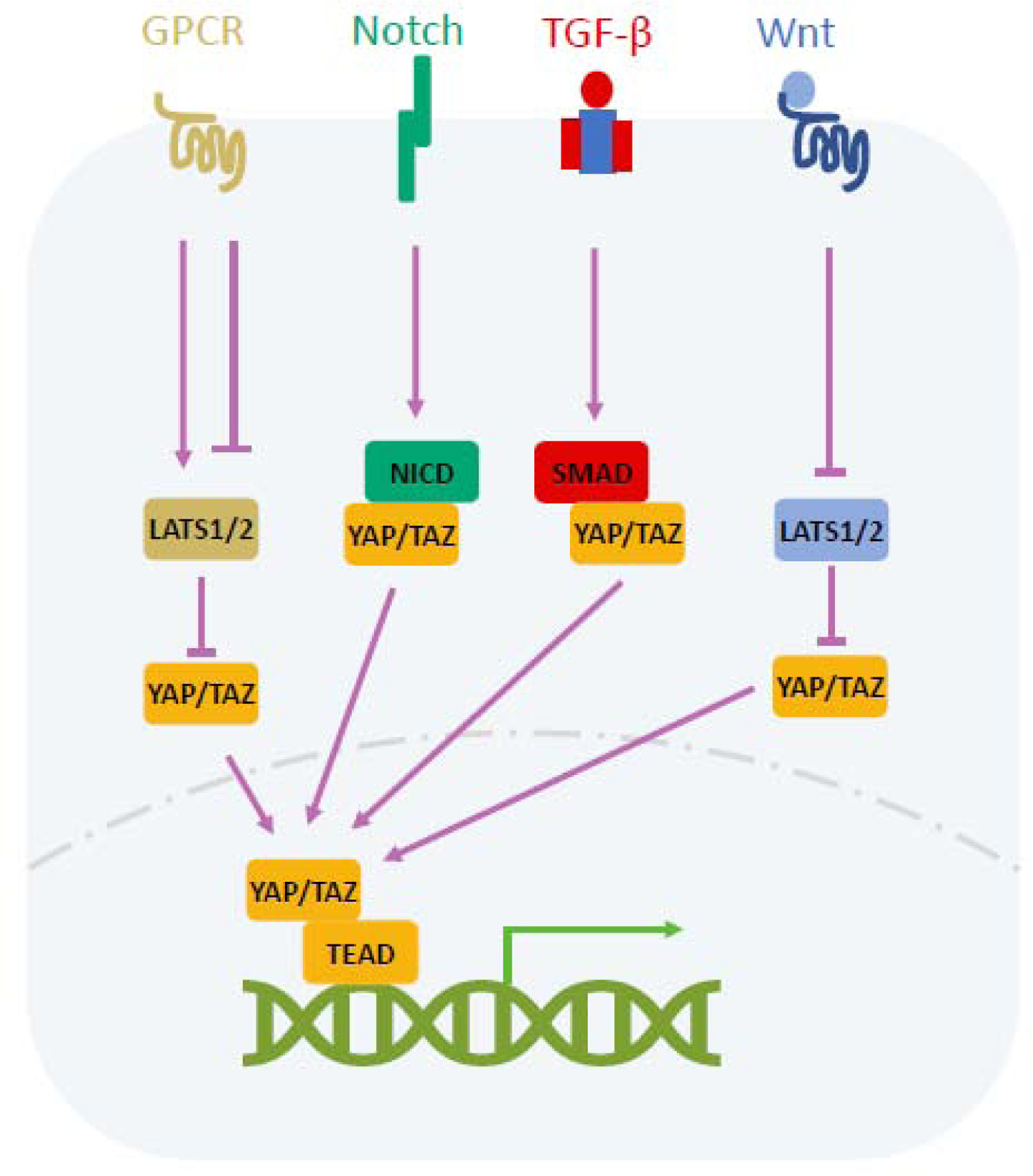
Crosstalk of the Hippo pathway with other developmental cues. Stimulation of GPCR can either positively or negatively affect YAP/TAZ activity in a manner dependent on the type of heterotrimeric G proteins coupled to the receptor. Notch intracellular domain (NICD), the effector of NOTCH signaling, enhances YAP/TAZ activity by promoting protein stability. TAZ binds SMAD, the effector of TGF-β signaling, to promote its nuclear translocation. YAP/TAZ are stimulated by Wnt ligands.
Wnt
Wnt signaling is known for its critical role in embryonic development [40]. The destruction complex, including Axin, APC (Adenomatous Polyposis Coli) and GSK3β (Glycogen Synthase Kinase 3 Beta) degrades β-catenin, the key effector of Wnt pathway, in the absence of Wnt ligands. Under Wnt stimulation, cytoplasmic β-catenin accumulates and enters the nucleus to activate transcription [40, 41]. Studies have revealed YAP activation by Wnt ligands, but the underlying mechanism is still controversial (Fig. 2). Some reports indicate that YAP/TAZ are released from the destruction complex and hence stabilized upon Wnt stimulation [42, 43]. One study suggests that APC interacts with Sav1 and LATS to facilitate the phosphorylation of YAP/TAZ [44]. Yet, another report shows that Wnt signaling inhibits YAP phosphorylation via the conventional Hippo pathway [45]. However, it is clear that YAP activation also leads to suppression of Wnt signaling.
Notch
The Notch pathway functions in development and homeostasis. When cells are in contact with each other, the Notch receptor interacts with its ligands in the neighboring cells. This interaction leads to cleavage of Notch receptor and the release of Notch intracellular domain (NICD), which translocates into the nucleus to induce target genes [46, 47]. The crosstalk between the two signaling pathways have been demonstrated (Fig. 2): NICD is reported to enhance YAP/TAZ activity by promoting protein stability and YAP/TAZ can activate the transcription of Notch receptors and its ligand, JAG1 (Jagged Canonical Notch Ligand 1) [48, 49].
TGF
The transforming growth factor-β (TGF-β) superfamily is involved in a large numbers of biological events in embryogenesis [50]. TGFβ ligands bind to their specific Ser/Thr kinase receptors to activate SMAD and gene transcription [50]. TAZ is reported to bind to SMAD, promoting its nuclear translocation under TGF-β stimulation [51] (Fig. 2). Conversely, TGF-β signaling can be inhibited via YAP/TAZ-mediated SMAD cytoplasmic sequestration [52].
Hippo pathway in the preimplantation stage
Embryogenesis in mammals is a complicated process that can be divided into several developmental stages. In the next few sections, we review the role of Hippo pathway in embryogenesis, with a focus on genetic data from mouse models (Fig. 3).
Figure 3.
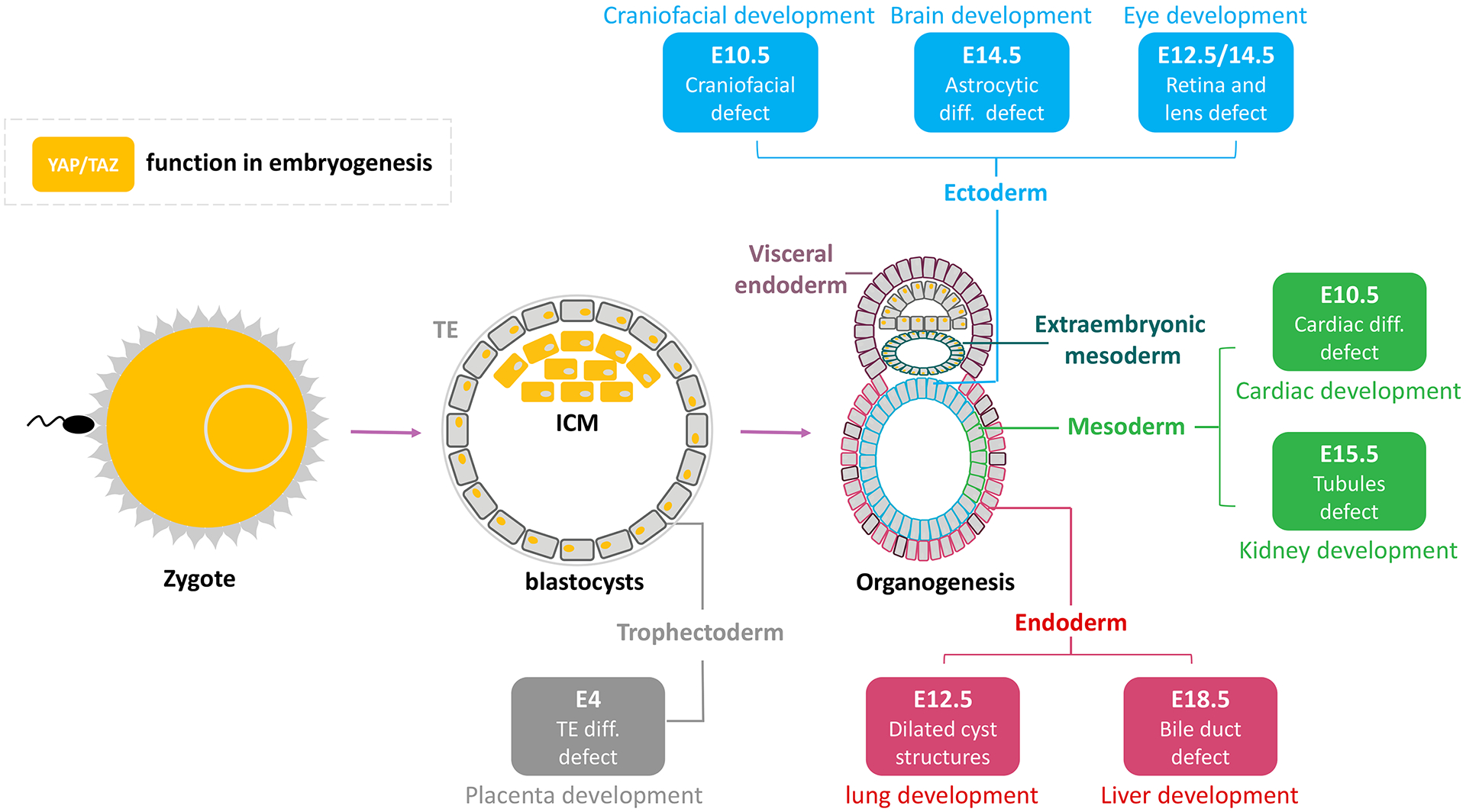
Functions of Hippo pathway in embryogenesis and development. This diagram shows the YAP/TAZ subcellular distributions and their functions at different embryonic stages. In zygotes, YAP/TAZ is critical in preventing the premature expression of SOX2 and abnormal ICM differentiation. In morula and blastocyst, cells are specialized depending on Hippo pathway activity in response to their position and polarity. The exterior cells with high YAP/TAZ activity develop into trophoblasts while the inner cells with low YAP/TAZ activity develop into ICM. YAP/TAZ is required for organogenesis in all three germ layers. The color of cell outlines and text boxes represent certain germ layers (gray, blue, green, and red for trophectoderm, ectoderm, mesoderm, and endoderm, respectively). The text in colored background denotes phenotypes of altered Hippo signaling at each indicated embryonic “E” stage.
During embryogenesis, the preimplantation stage refers to the time from fertilization to implantation. The preimplantation stage takes around 10 days in human and 4.5 days in mice. It begins with the formation of a zygote, which then undergoes several initial cell divisions to form a solid ball of cells called a morula . A morula undergoes additional cell division and morphogenesis to form a blastocyst . The preimplantation stage ends when the blastocyst implants in the uterus. Here, we will discuss the role of the Hippo pathway in zygote pluripotency, maternal-zygotic transition, and trophoblast differentiation.
Zygote and blastomere
A zygote is totipotent, meaning it has the ability to produce all the different cells types in an organism and the extra embryonic tissues. YAP/TAZ has been shown to have a role in totipotency. Zygotes and blastomeres without maternal YAP/TAZ perish before the blastocyst stage [53]. In the 4/8-cell blastomeres, YAP/TAZ inhibits the stem factor SOX2 (SRY-Box Transcription Factor 2) expression and the formation of inner cell mass (ICM), the cells of origin for all three germ layers of endoderm, ectoderm, and mesoderm [54]. Knockout of maternal YAP/TAZ results in the premature expression of the ICM marker SOX2 prior to the 16-cell stage, and eventually inhibits the expression of trophoblast factor CDX2 (Caudal Type Homeobox 2) [54]. This abnormal ICM differentiation and the inhibition of trophectoderm differentiation may explain the failure of blastocyst formation in zygotes and blastomeres without maternal YAP/TAZ.
Maternal-zygotic transition
After fertilization, in mammals, transcription of the newly formed embryo genome is inactivated. Development strictly depends on the maternal RNA and proteins in the ovum [55]. The start of embryonic transcription, called “zygotic genome activation (ZGA)”, occurs later during the preimplantation period, and in the meantime, zygotes begin to eliminate the contribution of maternal gene products by degrading the maternally-supplied mRNA. The event containing both ZGA and degradation of maternal products is called the “maternal to zygotic transition (MZT)”. After MZT, developmental process is controlled by zygote genomes [56].
The very early stage of development is controlled by maternally RNA and protein. Conventional YAP knockout mice (YAP−/−) from YAP−/+ parents are unable to achieve true YAP inactivation in early embryos, as the mother will produce oocytes with YAP protein. Oocyte-specific knockout can solve this problem and produce early embryos devoid of YAP. Maternal YAP has been found to play an important role in MZT. When fertilized by wild-type spermatozoa, the resulting maternal Yap1-knockout embryos (YAP♀−/♂+) display a prolonged two-cell stage and develop into the four-cell stage at a much slower rate when compared with wild-type maternal controls (YAP♀+/♂+) [57]. Transcriptomes of four-cell embryos derived from YAP♀−/♂+ and YAP♀+/♂+ show significant differences in thousands of genes. Many maternal transcripts are upregulated in YAP♀−/♂+ compared with YAP♀+/♂+, implying the evasion of maternal mRNA degradation. Meanwhile, early zygotic genes targeted by YAP/TAZ are downregulated, including Rpl13 and Rrm2, suggesting the failure of zygote genes activation in the YAP♀−/♂+ embryo [57]. These data suggest a critical role of YAP in MZT.
TE specification
In early embryogenesis, blastocysts are composed of the outer epithelial trophectoderm (TE) and the inner cell mass (ICM) (Fig. 3). TE cells form extraembryonic tissues including placenta, supporting the embryo proper. ICM further gives rise to primitive endoderm and epiblast. Although historical studies established the blastocyst atlas in the 20th century, the mechanism of fate decision for the initial embryonic totipotent cells to develop into the TE or ICM lineage have not been available until 2000s [58].
Hippo signaling activity, which is dependent on cell polarity, is a critical step for this first cell fate specification (Fig. 3). In the outer polar cells, Hippo signaling is suppressed: dephosphorylated YAP/TAZ translocate into nucleus, interact with TEAD, and directly induce TE specific transcriptional factors CDX2 and GATA3 (GATA Binding Protein 3), which are required to repress pluripotent genes and promote differentiation into TE [53, 59–61]. Perhaps, cell polarity and less cell contact play a role in Hippo inactivation in the out-layer cells during this very first cell specification in mammalian embryonic development. But in the inner apolar cells, Hippo pathway is active: LATS1/2 phosphorylates and promotes YAP/TAZ in the cytoplasm, thus preventing YAP/TAZ to induce target genes. The requirement of differential Hippo pathway activity for TE and ICM patterning is transient [62].
Consistently, loss of LATS1/2 results in a developmental bias toward the TE-like lineage [53, 62]. In contrast, TEAD4 knockout embryos show severe downregulation of CDX2 expression and aberrant high expression of ICM specific transcription factors like POU5F1 (POU Class 5 Homeobox 1) and Nanog in all blastomeres [59]. Besides Hippo core components, NF2 (Merlin) is also involved in establishing TE fate. Nuclear YAP localization and CDX2 expression can be observed in both outer and inner cells when NF2 is mutated [63]. Collectively, the Hippo pathway plays a major role in controlling TE differentiation.
In addition to CDX2, Hippo signaling also affects the expression of SOX2, which is a key transcription factor in ICM. Enforced cytoplasmic YAP in outer cells is sufficient to induce aberrant SOX2 expression [64]. A recent study has shown that YAP/TAZ-TEAD4 directly suppresses the SOX2 gene expression [54].
ICM specification
In the ICM, the activation of LATS kinases induces the cytoplasmic translocation of YAP/TAZ, thus relieving the transcriptional repression of SOX2 and promoting the differentiation of ICM [54]. Before the blastocyst is implanted, the ICM will further differentiate into epiblasts and hypoblasts, the former will develop into the three germ layers, and the latter will become the yolk sac. Epiblasts need to form an entire individual from a small number of cells, thus they are highly pluripotent. YAP-TEAD plays an important role in the formation of epiblasts: TEAD can activate expression of pluripotent genes and MYC simultaneously [65]. Cells that fail to activate YAP-TEAD signal will be eliminated by cell competition, which ensures the formation of uniform epiblasts with naive pluripotency [65].
Based on their ability to maintain pluripotent state upon MEK inhibition, embryonic stem cells can be grouped into naïve pluripotent or primed pluripotent ESc [66]. Usually, mouse embryonic stem cells are naïve pluripotent, similar to the pre-implantation epiblasts that have higher growth rate, easy for clonal expansion and invitro culture, whereas human embryonic stem cells are in a primed pluripotent state similar to the post-implantation epiblasts [66].
Some studies report that YAP/TAZ are required for mouse ESC self-renewal and differentiation in vitro [67]. YAP reduction leads to the loss of pluripotency in embryonic stem cell. Knockout of MST1/2 causes resistance to differentiation induced by LIF withdrawal [68]. Mechanistically, YAP/TAZ bind to TEAD and activate the transcription of POU5F1 and Nanog, which are the key transcriptional factors in ESC. YES1, a LIF stimulated Src family tyrosine kinase, can activate YAP via tyrosine phosphorylation [69]. But some other groups report that YAP is essential for differentiation [43, 70]. Cells lacking YAP/TAZ maintain aberrant pluripotency and show impaired induction of lineage specific genes during differentiation, as loss of YAP/TAZ compensates for lack of Wnt signaling and opposes ESC differentiation [43]. Conversely, overexpression of YAP in ES cells disrupts self-renewal and triggers differentiation by up-regulating lineage markers [70]. Furthermore, bioinformatics analyses have indicated that YAP-TEAD is not involved in the transcription factor network of naïve pluripotency [71, 72]. Further studies are needed to elucidate the precise functions of YAP/TAZ in ESC.
In human embryonic stem cells, besides interacting with the TEAD family, YAP/TAZ can also form a regulatory complex with SMAD2/3 and POU5F1, cooperating with the NuRD repressor complex to buffer pluripotency gene expression while suppressing differentiation genes [73]. A mechanism for YAP/TAZ activation in ESC/IPSc has been proposed. AKAP13 (A-Kinase Anchoring Protein 13), a Rho GEF highly expressed in ESC/IPSc, stimulates YAP/TAZ activity by modulating cytoskeleton, thereby maintaining the survival and pluripotency of ESCs [74]. A common regulator of YAP in mouse and human ESCs is RASSF1A (Ras Association Domain Family Member 1). When ESC cells differentiate, RASSF1A is induced and functions to prevent YAP from binding to TEAD, and thus abolishing POU5F1 transcription [75].
Finally, it is worth mentioning that although ESc/IPSc cells are considered to be pluripotent rather than totipotent, extra-embryonic cell differentiation protocols from ESc/IPSc are emerging [53, 76–79]. The potential of totipotency in ESc/IPSc is likely to be underestimated. In conventional in vivo chimera experiments, ES cells are often injected into the center of the morula/blastocyst. But it has been shown that the location and polarity of cells in the morula/blastocyst is critical for the embryonic/extra-embryonic differentiation. The exterior cells develop into trophoblasts while the inner cells develop into ICM. Therefore, the results of these chimera experiments should be interpreted carefully.
Hippo pathway in gastrulation and neurulation stage
Gastrulation and neurulation stages follow the preimplantation stage and are characterized by the formation of a multilayered structure known as the gastrula, as well as the folding process from the neural plate into the neural tube. It takes place around the 17th day after fertilization and ends at day 26 in human, and from mouse embryonic day 6 (E6) to E9 in mice.
Even though YAP plays an important role during preimplantation, conventional YAP−/− embryos from YAP+/− parents don’t show obvious developmental defects until E8.5 in mice [3]. In contrast, knockout of maternal YAP or YAP/TAZ causes a failure of blastocyst formation [54, 57]. This suggests that maternal YAP in oocytes from YAP+/− mothers is sufficient to support zygotes through development events like MZT. In addition, conventional YAP/TAZ knockout embryos from YAP+/−/TAZ+/− parents die before morula stage, which implies the expression of zygotic TAZ compensates the effects of YAP in conventional YAP−/− embryos [53]. Mouse embryos lacking YAP arrest development around E8.5, display defects in yolk sac vascular development, chorioallantoic fusion, and embryonic axis elongation [3]. Histologic analysis shows these defects are not due to problems with tissue specification, but rather, due to the requirement of YAP in morphogenesis and proliferation. These studies indicate that YAP has unique functions in vasculature which can’t be compensated by TAZ.
Vascularization begins in yolk sac and placenta. Loss of YAP/TAZ in mice endothelial cells (EC) leads to embryonic lethality, supporting a critical role of YAP/TAZ in vascularization [80–82]. YAP/TAZCDH5−CreERT2 mice show impaired vasculature [82]. VEGF-VEGFR signaling, the primary factor for vascularization, activates YAP/TAZ in endothelial cells by regulating Src family kinases, Rho GTPase, cytoskeleton, and Lats1/2 activity. This mechanism is critical in EC migration and angiogenesis mediated by VEGF[82–85]. YAP/TAZ is also responsible for some trafficking proteins induced by VEGF, participating in a positive feedback loop that help VEGFR2 translocate from the Golgi to the plasma membrane [82, 86].
Organogenesis stage
Organogenesis begins from three to eight weeks in humans and around E9.5 in mice. Organ development continues until birth in humans, with the exception of some organs like the mammary which occurs after birth. In this section, we will review the importance of the Hippo pathway in organogenesis in chronological order (see also Figure 3). Generally, the Hippo pathway plays an important role in cell survival, cell migration, and 3D structure formation.
Cardiac development
Accumulating data show that the Hippo pathway is closely involved in cardiac development. Loss of Hippo pathway components SAV1 early in development using SAV1Nkx2.5−Cre mice leads to substantial cardiomegaly [87]. Similar phenotypes can be observed in MST1/2Nkx2.5−Cre and LATS2Nkx2.5−Cre mice [87]. The change of myocardium thickness and heart size is due to over proliferation of cardiomyocytes rather than hypertrophy [87]. Consistently, loss of YAP in early development(YAPNkx2.5−Cre) or cardiac muscle (YAPTnnt2−Cre) results in severe myocardium hypoplasia and embryonic lethality [88, 89]. Despite the change of heart size, ectopic apoptosis is not observed. Instead, cardiomyocyte proliferation is severely reduced [88]. The dramatic myocardial overgrowth and cardiomegaly in embryos of active YAP conditional transgenic mice further support the function of Hippo pathway in regulating cardiomyocyte proliferation [88–90].
Craniofacial development
At E10.5 YAP/TAZ deletion in neural crest (YAP/TAZWnt1−Cre) causes overt craniofacial defects. Hemorrhages in the branchial arch regions are also observed. Embryos undergo fetal demise prior to E11.5. Histologic analysis shows that maxillary and mandibular branchial arches, which neural crest normally migrate and develop into, are deficient [91]. Mechanistically, PAX3, a critical transcription factor in pre-migratory neural crest, binds to YAP/TAZ and loss of YAP/TAZ leads to the downregulation of neural crest specific genes such as Mitf [91]. Furthermore, YAP is also required for smooth muscle differentiation of neural crest by coordinating with Notch signaling. YAP can interact with the NICD, recruited to the enhancer of the Notch ligand Jagged by the DNA binding protein Rbp-J independent of TEAD, thus to promote further Notch signaling and smooth muscle differentiation [92].
Lung development
From E12.5 to E15.5, YAP is reported to regulate proximal-distal patterning in lung development [93]. Proximal cells express YAP in cytoplasm while distal counterparts express YAP in the nucleus [93]. Lungs without YAP expression (YAPShh−Cre) are highly hypoplastic and show severe disruption in branching morphogenesis, resulting in dilated cyst-like structures, where distal cells expanded at the cost of the proximal progenitors which normally develop into airways [93]. TGF-β plays an essential role in lung development. YAP activity is required for epithelial progenitor cells to properly respond to TGF-β cues and initiate the differentiation program [93], in which YAP promotes Sox 2 expression to specify the airway epithelial differentiation transcriptome. Besides the Hippo pathway, many other signaling pathways are involved in the patterning of lung. In in vitro differentiation protocols, modulation of WNT signaling is sufficient to give rise to organoids with proximal and distal traits, suggesting that the Hippo pathway is likely a regulator of morphogenesis, rather than the lineage specification determinant [94].
Eye development
The retina is originated from the two-layered optic cup (OC) in the embryo. The outer layer develops into retinal pigment epithelium (RPE), which supports the neural inner cells called neural retina (NR) [95, 96]. The requirement of YAP activity during eye organogenesis was first found in zebrafish, where knockdown of YAP results in reduced eye size [97]. Mice harboring conditional retina knockout of YAP (YAPrx−Cre) display hypopigmentation and protrusion of retina-like epithelia and the overall morphology of eyes is severely impaired [98]. Histologic analysis in E12.5 shows pigmentation loss and organization change, suggesting a trans-differentiation of RPE to NR [98]. Furthermore, progressive degeneration is also observed in NR as YAP maintains NR polarity via crumbs polarity complex [98]. Notably, mutation in TEAD1 that cannot bind YAP causes Sveinsson chorioretinal atrophy in human, supporting a conserved role of YAP in retinal development.
At E14.5, deletion of YAP (YAPnestin−Cre) in the lens leads to severe atrophy due to lens fiber defect (LF) and the hypocellularity in the lens epithelium (LE). Anatomically, the lens is composed of LE and LF cells. LE cells are progenitor cells and LF cells are fully differentiated cells that constitute the majority of a lens [99, 100]. YAP cooperates with tight junction and polarity complex proteins to maintain self-renewal of LE cells. YAP-null LE cells exit from cell-cycle and differentiate to LF cells [101].
Brain development
In E14.5, YAP plays a critical role in neocortical astrocytic differentiation and proliferation. In developmental neural system, YAP is selectively expressed in neural stem cells (NSCs) and astrocytes while it is repressed in neurons. YAP astrocyte conditional knockout mice (YAPGFAP−Cre) display less neocortical astrocytes, whereas YAP-deficient NSCs (YAPnestin−Cre) shows normal self-renewal and neural differentiation ability. Mechanically, YAP is involved in the stabilization of SMAD1 induced by BMP2, to promote astrocytic specification [102, 103].
Kidney development
At E15.5, YAP/TAZ also play important roles in kidney development. Knockout of YAP(YAPsix2−Cre) in cap mesenchyme, which develops into nephron, leads to dramatic reductions in Henle’s loop, glomeruli and proximal tubules formation [104]. Conventional TAZ knockout mice are viable, but display renal cysts with dilatation of bowman’s capsules and proximal tubules [105]. These results suggest that YAP and TAZ play distinct roles during kidney development. Kidneys without YAP display early defects in nephron induction and the stereotypical morphogenesis. CDC42 is an activator of YAP/TAZ during kidney development, CDC42 knock mouse kidneys show phenotypes similar to YAP knockout [104].
Bile duct development
YAP is required for bile duct development through E18.5. Hepatoblasts are progenitors for both hepatocytes and intrahepatic biliary epithelial cells (BEC) in embryonic liver [106]. Conditional YAP knockout (YAPAlbumin−Cre) in biliary cells leads to the loss of tubular and non-tubular structures formed by WT BECs [107]. Knockout of NF2 (NF2Albumin−Cre) or LATS1/2 (LATS1/2Albumin−Cre) in the liver leads to a bias towards BECs at the expense of hepatocytes [107–109]. Furthermore, an in vitro differentiation assay shows that hepatocyte differentiation is compromised when LATS activity is abolished. Consistently, RNA-seq analysis shows an enrichment of BEC differentiation gene signature [110]. In an in vitro differentiation protocol, activation of TGF-β and NOTCH signaling is sufficient to transform hepatoblasts into BECs. Thus, the crosstalk between Hippo signaling and TGF-β/NOTCH signaling is possibly responsible for the differentiation bias towards BECs in the absence of LATS kinases [111].
Concluding remarks
Extensive genetic studies in the last decade, done primarily in mouse models, have revealed fundamentally important roles of the Hippo pathway and its effectors YAP/TAZ in mammalian development. YAP/TAZ have a broad function in early development in most, if not all, tissues and organs. These functions of YAP/TAZ in development are not simply due to their role in tissue size (cell number) control as YAP/TAZ can promote either lineage specific differentiation or stem cell maintenance/self-renewal in a context dependent manner. Another general conclusion is that YAP/TAZ often collaborate with other morphogens/development cues to control development. Moreover, in addition to TEAD, YAP/TAZ can interact with other DNA binding factors to regulate developmental programs. Although not discussed in this review, it should be noted that the Hippo pathway also plays a key role in wound healing and tissue homeostasis in adults.
Despite the tremendous progress made regarding the function of Hippo in early development, many key questions remain (see Outstanding Questions). Given the significant functional overlap between YAP and TAZ, many genetic studies with a single deletion of YAP or TAZ may not necessarily uncover the true function of the Hippo pathway. Moreover, due to the essential role in TE differentiation, the function of Hippo pathway in germ layer specification is difficult to study. Techniques like cell type specific inducible knockout in early embryos may be helpful to address these fundamental questions. The knowledge gained on the Hippo pathway in development may be translated for future tissue and organ engineering in vitro.
Outstanding Questions.
Are YAP and TAZ activity required for maintaining zygote totipotency in vivo?
Do YAP and TAZ play redundant roles in embryogenesis in addition to tissue specificity?
Is Hippo signaling essential in controlling germ layer specification?
What are the upstream cues regulating YAP/TAZ activity during each organ development and size control?
Is manipulating the Hippo pathway activity enough to induce certain tissue/cell development?
How can Hippo pathway activate different target gene transcription in different tissues during embryogenesis?
Highlights.
The evolutionary conserved Hippo pathway functions to limit tissue and organ growth, and its deregulation contributes to human cancer.
Hippo pathway cross talks with numerous developmental signaling pathways.
Hippo signaling plays critical roles in early embryonic development as low Hippo activity is required for trophoblast differentiation and high Hippo activity permits inner cell mass formation.
Hippo pathway positively or negatively regulates development of multiple tissues/organs.
Acknowledgments
This work was supported in part by the National Institutes of Health grant (CA217642).
glossary
- Adherens junctions (AJs)
The connection structure between neighboring cells in epithelial or endothelial tissues. They are formed by adherens molecules and connect to intra cellular actin filaments or intermediate filaments.
- Blastocyst
A structure formed at the end of cleavage stage of mammals. It is composed of an inner cell mass (ICM), which subsequently forms the embryo, and an outer layer called trophoblasts.
- Blastomere
Totipotent cells produced by the cleavage of the zygote. This term mainly refers the 2/4/8 - cells embryos.
- Inner cell mass (ICM)
Cells lies inside the blastocyst which will develop into the fetus. ICM is surrounded by trophoblasts which are the outer layers of the blastocyst.
- Morula
A 16-cell embryo formed by cell division of a zygote in early stage of animal embryo development.
- NuRD repressor complex
A repressive transcription complex with both ATP-dependent chromatin remodeling and histone deacetylase activities. Mi2 and HDAC1/2 are the two core subunits.
- Palmitoylation
A covalent modification of amino acid to cysteine (most common), serine, or threonine residues of proteins. Palmitoylation generally serves as a membrane attachment signal. However, TEAD palmitoylation does not serve as a membrane targeting, but rather is important for TEAD function.
- Rho family GTPases
a group of small (~21 kDa) GTP binding proteins with GTPase activity and belong to the Ras superfamily. Rho GTPase family members play major roles in actin dynamics, cell morphology, and migration.
- SMAD
A group of structurally related proteins that are the main downstream effectors of the TGF-B receptor superfamily. There are three SMAD sub-types: receptor-regulated SMAD (R-SMAD), common partner SMAD (Co-SMAD), and inhibitory SMAD (I-SMAD).
- Striatin-interacting phosphatase and kinase (STRIPAK)
Protein complexes containing both kinases and phosphatases. In these complexes, striatin serves as a key scaffold protein to tether both kinases and PP2A phosphatase. STRIPAK complexes are evolutionarily conserved and have critical roles in protein (de)phosphorylation, particularly in dephosphorylation and inactivation of the STE20 family kinases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
K.-L.G. is a co-founder and has equity interest in Vivace Therapeutics. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies.
References
- 1.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila Tumor-Suppressor Gene Warts Encodes a Homolog of Human Myotonic-Dystrophy Kinase and Is Required for the Control of Cell-Shape and Proliferation. Gene Dev. 1995;9(5):534–46. doi: DOI 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 2.Xu TA, Wang WY, Zhang S, Stewart RA, Yu W. Identifying Tumor Suppressors in Genetic Mosaics - the Drosophila Lats Gene Encodes a Putative Protein-Kinase. Development. 1995;121(4):1053–63. [DOI] [PubMed] [Google Scholar]
- 3.Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Molecular and Cellular Biology. 2006;26(1):77–87. doi: 10.1128/Mcb.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells (vol 17, pg 2054, 2007). Current Biology. 2007;17(23):2094-. doi: 10.1016/j.cub.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Ma SH, Meng ZP, Chen R, Guan KL. The Hippo Pathway: Biology and Pathophysiology. Annu Rev Biochem. 2019;88:577–604. doi: 10.1146/annurev-biochem-013118-111829. [DOI] [PubMed] [Google Scholar]
- 6.Calses PC, Crawford JJ, Lill JR, Dey A. Hippo Pathway in Cancer: Aberrant Regulation and Therapeutic Opportunities. Trends Cancer. 2019;5(5):297–307. doi: 10.1016/j.trecan.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Manmadhan S, Ehmer U. Hippo Signaling in the Liver - A Long and Ever-Expanding Story. Front Cell Dev Biol. 2019;7:33. doi: 10.3389/fcell.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R, Xie R, Meng Z, Ma S, Guan KL. STRIPAK integrates upstream signals to initiate the Hippo kinase cascade. Nat Cell Biol. 2019;21(12):1565–77. doi: 10.1038/s41556-019-0426-y. [DOI] [PubMed] [Google Scholar]
- 9.Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell. 2011;21(5):888–95. doi: 10.1016/j.devcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poon CL, Lin JI, Zhang X, Harvey KF. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev Cell. 2011;21(5):896–906. doi: 10.1016/j.devcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273(18):4264–76. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- 12.Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18(5):311–21. doi: 10.1016/j.cub.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hergovich A, Schmitz D, Hemmings BA. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem Bioph Res Co. 2006;345(1):50–8. doi: 10.1016/j.bbrc.2006.03.244. [DOI] [PubMed] [Google Scholar]
- 14.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. Identification of Happyhour/MAP4K as alternative Hpo/Mst-like kinases in the Hippo kinase cascade. Developmental cell. 2015;34(6):642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors merlin and expanded function cooperatively to modulate receptor endocytosis and signaling. Current Biology. 2006;16(7):702–9. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 17.Yin F, Yu JZ, Zheng YG, Chen Q, Zhang NL, Pan DJ. Spatial Organization of Hippo Signaling at the Plasma Membrane Mediated by the Tumor Suppressor Merlin/NF2. Cell. 2013;154(6):1342–55. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Gene Dev. 2007;21(21):2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, et al. The Hippo Tumor Pathway Promotes TAZ Degradation by Phosphorylating a Phosphodegron and Recruiting the SCF beta-TrCP E3 Ligase. Journal of Biological Chemistry. 2010;285(48):37159–69. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF beta-TRCP. Gene Dev. 2010;24(1):72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao B, Ye X, Yu JD, Li L, Li WQ, Li SM, et al. TEAD mediates YAP-dependent gene induction and growth control. Gene Dev. 2008;22(14):1962–71. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noland CL, Gierke S, Schnier PD, Murray J, Sandoval WN, Sagolla M, et al. Palmitoylation of TEAD Transcription Factors Is Required for Their Stability and Function in Hippo Pathway Signaling. Structure. 2016;24(1):179–86. doi: 10.1016/j.str.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Koontz LM, Liu-Chittenden Y, Yin F, Zheng YG, Yu JZ, Huang B, et al. The Hippo Effector Yorkie Controls Normal Tissue Growth by Antagonizing Scalloped-Mediated Default Repression. Developmental Cell. 2013;25(4):388–401. doi: 10.1016/j.devcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohno S Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13(5):641–8. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- 25.Hirate Y, Hirahara S, Inoue K, Suzuki A, Alarcon VB, Akimoto K, et al. Polarity-Dependent Distribution of Angiomotin Localizes Hippo Signaling in Preimplantation Embryos. Current Biology. 2013;23(13):1181–94. doi: 10.1016/j.cub.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei QY, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Gene Dev. 2011;25(1):51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YJ, Zhou H, Li FZ, Chan SW, Lin ZJ, Wei ZY, et al. Angiomotin binding-induced activation of Merlin/NF2 in the Hippo pathway. Cell Res. 2015;25(7):801–17. doi: 10.1038/cr.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirate Y, Sasaki H. The role of angiomotin phosphorylation in the Hippo pathway during preimplantation mouse development. Tissue Barriers. 2014;2(1). doi: ARTN e28127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540(7634):579–82. doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- 31.Elbediwy A, Vincent-Mistiaen ZI, Spencer-Dene B, Stone RK, Boeing S, Wculek SK, et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143(10):1674–87. doi: 10.1242/dev.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, et al. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell. 2013;154(5):1047–59. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 33.Zhao B, Li L, Wang L, Wang CY, Yu JD, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Gene Dev. 2012;26(1):54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, et al. Regulation of the Hippo-YAP Pathway by G-Protein-Coupled Receptor Signaling. Cell. 2012;150(4):780–91. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller E, Yang JY, DeRan M, Wu CL, Su AI, Bonamy GMC, et al. Identification of Serum-Derived Sphingosine-1-Phosphate as a Small Molecule Regulator of YAP. Chem Biol. 2012;19(8):955–62. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Yu FX, Luo J, Mo JS, Liu GB, Kim YC, Meng ZP, et al. Mutant Gq/11 Promote Uveal Melanoma Tumorigenesis by Activating YAP. Cancer Cell. 2014;25(6):822–30. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu G, Yu FX, Kim YC, Meng Z, Naipauer J, Looney DJ, et al. Kaposi sarcoma-associated herpesvirus promotes tumorigenesis by modulating the Hippo pathway. Oncogene. 2015;34(27):3536–46. doi: 10.1038/onc.2014.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou X, Wang SY, Wang Z, Feng X, Liu P, Lv XB, et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest. 2015;125(5):2123–35. doi: 10.1172/Jci79573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes Dev. 2012;26(19):2138–43. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nusse R, Clevers H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169(6):985–99. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Kim W, Kim M, Jho EH. Wnt/beta-catenin signalling: from plasma membrane to nucleus. Biochem J. 2013;450(1):9–21. doi: 10.1042/BJ20121284. [DOI] [PubMed] [Google Scholar]
- 42.Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, et al. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151(7):1443–56. [DOI] [PubMed] [Google Scholar]
- 43.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157–70. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Cai J, Maitra A, Anders RA, Taketo MM, Pan D. beta-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29(14):1493–506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 46.Struhl G, Fitzgerald K, Greenwald I. Intrinsic Activity of the Lin-12 and Notch Intracellular Domains in-Vivo. Cell. 1993;74(2):331–45. [DOI] [PubMed] [Google Scholar]
- 47.Bray SJ. Notch signalling in context. Nat Rev Mol Cell Bio. 2016;17(11):722–35. [DOI] [PubMed] [Google Scholar]
- 48.Kim W, Khan SK, Gvozdenovic-Jeremic J, Kim Y, Dahlman J, Kim H, et al. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. The Journal of clinical investigation. 2017;127(1):137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, et al. Yes-Associated Protein Up-regulates Jagged-1 and Activates the NOTCH Pathway in Human Hepatocellular Carcinoma. Gastroenterology. 2013;144(7):1530–U368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massague J TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10(7):837–48. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 52.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19(6):831–44. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, et al. The Hippo Signaling Pathway Components Lats and Yap Pattern Tead4 Activity to Distinguish Mouse Trophectoderm from Inner Cell Mass. Developmental Cell. 2009;16(3):398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Frum T, Watts JL, Ralston A. TEAD4, YAP1 and WWTR1 prevent the premature onset of pluripotency prior to the 16-cell stage. Development. 2019;146(17). doi: 10.1242/dev.179861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schier AF. The maternal-zygotic transition: Death and birth of RNAs. Science. 2007;316(5823):406–7. doi: 10.1126/science.1140693. [DOI] [PubMed] [Google Scholar]
- 56.Tadros W, Lipshitz HD. The maternal-to-zygotic transition: a play in two acts. Development. 2009;136(18):3033–42. doi: 10.1242/dev.033183. [DOI] [PubMed] [Google Scholar]
- 57.Yu C, Ji SY, Dang YJ, Sha QQ, Yuan YF, Zhou JJ, et al. Oocyte-expressed yes-associated protein is a key activator of the early zygotic genome in mouse. Cell Res. 2016;26(3):275–87. doi: 10.1038/cr.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sasaki H Mechanisms of trophectoderm fate specification in preimplantation mouse development. Dev Growth Differ. 2010;52(3):263–73. doi: 10.1111/j.1440-169X.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 59.Nishioka N, Yamamoto S, Kiyonari H, Sato H, Sawada A, Ota M, et al. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mechanisms of Development. 2008;125(3–4):270–83. doi: 10.1016/j.mod.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Ralston A, Cox BJ, Nishioka N, Sasaki H, Chea E, Rugg-Gunn P, et al. Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development. 2010;137(3):395–403. doi: 10.1242/dev.038828. [DOI] [PubMed] [Google Scholar]
- 61.Anani S, Bhat S, Honma-Yamanaka N, Krawchuk D, Yamanaka Y. Initiation of Hippo signaling is linked to polarity rather than to cell position in the pre-implantation mouse embryo. Development. 2014;141(14):2813–24. doi: 10.1242/dev.107276. [DOI] [PubMed] [Google Scholar]
- 62.Lorthongpanich C, Messerschmidt DM, Chan SW, Hong WJ, Knowles BB, Solter D. Temporal reduction of LATS kinases in the early preimplantation embryo prevents ICM lineage differentiation. Gene Dev. 2013;27(13):1441–6. doi: 10.1101/gad.219618.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cockburn K, Biechele S, Garner J, Rossant J. The Hippo Pathway Member Nf2 Is Required for Inner Cell Mass Specification. Current Biology. 2013;23(13):1195–201. doi: 10.1016/j.cub.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 64.Wicklow E, Blij S, Frum T, Hirate Y, Lang RA, Sasaki H, et al. HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst. Plos Genetics. 2014;10(10). doi: ARTN e1004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hashimoto M, Sasaki H. Epiblast Formation by TEAD-YAP-Dependent Expression of Pluripotency Factors and Competitive Elimination of Unspecified Cells. Dev Cell. 2019;50(2):139–54 e5. doi: 10.1016/j.devcel.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 66.Weinberger L, Ayyash M, Novershtern N, Hanna JH. Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat Rev Mol Cell Biol. 2016;17(3):155–69. doi: 10.1038/nrm.2015.28. [DOI] [PubMed] [Google Scholar]
- 67.Lian I, Kim J, Okazawa H, Zhao JG, Zhao B, Yu JD, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Gene Dev. 2010;24(11):1106–18. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li P, Chen Y, Mak KK, Wong CK, Wang CC, Yuan P. Functional role of Mst1/Mst2 in embryonic stem cell differentiation. PLoS One. 2013;8(11):e79867. doi: 10.1371/journal.pone.0079867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tamm C, Bower N, Anneren C. Regulation of mouse embryonic stem cell self-renewal by a Yes-YAP-TEAD2 signaling pathway downstream of LIF. J Cell Sci. 2011;124(7):1136–44. doi: 10.1242/jcs.075796. [DOI] [PubMed] [Google Scholar]
- 70.Chung H, Lee BK, Uprety N, Shen W, Lee J, Kim J. Yap1 is dispensable for self-renewal but required for proper differentiation of mouse embryonic stem (ES) cells. EMBO Rep. 2016;17(4):519–29. doi: 10.15252/embr.201540933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niwa H The principles that govern transcription factor network functions in stem cells. Development. 2018;145(6). doi: UNSP dev157420. [DOI] [PubMed] [Google Scholar]
- 72.Dunn SJ, Martello G, Yordanov B, Emmott S, Smith AG. Defining an essential transcription factor program for naive pluripotency. Science. 2014;344(6188):1156–60. doi: 10.1126/science.1248882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beyer TA, Weiss A, Khomchuk Y, Huang K, Ogunjimi AA, Varelas X, et al. Switch Enhancers Interpret TGF-beta and Hippo Signaling to Control Cell Fate in Human Embryonic Stem Cells. Cell Reports. 2013;5(6):1611–24. doi: 10.1016/j.celrep.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 74.Ohgushi M, Minaguchi M, Sasai Y. Rho-Signaling-Directed YAP/TAZ Activity Underlies the Long-Term Survival and Expansion of Human Embryonic Stem Cells. Cell Stem Cell. 2015;17(4):448–61. doi: 10.1016/j.stem.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Papaspyropoulos A, Bradley L, Thapa A, Leung CY, Toskas K, Koennig D, et al. RASSF1A uncouples Wnt from Hippo signalling and promotes YAP mediated differentiation via p73. Nature Communications. 2018;9 doi: ARTN 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang JL, Anguera MC. In Vitro Differentiation of Human Pluripotent Stem Cells into Trophoblastic Cells. Jove-J Vis Exp. 2017;(121). doi: ARTN 55268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bernardo AS, Faial T, Gardner L, Niakan KK, Ortmann D, Senner CE, et al. BRACHYURY and CDX2 Mediate BMP-Induced Differentiation of Human and Mouse Pluripotent Stem Cells into Embryonic and Extraembryonic Lineages. Cell Stem Cell. 2011;9(2):144–55. doi: 10.1016/j.stem.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei S, Zou QJ, Lai SS, Zhang QJ, Li L, Yan QM, et al. Conversion of embryonic stem cells into extraembryonic lineages by CRISPR-mediated activators. Scientific Reports. 2016;6 doi: ARTN1964810.1038/srep19648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giakoumopoulos M, Golos TG. Embryonic stem cell-derived trophoblast differentiation: a comparative review of the biology, function, and signaling mechanisms. J Endocrinol. 2013;216(3):R33–R45. doi: 10.1530/Joe-12-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi HJ, Zhang H, Park H, Choi KS, Lee HW, Agrawal V, et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat Commun. 2015;6:6943. doi: 10.1038/ncomms7943. [DOI] [PubMed] [Google Scholar]
- 81.Kim J, Kim YH, Kim J, Park DY, Bae H, Lee DH, et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J Clin Invest. 2017;127(9):3447–67. doi: 10.1172/Jci93825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang XH, Valls AF, Schermann G, Shen Y, Moya IM, Castro L, et al. YAP/TAZ Orchestrate VEGF Signaling during Developmental Angiogenesis. Developmental Cell. 2017;42(5):462−+. doi: 10.1016/j.devcel.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 83.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438(7070):937–45. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 84.Chappell JC, Wiley DM, Bautch VL. Regulation of blood vessel sprouting. Seminars in Cell & Developmental Biology. 2011;22(9):1005–11. doi: 10.1016/j.semcdb.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Azad T, van Rensburg HJJ, Lightbody ED, Neveu B, Champagne A, Ghaffari A, et al. A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nature Communications. 2018;9 doi: ARTN106110.1038/s41467-018-03278-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elaimy AL, Mercurio AM. Convergence of VEGF and YAP/TAZ signaling: Implications for angiogenesis and cancer biology. Science Signaling. 2018;11(552). doi: ARTN eaau1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, et al. Hippo Pathway Inhibits Wnt Signaling to Restrain Cardiomyocyte Proliferation and Heart Size. Science. 2011;332(6028):458–61. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.von Gise A, Lin ZQ, Schlegelmilch K, Honor LB, Pan GM, Buck JN, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. P Natl Acad Sci USA. 2012;109(7):2394–9. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xin M, Kim Y, Sutherland LB, Qi XX, McAnally J, Schwartz RJ, et al. Regulation of Insulin-Like Growth Factor Signaling by Yap Governs Cardiomyocyte Proliferation and Embryonic Heart Size. Science Signaling. 2011;4(196). doi: ART Nra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xin M, Kim Y, Sutherland LB, Murakami M, Qi XX, McAnally J, et al. Hippo pathway effector Yap promotes cardiac regeneration. P Natl Acad Sci USA. 2013;110(34):13839–44. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manderfield LJ, Engleka KA, Aghajanian H, Gupta M, Yang S, Li L, et al. Pax3 and Hippo Signaling Coordinate Melanocyte Gene Expression in Neural Crest. Cell Reports. 2014;9(5):1885–95. doi: 10.1016/j.celrep.2014.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Manderfield LJ, Aghajanian H, Engleka KA, Lim LY, Liu FY, Jain R, et al. Hippo signaling is required for Notch-dependent smooth muscle differentiation of neural crest. Development. 2015;142(17):2962−+. doi: 10.1242/dev.125807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The Hippo Pathway Effector Yap Controls Patterning and Differentiation of Airway Epithelial Progenitors. Developmental Cell. 2014;30(2):137–50. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN. Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling. Cell Stem Cell. 2017;20(6):844−+. doi: 10.1016/j.stem.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Bi. 2001;17:255–96. doi: DOI 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- 96.Heavner W, Pevny L. Eye Development and Retinogenesis. Csh Perspect Biol. 2012;4(12). doi: ARTN a008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang Q, Liu D, Gong YB, Wang YX, Sun SN, Gui YH, et al. yap is required for the development of brain, eyes, and neural crest in zebrafish. Biochem Bioph Res Co. 2009;384(1):114–9. doi: 10.1016/j.bbrc.2009.04.070. [DOI] [PubMed] [Google Scholar]
- 98.Kim JY, Park R, Lee JHJ, Shin J, Nickas J, Kim S, et al. Yap is essential for retinal progenitor cell cycle progression and RPE cell fate acquisition in the developing mouse eye. Developmental Biology. 2016;419(2):336–47. doi: 10.1016/j.ydbio.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cvekl A, Duncan MK. Genetic and epigenetic mechanisms of gene regulation during lens development. Prog Retin Eye Res. 2007;26(6):555–97. doi: 10.1016/j.preteyeres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Menko AS. Lens epithelial cell differentiation. Experimental Eye Research. 2002;75(5):485–90. doi: 10.1006/exer.2002.2057. [DOI] [PubMed] [Google Scholar]
- 101.Song JY, Park R, Kim JY, Hughes L, Lu L, Kim S, et al. Dual function of Yap in the regulation of lens progenitor cells and cellular polarity. Developmental Biology. 2014;386(2):281–90. doi: 10.1016/j.ydbio.2013.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang ZH, Hu JX, Pan JX, Wang Y, Hu GQ, Zhou JL, et al. YAP stabilizes SMAD1 and promotes BMP2-induced neocortical astrocytic differentiation. Development. 2016;143(13):2398–409. doi: 10.1242/dev.130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang ZH, Sun D, Hu JX, Tang FL, Lee DH, Wang Y, et al. Neogenin Promotes BMP2 Activation of YAP and Smad1 and Enhances Astrocytic Differentiation in Developing Mouse Neocortex. Journal of Neuroscience. 2016;36(21):5833–49. doi: 10.1523/Jneurosci.4487-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim DS, et al. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013;9(3):e1003380. doi: 10.1371/journal.pgen.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol-Renal. 2008;294(3):F542–F53. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- 106.Zhao R, Duncan SA. Embryonic development of the liver. Hepatology. 2005;41(5):956–67. doi: 10.1002/hep.20691. [DOI] [PubMed] [Google Scholar]
- 107.Zhang NL, Bai HB, David KK, Dong JX, Zheng YG, Cai J, et al. The Merlin/NF2 Tumor Suppressor Functions through the YAP Oncoprotein to Regulate Tissue Homeostasis in Mammals. Developmental Cell. 2010;19(1):27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Q, Zhang NL, Xie R, Wang W, Cai J, Choi KS, et al. Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Gene Dev. 2015;29(12):1285–97. doi: 10.1101/gad.264234.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yi J, Lu L, Yanger K, Wang WQ, Sohn BH, Stanger BZ, et al. Large tumor suppressor homologs 1 and 2 regulate mouse liver progenitor cell proliferation and maturation through antagonism of the coactivators YAP and TAZ. Hepatology. 2016;64(5):1757–72. doi: 10.1002/hep.28768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee DH, Park JO, Kim TS, Kim SK, Kim TH, Kim MC, et al. LATS-YAP/TAZ controls lineage specification by regulating TGFbeta signaling and Hnf4alpha expression during liver development. Nat Commun. 2016;7:11961. doi: 10.1038/ncomms11961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Clotman F, Jacquemin P, Plumb-Rudewiez N, Pierreux CE, Van der Smissen P, Dietz HC, et al. Control of liver cell fate decision by a gradient of TGF beta signaling modulated by Onecut transcription factors. Genes Dev. 2005;19(16):1849–54. doi: 10.1101/gad.340305. [DOI] [PMC free article] [PubMed] [Google Scholar]


