Abstract
The association between physical activity and risk of abdominal aortic aneurysm has been inconsistent with some studies reporting a reduced risk while others have found no association. We conducted a systematic review and meta-analysis of prospective studies to quantify the association. PubMed and Embase databases were searched up to 3 October 2020. Prospective studies were included if they reported adjusted relative risk (RR) estimates and 95% confidence intervals (CIs) of abdominal aortic aneurysm associated with physical activity. Summary RRs (95% CIs) were estimated using a random effects model. Nine prospective studies (2073 cases, 409,732 participants) were included. The summary RR for high vs. low physical activity was 0.70 (95% CI: 0.56–0.87, I2 = 58%) and per 20 metabolic equivalent task (MET)-hours/week increase of activity was 0.84 (95% CI: 0.74–0.95, I2 = 59%, n = 6). Although the test for nonlinearity was not significant (p = 0.09) the association appeared to be stronger when increasing the physical activity level from 0 to around 20–25 MET-hours/week than at higher levels. The current meta-analysis suggest that higher physical activity may reduce the risk of abdominal aortic aneurysm, however, further studies are needed to clarify the dose–response relationship between different subtypes and intensities of activity and abdominal aortic aneurysm risk.
Subject terms: Epidemiology, Cardiology, Medical research, Risk factors
Introduction
Aortic aneurysms are dilatations of the aorta which when ruptured have an 80% overall mortality rate, with about one third dying before reaching hospital1 and 25–50% of cases undergoing surgery being fatal1–4. Some studies have shown improved survival rates in more recent years1. According to the Global Burden of Disease Study aortic aneurysms was estimated to account for 167,200 deaths and 3 million disability-adjusted life years in 20175,6. The vast majority of aortic aneurysms are abdominal aortic aneurysms, with only 3% originating in the thorax7. In Caucasian populations the prevalence of abdominal aortic aneurysms is 4.7%8–10, while it is 0.45% in Asian populations11. Established risk factors for abdominal aortic aneurysm include age12–14, male sex15, family history of abdominal aortic aneurysm16, low education15, hypertension13–15,17, elevated lipid levels16, coronary heart disease12,15, peripheral artery disease12, chronic obstructive pulmonary disease and smoking13,15,18, while diabetes or diabetes medications appears to be protective12–16,19.
Physical activity has been shown to reduce blood pressure in randomized controlled trials20 and has been associated with reduced risk of hypertension in cohort studies21–24, and elevated blood pressure is a strong risk factor for abdominal aortic aneurysms17. Although there is strong evidence that physical activity reduces the risk of other vascular disorders such as coronary heart disease25, stroke25, and heart failure26, studies on physical activity and risk of abdominal aortic aneurysm have shown mixed results13,14,27–33. Some studies have shown inverse associations between higher physical activity and risk of abdominal aortic aneurysm27,29–33, however, other studies found no association13,14,28. The equivocal nature of these findings might be explained by relatively low numbers of events in some studies as abdominal aortic aneurysm are far less common than other cardiovascular events such as coronary heart disease and stroke.
Given the high mortality rates among patients with ruptured aortic aneurysm2, primary prevention is of major importance to reduce the public health burden of abdominal aortic aneurysms. We therefore conducted a systematic review and meta-analysis of prospective studies on physical activity and the risk of abdominal aortic aneurysm to clarify whether there is an association as well as the strength and shape of the dose–response relationship between the two.
Results
Out of a total of 9520 records retrieved by the search, 9473 were excluded based on title or abstract, and of the 47 publications that were assessed in more detail 9 cohort studies (2073 cases of abdominal aortic aneurysm among 409,732 participants) were included in the meta-analysis (Fig. 1, Table 1)13,14,27–33. Five of the studies were from Europe and four studies were from the US. Three studies were identified from searches on other risk factors and abdominal aortic aneurysm27,28,31. Eight studies reported on physical activity and abdominal aortic aneurysm13,14,27,29–33 and one reported on aortic aneurysm overall28, and the latter study was still included in the overall analysis since most aortic aneurysms are abdominal aortic aneurysms (in a sensitivity analysis this study was excluded). Physical activity was assessed by self-reported (most studies) or interviewer-administered (one study) questionnaires, and two studies used validated questionnaires (Table 1). Three studies reported physical activity measures in METs14,31,32 two reported the duration of physical activity30,33, one reported physical activity on a scale from none to heavy27, one used a physical activity index28 and two simply compared no activity with any activity13,29. For three studies27,30,33 the physical activity measures were converted to MET-hours/week as described in the methods section.
Figure 1.
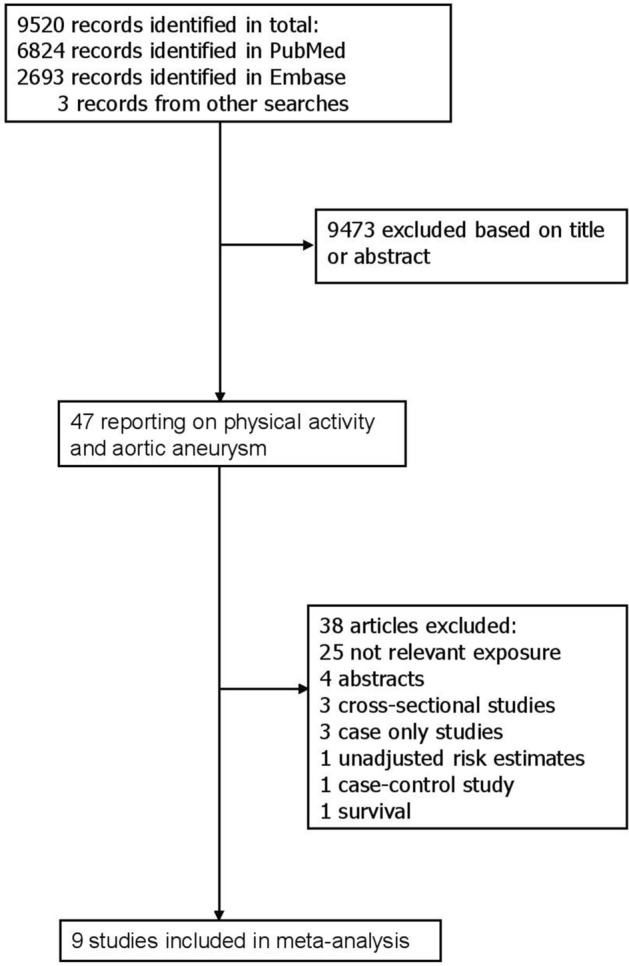
Flow-chart of study selection.
Table 1.
Prospective studies of physical activity and abdominal aortic aneurysm.
| References, country | Study name or description | Study period | Number of participants, number of cases | Identification of cases | Physical activity assessment | Type of activity, subgroup | Comparison | Relative risk (95% confidence interval) | Adjustment for confounders |
|---|---|---|---|---|---|---|---|---|---|
| Oyenuga et al.33, USA | The Atherosclerosis Risk in Communities (ARIC) study | 1987–1989–2011, 22.6 years follow-up | 14,375 men and women, age 45–64 years: 545 AAA cases | Self-report confirmed by hospitalization records and death records, linkage to hospital records | Interviewer administered Baecke questionnaire | Leisure-time physical activity | 0 min/wk |
1.00 0.75 (0.59–0.94) 0.78 (0.64–0.95) |
Age, sex, race |
| 1–149 | |||||||||
| ≥ 150 | |||||||||
| Hamer et al.32, United Kingdom | The Health Survey for England and the Scottish Health Surveys | 1994, 1995, 1997, 1998, 1999, 2003, 2004, 2006, 2008–2009/2011, 9.4 years follow-up | 65,093 men and women (59,122 without prevalent CVD), age ≥ 40 years: 113 (76) AAA deaths | British National Health Service Central Registry | Validated questionnaire | Leisure-time physical activity—meeting recommendations | Inactive | 1.00 | Age, sex, smoking, social occupational group, chronic illnesses, psychological distress |
| Insufficient | 0.51 (0.24–1.11) | ||||||||
| Sufficient | 0.41 (0.10–1.69) | ||||||||
| High | 0.77 (0.24–2.45) | ||||||||
| Leisure-time physical activity, all | < 1.64 MET-hrs/wk | 1.00 | |||||||
| 1.65–9.37 | 0.91 (0.55–1.52) | ||||||||
| 9.38–19.30 | 1.29 (0.75–2.20) | ||||||||
| 19.31–37.60 | 0.85 (0.45–1.61) | ||||||||
| > 37.60 | 0.88 (0.43–1.82) | ||||||||
| Leisure-time physical activity, excluding prevalent CVD at baseline | < 1.64 MET-hrs/wk | 1.00 | |||||||
| 1.65–9.37 | 0.69 (0.36–1.33) | ||||||||
| 9.38–19.30 | 1.00 (0.52–1.94) | ||||||||
| 19.31–37.60 | 0.78 (0.37–1.63) | ||||||||
| > 37.60 | 0.86 (0.37–1.96) | ||||||||
| Nordkvist et al.31, Sweden | Malmö Diet and Cancer Study | 1991–1996–NA, 20.7 years follow-up | 26,133 men and women, mean age 57.3 years: 353 AAA cases | Linkage to Inpatient and Outpatient Register, Cause of Death Register | Questionnaire | Leisure-time physical activity | 0–7.5 MET-hrs/wk | 1.00 | Age, sex |
| 7.5–15 | 0.72 (0.35–1.04) | ||||||||
| 15–25 | 0.50 (0.35–0.72) | ||||||||
| 25–50 | 0.54 (0.39–0.74) | ||||||||
| > 50 | 0.46 (0.31–0.68) | ||||||||
| Stackelberg et al.30, Sweden | Cohort of Swedish Men | 1998–2011, 13 years follow-up | 14,249 men, age 65–75 years: 156 AAA cases | Ultrasound screening | Validated questionnaire | Walking, bicycling | Almost never | 1.00 | Age, education, smoking status, pack-years, BMI, waist circumference, healthy diet score, alcohol, diabetes mellitus, cardiovascular disease, hypertension, hypercholesterolemia |
| < 20 min/day | 0.83 (0.53–1.32) | ||||||||
| 20–40 | 0.72 (0.45–1.16) | ||||||||
| ≥ 40 | 0.59 (0.36–0.97) | ||||||||
| Wong et al.14, USA | Health Professionals Follow-up Study | 1986–2002, ~ 14.6 years follow-up | 39,352 men, age 40–75 years: 376 AAA cases | Self-report confirmed by medical records, National Death Index | Questionnaire | Leisure-time physical activity | 0.1–5.9 METs/wk | 1.00 | Age, smoking, hypertension, diabetes, hypercholesterolemia, BMI |
| 6.0–13.7 | 0.98 (0.74–1.31) | ||||||||
| 13.8–24.2 | 1.15 (0.85–1.56) | ||||||||
| 24.3–40.8 | 0.95 (0.67–1.35) | ||||||||
| ≥ 40.9 | 1.02 (0.72–1.46) | ||||||||
| Lindblad et al.29, Sweden | Malmo Preventive Study | 1974–1991, 21 years follow-up |
22,444 men, mean age 43.7 years: Nested case–control study: 126 AAA cases 126 controls |
Hospital register data, SwedVasc quality control data, death certificates | Questionnaire | Physical inactivity | Yes vs. no | 2.67 (1.42–5.01) | Age, serum triglycerides, DBP, serum cholesterol, smoking |
| Tornwall et al.13, Finland | Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study | 1985–1988–1993, 5.8 years follow-up | 29,133 male smokers, age 50–69 years: 181 AAA cases | National hospital discharge register, national register of causes of death | Questionnaire | Leisure-time exercise | No vs. yes | 1.29 (0.95–1.73) | Age, cigarettes per day, years of smoking, BMI, SBP, DBP, total cholesterol, HDL cholesterol, diabetes mellitus, education, exercise, alpha-tocopherol and beta-carotene supplementation group |
| Goldberg et al.28, USA | Honolulu Heart Program | 1965–1968–1988, 23 years follow-up | 2710 Japanese American men, age 55–64 years: 119 AA cases | Medical, surgical and autopsy records | History of usual 24-h physical activity | Physical activity index | ≤ 29.6 | 1.00 | Age, ventricular rate, BMI, SBP, serum cholesterol, serum triglycerides, serum glucose, serum uric acid, hematocrit, forced expiratory volume, cigarettes per day, alcohol |
| 29.7–32.1 | 0.46 (0.19–1.13) | ||||||||
| 3.22–35.5 | 0.97 (0.47–2.01) | ||||||||
| ≥ 35.6 | 1.37 (0.69–2.72) | ||||||||
| Hammond et al.27, USA | Cancer Prevention Study 1 | 1959–1960–NA, 6 years follow-up | 218,435 men, age 50–69 years: 141 AAA deaths | Linkage to death records | Questionnaire | Exercise | Heavy | 1.00 | Age |
| Moderate | 1.43 (0.84–2.43) | ||||||||
| Slight | 1.87 (1.13–3.10) | ||||||||
| None | 1.83 (1.12–3.11) |
AAA abdominal aortic aneurysm, AA aortic aneurysm, BMI body mass index, DBP diastolic blood pressure, HDL high-density lipoprotein, SBP systolic blood pressure, wk week.
The summary RR for individuals with high compared to low physical activity was 0.70 (95% CI: 0.56–0.87, I2 = 58%, pheterogeneity = 0.01) (Fig. 2). There was no evidence of publication bias with Egger’s test (p = 0.56) or with Begg’s test (p = 0.60) or by inspection of the funnel plot (Supplementary Fig. 1). In sensitivity analyses excluding one study at a time the summary RR ranged from 0.66 (95% CI: 0.53–0.83) when excluding the study by Wong et al.14 to 0.75 (0.61–0.92) when excluding the study by Nordkvist et al.31 (Supplementary Table 2). Excluding one study (Honolulu Heart Program) reporting on total physical activity and aortic aneurysm in Japanese American men28 did not materially alter the results, and the summary RR was 0.67 (95% CI: 0.54–0.83, I2 = 56%). Further exclusion of one additional study which reported on bicycling/walking30 left seven studies on leisure-time physical activity/exercise and aortic aneurysm13,14,27,29,31–33 did also not alter the results, and the summary RR was 0.68 (95% CI: 0.54–0.86, I2 = 61%).
Figure 2.
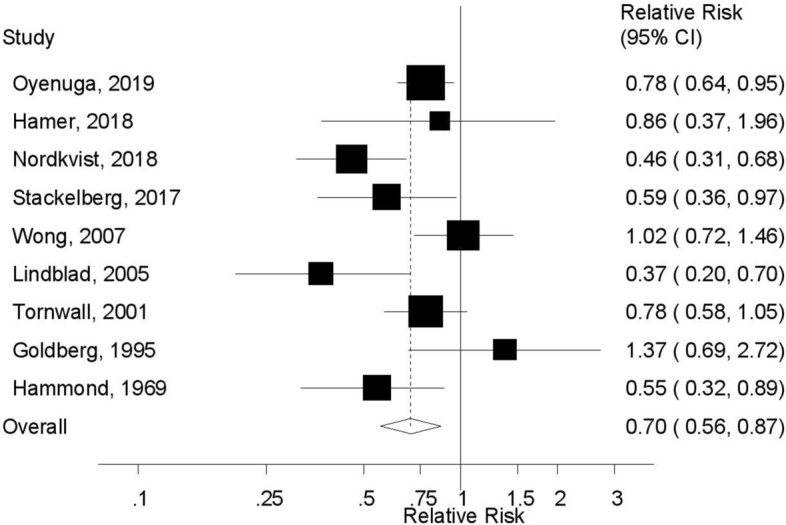
Physical activity and abdominal aortic aneurysm.
Six studies were included in the dose–response analysis14,27,30–33 including 1641 cases and 377,637 participants. The summary RR per 20 MET-hours/week increase in physical activity was 0.84 (95% CI: 0.74–0.95, I2 = 59%) (Fig. 3a). There was no indication of publication bias with Egger's test (p = 0.71) or with Begg's test (p = 0.63). Sensitivity analyses excluding the Cancer Prevention Study 127 (for which we approximated the physical activity level using data from the Cancer Prevention Study 234 did not substantially alter the results and the summary RR was 0.86 (95% CI: 0.77–0.97, I2 = 53%) (Supplementary Fig. 3). In influence analyses excluding one study at a time, the summary RR ranged from 0.80 (95% CI: 0.73–0.88) when excluding the study by Wong et al.14, to 0.86 (95% CI: 0.77–0.97) when excluding the study by Hammond et al.27 (Supplementary Fig. 3). We also repeated the analysis of the highest versus lowest level of activity and risk of abdominal aortic aneurysm using the same studies that were included in the dose–response analysis and the summary RR was 0.69 (95% CI: 0.54–0.88, I2 = 55%), which was very similar to the summary estimate among all studies.
Figure 3.
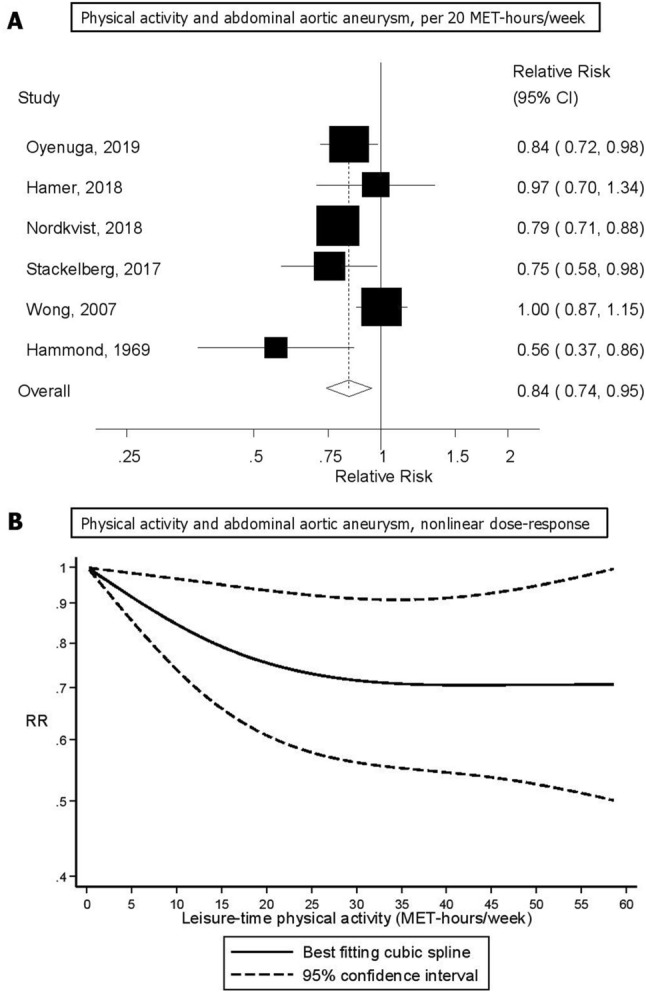
Physical activity and abdominal aortic aneurysm, per 20 MET-hours/week and nonlinear dose–response analysis.
Although the test for nonlinearity was not significant (p = 0.09) the association appeared to be stronger when increasing the physical activity level from 0 to around 20–25 MET-hours/week than at higher levels (Fig. 3b, Supplementary Table 2).
Subgroup and sensitivity analyses and study quality
Inverse associations were observed in most subgroup analyses defined by duration of follow-up, outcome type, outcome assessment, number of cases, study quality, and by whether studies excluded prevalent cases at baseline. The associations were often not significant when analyses were stratified by whether the studies adjusted for confounding factors or not, however, the number of studies in some subgroups were limited and there was no significant heterogeneity between any of the subgroup analyses (Table 2).
Table 2.
Subgroup analyses of physical activity and abdominal aortic aneurysm.
| Physical activity and abdominal aortic aneurysm | |||||
|---|---|---|---|---|---|
| n | Relative risk (95% CI) | I2 (%) | Ph1 | Ph2 | |
| All studies | 9 | 0.70 (0.56–0.87) | 58.3 | 0.01 | |
| Sex | |||||
| Men | 6 | 0.72 (0.53–0.98) | 61.9 | 0.02 | 0.74 |
| Women | 0 | ||||
| Men and women | 3 | 0.66 (0.44–0.98) | 65.3 | 0.06 | |
| Outcome type | |||||
| Incidence | 7 | 0.71 (0.55–0.91) | 66.3 | 0.007 | 0.58 |
| Mortality | 2 | 0.62 (0.40–0.96) | 0 | 0.37 | |
| Follow-up | |||||
| < 10 years | 3 | 0.73 (0.57–0.93) | 0 | 0.47 | 0.95 |
| ≥ 10 years | 6 | 0.69 (0.51–0.95) | 71.7 | 0.003 | |
| Geographic location | |||||
| Europe | 5 | 0.58 (0.43–0.79) | 48.3 | 0.10 | 0.13 |
| America | 4 | 0.85 (0.64–1.12) | 51.6 | 0.10 | |
| Number of cases | |||||
| Cases < 150 | 4 | 0.68 (0.39–1.18) | 64.7 | 0.04 | 0.81 |
| Cases ≥ 150 | 5 | 0.72 (0.57–0.91) | 59.9 | 0.04 | |
| Exclusion of prevalent cases at baseline | |||||
| Yes | 6 | 0.76 (0.60–0.96) | 61.1 | 0.03 | 0.24 |
| No | 3 | 0.53 (0.35–0.80) | 22.4 | 0.28 | |
| Outcome assessment | |||||
| Record linkage (hospital, death records) | 8 | 0.71 (0.56–0.91) | 62.0 | 0.01 | 0.67 |
| Ultrasound screening | 1 | 0.59 (0.36–0.97) | |||
| Study quality | |||||
| 0–3 stars | 0 | 0.82 | |||
| 4–6 stars | 4 | 0.68 (0.44–1.05) | 66.8 | 0.03 | |
| 7–9 stars | 5 | 0.71 (0.54–0.94) | 60.0 | 0.04 | |
| Adjustment for confounding factors3 | |||||
| Age | |||||
| Yes | 9 | 0.70 (0.56–0.87) | 58.3 | 0.01 | NC |
| No | 0 | ||||
| Education | |||||
| Yes | 2 | 0.72 (0.56–0.93) | 0 | 0.34 | 0.97 |
| No | 7 | 0.70 (0.52–0.94) | 67.2 | 0.006 | |
| Alcohol | |||||
| Yes | 3 | 0.85 (0.50–1.44) | 47.9 | 0.15 | 0.43 |
| No | 6 | 0.66 (0.52–0.86) | 66.8 | 0.01 | |
| Smoking | |||||
| Yes | 5 | 0.76 (0.54–1.07) | 65.1 | 0.02 | 0.49 |
| No | 4 | 0.60 (0.45–0.80) | 57.6 | 0.07 | |
| BMI or obesity | |||||
| Yes | 4 | 0.86 (0.65–1.14) | 43.3 | 0.15 | 0.12 |
| No | 5 | 0.58 (0.42–0.80) | 60.6 | 0.04 | |
| Diabetes mellitus | |||||
| Yes | 3 | 0.81 (0.61–1.06) | 38.7 | 0.20 | 0.45 |
| No | 6 | 0.65 (0.47–0.90) | 64.9 | 0.01 | |
| Hypertension | |||||
| Yes | 2 | 0.80 (0.47–1.36) | 67.8 | 0.08 | 0.57 |
| No | 7 | 0.67 (0.52–0.86) | 59.4 | 0.02 | |
| Systolic blood pressure | |||||
| Yes | 2 | 0.95 (0.56–1.60) | 54.2 | 0.14 | 0.23 |
| No | 7 | 0.64 (0.50–0.83) | 61.1 | 0.02 | |
| Diastolic blood pressure | |||||
| Yes | 2 | 0.57 (0.27–1.17) | 77.5 | 0.04 | 0.51 |
| No | 7 | 0.73 (0.57–0.94) | 58.3 | 0.03 | |
| Hypercholesterolemia or serum cholesterol | |||||
| Yes | 5 | 0.76 (0.54–1.07) | 65.1 | 0.02 | 0.49 |
| No | 4 | 0.63 (0.46–0.86) | 54.5 | 0.09 | |
| Triglycerides | |||||
| Yes | 2 | 0.71 (0.20–2.55) | 86.9 | 0.006 | 0.96 |
| No | 7 | 0.71 (0.58–0.87) | 47.4 | 0.08 | |
n denotes the number of studies.
1P for heterogeneity within each subgroup.
2P for heterogeneity between subgroups with meta-regression analysis.
BMI body mass index, NC not calculable because no studies were present in one of the subgroups.
The mean (median) study quality scores were 6.8 (7.0) out of 9.0 points possible for the studies included in the analysis. The main reasons for a less than optimal study quality score were lack of representativeness of the general population (n = 3), lack of validation or lack of reporting of validation of physical activity questionnaires (n = 7), lack of exclusion or lack of reporting of exclusion of prevalent AAA cases at baseline (n = 3), and lack of reporting on loss to follow-up (n = 6) (Supplementary Table 3).
Discussion
This meta-analysis of 9 cohort studies including 2073 cases of abdominal aortic aneurysm among 409,732 participants suggest that a high level of physical activity reduces the risk of abdominal aortic aneurysms by 30%. In the dose–response analysis there was a 16% reduction in the relative risk for each 20 MET-hours/week increase in physical activity (equivalent to approximately 2 h/week of vigorous running or bicycling, 3 h/week of moderate running/bicycling, or 6 h of brisk walking per week) and although the statistical test for nonlinearity was not significant, there was some indication of a stronger reduction in risk from 0 to 20–25 MET-hours/week than at higher levels of activity. The inverse association was observed in European studies, but was not significant in American studies. The association was in the direction of reduced risk, but not always significant across subgroups, most likely because of the limited number of studies in several subgroups. However, there was no evidence of between subgroup heterogeneity with meta-regression analyses.
As with any meta-analysis of epidemiological studies this meta-analysis has limitations. There was moderately high heterogeneity in the analysis across studies. Different studies used different types of questionnaires to assess physical activity, which likely differed in levels of validity that may have contributed to part of the observed heterogeneity. All the included studies assessed physical activity by self-report, and in some studies the self-reported measures were validated. In addition, different studies differed in the way physical activity was reported from a qualitative description of the level of activity to a quantitative measure reported using MET-hours. This appears to be a recurring issue in physical activity epidemiology as we in several previous meta-analyses on physical activity and different health outcomes only were able to include a fraction of the available studies in the dose–response analyses, if at all35–37. In the current analysis we were able to include six out of nine studies in the dose–response analysis. It seems less likely that the three studies which were excluded from the dose–response analysis would have substantially altered the overall association as the summary estimate for the highest versus lowest analysis was very similar to the overall analysis when restricted to these same six studies. Any future studies should emphasize reporting the results in a way that can be combined with the available data, for example by reporting the level of physical activity in MET-hours per week and/or hours per week.
We conducted several subgroup analyses to investigate potential sources of heterogeneity, but found no evidence of heterogeneity between subgroups. The association was not significant in among studies with adjustment for BMI or obesity, but whether this is a chance finding, or is an indication that reduced adiposity may be a mechanism that explains part of the association between physical activity and abdominal aortic aneurysm needs further exploration in future studies with models with and without adjustment for adiposity in the same dataset. The non-significant associations among studies with adjustment for hypertension, blood pressure or lipids also needs to be interpreted carefully as these factors potentially could be mediating variables for the association between physical activity and abdominal aortic aneurysm. In addition, we cannot exclude the possibility that the association may have been partly confounded by tobacco smoking, which is a rather strong risk factor for abdominal aortic aneurysm18, because the association was not statistically significant when the analyses were restricted to the studies with adjustment for tobacco smoking. However, the association was still in the direction of reduced risk among studies with adjustment for smoking and since there was no significant heterogeneity between these subgroups with meta-regression analyses, limited statistical power may be another potential explanation. Any further studies should adjust more comprehensively for tobacco smoking and analyses stratified by smoking status may be needed to completely rule out the potential for residual confounding.
Reverse causation or a temporal bias where patients with prevalent abdominal aortic aneurysms before baseline may have reduced their physical activity level because of the condition could also have biased the results. However, the association persisted among the six studies that excluded participants with prevalent disease at baseline. Publication bias can affect meta-analyses of published studies. Although we found no evidence of publication bias in this analysis, it is possible that we may have had too low power to detect such bias, however, there was also no evidence of asymmetry in the funnel plots, which might argue against this being the case.
Little is known about the potential underlying mechanisms that could explain a beneficial effect of physical activity on the risk of abdominal aortic aneurysms. Physical activity has been associated with lower blood pressure and a lower risk of hypertension20–24, lower lipid levels38,39, as well as a lower risk of coronary heart disease40, all of which are important risk factors for abdominal aortic aneurysms12,17,41. However, it is possible that the association is independent of traditional risk factors. A recent experimental study showed that low intensity exercise improved aortic wall structure and function in a mouse model of Marfan syndrome, while these benefits were attenuated at higher intensity exercise42. Reduced expression of matrix metalloproteinases 2 and 9 explained the reduced elastin fragmentation42. Whether these findings can be transferred to humans and individuals without Marfan syndrome is unclear and whether other mechanistic pathways are involved needs further study.
The current meta-analysis has several strengths including the relatively large sample size, comprehensive subgroup and sensitivity analyses, detailed linear and nonlinear dose–response analyses and moderately high study quality. Any further studies should investigate the dose–response relationship between different types and intensities of physical activity in relation to risk of abdominal aortic aneurysm, adjust more comprehensively for potential confounding factors, conduct analyses stratified by smoking, and could investigate potential mechanistic pathways that may explain the association between physical activity and lower risk of abdominal aortic aneurysm. In addition, more detailed reporting on the exposure ascertainment and whether it has been validated or not, exclusion of prevalent cases at baseline and/or clear reporting on whether prevalent cases have been excluded at baseline, as well as reporting on loss to follow-up seems to be areas for improving study quality.
The current findings are consistent with recommendations to increase the level of physical activity to reduce the risk of other chronic diseases such as coronary heart disease25, stroke25, type 2 diabetes35, several cancers43, and other conditions36,37, as well as premature mortality44, and suggest these benefits perhaps also may extend to abdominal aortic aneurysms. However, further research is needed before these findings can be considered conclusive.
Conclusion
In conclusion, this meta-analysis suggests that individuals who are physically active have a 30% reduction in the risk of abdominal aortic aneurysm, however, residual confounding cannot be entirely excluded. Additional studies from geographically diverse regions with better adjustment and stratification for smoking and other potential confounding factors are needed to clarify these associations. Further assessment of the dose–response relationship between different types and intensities of physical activity and risk of abdominal aortic aneurysm and the potential mechanistic pathways that may explain these findings is also needed.
Material and methods
Search strategy
Pubmed, and Embase databases were searched up to October 3rd 2020. The full search is described in the Supplementary Text. In addition, we searched the reference lists of the included publications for any additional studies. MOOSE criteria for reporting of meta-analyses were followed45.
Study selection and inclusion criteria
We included published retrospective and prospective cohort studies and nested case–control studies within cohorts which reported adjusted estimates of the relative risk (RR) with the 95% confidence intervals (CIs) for the association between physical activity and the risk of abdominal aortic aneurysm. The excluded studies and exclusion reasons can be found in Supplementary Table 1.
Data extraction
The following data were extracted from the included studies: The first author’s name, publication year, country where the study was conducted, study period, sample size, number of cases and participants, exposure and subgroup, RRs and 95% CIs and variables adjusted for in the analysis. The data were extracted by DA and checked for accuracy by EK.
Statistical methods
The random-effects model by DerSimonian and Laird46 was used to calculate summary relative risks (RRs) and 95% confidence intervals (CIs) of abdominal aortic aneurysm for the highest vs. lowest level of physical activity and per 20 MET-hours per week. The average of the natural logarithm of the RRs was estimated and the RR from each study was weighted using random effects weights. For the linear dose–response analysis we used the method by Greenland and Longnecker to estimate linear trends and (CIs) across categories of physical activity47. For studies that reported physical activity by ranges of activity we calculated the average of the upper and lower cut-off value to obtain an estimate of the midpoint for each category. For studies with open-ended extreme categories we used the width of the adjacent category to estimate an upper and lower cut-off for the highest and lowest category, respectively. Nonlinear dose–response analyses were conducted using restricted cubic splines with knots at 10, 50 and 90% percentiles of the distribution of physical activity, which were combined using multivariate meta-analysis48,49.
Because of differences in the way physical activity was reported between studies, conversions to the same scale (MET-hours/week) were made when possible. For one study which reported on walking/bicycling in minutes per day30 we used the average of 8 METs for bicycling (equivalent to bicycling at 12–13.9 mph with moderate effort) and 3.3 METs for walking at moderate pace (3.0 mph) 50, thus we used 5.65 METs as the average intensity for that study. For a second study we used the average of moderate (3–5.9 METs) and most vigorous (6–12 METs) activities to convert minutes/week of activity to METs (although some vigorous activities, e.g. bicycle racing, competitive skating, and ski racing, have METs up to 18 we chose 12 as an upper limit as most of the vigorous activities are in that range and because relatively few participants would be competitive sportsmen50). Thus we used 6.725 METs as intensity for that study. Another study which used a physical activity index28 which had a different scoring of the intensities than the MET values was excluded from the dose–response analysis because it was impossible to convert the reported data to METs. For one study (Cancer Prevention Study 1) which reported physical activity data as none, slight, moderate and heavy we used estimated METs from the Cancer Prevention Study 234, but excluded the study in a sensitivity analysis. For one study which reported on aneurysms and peripheral vascular disease combined32, the analyses were repeated but restricted to abdominal aortic aneurysm. Two additional studies were only included in the analysis of the highest versus lowest level of physical activity because they had < 3 categories of physical activity13,29. For two studies13,29 which compared no physical activity with activity we converted the risk estimates so the comparison became the highest vs. the lowest (consistent with the remaining studies) by inverting the risk estimates and confidence intervals.
Heterogeneity between studies was evaluated using Q and I2 statistics51. I2 measures how much of the heterogeneity is due to between study variation rather than chance. We conducted main analyses (all studies combined) and subgroup analyses stratified by study characteristics to investigate potential sources of heterogeneity. The subgroups included sex, outcome type (incidence vs. mortality), duration of follow-up, geographic location, number of cases, exclusion of prevalent cases at baseline, outcome assessment, study quality and by adjustment for confounding factors (age, education, alcohol, smoking, BMI/obesity, diabetes mellitus, hypertension, systolic blood pressure, diastolic blood pressure, hypercholesterolemia or serum cholesterol, and triglycerides. Study quality was assessed using the Newcastle Ottawa scale which rates studies a score from 0 to 9 based on the selection, comparability and outcome assessment of the studies52.
Publication bias was assessed using Egger’s test53 and Begg-Mazumdar’s test54 and by inspection of funnel plots. The statistical analyses were conducted using the software package Stata, version 13.1 software (StataCorp, Texas, US).
Supplementary information
Acknowledgements
This work has been supported by funding from Raagholtsstiftelsen and the South-East Regional Health Authority of Norway.
Author contributions
D.A. conducted the literature search, did the data extraction, analyses and wrote the first draft of the paper. D.A., A.S. contributed to the literature screening and E.K. checked the data extraction for accuracy. DA., A.S, E.K, M.H., T.N, E.R. interpreted the data, revised the subsequent drafts for important intellectual content, read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-76306-9.
References
- 1.Reimerink JJ, van der Laan MJ, Koelemay MJ, Balm R, Legemate DA. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br. J Surg. 2013;100:1405–1413. doi: 10.1002/bjs.9235. [DOI] [PubMed] [Google Scholar]
- 2.Verhoeven EL, et al. Mortality of ruptured abdominal aortic aneurysm treated with open or endovascular repair. J. Vasc. Surg. 2008;48:1396–1400. doi: 10.1016/j.jvs.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 3.Sweeting MJ, Balm R, Desgranges P, Ulug P, Powell JT. Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. Br. J Surg. 2015;102:1229–1239. doi: 10.1002/bjs.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karthikesalingam A, et al. Mortality from ruptured abdominal aortic aneurysms: clinical lessons from a comparison of outcomes in England and the USA. Lancet. 2014;383:963–969. doi: 10.1016/S0140-6736(14)60109-4. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet392, 1736–1788 (2018). [DOI] [PMC free article] [PubMed]
- 6.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet392, 1789–1858 (2018). [DOI] [PMC free article] [PubMed]
- 7.Orr N, Minion D, Bobadilla JL. Thoracoabdominal aortic aneurysm repair: current endovascular perspectives. Vasc. Health Risk Manag. 2014;10:493–505. doi: 10.2147/VHRM.S46452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994–2001. Circulation. 2009;119:2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 9.Vardulaki KA, et al. Incidence among men of asymptomatic abdominal aortic aneurysms: estimates from 500 screen detected cases. J. Med. Screen. 1999;6:50–54. doi: 10.1136/jms.6.1.50. [DOI] [PubMed] [Google Scholar]
- 10.Lederle FA, et al. Yield of repeated screening for abdominal aortic aneurysm after a 4-year interval. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch. Intern. Med. 2000;160:1117–1121. doi: 10.1001/archinte.160.8.1117. [DOI] [PubMed] [Google Scholar]
- 11.Salem MK, et al. Should Asian men be included in abdominal aortic aneurysm screening programmes? Eur. J. Vasc. Endovasc. Surg. 2009;38:748–749. doi: 10.1016/j.ejvs.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Lederle FA, et al. Abdominal aortic aneurysm events in the women's health initiative: cohort study. BMJ. 2008;337:a1724. doi: 10.1136/bmj.a1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tornwall ME, Virtamo J, Haukka JK, Albanes D, Huttunen JK. Life-style factors and risk for abdominal aortic aneurysm in a cohort of Finnish male smokers. Epidemiology. 2001;12:94–100. doi: 10.1097/00001648-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Wong DR, Willett WC, Rimm EB. Smoking, hypertension, alcohol consumption, and risk of abdominal aortic aneurysm in men. Am. J. Epidemiol. 2007;165:838–845. doi: 10.1093/aje/kwk063. [DOI] [PubMed] [Google Scholar]
- 15.Jahangir E, et al. Smoking, sex, risk factors and abdominal aortic aneurysms: a prospective study of 18 782 persons aged above 65 years in the Southern Community Cohort Study. J. Epidemiol. Community Health. 2015;69:481–488. doi: 10.1136/jech-2014-204920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 17.Kobeissi E, Hibino M, Pan H, Aune D. Blood pressure, hypertension and the risk of abdominal aortic aneurysms: a systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 2019;34:547–555. doi: 10.1007/s10654-019-00510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of abdominal aortic aneurysm: a systematic review and meta-analysis of prospective studies. Sci. Rep. 2018;8:14786. doi: 10.1038/s41598-018-32100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aune D, Schlesinger S, Norat T, Riboli E. Diabetes mellitus and the risk of abdominal aortic aneurysm: a systematic review and meta-analysis of prospective studies. J. Diabetes Complicat. 2018;32:1169–1174. doi: 10.1016/j.jdiacomp.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Cornelissen, V. A. & Smart, N. A. Exercise training for blood pressure: a systematic review and meta-analysis. J. Am. Heart Assoc. 2, e004473 (2013). [DOI] [PMC free article] [PubMed]
- 21.Barengo NC, et al. Low physical activity as a predictor for antihypertensive drug treatment in 25–64-year-old populations in eastern and south-western Finland. J. Hypertens. 2005;23:293–299. doi: 10.1097/00004872-200502000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Hu G, et al. Relationship of physical activity and body mass index to the risk of hypertension: a prospective study in Finland. Hypertension. 2004;43:25–30. doi: 10.1161/01.HYP.0000107400.72456.19. [DOI] [PubMed] [Google Scholar]
- 23.Williams PT. A cohort study of incident hypertension in relation to changes in vigorous physical activity in men and women. J. Hypertens. 2008;26:1085–1093. doi: 10.1097/HJH.0b013e3282fb81dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira MA, et al. Physical activity and incident hypertension in black and white adults: the Atherosclerosis Risk in Communities Study. Prev. Med. 1999;28:304–312. doi: 10.1006/pmed.1998.0431. [DOI] [PubMed] [Google Scholar]
- 25.Kyu HH, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857. doi: 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey A, et al. Dose–response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation. 2015;132:1786–1794. doi: 10.1161/CIRCULATIONAHA.115.015853. [DOI] [PubMed] [Google Scholar]
- 27.Hammond EC, Garfinkel L. Coronary heart disease, stroke, and aortic aneurysm. Factors in the etiology. Arch. Environ. Health. 1969;19:167–182. doi: 10.1080/00039896.1969.10666825. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg RJ, et al. Lifestyle and biologic factors associated with atherosclerotic disease in middle-aged men. 20-year findings from the Honolulu Heart Program. Arch. Intern. Med. 1995;155:686–694. doi: 10.1001/archinte.1995.00430070036004. [DOI] [PubMed] [Google Scholar]
- 29.Lindblad B, Borner G, Gottsater A. Factors associated with development of large abdominal aortic aneurysm in middle-aged men. Eur. J. Vasc. Endovasc. Surg. 2005;30:346–352. doi: 10.1016/j.ejvs.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Stackelberg O, et al. Lifestyle and risk of screening-detected abdominal aortic aneurysm in men. J. Am. Heart Assoc. 2017;6:e004725. doi: 10.1161/JAHA.116.004725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordkvist S, Sonestedt E, Acosta S. Adherence to diet recommendations and risk of abdominal aortic aneurysm in the Malmo Diet and Cancer Study. Sci. Rep. 2018;8:2017. doi: 10.1038/s41598-018-20415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamer M, O'Donovan G, Stamatakis E. Association between physical activity and sub-types of cardiovascular disease death causes in a general population cohort. Eur. J. Epidemiol. 2019;34:483–487. doi: 10.1007/s10654-018-0460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oyenuga, A. O., Folsom, A. R., Lutsey, P. L. & Tang, W. Association of Life's Simple 7 with reduced clinically manifest abdominal aortic aneurysm: the ARIC study. Vasc. Med. 1358863X19829226 (2019). [DOI] [PMC free article] [PubMed]
- 34.Patel AV, et al. The relationship between physical activity, obesity, and lung cancer risk by smoking status in a large prospective cohort of US adults. Cancer Causes Control. 2017;28:1357–1368. doi: 10.1007/s10552-017-0949-0. [DOI] [PubMed] [Google Scholar]
- 35.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur. J Epidemiol. 2015;30:529–542. doi: 10.1007/s10654-015-0056-z. [DOI] [PubMed] [Google Scholar]
- 36.Aune D, Leitzmann M, Vatten LJ. Physical activity and the risk of gallbladder disease: a systematic review and meta-analysis of cohort studies. J. Phys. Act. Health. 2016;13:788–795. doi: 10.1123/jpah.2015-0456. [DOI] [PubMed] [Google Scholar]
- 37.Aune D, Sen A, Henriksen T, Saugstad OD, Tonstad S. Physical activity and the risk of gestational diabetes mellitus: a systematic review and dose-response meta-analysis of epidemiological studies. Eur. J Epidemiol. 2016;31:967–997. doi: 10.1007/s10654-016-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelley GA, Kelley KS, Tran ZV. Aerobic exercise and lipids and lipoproteins in women: a meta-analysis of randomized controlled trials. J. Womens Health (Larchmt) 2004;13:1148–1164. doi: 10.1089/jwh.2004.13.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelley GA, Kelley KS. Aerobic exercise and lipids and lipoproteins in men: a meta-analysis of randomized controlled trials. J Mens. Health Gend. 2006;3:61–70. doi: 10.1016/j.jmhg.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sofi F, Capalbo A, Cesari F, Abbate R, Gensini GF. Physical activity during leisure time and primary prevention of coronary heart disease: an updated meta-analysis of cohort studies. Eur. J. Cardiovasc. Prev. Rehabil. 2008;15:247–257. doi: 10.1097/HJR.0b013e3282f232ac. [DOI] [PubMed] [Google Scholar]
- 41.Iribarren C, et al. Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: the Kaiser multiphasic health checkup cohort study. Ann. Epidemiol. 2007;17:669–678. doi: 10.1016/j.annepidem.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Gibson C, et al. Mild aerobic exercise blocks elastin fiber fragmentation and aortic dilatation in a mouse model of Marfan syndrome associated aortic aneurysm. J. Appl. Physiol. 2017;123:147–160. doi: 10.1152/japplphysiol.00132.2017. [DOI] [PubMed] [Google Scholar]
- 43.Moore SC, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern. Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose-response meta-analysis of cohort studies. Int. J. Epidemiol. 2011;40:1382–1400. doi: 10.1093/ije/dyr112. [DOI] [PubMed] [Google Scholar]
- 45.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 46.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 47.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 48.Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta-analyses. Stat. Med. 2010;29:1282–1297. doi: 10.1002/sim.3602. [DOI] [PubMed] [Google Scholar]
- 49.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ainsworth BE, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 51.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 52.Wells, G. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, Accessed August 8th 2018.
- 53.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


