Highlights
-
•
Endurance training has beneficial effects on red blood cell aging and function.
-
•
Improved hemorheological effects are observed in endurance-untrained individuals after a 6-week running training.
-
•
A shift to more young red blood cells is observed after the training.
-
•
Physical performance and blood parameters increased after the training.
Keywords: Cellular adaptation, Hemorheology, Nitric oxide, Performance, RBC physiology and aging
Abstract
Objective
To examine the impact of a 6-week endurance training on red blood cell (RBC) aging and deformability of healthy participants to detect possible improved hemorheological and performance-related adaptations.
Methods
A total of 31 participants (17 females and 14 males) performed a 6-week moderate training protocol (three 1-h running sessions per week at 70% of maximal heart rate). Blood was sampled before and after the training. RBCs from each participant were fractioned according to density and age into 4 RBC subfractions. Subfractions were examined for changes of RBC properties, including aging distribution, RBC deformability, RBC microparticles, and phosphatidylserine concentrations. RBC and plasma nitrite levels were measured as indicators of nitric oxide metabolism.
Results
Aerobic performance, peak oxygen consumption, ventilatory thresholds, velocity at the aerobic–anaerobic threshold, and lactate at exhaustion improved after training. The relative amount of both young RBCs and old RBCs increased, and the amount of the main RBC fraction decreased. Phosphatidylserine externalization and RBC-derived microparticles decreased. Overall deformability expressed as shear stress required to achieve half-maximum deformation to theoretical maximal elongation index at infinite shear stress improved in unfractioned RBCs (p < 0.001). Nitrite decreased in total (p = 0.001), young (p < 0.001), main (p < 0.001), and old (p = 0.020) aged RBCs and in plasma (p = 0.002), but not in very old RBCs.
Conclusion
These results indicate that non-endurance-trained healthy participants benefit from a regular moderate running training program because performance-related parameters improve and a younger RBC population with improved RBC properties is induced, which might support oxygen supply in the microcirculation.
1. Introduction
The major function of red blood cells (RBCs) relates to facilitating the exchange of oxygen and carbon dioxide with the surrounding tissue in the microcirculation.1 Indeed, RBCs need to deform to flow through small capillaries, which sometimes have a smaller diameter than the RBCs themselves.1 The average life span of RBCs is about 120 days but may vary with health condition, hormonal status, or physical activity (PA) level. Approximately 200 billion RBCs are newly formed each day, meaning that RBCs of different ages are present in the circulation.2
It has been shown previously that RBC deformability decreases with RBC aging.3, 4 Because RBCs lack a nucleus, their capacity of antioxidative defense and damage removal is limited. To maintain their functionality, RBCs can remove oxidized and damaged proteins by membrane shedding and vesiculation.5 Because the concomitant loss of enclosed water is proportionally greater than the loss of proteins, RBC volume decreases and RBC density increases with aging.5, 6 In addition to the removal of nonfunctioning cell components, microparticles (MPs) derived from RBCs can still exhibit functional proteins and are assumed to play a role in cell-to-cell communication and in the induction of adaptations through internal and external stimulations, such as exercise.7
PA is one factor that can modify the age distribution of the RBC population, and it has been shown that endurance athletes possess a greater number of more flexible and low-density young RBCs.8 Increased RBC deformability is suspected to play a crucial role for athletic performance.9 RBC deformability depends on the surface-to-volume ratio, internal viscosity, and membrane elasticity, which can be affected by other factors such as by dehydration, cytoskeletal protein distribution, hemoglobin concentration, or RBC aging.10, 11 It has been demonstrated that nitric oxide (NO) synthesized by a RBC–NO synthase may S-nitrosylate α- and β-spectrins of the RBC cytoskeleton, which ultimately leads to an improvement of membrane flexibility and thus whole RBC deformability.12 Therefore, improvement of RBC and plasma NO bioavailability has been proposed to play a major role in physical performance because it enhances vasodilatation and RBC deformability, which leads to a more efficient oxygen supply in the microcirculation.
The aim of this study was to investigate the effects of a 6-week endurance training program on the RBCs aging distribution and their deformability. Previous studies suggest that RBC deformability is positively influenced by endurance exercise, primarily by increased formation of new RBCs with higher deformability,4, 13 and that aerobic exercise leads to adaptations in the RBC aging proportion in patients with cardiovascular diseases.14 Because these studies showed improvements in aerobic performance, formation of a younger RBC population, and improved deformability, we aimed to examine these effects in a healthy population. We hypothesized that even a comparable short, 6-week training intervention would lead to fast adaptations in the RBC population, which in turn would lead to an increased numbers of young RBCs and an overall rejuvenation of the entire RBC population.
2. Methods
2.1. Ethics
The protocols used in this study were approved by the Ethics Committee of the German Sport University Cologne. These protocols align with the Declaration of Helsinki, and all participants gave written informed consent to participate in this study.
2.2. Participants
A total of 31 participants (17 females, 14 males) were included in this study. All participants were healthy, moderately fit, and nonsmokers. Further inclusion criteria were: age 18–32 years; body mass index 18–26 kg/m², central European, no severe cardiovascular diseases and <4 h of endurance training per week. Baseline anthropometric data were as follows: age = 23.9 ± 3.3 years, height = 174.2 ± 8.8 cm, and weight = 68.0 ± 11.5 kg (mean ± SD).
2.3. Performance diagnostics
To determine the endurance capacity of the participants, an exercise test was performed on a treadmill using a previously used incremental stepwise protocol.15 Lactate concentrations were measured using EBIOplus (EKF Diagnostic Sales GmbH, Magdeburg, Germany). Running speed at the 4 mmol/L lactate threshold was calculated via a calculation matrix.15 Peak oxygen consumption (VO2peak) was measured using a ZAN 300 spirometer (ZAN Austria E.U., Winkling, Austria) during the test. Heart rate (HRmax) was measured using a heart rate monitor (Polar Electro GmbH, Büttelborn, Germany). The individual maximal heart rate (HR) was determined as the highest value measured during the exercise test. The VO2peak was determined as the average of the highest values measured over the last 30 s. The following classical criteria were used to check that all participants reached their VO2peak: (1) plateau of VO2 achieved, (2) HRmax achieved (220 – age (±10%)), (3) inability to maintain the effort, and (4) VCO2/O2 ratio of >1:1. Performance diagnostics were conducted before and after the training. First ventilatory threshold (VT1) and second ventilatory threshold (VT2) were determined based on the protocol of Beaver et al.16 and Schneider et al.17 and were expressed as a percentage of VO2peak. Time to exhaustion (TTE) was determined as the time from the start of the exercise test until the stoppage of the exercise test (when participants were completely exhausted).
2.4. Training protocol
Participants were not endurance trained but practiced combat sports, horse riding, court sports, and recreational activities such as yoga and surfing. In addition to their normal activities and training, participants were asked to run for 1 h 3 times a week at 70% of HRmax for 6 weeks. Total training volume of the participants thus ranged 3–10 h per week, depending on their other recreational activities (3–5 days per week). A 1-day rest period between the endurance training sessions was predefined for regeneration and adaptation purposes. To ensure that participants followed training intensity protocols, HR monitors (Polar Electro GmbH) were provided. All participants completed the training protocol over the training period with high compliance, which covered all exercise activities, including the type of sport and its duration, as well as subjective exertion (Borg scale) to control and monitor all training and recreational exercise activities. Additionally, the training protocol enabled participants to control their individual progress at the predefined heart rate and running time. Exercise sessions were performed individually or in training groups having similar endurance capacity. In addition, a professional trainer was provided, who offered associated training such as training advice or individual training.
2.5. Blood sampling and experimental protocol
2.5.1. Blood sample collection
Venous blood samples were taken before and after training. Blood was anticoagulated using either sodium heparin or Na3-citrate (BD Vacutainer; Becton Dickinson, Franklin Lakes, NJ, USA). Pretraining samples were taken within 1 week before training. Post-training samples were taken within 1 week after training. Blood removal was performed so that every participant had the same time interval between the pretraining and post-training blood removal. RBCs were separated according to age using a discontinuous Percoll density centrifugation (Percoll; Amersham Biosciences, Uppsala, Sweden) according to the protocol of Bizjak et al.18 Briefly, heparinized blood samples were centrifuged at 3500 g for 1 min and the buffy coat was removed.
2.5.2. RBC fractionation
Plasma supernatant was collected into 1.5 mL microcentrifuge tubes (Eppendorf GmbH, Wesseling-Berzdorf, Germany) for plasma nitrite analysis and stored at –80°C until measurement. RBCs were isolated, washed with a 9-fold volume of isotonic buffer originating from an established protocol by Bosch et al.5 (GASP-buffer containing albumin and glucose: 9 mmol/L Na2HPO4, 1.3 mmol/L NaH2PO4, 140 mmol/L NaCl, 5.5 mmol/L glucose, and 0.8 g/L BSA pH 7.4), and centrifuged (3500 g, 1 min, at 4°C). The supernatant was removed, reference samples of unfractioned RBCs were taken (total RBCs), and the RBC pellet was resuspended 1:1 in SAH-buffer 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer containing BSA: 26.3 g/L BSA, 132 mmol/L NaCl, and 4.6 mmol/L KCl, and 10 mmol/L HEPES; pH = 7.1). A total of 600 µL of washed and resuspended RBCs were cautiously applied on 5 Percoll layers diluted in SAH buffer. Percoll layers were built up as previously described by Bosch et al.,5 and the density of Percoll solutions (Amersham Biosciences) was adapted to yield the optimal fractionation success. Briefly, 2 mL of the respective Percoll solutions with an increasing density of 1.064 g/mL, 1.066 g/mL, 1.068 g/mL, 1.072 g/mL, and 1.076 g/mL were layered manually, beginning with the densest solution. Layers were built cautiously and dropwise to minimize mingling of the different dense solutions. Centrifugation was performed at 3700 g for 25 min at 4°C, with the lowest possible slowing-down process of the centrifuge used to minimize RBCs clumping or mingling. After centrifugation, 4 different RBC fractions assembled in the respective Percoll phases and were obtained by careful collection of each fraction. Obtained fractions were classed into young (≤1.064 g/mL), main (1.065–1.071 g/mL), old (1.072–1.076 g/mL), and very old (>1.076 g/mL) RBCs. The volume percentage of each fraction was determined, and isolated RBCs were subsequently used for the following analyses.
2.5.3. Aging-related parameters
Hemograms were performed to determine the mean cellular volume (MCV) in whole blood after blood removal as well as in the subpopulations after fractionation using the hematology analyzer Sysmex Digitana KX-21N (Sysmex, Horgen, Switzerland).
Concentrations of externalized phosphatidylserine (PS) were determined with an Annexin V enzyme-linked immunosorbent assay (IBL International, Hamburg, Germany) for the main fraction and the very old fraction according to the manufacturer's kit manual.
2.5.4. RBC deformability
RBC deformability was measured using the Laser-Assisted Optical Rotational Cell Analyzer (LORCA, RR Mechatronics, Hoorn, the Netherlands) as described in detail elsewhere.18, 19 Briefly, RBCs were mixed with a viscous polyvinylpyrrolidone solution (RR Mechatronics, Hoorn, the Netherlands; viscosity of 30 cP; 1:250 ratio), and deformability was measured at 37°C at 9 increasing shear stresses (0.3–50.0 Pa). An elongation index (EI) was calculated by the LORCA software (RR Mechatronics) from the vertical and horizontal radius of the ellipsoidal diffraction pattern. The theoretical maximal EI at infinite shear stress (EImax) and the shear stress that is required to achieve half-maximum deformation (SS½) were calculated using a nonlinear regression according to Baskurt et al.20 Any increase in EImax and/or decrease in SS½ indicates increased RBC deformability.20 The SS½ to EImax ratio, which is a more robust approach in reflecting alterations in deformability,19 was calculated to complete deformability outcomes. A low ratio represents high deformability.19
2.5.5. Nitrite analysis
Measurement and analysis of nitrite were performed with a chemiluminescence detector (CLD 88e; EcoPhysics, Duernten, Switzerland) and the Chart FIA software (EcoPhysics) described in detail elsewhere.14, 18 For RBC nitrite measurement, total RBCs and all subfractions were immediately mixed with preservation solution (800 mmol/L K3[Fe(CN)6], 100 mmol/L N-ethylmaleimide, 10 V-% Nonidet P-40, 90 V-% aqua dest.) in a 5:1 ratio, snap frozen, and stored at –80°C until measurement. Measurement of nitrite content was performed according to Hendgen-Cotta et al.21 Briefly, for measurement of RBC nitrite content, samples were mixed with methanol in a 1:2 ratio for protein precipitation and centrifuged at 21,000 g for 10 min at 4°C. Clear supernatant was separated from the pellet and collected in 1.5-mL microcentrifuge tubes (Eppendorf GmbH). The concentration of RBC nitrite was determined by injecting triplicates of 100 μL of each supernatant into an acidified tri-iodide solution that reduced nitrite to NO gas. The resulting NO was purged by a NaOH trap and transported into an ozone-based chemiluminescence NO detector via a constant helium gas-stream. Measured NO was analyzed with the Chart FIA software (EcoPhysics), and a calibration curve derived from an aqueous solution with a known nitrite solution was used to calculate the RBC nitrite concentration. Plasma nitrite samples were directly injected without further processing and analyzed as described. RBC nitrite concentration of the sample was corrected for nitrite levels of methanol and preservation solution.
2.5.6. RBC MP analysis
For RBC MP isolation, anticoagulated Na3-citrate blood was prepared according to the method of Jansen et al.22 and MP concentration was measured via flow cytometry as described in detail elsewhere.23 Briefly, after purification using sequential centrifugations, MPs were incubated with fluorescein isothiocyanate–annexin V (Beckman Coulter, Brea, CA, USA) and phycoerythrin-conjugated anti-CD235a (glycophorin A, clone 11E4B-7-6, IgG1; Beckman Coulter) monoclonal antibodies to identify MPs derived from RBCs. As an isotypic control, immunoglobulin G1 (IgG1) was used (679.1Mc7; Beckman Coulter). The Megamix kit (size-calibrated fluorescent microspheres of 0.5μm, 0.9 μm, and 3.0 µm (Biocytex, Marseille, France)) was used to obtain a reproducible gating for MPs, and Flow-CountTM fluorospheres (Beckman Coulter) were used to determine absolute concentration of MPs. Sample data were acquired on an FC500 MCL flow cytometer (Beckman Coulter) using the CXP acquisition software (Beckman Coulter). Both forward scatter and side scatter were set in a logarithmic gain and acquired at a low flow rate for 5 min for all samples.
2.6. Statistics
All data were tested for normality of distribution using the Shapiro-Wilk test. If data fulfilled the requirements for a normal distribution, a paired t test was conducted to reveal differences between pre- and post-testing. If requirements for a normal distribution were not met, Wilcoxon tests were conducted to reveal differences between pre- and post-testing. Statistical analyses were performed using the GraphPad Prism 5 software package (La Jolla, CA, USA). Differences were considered as significant with p < 0.05, unless otherwise marked. All data are expressed as mean ± SD.
3. Results
3.1. Performance data
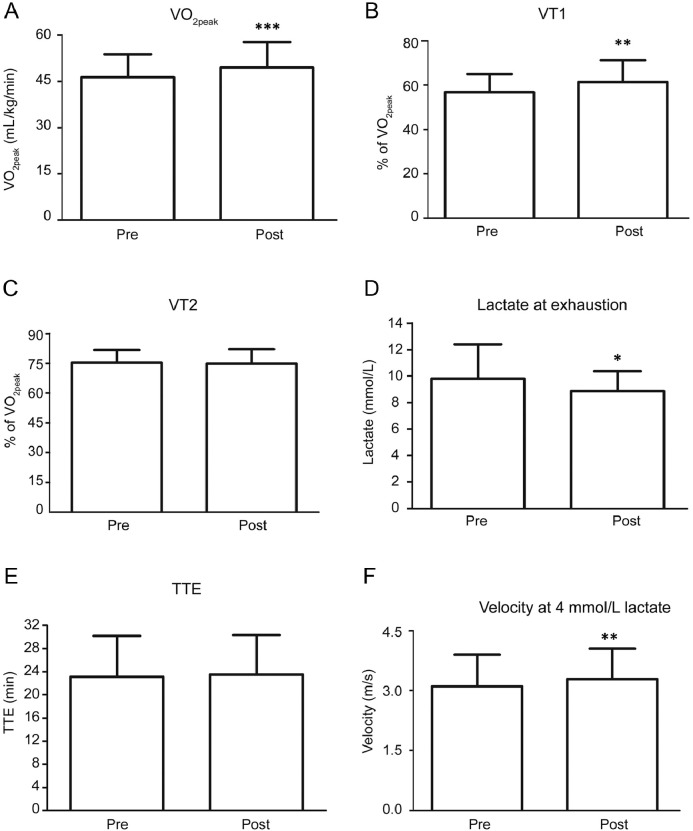
Endurance training significantly improved VO2peak (p < 0.001; Fig. 1A) and VT1 (p = 0.0046; Fig. 1B). Changes in VT2 (Fig. 1C) were not statistically significant. Peak lactate values at exhaustion significantly decreased after training compared with before (p = 0.025; Fig. 1D). TTE (Fig. 1E) was not significantly affected by the training. Running velocity at the 4 mmol/L lactate threshold significantly increased after training compared to before training (p = 0.004; Fig. 1F).
Fig. 1.
Performance parameters before (Pre) and after (Post) a 6-week endurance training. (A) VO2peak increased significantly; (B) Percentage of VT1 in relation to VO2peak increased significantly; (C) Percentage of VT2 in relation to VO2peak remained unchanged; (D) Lactate concentrations at exhaustion decreased significantly; (E) Paired t tests revealed no change in running duration until exhaustion; (F) Participants could maintain higher velocities at the 4 mmol/L threshold. *p < 0.05, **p < 0.01, ***p < 0.001, compared to pre-training. TTE = time to exhaustion; VO2peak = peak oxygen consumption; VT1 = first ventilatory threshold; VT2 = second ventilatory threshold.
3.2. RBC fractions
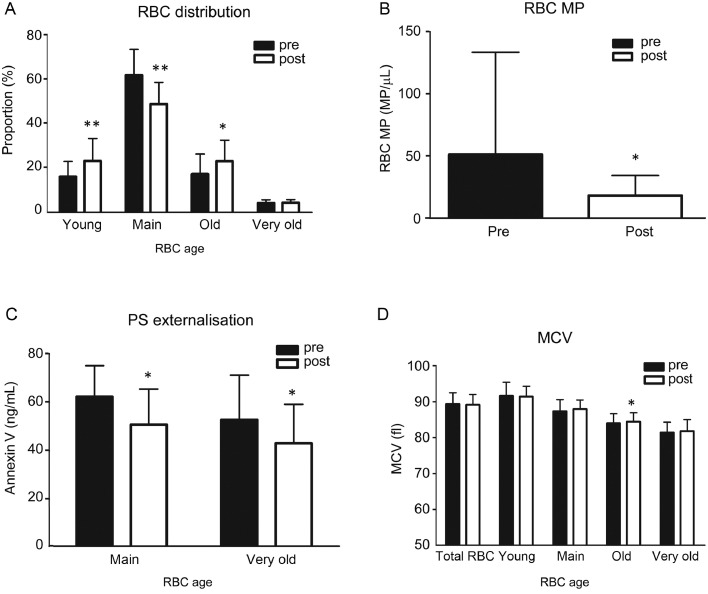
The relative distribution of the 4 different RBC subfractions that were determined according to their densities revealed that the relative amount of young RBCs (<1.064 g/mL) significantly increased after training from pretraining to post-training (p = 0.002; Fig. 2A). The relative amount of the main fraction (1.065–1.071 g/mL) significantly decreased (p < 0.001), and the relative amount of the old fraction (1.072–1.076 g/mL) significantly increased after training (p = 0.021). The relative amount of the very old fraction (>1.076 g/mL) did not show any difference between the pre- and post-training groups (p = 0.799).
Fig. 2.
RBC aging parameters before (Pre) and after (Post) a 6-week endurance training. (A) For RBC subfraction distribution, statistical analyses revealed that training resulted in a significant increase in the young and old fractions with a concomitant decrease of the main fraction. The very old fraction was not affected by the training; (B) Microparticles derived from RBCs decreased after training compared with Pre; (C) PS externalization represented by Annexin V concentration decreased in the main fraction as well as in the very old fraction; (D) MCV of the old fraction significantly increased. *p < 0.05, **p < 0.01, compared to pretraining. MCV = mean cellular volume; MP = microparticle; PS = phosphatidylserine; RBC = red blood cell.
3.3. RBC MPs, PS externalization, and MCV
MPs deriving from RBCs significantly decreased from before to after training (p = 0.044; Fig. 2B). Annexin V concentrations as a 1:1 determinant for PS externalization decreased in the main RBC population (p = 0.028) and in the very old RBC fraction (p = 0.022) from pretraining to post-training (Fig. 2C). The MCV was highest in the young fraction and lowest in the very old fraction, whereas MCV in the old fraction increased after training (p = 0.035; Fig. 2D). The MCV of the total RBCs did not change after training.
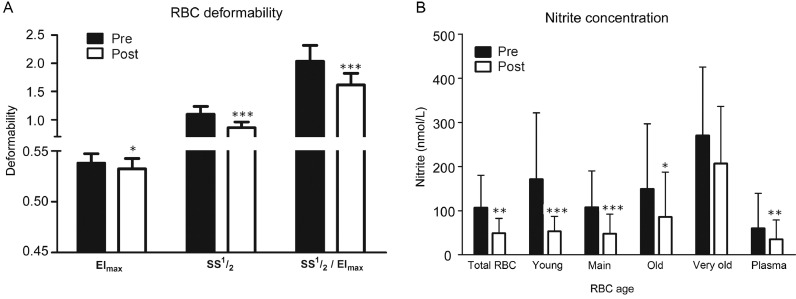
3.4. RBC deformability
The EImax of the total RBCs decreased after training compared with pretraining values (p = 0.039; Fig. 3A). The SS½ of the total RBCs significantly decreased after training (p < 0.001), as did the SS½ to EImax ratio (p < 0.001), indicating greater RBC deformability. The deformability of subfractions did not show any significant difference between the pretraining and post-training groups (data not shown).
Fig. 3.
RBC deformability and nitrite concentrations of subfractions before (Pre) and after (Post) a 6-week endurance training. (A) For the EImax, statistical analyses revealed a decrease in total RBCs. The shear stress required to achieve SS½ was markedly decreased in total RBCs. The calculated SS½ to EImax ratio decreased significantly between pre- and post-training; (B) Nitrite decreased in post-training in total RBCs and in all RBC samples except for the very old fraction and for the plasma nitrite. *p < 0.05, **p < 0.01, *** p< 0.001, compared with pretraining. EImax = theoretical maximal elongation index at infinite shear stress; RBC = red blood cell; SS½ = half-maximum deformation.
3.5. NO metabolism
In total RBCs, the nitrite concentration significantly decreased from pre- to post-training data (p = 0.001; Fig. 3B). Nitrite concentrations decreased in young RBCs (p < 0.001), main RBC fraction (p < 0.001), and old fraction (p = 0.020) in the post-training group. Nitrite levels remained unaltered in the very old RBC fraction (p = 0.065). Fig. 3B shows that plasma nitrite decreased from pretraining to post-training data (p = 0.002).
4. Discussion
This study sought to evaluate the effects of a 6-week running training program on RBC aging and distribution, and on RBC deformability in young, moderately fit, and moderately healthy untrained participants. RBC deformability is usually thought to be of major importance for athletic performance in providing sufficient oxygen to the working muscle because RBCs have to pass through capillaries with diameters smaller than their own.1, 9 In contrast with resting and nonexercising healthy individuals, whose blood profiles and aging parameters do not change significantly over time,24 trained individuals usually exhibit increased RBC deformability,8, 15 increased RBC turnover (with a decreased RBC life span), and a concomitantly greater proportion of young RBCs.25, 26
However, the interdependence of possible training-induced adaptations on the RBC population and hemorheological properties has not been examined thus far. The present study shows for the first time that a 6-week endurance training program involving submaximal running leads to a significant increase in the number of young RBCs, improved aerobic performance, and improved overall RBC deformability in individuals who are not endurance trained. The 6-week training period resulted in increased aerobic endurance performance with respect to VO2peak, VT1, and velocity measured at the 4 mmol/L lactate threshold. Furthermore, lactate concentration measured at exhaustion decreased. These results were very much expected, because the positive effects of regular aerobic endurance training are well-documented.24
Regular endurance exercise leads to an upregulation of catecholamines, cortisol, growth hormones, and insulin-like factors that stimulate bone marrow activity and increase erythropoiesis.1 Erythropoiesis leads to a greater number of reticulocytes, which in turn increases the number of young RBCs.24 This adaptation in response to the endurance training could be observed with an increased proportion of the young as well as the old RBC subpopulation. Interestingly, the relative proportion of the main RBC fraction significantly decreased concomitantly after the training in the present study, which was also observed in a previous training study with participants who had type 2 diabetes.14 A possible explanation might be related to the fact that regular endurance training accelerates RBC aging; thus, the formation of both young and old RCBs is favored by an overall increased RBC turnover. Mairbäurl et al.4 showed that trained individuals have more young, low-density cells with high 2,3-diphosphoglycerate concentrations and fewer old, high-density RBCs with impaired functionality. A greater proportion of young RBCs might contribute to improved oxygen transport and release in the microcirculation. These observations were recently confirmed by Tomschi et al.,9 whose study tested highly trained, moderately trained, and low-trained individuals in relation to their RBC age distribution. Highly trained individuals exhibited more young RBCs and overall improved hemorheological properties. Thus, the relatively large number of RBCs in the main fraction that was measured before training in the present study might have matured faster in response to the regular endurance exercise. This finding might explain the significantly increased number of old RBCs. Weight et al.25 observed an increased RBC turnover and reduced RBC life span (65–75 days) in highly active runners, indicating an overall rejuvenated RBC population in endurance-trained athletes.27 Therefore, although a nonexercising control was not integrated into the design of the current study, regular endurance training seems to accelerate the selective elimination of rigid and old RBCs via the reticuloendothelial macrophage system.14, 27
Adaptations of RBCs could be observed already after 6 weeks of training in the present study. RBC-derived MPs, whose formation can, among other things, be induced by high shear stress,28 decreased after training, as did phosphatidylserine externalization, which acts as a removal signal for the reticuloendothelial system and RBC degradation.29 Both reduced RBC-derived MPs and decreased phosphatidylserine externalization indicate reduced RBC senescence and later removal with chronic endurance exercise. These findings suggest that RBC adaptations during exercise possibly lead to less frequent membrane shedding, which could reduce circulating MP.7, 30 Regular endurance training could, therefore, improve the MP profile, as was assumed in a previous cross-sectional study.31
Increased formation of young RBCs and the faster removal of high-density old RBCs likely strengthens the entire RBC system, because old RBCs show increased membrane exocytosis to discard oxidative waste products.32, 33 This process in turn leads to a loss of cytosolic components, decreased MCV, decreased RBC deformability, and overall weakened RBC function.33 The successful separation with intact RBCs was indicated by MCV levels of total RBCs being in the normal range. The MCV decreased from the young to very old RBCs, thus confirming previous findings.18 The MCV increased in the old fraction after training. It was speculated that this increase in RBC volume could be favorable14, 18 because of the concomitant loss of volume during the aging process. A cell with an increased MCV might be capable of removing an increased amount of damaged and oxidized protein through an increased capacity of vesiculation. However, this hypothesis remains to be tested.
Total RBCs showed higher deformability values after training. Smith et al.8 found increased RBC deformability in world-class endurance athletes and ascribed this finding to the increased RBC turnover with a higher proportion of younger RBCs. The EImax describes the theoretical maximum deformability at infinite shear stress.34 The EImax is generally higher in endurance-trained athletes,8 but can temporarily decrease with unusually high training loads.35 In this study, the EImax values of total RBCs decreased after the training, indicating impaired maximum deformability, which is in accordance with previous studies.11 Increased lactic acid production and/or enhanced oxidative stress during training sessions could have contributed to the decrease in the EImax.11, 36 It is known that longer endurance training is needed to improve erythrocyte metabolism.36 Previous observations showed that a 12-week moderately intense treadmill walking program increased overall deformability.37 However, although our training program decreased the EImax, we also observed a decrease in the SS½, which indicated an overall improved deformability. This apparently inconsistent finding could be explained by the faster RBC aging process that was induced by training. Both results might point to more young RBCs in the circulation, which are more flexible (reduced SS1/2), but might show overall reduced EImax because of an altered surface-to-volume ratio. The increase of the young fraction lowered overall EImax, as was observed in previous studies, which showed that young RBCs exhibit a lower EImax compared with the main fraction owing to high cellular volume and ongoing membrane remodelling.14, 18 Therefore, an SS½ to EImax ratio was calculated, which is a more robust approach in reflecting alterations of deformability and covers 2 of the important parameters of RBC rheological properties.19 The markedly reduced ratio, indicating improved deformability, supports the assumption of training-induced positive effects on the hemorheological system.
A crucial parameter for increasing endothelial and hemorheological functionality is the main vasodilator NO.12, 38 Nitrite represents the primary oxidation product of NO39 and, thus, functions as a valid marker of NO synthesis. In the present study, NO concentrations significantly decreased in plasma, total RBCs, and the young, main, and old subfractions. Multivarious reaction pathways of NO exist in the RBCs, in which NO can be oxidized to form nitrite and finally nitrate39 or can result in the formation of RxNOs, which includes S-nitrosothiols or N-nitrosamines.21 Because bioactive NO in the microcirculation could be produced by several cells,40 the reason for the observed decrease in plasma nitrite can only be speculated. A decrease might result either by endothelial or RBC adaptations, but a specific interdependence with observed decreased RBC nitrite remains speculative. It was previously shown that especially very old and barely functioning RBCs exhibit high NO concentrations.18 It was suggested that the increased NO concentrations found in RBCs could counteract impaired RBC deformability, possibly by increasing NO-mediated modification of the cytoskeleton, which was shown to improve RBC deformability and mechanical properties.12 The findings of reduced nitrite and improved deformability might, therefore, be explained by 2 hypotheses: (1) the hemorheological adaptations caused by the endurance training are independent of NO adaptations in the RBC metabolism, or (2) less NO is needed to improve deformability owing to an improvement of antioxidative defenses with training and overall better RBC health, which might be caused by a rejuvenated and younger RBC population. To address these questions, further studies are needed to solve the possible interactions and elucidate the complex in vivo situation.
Different techniques exist to separate RBCs according to age, which exhibit different advantages and disadvantages. The use of Percoll density centrifugation as a separation medium is one established method to separate RBCs, but continuous or discontinuous gradient, as well as low-speed,18 high-speed,29 or counterflow centrifugation,5 are applied. Therefore, an established and widely accepted protocol is missing. This factor limits the transferability of the present study to other RBC aging studies. In addition, the only reliable method of measuring the exact RBC aging is in vivo labelling and calculation of the biological half-life of RBCs, whereas the separation in this study only yielded “young to very old” RBCs, which could disguise further RBC aging properties after training. This study used buffer systems established by Bosch et al.,5 which led to RBCs with different buoyant densities, normally expected to be around 1.07–1.11 g/mL.4 Therefore, although blood profile and microscopic approaches were used to determine cell integrity, an impact of these buffers on in vitro RBCs shrinking or swelling cannot fully be excluded. In addition, although previous studies did not show any aging effects in the untrained populations,4, 18,24 a nonexercising control group might be integrated in prospective studies to determine the differences in RBC aging alterations between training and resting individuals.
5. Conclusion
It was previously shown that highly endurance-trained athletes have a younger RBC population with improved deformability and oxygen transport/release properties compared to the average population.3, 9 In endurance-trained athletes, younger RBC populations with a lower mean age are induced by enhanced shear stress in the cardiovascular system and by increased oxidative stress, which limit the average RBC aging and increase RBC turnover.24 Therefore, faster RBC aging seems like a promising way for the blood system to adapt faster to changed exercise activity by increasing old RBC removal and continuously increasing the number of younger RBCs with improved properties. However, the time needed for erythropoietic adaptations of RBCs, the impact of the exercise intensity or form of endurance sport, and the influence of a younger RBC population on athletic performance are still under debate. The present study found that a 6-week, moderately intensive endurance training program leads to improved aerobic capacity in young, moderately fit participants who had not previously had endurance training. The benefits of the program included a generally younger RBC population and increased overall RBC deformability. Therefore, the present study supports the idea that blood rheology and the distribution of the RBC population can be rapidly influenced by endurance training, but their interdependence with increased aerobic capacities remains to be investigated. It should be noted that, although the present study supports beneficial RBC adaptations through moderate endurance running, research involving prolonged training periods, different training intensities (e.g., high-intensity marathon running), and alternative exercises with weight-bearing activities (e.g., floor exercises in gymnastics) that may impact increased foot hemolysis are needed to give more insights into the long-term adaptation processes.
Acknowledgments
Acknowledgments
The authors thank Bianca Collins, Anika Voß, Thomas Dietz, Kristina Oden, and Anke Schmitz for indispensable technical assistance.
This article is supported by the Hochschulinterne Forschungsförderung (HIF) of the German Sport University Cologne. The sponsor of this study is a non-profit organization that supports science in general. The sponsor had no role in gathering, analyzing, or interpreting the data; in writing the manuscript; or in deciding to submit the manuscript for publication.
Authors’ contribution
DAB and FT designed the study, performed the experiments, data collection, and data analysis, and drafted the manuscript; GB performed the experiments and helped to coordinate the training; EN and MR performed MP experiments and analysis; PC performed MP analysis and helped to draft the manuscript; WB helped to draft and to edit the manuscript; MG designed the study and helped to draft the manuscript. All authors read and approved the final version of the manuscript, and agreed with the order of presentation of the authors.
Competing interest
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Mairbäurl H. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front Physiol. 2013;4:332. doi: 10.3389/fphys.2013.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palis J. Primitive and definitive erythropoiesis in mammals. Front Physiol. 2014;5:3. doi: 10.3389/fphys.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohandas N., Groner W. Cell membrane and volume changes during red cell development and aging. Ann N Y Acad Sci. 1989;554:217–224. doi: 10.1111/j.1749-6632.1989.tb22423.x. [DOI] [PubMed] [Google Scholar]
- 4.Mairbäurl H., Humpeler E., Schwaberger G., Pessenhofer H. Training-dependent changes of red cell density and erythrocytic oxygen transport. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1403–1407. doi: 10.1152/jappl.1983.55.5.1403. [DOI] [PubMed] [Google Scholar]
- 5.Bosch F.H., Werre J.M., Roerdinkholder-Stoelwinder B., Huls T.H., Willekens F.L., Halie M.R. Characteristics of red blood cell populations fractionated with a combination of counterflow centrifugation and Percoll separation. Blood. 1992;79:254–260. [PubMed] [Google Scholar]
- 6.Linderkamp O., Meiselman H.J. Geometric, osmotic, and membrane mechanical properties of density-separated human red cells. Blood. 1982;59:1121–1127. [PubMed] [Google Scholar]
- 7.Sossdorf M., Otto G.P., Claus R.A., Gabriel H.H., Losche W. Cell-derived microparticles promote coagulation after moderate exercise. Med Sci Sports Exerc. 2011;43:1169–1176. doi: 10.1249/MSS.0b013e3182068645. [DOI] [PubMed] [Google Scholar]
- 8.Smith J.A., Martin D.T., Telford R.D., Ballas S.K. Greater erythrocyte deformability in world-class endurance athletes. Am J Physiol. 1999;276:H2188–H2193. doi: 10.1152/ajpheart.1999.276.6.H2188. [DOI] [PubMed] [Google Scholar]
- 9.Tomschi F., Bizjak D., Bloch W., Latsch J., Predel H.G., Grau M. Deformability of different red blood cell populations and viscosity of differently trained young men in response to intensive and moderate running. Clin Hemorheol Microcirc. 2018;69:503–514. doi: 10.3233/CH-189202. [DOI] [PubMed] [Google Scholar]
- 10.El-Sayed M.S., Ali N., El-Sayed Ali Z. Haemorheology in exercise and training. Sports Med. 2005;35:649–670. doi: 10.2165/00007256-200535080-00001. [DOI] [PubMed] [Google Scholar]
- 11.Connes P., Simmonds M.J., Brun J.F., Baskurt O.K. Exercise hemorheology: classical data, recent findings and unresolved issues. Clin Hemorheol Microcirc. 2013;53:187–199. doi: 10.3233/CH-2012-1643. [DOI] [PubMed] [Google Scholar]
- 12.Grau M., Pauly S., Ali J., Walpurgis K., Thevis M., Bloch W. RBC-NOS-dependent S-nitrosylation of cytoskeletal proteins improves RBC deformability. PLoS One. 2013;8:e56759. doi: 10.1371/journal.pone.0056759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linderkamp O., Friederichs E., Meiselman H.J. Mechanical and geometrical properties of density-separated neonatal and adult erythrocytes. Pediatr Res. 1993;34:688–693. doi: 10.1203/00006450-199311000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Brinkmann C., Bizjak D.A., Bischof S., Latsch J., Brixius K., Bloch W. Endurance training alters enzymatic and rheological properties of red blood cells (RBC) in type 2 diabetic men during in vivo RBC aging. Clin Hemorheol Microcirc. 2016;63:173–184. doi: 10.3233/CH-151957. [DOI] [PubMed] [Google Scholar]
- 15.Suhr F., Brenig J., Müller R., Behrens H., Bloch W., Grau M. Moderate exercise promotes human RBC-NOS activity, NO production and deformability through Akt kinase pathway. PLoS One. 2012;7:e45982. doi: 10.1371/journal.pone.0045982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaver W.L., Wasserman K., Whipp B.J. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 17.Schneider D.A., Phillips S.E., Stoffolano S. The simplified V-slope method of detecting the gas exchange threshold. Med Sci Sports Exerc. 1993;25:1180–1184. [PubMed] [Google Scholar]
- 18.Bizjak D.A., Brinkmann C., Bloch W., Grau M. Increase in red blood cell-nitric oxide synthase dependent nitric oxide production during red blood cell aging in health and disease: a study on age dependent changes of rheologic and enzymatic properties in red blood cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baskurt O.K., Meiselman H.J. Data reduction methods for ektacytometry in clinical hemorheology. Clin Hemorheol Microcirc. 2013;54:99–107. doi: 10.3233/CH-2012-1616. [DOI] [PubMed] [Google Scholar]
- 20.Baskurt O.K., Boynard M., Cokelet G.C., Connes P., Cooke B.M., Forconi S. New guidelines for hemorheological laboratory techniques. Clin Hemorheol Microcirc. 2009;42:75–97. doi: 10.3233/CH-2009-1202. [DOI] [PubMed] [Google Scholar]
- 21.Hendgen-Cotta U., Grau M., Rassaf T., Gharini P., Kelm M., Kleinbongard P. Reductive gas-phase chemiluminescence and flow injection analysis for measurement of the nitric oxide pool in biological matrices. Methods Enzymol. 2008;441:295–315. doi: 10.1016/S0076-6879(08)01216-0. [DOI] [PubMed] [Google Scholar]
- 22.Jansen F., Wang H., Przybilla D., Franklin B.S., Dolf A., Pfeifer P. Vascular endothelial microparticles-incorporated microRNAs are altered in patients with diabetes mellitus. Cardiovasc Diabetol. 2016;15:49. doi: 10.1186/s12933-016-0367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hierso R., Lemonne N., Villaescusa R., Lalanne-Mistrih M.L., Charlot K., Etienne-Julan M. Exacerbation of oxidative stress during sickle vaso-occlusive crisis is associated with decreased anti-band 3 autoantibodies rate and increased red blood cell-derived microparticle level: a prospective study. Br J Haematol. 2017;176:805–813. doi: 10.1111/bjh.14476. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt W., Maassen N., Trost F., Boning D. Training induced effects on blood volume, erythrocyte turnover and haemoglobin oxygen binding properties. Eur J Appl Physiol Occup Physiol. 1988;57:490–498. doi: 10.1007/BF00417998. [DOI] [PubMed] [Google Scholar]
- 25.Weight L.M., Byrne M.J., Jacobs P. Haemolytic effects of exercise. Clin Sci (Lond) 1991;81:147–152. doi: 10.1042/cs0810147. [DOI] [PubMed] [Google Scholar]
- 26.Smith J.A. Exercise, training and red blood cell turnover. Sports Med. 1995;19:9–31. doi: 10.2165/00007256-199519010-00002. [DOI] [PubMed] [Google Scholar]
- 27.Robinson Y., Cristancho E., Böning D. Intravascular hemolysis and mean red blood cell age in athletes. Med Sci Sports Exerc. 2006;38:480–483. doi: 10.1249/01.mss.0000188448.40218.4c. [DOI] [PubMed] [Google Scholar]
- 28.Burnier L., Fontana P., Kwak B.R., Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009;101:439–451. [PubMed] [Google Scholar]
- 29.Huang Y.X., Wu Z.J., Mehrishi J., Huang B.T., Chen X.Y., Zheng X.J. Human red blood cell aging: correlative changes in surface charge and cell properties. J Cell Mol Med. 2011;15:2634–2642. doi: 10.1111/j.1582-4934.2011.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fruhbeis C., Helmig S., Tug S., Simon P., Kramer-Albers E.M. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles. 2015;4:28239. doi: 10.3402/jev.v4.28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bittencourt C.R.O., Izar M.C.O., Franca C.N., Schwerz V.L., Póvoa R.M.D.S., Fonseca F.A.H. Effects of chronic exercise on endothelial progenitor cells and microparticles in professional runners. Arq Bras Cardiol. 2017;108:212–216. doi: 10.5935/abc.20170022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzanti L., Faloia E., Rabini R.A., Staffolani R., Kantar A., Fiorini R. Diabetes mellitus induces red blood cell plasma membrane alterations possibly affecting the aging process. Clin Biochem. 1992;25:41–46. doi: 10.1016/0009-9120(92)80044-h. [DOI] [PubMed] [Google Scholar]
- 33.Antonelou M.H., Kriebardis A.G., Papassideri I.S. Aging and death signalling in mature red cells: from basic science to transfusion practice. Blood Transfus. 2010;8(Suppl. 3):S39–S47. doi: 10.2450/2010.007S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardeman M.R., Goedhart PT, Dobbe JGG, Lettinga KP. Laser-assisted optical rotational cell analyser (L.O.R.C.A.); I. A new instrument for measurement of various structural hemorheological parameters. Clin Hemorheol Microcirc. 1994;14:605–618. [Google Scholar]
- 35.Suhr F., Porten S., Hertrich T., Brixius K., Schmidt A., Platen P. Intensive exercise induces changes of endothelial nitric oxide synthase pattern in human erythrocytes. Nitric Oxide. 2009;20:95–103. doi: 10.1016/j.niox.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Connes P., Bouix D., Py G., Prefaut C., Mercier J., Brun J.F. Opposite effects of in vitro lactate on erythrocyte deformability in athletes and untrained subjects. Clin Hemorheol Microcirc. 2004;31:311–318. [PubMed] [Google Scholar]
- 37.Simmonds M.J., Minahan C.L., Serre K.R., Gass G.C., Marshall-Gradisnik S.M., Haseler L.J. Preliminary findings in the heart rate variability and haemorheology response to varied frequency and duration of walking in women 65-74 yr with type 2 diabetes. Clin Hemorheol Microcirc. 2012;51:87–99. doi: 10.3233/CH-2011-1514. [DOI] [PubMed] [Google Scholar]
- 38.Simmonds M.J., Detterich J.A., Connes P. Nitric oxide, vasodilation and the red blood cell. Biorheology. 2014;51:121–134. doi: 10.3233/BIR-140653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grau M., Hendgen-Cotta U.B., Brouzos P., Drexhage C., Rassaf T., Lauer T. Recent methodological advances in the analysis of nitrite in the human circulation: nitrite as a biochemical parameter of the L-arginine/NO pathway. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:106–123. doi: 10.1016/j.jchromb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Shu X., Keller T.C., 4th, Begandt D., Butcher J.T., Biwer L., Keller A.S. Endothelial nitric oxide synthase in the microcirculation. Cell Mol Life Sci. 2015;72:4561–4575. doi: 10.1007/s00018-015-2021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]