Highlights
-
•
In adolescents, there is a relationship between measures of adiposity and parental weight that involves factors other than cardiovascular fitness.
-
•
Adolescent boys have relatively more abdominal fat than girls and a proinflammatory profile of biomarkers.
-
•
Family and social environmental interventions to prevent obesity are best undertaken early in childhood, particularly in boys.
Keywords: Adiposity, Cardiovascular fitness, Cardiovascular risk, Inflammation, Puberty
Abstract
Background
Puberty is a critical time in the development of overweight and obesity. The aim of this study was to examine relationships between measures of adiposity, cardiovascular fitness, and biomarkers of cardiovascular disease risk in adolescents.
Methods
In a cross-sectional study design, 129 girls and 95 boys aged 12.9–14.4 years at various stages of puberty were included, along with their mothers (n = 217) and fathers (n = 207). Anthropometric assessments of adiposity were made, along with cardiovascular physical fitness, using the 20-m shuttle run test, and biomarkers associated with cardiovascular risk, including glucose, insulin, triglyceride, fibrinogen, and C-reactive protein (CRP) concentrations.
Results
Waist-to-height ratio values were similar in boys and girls and correlated positively with diastolic blood pressure, insulin, triglyceride, fibrinogen, and CRP concentrations, and inversely with cardiovascular fitness scores. Skinfold thickness measurements were higher in girls. High-molecular-weight adiponectin concentrations were lower in boys than girls, particularly in late puberty, and CRP levels were higher. Cardiovascular fitness, maternal body mass index (BMI), and paternal BMI contributed independently to the variance in waist measurements in girls and boys. Gender, triceps skinfold thickness, and weight-to-height ratio, but not parental BMI, contributed independently to the variance in cardiovascular fitness.
Conclusion
There is a relationship between measures of adolescent adiposity and parental weight that involves factors other than cardiovascular fitness. Adolescent boys have relatively more abdominal fat than girls and a tendency to have a proinflammatory profile of biomarkers. These observations suggest that family and social environmental interventions are best undertaken earlier in childhood, particularly among boys.
1. Introduction
Childhood obesity predicts obesity later in adulthood1 and is associated with adult cardiovascular disease and related mortality.2 The increasing prevalence of obesity in childhood is, therefore, likely to have pathophysiological consequences and translate to an increased incidence of cardiovascular events in adulthood.3 It is now recognized that there is an interaction between overweight and fitness that contributes to cardiovascular risk and prognosis.4 Adolescence is associated with increases in overweight and obesity5 and a decrease in levels of physical activity.6 Puberty is, therefore, a stage when the promotion of healthy behaviors may be crucial. Family environment is likely to play an important role; for example, an overweight mother and a single-parent family are associated with an increased likelihood of a child being overweight or obese.7
In adolescence, calculation of the body mass index (BMI) does not fully adjust for the effect of height.8, 9 The waist-to-height ratio (WHtR) is regarded as a better marker of adiposity10 and may be a stronger predictor of cardiovascular disease risk factors.11 Increasing adiposity in childhood, however, is not necessarily accompanied by a deteriorating metabolic profile.12 It is likely that a combination of adiposity measures and proinflammatory markers is a better predictor than measurement of adiposity alone.13 There is an effect of gender on these measures; for example, it is reported that high-sensitivity C-reactive protein (hsCRP) concentrations are higher in girls.14
The aim of this study was to examine the effect of gender and puberty on the relationship between measures of adiposity and cardiovascular fitness and (1) inflammatory and metabolic biomarkers known to be associated with disease risk in adults, including serum CRP, insulin, and lipid concentrations and (2) parental BMI and waist circumference. We have previously shown that aerobic fitness, estimated with a 20-m shuttle run test, is inversely related to measures of adiposity in adolescent boys and girls with no significant effect of pubertal status on the relationship.8 It was hypothesized that, in this group of adolescents, those with greater adiposity and lower cardiovascular fitness are more likely to have a biomarker profile that is associated with increased cardiovascular risk. Furthermore, it was hypothesized that an increased cardiovascular risk profile is more likely in boys and in children whose parents are overweight or obese.
2. Methods
2.1. Participants
In a cross-sectional study of children in year 8 (age range: 12.9–14.4 years) in 3 schools in Carmarthenshire, Wales,15 information letters were sent to parents and guardians of potential participants. The response rate was 86%. The number of children registered as receiving free school meals was lower than the national average (1% vs. 16%),15 and was similar in all 3 schools, indicating similar socioeconomic status.16 Signed consent was obtained from parents or guardians and assent was obtained from children. The study was conducted in accordance with the Declaration of Helsinki and approved by the National Health Service Research Ethics Committee (Dyfed Powys; 07/WMW01/12).
Data on self-assessed pubertal status, along with measurements of weight, waist, height, and skinfold thickness (SKF) were available from 247 participants. The relationship between weight, waist, height, and cardiovascular fitness measurements in that group were presented in a previous publication.8 For inclusion in the present study, those participants who had a measure of cardiovascular fitness and blood analysis that included insulin concentration were selected for inclusion. A total of 224 participants (129 girls and 95 boys) met the inclusion criteria. Weight, waist, height, and SKF measurements for this group were similar to the larger cohort (data not shown).
2.2. Physical measurements
All measurements were carried out by the same researcher, an experienced pediatric exercise physiologist. Children wore light clothing and were barefoot. Privacy was ensured and 2 gender-concordant adults were present at all times. Body height was measured using a portable stadiometer (Holtain Ltd., Crymych, Pembrokeshire, UK) and weight was measured using a calibrated Philips HP 5320 electronic scale (Philips N.V., Amsterdam, the Netherlands). Waist circumference was measured directly over the skin at the smallest circumference between the lower costal margin and iliac crest using anthropometric tape (Holtain Ltd., Crymych, Pembrokeshire, UK). Waist is also reported as a percentage of height (WHtR) when appropriate. SKF measurements were made at the triceps, biceps, subscapular, and suprailiac sites on the right side using Harpenden skin-fold callipers (Holtain Ltd., Brynberian, UK) and standard techniques. Anthropometric measurements were made in duplicate and if the values differed by >1.0 mm or >0.1 kg, a third measurement was taken. The intra-observer technical error of measurement (TEM)16 was determined in a study of 20 measurements. The TEM was <1.0 mm (coefficient of reliability (R) > 0.99) for height measurement. The TEM for waist was 0.98 mm (R = 0.973), and TEMs for SKF were 0.42 mm (R = 0.996), 0.42 mm (R = 0.989), 0.51 mm (R = 0.993), and 0.54 mm (R = 0.992), respectively, for the triceps, biceps, subscapular, and suprailiac sites. Systolic and diastolic blood pressure was taken 3 times, after the child had been sitting quietly for 5 min (Dinamap IL, Critikron, Inc., Tampa, FL, USA). The average of the second and third readings was recorded.
Parents were asked to complete a form reporting the child's birth weight, as well as their own date of birth and adult height, weight, and waist measurements. Parental self-reported height and weight and/or waist measurements were available from parents of 218 of the 224 children: 217 mothers (mean age = 41.7 years; 95% confidence interval (CI): 41.0–42.3 years) and 207 fathers (mean age = 44.0 years; 95%CI: 43.2–44.9 years). Maternal BMI, calculated from self-reported weight and height, was 26.6 kg/m2 (95%CI: 25.9–27.3 kg/m2; n = 209) and paternal BMI was 28.0 kg/m2 (95%CI: 27.5–28.6 kg/m2; n = 196). Because self-reported height tends to be overestimated and weight underestimated, published equations were used to adjust parental BMI values.17 The formula used for the mother's value was BMIcorrected = 0.12 + 1.05 × BMIself-reported, and for the father's it was BMIcorrected = 0.12 + 1.05 × BMIself-reported. Corrected maternal BMI was 27.8 kg/m2 (95%CI: 27.1–28.5 kg/m2), and corrected paternal BMI was 29.2 kg/m2 (95%CI: 28.6–29.8 kg/m2).
2.3. Pubertal assessment
Pubertal status was determined with self-assessment questionnaires using gender-specific line drawings of the stages18 based on those described by Tanner.19 Children completed the questionnaire alone and in private at home. Within each gender group, those reporting being in Tanner stages 1–3 (T1–3) and in Tanner stages 4–5 (T4–5), were combined for data analysis.8 All children reporting being in Tanner stage 1 (T1) for breast or genital development reported being in Tanner stages 2–4 (T2–4) for pubic hair development (Table 1). Status assessed by breast development or genital development was used for the correlations. Similar patterns were observed in correlations using pubic hair development. Because stages of pubertal development are not equivalent, analyses were performed separately for boys and girls.
Table 1.
Stage of puberty in girls and boys: children's estimates of pubertal stages using line drawings described in Methods.
| Girls (breast) |
Boys (genitalia) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pubic hair | 1 | 2 | 3 | 4 | 5 | Total | 1 | 2 | 3 | 4 | 5 | Total |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 1 | 8 | 8 | 1 | 0 | 18 | 1 | 5 | 9 | 2 | 2 | 19 |
| 3 | 1 | 6 | 29 | 10 | 8 | 54 | 0 | 3 | 12 | 9 | 0 | 24 |
| 4 | 0 | 3 | 14 | 20 | 9 | 46 | 1 | 1 | 9 | 27 | 7 | 45 |
| 5 | 0 | 0 | 3 | 5 | 3 | 11 | 0 | 0 | 1 | 3 | 3 | 7 |
| Total | 2 | 17 | 54 | 36 | 20 | 129 | 2 | 9 | 31 | 41 | 12 | 95 |
2.4. Cardiovascular fitness
A 20-m shuttle run test was used as an indirect measurement of maximal aerobic power.20 Participants ran between 2 parallel lines 20 m apart. A commercially available audio tape that emits a beep at the point where the runner should be pivoting at the next line was used. The pacer started at 8.5 km/h and increased by 8.5 km/h each minute and was timed for accuracy before each session. Boys and girls performed separately. Testing took place at the same time of day, supervised by a person trained in the method, who gave consistent verbal encouragement. All participants were fully familiarized with testing procedures before data collection. The test ended if running could not be maintained 2 laps in succession, or voluntarily if the participant was exhausted. The number of laps completed was used as the cardiovascular fitness score.
2.5. Biomarkers
Serum was prepared from blood samples collected between 9:00 a.m. and 12:00 noon after an overnight fast and after the child had been sitting for ≥30 min. Glucose was determined by the glucose oxidase method (Randox Laboratories LTD, Crumlin, CO Antrim, UK). Total cholesterol and triglyceride concentrations were determined by routine enzymatic techniques (Vitros 950 System, Ortho-Clinical Diagnostics, Amersham, Bucks, UK). Laboratory analytical variances were 1.5%, 1.6%, and 2.0%, respectively, for these 3 measurements. High-density lipoprotein (HDL) cholesterol was determined after precipitation of very-low-density and LDL with dextran sulphate and magnesium chloride (coefficient of variation (CV): 5.3%). LDL cholesterol concentrations were estimated by the Friedwald formula. Insulin concentration was determined using an enzyme-linked immunosorbent assay (ALPCO Diagnostics, Salem, NH, USA; CV: 7.5%). Interleukin 6 (IL-6) and high-molecular weight adiponectin were measured using enzyme-linked immunosorbent assay kits from R&D Systems (Abingdon, UK; CV of 8.9% and 8.4%, respectively). Concentrations of hsCRP were measured using latex-enhanced immunoturbimetric assay (Randox Laboratories) on a Cobas FARA bioanalyzer (Roche Products Ltd., Herts, UK; lower detection limit, 0.1 mg/L; CV: 5.5%). Fibrinogen was determined using an automated coagulation analyzer (ACL Futura; Instrumentation Laboratory Company, Lexington, MA, USA; CV: 1.6%).
2.6. Statistics
Data were tested for normality by examining histograms of values and using the normality plot, Skewness value, and Shapiro-Wilk test. Waist, SKF, and biochemical variables were not normally distributed and were normalized by log-transformation before analysis and are presented as geometric means. The homeostatic model assessment index was calculated using insulin × glucose/22.5.21
Sex differences were analyzed by independent (unpaired) Student's t tests. The effects of sex and puberty, or sex and waist measurement, were analyzed by two-way analysis of variance. Log–log regression analysis was used to estimate the power with which to raise the height to correct SKF measurements.8, 22 Pearson correlation coefficients were calculated between pairs of variables. Linear and nonlinear regression analyses were used, as appropriate, to determine the relationship between variables. Hierarchical multiple regression analysis was used to identify independent predictor variables, after ensuring no violation of the assumptions of normality, linearity, multicollinearity, and homoscedasticity. Because patterns differed by gender, genders were also considered separately. Statistical analyses were performed using SPSS Statistics Version 20.0 (IBM Corp., Armonk, NY, USA) and Statistica Version 10.0 (StatSoft Inc., Tulsa, OK, USA). To take into account multiple measurements, the level of statistical significance was determined using the Bonferroni correction. Uncorrected p values are shown. A level of significance of p < 0.05 was set for multiple regression.
3. Results
3.1. Biomarkers associated with metabolic and cardiovascular risk in girls and boys
Biomarkers and cardiovascular fitness measurements were available from 129 girls and 95 boys (Table 2). In this cohort of 13-year-olds, boys had higher cardiovascular fitness scores than girls. They also tended to be taller and have a higher birth weight. Height in girls correlated with birth weight (r = 0.395, p < 0.01; n = 129), but this was not significant in boys (r = 0.146, p = 0.17; n = 92), and there was no relationship between birth weight and any other measurement among girls or boys or among their parents (data not shown). Girls had higher SKF measurements than boys; however, waist circumference expressed as a percentage of height (WHtR) and the ratio of trunk-to-extremity SKF (T/E SKF) did not differ between genders. The small difference in age between T1–3 compared with T4–5 was statistically significant in girls and not in boys (p = 0.016; Table 3).
Table 2.
Gender differences in markers of metabolic and cardiovascular risk in adolescent children (mean ± SD) (95%CI).
| Girls | Boys | ||
|---|---|---|---|
| (n = 129) | (n = 95) | pa | |
| Age (year) | 13.48 ± 0.30 (13.43–13.53) | 13.53 ± 0.33 (13.46–13.60) | 0.231 |
| Anthropometry | |||
| Height (cm) | 158.1 ± 6.7 (156.9–159.3) | 160.1 ± 9.0 (158.3–161.9) | 0.056 |
| Waist (cm)b | 65.2 ± 1.1 (63.9–66.6) | 67.7 ± 1.1 (66.0–69.5) | 0.023 |
| Weight (kg) | 53.3 ± 11.0 (51.4–55.2) | 53.2 ± 13.1 (50.5–55.8) | 0.921 |
| WHtR (%)b | 41.3 ± 1.1 (40.5–42.1) | 42.4 ± 1.1 (41.4–43.4) | 0.088 |
| Birth weight (kg) | 3.33 ± 0.36 (3.23–3.43) | 3.52 ± 0.59 (3.40–3.64)b | 0.017 |
| Subscapular SKF (mm)b | 10.8 ± 1.6 (10.0–11.7) | 8.8 ± 1.7 (7.9–9.8) | <0.001 |
| Suprailiac SKF (mm)b | 12.2 ± 1.6 (11.3–13.3) | 9.1 ± 1.9 (8.0–10.4) | <0.001 |
| Triceps SKF (mm)b | 16.1 ± 1.4 (15.1–17.2) | 12.7 ± 1.6 (11.6–13.9) | <0.001 |
| Biceps SKF (mm)b | 10.2 ± 1.5 (9.5–11.0) | 7.9 ± 1.6 (7.1–8.7) | 0.001 |
| 4SKF (mm)b,c | 50.2 ± 1.5 (47.1–53.6) | 39.2 ± 1.6 (35.4–43.4) | <0.001 |
| T/E SKFb,d | 0.87 ± 1.30 (0.84–0.91) | 0.87 ± 1.36 (0.82–0.93) | 0.915 |
| Blood pressure | |||
| Systolic (mmHg) | 115.6 ± 10.1 (113.8–117.3) | 116.8 ± 12.6 (114.2–119.3) | 0.422 |
| Diastolic (mmHg) | 65.7 ± 10.2 (63.9–67.5) | 64.7 ± 11.0 (62.4–66.9) | 0.472 |
| Cardiovascular fitness (laps) | 44.5 ± 1.4 (41.9–47.2) | 62.6 ± 1.4 (58.4–67.0) | <0.001 |
| Biomarkers | |||
| Total cholesterol (mmol/L)b | 4.01 ± 1.15 (3.90–4.12) | 3.76 ± 1.18 (3.63–3.89) | 0.003 |
| LDL cholesterol (mmol/L)b | 1.95 ± 1.33 (1.86–2.05) | 1.81 ± 1.35 (1.70–1.92) | 0.052 |
| HDL cholesterol (mmol/L)b | 1.67 ± 1.21 (1.62–1.73) | 1.60 ± 1.24 (1.53–1.67) | 0.114 |
| Triglycerides (mmol/L)b | 0.71 ± 1.43 (0.66–0.75) | 0.59 ± 1.53 (0.54–0.64) | <0.001 |
| Glucose (mmol/L)b | 4.80 ± 0.27 (4.75–4.85)e | 4.92 ± 0.35 (4.85–4.99)f | 0.006 |
| Insulin (mIU/L)b | 10.02 ± 1.47 (9.37–10.71) | 6.96 ± 1.58 (6.34–7.63) | <0.001 |
| HOMA (insulin×glucose/22.5)b | 2.16 ± 1.49 (2.01–2.32)e | 1.53 ± 1.63 (1.38–1.69)f | <0.001 |
| Fibrinogen (g/L)b | 2.62 ± 1.19 (2.54–2.70)g | 2.63 ± 1.18 (2.54–2.73)g | 0.807 |
| hsCRP (mg/L)b | 0.29 ± 2.19 (0.26–0.34)f | 0.43 ± 2.38 (0.36–0.52) | <0.001 |
| IL-6 (ng/L)b | 0.57 ± 2.09 (0.50–0.64)f | 0.69 ± 2.14 (0.59–0.81)g | 0.047 |
| HMW Adiponectin (mg/L)b | 2.93 ± 1.77 (2.65–3.24)f | 2.11 ± 1.89 (1.85–2.40)g | <0.001 |
Notes: a Unpaired t test girls vs. boys Bonferroni-corrected α = 0.002; b Geometric mean ± SD; c Sum of triceps, subscapular, biceps, and suprailiac SKF; d Ratio of trunk (sum of subscapular and suprailiac)-to-extremity (sum of triceps and biceps) SKF; e Data missing from 3 participants; f Data missing from 2 participants; g Data missing from 1 participant.
Abbreviations: CI = confidence interval; HDL = high-density lipoprotein; HMW = high-molecular weight; HOMA = homeostatic model assessment; hsCRP = high-sensitivity C-reactive protein; IL-6 = interleukin-6; LDL = low-density lipoprotein; SKF = skinfold thickness; T/E SKF = the ratio of trunk-to-extremity skinfold thicknesses; WHtR = waist-to-height ratio.
Table 3.
Gradient b (SE) derived from log–log relationships between height and SKF in children who reported being in Tanner stages 1–3 (T1–3) and in Tanner stages 4–5 (T4–5) for breast development in girls and genital development in boys, using line drawings (Taylor et al., 2001)19 based on Tanner (1962)48 (mean ± SD).
| Girls |
Boys |
|||
|---|---|---|---|---|
| Pubertal stage | T1–3 (n = 73) | T4–5 (n = 56) | T1–3 (n = 42) | T4–5 (n = 53) |
| Agea | 13.4 ± 0.30 (13.4–13.5) | 13.6 ± 0.29 (13.5–13.6) # | 13.5 ± 0.36 (13.4–13.6) | 13.6 ± 0.30 (13.5–13.7) |
| 4SKFb | 2.14 ± 0.93 | 0.70 ± 1.34 | 4.66 ± 1.19*** | –1.28 ± 0.26 |
| Triceps | 1.77 ± 0.92 | 0.11 ± 1.28 | 2.73 ± 1.08 | –2.23 ± 1.19 |
| Biceps | 0.49 ± 0.99 | 0.51 ± 1.59 | 3.53 ± 1.14** | –2.26 ± 1.36 |
| Subscapular | 2.84 ± 1.08* | 0.27 ± 1.67 | 6.05 ± 1.27*** | 0.18 ± 1.21 |
| Suprailiac | 3.51 ± 1.15** | 2.11 ± 1.61 | 6.66 ± 1.59*** | –0.43 ± 1.66 |
| T/E SKFc | 1.91 ± 0.58** | 1.10 ± 0.93 | 3.37 ± 0.64*** | 2.08 ± 0.75** |
Notes: a Data presented as mean ± SD (95%CI); b Sum of triceps, subscapular, biceps, and suprailiac SKF; c Trunk-to-extremity SKF ratio ((subscapular+suprailiac)/(triceps+biceps)); Bonferroni-corrected α = 0.01.
* p ≤ 0.01, ** p ≤ 0.005, *** p ≤ 0.001, significant relationship between height and SKF; #p = 0.016, compared with girls in T1–3 (independent t test).
Abbreviations: CI = confidence interval; SKF = skinfold thickness; T/E SKF = the ratio of trunk-to-extremity skinfold thicknesses SKF.
There was a relationship between height and the sum of the SKFs (4SKF) in children in T1–3 (r = 0.279, p = 0.017, n = 73 for girls; and r = 0.563, p < 0.001, n = 42 for boys), but not in T4–5 (r = 0.078, p = 0.570, n = 56 for girls; and r = –0.156, p = 0.265, n = 53 for boys). Because 4SKF is dependent on height, the relationship between height and each SKF was also determined. Gradients obtained from log–log linear regression analysis of height and SKF (Table 3) indicate that most of the effect of puberty on the relationship between height and 4SKF was from the contribution of subscapular SKF and suprailiac SKF measurements. This finding was markedly apparent in T1–3 boys. There was no significant relationship between triceps SKF and height in any group. There was a positive relationship between T/E SKF and height in girls in T1–3 and in boys in T1–3 and T4–5.
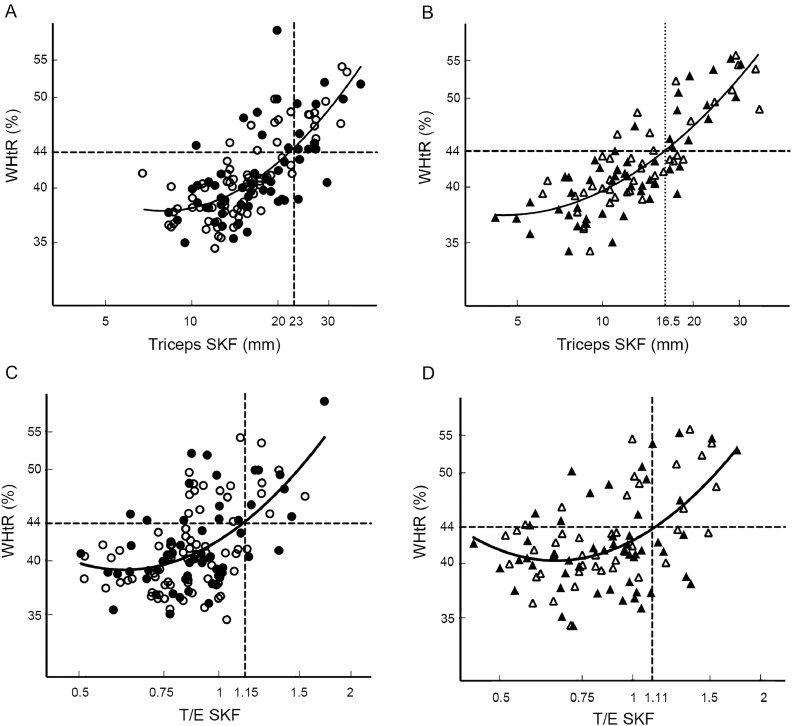
There was a similar relationship between WHtR and triceps SKF or T/E SKF irrespective of gender or pubertal status (Fig. 1). Nonlinear regression lines and correlations did not differ significantly in slope; however, in boys with a triceps SKF of ≥10 mm, the mean deviation of the WHtR was 7.5% (6.3%–8.6%) above the girls’ curvilinear regression line (p < 0.001). The regression lines in girls and boys crossed a WHtR of 44% at triceps SKF measurements of 23 mm and 16.5 mm, respectively (i.e., values were 42% higher in girls), whereas the regression lines crossed a WHtR of 44% at similar T/E SKF measurements in girls and boys.
Fig. 1.
Relationship between waist-to-height ratio (WHtR) and triceps skinfold thickness (SKF) or the ratio of trunk-to-extremity SKF (T/E SKF), in girls (A, C) and boys (B, D). The open symbols represent those self-reporting being in Tanner stages 1–3 for breast development in girls and genital development in boys. The closed symbols represent Tanner stages 4–5. Nonlinear regression lines and correlations for the relationships are indicated by the solid lines. (A) lnWHtR = 4.264 – 0.601 × lnTricepsSKF + 0.144 × lnTricepsSKF2, r2 = 0.538, p < 0.001 (129 girls); (B) lnWHtR = 3.842 – 0.287 × lnTricepsSKF + 0.095 × lnTricepsSKF2, r2 = 0.690, p < 0.001 (95 boys); (C) lnWHtR = 3.738 + 0.294 × lnT/E SKF + 0.298 × lnT/ESKF2, r2 = 0.308, p < 0.001 (129 girls); (D) lnWHtR = 3.746 + 0.247 × lnT/ESKF + 0.308 × lnT/ESKF2, r2 = 0.271, p < 0.001 (95 boys). SKF = skinfold thickness; T/E SKF = the ratio of trunk-to-extremity skinfold thicknesses; WHtR = waist-to-height ratio.
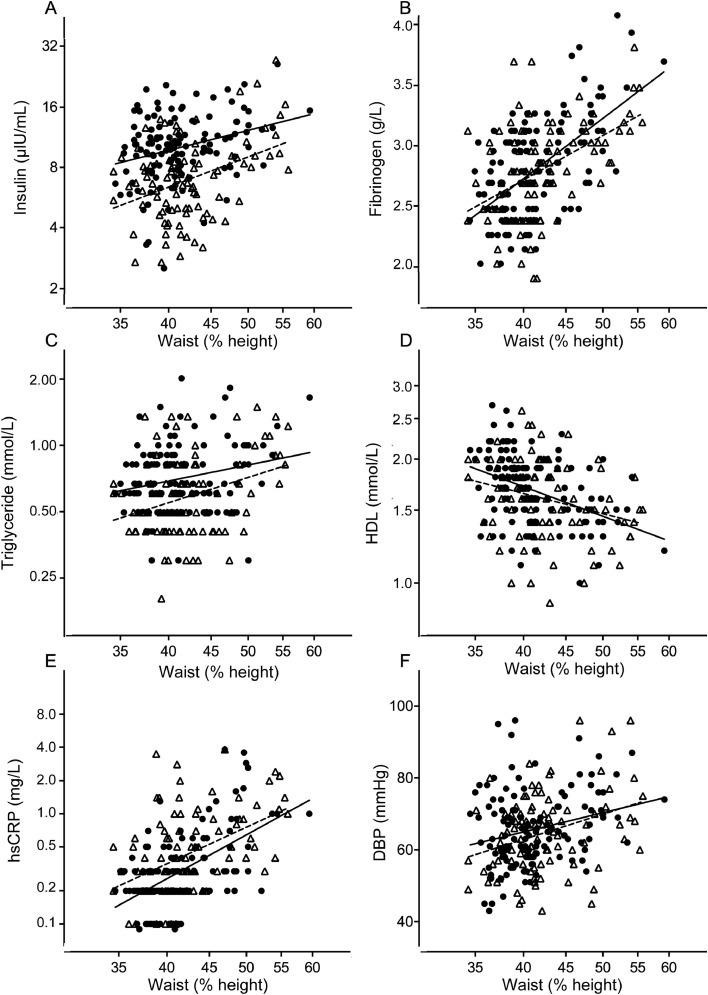
Serum insulin concentrations were higher in girls than in boys (Table 2) and correlated positively with WHtR in girls and boys (Fig. 2), regardless of pubertal status (data not shown). Glucose levels were lower in girls than in boys, and correlated positively with insulin (r = 0.235, p = 0.008, n = 126 for girls; and r = 0.398, p < 0.001, n = 93 for boys). In boys, insulin concentrations correlated with height z scores (r = 0.451, p < 0.001, n = 95 for boys; and r = 0.119, p = 0.181, n = 129 for girls). In hierarchical multiple regression analysis, glucose and WHtR were entered at Step 1 and explained 11% of the variance in insulin. After entry of gender and cardiovascular fitness at Step 2, the total variance in insulin explained by the model was 34%, F(4, 214) = 27.70, p < 0.001. Gender and cardiovascular fitness explained an additional 23% of the variance in insulin after controlling for glucose and WHtR, F(2, 214) = 38.15, p < 0.001. In the final model, the following were statistically significant: glucose (β = 0.29, p < 0.001), WHtR (β = 0.26, p < 0.001), and gender (β = 0.45, p < 0.001).
Fig. 2.
Relationship between waist-to-height ratio (WHtR, %) and other markers of metabolic and cardiovascular risk in adolescent girls (closed symbols, intact line) and boys (open symbols, broken line). Linear regression lines and correlations for the relationships are as follows. (A) lnInsulin = 1.041 × lnWHtR – 1.568; r = 0.288, p < 0.001 (129 girls) and lnInsulin = 1.572 × lnWHtR–3.949; r = 0.394, p < 0.001 (95 boys); (B) lnFibrinogen = 0.948 × lnWHtR–2.565; r = 0.585, p < 0.001 (128 girls) and lnFibrinogen = 0.702 × lnWHtR – 1.662; r = 0.477, p < 0.001 (94 boys); (C) lnTriglyceride = 0.771 × lnWHtR–3.219; r = 0.226, p = 0.010 (129 girls) and lnTriglyceride = 1.186 × lnWHtR–4.977; r = 0.322, p = 0.002 (95 boys); (D) lnHDL = 3.309–0.751 × lnWHtR; r = –0.411, p < 0.001 (129 girls) and lnHDL = 2.407–0.517 × lnWHtR; r = –30.273, p = 0.008 (95 boys); (E) lnCRP = 4.14 × lnWHtR–16.62; r = 0.553, p < 0.001 (127 girls) and lnCRP = 3.46 × lnWHtR–13.80; r = 0.456, p < 0.001 (95 boys); (F) DBP=24.29 × lnWHtR–24.66; r = 0.252, p = 0.004 (129 girls) and DBP = 31.80 × lnWHtR–54.46; r = 0.330, p < 0.001 (95 boys). DBP = diastolic blood pressure; HDL = high-density lipoprotein; hsCRP = high-sensitivity C-reactive protein.
Serum concentrations of triglycerides and total cholesterol were higher in girls (Table 2), regardless of pubertal status (data not shown). Fibrinogen and triglyceride concentrations, and diastolic blood pressure levels, each correlated positively with WHtR in boys and girls, while HDL cholesterol was inversely correlated (Fig. 2).
HMW adiponectin concentrations were lower in boys (Table 2) and were lower in late puberty, analysis of variance (ANOVA) F(3, 217) = 8.201; gender p < 0.001 and puberty p = 0.012; interaction p = 0.138. Concentrations of HMW adiponectin were 2.52 mg/L (95%CI: 2.09–3.04 mg/L) in the 42 T1–3 boys and 1.82 mg/L (95%CI: 1.53–2.17 mg/L) in the 52 T4–5 boys. In girls, values were 2.80 mg/L (95%CI: 2.36–3.31 mg/L) in the 71 T1–3 girls and 3.04 mg/L (95%CI: 2.69–3.45 mg/L) in the 56 T4–5 girls. In hierarchical multiple regression analysis, gender and puberty were entered at Step 1 and explained 9% of the variance in HMW adiponectin. After entry of WHtR, insulin and triceps SKF at Step 2, the total variance in HMW adiponectin explained by the model was just 15%, F(5, 215) = 7.65, p < 0.001. WHtR, insulin and T/E SKF explained an additional 6% of the variance in HMW adiponectin after controlling for gender and puberty, F(3, 215) = 4.94, p = 0.002. In the final model, the following were statistically significant: gender (β = 0.30, p < 0.001), puberty (β = 0.14, p = 0.032), and T/E SKF (β = –0.15, p = 0.035).
hsCRP concentrations were significantly lower in the girls compared with the boys (Table 2) and correlated with IL-6 (r = 0.360, p < 0.001 in 125 girls; and r = 0.466, p < 0.001 in 94 boys). There was an association between CRP and WHtR in girls and boys (Fig. 2). In hierarchical multiple regression analysis, gender, IL-6, and WHtR were entered at Step 1 and explained 37% of the variance in CRP. After entry of puberty, insulin, T/E SKF, and cardiovascular fitness at Step 2, the total variance in CRP explained by the model was 36%, F(4, 211) = 18.58, p < 0.001. In the final model the following were statistically significant: WHtR (β = 0.39, p < 0.001), IL-6 (β = 0.29, p < 0.001), and gender (β = 0.14, p = 0.045).
3.2. Characteristics of adolescents with higher WHtR
We compared children with the highest waist circumferences (>44% of height; 35 girls and 26 boys) with the rest of the cohort (94 girls and 69 boys; Table 4). There were no differences in pubertal status among the 4 groups (data not shown). The T/E SKF was higher in the group with a WHtR of >44% in both girls and boys. Most children with a WHtR of >44% had a triceps SKF measurement of >23 mm in girls and >16.5 mm in boys (Fig. 1A and 1B). These children also had higher diastolic blood pressure, insulin concentrations, and homeostatic model assessment measurements, and CRP and fibrinogen concentrations, and lower HDL cholesterol concentrations, than the rest of the cohort. The cardiovascular fitness score was lower in girls and boys with the higher WHtR. In boys with a WHtR of >44%, there was an inverse correlation with insulin concentrations (lnInsulin = 6.251 – 1.050 × lnFitnessScore; r = –0.727, p < 0.001), which was not seen in thinner boys or in girls (data not shown).
Table 4.
Characteristics of children with waist measurements that were >44% or ≤44% height (mean (95%CI)).
| WHtR (%) | >44% |
≤44% |
pa |
||||
|---|---|---|---|---|---|---|---|
| Girls | Boys | Girls | Boys | Waist | Sex | Interaction | |
| Gender group (n (%)) | 35 (27%) | 26 (27%) | 94 (73%) | 69 (73%) | |||
| Anthropometry | |||||||
| Height (cm) | 159.3 (157.1–161.5) | 160.4 (156.3–164.5) | 157.7 (156.3–159.1) | 160.0 (157.9–162.1) | 0.393 | 0.145 | 0.598 |
| WHtR (%)b | 47.8 (46.8–49.0) | 49.4 (47.9–50.9) | 39.1 (38.7–39.5) | 40.0 (39.4–40.6) | <0.001 | 0.003 | 0.584 |
| 4SKF (mm)b,c | 78.5 (72.7–84.7) | 71.4 (60.2–84.7) | 42.6 (40.3–45.0) | 31.3 (29.1–33.6) | <0.001 | <0.001 | 0.017 |
| T/E SKFb,d | 1.06 (0.98–1.15) | 1.02 (0.90–1.17) | 0.81 (0.77–0.85) | 0.82 (0.76–0.87) | <0.001 | 0.741 | 0.644 |
| Triceps SKF (mm)b | 23.3 (21.1–25.7) | 20.7 (19.0–23.7) | 14.0 (13.3–14.9) | 10.6 (9.7–11.4) | <0.001 | <0.001 | 0.078 |
| Weight (kg) | 66.4 (63.2–69.5) | 65.7 (59.3–72.2) | 48.5 (47.1–49.8) | 48.4 (46.5–50.3) | <0.001 | 0.803 | 0.824 |
| Birth weight (kg) | 3.37 (3.14–3.60) | 3.46 (3.19–3.73)e | 3.31 (3.20–3.43) | 3.54 (3.40–3.68)e | 0.613 | 0.044 | 0.976 |
| Blood pressure | |||||||
| Systolic (mmHg) | 117.3 (114.7–120.0) | 117.5 (111.2–123.9) | 114.9 (112.7–117.1) | 116.5 (113.8–119.2) | 0.307 | 0.599 | 0.681 |
| Diastolic (mmHg) | 70.5 (67.4–73.6) | 68.3 (63.0–73.7) | 63.9 (61.9–66.0) | 63.3 (61.0–65.6) | <0.001 | 0.375 | 0.629 |
| Cardiovascular fitness (laps) | 35.3 (31.3–39.2) | 52.8 (44.6–61.0) | 51.4 (48.4–54.5) | 70.7 (66.5–74.9) | <0.001 | <0.001 | 0.711 |
| Biomarkers | |||||||
| Total cholesterol (mmol/L)b | 3.97 (3.75–4.21) | 3.69 (3.43–3.98) | 4.02 (3.90–4.15) | 3.78 (3.64–3.93) | 0.468 | 0.006 | 0.809 |
| LDL cholesterol (mmol/L)b | 2.05 (1.86–2.27) | 1.81 (1.58–2.06) | 1.92 (1.81–2.03) | 1.81 (1.68–1.94) | 0.449 | 0.039 | 0.454 |
| HDL cholesterol (mmol/L)b | 1.52 (1.43–1.62) | 1.49 (1.37–1.62) | 1.73 (1.67–1.80) | 1.64 (1.56–1.73) | <0.001 | 0.223 | 0.627 |
| Triglycerides (mmol/L)b | 0.76 (0.66–0.87) | 0.67 (0.56–0.81) | 0.69 (0.64–0.73) | 0.56 (0.50–0.61) | 0.012 | 0.005 | 0.410 |
| Glucose (mmol/L)b,f | 4.81 (4.72–4.90) | 4.89 (4.76–5.01) | 4.80 (4.74–4.85) | 4.93 (4.84–5.01) | 0.783 | 0.027 | 0.569 |
| Insulin (mIU/L)b | 11.4 (10.1–12.9) | 8.7 (7.0–10.7) | 9.5 (8.8–10.3) | 6.4 (5.8–7.1) | <0.001 | <0.001 | 0.314 |
| HOMA (insulin×glucose/22.5) b,f | 2.50 (2.20–2.84) | 1.93 (1.53–2.42) | 1.97 (1.78–2.20) | 1.37 (1.21–1.55) | <0.001 | <0.001 | 0.482 |
| Fibrinogen (g/L)b,g | 3.04 (2.86–3.23) | 2.96 (2.82–3.12) | 2.48 (2.41–2.55) | 2.52 (2.42-2.62) | <0.001 | 0.848 | 0.355 |
| hsCRP (mg/L)b | 0.62 (0.45–0.85) | 0.74 (0.54–1.03) | 0.23 (0.20–0.25) | 0.35 (0.29–0.43) | <0.001 | 0.005 | 0.240 |
| IL-6 (ng/L)b,h | 0.78 (0.63–0.95) | 0.76 (0.55–1.04) | 0.50 (0.43–0.59) | 0.67 (0.56–0.81) | 0.016 | 0.241 | 0.157 |
| HMW adiponectin (mg/L)b,h | 2.75 (2.25–3.37) | 1.88 (1.41–2.51) | 3.00 (2.67–3.38) | 2.19 (1.89–2.54) | 0.188 | <0.001 | 0.707 |
| Parental data (mean (95%CI; n)) | |||||||
| Maternal BMI (kg/m2) | 28.8 (26.7–30.9; 33) | 28.3 (26.5–30.0; 26) | 25.6 (24.6–26.5; 85) | 26.1 (25.1–27.2; 65) | 0.001 | 0.677 | 0.231 |
| Maternal waist2/htx10b | 4.37 (3.91–4.89; 29) | 4.31 (3.82–4.85; 24) | 3.78 (3.55–4.03; 79) | 3.75 (3.51–4.01; 63) | 0.001 | 0.774 | 0.838 |
| Paternal BMI (kg/m2) | 28.6 (26.9–30.3; 28) | 30.6 (28.7–32.5; 22) | 27.4 (26.5–28.2; 81) | 27.8 (26.7–28.7; 66) | 0.004 | 0.094 | 0.225 |
| Paternal waist2/htx10b | 4.61 (4.34–4.90; 27) | 5.02 (4.59–5.49; 21) | 4.48 (4.33–4.65; 78) | 4.63 (4.45–4.82; 66) | 0.070 | 0.033 | 0.293 |
Notes: a Two-way analysis of variance, Bonferroni-corrected α = 0.002; b Geometric mean; c Sum of triceps, subscapular, biceps and suprailiac SKF; d Ratio of trunk (sum of subscapular and suprailiac)-to-extremity (sum of triceps and biceps) SKF; e Data missing for 1 individual; f Data missing for 3 girls ≤ 44% and 2 boys ≤ 44%; g Data for 1 girl ≤ 44% and 1 boy ≤ 44%; h Data missing for 1 boy > 44%, 2 girls ≤ 44%.
Abbreviations: BMI = body mass index; CI = confidence interval; HMW = high-molecular weight; HDL = high-density lipoprotein; HOMA = homeostatic model assessment; hsCRP = high-sensitivity C-reactive protein; IL-6 = interleukin 6; LDL = low-density lipoprotein; SKF = skinfold thickness; T/E SKF = trunk-to-extremity SKF; WtHR = waist-to-height ratio.
3.3. Parental weight and waist measurements
Parental weight measurements correlated with height (mothers: r = 0.315, p < 0.001; fathers: r = 0.381, p < 0.001) and correction of weight by height2 (BMI) removed the effect of height. In men, the waist and WHtR correlated with height (r = 0.177, p = 0.014). When the formula23 waist2× 10/ht was used, there was no relationship between waist and height in mothers or fathers. According to World Health Organization criteria,24 and using corrected BMI values, 33% of mothers were normal weight and 29% were obese (BMI ≥ 30), and 11% of fathers were normal weight and 35% were obese.
3.4. Relationship between child and parental anthropometric variables
In the children with a WHtR of >44%, maternal BMI and waist2 × 10/ht were higher than for mothers of children with a WHtR of ≤44% (Table 4). Paternal BMI was also significantly higher. Values from children for whom both maternal and paternal BMI were available were included in the following analyses (93 girls and 80 boys).
Correlations between anthropometric variables in children and their parents are shown in Table 5. In girls, WHtR correlated significantly with maternal BMI. There was no correlation between maternal and paternal BMI; however, there was a significant relationship between maternal and paternal waist2 × 10/ht (data not shown).
Table 5.
Correlations between anthropometric variables in parents and children (Pearson's r).
| Girls (n = 93) |
Boys (n = 80) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Height | BMI | WHtRa | T/E SKFa | Triceps SKFa | Height | BMI | WHtRa | T/E SKFa | Triceps SKFa | |
| Child BMI | 0.196 | — | — | — | — | 0.304*** | — | — | — | — |
| Child WHtRa | –0.023 | 0.897*** | — | — | — | –0.002 | 0.881*** | — | — | — |
| Child T/E SKFa | 0.268*** | 0.509*** | 0.497*** | — | — | 0.454*** | 0.573*** | 0.433*** | — | — |
| Child Triceps SKFa | 0.133 | 0.748*** | 0.700*** | 0.125 | — | 0.019 | 0.764*** | 0.797*** | 0.076 | — |
| Maternal height | 0.359*** | –0.029 | –0.050 | 0.151 | –0.075 | 0.337*** | 0.132 | 0.056 | –0.079 | 0.209 |
| Paternal height | 0.297*** | 0.004 | –0.042 | –0.063 | 0.111 | 0.145 | 0.068 | 0.034 | 0.012 | 0.182 |
| Midparental heightb | 0.423*** | –0.014 | –0.060 | 0.044 | 0.035 | 0.324*** | 0.134 | 0.060 | –0.047 | 0.259* |
| Maternal BMI | 0.094 | 0.351*** | 0.324*** | 0.172 | 0.350*** | 0.181 | 0.286*** | 0.225* | 0.129 | 0.279* |
| Paternal BMI | –0.160 | 0.215* | 0.204* | 0.291*** | 0.011 | 0.066 | 0.315*** | 0.263* | 0.254* | 0.133 |
| Maternal W2HtRa | 0.123 | 0.189 | 0.199 | 0.201 | 0.211* | 0.271* | 0.307*** | 0.261* | 0.236* | 0.301*** |
| Paternal W2HtRa | –0.087 | 0.068 | 0.108 | 0.198 | –0.065 | 0.070 | 0.210 | 0.198 | 0.192 | 0.120 |
Notes: a Log-transformed; b For girls = (father's height – 13 cm + mother's height)/2; For boys = (mother's height + 13 cm + father's height)/2. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001; Bonferroni-corrected α = 0.004.
Abbreviations: BMI = body mass index; SKF = skinfold thickness; T/E SKF = trunk-to-extremity SKF; W2HtR = waist2 × 10/ht; WHtR = waist-to-height ratio.
Hierarchical multiple regression analysis was used to determine the impact of parental BMI on children's WHtR, triceps SKF, and T/E SKF. Gender and cardiovascular fitness were entered at Step 1 and explained 25% of the variance in child WHtR. After entry of maternal and paternal BMI at Step 2, the total variance in child WHtR explained by the model was 33%, F(4, 184) = 22.22, p < 0.001. Maternal and paternal BMI explained an additional 8% of the variance in child WHtR after controlling for gender and cardiovascular fitness, F(2, 184) = 10.73, p < 0.001. In the final model, the following independent variables were statistically significant: gender (β = 0.31, p < 0.001), cardiovascular fitness (β = –0.49, p < 0.001), maternal BMI (β = 0.22, p < 0.001), and paternal BMI (β = 0.17, p = 0.007). For child triceps SKF, gender and cardiovascular fitness, entered at Step 1, explained 32.5% of the variance. After entry of maternal and paternal BMI at Step 2, the total variance in child triceps SKF explained by the model was 37.3%, F(4, 184) = 27.38, p < 0.001. Maternal and paternal BMI explained an additional 4.8% of the variance, F(2, 184) = 7.09, p < 0.001. In the final model, the following were statistically significant: cardiovascular fitness (β = –0.52, p < 0.001) and maternal BMI (β = 0.22, p < 0.001). For child T/E SKF, gender and cardiovascular fitness, entered at Step 1, explained 7% of the variance. After entry of maternal and paternal BMI at Step 2, total variance in child triceps SKF explained by the model was 14%, F(4, 184) = 7.47, p < 0.001. Maternal and paternal BMI explained an additional 7% of the variance, F(2, 184) = 7.38, p = 0.001. In the final model, the following were statistically significant: cardiovascular fitness (β = –0.25, p = 0.001) and paternal BMI (β = 0.22, p = 0.002).
3.5. Cardiovascular fitness
We have previously shown that the cardiovascular fitness score is inversely related to WHtR and to 4SKF in girls and boys in early/mid and late puberty.8 Because 4SKF depends on height, we explored the relationship between cardiovascular fitness and triceps SKF. There is an inverse relationship, with no difference between those in T1–3 and T4–5 for each gender, and with similar combined slopes for girls (fitness score = 107.7–21.82 × lnTricepsSKF, r = 0.497, p < 0.001, n = 129) and boys (lnWHtR = 3.842–0.287 × lnTricepsSKF + 0.095 × lnTricepsSKF2, r = 0.752, p < 0.001, n = 95). However, the combined regression line was significantly higher for boys compared with girls, F(1, 221) = 39.477, p < 0.01. In a hierarchical multiple regression analysis, gender and WHtR were entered at Step 1 and explained 39% of the variance in cardiovascular fitness. After entry of maternal and paternal BMI at Step 2, the total variance in cardiovascular fitness explained by the model was 39%, F(4, 184) = 29.58, p < 0.001. In the final model, the following were statistically significant: gender (β = 0.50, p < 0.001) and WHtR (β = –0.45, p < 0.001), with no independent contribution of maternal BMI or paternal BMI.
4. Discussion
In this study of adolescents, measures of adiposity correlated with biomarkers that are associated with cardiovascular disease risk in adults. Waist and SKF measurements correlate with height in children in early or mid-puberty; however, the influence of height is lost when waist measurement is expressed as a percentage of height (WHtR). Gender differences were observed. Although girls seemed to have more subcutaneous fat and decreased levels of cardiovascular fitness, boys had relatively greater central adiposity and higher levels of proinflammatory markers. Parental overweight and obesity were associated with greater adiposity in adolescent girls and boys, suggesting that strategies for decreasing cardiovascular risk should be family focused.
The cohort was well-controlled for age and socioeconomic status, and we were therefore able to estimate the independent effects of puberty and parental adiposity. There were, however, limitations. This was a cross-sectional design, and a comparison of children in T1–3 and T4–5 in longitudinal studies would be required to predict the effect of puberty in individual subjects. Furthermore, pubertal status and parental anthropometry (height, weight, and waist measurements) were self-reported by participants. Self-reported height tends to be overestimated and weight underestimated,18 leading to an underestimation of obesity prevalence and an exaggeration of the relationship with cardiovascular disease risk.25 We used the protocol developed by Taylor et al.19 to estimate pubertal development. It should also be noted that children tend to underestimate pubertal development when using these line drawings.19
Sex hormones influence adipocyte deposition and function,26 and adolescence has been considered a critical time in the development of obesity.27, 28 However, although there is a relationship between obesity and the early onset of puberty in girls, the data for boys are inconclusive.29 There is also insufficient evidence that puberty is causally linked to obesity development. In a large cross-sectional study, sexual dimorphism in fat patterning was present before puberty and increased across puberty, with girls having less waist fat.28 The trend in British adolescents over recent decades for having increases in waist circumference exceed increases in BMI, particularly in girls, suggests that abdominal fatness is increasing at a greater rate than whole body fatness.30 In our study, despite having triceps SKF that was lower, boys had similar WHtR compared with girls. This observation could be explained by a relatively greater contribution of visceral fat to the abdominal measurement in boys.31 Assessing the value of using WHtR and SKF measurements (triceps and T/E ratios) to track adiposity across puberty would require longitudinal studies with larger cohorts.
We identified gender differences in circulating concentrations of biomarkers that are associated with cardiovascular disease. Fasting insulin and triglyceride concentrations were higher in girls than in boys. In boys with a higher WHtR, there was an inverse relationship between cardiovascular fitness and insulin levels. It is now recognized that cardiorespiratory fitness influences the relationship between adiposity and cardiovascular prognosis.4, 32 In boys, fat mass accrual during emerging adulthood is mitigated by physical activity.33 It, therefore, could be speculated that encouraging physical fitness in this group might have a significant impact in decreasing cardiometabolic risk. Higher hsCRP levels were observed in boys, which is consistent with a previous study of obese adolescents.14 In contrast, in prepubertal children, CRP is reported to be higher in girls34 and adult women independent of exogenous estrogen.35 In adults, there is a strong inverse relationship between hsCRP levels and adiposity, and these relationships independently predict cardiovascular disease events.36 Although CRP levels in children predict values in adults37 and predict adult obesity,38 evidence that CRP in children is a predictor of cardiovascular risk in adulthood is not strong.39 In obese girls, high hsCRP concentrations are associated with insulin resistance.33 In our study, although hsCRP correlated with WHtR in boys and girls, there was no relationship to fasting insulin. In a linear regression analysis, there was an influence of WHtR on hsCRP concentrations that is independent of the ratio of T/E SKF. HMW adiponectin, which has both anti-inflammatory and insulin-sensitizing properties, is reported to be inversely related to adiposity and insulin resistance.36 These relationships were not present in our study. However, we did observe that concentrations were lower in boys, particularly in the late puberty group, which may be explained by a direct effect of testosterone on adipocyte production.40 Lower adiponectin concentrations, taken together with relatively greater waist circumference measurements and higher CRP levels, suggest that adolescent boys have more visceral adiposity and associated systemic inflammation.
It has been suggested that a WHtR of >0.5 in children should be used to predict health risk when those children become adults.41 A study of Australian children aged 8–16 years showed that a WHtR of ≥0.46 for boys and ≥0.45 for girls identified those with percentage body fat in the ≥85th percentile.42 In black South African adolescents, a lower WHtR cutoff of 0.41 indicated metabolic risk.43 In our study, adolescents with a WHtR of >0.44 had higher levels of cardiovascular biomarkers. It has been suggested that WHtR further specifies cardiometabolic risk within classifications that are based on BMI percentiles.44 We observed that parental BMI measurements were also higher in those with a WHtR of >0.44. Although this finding may indicate that childhood overweight and obesity are strongly related to the social environment, genetic factors may also play a role.45 Birth weight is reported to predict later obesity.46 In our study, the child's birth weight reported by the parents was associated with height in girls; however, an association with markers of adiposity was not observed, suggesting that birth weight may not be a consistent predictor of later overweight and obesity.
In our group of 12-year-olds, pubertal status had no impact on the level of physical fitness in girls or boys. In a recent longitudinal study of younger children, persistently low, or a decrease in, cardiorespiratory fitness was associated with later cardiovascular disease risk.47 Although cardiovascular fitness was not related to parental BMI in our study, the observation that parental overweight and obesity is associated with higher waist measurements in children suggests that strategies for reducing cardiovascular risk should be family focused.
5. Conclusion
In adolescents, there is a relationship between measures of adiposity and parental weight that involves factors other than cardiovascular fitness. Adolescent boys have relatively more abdominal fat than girls and tend to have a proinflammatory profile of biomarkers. These observations suggest that family and social environmental interventions to prevent obesity are best undertaken early in childhood, particularly in boys.
Acknowledgments
Acknowledgments
The authors thank the parents, teachers, and, most important, the children who participated in this study. Professor Non Thomas, Swansea University, who led the original study design and data collection, died before analysis of the data. Professor Kerstin Hall, Karolinksa Institutet, who contributed to the statistical analysis of data, died on March 29, 2017. Professor Thomas and Professor Hall are greatly missed by academic colleagues and friends. Laboratory measurements were performed by the Biochemistry Departments of the Royal Glamorgan Hospital, Llantrisant, South Wales, UK, and Llandough Hospital, Penarth, South Wales, UK.
Authors' contributions
MSL carried out the statistical analyses and drafted the manuscript; JSB conceived the study, helped collect and analyze the data, and helped draft the manuscript. Both authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
Both authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Singh A.S., Mulder C., Twisk J.W., van Mechelen W., Chinapaw M.J. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9:474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 2.Baker J.L., Olsen L.W., Sørensen T.I. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayer J., Charakida M., Deanfield J.E., Celermajer D.S. Lifetime risk: childhood obesity and cardiovascular risk. Eur Heart J. 2015;36:1371–1376. doi: 10.1093/eurheartj/ehv089. [DOI] [PubMed] [Google Scholar]
- 4.Oktay A.A., Lavie C.J., Kokkinos P.F., Parto P., Pandey A., Ventura H.O. The interaction of cardiorespiratory fitness with obesity and the obesity paradox in cardiovascular disease. Prog Cardiovasc Dis. 2017;60:30–44. doi: 10.1016/j.pcad.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Kimm S.Y., Barton B.A., Obarzanek E., McMahon R.P., Kronsberg S.S., Waclawiw M.A. Obesity development during adolescence in a biracial cohort: the NHLBI Growth and Health Study. Pediatrics. 2002;110:e54. doi: 10.1542/peds.110.5.e54. [DOI] [PubMed] [Google Scholar]
- 6.Nader P.R., Bradley R.H., Houts R.M., McRitchie S.L., O'Brien M. Moderate-to-vigorous physical activity from ages 9 to 15 years. JAMA. 2008;300:295–305. doi: 10.1001/jama.300.3.295. [DOI] [PubMed] [Google Scholar]
- 7.Gibson L.Y., Byrne S.M., Davis E.A., Blair E., Jacoby P., Zubrick S.R. The role of family and maternal factors in childhood obesity. Med J Aust. 2007;186:591–595. doi: 10.5694/j.1326-5377.2007.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 8.Lewitt M.S., Baker J.S., Mooney G.P., Hall K., Thomas N.E. Pubertal stage and measures of adiposity in British schoolchildren. Ann Hum Biol. 2012;39:440–447. doi: 10.3109/03014460.2012.704070. [DOI] [PubMed] [Google Scholar]
- 9.Sugiura R., Murata M. Problems with body mass index as an index to evaluate physical status of children in puberty. Pediatr Int. 2011;53:634–642. doi: 10.1111/j.1442-200X.2010.03312.x. [DOI] [PubMed] [Google Scholar]
- 10.Brambilla P., Bedogni G., Heo M., Pietrobelli A. Waist circumference-to-height ratio predicts adiposity better than body mass index in children and adolescents. Int J Obes (Lond) 2013;37:943–946. doi: 10.1038/ijo.2013.32. [DOI] [PubMed] [Google Scholar]
- 11.Savva S.C., Tornaritis M., Savva M.E., Kourides Y., Panagi A., Silikiotou N. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Relat Metab Disord. 2000;24:1453–1458. doi: 10.1038/sj.ijo.0801401. [DOI] [PubMed] [Google Scholar]
- 12.Murphy M.J., Hosking J., Metcalf B.S., Voss L.D., Jeffery A.N., Sattar N. Distribution of adiponectin, leptin, and metabolic correlates of insulin resistance: a longitudinal study in British children; 1: prepuberty (EarlyBird 15) Clin Chem. 2008;54:1298–1306. doi: 10.1373/clinchem.2008.103499. [DOI] [PubMed] [Google Scholar]
- 13.Muller M.J., Lagerpusch M., Enderle J., Schautz B., Heller M., Bosy-Westphal A. Beyond the body mass index: tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes Rev. 2012;13(Suppl. 2):S6–S13. doi: 10.1111/j.1467-789X.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 14.Alemzadeh R., Kichler J. Gender differences in the association of insulin resistance and high-sensitivity C-reactive protein in obese adolescents. J Diabetes Metab Disord. 2014;13:35. doi: 10.1186/2251-6581-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas N.E., Jasper M., Williams D.R., Rowe D.A., Malina R.M., Davies B. Secular trends in established and novel cardiovascular risk factors in Welsh 12-13 year olds: a comparison between 2002 and 2007. Ann Hum Biol. 2011;38:22–27. doi: 10.3109/03014460.2010.482540. [DOI] [PubMed] [Google Scholar]
- 16.Shuttleworth I. The relationship between social deprivation, as measured by individual free school meal eligibility, and educational attainment at GCSE in Northern Ireland: a preliminary investigation. Br Educ Res J. 1995;21:487–504. [Google Scholar]
- 17.Ulijaszek S.J., Kerr D.A. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr. 1999;82:165–177. doi: 10.1017/s0007114599001348. [DOI] [PubMed] [Google Scholar]
- 18.Connor Gorber S., Shields M., Tremblay M.S., McDowell I. The feasibility of establishing correction factors to adjust self-reported estimates of obesity. Health Rep. 2008;19:71–82. [PubMed] [Google Scholar]
- 19.Taylor S.J., Whincup P.H., Hindmarsh P.C., Lampe F., Odoki K., Cook D.G. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 20.Ortega F.B., Artero E.G., Ruiz J.R., Vicente-Rodriguez G., Bergman P., Hagströmer M. Reliability of health-related physical fitness tests in European adolescents. The HELENA Study. Int J Obes (Lond) 2008;32(Suppl. 5):S49–S57. doi: 10.1038/ijo.2008.183. [DOI] [PubMed] [Google Scholar]
- 21.Turner R.C., Holman R.R., Matthews D., Hockaday T.D., Peto J. Insulin deficiency and insulin resistance interaction in diabetes: estimation of their relative contribution by feedback analysis from basal plasma insulin and glucose concentrations. Metabolism. 1979;28:1086–1096. doi: 10.1016/0026-0495(79)90146-x. [DOI] [PubMed] [Google Scholar]
- 22.Wells J.C., Cole T.J. ALSPAC study steam. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord. 2002;26:947–952. doi: 10.1038/sj.ijo.0802027. [DOI] [PubMed] [Google Scholar]
- 23.Lewitt M.S., Hilding A., Brismar K., Efendic S., Ostenson C.G., Hall K. IGF-binding protein-1 and abdominal obesity in the development of type 2 diabetes in women. Eur J Endocrinol. 2010;163:233–242. doi: 10.1530/EJE-10-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organisation. Obesity and overweight: Key facts. 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [accessed 20.11.2020].
- 25.Yannakoulia M., Panagiotakos D.B., Pitsavos C., Stefanadis C. Correlates of BMI misreporting among apparently healthy individuals: the ATTICA study. Obesity (Silver Spring) 2006;14:894–901. doi: 10.1038/oby.2006.103. [DOI] [PubMed] [Google Scholar]
- 26.Palmer B.F., Clegg D.J. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietz W.H. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59:955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- 28.Taylor R.W., Grant A.M., Williams S.M., Goulding A. Sex differences in regional body fat distribution from pre- to postpuberty. Obesity (Silver Spring) 2010;18:1410–1416. doi: 10.1038/oby.2009.399. [DOI] [PubMed] [Google Scholar]
- 29.Li W., Liu Q., Deng X., Chen Y., Liu S., Story M. Association between obesity and puberty timing: a systematic review and meta-analysis. Int J Environ Res Public Healt. 2017;14:1266. doi: 10.3390/ijerph14101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy H.D., Ellis S.M., Cole T.J. Central overweight and obesity in British youth aged 11–16 years: cross sectional surveys of waist circumference. BMJ. 2003;326:624. doi: 10.1136/bmj.326.7390.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verduin W.M., Van Den Helder R., Doodeman H.J., Struijf E., Houdijk A.P. DEXA body composition assessment in 10–11 year healthy children. PloS One. 2016;11 doi: 10.1371/journal.pone.0165275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crump C., Sundquist J., Winkleby M.A., Sundquist K. Interactive effects of obesity and physical fitness on risk of ischemic heart disease. Int J Obes (Lond) 2017;41:255–261. doi: 10.1038/ijo.2016.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbour-Tuck E., Erlandson M., Muhajarine N., Foulds H., Baxter-Jones A. Influence of childhood and adolescent fat development on fat mass accrual during emerging adulthood: a 20-year longitudinal study. Obesity (Silver Spring) 2018;26:613–620. doi: 10.1002/oby.22111. [DOI] [PubMed] [Google Scholar]
- 34.Cook D.G., Mendall M.A., Whincup P.H., Carey I.M., Ballam L., Morris J.E. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149:139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 35.Lakoski S.G., Cushman M., Criqui M., Rundek T., Blumenthal R.S., D'Agostino R.B., Jr Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152:593–598. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Balagopal P.B., de Ferranti S.D., Cook S., Daniels S.R., Gidding S.S., Hayman L.L. Nontraditional risk factors and biomarkers for cardiovascular disease: mechanistic, research, and clinical considerations for youth: a scientific statement from the American Heart Association. Circulation. 2011;123:2749–2769. doi: 10.1161/CIR.0b013e31821c7c64. [DOI] [PubMed] [Google Scholar]
- 37.Juonala M., Viikari J.S., Rönnemaa T., Taittonen L., Marniemi J., Raitakari O.T. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2006;26:1883–1888. doi: 10.1161/01.ATV.0000228818.11968.7a. [DOI] [PubMed] [Google Scholar]
- 38.Juonala M., Juhola J., Magnussen C.G., Würtz P., Viikari J.S., Thomson R. Childhood environmental and genetic predictors of adulthood obesity: the Cardiovascular Risk in Young Finns Study. J Clin Endocrinol Metab. 2011;96:E1542–E1549. doi: 10.1210/jc.2011-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeBoer M.D. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: a need for screening tools to target interventions. Nutrition. 2013;29:379–386. doi: 10.1016/j.nut.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu A., Chan K.W., Hoo R.L., Wang Y., Tan K.C., Zhang J. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–18080. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 41.Browning L.M., Hsieh S.D., Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr Res Rev. 2010;23:247–269. doi: 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]
- 42.Nambiar S., Hughes I., Davies P.S. Developing waist-to-height ratio cut-offs to define overweight and obesity in children and adolescents. Public Health Nutr. 2010;13:1566–1574. doi: 10.1017/S1368980009993053. [DOI] [PubMed] [Google Scholar]
- 43.Kruger H.S., Faber M., Schutte A.E., Ellis S.M. A proposed cutoff point of waist-to-height ratio for metabolic risk in African township adolescents. Nutrition. 2013;29:502–507. doi: 10.1016/j.nut.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Khoury M., Manlhiot C., McCrindle B.W. Role of the waist/height ratio in the cardiometabolic risk assessment of children classified by body mass index. J Am Coll Cardiol. 2013;62:742–751. doi: 10.1016/j.jacc.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 45.Dubois L., Ohm Kyvik K., Girard M., Tatone-Tokuda F., Pérusse D., Hjelmborg J. Genetic and environmental contributions to weight, height, and BMI from birth to 19 years of age: an international study of over 12,000 twin pairs. PloS One. 2012;7:e30153. doi: 10.1371/journal.pone.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baird J., Fisher D., Lucas P., Kleijnen J., Roberts H., Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castro-Pinero J., Perez-Bey A., Segura-Jiménez V., Aparicio V.A., Gómez-Martínez S., Izquierdo-Gomez R. Cardiorespiratory fitness cutoff points for early detection of present and future cardiovascular risk in children: a 2-year follow-up study. Mayo Clin Proc. 2017;92:1753–1762. doi: 10.1016/j.mayocp.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Tanner J.M. 2nd ed. Blackwell; Oxford: 1962. Growth at adolescence. [Google Scholar]