Abstract
Objective
To assess the proportion of patients with cirrhosis up to date with vaccinations and associations of vaccination with age, sex, race, ethnicity, marital status, and type of provider follow-up.
Patients and Methods
Patients with cirrhosis diagnosed at Mayo Clinic in Rochester and Mayo Clinic Health System in Minnesota from January 1, 2007, to December 31, 2009, were followed up from diagnosis until May 31, 2015. Data were abstracted from Mayo Clinic and Minnesota State records. Factors determining vaccination coverage were assessed.
Results
At the end of the study period (8 years follow-up), 26.4% (95 of 360), 24.7% (82 of 332), 63.2% (180 of 285), and 25.5% (54 of 212) of patients with cirrhosis were up to date with hepatitis A virus (HAV), hepatitis B virus, pneumococcal pneumonia (PN), and herpes zoster vaccinations, respectively. Influenza (FLU) vaccine coverage increased from 36.1% (57 of 158) in 2007 to 2008 to 65.8% (106 of 161) in 2014 to 2015. Of those unvaccinated for HAV and hepatitis B virus before cirrhosis diagnosis, 18.6% (59 of 318) and 23.4% (71 of 304) completed vaccination. For HAV, more whites than nonwhites (28.3% [91 of 322] vs 10.5% [4 of 38]; odds ratio [OR], 3.35; 95% CI, 1.29 to 11.45; P=.02) and more non-Hispanics than Hispanics (27.4% [95 of 347] vs 0% [0 of 13]; OR, 0.00; 95% CI, 0.00 to 0.43; P=.03) were vaccinated. For PN, more younger than elderly people (66.8% [135 of 202] vs 54.2% [45 of 83]; OR, 1.70; 95% CI, 1.01 to 2.87; P=.04) and married vs single people (56.8% [100 of 176] vs 73.4% [80 of 109]; OR, 2.10; 95% CI, 1.26 to 3.56; P=.005) were vaccinated. For FLU, in 2013 to 2014, more elderly (72.0% [54 of 75] vs 58.0% [69 of 119]; OR, 0.54; 95% CI, 0.28 to 0.99; P=.05); in 2008 to 2009, more Hispanics (100% [4 of 4] vs 41.6% [116 of 279]; OR, ∞; 95% CI, 2.25 to ∞; P=.02); and in 2011 to 2012, more married people (62.4% [101 of 162] vs 50.5% [56 of 111]; OR, 1.63; 95% CI, 0.1.0 to 2.66; P=.05) were vaccinated. For FLU in 2008 to 2009, coverage was higher in the primary care than the specialist setting (55.8% [48 of 86] vs 36.6% [72 of 197]; P=.003).
Conclusion
Except for PN and FLU, vaccination coverage in patients with cirrhosis falls short of Healthy People 2020 target. Specific interventions are needed to improve vaccination coverage in patients with cirrhosis.
Abbreviations and Acronyms: ACIP, Advisory Committee on Immunization Practices; CLD, chronic liver disease; EMR, electronic medical record; FLU, influenza; GIH, gastroenterologist and/or hepatologist; HAV, hepatitis A virus; HBV, hepatitis B virus; HR, high-risk people; HZ, herpes zoster; LT, liver transplant specialist; OR, odds ratio; PCP, primary care provider; PN, pneumococcal pneumonia
Vaccination of patients with cirrhosis against hepatitis A (HAV), hepatitis B (HBV), pneumococcal pneumonia (PN), herpes zoster (HZ), and influenza (FLU) are recommended by the Advisory Committee on Immunization Practices (ACIP).
Chronic liver disease (CLD) including cirrhosis is the 12th leading cause of death in the United States, accounting for 34,000 to 38,000 annual deaths recently.1,2 However, considering additional liver-related causes of death such as hepatorenal syndrome, viral hepatitis, hepatic encephalopathy, and hepatobiliary cancers, liver-related mortality was found to rank as high as fourth for some age groups and eighth overall.3
Patients with cirrhosis are considered to be immunocompromised and are more susceptible to infections with an increased risk for subsequent complications and death, especially when the liver disease is more advanced and decompensated.4, 5, 6, 7, 8, 9, 10, 11, 12 The cost of managing liver disease is significant. The ACIP recommends vaccinating all people with CLD and cirrhosis against HAV, HBV, PN, HZ, and FLU.13 The US Preventive Services Task Force has recommended that health care providers incorporate vaccination needs assessment, recommendation, and offer of vaccination into routine clinical practice for adult patients to improve vaccination rates.14,15 Studies have stressed the importance of such efforts.9,16, 17, 18 Despite these recommendations, data reported during the past one and a half decades indicate that immunization in this group of patients remains low (<20% for HAV, 32%-34% for HBV, and 20% for PN16,18, 19, 20, 21; Table 1). Lack of physician enthusiasm, missed opportunities, and the proliferation of other quality measures have been suggested as potential reasons for nonadherence.22, 23, 24 With the present trends, current strategies are unlikely to achieve the immunization goals of Healthy People 2020.25
Table 1.
Summary of 2007 to 2014 National Adult Immunization Coverage (Centers for Disease Control and Prevention/National Health Interview Survey)a
| Vaccine | Age Group (y) | 2007, % | 2008, % | 2009, % | 2010, % | 2011, % | 2012, % | 2013, % | 2014, % | 2015, % |
|---|---|---|---|---|---|---|---|---|---|---|
| PN | 19-49 | 32.3 | 24.9 | 17.5 | 18.5 | 20.1 | 20.0 | |||
| 18-64 | 19.4 | 16.6 | 17.4 | 18.3 | 20.0 | |||||
| ≥65 | 65.6 | 60.0 | 60.6 | 59.5 | 62.3 | 59.9 | ||||
| FLU | ≥19 | 33.6 | 40.4 | 40.5 | 38.8 | 41.5 | 42.2 | 43.6 | ||
| 19-49 | 30.5 | 28.6 | 31.1 | 32.3 | ||||||
| 19-49 HR | 37.3 | 30.0 | 33.4 | 39.0 | 36.8 | 39.8 | ||||
| 19-49 non-HR | 17.4 | 19.7 | ||||||||
| 50-64 total | 38.7 | 44.5 | 42.7 | 45.1 | 45.3 | |||||
| 50-64 HR | 48.8 | 51.5 | ||||||||
| 50-64 non-HR | 34.4 | 34.2 | ||||||||
| ≥65 | 66.6 | 65.6 | 66.6 | 64.9 | 66.2 | 65.0 | ||||
| 18-64 | 24.9 | 34.8 | 33.1 | 35.7 | 36.7 | |||||
| 18-64 HR | 38.6 | 46.7 | 45.2 | 47.0 | 46.3 | |||||
| 18-64 non-HR | 33.9 | |||||||||
| HAV total | 19-49 | 12.1 | 8.8 | 9.8 | 10.7 | 12.5 | 12.2 | |||
| HAV HR | 14.6 | |||||||||
| HAV CLD | 19.7 | 17.1 | ||||||||
| HBV total | 19-49 | 23.4 | 35.9 | 35.3 | ||||||
| HBV HR | 31.6 | 41.8 | 42.0 | |||||||
| HBV non-HR | 33.8 | 33.7 | 33.1 | |||||||
| HBV CLD | ||||||||||
| HBV | ≥ 65 | |||||||||
| HZ | ≥ 60 | 1.9 | 6.7 | 10.0 | 14.4 | 15.8 | 20.1 |
aCLD = chronic liver disease; FLU = influenza; HAV = hepatitis A virus; HBV = hepatitis B virus; HR = high-risk people; HZ = herpes zoster; PN = pneumococcal pneumonia.
Hepatitis A and B virus infection or superinfection in CLD is associated with severe disease, including fulminant hepatic failure and increased morbidity and mortality.26, 27, 28, 29 Vaccination for HAV and HBV is safe and effective in patients with CLD and cirrhosis.30, 31, 32, 33, 34, 35, 36
Bacterial infections occur in 32% to 34% of hospitalized patients with cirrhosis and in approximately 44% of those admitted with gastrointestinal hemorrhage.10,37,38 Pneumonia is one of the major infectious diseases in patients with cirrhosis, beside spontaneous bacterial peritonitis and urinary tract infections, and carries the highest inpatient mortality risk of the 3 infections.6,10,39, 40, 41, 42 Invasive pneumonia can lead to severe sepsis with multiorgan failure and higher case fatality in hospitalized patients with cirrhosis than in patients with other chronic conditions.29 Community-acquired pneumonia is also associated with higher mortality in patients with cirrhosis compared with those without cirrhosis.42 Thus vaccination against PN is a critical preventative measure in patients with CLD.
Herpes zoster vaccine is associated with a significantly reduced risk (>50%) for HZ disease, including in patients with chronic medical conditions (hazard ratio, 0.45; 95% CI, 0.42 to 0.48).43
Patients with CLD, including those with cirrhosis, especially those who are decompensated, are at increased risk for severe disease and poor outcomes in the setting of FLU infection. Thus, preventative vaccination and early detection and treatment of FLU are recommended.44, 45, 46, 47, 48 In addition, FLU vaccine has been shown to be safe and effective in CLD and cirrhosis.49, 50, 51, 52, 53, 54, 55, 56 For example, FLU vaccination during the 2012 to 2013 season resulted in an estimated 79,260 fewer hospitalizations, 6.6 million fewer cases of FLU, and 3.2 million fewer medically attended cases.49 These results support the importance of FLU vaccination and highlight the need for increasing vaccination coverage rates. Recent statistics and studies revealed that current trends are far from reaching the goals of achieving the target vaccination coverage goals of 2020.57
Unfortunately, the immunization coverage of patients with CLD and especially with cirrhosis has not been widely studied. There have been only a few high-quality studies.16,18,19,21 A study done in 200558 revealed that patients seen in primary care received more FLU and pneumonia vaccinations. Those seen in specialist centers were predominantly vaccinated for HAV and HBV. It also concluded that patients vaccinated on site have higher coverage. Furthermore, there are no specific recent data for vaccination trends against FLU and HZ in this group. None of these reports studied all 5 recommended adult vaccinations (FLU, PN, HAV, HBV, and HZ). In addition, none of the studies for HAV and HBV vaccination provided details of whether they were up to date with their vaccine series at the end of follow-up of the studies.
Patients and Methods
The study population included patients with cirrhosis diagnosed at Mayo Clinic and Mayo Clinic Health System clinics in Minnesota from January, 1 2007, to December 31, 2009, and who were followed up at Mayo Clinic and Mayo Clinic Health System from the time of diagnosis until the end of the study period on May 31, 2015, or until they died, underwent liver transplant, or were lost to follow-up.
The diagnosis of cirrhosis was made based on International Classification of Diseases, Ninth Revision codes 571.2, 571.5, and 571.6 and/or keywords such as liver/hepatic cirrhosis and further confirmed by histopathology; radiology (shrunken, irregular, or nodular liver suggestive of cirrhosis); clinical features such as ascites, splenomegaly, esophageal or abdominal varices, spontaneous bacterial peritonitis, hepatic encephalopathy, hepatocellular carcinoma, or hepatorenal syndrome; or through documented expert diagnosis obtained from the electronic medical record (EMR) of Mayo Clinic. The immunization status of HBV, HAV, PN, FLU, and HZ in both primary care and specialty groups was obtained from the EMR and/or from the Minnesota Immunization Information Connection (Minnesota State Records). Demographic characteristics were obtained from the EMR. The provider was either a primary care provider (PCP) or a specialist, that is, gastroenterologist and/or hepatologist (GIH) including liver transplant hepatologist. If the patient was equally frequently followed up by a PCP and GIH, they were considered followed up by a GIH (N=3)
The primary end point of the study was to determine the proportion of patients with cirrhosis who received and were uptodate with immunization against HAV, HBV, PN, FLU, and HZ. Secondary end points were to determine the effects of age, sex, race, ethnicity, marital status, and type of provider follow-up on vaccination coverage, as well as to determine the time from eligibility to the first dose of vaccine, excluding FLU vaccine.
Patients who completed vaccinations before the cirrhosis diagnosis, those with a history of HAV or HBV infection before diagnosis, those who died or were lost to follow-up within the first 6 months of the follow-up period, and those who underwent liver transplant within the first 6 months of the follow-up period were excluded.
Data Analyses
We used mean ± SD and Wilcoxon nonparametric test for continuous variables (eg, age) and χ2 test for categorical (nominal) variables. Statistical significance for comparisons was designated as P<.05. We also obtained odds ratios (ORs) and area under the receiver operating characteristic curve using univariate logistic regression. Data analysis was performed using JMP, version 10 (SAS Institute Inc).
Results
Study Sample
A total cohort of 398 patients with cirrhosis diagnosed between January 1, 2007, and December 31, 2009, was obtained. Demographic and patient characteristics are summarized in Table 2. A total of 279 (70%) were younger than 65 years, 230 (58%) were males, 357 (90%) were white, and 236 (59%) were married. A total of 265 (66.6) were followed up mainly by a GIH or LT (specialist) and 133 (33.4%) by a PCP.
Table 2.
Demographic Characteristics of Study Population
| Characteristic | N=398 |
|---|---|
| Age (y), no. (%) | |
| <65 | 279 (70.1) |
| ≥65 | 119 (29.9) |
| Sex, no. (%) | |
| Male | 230 (57.8) |
| Female | 168 (42.2) |
| Race, no. (%) | |
| White | 357 (89.7) |
| African American | 11 (2.8) |
| Asian | 8 (2.0) |
| American Indian/Alaskan Native | 6 (1.5) |
| Other | 11 (2.8) |
| Unknown | 5 (1.3) |
| Ethnicity, no. (%) | |
| Not Hispanic or Latino | 351 (88.2) |
| Hispanic or Latino | 14 (3.5) |
| Unknown | 33 (8.3) |
| Marital status, no. (%) | |
| Married | 236 (59.3) |
| Divorced | 68 (17.1) |
| Single | 61 (15.3) |
| Widowed | 33 (8.3) |
| Type of cirrhosis, no. (%) | |
| Alcoholic cirrhosis of liver | 129 (32.4) |
| Cirrhosis of liver without mention of alcohol | 239 (60.1) |
| Biliary cirrhosis | 30 (7.5) |
| Provider, no. (%) | |
| Gastroenterologist/hepatologist | 265 (66.6) |
| Primary care provider | 133 (33.4) |
HAV Vaccination
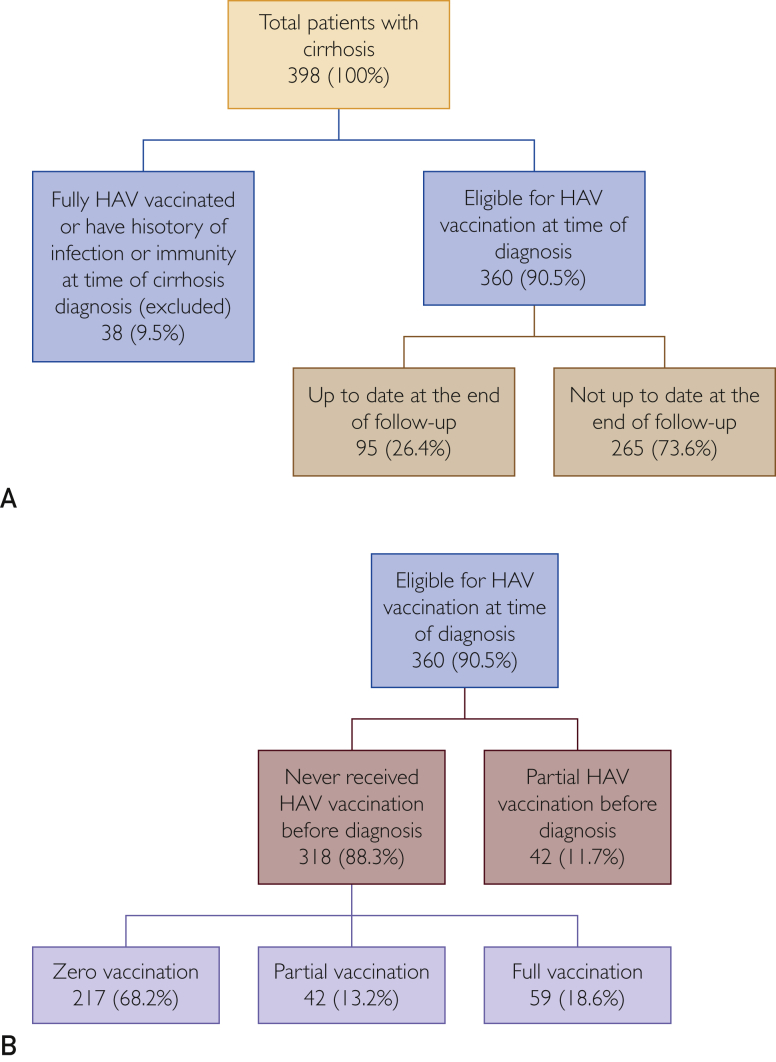
Of the 398 patients, 38 (9.5%) were excluded because they were either fully vaccinated or had a history of HAV infection/immunity at the time they had cirrhosis diagnosed. A total of 360 (90.5%) patients were eligible for vaccination at the time of cirrhosis diagnosis. The eligible patients either never received vaccination before cirrhosis diagnosis (n=318; 88%) or received only partial vaccination (n=42; 12%). Of the 360 patients eligible for vaccination, only 95 (26.4%) were up to date with their HAV vaccination at the end of the follow-up period (Figure 1A).
Figure 1.
Hepatitis A virus (HAV) vaccination coverage from 2007 to 2015 in Minnesotans with cirrhosis diagnosed between 2007 and 2009 at Mayo Clinic.
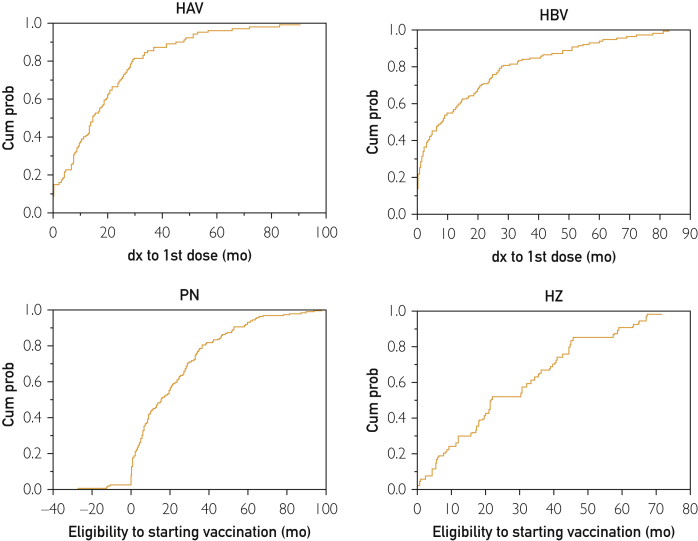
Of those who never received HAV vaccination before the cirrhosis diagnosis (n=318), 217 (68.2%) received zero vaccination, 42 (13.2%) received partial vaccination, and only 59 (18.6%) completed their vaccination series during the follow-up period (Figure 1B). Of the 101 patients who received vaccination during follow-up, only 40 (39.6%) received their first dose within the first year after the cirrhosis diagnosis (Figure 2).
Figure 2.
Time from diagnosis (dx) of cirrhosis to start of vaccination (hepatitis A virus [HAV] and hepatitis B virus [HBV]) or time from vaccine eligibility to start of vaccinations (pneumococcal pneumonia [PN] or herpes zoster [HZ]). Cum prob = cumulative probability.
In terms of demographic characteristics, 27.5% (69 of 251) of those who were younger than 65 years vs 24.1% (26 of 108) of those 65 years or older (OR, 1.20; 95% CI, 0.72 to 2.04; P=.50), 24.2% (50 of 207) of males vs 29.4% (45 of 153) of females (OR, 0.76; 95% CI, 0.48 to 1.23; P=.26), 28.3% (91 of 322) of whites vs 10.5% (4 of 38) of nonwhites (OR, 3.35; 95% CI, 1.29 to 11.45; P=.02), 0.0% (0 of 13) of Hispanics or Latinos vs 27.4 (95 of 347) of other ethnicities (OR, 0.00; 95% CI, 0.00 to 0.43; P=.03), 25.6% (54 of 211) of those who are married vs 27.5% (41 of 149) of singles (OR, 0.91; 95% CI, 0.56 to 1.46; P=.68), 26.3% (64 of 243) of those who were followed up by a specialist vs 26.5% (31 of 117) followed up by a PCP (OR, 0.99; 95% CI, 0.60 to 1.64; P=.97) were up to date with their HAV vaccination at the end of follow-up (Table 3)
Table 3.
Vaccination Coverage for HAV, HBV, PN, and HZ by Demographics and Provider From 2007 to 2015 in Minnesotans With Cirrhosis Diagnosed Between 2007 and 2009 at Mayo Clinica
| Age (y) |
Sex |
Race |
Ethnicity |
Marital Status |
Provider |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <65 | ≥65 | Male | Female | White | Nonwhite | Hispanic or Latino | Other | Married/Partner | Single | Specialist | PCP | |
| HAV | ||||||||||||
| Up to date, no. (%) | 69 (27.5) | 26 (24.1) | 50 (24.2) | 45 (29.4) | 91 (28.3) | 4 (10.5) | 0 (0.0) | 95 (27.4) | 54 (25.6 ) | 41 (27.5) | 64 (26.3) | 31 (26.5) |
| Not up to date, no. (%) | 182 (72.5) | 82 (75.9) | 157 (75.8) | 108 (70.6) | 231 (71.7) | 34 (89.5) | 13 (100.0) | 252 (72.6) | 157 (74.4) | 108 (72.5) | 179 (73.7) | 86 (73.5) |
| Total | 251 | 108 | 207 | 153 | 322 | 38 | 13 | 347 | 211 | 149 | 243 | 117 |
| P | .50 | .26 | .02 | .03 | .68 | .97 | ||||||
| HBV | ||||||||||||
| Up to date, no. (%) | 59 (26.2) | 23 (21.5) | 39 (20.9) | 43 (29.7) | 77 (25.3) | 5 (17.9) | 1 (9.1) | 81 (25.2) | 50 (26.0) | 32 (22.9) | 54 (24.0) | 28 (26.2) |
| Not up to date, no. (%) | 166 (73.8) | 84 (78.5) | 148 (79.1) | 102 (70.3) | 227 (74.7) | 23 (82.1) | 10 (90.9) | 240 (74.8) | 142 (74.0) | 108 (77.1) | 171 (76.0) | 79 (73.8) |
| Total | 225 | 107 | 187 | 145 | 304 | 28 | 11 | 321 | 192 | 140 | 225 | 107 |
| P | .35 | .07 | .38 | .22 | .51 | .67 | ||||||
| PN | ||||||||||||
| Up to date, no. (%) | 135 (66.8) | 45 (54.2) | 110 (62.9) | 70 (63.6) | 166 (64.3) | 14 (51.9) | 5 (55.6) | 175 (63.4) | 100 (56.8 ) | 80 (73.4) | 121 (61.4) | 59 (67.0) |
| Not up to date, no. (%) | 67 (33.2) | 38 (45.8) | 65 (37.1) | 40 (36.4) | 92 (35.7) | 13 (48.1) | 4 (44.4) | 101 (36.6) | 76 (43.2) | 29 (26.6) | 76 (38.6) | 29 (33.0) |
| Total | 202 | 83 | 175 | 110 | 258 | 27 | 9 | 276 | 176 | 109 | 197 | 88 |
| P | .04 | .89 | .20 | .63 | .005 | .36 | ||||||
| HZ | ||||||||||||
| Up to date, no. (%) | 31 (29.5) | 23 (21.5) | 25 (22.3) | 29 (29.0) | 49 (25.7) | 5 (23.8) | 1 (14.3) | 53 (25.9) | 38 (26.6 ) | 16 (23.2) | 31 (22.0) | 23 (32.4) |
| Not up to date ,no. (%) | 74 (70.5) | 84 (78.5) | 87 (77.7) | 71 (71.0) | 142 (74.4) | 16 (76.2) | 6 (85.7) | 152 (74.1) | 105 (73.4 ) | 53 (76.8) | 110 (78.0) | 48 (67.6) |
| Total | 105 | 107 | 112 | 100 | 191 | 21 | 7 | 205 | 143 | 69 | 141 | 71 |
| P | .18 | .27 | .85 | .49 | .60 | .10 | ||||||
HAV = hepatitis A virus; HBV = hepatitis B virus; HZ = herpes zoster; PCP = primary care provider; PN = pneumococcal pneumonia.
HBV Vaccination
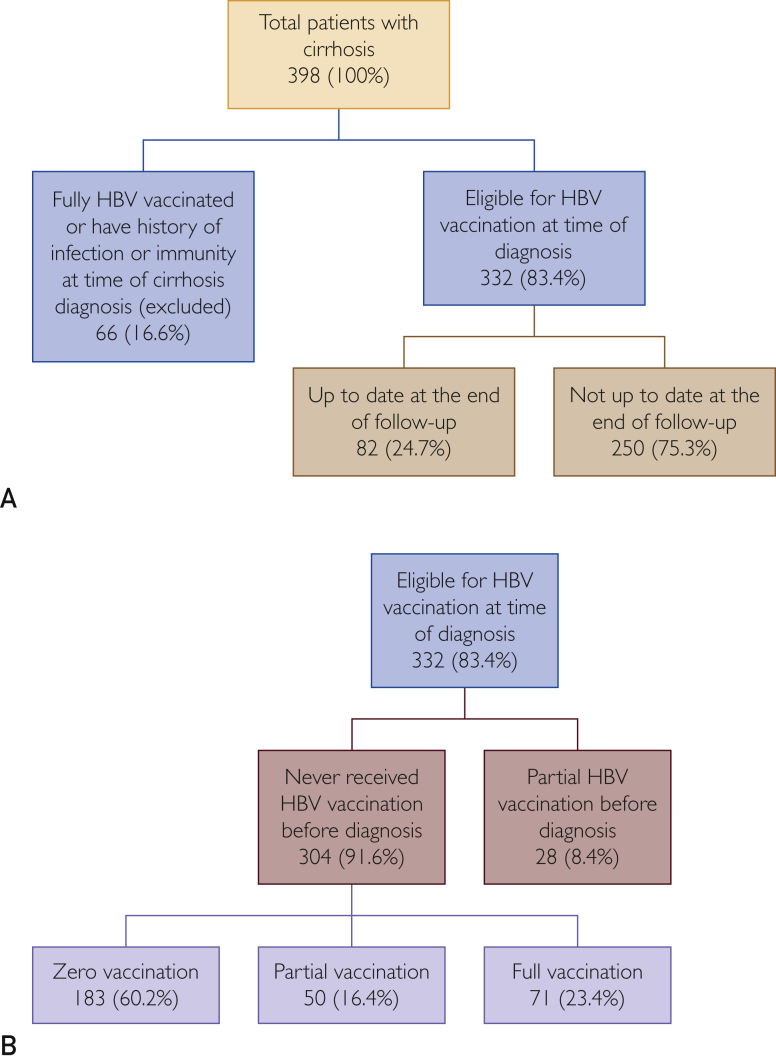
Of the 398 patients, 66 (16.6%) were excluded because they were either fully vaccinated or had a history of HBV infection/immunity at the time they had cirrhosis diagnosed. A total of 332 patients (83.4%) were eligible for vaccination at the time of cirrhosis diagnosis, of whom only 82 (24.7%) were up to date with their HBV vaccination at the end of follow-up (Figure 3A).
Figure 3.
Hepatitis B virus (HBV) vaccination coverage from 2007 to 2015 in Minnesotans with cirrhosis diagnosed between 2007 and 2009 at Mayo Clinic.
The 332 eligible patients had either never received HBV vaccination before diagnosis (n=304; 91.6%) or had received only partial vaccination (n=28; 8.4%). Of the 304 patients who had never received HBV vaccination before diagnosis, 183 (60.2%) received zero vaccination, 50 (16.4%) received partial vaccinations, and only 71 (23.4%) completed their vaccination series during the follow-up period (Figure 3B). Of the 121 patients who received vaccinations during follow-up, only 67 (55.4%) received their first dose within the first year of cirrhosis diagnosis (Figure 2).
In terms of demographic characteristics, 26.2% (59 of 225) of those who were younger than 65 years vs 21.5% (23 of 107) of those 65 years or older (OR, 1.30; 95% CI, 0.76 to 2.28; P=.35), 20.9% (39 of 187) of males vs 29.7% (43 of 145) of females (OR, 0.63; 95% CI, 0.38 to 1.03; P=.07), 25.3% (77 of 304) of whites vs 17.9% (5 of 28) of nonwhites (OR, 1.56; 95% CI, 0.62 to 4.77; P=.38), 9.1% (1 of 11) of Hispanics or Latinos vs 25.2% (81 of 321) of other ethnicities (OR, 0.18; 95% CI, 0.02 to 1.58; P=.22), 26.0% (14250 of 192) of those who were married vs 22.9% (32 of 140) of singles (OR, 1.19; 95% CI, 0.72 to 1.99; P=.51), and 24.0% (54 of 225) of those who were followed up by a specialist vs 26.2% (28 of 107) of those followed up by a PCP (OR, 0.89; 95% CI, 0.53 to 1.51; P=.67) were up to date with their HBV vaccination at the end of follow-up (Table 3).
PN Vaccination
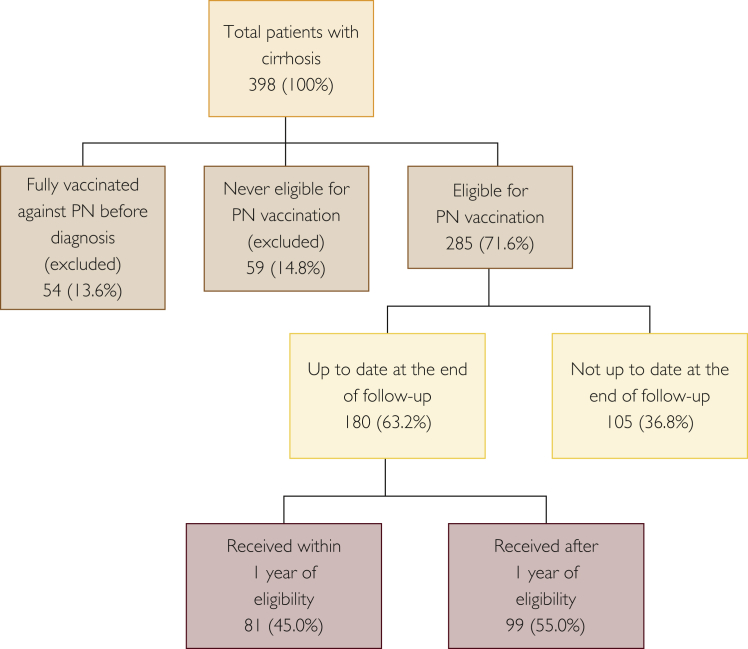
Of the 398 patients, 113 (28.4%) were excluded because they were either fully vaccinated for PN at the time of cirrhosis diagnosis (n=54; 13.6%) or never been eligible during the study period, that is, received a dose before diagnosis and hence their vaccination had been current because they never reached age 65 years during the study period (n=59; 14.8%). Of the remaining 285 (71.6%) eligible patients, 180 (63.2%) were up to date with their PN vaccination at the end of the study, of whom 81 (45.0%) received their vaccination within the first year of eligibility (Figures 2 and 4).
Figure 4.
Pneumococcal pneumonia (PN) vaccination coverage from 2007 to 2015 in Minnesotans with cirrhosis diagnosed between 2007 and 2009 at Mayo Clinic.
In terms of demographic characteristics, 66.8% (135 of 202) of those who were younger than 65 years vs 54.2% (45 of 83) of those 65 years or older (OR, 1.70; 95% CI, 1.01 to 2.87; P=.04), 62.9% of males (110 of 175) vs 63.6% (70 of 110) of females (OR, 0.97; 95% CI, 0.59 to 1.58; P=.89), 64.3% (166 of 258) of whites vs 51.9% (14 of 27) of nonwhites (OR, 1.68; 95% CI, 0.74 to 3.73; P=.20), 55.6% (5 of 9) of Hispanics or Latinos vs 63.4% (175 of 276) of other ethnicities (OR, 0.72; 95% CI, 0.19 to 2.97; P=.63), 56.8% (100 of 176) of those who are married vs 73.4% (80 of 109) of singles (OR, 2.10; 95% CI, 1.26 to 3.56; P=.005), and 61.4% (121 of 197) of those who are followed up by a specialist vs 67.1% (59 of 88) of those followed up by a PCP (OR, 0.78; 95% CI, 0.46 to 1.33; P=.36) were up to date with their PN vaccination at the end of the study (Table 3).
HZ Vaccination
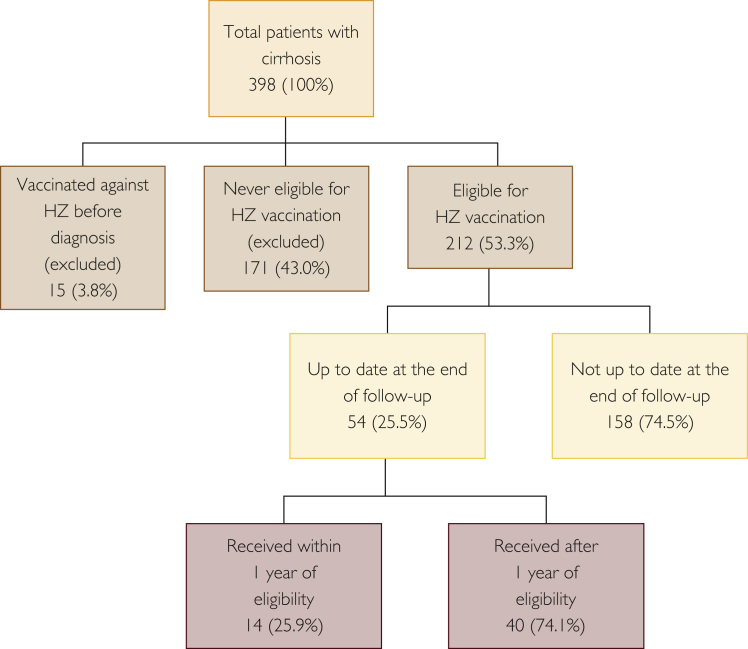
Of the 398 patients, 186 (46.8%) were excluded because they were either vaccinated for HZ at the time of cirrhosis diagnosis (n=15; 3.8%) or had never been eligible during the study period, that is, they never reached age 60 years during the study period (n=171; 43.0%). Of the 212 (53.3%) eligible patients, 54 (25.5%) were up to date with their HZ vaccination at the end of the study, of whom 14 (25.9%) received their vaccination within the first year of eligibility (Figures 2 and 5).
Figure 5.
Herpes zoster (HZ) vaccination coverage from 2007 to 2015 in Minnesotans with cirrhosis diagnosed between 2007 and 2009 at Mayo Clinic.
In terms of demographic characteristics, 29.5% (31 of 105) of those who were younger than 65 years vs 21.5% (23 of 107) of those 65 years or older (OR, 1.53; 95% CI, 0.82 to 2.88; P=.18), 22.3% (25 of 112) of males vs 29.0% (29 of 100) of females (OR, 0.70; 95% CI, 0.38 to 1.31; P=.26), 25.7% (49 of 191) of whites vs 23.8% (5 of 21) of nonwhites (OR, 1.10; 95% CI, 0.41 to 3.52; P=.85), 14.3% (1 of 7) of Hispanics or Latinos vs 25.9% (53 of 205) of other ethnicities (OR, 0.48; 95% CI, 0.03 to 2.89; P=.49), 26.6% (38 of 143) of those who are married vs 23.2% (16 of 69) of singles (OR, 1.20; 95% CI, 0.62 to 2.39; P=.60), and 22.0% (31 of 141) of those who are followed up by a specialist vs 32.4% (23 of 71) of those followed up by a PCP (OR, 0.59; 95% CI, 0.31 to 1.11; P=.10) were up to date with their HZ vaccinations at the end of the study (Table 3).
FLU Vaccination
For the 8 seasons from 2007 to 2014, the FLU vaccination rate was 36.1%, 42.4%, 46.3%, 48.3%, 57.5%, 64.8%, 63.4%, and 65.8%, respectively, indicating a progressive increase in vaccination rates from 36.1% to 65.8%. This steady improvement was also reflected in the demographic characteristics (age, sex, race, ethnicity, and marital status) and provider follow-up (Table 4). There were only 2 to 8 patients of Hispanic or Latino ethnicity per year, among whom coverage ranged between 42.9% (3 of 7) and 61.5% (8 of 13), except for the 2008 to 2009 season, when all 4 patients were vaccinated. For all other ethnic groups, coverage steadily increased from 35.7% (55 of 154) in 2007 to 2008 to 66.5% (103 of 155) in 2014 to 2015.
Table 4.
Influenza Vaccination Coverage by Demographic Characteristics and Provider From 2007 to 2015 in Minnesotans With Cirrhosis Diagnosed Between 2007 and 2009 at Mayo Clinica
| Season | Total Patients | Total Received, no. (%) | Age (y) |
Sex |
Race |
Ethnicity |
Marital Status |
Provider |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <65, no. (%) | ≥65, no. (%) | Male, no. (%) | Female, no. (%) | White, no. (%) | Nonwhite, no. (%) | Hispanic or Latino, no. (%) | Other, no. (%) | Married/Partner, no. (%) | Single, no. (%) | Specialist, no. (%) | PCP, no. (%) | |||
| 2007-2008 | 158 | 57 (36.1) | 40 (35.4) | 17 (37.8) | 29 (31.2) | 28 (43.1) | 51 (35.7) | 6 (40.0) | 2 (50.0) | 55 (35.7) | 35 (37.2) | 22 (34.4) | 38 (32.8) | 19 (45.2) |
| P=.78 | P=.13 | P=.74 | P=.56 | P=.71 | P=.15 | |||||||||
| 2008-2009 | 283 | 120 (42.4) | 79 (41.2) | 41 (45.1) | 66 (40.5) | 54 (45.0) | 107 (41.5) | 13 (52.0) | 4 (100.0) | 116 (41.6) | 73 (40.6) | 47 (45.6) | 72 (36.6) | 48 (55.8) |
| P=.53 | P=.45 | P=.31 | P=.02 | P=.41 | P=.003 | |||||||||
| 2009-2010 | 365 | 169 (46.3) | 109 (45.0) | 60 (48.8) | 90 (44.3) | 79 (48.8) | 149 (45.4) | 20 (54.1) | 8 (61.5) | 161 (45.7) | 95 (44.2) | 74 (49.3) | 107 (44.6) | 62 (49.6) |
| P=.50 | P=.40 | P=.42 | P=.26 | P=.33 | P=.36 | |||||||||
| 2010-2011 | 321 | 155 (48.3) | 96 (46.2) | 59 (52.2) | 90 (51.1) | 65 (44.8) | 140 (48.4) | 15 (46.9) | 7 (58.3) | 148 (47.9) | 99 (51.6) | 56 (43.4) | 98 (46.0) | 57 (52.8) |
| P=.30 | P=.26 | P=.87 | P=.48 | P=.15 | P=.25 | |||||||||
| 2011-2012 | 273 | 157 (57.5) | 93 (54.4) | 64 (62.8) | 88 (58.7) | 69 (56.1) | 142 (57.7) | 15 (55.6) | 5 (45.5) | 152 (58.0) | 101 (62.4) | 56 (50.5) | 101 (56.7) | 56 (59.0) |
| P=.18 | P=.67 | P=.83 | P=.41 | P=.05 | P=.73 | |||||||||
| 2012-2013 | 230 | 149 (64.8) | 89 (62.2) | 60 (69.0) | 78 (64.5) | 71 (65.1) | 131 (63.3) | 18 (78.3) | 5 (55.6) | 144 (65.2) | 92 (67.2) | 57 (61.3) | 94 (62.7) | 55 (68.8) |
| P=.30 | P=.91 | P=.15 | P=.55 | P=.36 | P=.36 | |||||||||
| 2013-2014 | 194 | 123 (63.4) | 69 (58.0) | 54 (72.0) | 62 (61.4) | 61 (65.6) | 111 (62.4) | 12 (75.0) | 3 (42.9) | 120 (64.2) | 71 (64.6) | 52 (61.9) | 78 (63.4) | 45 (63.4) |
| P=.05 | P=.54 | P=.31 | P=.25 | P=.71 | P=.996 | |||||||||
| 2014-2015 | 161 | 106 (65.8) | 60 (61.9) | 46 (71.9) | 53 (65.4) | 53 (66.3) | 97 (66.0) | 9 (64.3) | 3 (50.0) | 103 (66.5) | 61 (67.8) | 45 (63.4) | 67 (68.4) | 39 (61.9) |
| P=.19 | P=.91 | P=.90 | P=.40 | P=.56 | P=.40 | |||||||||
PCP = primary care provider; PN = pneumococcal pneumonia.
Effect of Demographics on Vaccination
Age
No significant difference in vaccination coverage of the young population (aged <65 years) vs the elderly (aged ≥65 years) was found for HAV (27.5% [69 of 251] vs 24.1% [26 of 108]; OR, 1.20; 95% CI, 0.72 to 2.04; P=.50), HBV (26.2% [59 of 225] vs 21.5% [23 of 107]; OR, 1.30; 95% CI, 0.76 to 2.28; P=.35), and HZ (29.5% [31 of 105] vs 21.5% [23 of 107]; OR, 1.53; 95% CI, 0.82 to 2.88; P=0.18). For PN, more young persons were vaccinated (66.8% [135 of 202] vs 54.2% [45 of 83]; OR, 1.70; 95% CI, 1.01 to 2.87; P=.0.4; Table 3).
Sex
No statistically significant difference was found in the vaccination coverage of males and females for HAV (24.2% [50 of 207] vs 29.4% [45 of 153]; OR, 1.20; 95% CI, 0.48 to 1.23; P=.26), HBV (20.9% [39 of 187] vs 29.7% [43 of 145]; OR, 0.63; 95% CI, 0.38 to 1.03; P=.07), PN (62.9% [110 of 175] vs 63.6% [70 of 110]; OR, 0.97; 95% CI, 0.59 to 0.58; P=.89), or HZ (22.3% [25 of 112] vs 29.0% [29 of 100]; OR, 0.70; 95% CI, 0.38 to 1.31; P=.27; Table 3).
Race
No significant difference in the vaccination coverage of whites vs nonwhites was found for HBV (25.3% [77 of 304] vs 17.9% [5 of 28]; OR, 1.56; 95% CI, 0.62 to 4.77; P=.38), PN (64.3% [166 of 258] vs 51.9% [14 of 27]; OR, 1.68; 95% CI, 0.74 to 3.73; P=.20), or HZ (25.7% [49 of 191] vs 23.8% [5 of 21]; OR, 1.10; 95% CI, 0.41 to 3.52; P=.85). For HAV, there were more whites vaccinated than nonwhites (28.3% [91 of 322] vs 10.5% [4 of 38]; OR, 3.35; 95% CI, 1.29 to 11.45; P=.02).
Ethnicity
Given the small number of Hispanics/Latinos, statistical significance was unlikely to reflect the outcome. For HAV vaccination, none of the 13 eligible Hispanics/Latinos was vaccinated (0.0%) compared with 27.4% (95 of 347) of non-Hispanics or Latinos (OR, 2.44; 95% CI, 0.00 to 0.43; P=.03). One of 11 Hispanics/Latinos was vaccinated against HBV (9.1%) vs 81 of 321 (25.2%) of non-Hispanics or Latinos (OR, 0.18; 95% CI, 0.02 to 1.58; P=0.22). For PN, the coverage was 5 of 9 (55.6%) vs 175 of 276 (63.4%; OR, 0.72; 95% CI, 0.19 to 2.97; P=0.63), and for HZ, it was1 of 7 (14.3%) vs 53 of 205 (25.9%; OR, 0.48; 95% CI, 0.03 to 2.89; P=.49). Although a number of these results between ethnic groups are statistically not significant due to small numbers, the differences between HAV, HBV, and HZ vaccine coverage are likely to reflect clinically relevant differences (Table 3).
Marital Status
Comparing vaccination coverage of those who were married or had partners with those who were single, there was no statistical significance in vaccination against HAV (25.6% [54 of 211] vs 27.5% [41 of 149]; OR, 0.91; 95% CI, 0.56 to 1.46; P=.68), HBV (26.0% [50 of 192] vs 22.9% [32 of 140]; OR, 1.19; 95% CI, 0.72 to 1.99; P=.51), or HZ (26.6% [38 of 143] vs 23.2% [16 of 69]; OR, 1.20; 95% CI, 0.62 to 2.39; P=.60). For PN, there was a significant difference in coverage between those who were married or had partners vs singles (56.8% [100 of 176] vs 73.4% [80 of 109]; OR, 2.10; 95% CI, 1.26 to 3.56; P=.005; Table 3).
For FLU, there was no significant difference in demographic parameters for vaccination by season except for age in the 2013 to 2014 season, in which 72.0% (54 of 75) of elderly (aged ≥65 years) were vaccinated vs 58.0% (69 of 119) of those younger than 65 years (OR, 0.54; 95% CI, 0.28 to 0.99; P=.05∗); for ethnicity in the 2008 to 2009 season, in which all Hispanics or Latinos (100% [4 of 4]) vs 41.6% (116 of 279) of non-Hispanics or Latinos (OR, ∞; 95% CI, 2.25 to ∞; P=.02) were vaccinated; and for marital status in the 2011 to 2012 season, in which 62.4% (101 of 162) of those who were married or had partners were vaccinated vs 50.5% (56 of 111) of singles (OR, 1.63; 95% CI, 1.00 to 2.66; P=.05; Table 4).
Effect of Provider Specialty on Vaccination
There was no significant difference in vaccination coverage for those who were usually seen by a GIH vs those usually seen by a PCP for HAV (26.3% [64 of 243] vs 26.5% [31 of 117]; OR, 0.99; 95% CI, 0.60 to 1.64; P=0.97), HBV (24.0% [54 of 225] vs 26.2% [28 of 107]; OR, 0.89; 95% CI, 0.53 to 1.51; P=.67), PN (61.4% [121 of 197] vs 67.1% [59 of 88]; OR, 0.78; 95% CI, 0.46 to 1.33; P=.36), or HZ (22.0% [31 of 141] vs 32.4% [23 of 71]; OR, 0.59; 95% CI, 0.31 to 1.11; P=.10; Table 3).
For FLU, there was no significant difference in vaccination coverage by provider except for the 2008 to 2009 season, for which results revealed more coverage in the primary care setting (55.8% [48 of 86] vs 36.6% [72 of 197]; OR, 0.46; 95% CI, 0.27 to 0.76; P= .003; Table 4).
On further separating specialist group into GIH and liver transplant specialist (Tables 5 and 6), we found that more vaccination coverage for HAV (12.6% [28 of 222] vs 30.4% [24 of 79]; OR, 3.02; 95% CI, 1.62 to 5.63; P=.0003) and HBV (21.5% [44 of 205] vs 34.3% [24 of 70]; OR, 1.91; 95% CI, 1.05 to 3.46; P=.03) in patients who follow up with a liver transplant specialist. There was no significant difference for PN (61.2% [109 of 178] vs 73.9% [51 of 69]; OR, 1.79; 95% CI, 0.97 to 3.32; P=.06) and HZ (21.7% [28 of 129] vs 18.9% [7 of 37]; OR, 0.84; 95% CI, 0.33 to 2.12; P=.71) vaccination coverage between the 2 specialties. For FLU vaccination, there was no difference between the 2 specialties throughout the years of the study.
Table 5.
Vaccination Coverage for HAV, HBV, PN, and HZ by GIH vs LT from 2007 to 2015 in Minnesotans With Cirrhosis Diagnosed Between 2007 and 2009 at Mayo Clinica
| Specialist |
||
|---|---|---|
| GIH, no. (%) | LT, no. (%) | |
| HAV | ||
| Up to date | 28 (12.6) | 24 (30.4) |
| Not up to date | 194 (87.4) | 55 (69.6) |
| Total | 222 | 79 |
| P | .0003 | |
| HBV | ||
| Up to date | 44 (21.5) | 24 (34.3) |
| Not up to date | 161 (78.5) | 46 (65.7) |
| Total | 205 | 70 |
| P | .03 | |
| PN | ||
| Up to date | 109 (61.2) | 51 (73.9) |
| Not up to date | 69 (38.8) | 18 (26.1) |
| Total | 178 | 69 |
| P | .06 | |
| HZ | ||
| Up to date | 28 (21.7) | 7 (18.9) |
| Not up to date | 101 (78.3) | 30 (81.1) |
| Total | 129 | 37 |
| P | .71 | |
GIH = gastroenterologist and/or hepatologist; HAV = hepatitis A virus; HBV = hepatitis B virus; HZ = herpes zoster; LT = liver transplant specialist; PN = pneumococcal pneumonia.
Table 6.
Influenza Vaccination Coverage by GIH vs LT from 2007 to 2015 in Minnesotans With Cirrhosis Diagnosed Between 2007 and 2009 at Mayo Clinic
| Season | Total Patients | Specialist |
|
|---|---|---|---|
| GIH, no. (%) | LT, no. (%) | ||
| 2007-2008 | 153 | 73 (67.6) | 30 (66.7) |
| P=.91 | |||
| 2008-2009 | 243 | 65 (36.1) | 25 (39.7) |
| P=.61 | |||
| 2009-2010 | 292 | 139 (64.1) | 50 (66.7) |
| P=.68 | |||
| 2010-2011 | 254 | 124 (64.9) | 39 (61.9) |
| P=.67 | |||
| 2011-2012 | 211 | 90 (56.6) | 30 (57.7) |
| P=.90 | |||
| 2012-2013 | 177 | 83 (62.4) | 32 (72.7) |
| P=.21 | |||
| 2013-2014 | 144 | 67 (62.0) | 23 (63.9) |
| P=.84 | |||
| 2014-2015 | 108 | 56 (66.7) | 17 (70.8) |
| P=.70 | |||
GIH = gastroenterologist and /or hepatologist; LT = liver transplant specialist.
Discussion
Chronic liver disease and cirrhosis are one of the leading causes of death in the United States.1, 2, 3 Patients with CLD and cirrhosis are considered immunocompromised and susceptible to certain severe infections, including HAV, HBV, PN, HZ, and FLU, with subsequent major complications, morbidity, and mortality.4, 5, 6, 7, 8, 9, 10, 11, 12 The ACIP recommends vaccinating patients with CLD against these infections. Studies and national data surveys revealed that vaccination in this group of patients is suboptimal18,19,21 (Table 1), and few studies have been conducted in patients with cirrhosis.
Our study examined a reasonably sized cohort compared with similar previous studies.19 Recent studies of larger populations did not study all 5 vaccines at the meticulous level of detail that we achieved.16,18,21 We also followed a different methodology than prior studies with the aim of obtaining more precise results. For example, we determined the vaccine up-to-date status at the end of follow-up based on the time frame within which each vaccine was scheduled to be delivered, instead of relying solely on criteria such as whether the patient received at least 1 vaccination to determine their vaccination status, as had been used in some previous studies. Addressing the details of vaccine delivery by dose specifically interrogates the quality of care delivery.
Our secondary objectives also included determining the time within which the patients received their vaccination after they became eligible for those who ever received vaccination in the follow-up period, as well as those who were vaccination naive at cirrhosis diagnosis. In addition, we addressed secondary questions examining the influences of demographic characteristics and type of care provider on vaccination coverage.
We included all Minnesotans with cirrhosis diagnosed between 2007 and 2009 at Mayo Clinic in Rochester, Minnesota, and its regional Health Care System. The vaccination status of patients was followed up from the time of diagnosis of cirrhosis until they were lost to follow-up or the end of the study in May 2015. We obtained detailed vaccination data and patient characteristics from facility medical records, with additional vaccination information obtained from the Minnesota Immunization Information Connection.
A total of 398 patients were included, with further exclusions for each vaccine independently. At the end of their follow-up, 26.4% (95 of 360), 24.7% (82 of 332), 63.2% (180 of 285), and 25.5% (54 of 212) were up to date with their vaccination for HAV, HBV, PN, and HZ, respectively. For FLU, our study revealed a progressive improvement from 36.1% (57 of 158) in the 2007 to 2008 season to 65.8% (106 of 161) in the 2014 to 2015 season. For HAV, PN, HZ, and FLU, these results reflect improvements from previous studies and surveys18,19,21 (Table 1).
Of those who never received HAV or HBV vaccination before the cirrhosis was diagnosed, 13.2% (42 of 318) and 16.4% (50 of 304) received partial vaccination and 18.6% (59 of 318) and 23.4% (71 of 304) completed their vaccination series for HAV and HBV, respectively, during the follow-up period. Of those who received vaccination during the follow-up, only 39.6% (HAV) and 55.4% (HBV) received their first dose within the first year of cirrhosis diagnosis.
Only 45.0% (81 of 180) and 25.9% (14 of 54) of those who were up to date for PN and HZ, respectively, received their vaccination within the first year of eligibility.
Regarding the effect of demographic characteristics on vaccination coverage, our study revealed that more younger people and more unmarried singles were vaccinated for PN, and more whites, for HAV. For FLU, more elderly in the 2013 to 2014 season and more married people in the 2011 to 2012 season were vaccinated. Regarding effects of ethnicity, there were relatively few Hispanics/Latinos in Minnesota and consequently few differences reached statistical significance, even when they appeared potentially clinically relevant.
No significant difference in specialist vs primary care provider coverage was seen except for FLU in the 2008 to 2009 season, when more vaccinations were done in the primary care setting (55.8% [48 of 86] vs 36.6% [72 of 197]; P=.003). Compared with a previous study, patients in primary care had more often received FLU (47.0% vs 32.0%; P<.001) and PN vaccines (39.0% vs 19.0%; P<.001), whereas those seeing specialists had more often completed HAV (28.0% vs 5.0%; P<.001) and HBV (29.0% vs 14.0%; P<.001) vaccinations.58
Potential solutions and suggestions to improve vaccination rates in this population include but are not limited to the education of patients about the importance of adult vaccinations, especially in debilitating conditions such as cirrhosis; handling of financial barriers; easing vaccination delivery (eg, through retail clinics); setting vaccination protocols; and reminders for health care professionals, as well as follow-up protocols with patients to make sure they have completed the vaccines and serologic tests are routinely checked and repeated when appropriate. Also, it is important to address any demographic factors, such as age, race, ethnicity, and marital status, as potential barriers to vaccination.
Healthy People 2020 Target (for PN, FLU, and HZ vaccination)
Our study revealed that PN vaccination has already reached the Healthy People 2020 target for high-risk adults (63.2% [180 of 285] in our study vs a target of 60.0%). Vaccination for FLU steadily improved in our study period from 36.1% (57 of 158) in the 2007 to 2008 season to 65.8% (106 of 161) in the 2014 to 2015 season. Vaccination for HZ was 25.5% (54 of 212) at the end of our study (2015), improving from a national rate of 6.7% in 2008 but still far from the Healthy People 2020 target of 60.0%.
Limitations
The study was conducted in a single state but in a large referral center and its local outreach facilities. Also, because we were concentrating on the quality of care regarding vaccination coverage, because of the retrospective nature of the study and the difficulty obtaining such information for every patient, we were unable to obtain serologic/laboratory confirmation of patient immunity for most of the vaccines.
Conclusion
We evaluated vaccination trends for HAV, HBV, PN, HZ, and FLU in patients with liver cirrhosis. Except for PN vaccination and steady improvement in FLU vaccination, it appears that current vaccination coverage figures in patients with cirrhosis are suboptimal and far away from the Healthy People 2020 target. More interventions and vaccination trends will need to be considered to improve vaccination coverage. Although our study was conducted in a single state, it might represent a nationwide outcome. Further studies are needed to confirm this.
Acknowledgments
We thank the biostatistics consultative service of the Center for Clinical and Translational Science for their assistance with data analyses.
Footnotes
Grant Support: This work was supported in part by funds from the National Institutes of Health, USA, to the Mayo Clinic Center for Clinical and Translational Science (UL1 TR002377) and the Mayo Clinic Hepatobiliary SPORE (P50 CA210964).
Potential Competing Interests: The authors declare no competing interests.
References
- 1.Kochanek K.D., Murphy S.L., Xu J., Tejada-Vera B. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65(4):1–122. [PubMed] [Google Scholar]
- 2.Xu J., Murphy S.L., Kochanek K.D., Bastian B.A. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1–119. [PubMed] [Google Scholar]
- 3.Asrani S.K., Larson J.J., Yawn B., Therneau T.M., Kim W.R. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145(2):375–382.e1-382.e2. doi: 10.1053/j.gastro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvaniti V., D'Amico G., Fede G., et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139(4):1246–1256. doi: 10.1053/j.gastro.2010.06.019. 1256.e1-1256.e5. [DOI] [PubMed] [Google Scholar]
- 5.Bonnel A.R., Bunchorntavakul C., Reddy K.R. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(9):727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Christou L., Pappas G., Falagas M.E. Bacterial infection-related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007;102(7):1510–1517. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 7.Fiuza C., Salcedo M., Clemente G., Tellado J.M. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis. 2000;182(2):526–533. doi: 10.1086/315742. [DOI] [PubMed] [Google Scholar]
- 8.Ghassemi S., Garcia-Tsao G. Prevention and treatment of infections in patients with cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21(1):77–93. doi: 10.1016/j.bpg.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Mehta G., Rothstein K.D. Health maintenance issues in cirrhosis. Med Clin North Am. 2009;93(4):901–915. doi: 10.1016/j.mcna.2009.03.005. viii-ix. [DOI] [PubMed] [Google Scholar]
- 10.Tandon P., Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28(1):26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 11.Wang S.S., Lee F.Y., Chan C.C., et al. Sequential changes in plasma cytokine and endotoxin levels in cirrhotic patients with bacterial infection. Clin Sci (Lond) 2000;98(4):419–425. [PubMed] [Google Scholar]
- 12.Wiest R., Lawson M., Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60(1):197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Recommended adult immunization schedule -- United States, 2014. J Midwifery Womens Health. 2014;59(2):205–209. doi: 10.1111/jmwh.12184. [DOI] [PubMed] [Google Scholar]
- 14.Williams W.W., Lu P.J., O'Halloran A., et al. Centers for Disease Control and Prevention (CDC) Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(4):95–102. [PMC free article] [PubMed] [Google Scholar]
- 15.Williams W.W., Lu P.J., O'Halloran A., et al. Centers for Disease Control and Prevention (CDC) Noninfluenza vaccination coverage among adults - United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(5):95–102. [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) Hepatitis B vaccination coverage among adults--United States, 2004. MMWR Morb Mortal Wkly Rep. 2006;55(18):509–511. [PubMed] [Google Scholar]
- 17.Uscher-Pines L., Harris K.M., Burns R.M., Mehrotra A. The growth of retail clinics in vaccination delivery in the U.S. Am J Prev Med. 2012;43(1):63–66. doi: 10.1016/j.amepre.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waghray A., Waghray N., Khallafi H., Menon K.V. Vaccinating adult patients with cirrhosis: trends over a decade in the United States. Gastroenterol Res Pract. 2016;2016:5795712. doi: 10.1155/2016/5795712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arguedas M.R., McGuire B.M., Fallon M.B. Implementation of vaccination in patients with cirrhosis. Dig Dis Sci. 2002;47(2):384–387. doi: 10.1023/a:1013734525348. [DOI] [PubMed] [Google Scholar]
- 20.Wörns M.A., Teufel A., Kanzler S., et al. Incidence of HAV and HBV infections and vaccination rates in patients with autoimmune liver diseases. Am J Gastroenterol. 2008;103(1):138–146. doi: 10.1111/j.1572-0241.2007.01609.x. [DOI] [PubMed] [Google Scholar]
- 21.Younossi Z.M., Stepanova M. Changes in hepatitis A and B vaccination rates in adult patients with chronic liver diseases and diabetes in the U.S. population. Hepatology. 2011;54(4):1167–1178. doi: 10.1002/hep.24510. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez B., Hasson N.K., Cheung R. Hepatitis C performance measure on hepatitis A and B vaccination: missed opportunities? Am J Gastroenterol. 2009;104(8):1961–1967. doi: 10.1038/ajg.2009.252. [DOI] [PubMed] [Google Scholar]
- 23.Kramer J.R., Hachem C.Y., Kanwal F., Mei M., El-Serag H.B. Meeting vaccination quality measures for hepatitis A and B virus in patients with chronic hepatitis C infection. Hepatology. 2011;53(1):42–52. doi: 10.1002/hep.24024. [DOI] [PubMed] [Google Scholar]
- 24.Lee W.M. Hepatitis B virus infection. N Engl J Med. 1997;337(24):1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 25.Office of Disease Prevention and Health Promotion. Immunization and Infectious Diseases. ODPHP Website. https://www.healthypeople.gov/2020/data-search/Search-the-Data#topic-area=3527. Accessed September 15, 2020.
- 26.Keeffe E.B. Is hepatitis A more severe in patients with chronic hepatitis B and other chronic liver diseases? Am J Gastroenterol. 1995;90(2):201–205. [PubMed] [Google Scholar]
- 27.Keeffe E.B. Hepatitis A and B superimposed on chronic liver disease: vaccine-preventable diseases. Trans Am Clin Climatol Assoc. 2006;117:227–237. discussion 237-238. [PMC free article] [PubMed] [Google Scholar]
- 28.Liaw Y.F., Yeh C.T., Tsai S.L. Impact of acute hepatitis B virus superinfection on chronic hepatitis C virus infection. Am J Gastroenterol. 2000;95(10):2978–2980. doi: 10.1111/j.1572-0241.2000.02337.x. [DOI] [PubMed] [Google Scholar]
- 29.Vento S., Garofano T., Renzini C., et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338(5):286–290. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 30.Almasio P.L., Amoroso P. HAV infection in chronic liver disease: a rationale for vaccination. Vaccine. 2003;21(19-20):2238–2241. doi: 10.1016/s0264-410x(03)00139-7. [DOI] [PubMed] [Google Scholar]
- 31.André F., Van Damme P., Safary A., Banatvala J. Inactivated hepatitis A vaccine: immunogenicity, efficacy, safety and review of official recommendations for use. Expert Rev Vaccines. 2002;1(1):9–23. doi: 10.1586/14760584.1.1.9. [DOI] [PubMed] [Google Scholar]
- 32.Keeffe E.B. Acute hepatitis A and B in patients with chronic liver disease: prevention through vaccination. Am J Med. 2005;118(suppl 10A):21s–27s. doi: 10.1016/j.amjmed.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Koff R.S. Risks associated with hepatitis A and hepatitis B in patients with hepatitis C. J Clin Gastroenterol. 2001;33(1):20–26. doi: 10.1097/00004836-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Reiss G., Keeffe E.B. Review article: hepatitis vaccination in patients with chronic liver disease. Aliment Pharmacol Ther. 2004;19(7):715–727. doi: 10.1111/j.1365-2036.2004.01906.x. [DOI] [PubMed] [Google Scholar]
- 35.Szmuness W., Stevens C.E., Harley E.J., et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303(15):833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- 36.Zuckerman J.N. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J Med Virol. 2006;78(2):169–177. doi: 10.1002/jmv.20524. [DOI] [PubMed] [Google Scholar]
- 37.Borzio M., Salerno F., Piantoni L., et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33(1):41–48. doi: 10.1016/s1590-8658(01)80134-1. [DOI] [PubMed] [Google Scholar]
- 38.Fernández J., Navasa M., Gómez J., et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35(1):140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 39.Caly W.R., Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18(3):353–358. doi: 10.1016/s0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 40.Feikin D.R., Schuchat A., Kolczak M., et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995-1997. Am J Public Health. 2000;90(2):223–229. doi: 10.2105/ajph.90.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hung T.H., Tseng C.W., Hsieh Y.H., Tseng K.C., Tsai C.C., Tsai C.C. High mortality of pneumonia in cirrhotic patients with ascites. BMC Gastroenterol. 2013;13:25. doi: 10.1186/1471-230X-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viasus D., Garcia-Vidal C., Castellote J., et al. Community-acquired pneumonia in patients with liver cirrhosis: clinical features, outcomes, and usefulness of severity scores. Medicine (Baltimore) 2011;90(2):110–118. doi: 10.1097/MD.0b013e318210504c. [DOI] [PubMed] [Google Scholar]
- 43.Tseng H.F., Smith N., Harpaz R., Bialek S.R., Sy L.S., Jacobsen S.J. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305(2):160–166. doi: 10.1001/jama.2010.1983. [DOI] [PubMed] [Google Scholar]
- 44.Bal C.K., Bhatia V., Kumar S., et al. Influenza A/H1/N1/09 infection in patients with cirrhosis has a poor outcome: a case series. Indian J Gastroenterol. 2014;33(2):178–182. doi: 10.1007/s12664-014-0443-5. [DOI] [PubMed] [Google Scholar]
- 45.Duchini A., Viernes M.E., Nyberg L.M., Hendry R.M., Pockros P.J. Hepatic decompensation in patients with cirrhosis during infection with influenza A. Arch Intern Med. 2000;160(1):113–115. doi: 10.1001/archinte.160.1.113. [DOI] [PubMed] [Google Scholar]
- 46.Guralnick L., Jackson A. An index of unnecessary deaths. Public Health Rep. 1967;82(2):180–182. [PMC free article] [PubMed] [Google Scholar]
- 47.Marzano A., Marengo A., Ruggiero T., et al. Clinical impact of A/H1/N1/09 influenza in patients with cirrhosis: experience from a nosocomial cluster of infection. J Med Virol. 2013;85(1):1–7. doi: 10.1002/jmv.23454. [DOI] [PubMed] [Google Scholar]
- 48.Song J.Y., Cheong H.J., Ha S.H., et al. Clinical impact of influenza immunization in patients with liver cirrhosis. J Clin Virol. 2007;39(3):159–163. doi: 10.1016/j.jcv.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention (CDC) Estimated influenza illnesses and hospitalizations averted by influenza vaccination - United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2013;62(49):997–1000. [PMC free article] [PubMed] [Google Scholar]
- 50.Cheong H.J., Song J.Y., Park J.W., et al. Humoral and cellular immune responses to influenza vaccine in patients with advanced cirrhosis. Vaccine. 2006;24(13):2417–2422. doi: 10.1016/j.vaccine.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 51.Duchini A., Hendry R.M., Nyberg L.M., Viernes M.E., Pockros P.J. Immune response to influenza vaccine in adult liver transplant recipients. Liver Transpl. 2001;7(4):311–313. doi: 10.1053/jlts.2001.23010. [DOI] [PubMed] [Google Scholar]
- 52.Gaeta G.B., Pariani E., Amendola A., et al. Influenza vaccination in patients with cirrhosis and in liver transplant recipients. Vaccine. 2009;27(25-26):3373–3375. doi: 10.1016/j.vaccine.2009.01.077. [DOI] [PubMed] [Google Scholar]
- 53.Gaeta G.B., Stornaiuolo G., Precone D.F., Amendola A., Zanetti A.R. Immunogenicity and safety of an adjuvanted influenza vaccine in patients with decompensated cirrhosis. Vaccine. 2002;20(suppl 5):B33–B35. doi: 10.1016/s0264-410x(02)00510-8. [DOI] [PubMed] [Google Scholar]
- 54.Ohfuji S., Fukushima W., Sasaki Y., et al. Influenza A(H1N1)pdm09 vaccine effectiveness and other characteristics associated with hospitalization in chronic liver disease patients. Liver Int. 2014;34(5):700–706. doi: 10.1111/liv.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohfuji S., Fukushima W., Tamori A., Maeda K., Maeda A., Hirota Y. Immunogenicity of influenza A(H1N1)pdm09 vaccine and the associated factors on lowered immune response in patients with hepatitis C. Influenza Other Respir Viruses. 2013;7(3):456–465. doi: 10.1111/j.1750-2659.2012.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soesman N.M., Rimmelzwaan G.F., Nieuwkoop N.J., et al. Efficacy of influenza vaccination in adult liver transplant recipients. J Med Virol. 2000;61(1):85–93. [PubMed] [Google Scholar]
- 57.Antonova E., Ambrose C.S., Kern D., Block S.L., Caspard H., Tunceli O. Seasonal influenza vaccination trends from 2007-2011 in privately insured children and adults in the United States. Vaccine. 2014;32(48):6563–6568. doi: 10.1016/j.vaccine.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Jacobs R.J., Meyerhoff A.S., Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci. 2005;50(8):1525–1531. doi: 10.1007/s10620-005-2873-5. [DOI] [PubMed] [Google Scholar]