Highlights
-
•
Exposure to hypoxia impaired attentional ability, executive function, and memory function but not information processing.
-
•
The effects of hypoxia on cognition were significantly moderated by sex and cognitive task type but not by other variables.
-
•
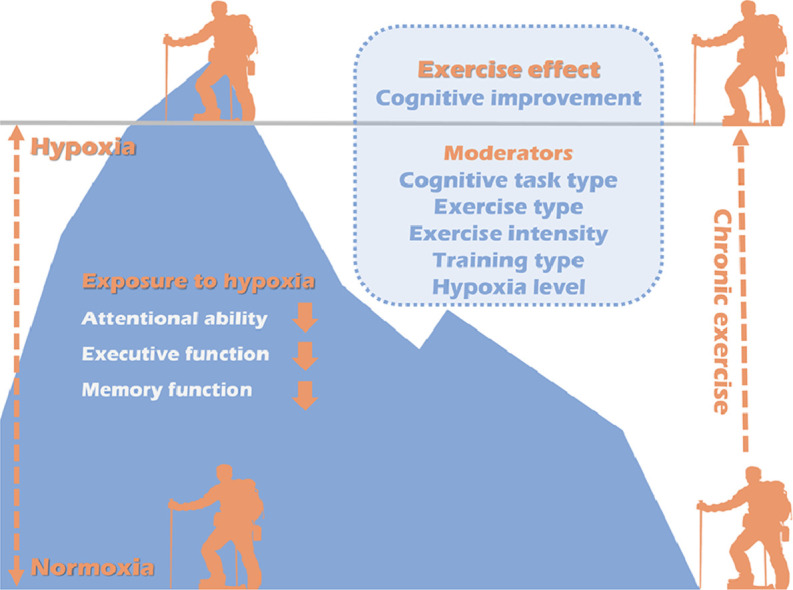
Performing exercise under a hypoxia setting has significant effects on cognitive improvement, which was moderated by cognitive task type, exercise type, exercise intensity, training type, and hypoxia level.
Keywords: Cognition, Executive function, Exercise, Hypoxia, Memory
Abstract
Objective
This study aimed to examine (1) the independent effects of hypoxia on cognitive function and (2) the effects of exercise on cognition while under hypoxia.
Methods
Design: Systematic review with meta-analysis. Data sources: PubMed, Scopus, Web of Science, PsychInfo, and SPORTDiscus were searched. Eligibility criteria for selecting studies: randomized controlled trials and nonrandomized controlled studies that investigated the effects of chronic or acute exercise on cognition under hypoxia were considered (Aim 2), as were studies investigating the effects of hypoxia on cognition (Aim 1).
Results
In total, 18 studies met our inclusionary criteria for the systematic review, and 12 studies were meta-analyzed. Exposure to hypoxia impaired attentional ability (standardized mean difference (SMD) = –0.4), executive function (SMD = –0.18), and memory function (SMD = –0.26), but not information processing (SMD = 0.27). Aggregated results indicated that performing exercise under a hypoxia setting had a significant effect on cognitive improvement (SMD = 0.3, 95% confidence interval: 0.14 – 0.45, I2 = 54%, p < 0.001). Various characteristics (e.g., age, cognitive task type, exercise type, exercise intensity, training type, and hypoxia level) moderated the effects of hypoxia and exercise on cognitive function.
Conclusion
Exercise during exposure to hypoxia improves cognitive function. This association appears to be moderated by individual and exercise/hypoxia-related characteristics.
Graphical Abstract
1. Introduction
Cognitive functions are brain-based skills that allow humans to carry out tasks at various levels of difficulty and that are critical in day-to-day life.1,2 Notably, cognitive performance is possibly affected by environmental cues such as ambient temperature and altitude.3,4 For instance, increasing altitude and the ensuing severity of hypoxia may attenuate oxygen delivery to the brain tissue. Such exponentially reduced oxygen fraction during inspiration may result in impairment of brain function and cognitive abilities, including executive function, attention, episodic memory, and information processing.5, 6, 7 This occurrence may be detrimental for particular populations, including those in the armed forces, athletes, mountaineers, mountain rescuers, and other high-altitude residents who are repeatedly exposed to hypoxic conditions.8, 9, 10
Findings from several primary studies11, 12 and a previously published review13 have indicated hypoxia-induced cognitive deficits. Regardless of cognitive task types (i.e., central executive vs. nonexecutive tasks) and hypoxic conditions (i.e., hypobaric vs. normobaric hypoxia), low partial pressure of oxygen (PaO2) levels are strongly associated with greater reductions in cognitive function. In contrast, accumulating evidence suggests that hypoxia has no negative effects on cognitive function.14, 15, 16, 17 For instance, an experimental study by Lefferts et al.9 showed that response accuracy on cognitive tasks was similar in normoxia compared to hypoxia in a mixed sample of young men and women. Sun et al.18 reported that moderate hypoxia did not alter either reaction time or accuracy in sedentary young adults. Given the conflicting findings in previous studies, along with new publications on this topic, an updated systematic review is needed to evaluate and synthesize the current evidence on the effects of hypoxia on cognition.
Behavioral approaches such as exercise have previously been shown to enhance cognitive function and prevent neurocognitive disorders.19, 20, 21, 22 Notably, the majority of these previous studies investigating the cognitive benefits of exercise were conducted under normoxic conditions. Recent exploratory studies have suggested that moderate exercise has the potential to improve cognitive function during exposure to moderate or severe hypoxia,3,10,16,23 whereas others have reported that cognitive function may be impaired if exercise occurred under hypoxic conditions.8,24 In 2019, a narrative review qualitatively examined the combined effects of exercise and hypoxia on cognitive function, suggesting that these effects are determined largely by the interaction of moderators, such as exercise duration and intensity, hypoxia level and duration, and cognitive task type.25
Despite this recent narrative review,25 current data investigating the quantitative effects of hypoxia on cognition and whether exercise modifies these effects are inconsistent, which may be due to differences in methodological and experimental conditions. Given the heterogeneity of these aforementioned results, further studies are required to test the moderator effects of experimental conditions on cognitive function in relation to hypoxia and exercise.26,27 Therefore, to provide a comprehensive review of the extant research on this topic, the current meta-analysis addresses 2 primary aims. The 1st aim was to investigate the independent effects of hypoxia on cognitive function. The 2nd aim was to examine the effects of exercise on cognition while under hypoxia. Given these aims, we further performed subgroup analyses to evaluate potential moderators of the direction and magnitude of the effect sizes. The potential moderators included key study characteristics (e.g., hypoxia protocol, exercise protocol, and specific cognitive task), and participant attributes (e.g., sex, age) were selected because they have been shown to affect cognitive function or influence the effects of exercise on cognitive function while under hypoxia.20,25
2. Methods
2.1. Protocol and registration
This study protocol was registered with PROSPERO and approved with registration number CRD42019145773.
2.2. Search strategy
To obtain adequate and efficient coverage of relevant literature, we used PubMed, Scopus, Web of Science, PsychInfo, and SPORTDiscus for the literature search. All documents were retrieved from inception to August 2019. Three groups of search terms were combined to locate studies: (1) “exercise” OR “training” OR “sport” OR “physical activity” OR “strength”; (2) “cognition” OR “cognitive function” OR “executive function” OR “cognitive flexibility” OR “cognitive task” OR “neuropsychological test” OR “perception” OR “reaction time” OR “memory” OR “mental processing” OR “inhibition”; and (3) “hypoxia” OR “hypobaric” OR “normobaric” OR “high altitude”. Reference lists of selected studies were further investigated to avoid missing any relevant article. We performed this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.3. Eligibility criteria
First, in this review we included only English-language studies published in peer-reviewed journals obtainable in full text. Second, given that this is the 1st systematic review of this topic, studies that investigated the effects of hypoxia on cognition or experimental studies of randomized controlled trials (RCTs) and non-randomized controlled studies (NRSs) that investigated the effects of chronic or acute exercise on cognition under hypoxia were considered (Aim 2). Third, sufficient information (e.g., arterial oxygen saturation and/or altitude) had to be provided so that PaO2 could be estimated. Fourth, at least 1 outcome measure of cognition had to be reported in order to be extracted for meta-analyses.
2.4. Collection of studies
All records from the searched databases were managed with an EndNote library. This literature-management tool helped to remove record duplications from the various searched databases. Based on the predetermined eligibility criteria, titles and abstracts of the remaining records were independently screened by 2 review authors (LZ and JJY) in order to exclude ineligible studies. If a study met eligibility requirements, it received a full-text article assessment. If any disagreement occurred, a 3rd review author (PDL) was invited to achieve consensus through discussion.
2.5. Data extraction
We used a customized form for data extraction. Detailed information included the 1st author of the article and publication year, characteristics of participants (sample size and mean age/age range), study design, normoxia/hypoxia protocol, hypoxia-exercise temporality, exercise protocol, the instrument measuring and method of reporting the outcomes, and statistical analyses and results. To obtain pooled effect size of outcomes, we also extracted the following quantitative data: (1) mean and standard deviation (SD) of cognitive function between performing tasks under hypoxia and normoxia at rest and its corresponding sample size (Aim 1); (2) mean and SD of cognitive function between performing tasks under hypoxia during exercise and rest and its corresponding sample size (Aim 2); (3) mean and SD of pre-to-post difference, along with sample size of both experimental and control groups.
2.6. Methodological quality assessment
Two authors (MJ and SR) used the modified Downs and Black checklist to independently perform methodological quality assessment of both RCTs and NRSs.28 This assessment consisted of 24 items across 4 domains (reporting, external validity, internal validity, and power), and scoring ranged from 0 to 25 (1 point per each question except Question 5 (2 points)), with higher scores representing better methodological quality.
2.7. Classification of study aims
The study had 2 specific aims regarding the association between hypoxia and exercise on cognitive function. The 1st aim was to explore the effects of hypoxia on cognitive function by comparing cognitive performance between 2 groups, with 1 group performing the cognitive task during exposure to hypoxia and the other group completing the cognitive task during normoxia. The 2nd aim was to examine the effects of exercise on cognitive function while under hypoxia by comparing cognitive performance between 2 groups, with 1 group performing the cognitive task and exercise under a hypoxic condition and the other group completing the cognitive task under hypoxia (no exercise).
2.8. Statistical analysis
Comprehensive Meta-Analysis software (Version 3.0; Biostat Inc., Englewood, NJ, USA) was used to calculate effect sizes (standardized mean difference (SMD)) representing the magnitude of the exercise intervention on cognition. SMD was considered as small (0.20–0.49), moderate (0.50–0.79), or large (≥0.80). Considering that the outcomes and unit of cognitive measures varied across the selected studies, the random-effects model was used along with a 95% confidence interval (CI). We used I-squared (I2) to check heterogeneity, with 25%, 50%, and 75% reflecting small, medium, and large heterogeneity, respectively. Publication bias was assessed by the Egger's test, along with a visual funnel plot.
The heterogeneity of effect sizes (ESs) was assessed by Q statistics. Q statistics evaluate the null hypothesis that all individual ESs compute the same population ES. Statistically significant Q statistics indicate the existence of possible moderator variables.29 The moderator analyses were conducted to test whether the above-mentioned moderators influenced the overall ESs.20,25 Meta-regression was used for continuous variables (hypoxia duration, hypoxia level, hypoxia dose (determined by multiplying hypoxia duration by hypoxia level), and exercise duration) to quantify the relationship between the magnitude of the moderator and cognitive function,30 whereas subgroup analyses were performed based on categorical variables, including sex, age (young adults = 20–24 years vs. older adults = 60 years and older), study design (RCTs or NRSs), exercise type, exercise intensity, exercise temporality, training type, and cognitive task type. We computed a mean ES and SE for each group, and then tested whether these mean ESs were significantly different from one another, a model that is analogous to a one-way random effects analysis of variance model.31
3. Results
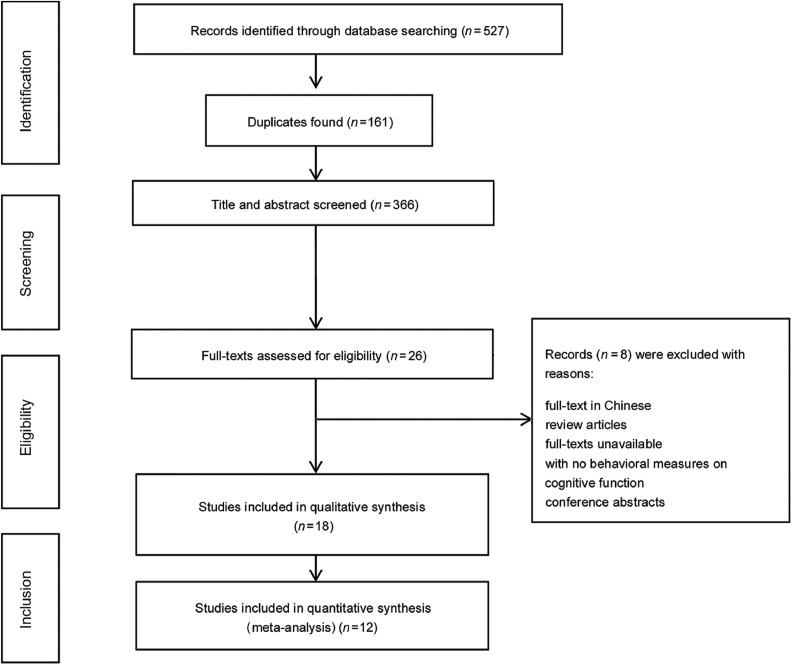
Fig. 1 displays the flow chart for the literature search process. The computerized searches yielded 527 articles. Among the 527 articles, 161 were eliminated due to duplication, and 366 articles were screened. After initial screening of 366 titles and abstracts, 26 full-text articles were reviewed. Among these 26 articles, 8 were ineligible because they did not meet the inclusion criteria or did not provide sufficient data to calculate ESs. Therefore, in total, 18 studies met our inclusion criteria for the systematic review, and 12 studies were eligible for the quantitative meta-analysis.
Fig. 1.
Flow chart of literature search.
3.1. Study characteristics
Detailed information on study characteristics is displayed in Table 1. Sample sizes ranged from 8 to 80 participants, with mean age varying from 20 to 92 years old.32,33 Among the 18 studies, 6 (33%) were RCTs, and 12 (67%) were NRSs. The hypoxia protocol varied, including, for example, hypoxia duration (e.g., ranging from 5 min to 180 min), hypoxia level (e.g., fraction of inspired oxygen (FIO2) ranging from 10% to 18%), and hypoxia dose (e.g., exercise duration (min) × hypoxia level (%) ranging from 0.73 to 25.38). The exercise protocol varied by exercise type (e.g., cycle ergometer, a full-body strength-endurance program, multimodal training program, and repeated sprint running), exercise intensity (e.g., light, moderate, and high), exercise duration (e.g., ranging from 5 min to 165 min), exercise temporality (e.g., exercise occurred during hypoxia or both before and during hypoxia), and training type (e.g., acute exercise (also referred to as a single bout of exercise) and chronic exercise (also referred to as a training)). Of the 18 studies, 12 (67%) employed acute exercise interventions and 6 (33%) employed chronic exercise interventions. Cognitive tasks were categorized into information processing, attention-based ability, executive function, memory, and reaction time. A listing of these tasks is presented in Table 2.
Table 1.
Extraction table of the evaluated studies.
| Study | Subject characteristics Age (mean ± SD or range) | Study design | Normoxia/Hypoxia protocol | Hypoxia-exercise temporality | Exercise protocol | Cognitive function assessment | Results |
|---|---|---|---|---|---|---|---|
| Ando et al. (2013)3 | 12 adult males 22.9 ± 1.5 years |
Within-subject, Pre–post comparison | Performed cognitive tasks under either normoxia (20.9%) or normobaric hypoxia (18%, 15%) 25 min of hypoxia |
Hypoxia/Normoxia both before and during exercise | 5 min of cycle ergometer exercise at 20% peak VO2max and 10 min of cycle ergometer exercise at 60% peak VO2max/Acute exercise | GNG (Go/No-Go) task | GNG–RT Main effect EX: F (1, 11) = 18.73, p < 0.01 Main effect HY: F (2, 22) = 0.06, p = 0.94 EX × HY: F (1.41, 15.53) = 0.06, p = 0.69 GNG–Accuracy Main effect EX: F (1, 11) = 1.49, p = 0.25 Main effect HY: F (2, 22) = 2.14, p = 0.14 EX × HY: N/A |
| Schega et al. (2013)32 | 34 retired older adults 60–70 years |
Between-subject, RCT, Pre–post comparison |
The CG was supplied with a placebo air mixture and the EG was supplied with an IHT (intermittent hypoxia training) 10 min of hypoxia (10%) |
Hypoxia/Normoxia during exercise | A full-body strength-endurance exercise program at 50% of maximum force/Chronic exercise | d2 test: GZ (quantitative performance index; the rate at which participants mark off each d2 and the overall number of marked letters within the d2 test), SKL (concentration performance index; the standardized number of accurate answers minus confusion errors) and ZVT |
GZ Main effect of EX: F (1, 32) = 36.325, p = 0.00, η² = 0.532 Main effect HY: F (1, 32) = 0.08, p = 0.78, η² = 0.002 EX × HY: F (1, 32) = 4.59, p = 0.04, η² = 0.125 SKL Main effect EX: F (1, 32) = 23.565, p = 0.00, η² = 0.424 Main effect HY: F (1, 32) = 0.047, p = 0.831, η² = 0.001 EX × HY: F (1, 32) = 4.65, p = 0.034, η² = 0.127 ZVT Main effect EX: F (1, 32) = 32.065, p = 0.00, η²= 0.501 Main effect HY: F (1, 32) = 1.906, p = 0.177, η² = 0.056 EX × HY: F (1, 32) = 0.21, p = 0.649, η² = 0.007 |
| Kim et al. (2015)8 | 8 healthy adult males 41± 2 years |
Within-subject, Counterbalanced, Pre–post comparison |
In one of the experimental trials (HY, 12.5%), subjects remained resting in a seated position the entire 5 h; in the other experimental trial (HY + EX), subjects rested 2 h, cycled for 1 h, then rested the last 2 h. | Hypoxia/Normoxia both before and during exercise | 1 h of a Lode cycle exercise (workload equivalent to 50% altitude-adjusted VO2max) /Acute exercise |
Trail Making Test A and B |
Main effect EX: N/A Main effect HY: N/A EX × HY: N/A |
| Komiyama et al. (2015)16 | 16 adult males 23.0 ± 2.3 years |
Within-subject, Pre–post comparison |
Performed cognitive tasks at rest and during exercise under normoxic and hypoxic conditions (15%) 45 min of hypoxia |
Hypoxia/Normoxia both before and during exercise | 30 min of cycle ergometer exercise until heart rate was 140 beats/min /Acute exercise |
Spatial DR GNG task |
Spatial DR–Accuracy Main effect EX: F (2, 30) = 1.34, p = 0.28, η² = 0.08 Main effect HY: F (1, 15) = 0.81, p = 0.38, η² = 0.05 EX × HY: F (2, 30) = 1.69, p = 0.20, η² = 0.10 GNG–Accuracy Main effect EX: F (2, 30) = 2.79, p = 0.08, η² = 0.16 Main effect HY: F (1, 15) = 2.5, p = 0.14, η² = 0.14 EX × HY: F (2, 30) = 1.13, p = 0.34, η² = 0.07 GNG–RT Main effect EX: F (2, 30) = 8.02, p < 0.01, η² = 0.35 Main effect HY: F (1, 15) = 0.63, p = 0.44, η² = 0.04 EX × HY: F (2, 30) = 0.10, p = 0.91, η² = 0.006 |
| Seo (2015)45 | 16 young healthy men 24 ± 4 years |
Within-subject, counterbalanced, Pre–post comparison |
Performed cognitive tasks at rest and during exercise under normoxic and hypoxic conditions (12.5%) 90-min of hypoxia |
Hypoxia/Normoxia both before and during exercise | Two 15-min bouts of cycle ergometer exercise at 40% and 60% of adjusted VO2max with 15-min recovery period between bouts/Acute exercise | GNG task, RMCPT (running memory continuous performance task) |
GNG–RT Main effect EX: N/A Main effect HY: F = 1.8, p = 0.168 EX × HY: N/A GNG–Accuracy Main effect EX: N/A Main effect HY: F = 2.2, p = 0.098 EX × HY: N/A RMCPT–RT Main effect EX: 40% (p = 0.028), 60% ( p = 0.009) Main effect HY: F = 3.4, p = 0.025 EX × HY: N/A RMCPT - Accuracy Main effect EX: p > 0.262 Main effect HY: F = 7.6, p ≤ 0.001 EX × HY: N/A RMCPT–Throughout score Main effect EX: 40% (p = 0.023), 60% (p = 0.006) Main effect HY: F = 5.0, p = 0.005 EX × HY: N/A |
| Dobashi et al. (2016)24 | 8 healthy males 23.7 ± 2.1 years |
Within-subject, Counterbalanced, Pre–post comparison |
Performed cognitive tasks before, during, and 60 min after exercise under normoxic and hypoxic conditions (14.1%) 180 min of hypoxia |
Hypoxia/Normoxia both before and during exercise | Four 30-min bouts of cycle ergometer exercise at moderate intensity with a 15-min interval rest between each set /Acute exercise |
CWST: Task 1 (Reverse-Stroop control) Task 2 (Reverse-Stroop interference) Task 3 (Stroop control) Task 4 (Stroop interference) |
Task 1–Number of Achievement Main effect EX: N/A Main effect HY: N/A EX × HY: F (3, 21) = 4.53, p < 0.05, η² = 0.39 Task 3–Number of achievement Main effect EX: F (3, 14) = 4.09, p < 0.05, η² = 0.37 Main effect HY: F (3, 21) = 0.24, p > 0.05, η² = 0.03 EX × HY: F (3, 21) = 2.97, p > 0.05, η² = 0.30 Tasks 2, 4–Number of achievement Main effect EX: p > 0.05 Main effect HY: p > 0.05 EX × HY: p > 0.05 Task 1–Number of correct response Main effect EX: N/A Main effect HY: N/A EX × HY: F (2, 14) = 5.63, p < 0.05, η² = 0.45 Task 3–Number of correct response Main effect EX: F (2, 14) = 3.2, p > 0.05, η² = 0.31 Main effect HY: F (1, 8) = 9.1, p < 0.05, η² = 0.57 EX × HY: F (2, 14) = 1.77, p > 0.05, η² = 0.20 Tasks 2,4 – Number of correct response Main effect EX: p > 0.05 Main effect HY: p > 0.05 EX × HY: p > 0.05 |
| Lefferts et al. (2016)9 | 30 adults M = 15 22 ± 4 years W = 15 20 ± 3 years |
Within-subject, Pre–post comparison |
Performed cognitive tasks during exercise under normoxic and hypoxic conditions (12.5%) 120 min of hypoxia |
Hypoxia/Normoxia both before and during exercise | 25-min bouts of cycle ergometer exercise at moderate intensity/Acute exercise | Memory recognition, N-Back, Flanker tasks |
Main effect EX: N/A Main effect HY: N/A EX × HY: N/A |
| Schega et al. (2016)33 | 33 older adults 60–75 years |
Between-subject, RCT, Pre–post comparison |
The CG breathed ambient air and the EG was supplied with IHT (10%) 120-min of hypoxia |
Hypoxia/Normoxia both before and during exercise | 30-min bouts of bicycle ergometer at moderate intensity/Chronic exercise | Stroop test | Color task Main effect EX: N/A Main effect HY: p = 0.004 EX × HY: F (1, 31) = 1.833, p = 0.178, η² = 0.056 Word-color-tasks Main effect EX: N/A Main effect HY: p = 0.005 EX × HY: F (1, 30) = 1.506, p = 0.23, η² = 0.048 |
| Bayer et al. (2017)58 | 34 older adults 64–75 years |
Between-subject, RCT, Pre–post comparison |
EG received MTP and IHHT (12%); CG received MTP during the IHHT. Both performed cognitive tasks before and after the training. 35–45 min of hypoxia |
Hypoxia/Normoxia during exercise | 2 h of MTP (2–3 times/week, 14–15 trainings in 5–6 weeks) /Chronic exercise |
DemTect, CDT | DemTect Main effect EX: 16.7% vs. –0.39%, p < 0.001 Main effect HY: N/A EX × HY: N/A CDT Main effect EX: 10.7% vs. –8%, p = 0.031 Main effect HY: N/A EX × HY: N/A |
| Bayer et al. (2017)59 | 34 older adults 64–92 years |
Between-subject, RCT, Pre–post comparison |
EG received MTP and IHHT (10%–14%), and CG received MTP during the simulation of IHHT. Both performed cognitive tasks before and after the training. 30–40 min of hypoxia |
Hypoxia/Normoxia during exercise | 2 h of MTP (2–3 times/week, 12–15 trainings in 5–7 weeks) /Chronic exercise |
DemTect, CDT | DemTect Main effect EX: 16.7% vs. –0.39%, p < 0.001 Main effect HY: N/A EX × HY: N/A CDT Main effect EX: 10.7% vs. –8%, p = 0.031 Main effect HY: N/A EX × HY: N/A |
| Komiyama et al. (2017)23 | 13 adult males 21.5 years (mean) |
Within-subject, Pre–post comparison |
Performed cognitive tasks at rest and during exercise under either normoxic or hypoxic conditions (13%) 30 min of hypoxia |
Hypoxia/Normoxia both before and during exercise | 20 min of cycle ergometer exercise at moderate intensity/Acute exercise | Spatial DR, GNG task |
GNG–RT Main effect EX: F (1, 12) = 29.52, p < 0.001 Main effect HY: F (1, 12) = 0.16, p = 0.69 EX × HY: N/A GNG–Accuracy Main effect EX: p > 0.14 Main effect HY: p > 0.14 EX × HY: N/A Spatial DR–Accuracy Main effect EX: p > 0.14 Main effect HY: p > 0.14 EX × HY: N/A |
| Seo et al. (2017)10 | 15 healthy women 22 ± 2 years |
Within-subject, Counterbalanced, Pre–post comparison |
Performed cognitive tasks at rest and during exercise under either normoxia or hypoxia (12.5%) 105 min of hypoxia |
Hypoxia/Normoxia both before and during exercise | Two 15-min bouts of cycle ergometer exercise at 40% and 60% VO2max with a 15-min recovery between bouts/Acute exercise | RMCPT | Main effect EX: F (4, 56) = 2.6, p = 0.047, η² = 0.2 Main effect HY: N/A EX × HY: N/A |
| Stavres et al. (2017)60 | 18 adults M = 9 22 ± 3 years W = 9 23 ± 2 years |
Within-subject, Counterbalanced, Pre–post comparison |
Performed cognitive tasks at rest and during exercise under normoxic and hypoxic conditions (12.5%) 100 min of hypoxia |
Hypoxia/Normoxia both before and during exercise | 20 min of cycle ergometer exercise at moderate intensity/Acute exercise | MATH, RMCPT | MATH Main effect EX: F(6, 102) = 3.67, p = 0.002 Main effect HY: N/A EX × HY: N/A RMCPT Main effect EX: F(6, 102) = 9.64, p < 0.001 Main effect HY: N/A EX × HY: N/A |
| Limmer et al. (2018)61 | 80 adults M = 51, 25.5 ± 6.0 years W = 29 24.8 ± 5.9 years |
Between-subject, Pre–post comparison |
Group A: HP + EX (n = 15) Group B: HP (n = 25) Group C: EX (n = 19) Group D: NOR (n = 21) |
Hypoxia/Normoxia during exercise | Group A: mountain climbing (7 days) Group B: rest in a hypoxic state Group C: ski hiking (7 days) Group D: rest in a normoxia state / Chronic exercise |
FAIR-2 | FAIR-2 Main effect EX: N/A Main effect HY: N/A EX × HY: N/A |
| Bayer et al. (2019)62 | 34 older adults 64–92 years |
Between-subject, RCT, Pre–post comparison |
EG received MTP and IHHT (10%–14%), and CG received MTP during the simulation of IHHT. Both performed cognitive tasks before and after the training. 30–40 min of hypoxia |
Hypoxia/Normoxia during exercise | 30 min of MTP (2–3 times/week, 12–15 trainings in 5–7 weeks) /Chronic exercise |
DemTect, CDT | Main effect EX: N/A Main effect HY: N/A EX × HY: N/A |
| Lei et al. (2019))17 |
30 healthy inactive women 22.6 ± 3.2 years |
Within-subject, Counterbalanced, Pre–post comparison |
Performed cognitive tasks at rest and during exercise under normoxic and hypoxic conditions (12%) 20 min of hypoxia |
Hypoxia/Normoxia both before and during exercise | 10-min bouts of cycle ergometer exercise at moderate intensity /Acute exercise |
Interference control task | Interference control task — RT Main effect EX: F (1, 29) = 8.336, p = 0.011, η²p = 0.203 Main effect HY: F (1, 29) = 5.425, p = 0.043, η²p = 0.134 EX × HY: F (1, 29) = 0.524, p = 0.573, η²p = 0.011 Interference control task — Accuracy Main effect EX: F (1, 29) = 0.487, p = 0.445, η²p = 0.02 Main effect HY: F (1, 29) = 0.777, p = 0.796, η²p = 0.002 EX × HY: F (1, 29) = 0.03, p = 0.798, η²p = 0.002 |
| Morrison et al. (2019)63 | 11 amateur team-sport athletes 22.8 ± 3.6 years |
Within-subject, Counterbalanced, Pre–post comparison |
Performed cognitive tasks before and after exercise under normoxic and hypoxic conditions (14.5%) 5 min of hypoxia |
Hypoxia/Normoxia during exercise | A repeated sprint-running protocol (4 sets of 4, 4-s all-out sprints)/Acute exercise |
DET IDN OCL |
DET Main effect EX: N/A Main effect HY: N/A EX × HY: N/A IDN Main effect EX: N/A Main effect HY: N/A EX × HY: N/A OCL–RT Main effect EX: F = 10.62, p = 0.01, η² = 0.52 Main effect HY: F = 0.3, p = 0.56, η² = 0.03 EX × HY: F = 0.29, p = 0.60, η² = 0.03 OCL–Accuracy Main effect EX: N/A Main effect HY: p = 0.20 EX × HY: N/A |
| Sun et al. (2019)18 | 20 inactive adults M = 10, W = 10 23.9 ± 2.5 years |
Between-subject, RCT, Pre–post comparison |
Performed cognitive tasks before and after exercise under normoxia and hypoxia (15.4%) 30–40 min of hypoxia |
Hypoxia/Normoxia both before and during exercise | 6 min of cycle ergometer exercise at high intensity (10 repetitions of 6 s with 30 s of recovery) /Acute exercise |
GNG task | GNG–RT Main effect EX: p = 0.204, η² = 0.083 Main effect HY: p = 0.782, η² = 0.004 EX × HY: p = 0.514, η² = 0.023 GNG–Accuracy Main effect EX: p = 0.001, η² = 0.467 Main effect HY: p = 0.972, η² = 0.001 EX × HY: p = 0.537, η² = 0.02 |
Abbreviations: CDT = clock-drawing test; CG = control group; CWST = color-word stroop task; DemTect = dementia detection test; DET = detection task; EG = experimental group; EX = exercise; EX × HY = interaction effect between exercise and hypoxia; FAIR-2 = Frankfurt attention inventory-2; GNG = Go/No-Go; GZ = quantitative performance index; HY = hypoxia; IDN = identification task; IHHT = interval hypoxic–hyperoxic training; M = male; MATH = mathematical; MTP = multimodal training programs; N/A = not assessed; NOR = normoxia; OCL = one card learning task; RCT = randomized controlled trial; RMCPT = running memory continuous performance task; performance task; RT = reaction time; SKL = concentration performance index; Spatial DR = spatial delayed response; VO2max = maximum oxygen uptake; W = woman; ZVT = Zahlen Verbindungs test.
Table 2.
Cognitive tasks and cognitive task categories.
| Category | Task |
|---|---|
| Information processing | Color-word stroop task |
| Attention | d2 test (GZ, SKL) |
| Frankfurt attention inventory-2 | |
| Executive function | Go/No-Go task |
| Zahlen Verbindungs test | |
| Trail making test A and B | |
| Flanker task | |
| Mathematical performance | |
| Interference control task | |
| Memory | Spatial delayed response |
| Running memory continuous performance task | |
| Memory recognition | |
| N-back | |
| One cardlearning task | |
| Dementia detection test | |
| Clock-drawing test | |
| Reaction time | Detection task |
| Identificationtask |
Abbreviations: GZ = quantitative performance index; the rate at which participants mark off each d2 and the overall number of marked letters within the d2 test); SKL = concentration performance index (the standardized number of accurate answers minus confusion errors).
3.2. Study quality
Based on the modified Downs and Black checklist, the methodological quality of the included studies was robust (21.31 ± 1.49, mean ± SD), ranging from 19 to 24, with the total maximum score of 25. All studies were within 3 SD of the mean scores and, thus, were included in the meta-analysis.34
3.3. Reasons for exclusions in each study screened by full text
Detailed reasons for the exclusion of participants in each study that received a full-text screening were extracted and are presented in Table 3. Examples included history of chronic diseases and physical problems during exercise or hypoxia, experience of smoking, medication use, cognitive disorders, exposured to hypoxia or high altitude within several months (3 months) prior to the study, and engagement in regular exercise training prior to the study.
Table 3.
Exclusionary criteria table for each of the included studies.
| Study | Exclusionary criteria |
|---|---|
| Ando et al. (2013)3 | • Participants who were currently engaged in regular training. • Participants who had any history of cardiovascular, cerebrovascular, or respiratory disease. |
| Schega et al. (2013)32 | • Subjects who were physically active and did not pass a medical examination by a medical doctor. |
| Kim et al. (2015)8 | • Participants who had been exposed to normobaric hypoxia or altitudes over 2500 m within previous 2 months. • Participants who had any history of smoking or had signs of cardiovascular, metabolic, or respiratory disease. • Participants who had experienced syncope, anemia, or fainting during exercise. |
| Komiyama et al. (2015)16 | • Participants who had any history of cardiovascular, cerebrovascular, or respiratory diseases. |
| Seo et al. (2015)45 | • Subjects who reported presence or history of pulmonary disease, cardiovascular disease, postural orthostatic tachycardia syndrome, skeletal muscle injury in the lower limbs, and metabolic syndrome. • Subjects who were exposed to normobaric hypoxia or an altitude above 2500 m within 2 months before study. |
| Dobashi et al. (2016)24 | • Participants who had any cardiovascular, cerebrovascular, or respiratory diseases. • Participants who had smoked, taken medications, or performed exercise training in the 6 months prior to study. |
| Lefferts et al. (2016)9 | • Participants who had experienced smoking, hypertension, diabetes mellitus, hyperlipidemia, pulmonary disease, renal disease, neurological disease, or peripheral artery disease. |
| Schega et al. (2016)33 | • Participants who had stayed in an altitude above 1800 m, as well as those who gave blood donations, in the past 2 months. • Participants who had chronic or acute renal, cardiovascular, metabolic, neuronal, or orthopaedic diseases. |
| Bayer et al. (2017)58 | • Individuals who were not able to move unaided or who had uncontrolled hypertension (systolic BP >180 mmHg), chronic bronchopulmonary diseases, decompensated heart failure (NYHA, III-IV FC), previous intracerebral hemorrhages, or marked cognitive disorders (MMSE < 12 points). |
| Bayer et al. (2017)59 | • Subjects who were not able to walk without any staff assistance or suffered from severe dementia with a score of less than 12 points on the MMSE, uncontrolled hypertension (systolic BP >180 mmHg and/or diastolic BP >100 mmHg),COPD III–IV, decompensated heart failure (NYHA III–IV), or previous intracerebral bleeding. |
| Komiyama et al. (2017)23 | • Participants who had any history of cardiovascular, cerebrovascular, or respiratory disease. |
| Seo et al. (2017)10 | • Subjects who not were physically active and had any history of pulmonary disease, cardiovascular disease, postural orthostatic tachycardia syndrome, or skeletal muscle injury in the lower limbs. • Subjects who were exposed to hypoxia or an altitude above 2500 m (8202 feet) within 2 months prior to study. |
| Stavres et al. (2017)60 | • Subjects who had any history of any cardiac, metabolic, or respiratory disease; any musculoskeletal issues prohibiting exercise; or any previous adverse reaction to altitude exposure. • Subjects who had been at altitudes above 2500 m within 3 months prior to participating in study. |
| Limmer et al. (2018)61 | • Subjects who had previous experience with the Frankfurt attention inventory-2 (FAIR-2) test, altitude sojourns above 2000 m in the 4 weeks prior to the investigation, neurological disease, psychiatric illness, learning disabilities, alcohol or drug use, or any difficulty that could interfere with behavioral or cognitive testing. |
| Bayer et al. (2019)62 | • Patients of the Geriatric Day Clinic who did not suffer from any diseases. |
| Lei et al. (2019)17 | • Subjects who had not lived at an altitude below 1300 m. • Subjects who had previous experience with hypoxic training or prior engagement in any regular exercise. • Subjects who had experienced smoking and alcohol-drinking habits. • Subjects who had a self-reported regular menstrual cycle of 28–34 days in length. • Subjects who were taking any form of the contraceptive pill or other drugs. |
| Morrison et al. (2019)63 | • N/A |
| Sun et al. (2019)18 | • Subjects who had not lived at an altitude lower than 1000 m. • Subjects who had experience with hypoxic training and were currently engaged in any structured exercise. • Subjects who were smokers and had taken oral contraceptives or any medication during the past 6 months. • Subjects who had musculoskeletal problems. |
Abbreviations: BP = blood pressure; COPD = chronic obstructive pulmonary disease; MMSE = Mini-Mental State Examination; N/A = not available; NYHA III–IV FC = New York Heart Association Functional Class III–IV.
3.4. Meta-analysis of effects on cognitive outcomes
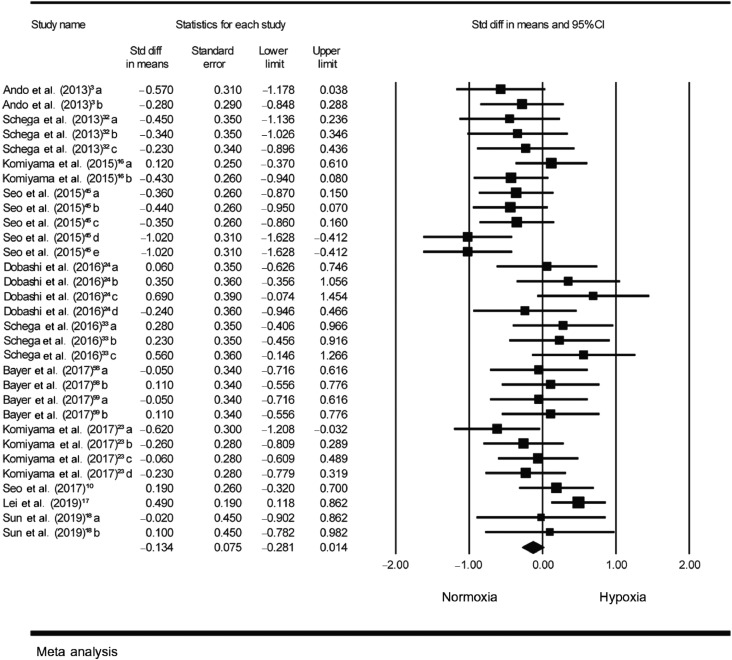
Among these 12 studies, 31 ESs examining the effect of hypoxia on cognition were calculated. As illustrated in Fig. 2 (Aim 1), aggregated results indicated a non-statistically significant association between the presence of hypoxia and an overall lower cognition (SMD = –0.13, 95%CI: –0.28 to 0.01, I2 = 45%, p = 0.075).
Fig. 2.
Forest plot depicting the standardized mean difference effect sizes (hypoxia vs. normoxia) and 95%CI for Aim 1. Ando et al. (2013): a = response time in Go/No-Go task; b = response accuracy in Go/No-Go task. Schega et al. (2013): a = SKL in d2 test; b = GZ in d2 test; c = Zahlen Verbindungs test. Komiyama et al. (2015): a = accuracy in spatial delayed response; b = response accuracy in Go/No-Go task. Seo et al. (2015): a = response time in Go/No-Go task; b = response accuracy in Go/No-Go task; c = response time in running memory continuous performance task; d = response accuracy in running memory continuous performance task; e = total score in running memory continuous performance task. Dobashi et al. (2016): a = Reverse-Stroop control task; b = Reverse-Stroop interference task; c = Stroop control task; d = Stroop interference task. Schega et al. (2016): a = Word-task of the Stroop test; b = Color-task of the Stroop test; c = Word-color-task of the Stroop test. Bayer et al. (2017)58: a = Dementia detection test; b = Clock-drawing test. Bayer et al. (2017)59: a = Dementia detection test; b = Clock-drawing test. Komiyama et al. (2017): a = accuracy in spatial delayed response; b = response accuracy on Go trial in the Go/No-Go task; c = response accuracy on No-Go trial in the Go/No-Go task; d = response time in Go/No-Go task. Seo et al. (2017): total score in running memory continuous performance task. Lei et al. (2019): response time in Go/No-Go task. Sun et al. (2019): a = response time in Go/No-Go task; b = response accuracy in Go/No-Go task. CI = confidence interval; diff = difference; Std = standardized.
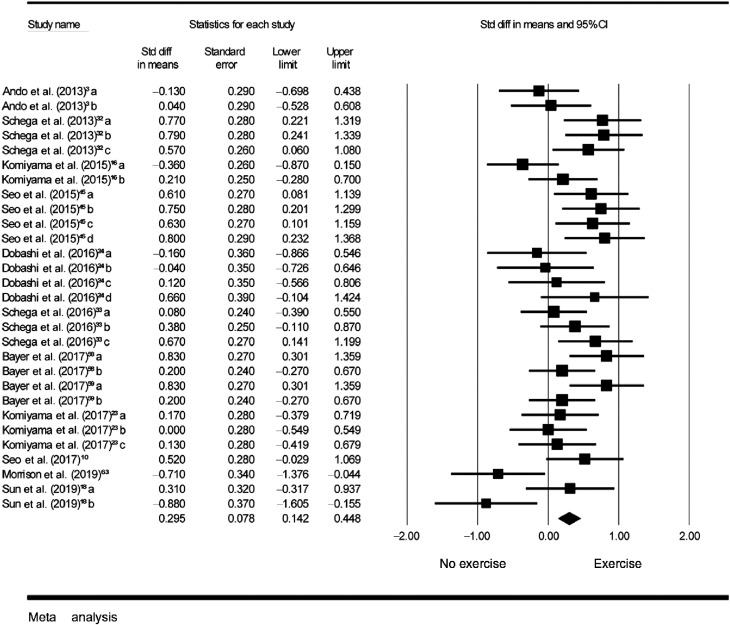
Among these 12 studies, 29 ESs examining the effect of exercise on cognition under hypoxia were calculated. As illustrated in Fig. 3 (Aim 2), the aggregated results indicated that performing exercise under a hypoxia setting has significant effects on cognitive improvement (SMD = 0.30, 95%CI: 0.14–0.45, I2 = 54%, p < 0.001).
Fig. 3.
Forest plot depicting the standardized mean difference effect sizes (exercise vs. no exercise) and 95%CI for Aim 2. Ando et al. (2013): a = response time in Go/No-Go task; b = response accuracy in Go/No-Go task. Schega et al. (2013): a = SKL in d2 test; b = GZ in d2 test; c = Zahlen Verbindungs test. Komiyama et al. (2015): a = accuracy in spatial delayed response; b = response accuracy in Go/No-Go task. Seo et al. (2015): a = response time in running memory continuous performance; b = response time in running memory continuous performance; c = total score in running memory continuous performance task; d = Total score in running memory continuous performance task. Dobashi et al. (2016): a = Reverse-Stroop control task; b = Reverse-Stroop interference task; c = Stroop control task; d = Stroop interference task. Schega et al. (2016): a = Word-task of the Stroop test; b = Color-task of the Stroop test; c = Word-color-task of the Stroop test. Bayer et al. (2017)58: a = Dementia detection test; b = Clock-drawing test. Bayer et al. (2017)59: a = Dementia detection test; b = Clock-drawing test. Komiyama et al. (2017): a = accuracy in spatial delayed response; b = response accuracy on Go trial in the Go/No-Go task; c = response accuracy on No-Go trial in the Go/No-Go task. Seo et al. (2017): total score in running memory continuous performance task. Morrison et al. (2019): accuracy in one card learning task. Sun et al. (2019): a = response time in Go/No-Go task; b = response accuracy in Go/No-Go task. CI = confidence interval; diff = difference; Std = standardized.
3.5. Moderator analyses
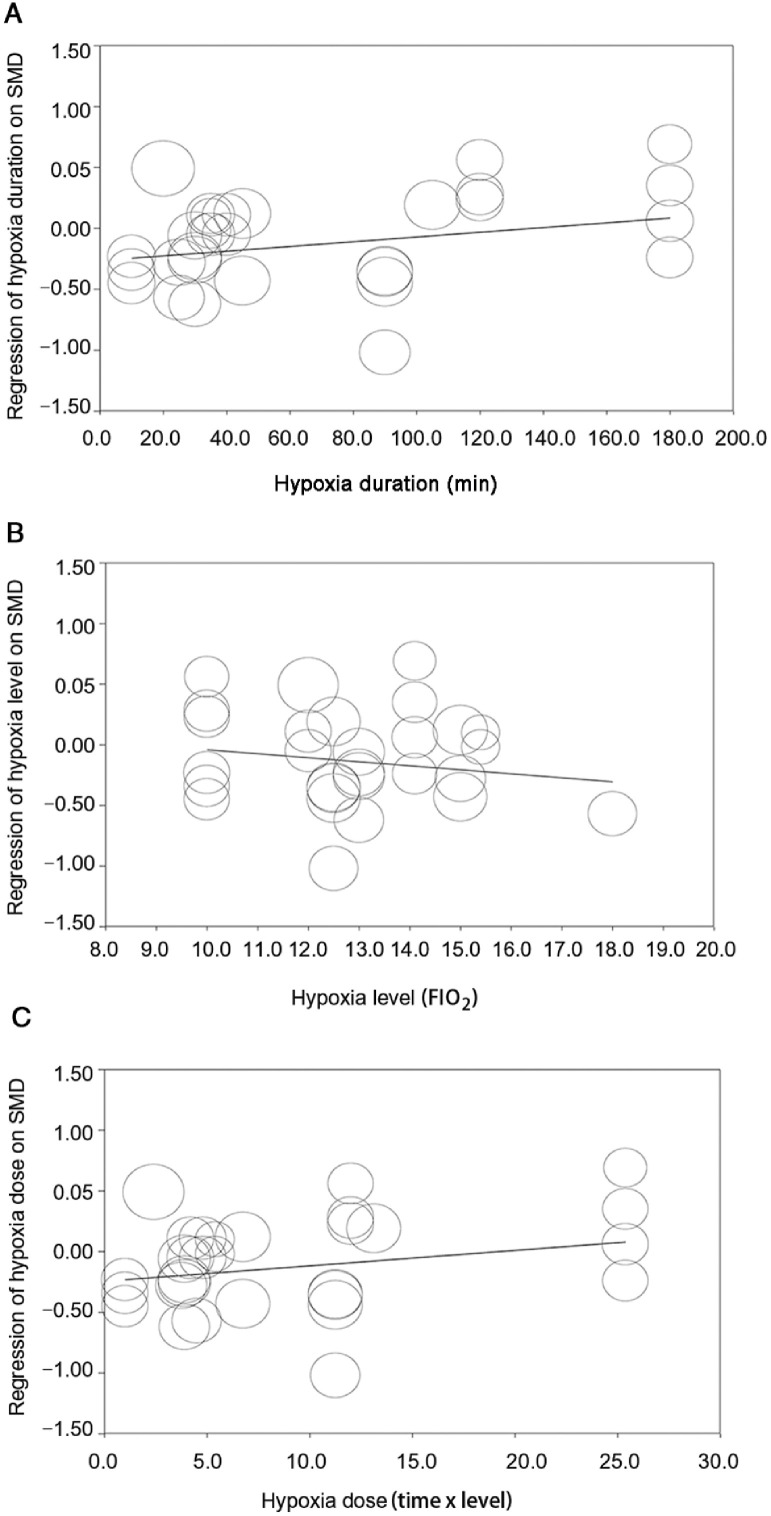
For Aim 1, in the subgroup analyses (Table 4), the effect of hypoxia on cognition was significantly moderated by sex (Qb = 15.22, df = 2, p < 0.001) and cognitive task type (Qb = 10.33, df = 3, p = 0.016) but not by other variables. Furthermore, males (SMD = –0.30, 95%CI: –0.48 to –0.11) suffered significant cognitive impairment when being exposed to hypoxia, as compared with females (SMD = 0.39, 95%CI: 0.09–0.69) and mixed sexes (SMD = 0.02, 95%CI: –0.19 to 0.22). Exposure to hypoxia impaired attentional ability (SMD = –0.40, 95%CI: –0.88 to 0.09), executive function (SMD = –0.18, 95%CI: –0.39 to 0.02), and memory function (SMD = –0.26, 95%CI: –0.55 to 0.04) but improved information processing (SMD = 0.27, 95%CI: 0.00–0.53). In the meta-regressions, the hypoxia-cognition associations did not differ by these hypoxia characteristics (hypoxia duration (β = 0.002, 95%CI: –0.001 to 0.005, p = 0.19), hypoxia level (β = –0.03, 95%CI: –0.11 to 0.05, p = 0.42), and hypoxia dose (β = 0.01, 95%CI: 0.00–0.03, p = 0.25)). Results of the meta-regressions are displayed in Fig. 4.
Table 4.
Effect sizes by moderator variables in meta-analysis of variance for Aim 1.
| Category | Effect size | SMD | 95%CI | Qb |
|---|---|---|---|---|
| Sex | 15.22⁎⁎ | |||
| Male | 17 | –0.30⁎⁎ | –0.48 to –0.11 | |
| Female | 2 | 0.39* | 0.09 to 0.69 | |
| Mixed | 12 | 0.02 | –0.19 to 0.22 | |
| Age | 2.01 | |||
| Young Adults | 21 | –0.20* | –0.39 to 0.00 | |
| Older Adults | 10 | 0.01 | –0.20 to 0.23 | |
| Cognitive task type | 10.33* | |||
| Information processing | 7 | 0.27 | 0.00 to 0.53 | |
| Attention | 2 | –0.40 | –0.88 to 0.09 | |
| Executive function | 12 | –0.18 | –0.39 to 0.02 | |
| Memory | 10 | –0.26 | –0.55 to 0.04 | |
| Study design | 2.38 | |||
| RCT | 12 | 0.02 | –0.19 to 0.22 | |
| NRS | 19 | –0.21* | –0.41 to −0.01 |
p < 0.05; ** p < 0.01.
Abbreviations: CI = confidence interval; NRS = non-randomized controlled study; RCT = randomized controlled trial; SMD = standardized mean difference; Qb = Cochran's Q statistics.
Fig. 4.
Effect sizes by moderator variables in meta-regression for Aim 1. (A) Regression of hypoxia duration on SMD; (B) Regression of hypoxia level (FIO2) on SMD; (C) Regression of hypoxia dose on SMD. FIO2 = fraction of inspired oxygen; SMD = standardized mean difference.
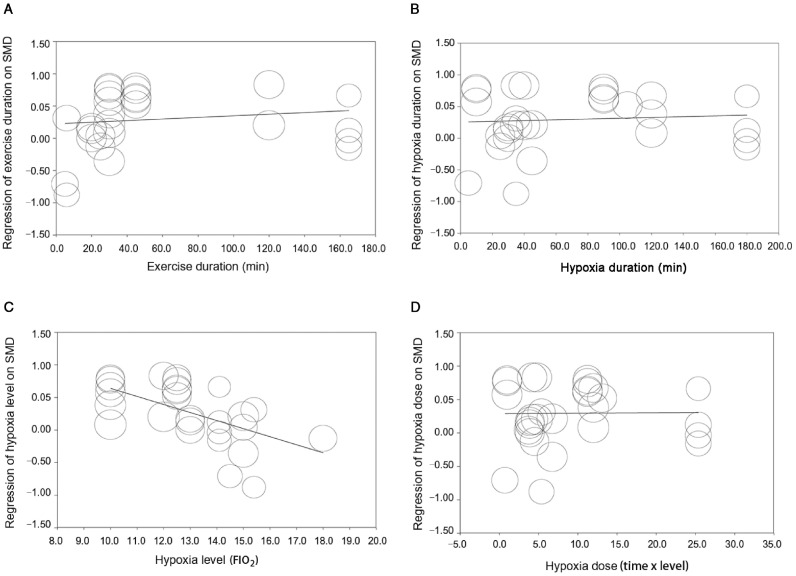
For Aim 2, in the subgroup analyses (Table 5), the effect of exercise on cognition under hypoxia was significantly moderated by age (Qb = 6.30, df = 1, p = 0.012), cognitive task type (Qb = 9.34, df = 3, p = 0.025), exercise type (Qb = 17.12, df = 3, p = 0.001), exercise intensity (Qb = 3.98, df = 1, p = 0.046), and training type (Qb = 6.30, df = 1, p = 0.012). Furthermore, older adults (SMD = 0.51, 95%CI: 0.32–0.69) benefited more from exercise under hypoxia than did young adults (SMD = 0.16, 95%CI: –0.04 to 0.36). Exercising under hypoxia had the greatest (favorable) impact on attention (SMD = 0.78, 95%CI: 0.39–1.17) compared to information processing (SMD = 0.26, 95%CI: 0.03–0.49), executive function (SMD = 0.07, 95%CI: –0.19 to 0.33), and memory (SMD = 0.38, 95%CI: 0.12–0.64). Full-body strength-endurance programs (SMD = 0.70, 95%CI: 0.39–1.01) were more effective than cycle ergometer (SMD = 0.23, 95%CI: 0.06–0.40), multimodal training program (SMD = 0.50, 95%CI: 0.14–0.85), and repeated sprint running (SMD = –0.71, 95%CI: –1.38 to –0.04) in improving cognitive function. Exercising at moderate intensity (SMD = 0.36, 95%CI: 0.22–0.50) had greater effects on cognitive improvement when compared to high-intensity exercise (SMD = –0.41, 95%CI: –1.16 to 0.34). Chronic exercise (SMD = 0.51, 95%CI: 0.32–0.69) was more effective than acute exercise (SMD = 0.16, 95%CI: –0.04 to 0.36) in enhancing cognitive function. In the meta-regressions, although no significant moderator effect was present for exercise duration (β = 0.001, 95%CI: –0.002 to 0.004, p = 0.42), hypoxia duration (β = 0.001, 95%CI: –0.002 to 0.004, p = 0.68), and hypoxia dose (β = 0.001, 95%CI: –0.02 to 0.02, p = 0.96), there was evidence of moderation effect for hypoxia level (β = –0.12, 95%CI: –0.19 to –0.06, p < 0.001), indicating that exercising while at greater hypoxia level was negatively associated with cognitive performance. Results of the meta-regressions are presented in Fig. 5.
Table 5.
Effect sizes by moderator variables in meta-analysis of variance for Aim 2.
| Category | Effect size | SMD | 95%CI | Qb |
|---|---|---|---|---|
| Sex | 2.96 | |||
| Male | 16 | 0.18 | –0.03 to 0.38 | |
| Female | 1 | 0.52 | –0.03 to 1.07 | |
| Mixed | 12 | 0.42⁎⁎ | 0.19 to 0.65 | |
| Age | 6.30* | |||
| Young adults | 19 | 0.16 | –0.04 to 0.36 | |
| Older adults | 10 | 0.51⁎⁎ | 0.32 to 0.69 | |
| Cognitive task type | 9.34* | |||
| Information processing | 7 | 0.26* | 0.03 to 0.49 | |
| Attention | 2 | 0.78⁎⁎ | 0.39 to 1.17 | |
| Executive function | 8 | 0.07 | –0.19 to 0.33 | |
| Memory | 12 | 0.38⁎⁎ | 0.12 to 0.64 | |
| Study design | 1.98 | |||
| RCT | 12 | 0.42⁎⁎ | 0.19 to 0.65 | |
| NRS | 17 | 0.20* | 0.00 to 0.40 | |
| Exercise type | 17.12⁎⁎ | |||
| Cycle ergometer | 21 | 0.23⁎⁎ | 0.06 to 0.40 | |
| Full-body strength-endurance program | 3 | 0.70⁎⁎ | 0.39 to 1.01 | |
| Multimodal training program | 4 | 0.50⁎⁎ | 0.14 to 0.85 | |
| Repeated sprint running | 1 | –0.71* | –1.38 to −0.04 | |
| Exercise intensity | 3.98* | |||
| Moderate | 26 | 0.36⁎⁎ | 0.22 to 0.50 | |
| High | 3 | –0.41 | –1.16 to 0.34 | |
| Exercise temporality | 1.43 | |||
| During hypoxia | 8 | 0.45⁎⁎ | 0.13 to 0.78 | |
| Both before and during hypoxia | 21 | 0.23⁎⁎ | 0.06 to 0.40 | |
| Training type | 6.30* | |||
| Acute exercise | 19 | 0.16 | –0.04 to 0.36 | |
| Chronic exercise | 10 | 0.51⁎⁎ | 0.32 to 0.69 |
p < 0.05; ** p < 0.01.
Abbreviations: CI = confidence interval; NRS = non-randomized controlled study; RCT = randomized controlled trial; SMD = standardized mean difference; Qb = Cochran Q statistics.
Fig. 5.
Effect sizes by moderator variables in meta-regression for Aim 2. (A) Regression of exercise duration on SMD; (B) Regression of hypoxia duration on SMD; (C) Regression of hypoxia level (FIO2) on SMD; (D) Regression of hypoxia dose on SMD. FIO2 = fraction of inspired oxygen; SMD = standardized mean difference.
3.6. Publication bias
Based on the funnel plot, the Egger's test of regression intercept was not statistically significant in Aim 1 (intercept = –0.22, p = 0.87) or Aim 2 (intercept = –3.37, p = 0.13), indicating that there was no evidence of risk of bias across studies.
4. Discussion
4.1. Main study findings
This review included both RCTs and NRSs, with consideration of the following potential moderating variables: (1) the effects of hypoxia on cognitive function and (2) the effects of exercise under hypoxia on cognitive function. First, we found that hypoxia exposure impaired some but not all aspects of cognitive function. The effect of hypoxia on cognition was moderated by sex and cognitive task type. Second, exercising under hypoxia may have had small to medium positive effects on selected aspects of cognitive function. When being exposed to hypoxia, exercise-cognition association was moderated by age, cognitive task type, exercise type, exercise intensity, training type, and hypoxia level.
4.2. Comparisons with previous reviews
As indicated in recent narrative and meta-analytic reviews, cognitive function may be compromised during exposure to hypoxia.13 In alignment with these previous reviews, our meta-analysis demonstrates that hypoxia had a selective effect on cognition, in that hypoxia enhanced information processing but impaired cognition-based attention, executive function, and memory. Even though this result supports the conclusions of other related studies, the magnitude of effect was smaller than in previous work. Notably, Taylor et al.11 reported that hypoxia was more negatively associated with central executive tasks compared with nonexecutive tasks. This, however, is in contrast to the review by McMorris et al.,13 which suggested that there are no significant hypoxia-induced differences between executive and nonexecutive tasks. Moreover, although the meta-analytic review by McMorris et al.13 was for within-group studies, our work utilized both within- and between-group studies. These conflicting findings across the literature render challenges in the interpretation of our observations regarding Aim 1. If our findings are replicated and prove to be reliable effects, then future research should aim to evaluate the underlying reasons for hypoxia’s having a unique effect on selective cognitions. Next, our main findings (Aim 2) demonstrate that exercise under hypoxia had a positive effect on cognitive function, which extends a recently published narrative review on this topic.25
4.3. Possible explanation for study findings
Notably, for Aim 1, we found a significant sex-specific effect. That is, performing cognitive tasks under hypoxia was advantageous in improving cognitive function for females. This may, in part, be attributed to females’ having relatively higher peripheral oxygen saturation (SpO2) and estrogen hormones than males, which provide a greater resistance to hypoxia.35, 36, 37, 38 However, this neuroprotective effect of estrogen in response to hypoxia should be interpreted with caution, because few studies that investigated the hypoxia-cognition interaction for women during a specific timing of the menstrual cycles were included in this review. We also observed significant differences among cognitive task types. That is, hypoxia had a favorable effect on information processing as opposed to the observed impairment effect on attention, executive function, and memory. Such selected aspects of cognitive impairment are supported by a previous review suggesting that arterial PaO2 is a strong predictor of reduced cognitive performance. If PaO2 level is low (35–60 mmHg), increased cerebral blood flow may not compensate adequately for the lack of oxygen required to maintain cognitive ability. Ochi et al.39 indicated that SpO2 gradually decreases as the severity of hypoxia increases, and low levels of SpO2 may induce cerebral deoxygenation. Hence, it is plausible that hypoxia may be responsible for negative cognitive-related outcomes due to neurological and structural alterations of the brain tissue.40
Regarding Aim 2, we observed moderation effects for age, cognitive task type, exercise type and intensity, and hypoxia level. It is challenging to explain the moderation effects of age. If hypoxia has a greater negative effect on cognition for older adults (vs. young adults), it is conceivable that exercise may help attenuate this effect in older adults. However, for our Aim 1, we did not observe an age-moderation effect. Our findings also demonstrate that attention tends to be more positively influenced by exercise during exposure to hypoxia than other cognitive task types (e.g., information-processing, executive function, and memory). Notably, however, all cognitions were enhanced with exercise. Although Chang et al.20 observed that acute exercise under normoxia helped to improve executive function, there are few studies that investigate which types of cognitions are most sensitive to exercise in hypoxic conditions. Thus, this is an area in need of future research.
In addition, a notable finding was that full-body strength-endurance exercise had a greater impact on cognition improvement. This finding may be related to the cognitive enhancement effects of complex movement patterns, which we have detailed elsewhere.41 Encouragingly, it is anticipated that more complicated movement patterns stimulate regional cerebral flow and cortical excitability, resulting in enhancing cognitive function;42, 43, 44 thus, full-body strength-endurance exercise, consisting of aerobic exercise and strength training, may help to exert cognitive ability under hypoxia. Furthermore, we detected a significant difference in effect size for exercise intensity (moderate vs. high). Our findings indicated that moderate-intensity exercise under hypoxia increases cognitive function. This improvement effect is consistent with the moderation results from several previous studies.17,18,45 Importantly, however, under normoxia, exercise intensity may differentially influence cognition-based reaction time and accuracy.46 Future work should evaluate this under hypoxic conditions. However, when exposed to hypoxia, moderate-intensity exercise may increase cerebral blood flow and compensate for decreased SpO2.47,48 Indeed, exercising under moderate hypoxia favored cognitive benefits when compared to severe hypoxia. This effect may be a result of the potential additive effects of exercise and moderate hypoxia on cognition. As fully discussed elsewhere,18,49 moderate levels of hypoxia and exercise may enhance synaptic plasticity via an increased expression of brain-derived neurotrophic factor. The hypoxia- and exercise-induced upregulation of brain-derived neurotrophic factor can facilitate cerebral neural activation and neurogenesis and, therefore, lead to cognition improvements.50 Last, chronic exercise had a more positive effect on cognition than acute exercise under hypoxia. This finding is consistent with previous work demonstrating that acute exercise under normoxia was not as beneficial in enhancing cognitive function when compared to chronic exercise.51 This may be attributed partially to adaptations of physiological and neurological parameters induced by chronic exercise.22,52,53 To provide definitive conclusions, longitudinal studies of the effects of exercise training on cognition while under hypoxia are needed, because there are limited studies comparing how the length of training intervention affects cognition at different levels of hypoxia.
4.4. Strengths and limitations
This meta-analytic review has several strengths. First, this is the 1st meta-analytic review that investigated the combined effects of exercise and hypoxia on cognitive function. Second, we simultaneously demonstrated the independent effects of hypoxia on cognitive function and the interactive effects of exercise and hypoxia on cognitive function. Third, we tested a variety of moderating variables to determine the cause of heterogeneity, which provides a much clearer picture regarding the effects of exercise under hypoxia on cognitive function. Despite these strengths, there are several limitations in our study. First, several moderator effects should be interpreted with caution because small cell sizes in this review evaluated the relationship between exercise and cognition under hypoxia. Although there are limited cell sizes for moderators, the identification of potential moderators will help future work to demonstrate the complexity of these interactions. Second, timing of the cognitive task in relation to exercise (e.g., before, during, or after exercise) was not considered; thus, future studies should establish whether different cognitive assessment periods alter our observations. Third, training periods (e.g., short, medium, and long) should be included in further studies to determine the optimal training length for cognition improvement under hypoxia. Moreover, physiological parameters should be investigated to evaluate the underlying mechanisms of our observed effects. Fourth, although meta-analyses provide a rational way to summarize and quantitatively synthesize a large number of previous empirical studies, it is challenging to account for the unique design characteristics of individual experiments.54,55 Therefore, the results may be biased by systematic confounding factors that correlate with effect size. For example, this problem may be applied to moderation analyses that include small numbers of studies and to between-study comparisons when we are actually interested in within-subject correlations.56,57
5. Conclusion
This meta-analysis demonstrates 2 important findings. First, cognitive function is impaired during hypoxia in resting conditions, particularly for attention, executive function, and memory. Second, exercise during exposure to hypoxia plays a key role in improving cognitive function. Various characteristics (e.g., exercise modality) are likely to moderate the relationship between exercise and cognition under hypoxia.
Acknowledgments
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (Grant #31671150) and Guangdong Province Key Project (Grant #2018B030335001).
Authors’ contributions
While conducting this review, LZ played a role in conceiving, designing, overseeing data collection, and editing this paper; PDL played a role in conceiving, designing, overseeing data collection/analysis, and editing this manuscript; JJY played a role in collecting data and editing this manuscript; SR played in a role data collection/analysis; ZK, LY, MK, JL, HL, and LS played roles in editing this manuscript; and MJ played a role in analyzing the data and prepared the initial draft of the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2020.04.004.
Appendix. Supplementary materials
References
- 1.Korol D.L. Enhancing cognitive function across the life span. Ann N Y Acad Sci. 2002;959:167–179. doi: 10.1111/j.1749-6632.2002.tb02091.x. [DOI] [PubMed] [Google Scholar]
- 2.McMorris T., Sproule J., Turner A., Hale B.J. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: a meta-analytical comparison of effects. Physiol Behav. 2011;102:421–428. doi: 10.1016/j.physbeh.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Ando S., Hatamoto Y., Sudo M., Kiyonaga A., Tanaka H., Higaki Y. The effects of exercise under hypoxia on cognitive function. PLoS One. 2013;8:e63630. doi: 10.1371/journal.pone.0063630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knicker A.J., Renshaw I., Oldham A.R., Cairns S.P. Interactive processes link the multiple symptoms of fatigue in sport competition. Sports Med. 2011;41:307–328. doi: 10.2165/11586070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Crow T.J., Kelman G.R. Psychological effects of mild acute hypoxia. Br J Anaesth. 1973;45:335–337. doi: 10.1093/bja/45.4.335. [DOI] [PubMed] [Google Scholar]
- 6.Yan X. Cognitive impairments at high altitudes and adaptation. High Alt Med Biol. 2014;15:141–145. doi: 10.1089/ham.2014.1009. [DOI] [PubMed] [Google Scholar]
- 7.Roach E.B., Bleiberg J., Lathan C.E., Wolpert L., Tsao J.W., Roach R.C. AltitudeOmics: decreased reaction time after high altitude cognitive testing is a sensitive metric of hypoxic impairment. Neuroreport. 2014;25:814–818. doi: 10.1097/WNR.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim E.J., Kim C.H., Ryan E.J., Seo Y., Peacock C., Gunstad J. Low-intensity exercise does not impact cognitive function during exposure to normobaric hypoxia. Physiol Behav. 2015;151:24–28. doi: 10.1016/j.physbeh.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Lefferts W.K., Babcock M.C., Tiss M.J., Ives S.J., White C.N., Brutsaert T.D. Effect of hypoxia on cerebrovascular and cognitive function during moderate intensity exercise. Physiol Behav. 2016;165:108–118. doi: 10.1016/j.physbeh.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Seo Y., Gerhart H.D., Stavres J., Fennell C., Draper S., Glickman E.L. Normobaric hypoxia and submaximal exercise effects on running memory and mood state in women. Aerosp Med Hum Perform. 2017;88:627–632. doi: 10.3357/AMHP.4798.2017. [DOI] [PubMed] [Google Scholar]
- 11.Taylor L., Watkins S.L., Marshall H., Dascombe B.J., Foster J. The impact of different environmental conditions on cognitive function: a focused review. Front Physiol. 2015;6:372. doi: 10.3389/fphys.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virués-Ortega J., Buela-Casal G., Garrido E., Alcázar B. Neuropsychological functioning associated with high-altitude exposure. Neuropsychol Rev. 2004;14:197–224. doi: 10.1007/s11065-004-8159-4. [DOI] [PubMed] [Google Scholar]
- 13.McMorris T., Hale B.J., Barwood M., Costello J., Corbett J. Effect of acute hypoxia on cognition: a systematic review and meta-regression analysis. Neurosci Biobehav Rev. 2017;74:225–232. doi: 10.1016/j.neubiorev.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Davranche K., Casini L., Arnal P.J., Rupp T., Perrey S., Verges S. Cognitive functions and cerebral oxygenation changes during acute and prolonged hypoxic exposure. Physiol Behav. 2016;164:189–197. doi: 10.1016/j.physbeh.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Hu F., Liu H., Xu L., Li Y., Liu X., Shi L. Hypoxia-inducible factor-1α perpetuates synovial fibroblast interactions with T cells and B cells in rheumatoid arthritis. Eur J Immunol. 2016;46:742–751. doi: 10.1002/eji.201545784. [DOI] [PubMed] [Google Scholar]
- 16.Komiyama T., Sudo M., Higaki Y., Kiyonaga A., Tanaka H., Ando S. Does moderate hypoxia alter working memory and executive function during prolonged exercise? Physiol Behav. 2015;139:290–296. doi: 10.1016/j.physbeh.2014.11.057. [DOI] [PubMed] [Google Scholar]
- 17.Lei O.K., Kong Z., Loprinzi P.D., Shi Q., Sun S., Zou L. Severe hypoxia does not offset the benefits of exercise on cognitive function in sedentary young women. Intl J Environ Res Public Health. 2019;16:1003. doi: 10.3390/ijerph16061003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun S., Loprinzi P.D., Guan H., Zou L., Kong Z., Hu Y. The effects of high-intensity interval exercise and hypoxia on cognition in sedentary young adults. Medicina (Kaunas) 2019;55:43. doi: 10.3390/medicina55020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brisswalter J., Collardeau M., René A. Effects of acute physical exercise characteristics on cognitive performance. Sports Med. 2002;32:555–566. doi: 10.2165/00007256-200232090-00002. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y.K., Labban J.D., Gapin J.I., Etnier J.L. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. 2012;1453:87–101. doi: 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 21.Dickerson B.C., Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambourne K., Tomporowski P. The effect of exercise-induced arousal on cognitive task performance: a meta-regression analysis. Brain Res. 2010;1341:12–24. doi: 10.1016/j.brainres.2010.03.091. [DOI] [PubMed] [Google Scholar]
- 23.Komiyama T., Katayama K., Sudo M., Ishida K., Higaki Y., Ando S. Cognitive function during exercise under severe hypoxia. Sci Rep. 2017;7:10000. doi: 10.1038/s41598-017-10332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobashi S., Horiuchi M., Endo J., Kiuchi M., Koyama K. Cognitive function and cerebral oxygenation during prolonged exercise under hypoxia in healthy young males. High Alt Med Biol. 2016;17:214–221. doi: 10.1089/ham.2016.0036. [DOI] [PubMed] [Google Scholar]
- 25.Ando S., Komiyama T., Sudo M., Higaki Y., Ishida K., Costello J.T. The interactive effects of acute exercise and hypoxia on cognitive performance: a narrative review. Scand J Med Sci Sports. 2019;30:384–398. doi: 10.1111/sms.13573. [DOI] [PubMed] [Google Scholar]
- 26.Loprinzi P.D., Blough J., Crawford L., Ryu S., Zou L., Li H. The temporal effects of acute exercise on episodic memory function: systematic review with meta-analysis. Brain Sci. 2019;9:87. doi: 10.3390/brainsci9040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loprinzi P.D., Blough J., Ryu S., Kang M. Experimental effects of exercise on memory function among mild cognitive impairment: systematic review and meta-analysis. Phys Sportsmed. 2019;47:21–26. doi: 10.1080/00913847.2018.1527647. [DOI] [PubMed] [Google Scholar]
- 28.Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipsey M.W., Wilson D.B. Sage Publications Inc; Thousand Oaks, CA: 2001. Pratical Meta-analysis. [Google Scholar]
- 30.Cooper H., Hedges L.V., Valentine J.C. The handbook of research synthesis and meta-analysis. 2nd ed. Russell Sage Foundation; New York, NY: 2009. Effect sizes for continuous data; pp. 221–235. [Google Scholar]
- 31.Borenstein M., Higgins J.P.T. Meta-analysis and subgroups. Prev Sci. 2013;14:134–143. doi: 10.1007/s11121-013-0377-7. [DOI] [PubMed] [Google Scholar]
- 32.Schega L., Peter B., Törpel A., Mutschler H., Isermann B., Hamacher D. Effects of intermittent hypoxia on cognitive performance and quality of life in elderly adults: a pilot study. Gerontology. 2013;59:316–323. doi: 10.1159/000350927. [DOI] [PubMed] [Google Scholar]
- 33.Schega L., Peter B., Brigadski T., Lessmann V., Isermann B., Hamacher D. Effect of intermittent normobaric hypoxia on aerobic capacity and cognitive function in older people. J Sci Med Sport. 2016;19:941–945. doi: 10.1016/j.jsams.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Kim K., Ok G., Jeon S., Kang M., Lee S. Sport-based physical activity intervention on body weight in children and adolescents: a meta-analysis. J Sports Sci. 2017;35:369–376. doi: 10.1080/02640414.2016.1166389. [DOI] [PubMed] [Google Scholar]
- 35.Heyer A., Hasselblatt M., von Ahsen N., Häfner H., Sirén A.-L., Ehrenreich H. In vitro gender differences in neuronal survival on hypoxia and in 17β-estradiol-mediated neuroprotection. J Cereb Blood Flow Metab. 2005;25:427–430. doi: 10.1038/sj.jcbfm.9600056. [DOI] [PubMed] [Google Scholar]
- 36.Mage D.T., Donner M. Female resistance to hypoxia: does it explain the sex difference in mortality rates? J Womens Health (Larchmt) 2006;15:786–794. doi: 10.1089/jwh.2006.15.786. [DOI] [PubMed] [Google Scholar]
- 37.Levental S., Picard E., Mimouni F., Joseph L., Samuel T.Y., Bromiker R. Sex-linked difference in blood oxygen saturation. Clin Respir J. 2018;12:1900–1904. doi: 10.1111/crj.12753. [DOI] [PubMed] [Google Scholar]
- 38.Loprinzi P.D., Frith E. The role of sex in memory function: considerations and recommendations in the context of exercise. J Clin Med. 2018;7:132. doi: 10.3390/jcm7060132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ochi G., Yamada Y., Hyodo K., Suwabe K., Fukuie T., Byun K. Neural basis for reduced executive performance with hypoxic exercise. Neuroimage. 2018;171:75–83. doi: 10.1016/j.neuroimage.2017.12.091. [DOI] [PubMed] [Google Scholar]
- 40.Gibson G.E., Pulsinelli W., Blass J.P., Duffy T.E. Brain dysfunction in mild to moderate hypoxia. Am J Med. 1981;70:1247–1254. doi: 10.1016/0002-9343(81)90834-2. [DOI] [PubMed] [Google Scholar]
- 41.Gu Q., Zou L., Loprinzi P.D., Quan M., Huang T. Effects of open versus closed skill exercise on cognitive function: a systematic review. Front Psychol. 2019;10:1707. doi: 10.3389/fpsyg.2019.01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carey J.R., Bhatt E., Nagpal A. Neuroplasticity promoted by task complexity. Exerc Sport Sci Rev. 2005;33:24–31. [PubMed] [Google Scholar]
- 43.Gur R.C., Jaggi J.L., Ragland J.D., Resnick S.M., Shtasel D., Muenz L. Effects of memory processing on regional brain activation: cerebral blood flow in normal subjects. Int J Neurosci. 1993;72:31–44. doi: 10.3109/00207459308991621. [DOI] [PubMed] [Google Scholar]
- 44.Winstein C.J., Grafton S.T., Pohl P.S. Motor task difficulty and brain activity: investigation of goal-directed reciprocal aiming using positron emission tomography. J Neurophysiol. 1997;77:1581–1594. doi: 10.1152/jn.1997.77.3.1581. [DOI] [PubMed] [Google Scholar]
- 45.Seo Y., Burns K., Fennell C., Kim J.H., Gunstad J., Glickman E. The influence of exercise on cognitive performance in normobaric hypoxia. High Alt Med Biol. 2015;16:298–305. doi: 10.1089/ham.2015.0027. [DOI] [PubMed] [Google Scholar]
- 46.McMorris T., Hale B.J. Differential effects of differing intensities of acute exercise on speed and accuracy of cognition: a meta-analytical investigation. Brain Cogn. 2012;80:338–351. doi: 10.1016/j.bandc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Curtelin D., Morales-Alamo D., Torres-Peralta R., Rasmussen P., Martin-Rincon M., Perez-Valera M. Cerebral blood flow, frontal lobe oxygenation and intra-arterial blood pressure during sprint exercise in normoxia and severe acute hypoxia in humans. J Cereb Blood Flow Metab. 2018;38:136–150. doi: 10.1177/0271678X17691986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore L.G., Niermeyer S., Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Am J Phys Anthropol. 1998;(Suppl. 27):S25–64. doi: 10.1002/(sici)1096-8644(1998)107:27+<25::aid-ajpa3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 49.Dale E.A., Ben Mabrouk F., Mitchell G.S. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 2014;29:39–48. doi: 10.1152/physiol.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loprinzi P.D., Edwards M.K., Frith E. Potential avenues for exercise to activate episodic memory-related pathways: a narrative review. Eur J Neurosci. 2017;46:2067–2077. doi: 10.1111/ejn.13644. [DOI] [PubMed] [Google Scholar]
- 51.Colcombe S., Kramer A.F. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 52.Knaepen K., Goekint M., Heyman E.M., Meeusen R. Neuroplasticity-exercise-induced response of peripheral brain-derived neurotrophic factor. Sports Med. 2010;40:765–801. doi: 10.2165/11534530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 53.El-Sayes J., Harasym D., Turco C.V., Locke M.B., Nelson A.J. Exercise-induced neuroplasticity: a mechanistic model and prospects for promoting plasticity. Neuroscientist. 2019;25:65–85. doi: 10.1177/1073858418771538. [DOI] [PubMed] [Google Scholar]
- 54.Esterhuizen T.M., Thabane L. Con: Meta-analysis: some key limitations and potential solutions. Nephrol Dial Transplant. 2016;31:882–885. doi: 10.1093/ndt/gfw092. [DOI] [PubMed] [Google Scholar]
- 55.Spector T.D., Thompson S.G. The potential and limitations of meta-analysis. J Epidemiol Commun Health. 1991;45:89–92. doi: 10.1136/jech.45.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamaker E.L., Dolan C.V., Molenaar P.C.M. Statistical modeling of the individual: rationale and application of multivariate stationary time series analysis. Multivariate Behav Res. 2005;40:207–233. doi: 10.1207/s15327906mbr4002_3. [DOI] [PubMed] [Google Scholar]
- 57.Molenaar P.C.M., Campbell C.G. The new person-specific paradigm in psychology. Cur Dir Psychol Sci. 2009;18:112–117. [Google Scholar]
- 58.Bayer U., Glazachev O.S., Likar R., Burtscher M., Kofler W., Pinter G. Adaptation to intermittent hypoxia-hyperoxia improves cognitive performance and exercise tolerance in the elderly. Adv Gerontol. 2017;30:214–220. [PubMed] [Google Scholar]
- 59.Bayer U., Likar R., Pinter G., Stettner H., Demschar S., Trummer B. Intermittent hypoxic-hyperoxic training on cognitive performance in geriatric patients. Alzheimers Dement (N Y) 2017;3:114–122. doi: 10.1016/j.trci.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stavres J., Gerhart H.D., Kim J.H., Glickman E.L., Seo Y. Cerebral hemodynamics and executive function during exercise and recovery in normobaric hypoxia. Aerosp Med Hum Perform. 2017;88:911–917. doi: 10.3357/AMHP.4830.2017. [DOI] [PubMed] [Google Scholar]
- 61.Limmer M., Platen P. The influence of hypoxia and prolonged exercise on attentional performance at high and extreme altitudes: a pilot study. PloS One. 2018;13 doi: 10.1371/journal.pone.0205285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bayer U., Likar R., Pinter G., Stettner H., Demschar S., Trummer B. Effects of intermittent hypoxia-hyperoxia on mobility and perceived health in geriatric patients performing a multimodal training intervention: a randomized controlled trial. BMC Geriatr. 2019;19:167. doi: 10.1186/s12877-019-1184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrison J.D., Quinn K., MacDonald L.A., Billaut F., Minahan C. Repeated treadmill sprints impair cognitive performance in amateur team-sport athletes when performed in normobaric hypoxia. J Sports Sci Med. 2019;18:369–375. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.