Abstract
A 71-year-old Japanese woman with a history of rheumatoid arthritis of 50 years’ duration was admitted to our hospital with refractory diarrhea. Endoscopic biopsy revealed AA amyloid deposition in the large intestine. Although the patient had been prescribed 5 tumor necrosis factor inhibitors over the past 10 years, rheumatoid arthritis was poorly controlled, with a Disease Activity Score 28 using C-reactive protein score of 6.52 on admission. Treatment with tocilizumab (8 mg/kg every 2 weeks) was initiated, but this was ineffective. After 3 months, abatacept (cytotoxic T-lymphocyte–associated antigen 4 immunoglobulin) was initiated (750 mg/mo) and the patient’s diarrhea began to improve. After 3 months of abatacept treatment, serum albumin, C-reactive protein, and serum amyloid A levels had all decreased to within normal ranges. After 3 years of abatacept treatment, a repeat biopsy of the large intestine revealed a marked improvement in amyloid deposition. Interleukin 6 is a key factor in AA amyloid formation, but this case suggests that T-cell activation increases the production of cytokines (including interleukin 6) via a mechanism involving cytotoxic T-lymphocyte–associated antigen 4, resulting in a second key factor of AA amyloid formation.
Abbreviations and Acronyms: ABT, abatacept; CRP, C-reactive protein; IL, interleukin; RA, rheumatoid arthritis; SAA, serum amyloid A; TCZ, tocilizumab; TNF, tumor necrosis factor
AA amyloidosis is characterized by insoluble plaque deposits in organs; these plaques are composed mainly of serum amyloid A (SAA).1 The most rational treatment of AA amyloidosis is to inhibit the production of SAA.2 Okuda3 and Hattori et al4 reported that tocilizumab (TCZ) can decrease AA amyloidosis in the gastrointestinal system in patients with rheumatoid arthritis (RA). However, we encountered a case that exhibited the effectiveness of abatacept (ABT) for protein-losing enteropathy secondary to AA amyloidosis refractory to 5 tumor necrosis factor (TNF) inhibitors and TCZ.
Case Report
A 71-year-old Japanese woman with RA was admitted to our hospital with refractory diarrhea. Rheumatoid arthritis had been diagnosed at the age of 21, and nonsteroidal anti-inflammatory drugs had been the main treatment. At the age of 59, the patient began taking prednisolone (20 mg/d) and methotrexate (8 mg/wk). At the age of 60, she underwent bilateral knee replacement surgery, after which infliximab (3 mg/kg every 2 months) was initiated but infliximab was discontinued because of facial flushing and shortness of breath. At the age of 63, the patient began taking etanercept (25 mg twice a week); disease activity seemed to subside, but the patient complained of pain in her fingers. Therefore, when the patient was 69 years old, she was switched from etanercept to certolizumab pegol (400 mg/mo), but this was not effective. At the age of 70, she began taking golimumab (100 mg/mo) and adalimumab (40 mg every 2 weeks), but these were also ineffective. At the age of 71 years, the patient tried certolizumab pegol again, but refractory diarrhea occurred (Figure 1).
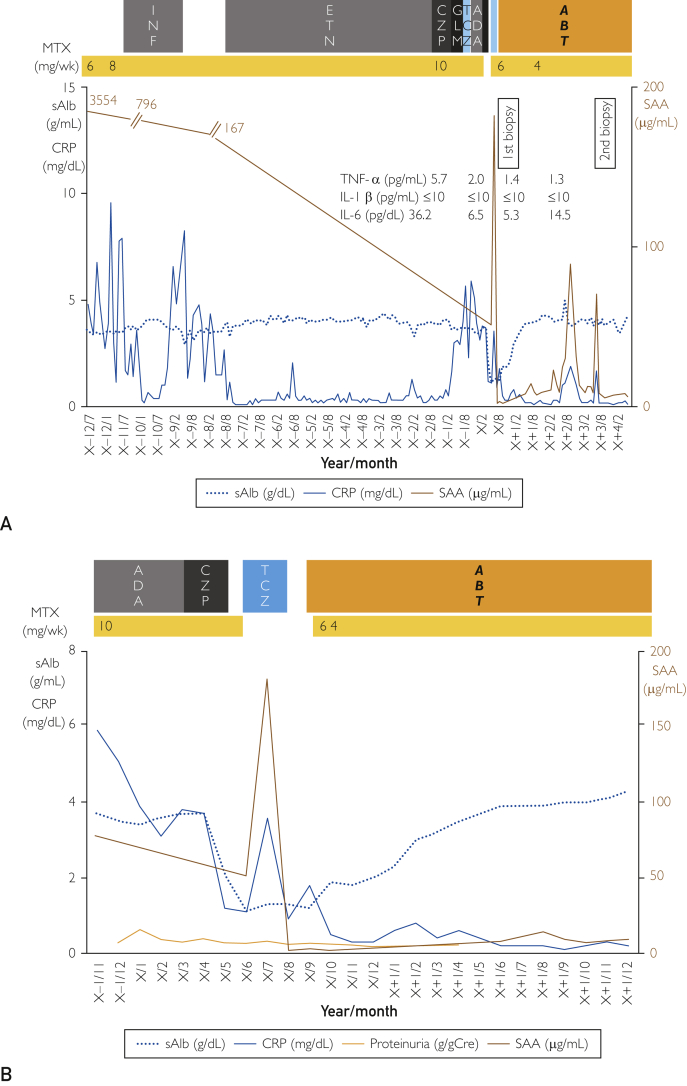
Figure 1.
A, Entire clinical course of the patient. B, Clinical course over the past 3 years. ABT = abatacept; ADA = adalimumab; CRP = C-reactive protein(normal range, <0.14 mg/dL); CZP = certolizumab pegol; ETN = etanercept; GLM = golimumab; IL = interleukin; IL-1β(normal range, <10 pg/mL); IL-6(normal range, <4.0pg/mL); INF = infliximab; MTX = methotrexate; SAA = serum amyloid A(normal range, <8.0 μg/mL); sAIb = serum albumin(normal range, 4.1-5.1 g/dL); TNF-α = tumor necrosis factor-α(normal range, 1.5-12.0 pg/mL); TCZ = tocilizumab.
On admission, the patient, a nonsmoker, was 154 cm tall and weighed 81.8 kg (body mass index, 40 kg/m2) with a blood pressure of 136/72 mm Hg and a temperature of 36.7°C. Her hands, feet, and ankles were deformed and severely swollen bilaterally, with edema in the lower extremities.
Laboratory findings were as follows: white blood cell count, 10,300/μL(normal range, 3,300-8,600/μL); red blood cell count, 390×104/μL(normal range, 386 to 492×104/μL); hemoglobin level, 12.5 g/dL(normal range, 11.6 to 14.8 g/dL); platelet count, 33.9×103/μL(normal range, 15.8 to 34.8×103/μL); total protein level, 2.9 g/dL(normal range, 6.6 to 8.1 g/dL); albumin level, 1.1 g/dL(normal range, 4.1 to 5.1 g/dL); serum urea nitrogen level, 19 mg/dL; serum creatinine level, 0.6 mg/dL(normal range, 0.4 to 0.8 mg/dL); erythrocyte sedimentation rate, 60 mm/h(normal range, 3 to 15 mm/h), C-reactive protein (CRP) level, 1.1 mg/dL(normal range, <0.14 mg/dL); rheumatoid factor, 20 U/mL (normal range, 0 to 15 U/mL); matrix metalloprotease-3(MMP-3) level, 15 μg/mL(normal range, 4.1-5.1 g/dL); anti–cyclic citrullinated peptide antibody level, 175 U/mL (normal range, <4.5 U/mL); SAA level, 51.3 μg/mL (normal range, <8.0 μg/mL); IgG level, 192 mg/dL(normal range, 861-1,747 mg/dL); IgA level, 87.1 mg/dL(normal range, 93-393 mg/dL); and IgM level, 23.8 mg/dL(normal range, 50 to 269 mg/dL).
Urinary protein excretion was 0.3 g/d, and urinary sediment contained 11 to 30 erythrocytes per high power field.
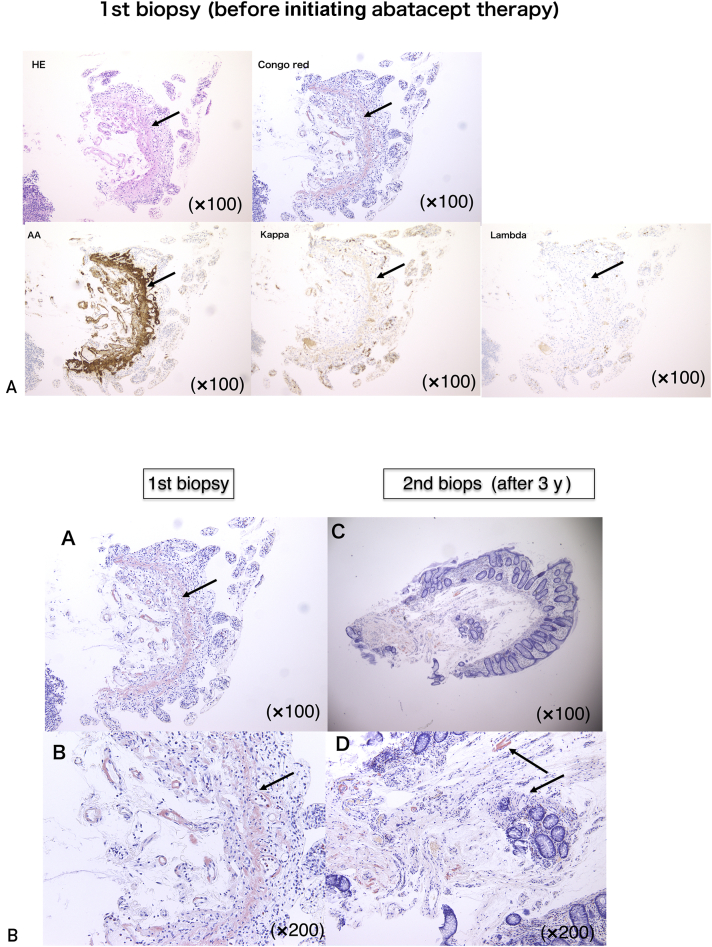
We performed an endoscopic biopsy of the large intestine. Congo red staining was positive for the thickened walls of small arteries, muscularis mucosae, and surrounding tissues in the subserosal layerand revealed apple green birefringence under polarizing light. Immunohistochemical staining was positive for AA but negative for kappa and lambda chains, β2 microglobulin, and transthyretin (Figure 2A). Protein-losing enteropathy secondary to AA amyloidosis was diagnosed. Because RA was poorly controlled, with a Disease Activity Score 28 using CRP score of 6.52 on admission, we initiated treatment with TCZ (8 mg/kg every 2 weeks). Neither RA disease activity nor intractable diarrhea subsided, and SAA levels increased to 182 μg/mL. After 3 months, TCZ was discontinued after the development of a severe systemic allergic reaction. We then tried ABT (750 mg/mo). Intractable diarrhea began to improve (Figure 1B). After 3 months, serum albumin, CRP, and SAA levels had all normalized. A repeat biopsy of the large intestine 3 years since the initiation of ABT therapy revealed a marked reduction in amyloid deposition (Figure 2B). Cytokines (TNF-α and interleukin [IL] 6) also reflect disease activity and are improved (Figure 1A).
Figure 2.
Endoscopic biopsy (second biopsy) of the large intestine at 3 years’ follow-up since the initiation of abatacept therapy was performed. Amyloid deposition resolved as compared with the first biopsy. Panel A shows first biopsy. Panel C shows second biopsy. Panel B shows the magnification of panel A. Panel D shows the magnification of panel C. HE = hematoxylin and eosin.
Discussion
Gillmore et al2 reported that when SAA concentrations in the blood were controlled at less than 10 mg/L, amyloid deposits in the organs decreased and the 10-year survival rate was good (~90%), whereas in patients with SAA concentrations 10 mg/L or higher, the 10-year survival rate was approximately 40%. Inhibiting SAA production is therefore the most rational treatment of AA amyloidosis. Treatments of AA amyloidosis involving biologics have recently shown promise.3 Several studies and case reports3, 4, 5, 6 have suggested that TCZ is useful in treating RA complicated by AA amyloidosis. Okuda et al7 conducted a nationwide survey of 199 Japanese patients with AA amyloidosis. Biologics were used to treat 97 patients (48.7%): TCZ was administered to 66 patients, with 95.5% exhibiting good responses; anti-TNF agents were administered to 27 patients, with 74.1% exhibiting good responses; and ABT was administered to 4 patients, with 75% exhibiting good responses. The efficacy of TCZ was significantly better than that of TNF inhibitors (P=.007). In addition, Hagihara et al8 found that IL-6 is necessary for the synergistic induction of the SAA gene by IL-6, IL-1β, and TNF-α.
Only 1 study9 to date has examined the effectiveness of ABT for AA amyloidosis refractory to TNF inhibitors with or without TCZ. Nakamura et al reported that ABT was effective against AA amyloidosis secondary to RA in 2 patients. In one patient, refractory diarrhea occurred 5 years after initiating etanercept and endoscopic biopsy revealed AA amyloid deposition in the gastrointestinal tract. After ABT administration, disease activity of RA and diarrhea subsided and amyloid deposition was reduced after 1 year. In the other patient, renal dysfunction and proteinuria occurred and renal biopsy revealed AA amyloid deposition. Etanercept and TCZ were administered sequentially, but renal function continued to decline. After switching to ABT, proteinuria decreased and renal function deterioration halted. After 2 years, a biopsy of the gastrointestinal tract revealed deposition of AA amyloid equal to that revealed by the first biopsy.9
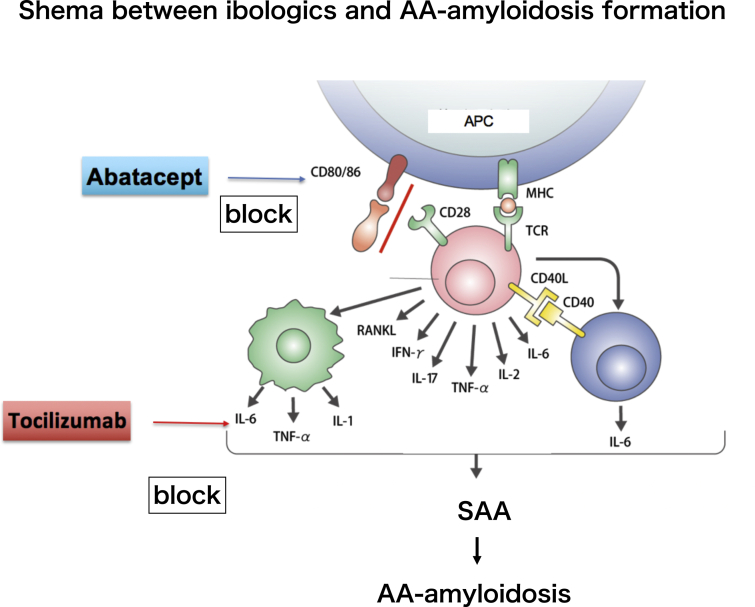
Abatacept consists of the extracellular domain of human cytotoxic T-lymphocyte–associated antigen 4. It inhibits T-cell activation by selectively binding to CD80 and CD86, thereby blocking interaction with CD28 and suppressing the production of cytokines such as IL-6 and TNF-α, resulting in effective treatment of RA with AA amyloidosis (Figure 3).10, 11, 12
Figure 3.
Schematic illustrating the relationship between biologics and AA amyloidosis. Abatacept contains the extracellular domain of human cytotoxic T-lymphocyte–associated antigen 4. It inhibits T-lymphocyte activation by selectively binding to CD80 and CD86, thereby blocking interaction with CD28 and suppressing the production of cytokines such as interleukin (IL) 6 and tumor necrosis factor-α (TNF-α). APC = antigen presenting cell; IFN-γ = interferon-gamma; MHC = major histocompatibility complex; RANKL = Receptor activator of nuclear factor kappa-Β ligand; SAA = serum amyloid A; TCR = T cell receptor.
Statement of Ethics
The present report was produced in conformity with the Declaration of Helsinki, and the patient gave consent for this case report to be published.
Conclusion
We encountered a case of AA amyloidosis–related protein-losing enteropathy resulting in diarrhea and hypoalbuminemia, which was refractory to TCZ. Soon after the administration of ABT, diarrhea and hypoalbuminemia subsided. After 3 years, endoscopic biopsy confirmed that amyloid deposition in the large intestine had resolved. The IL-6 receptor antibody derivative TCZ can treat AA amyloidosis by markedly decreasing SAA by blocking IL-6, which may be a key factor in amyloidosis. However, the present case suggests that T-cell activation via a mechanism involving cytotoxic T-lymphocyte–associated antigen 4 increases the production of cytokines including IL-6, resulting in the second key factor of AA amyloid formation (Figure 3).
ORCID
Masato Sawamura: https://orcid.org/0000-0002-4763-6124
Noriko Hayami: https://orcid.org/0000-0003-4461-9721
Tatsuya Suwabe: https://orcid.org/0000-0003-0825-2512
Junichi Hoshino: https://orcid.org/0000-0002-0444-101X
Yoshifumi Ubara: https://orcid.org/0000-0003-2322-8203
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Jensen L.E., Whitehead A.S. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J. 1998;334(pt 3):489–503. doi: 10.1042/bj3340489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillmore J.D., Lovat L.B., Persey M.R., Pepys M.B., Hawkins P.N. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet. 2001;358(9275):24–29. doi: 10.1016/S0140-6736(00)05252-1. [DOI] [PubMed] [Google Scholar]
- 3.Okuda Y. AA amyloidosis—benefits and prospects of IL-6 inhibitors. Mod Rheumatol. 2019;29(2):268–274. doi: 10.1080/14397595.2018.1515145. [DOI] [PubMed] [Google Scholar]
- 4.Hattori Y., Ubara Y., Sumida K., et al. Tocilizumab improves cardiac disease in a hemodialysis patient with AA amyloidosis secondary to rheumatoid arthritis. Amyloid. 2012;19(1):37–40. doi: 10.3109/13506129.2011.636460. [DOI] [PubMed] [Google Scholar]
- 5.Courties A., Grateau G., Philippe P., et al. Club Rhumatismes Inflammation and the REGATE Registry. AA amyloidosis treated with tocilizumab: case series and updated literature review. Amyloid. 2015;22(2):84–92. doi: 10.3109/13506129.2014.1002031. [DOI] [PubMed] [Google Scholar]
- 6.Lane T., Gillmore J.D., Wechalekar A.D., Hawkins P.N., Lachmann H.J. Therapeutic blockade of interleukin-6 by tocilizumab in the management of AA amyloidosis and chronic inflammatory disorders: a case series and review of the literature. Clin Exp Rheumatol. 2015;33(6 suppl 94):S46–S53. [PubMed] [Google Scholar]
- 7.Okuda Y., Yamada T., Ueda M., Ando Y. First nationwide survey of 199 patients with amyloid A amyloidosis in Japan. Intern Med. 2018;57(23):3351–3355. doi: 10.2169/internalmedicine.1099-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagihara K., Nishikawa T., Isobe T., Song J., Sugamata Y., Yoshizaki K. IL-6 plays a critical role in the synergistic induction of human serum amyloid A (SAA) gene when stimulated with proinflammatory cytokines as analyzed with an SAA isoform real-time quantitative RT-PCR assay system. Biochem Biophys Res Commun. 2004;314(2):363–369. doi: 10.1016/j.bbrc.2003.12.096. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T., Kumon Y., Hirata S., Takaoka H. Abatacept may be effective and safe in patients with amyloid A amyloidosis secondary to rheumatoid arthritis. Clin Exp Rheumatol. 2014;32(4):501–508. [PubMed] [Google Scholar]
- 10.Adams A.B., Ford M.L., Larsen C.P. Costimulation blockade in autoimmunity and transplantation: the CD28 pathway. J Immunol. 2016;197(6):2045–2050. doi: 10.4049/jimmunol.1601135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choy E.H., Panayi G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344(12):907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 12.Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2012;51(suppl 5):v3–v11. doi: 10.1093/rheumatology/kes113. [DOI] [PubMed] [Google Scholar]