Abstract
Systemic or localized application of glucocorticoids (GCs) can lead to iatrogenic ocular hypertension, which is a leading cause of secondary open-angle glaucoma and visual impairment. Previous work has shown that dexamethasone increases zonula occludens-1 (ZO-1) protein expression in trabecular meshwork (TM) cells, and that an antisense oligonucleotide inhibitor of ZO-1 can abolish the dexamethasone-induced increase in trans-endothelial flow resistance in cultured Schlemm’s canal (SC) endothelial and TM cells. We have previously shown that intracameral inoculation of small interfering RNA (siRNA) targeting SC endothelial cell tight junction components, ZO-1 and tricellulin, increases aqueous humor outflow facility ex vivo in normotensive mice by reversibly opening SC endothelial paracellular pores. In this study, we show that targeted siRNA downregulation of these SC endothelial tight junctions reduces intraocular pressure (IOP) in vivo, with a concomitant increase in conventional outflow facility in a well-characterized chronic steroid-induced mouse model of ocular hypertension, thus representing a potential focused clinical application for this therapy in a sight-threatening scenario.
Keywords: glucocorticoid, ocular hypertension, primary open angle glaucoma, trabecular meshwork, tight junctions, Schlemm’s canal, pores, intracameral, intraocular pressure, outflow facility
Graphical Abstract
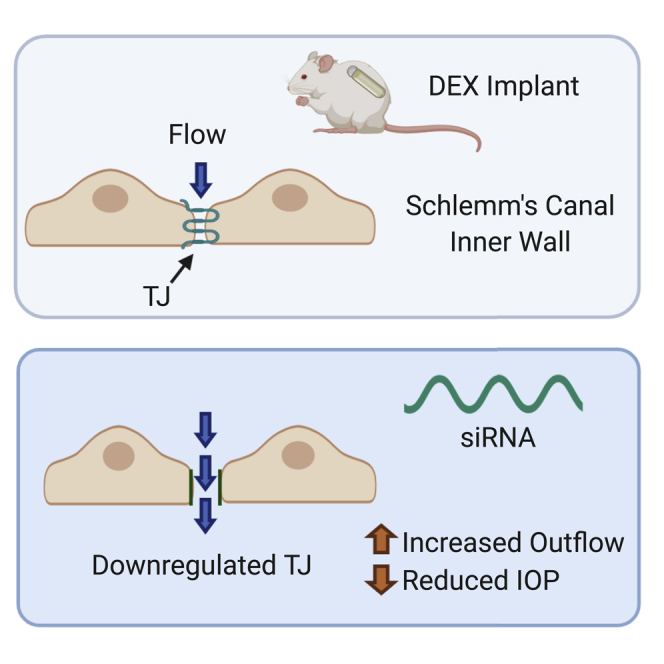
Cassidy and colleagues show that intracameral inoculation of siRNA targeting Schlemm’s canal endothelial cell tight junction proteins, ZO-1 and tricellulin, can reduce intraocular pressure and increase outflow facility in a murine model of steroid-induced ocular hypertension.
Introduction
Glucocorticoids (GCs) have been widely used in controlling ocular inflammation in uveitis; however, ocular hypertension (OHT) with the risk of secondary open-angle glaucoma is a significant complication of GC therapy.1 While most patients undergo short courses of GC therapy, in the UK an estimated up to 5% of adults undergo long-term systemic GC use daily for more than 5 years, while up to 20% undergo GC treatment for more than 6 months.2 One third of ophthalmic topical GC-treated patients exhibit a moderate increase in intraocular pressure (IOP), while approximately 5% of patients experience severe IOP increases. With intravitreal injection, these rates are greatly increased, with OHT resulting in 25% of cases.3 In those instances where acute steroid-induced elevations in IOP occur in GC-dependent patients, and in which OHT cannot be controlled with conventional pressure-reducing medications, trabeculectomy, laser trabeculoplasty, or shunt surgery may be required.4 These procedures all have associated risks of complications, warranting exploration of alternative, less invasive methods of IOP control.
Most resistance to aqueous outflow is generated within the juxtacanalicular tissues (JCTs), comprising the trabecular meshwork (TM) and inner wall endothelium of Schlemm’s canal (SC). Endothelial cells of the inner wall of SC form loosely organized tight junctions (TJs), leaving discrete paracellular clefts through which aqueous can enter the canal from the TM. A synergistic model of outflow resistance generation has been proposed, in which the inner wall of SC and the JCT region together are responsible for the bulk of conventional outflow resistance generation.5 Under this model, referred to as the funneling model of outflow resistance generation, due to the spatial separation of SC inner wall pores, aqueous humor (AH) moving from the JCTs into these pores must converge, or funnel, from wide areas of tissue to discrete pores on the inner wall through the tortuous extracellular spaces of the JCTs.5 Paracellular pores may be the preferential flow path, but transcellular pores also contribute to the overall outflow along the inner wall of SC.6 Increased pore density is associated with regions of higher segmental outflow, while glaucomatous eyes are reported to have reduced SC inner wall porosity.7,8 As such, the pore density of a region of the SC inner wall can directly influence conventional outflow resistance generation and hydraulic conductivity. GC treatment can have a range of effects on the outflow pathway, including altering TM cell functions, extracellular matrix metabolism, and gene expression, which may be responsible for the increase in outflow resistance associated with GC treatment.9
We have previously shown in normotensive mice that small interfering RNA (siRNA)-mediated downregulation of TJ proteins zonula occludens-1 (ZO-1) (encoded by the TJP1 gene) and tricellulin (encoded by the MARVELD2 gene) in SC endothelial cells results in a visible opening of paracellular clefts as observed by transmission electron microscopy, increasing cleft porosity and enhancing flow of aqueous from the TM into the lumen, thus resulting in an increase in conventional outflow facility.10 However, it remained to be demonstrated whether and to what extent this approach to IOP control might be effective in an animal model displaying features of steroid-induced OHT, where significant molecular pathological changes occur within the TM to reduce outflow, and whether the concomitant elevation in IOP could also be reduced. We investigated the efficacy of this approach in a mouse model of dexamethasone (DEX)-induced OHT. Sustained elevation of IOP can be induced in mice through long-term systemic delivery of DEX using micro-osmotic pumps. This method of inducing GC OHT has been shown to elevate IOP significantly within 2 weeks of treatment, with a concomitant reduction in conventional outflow facility, while preserving open-angled morphology within the anterior chamber.11, 12, 13, 14 We have previously shown how treatment with siRNA does not impact the open-angle structure in the anterior chamber in rodents. We have assumed that the same mode of action is occurring in these hypertensive animals treated with the same siRNA as was used in our previous work.10 This is inferred based on findings from Tam et al.10 that siRNA targeting both ZO-1 and tricellulin results in anatomical changes to the outflow tissue in normotensive animals.
Mice treated with DEX have also been shown to have increased deposition of extracellular matrix material within the TM, with elevated levels of extracellular collagen I, fibronectin, and mucopolysaccharides, and the formation of cross-linked α-smooth muscle actin networks.15,16 Also, of particular relevance to our TJ modulation-based approach, GC treatment of cultured human SC endothelial cells (SCECs) has been shown to significantly increase ZO-1 expression, and to increase the number of TJ-containing intercellular contacts.17 Hence, this model represents a suitable means for assessment of the modulatory effect of our approach on outflow facility, serving as a model of OHT in POAG in general and, more specifically, of human GC-induced glaucoma. We show herein that siRNA-mediated TJ protein suppression enhances conventional outflow facility and reduces IOP in this murine model of GC-induced OHT. Potential adaptation of this approach to enable clinical deployment is also discussed.
Results
Characterization of IOP elevation in DEX-treated mice
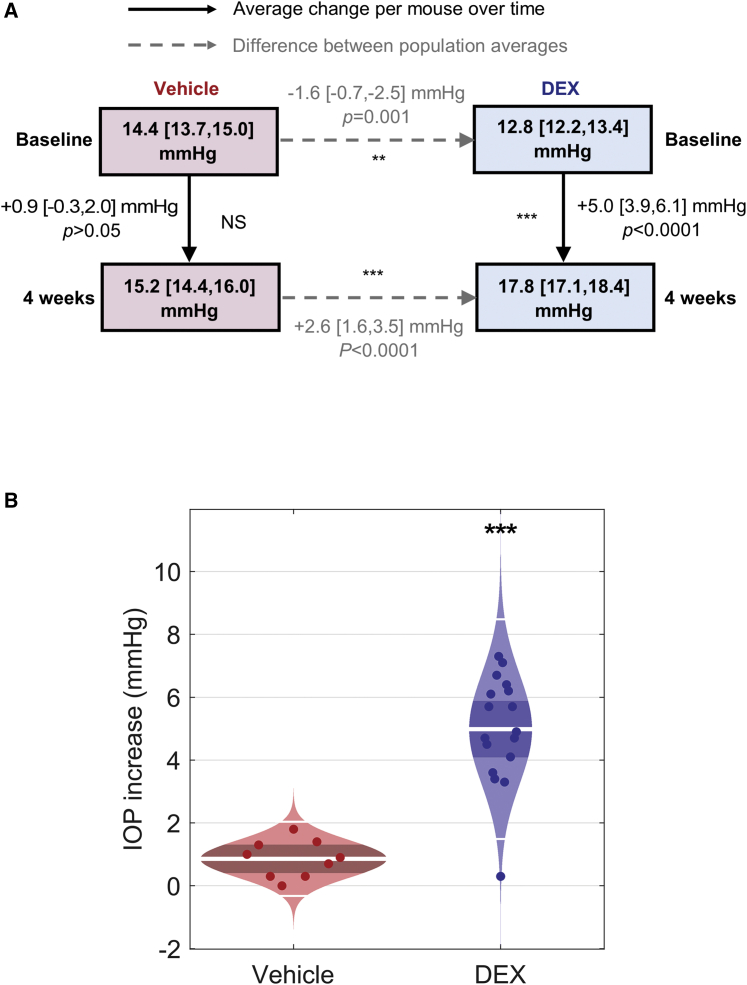
Wild-type C57BL/6J mice were implanted subcutaneously with micro-osmotic pumps delivering DEX at a dose of 2 mg/kg/day (n = 17), while a separate cohort of wild-type C57BL/6J mice designated as controls were implanted with pumps containing cyclodextrin as a vehicle control (n = 9). One day prior to pump implantation, mice were anesthetized with isoflurane, and IOP was measured by rebound tonometry in one eye. These measurements were repeated weekly thereafter for 4 weeks. The changes in IOP arising from this treatment, and differences in population averages, are summarized in Figure 1. The baseline IOP measurement for the vehicle-treated group was 14.4 [13.7, 15.0] (mean [confidence interval (CI)]), and final IOP measurement was 15.2 [14.4, 16.0], corresponding to a non-significant change of 0.9 [−0.3, 2.0] mmHg (p > 0.05, one-way ANOVA with Tukey’s post-test, n = 9, Figure 1A, red). For DEX-treated animals, baseline IOP was measured as 12.8 [12.2, 13.4], and final IOP was 17.8 [17.1, 18.4], corresponding to a significant increase in IOP of 5.0 [3.9, 6.1] mmHg (p < 0.0001, one-way ANOVA with Tukey’s post-test, n = 17, Figure 1A, blue). After 4 weeks of treatment, the final IOP difference between the DEX and control groups was 2.6 [1.6, 3.5] mmHg (p < 0.0001, unpaired t test, Figure 1A), compared to a 1.6 [−0.7, −2.5] mmHg (p = 0.001, unpaired t test, Figure 1A) difference in IOP between the DEX and vehicle group at the beginning of the study, before any treatment was administered. These data confirm that DEX treatment results in a significant elevation in IOP in these animals as compared to the vehicle controls (Figure 1B).
Figure 1.
Summary of IOP changes in vehicle and dexamethasone (DEX)-treated mice
(A) Change in IOP after 4 weeks of vehicle (red, n = 9) or DEX (blue, n = 17) delivery via a micro-osmotic pump. Dashed arrows represent the differences between population average at baseline and after treatment, while solid arrows represent the average change in IOP per mouse after treatment. (B) Cello plots show the IOP increase (mmHg) following treatment with either cyclodextrin vehicle (red) or DEX (blue) for 4 weeks. The white line represents the geometric mean, the dark red/blue bands indicate the 95% CI, and the light red/blue regions are the distributions of the data. Each data point represents the IOP increase (mmHg) for each individual animal. p values are represented by asterisks.
Targeted downregulation of TJ proteins ZO-1 and tricellulin in normotensive C57BL/6J mice
In order to validate the efficacy of siRNA-mediated transcript suppression of our target TJ proteins ZO-1 and tricellulin, we first carried out our intraocular siRNA delivery protocol that we have previously shown to be effective in the enhancement of outflow facility in normotensive mice.10 Briefly, C57BL/6J normotensive animals were treated with an intracameral injection containing 1 μg each of ZO-1 and tricellulin targeting siRNA (T-siRNA) in one eye, while contralateral control eyes received 2 μg of non-targeting siRNA (NT-siRNA) injections. Then, at 48 h post-injection, enucleated tissue was homogenized, RNA was extracted, and qPCR analysis was carried out on both NT-siRNA- and T-siRNA-treated eyes.
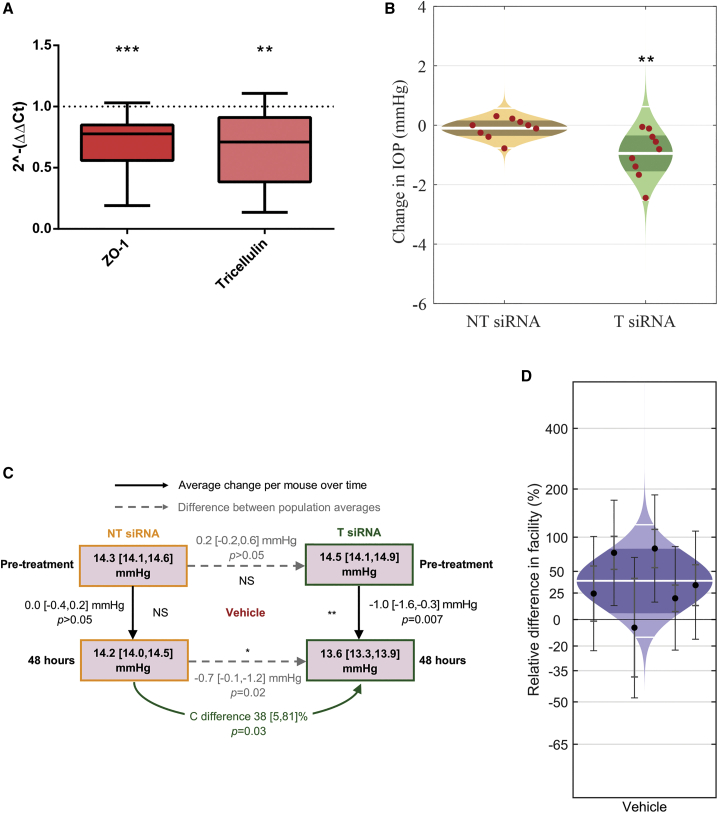
T-siRNA-injected eyes showed significant downregulation of both ZO-1 and tricellulin transcript levels, when compared to NT-siRNA-treated eyes. 2−ΔΔCt values are presented below, representing fold change in relative gene expression of these TJ proteins of interest. For ZO-1, intracameral injection with T-siRNA led to a significant mean fold change in relative gene expression of 0.7 [0.5, 0.8] (mean [CI) (p < 0.001, n = 12, Figure 2A), and tricellulin showed a significant mean fold change in relative gene expression of 0.6 [0.4, 0.9] (p < 0.01, one-sample t test to theoretical mean of 1, n = 11, Figure 2A), representing a 30% and 40% average reduction in transcript levels, respectively. These data suggest that the TJs ZO-1 and tricellulin are successfully downregulated at the outflow tissue following a single intracameral injection with T-siRNA.
Figure 2.
Summary of relative fold gene expression of TJ proteins, IOP, and facility changes after intracameral siRNA injection in vehicle control animals
(A) A mean fold change in relative gene expression of 0.7 [0.5, 0.8] (p < 0.001, one-sample t test to theoretical mean of 1, n = 12) and 0.6 [0.4, 0.9] (p < 0.01, one-sample t test to theoretical mean of 1, n = 11) is shown in both ZO-1 and tricellulin protein transcripts, respectively. Error bars represent minimum/maximum values, the 95% CI is shown by bounds of box, and the horizontal line represents the median. (B) Cello plots showing the change in IOP (mmHg) 48 h post-treatment with either non-targeting siRNA (NT-siRNA) (orange) or siRNA targeting ZO-1 and tricellulin (T-siRNA) (green). The white line represents the geometric mean, the dark orange/green bands indicate the 95% CI, and the light orange/green regions are the distributions of the data. Each data point represents the change in IOP (mmHg) for each individual animal. (C) Summary of changes in IOP and outflow facility after treatment with intracameral injection of T-siRNA (green, n = 9) or NT-siRNA (orange, n = 9) in vehicle-treated mice. Differences in ex vivo outflow facility (C) 72 h after treatment are shown via green arrows. Dashed arrows represent the differences between population average pre-treatment and 48 h post-treatment, while solid arrows represent the average change in IOP per mouse after treatment. (D) Intracameral injection of T-siRNA targeting ZO-1 and tricellulin is shown to significantly increase conventional outflow facility in treated eyes as compared to contralateral control eyes receiving NT-siRNA in vehicle normotensive animals, with an increase of 38 [5, 81] % (mean [95% CI]) (p = 0.029, n = 6 pairs). The white line represents the geometric mean, the dark blue bands indicate the 95% CI, and the light blue regions are the distribution of the data. Each data point represents the difference in C between contralateral eyes with 95% CI. p values are represented by asterisks.
Effect of TJ downregulation on IOP in vehicle-treated mice
To evaluate whether downregulation of ZO-1 and tricellulin was an effective means of lowering IOP in normotensive mice, all mice were anesthetized and IOP was measured again in both eyes to acquire baseline measurements after vehicle micro-osmotic pump treatment and prior to injection with siRNA. Mice were then treated with an intracameral injection containing T-siRNA in one eye, while contralateral control eyes received NT-siRNA injections. At 48 h post-injection, mice were anesthetized and IOP was once again measured by rebound tonometry. To account for the variation in the pre-treatment baseline IOP values, IOP data are presented as the change in IOP between pre- and post-treatment measurements in both NT-siRNA- and T-siRNA-treated eyes. The changes in IOP arising from this siRNA injection, and differences in population averages before and 48 h after treatment, are summarized in Figure 2 for vehicle-treated animals and Figure 3 for DEX-treated animals.
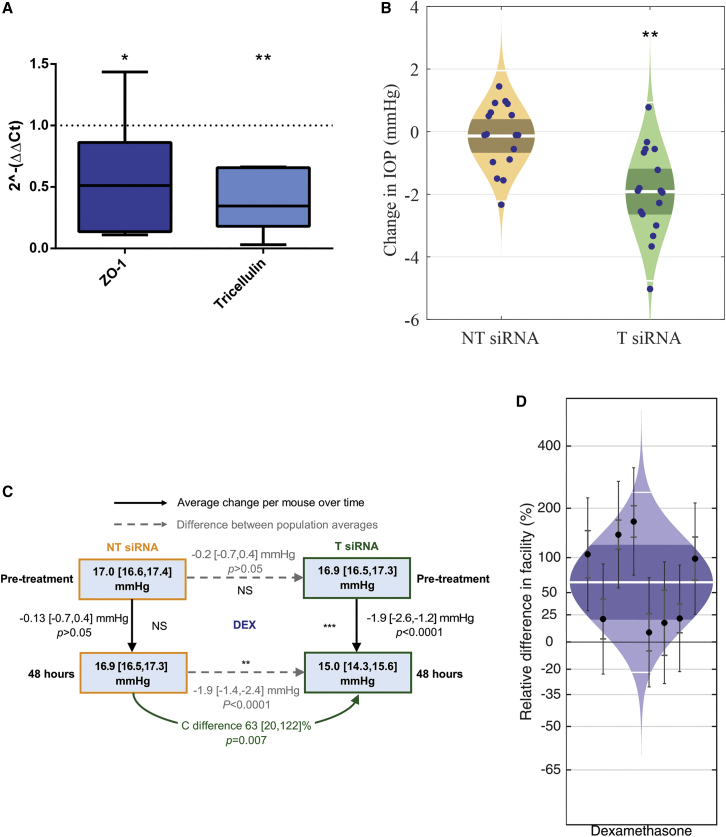
Figure 3.
Summary of relative fold gene expression of TJ proteins, IOP, and facility changes after intracameral siRNA injection in DEX-treated animals
(A) A mean fold change in relative gene expression of 0.6 [0.2, 1.0] (p < 0.05, one-sample t test to theoretical mean of 1, n = 8) and 0.4 [0.1, 0.6] (p < 0.01, one sample t test to theoretical mean of 1, n = 6) was shown in both ZO-1 and tricellulin protein transcripts, respectively. Error bars represent minimum/maximum values, the 95% CI is shown by bounds of box, and the horizontal line represents the median. (B) Cello plots showing the change in IOP (mmHg) 48 h post-treatment with either NT-siRNA (orange) or T-siRNA (green). The white line represents the geometric mean, the dark orange/green bands indicate the 95% CI, and the light orange/green regions are the distributions of the data. Each data point represents the change in IOP (mmHg) for each individual animal. (C) Summary of changes in IOP and outflow facility after treatment with intracameral injection of siRNA targeting ZO-1 and tricellulin (T-siRNA, green, n = 17) or NT-siRNA (orange, n = 17) in DEX-treated mice. (D) These cello plots show the significant increase in conventional outflow facility following treatment with T-siRNA compared to contralateral control eyes receiving NT-siRNA in DEX-treated animals, with an increase of 63 [20, 122] % (mean [95% CI]) (p = 0.0071, n = 8 pairs). The white line represents the geometric mean, the dark blue bands indicate the 95% CI, and the light blue regions are the distribution of the data. Each data point represents the difference in C between contralateral eyes with 95% CI. p values are represented by asterisks.
T-siRNA-injected eyes showed a significant average reduction in IOP of 1.0 [−1.6, −0.3] mmHg as compared to pre-treatment IOP measurement averages (13.6 [13.3, 13.9] versus 14.5 [14.2, 14.9] mmHg, p < 0.01, n = 9, Figures 2B and 2C, green). NT-siRNA-injected eyes showed no significant average change in IOP in these animals, −0.0 [−0.4, 0.2] mmHg, as compared to pre-treatment IOP measurement averages of (14.2 [14.0, 14.5] versus 14.3 [14.1, 14.6] mmHg, p > 0.05, n = 9, Figures 2B and 2C, orange). At 48 h post-treatment, a significant difference in IOP of −0.7 [−0.1, −1.2] mmHg (p = 0.02, two-way unpaired t test, Figure 2C) was shown between the NT-siRNA and T-siRNA groups compared to a non-significant difference in IOP of 0.2 [−0.2, 0.6] mmHg (p > 0.05, two-way unpaired t test, Figure 2C) between the groups at the beginning of the study, before treatment was administered. These data confirm that T-siRNA treatment in this vehicle-treated group results in a significant reduction in IOP compared to the NT-siRNA group (Figure 2B).
Effect of TJ downregulation on ex vivo conventional outflow facility in vehicle-treated mice
In normotensive vehicle control mice, T-siRNA induced an increase in conventional outflow facility (C) of 38 [5, 81] % (p = 0.029, Figure 2D) as compared to NT-siRNA. Changes in facility were determined by performing ex vivo perfusions using the iPerfusion system.17 This facilitated comparison of C between paired contralateral T-siRNA- and NT-siRNA-treated eyes in normotensive vehicle (n = 6) mice.
Targeted downregulation of TJ proteins ZO-1 and tricellulin in DEX-treated mice
After demonstrating above that siRNA-mediated transcript suppression of our target TJ proteins ZO-1 and tricellulin was effective in normotensive mice, we wanted to also validate this approach in siRNA-treated hypertensive DEX-treated animals. A cohort of mice was administered DEX daily for 4 weeks in order to induce OHT, and then individuals were administered a single intracameral injection of both T-siRNA and NT-siRNA as above. At 48 h post-injection, enucleated tissue was homogenized, RNA was extracted, and qPCR analysis was carried out, as in the normotensive cohort of mice.
T-siRNA-injected eyes showed significant downregulation of both ZO-1 and tricellulin gene expression, when compared to NT-siRNA-treated eyes. Following a once-off intracameral injection of T-siRNA, ZO-1 showed a significant mean fold change in relative gene expression of 0.6 [0.2, 1.0] (mean [CI]) (p < 0.05, one-sample t test to theoretical mean of 1, n = 8, Figure 3A). and tricellulin showed a significant mean fold change in relative gene expression of 0.4 [0.1, 0.6] (p < 0.01, n = 6, Figure 3A), representing a 40% and 60% mean reduction in transcript levels, respectively. These data suggest that T-siRNA has greater potential for TJ downregulation in the DEX-treated animals as compared to the normotensive control group.
Effect of TJ downregulation on IOP in DEX-treated mice
To evaluate whether downregulation of ZO-1 and tricellulin was an effective means of lowering IOP in OHT mice, all mice were anesthetized and IOP was measured again in both eyes to acquire baseline measurements after DEX micro-osmotic pump treatment and prior to injection with siRNA. Mice were then treated with an intracameral injection containing T-siRNA in one eye, while contralateral control eyes received NT-siRNA injections. At 48 h post-injection, mice were anesthetized and IOP was once again measured by rebound tonometry. DEX-treated mice showed a significant average reduction in IOP in T-siRNA-injected eyes of 1.9 [−2.6, −1.2] mmHg as compared to pre-treatment IOP measurement averages (15.0 [14.3, 15.6] versus 16.9 [16.5, 17.3] mmHg, p < 0.0001, n = 17, Figures 3B and 3C green). NT-siRNA-injected eyes showed no significant average change in IOP in these animals of 0.13 [-0.7, 0.4] mmHg, as compared to pre-treatment IOP measurement averages (16.9 [16.5, 17.3] versus 17[16.6, 17.4] mmHg, p > 0.05, n = 17, Figures 3B and 3C, orange). At 48 h post-treatment in the DEX-treated animals, the final IOP difference between the NT and T-siRNA groups was −1.9 [−1.4, −2.4] mmHg (p < 0.0001, two-way unpaired t test, Figure 3C), compared to −0.2 [−0.7, 0.4] mmHg (p < 0.05, two-way unpaired t test, Figure 3C) difference in IOP between the groups at the beginning of the study, before treatment was administered. These data confirm that siRNA treatment in these DEX-treated animals results in a significant reduction in IOP compared to the NT-siRNA group (Figure 3B).
Effect of TJ downregulation on ex vivo conventional outflow facility in DEX-treated mice
In DEX mice, eyes injected with T-siRNA had a significant increase in C of 63 [20, 122] % (p = 0.0071, n = 8 pairs, Figure 3D) over contralateral NT-siRNA-injected control eyes.
Discussion
We have previously shown that downregulation of the TJ-associated proteins ZO-1 and tricellulin at the SC inner wall leads to an increase in the number of open SC endothelial paracellular clefts, as well as an increase in C in normotensive wild-type mice.10 In the current study, we aimed to validate this approach in a disease setting using a murine model of steroid-induced OHT. Specifically, we wanted to assess the utility of this approach for IOP reduction in a model of chronic steroid-induced elevation in IOP, as is often observed in GC-dependent patients resistant to the use of conventional pressure-reducing topical medications, and where surgical intervention may be the only current option to reduce IOP. We wanted to determine whether C could be increased in the DEX model and, additionally, to investigate whether downregulation of ZO-1 and tricellulin reduced IOP.
We have shown significant downregulation of TJ protein transcript levels in both normotensive and hypertensive DEX-treated mice following a single intracameral injection of T-siRNA. A greater reduction in both ZO-1 and tricellulin relative gene expression is seen in the DEX-treated cohort in response to T-siRNA compared to that in normotensive animals. We have shown in the DEX model of OHT that such animals also exhibit a greater reduction in IOP than do normotensive animals after siRNA treatment. We additionally demonstrated that this therapeutic approach significantly increases C in both this hypertensive model and in normotensive vehicle controls, with a greater relative change seen in hypertensive animals. The data suggest that higher IOP may lead to a greater efficacy of this approach, as an increased pressure gradient across the SC inner wall may facilitate increased opening of paracellular pores upon TJ downregulation. The data also further emphasize the role that paracellular pores at the SC inner wall play in pathological reductions in outflow facility, and corresponding IOP elevation. T-siRNA-injected DEX-treated eyes show an average IOP decrease of approximately 2 mmHg, representing twice that measured in normotensive controls (approximately 1 mmHg). While the level of the mean increase in C in normotensive animals reported herein of 38% is lower than the mean increase of 113% previously reported in Tam et al.,10 perfusions were carried out 72 h post-treatment here as compared to 48 h previously to facilitate in vivo tonometric IOP readings carried out at 48 h post-treatment here. This lesser effect size is understandable, as the transient nature of siRNA-mediated downregulation results in reduced target downregulation with longer times post-treatment. As the DEX model is known to increase TJ protein expression, including ZO-1,18 we thus propose that siRNA-mediated knockdown of TJs is more effective in an environment with greater TJ expression, as there is a greater availability of TJ mRNA for siRNA to bind to. It is also possible that there is a greater number of closed or occluded paracellular pores with upregulated ZO-1, and therefore a greater potential number of pores that can be opened. We have also shown that T-siRNA has a greater effect in the DEX-treated mice compared to the normotensive C57BL/6J control group. There is a mean fold change in relative ZO-1 and tricellulin expression of 0.6 and 0.4, respectively, in the DEX mice, compared to 0.7 and 0.6 in the normotensive group. The data accrued in this study show that siRNA-mediated downregulation of TJs in the anterior chambers of this murine model of OHT has a greater effect than is observed in normotensive animals. Significant reduction in IOP is achieved through increasing the facility of the conventional outflow pathway, demonstrating that this potential approach to therapy could show efficacy in treatment of OHT.
In regard to the potential of this approach for clinical application, the downregulatory effects of siRNA are transient in nature, with levels of ZO-1 and tricellulin transcripts returning to normal over a number of days.10 Should repeated administration be required to reduce IOP appropriately, a minimally invasive approach has been reported for periodic retrograde introduction of low-molecular-weight compounds into SC via the episcleral veins, and this could be used for non-invasive delivery of siRNA without adaptation (Retroject, Chapel Hill, NC, USA). Moreover, Dillinger et al.19 have recently shown that coating nanoparticles with hyaluronan more efficiently targets siRNA to TM and SC endothelial cells in view of the fact that such particles bind to the cell surface antigen CD44, present in greater quantities on cells of the outflow tissues. In conclusion, this study demonstrates in a well-characterized model of steroid-induced OHT a proof of concept of an siRNA-based therapeutic approach for IOP reduction.
Materials and methods
Animal husbandry
Animals and procedures used in this study were carried out in accordance with the regulations set out by the Health Products and Regulatory Authority (HPRA) and the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research. C57BL/6J animals were used for DEX implantations. Both male and female animals were used in these studies. Animals were housed in specific pathogen-free environments in the University of Dublin, Trinity College, and all injections and IOP measurements complied with the HPRA project authorization no. AE19136/P017. Sample size was calculated for both control and treated animals separately. Treated animals had the potential to be exposed to varied levels of DEX, depending on how successfully the DEX was administered by the micro-osmotic pump during the 4-week treatment period. As this could cause variability in response to siRNA treatment, the effects of the T-siRNA would be expected to exhibit greater variation in the treated animals compared to the control group. To account for this and the fact that not all DEX mice would be expected to survive to the final 4-week time point due to DEX tolerability issues, the sample size for the treated group was twice that of the control group. The starting sample size was n = 10 for control animals and n = 20 for treated animals. The osmotic mini pumps are sold in packs of 10, and so n = 10 would not be sufficient for the DEX-treated group. The final sample size of n = 9 for control animals and n = 17 for treated animals was published below for the IOP and perfusion experiments. The sample size used for qPCR experiments was calculated separately. The number of normotensive animals used was n = 12 and n = 10 for the DEX-treated animals.
Tonometric IOP measurement
For analysis of the effect of DEX on IOP, weekly measurements were performed by rebound tonometry (TonoLab, Icare). Mice were anaesthetized with 3% isoflurane at 1 L/min for 2 min in an induction chamber, and then moved to a head holder delivering isoflurane at the same rate. At 3 min after induction of anesthesia, five consecutive IOP measurements (constituting an average of six readings each) were taken in the right eye and averaged. For measurement of IOP pre- and post-siRNA treatment, mice were anaesthetized again, as above, and three IOP measurements (constituting an average of six readings each) were made at three different time points in both eyes (eye 1, minutes 3, 5, and 7 after anesthesia; eye 2, minutes 4, 6, and 8 after anesthesia). The average IOP measurement at each time point was used to fit a line and interpolate IOP at minute 5 to allow for measurement of IOP in both eyes while accounting for the IOP lowering effect of anesthesia over time.
Micro-osmotic pump implantation
DEX micro-osmotic pumps (model 1004, Alzet) were implanted as in Whitlock et al.11 The DEX was water-soluble (D2915; Sigma-Aldrich), contained cyclodextrin (1.36 g per 100 mg of DEX), and was dissolved in PBS. Vehicle-treated mice received pumps containing cyclodextrin (C4555; Sigma-Aldrich) alone. DEX was delivered at 2 mg/kg/day. Adult C57BL/6J mice of 10–12 weeks of age were anaesthetized with 3% isoflurane at 1 L/min, and the surgical site was shaved and disinfected with chlorhexidine swabs. Mice were injected intramuscularly with 0.05 mg/kg buprenorphine (Buprecare, Animalcare) and subcutaneously with 5 mg/kg enrofloxacin (Enrocare, Animalcare). An incision was made between the scapulae, a subcutaneous pocket was created by blunt dissection, and the pump was inserted. The incision was closed using surgical glue (Surgibond, RayVet). After pump implantation, mice were housed singly and diet was supplemented with Complan (Nutricia Advanced Medical Nutrition) to prevent GC-induced weight loss. Weight was monitored weekly, with any mice losing more than 20% body weight overall, or 10% body weight in 1 week, required to be euthanized.
Intracameral injection of siRNA
Our method for intracameral delivery to the anterior chamber has been described in detail previously.10 Briefly, for siRNA injection, mice were anesthetized with 3% isoflurane at 1 L/min. Pupils were dilated with 2.5% tropicamide and 2.5% phenylephrine eye drops. Glass micro-capillaries (outer diameter, 1 mm; inner diameter, 0.58 mm; World Precision Instruments) were pulled using a micropipette puller (Narishige PB-7). Under microscopic control, a pulled blunt-ended micro-glass needle (tip diameter, ~100 μm) was first used to puncture the cornea to withdraw AH by capillarity. Immediately after puncture, a pulled blunt-ended micro-glass needle attached to a 10-μL syringe (Hamilton, Bonaduz) held in a micromanipulator (World Precision Instruments) was inserted through the puncture, and 1.5 μL of PBS containing 1 μg of ZO-1 siRNA and 1 μg of tricellulin siRNA was administered into the anterior chamber to give a final concentration of 16.84 μM. Contralateral eyes received an identical injection of 1.5 μL containing the same concentration of NT siRNA. Fusidic gel was applied topically to the eye as antibiotic and Vidisic gel was also applied topically as a moisturizer. Furthermore, 5 mg/kg enrofloxacin antimicrobial (Baytril, Bayer Healthcare) was injected subcutaneously.
siRNAs
All in vivo pre-designed siRNAs used in this study were synthesized by Ambion and reconstituted as per the manufacturer’s protocol. siRNA identification numbers are as follows: mouse ZO-1 siRNA (ID no. s75175), mouse MARVELD2 siRNA (ID no. ADCSU2H). Silencer negative control siRNA (Ambion) was used as a non-targeting control.
iPerfusion
Outflow facility measurements with iPerfusion were carried out as described in Sherwood et al.17 Mice were culled by cervical dislocation, and eyes were enucleated immediately and stored in PBS at room temperature to await perfusion (~20 min). Both eyes were perfused simultaneously using two independent perfusion systems as described previously. Briefly, each eye was affixed to a support using a small amount of cyanoacrylate glue and submerged in a PBS bath regulated at 35°C. The eye was cannulated via the anterior chamber with a 33G beveled needle (NanoFil, #NF33BV-2, World Precision Instruments) under a stereomicroscope using a micromanipulator. The iPerfusion system comprises an automated pressure reservoir, a thermal flow sensor (SLG64-0075, Sensirion), and a wet-wet pressure transducer (PX409, Omegadyne) in order to apply a desired pressure, measure flow rate out of the system, and measure the IOP, respectively. The perfusate was PBS containing Ca2+, Mg2+, and 5.5 mM glucose, and was filtered through a 0.22-μm filter (VWR International) prior to use. Following cannulation, eyes were perfused for 30 min at 8 mmHg to allow the eye to acclimatize. Subsequently, nine discrete pressure steps were applied from 4.5 to 21 mmHg, while flow and pressure were recorded. Stability was defined programmatically, and data were averaged over 5 min at steady state. A non-linear model was fit to flow-pressure data to account for the pressure dependence of outflow facility in mouse eyes. This model was of the form Q = CrP(P/Pr)β, where Q and P are the flow rate and pressure, respectively, and Cr is the outflow facility at reference pressure Pr, which is selected to be 8 mmHg (the approximate physiological pressure drop across the outflow pathway). The power law exponent β quantifies the non-linearity in the Q-P response and thus the pressure dependence of outflow facility. The data analysis methodology described in Sherwood et al.17 was applied in order to analyze the treatment effect, while accounting for measurement uncertainties, and statistical significance was evaluated using the paired weighted t test described therein.
qPCR
An optimized dissection method was used to enrich the number of endothelial cells from SC present in the homogenized tissue solution used for RNA extraction. The anterior segment was removed 2–3 mm anterior and posterior to the limbus, leaving a ring of tissue containing the outflow tissue at the iridocorneal angle, and any remnants of corneal and iris tissues. This results in minimal TJ transcripts arising from non-outflow tissues in our sample, such as from corneal endothelia and epithelia. Total RNA was extracted from this homogenized tissue solution using the RNeasy mini kit (QIAGEN) according to the manufacturer’s protocol. The RNA concentration of each sample was quantified using a NanoDrop ND-100 spectrophotometer, and equal concentrations were reverse transcribed into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems). A SensiFAST SYBR Hi-ROX kit (Bioline) was used according to manufacturer’s protocol along with ZO-1, tricellulin, and β-actin primer pairs and loaded onto a 96-well plate (Applied Biosystems). The plate was run on a StepOnePlus real-time PCR system (Applied Biosystems). Primer pair sequences can be found in Table 1. The threshold cycle (Ct) values of treated and untreated tissue samples from each sample were determined and averages were calculated. The mean normalized expression (ΔCt) of RNA encoding ZO-1 and tricellulin TJ proteins was determined and analyzed. Normalized gene expression was calculated by using the following equation: ΔCt = Ct(gene of interest) − Ct(housekeeping genes). Normalization was carried out with the β-actin housekeeping gene. ΔΔCt was calculated by subtracting the treated sample from the control sample: ΔΔCt = ΔCt(treated sample) − ΔCt(control sample). The 2−ΔΔCt method was then used to calculate relative fold gene expression for each sample pair. A 2−ΔΔCt value of 1 represents no change in relative fold gene expression. A value above 1 represents upregulation of the gene of interest, and a value below 1 represents downregulation of the gene of interest.
Table 1.
Primer sequences used in real-time PCR reactions
| Primer pair | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| ZO-1 | CGCTCTCGGGAGATGTTTAT | GTTTCCTCCATTGCTGTGCT |
| Tricellulin | AGGCAGCTCGGAGACATAGA | TCACAGGGTATTTTGCCACA |
| β-Actin | GGGAAATCGTGCGTGACAT | GTGATGACCTGGCCGTCAG |
Statistical analysis
A one-way ANOVA with Tukey’s post-test was performed to determine the statistical significance of IOP elevation in the DEX versus vehicle animals over time following intracameral injection with siRNAs. All IOP analysis was carried out using a two-tailed unpaired Student’s t test, unless otherwise stated. Outflow facility calculations were based on a weighted, paired, two-tailed t test. qPCR analysis was performed using a one-sample t test with a hypothetical value of 1, representing no change in relative gene expression.
Acknowledgments
Glaucoma research was supported at the Ocular Genetics Unit at the University of Dublin, Trinity College, by the European Research Council (ERC-2012-AdG) and by Science Foundation Ireland (SFI). Research at Duke University was supported by grants from the US National Institutes of Health (EY022359 and EY019696), and research at Imperial College London was supported by the US National Institutes of Health (EY022359 and EY019696) and the UK Engineering and Physical Sciences Research Council (EP/J010499/1). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Author contributions
Conceptualization, P.H.; Data Curation, P.S.C. and R.A.K.; Funding Acquisition, G.J.F. and P.H.; Investigation, P.S.C. and R.A.K.; Methodology, E.R.-T. and J.M.S.; Project Administration, M.M.H.; Supervision, P.H. and J.O.; Visualization, J.M.S. and J.O.; Writing – Original Draft, P.S.C., R.A.K., and J.O.; Writing – Review & Editing, E.R.-T., J.M.S., A.-S.K., C.O., M.C., W.D.S., D.R.O., and P.H.
Declaration of interests
The authors declare no competing interests.
References
- 1.Phulke S., Kaushik S., Kaur S., Pandav S.S. Steroid-induced glaucoma: an avoidable irreversible blindness. J. Curr. Glaucoma Pract. 2017;11:67–72. doi: 10.5005/jp-journals-l0028-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonough A.K., Curtis J.R., Saag K.G. The epidemiology of glucocorticoid-associated adverse events. Curr. Opin. Rheumatol. 2008;20:131–137. doi: 10.1097/BOR.0b013e3282f51031. [DOI] [PubMed] [Google Scholar]
- 3.Dada T., Nair S., Dhawan M. Steroid-induced glaucoma. J. Curr. Glaucoma Pract. 2009;3:33–38. [Google Scholar]
- 4.Razeghinejad M.R., Katz L.J. Steroid-induced iatrogenic glaucoma. Ophthalmic Res. 2012;47:66–80. doi: 10.1159/000328630. [DOI] [PubMed] [Google Scholar]
- 5.Overby D.R., Stamer W.D., Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp. Eye Res. 2009;88:656–670. doi: 10.1016/j.exer.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braakman S.T., Read A.T., Chan D.W., Ethier C.R., Overby D.R. Colocalization of outflow segmentation and pores along the inner wall of Schlemm’s canal. Exp. Eye Res. 2015;130:87–96. doi: 10.1016/j.exer.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson M., Chan D., Read A.T., Christensen C., Sit A., Ethier C.R. The pore density in the inner wall endothelium of Schlemm’s canal of glaucomatous eyes. Invest. Ophthalmol. Vis. Sci. 2002;43:2950–2955. [PubMed] [Google Scholar]
- 8.Overby D.R., Zhou E.H., Vargas-Pinto R., Pedrigi R.M., Fuchshofer R., Braakman S.T., Gupta R., Perkumas K.M., Sherwood J.M., Vahabikashi A. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc. Natl. Acad. Sci. USA. 2014;111:13876–13881. doi: 10.1073/pnas.1410602111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark A.F., Wordinger R.J. The role of steroids in outflow resistance. Exp. Eye Res. 2009;88:752–759. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Tam L.C.S., Reina-Torres E., Sherwood J.M., Cassidy P.S., Crosbie D.E., Lütjen-Drecoll E., Flügel-Koch C., Perkumas K., Humphries M.M., Kiang A.S. Enhancement of outflow facility in the murine eye by targeting selected tight-junctions of Schlemm’s canal endothelia. Sci. Rep. 2017;7:40717. doi: 10.1038/srep40717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitlock N.A., McKnight B., Corcoran K.N., Rodriguez L.A., Rice D.S. Increased intraocular pressure in mice treated with dexamethasone. Invest. Ophthalmol. Vis. Sci. 2010;51:6496–6503. doi: 10.1167/iovs.10-5430. [DOI] [PubMed] [Google Scholar]
- 12.Zode G.S., Sharma A.B., Lin X., Searby C.C., Bugge K., Kim G.H., Clark A.F., Sheffield V.C. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J. Clin. Invest. 2014;124:1956–1965. doi: 10.1172/JCI69774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overby D.R., Clark A.F. Animal models of glucocorticoid-induced glaucoma. Exp. Eye Res. 2015;141:15–22. doi: 10.1016/j.exer.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overby D.R., Bertrand J., Tektas O.Y., Boussommier-Calleja A., Schicht M., Ethier C.R., Woodward D.F., Stamer W.D., Lütjen-Drecoll E. Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Invest. Ophthalmol. Vis. Sci. 2014;55:4922–4933. doi: 10.1167/iovs.14-14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimoto T., Inoue T., Inoue-Mochita M., Tanihara H. Live cell imaging of actin dynamics in dexamethasone-treated porcine trabecular meshwork cells. Exp. Eye Res. 2016;145:393–400. doi: 10.1016/j.exer.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Yuan Y., Call M.K., Yuan Y., Zhang Y., Fischesser K., Liu C.Y., Kao W.W. Dexamethasone induces cross-linked actin networks in trabecular meshwork cells through noncanonical wnt signaling. Invest. Ophthalmol. Vis. Sci. 2013;54:6502–6509. doi: 10.1167/iovs.13-12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherwood J.M., Reina-Torres E., Bertrand J.A., Rowe B., Overby D.R. Measurement of outflow facility using iperfusion. PLoS ONE. 2016;11:e0150694. doi: 10.1371/journal.pone.0150694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Underwood J.L., Murphy C.G., Chen J., Franse-Carman L., Wood I., Epstein D.L., Alvarado J.A. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intercellular junctions. Am. J. Physiol. 1999;277:C330–C342. doi: 10.1152/ajpcell.1999.277.2.C330. [DOI] [PubMed] [Google Scholar]
- 19.Dillinger A.E., Guter M., Froemel F., Weber G.R., Perkumas K., Stamer W.D., Ohlmann A., Fuchshofer R., Breunig M. Intracameral delivery of layer-by-layer coated siRNA nanoparticles for glaucoma therapy. Small. 2018;14:e1803239. doi: 10.1002/smll.201803239. [DOI] [PMC free article] [PubMed] [Google Scholar]