Significance
Anxiety disorders and obsessive-compulsive disorder (OCD) are highly prevalent and debilitating psychiatric disorders commonly co-occurring in our stressful modern life. Yet the common effective therapeutic target is still unknown. Here we report that activation of the histaminergic afferent system, particularly the histamine H3 receptor, in the nucleus accumbens (NAc) core, a vital node in the limbic loop, inhibits glutamatergic synaptic transmission in the circuit from the prelimbic prefrontal cortex (PrL) to NAc and improves both anxiety- and obsessive-compulsive-like behaviors induced by restraint stress. Our results define a common glutamatergic PrL–NAc circuit involved in both anxiety- and obsessive-compulsive-like behaviors modulated by the H3 presynaptic heteroreceptor and pave a path for developing potential strategies for clinical treatment of anxiety and OCD.
Keywords: nucleus accumbens, prelimbic prefrontal cortex, histamine H3 receptor, anxiety, OCD
Abstract
Anxiety commonly co‐occurs with obsessive-compulsive disorder (OCD). Both of them are closely related to stress. However, the shared neurobiological substrates and therapeutic targets remain unclear. Here we report an amelioration of both anxiety and OCD via the histamine presynaptic H3 heteroreceptor on glutamatergic afferent terminals from the prelimbic prefrontal cortex (PrL) to the nucleus accumbens (NAc) core, a vital node in the limbic loop. The NAc core receives direct hypothalamic histaminergic projections, and optogenetic activation of hypothalamic NAc core histaminergic afferents selectively suppresses glutamatergic rather than GABAergic synaptic transmission in the NAc core via the H3 receptor and thus produces an anxiolytic effect and improves anxiety- and obsessive-compulsive-like behaviors induced by restraint stress. Although the H3 receptor is expressed in glutamatergic afferent terminals from the PrL, basolateral amygdala (BLA), and ventral hippocampus (vHipp), rather than the thalamus, only the PrL– and not BLA– and vHipp–NAc core glutamatergic pathways among the glutamatergic afferent inputs to the NAc core is responsible for co-occurrence of anxiety- and obsessive-compulsive-like behaviors. Furthermore, activation of the H3 receptor ameliorates anxiety and obsessive-compulsive-like behaviors induced by optogenetic excitation of the PrL–NAc glutamatergic afferents. These results demonstrate a common mechanism regulating anxiety- and obsessive-compulsive-like behaviors and provide insight into the clinical treatment strategy for OCD with comorbid anxiety by targeting the histamine H3 receptor in the NAc core.
Anxiety disorders and obsessive-compulsive disorder (OCD) are disabling psychiatric conditions and the major contributors to global burden of nonfatal illness (1). OCD is characterized by recurrent thoughts (obsessions) and/or repetitive behaviors (compulsions) that are aimed at reducing the anxiety caused by obsessions (2, 3), indicating a close correlation between anxiety and OCD. Indeed, anxiety disorders have been reported epidemiologically as the most frequent comorbid conditions with OCD (3, 4). Therefore, common pathologies may be present in anxiety disorders and OCD, and elucidation of the shared neural substrates will lead to greater insight into their pathophysiology and treatment.
The nucleus accumbens (NAc) is a main component of the ventral striatum and a pivotal node in limbic basal ganglia loop, whose dysfunction may result in psychiatric diseases such as anxiety and OCD (5, 6). Accumulating experimental and clinical evidence indicates that the NAc, particularly the core compartment, holds a key position in motivation, emotion, and cognition and is strongly implicated in the psychopathology and treatment of anxiety and OCD. It has been reported that trait anxiety and OCD risk are positively correlated with the volume of NAc (7, 8). Functional neuroimaging reveals that the NAc activation correlates positively with the severity of human anxiety and obsessive-compulsive symptoms in OCD patients (9, 10). More importantly, deep brain stimulation (DBS) targeting the NAc core has been found to improve obsessive-compulsive symptoms and decrease ratings of anxiety in patients suffering from treatment-resistant OCD or depression (11, 12). Therefore, NAc core may be a potential common neural substrate for the clinical and neuropathological overlap between anxiety and OCD.
The NAc core receives dense glutamatergic projections from the limbic system, including the prefrontal cortex, basolateral amygdala (BLA), and ventral hippocampus (vHipp), and integrates cognitive and affective information to instigate motivational approach behaviors (13, 14). In addition, the NAc core is regulated by various neuromodulators, such as orexin, serotonin, and histamine, from several brain regions (15–17). Among them, central histamine is synthesized and released by the histaminergic neurons restrictedly concentrated in the tuberomammillary nucleus (TMN) of the hypothalamus and serves as a general modulator for whole-brain activity via the mediation of histamine H1 to H4 receptors (18, 19). Accordingly, the aberrant histamine signaling is closely associated with sleep, motor, cognitive, and psychiatric conditions (18, 20, 21). In the clinic, drugs targeting the presynaptic H3 receptor have been used for prescribed treatment of various psychiatric and neurologic disorders (22). Interestingly, a high density of the H3 receptor has been found in NAc (23, 24). Therefore, in the present study, we create a transgenic rat strain expressing Cre recombinase in histidine decarboxylase (HDC, the histamine-synthesizing enzyme) neurons and employ anterograde axonal tract tracings, whole-cell patch clamp recordings, optogenetic and chemogenetic manipulation, and behavioral tests to explore the role of hypothalamic histaminergic afferents and the H3 receptor in the NAc core in regulation of anxiety and obsessive-compulsive-like behaviors. We find that optogenetic activation of hypothalamic TMN–NAc core histaminergic projections produces an anxiolytic effect and ameliorates obsessive-compulsive-like behaviors induced by restraint stress, which is due to H3 receptor–mediated suppression of glutamatergic transmission in a common prelimbic prefrontal cortex (PrL)–NAc core pathway.
Results
Histaminergic Afferents in the NAc Core and Involvement of Histamine H3 Receptor in Anxiety-Related Behaviors.
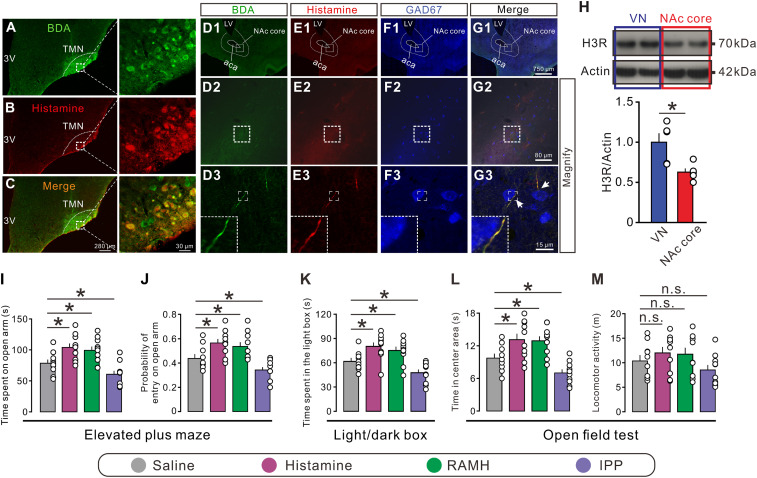
To determine whether the hypothalamic histaminergic neurons directly project to the NAc core, we delivered anterograde tracer BDA by iontophoresis into the hypothalamic TMN, the sole origin of the central histaminergic system (Fig. 1 A–C). As shown in Fig. 1D, 1 to G, 3, BDA/histamine double-labeled fibers were observed in the NAc core, which possessed prominent varicosities and passed around GAD67-labeled NAc core neurons. The result indicates a possible modulation of histamine released from varicosities of hypothalamic histaminergic afferents on GABAergic principle/projection neurons in the NAc core.
Fig. 1.
Histaminergic afferents in the NAc core and an involvement of the H3 receptor in anxiety-related behaviors. (A–C) Immunostaining micrographs showing the identification of histaminergic neurons with injections of BDA into the TMN. (D1 to G3) Triple immunostaining shows that the anterogradely labeled BDA fibers (green) in the NAc core contain immunoreactivity for histamine (red). Note that these histaminergic fibers pass around GAD67-labeled GABAergic neurons (blue) in the NAc core. LV, lateral ventricle; 3V, third ventricle; aca, anterior commissure, anterior part. (H) Western blot analysis indicates that the histamine H3 receptor is expressed in the NAc core (n = 5). The vestibular nuclei (VN), which have abundant expression of the H3 receptor, were taken as a positive control (n = 5). (I and J) The time and probability of entry into the open arm of rats with bilateral microinjection of saline (n = 10), histamine (n = 10), RAMH (a selective agonist for the H3 receptor, n = 10), and IPP (a selective antagonist for the H3 receptor, n = 10) in the elevated plus maze test. (K) The time spent in the light box of rats with bilateral microinjection of saline (n = 10), histamine (n = 10), RAMH (n = 10), and IPP (n = 10) in the light/dark box test. (L and M) Time in center area and locomotor activity of rats treated by saline (n = 10), histamine (n = 10), RAMH (n = 10), and IPP (n = 10) and in the open field test. Data are shown as means ± SEM; *P < 0.05, n.s., no statistical difference.
Next, we employed Western blot to detect the expression of histamine H3 receptor proteins in the NAc core. We found that the expression of the H3 receptor in the NAc core was only slightly less than that in the vestibular nuclei (Fig. 1H), where the H3 receptor is expressed very highly (24), indicating a high expression of the H3 receptor in the NAc core. Furthermore, bilateral microinjection of histamine into the NAc core significantly increased the time spent in the open arm and the probability of open-arm entry in the elevated plus maze test (Fig. 1 I and J). In addition, histamine remarkably increased the time spent in both the light compartment of the light/dark box (Fig. 1K) and the center square in an open field (Fig. 1L). Microinjection of the selective H3 receptor agonist RAMH into the bilateral NAc cores mimicked the behavioral effects of histamine, whereas the selective H3 receptor antagonist IPP produced the opposite effects (Fig. 1 I–L). Moreover, there was no significant difference between treatments on the locomotor activity (Fig. 1M). Thus, the results indicate that H3 receptor-mediated histamine signaling in the NAc core may hold a key position in anxiolytic-like response. The reconstructed microinjection sites are presented in SI Appendix, Fig. S1.
Optogenetic Activation of TMN–NAc Core Histaminergic Projections Improves the Anxiogenic and Obsessive-Compulsive-like Behaviors Induced by Restraint Stress via the H3 Receptor.
Stress is an important risk factor for the development of anxiety (25, 26) and OCD (27, 28). As shown in SI Appendix, Fig. S2A, rats were exposed to an acute restraint stress before elevated plus maze or marble-burying test. We found that acute restraint stress resulted in a significant decrease in open-arm time and entries in the elevated plus maze (SI Appendix, Fig. S2 B–D) and a remarkable increase in grooming, digging, and marble-burying behaviors (SI Appendix, Fig. S2 E–G), indicating anxiogenic and obsessive-compulsive-like phenotypes induced by acute restraint stress.
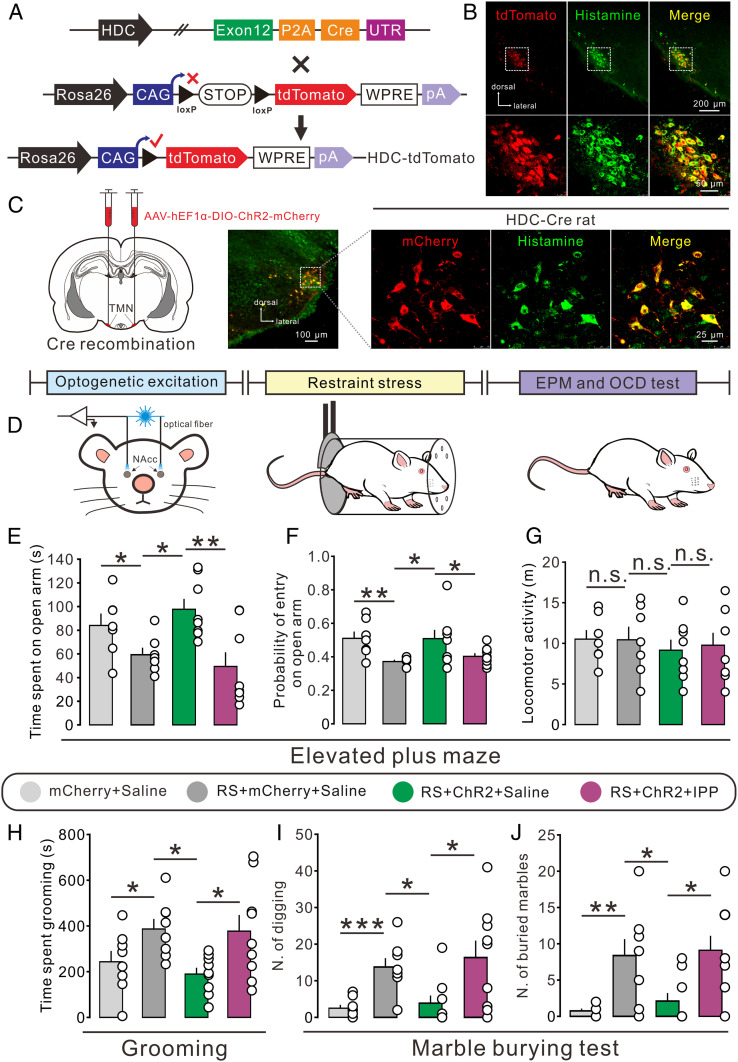
To determine the role of histaminergic afferent inputs in the NAc core in the stress-induced anxiogenic and obsessive-compulsive-like behaviors, we generated a transgenic rat line expressing Cre recombinase driven by the promoter of HDC, the primary enzyme responsible for histamine synthesis. Genotyping and correct recombination were confirmed by PCR and Southern blot analysis (SI Appendix, Fig. S3). Moreover, this HDC-Cre rat line was validated by crossing with a Rosa26-tdTomato reporter strain (Fig. 2A). As shown in Fig. 2B, tdTomato-positive neurons were distributed restrictedly to the hypothalamic TMN and colocalized with the histamine-labeled neurons in the HDC-Cre::tdTomato rats, indicating that the expression of Cre is highly specific to central histaminergic neurons. Next, the TMN histaminergic neurons of HDC-Cre rats were selectively transduced with ChR2-mCherry to examine the effect of optogenetic excitation of TMN–NAc histaminergic projections on anxiety- and obsessive-compulsive-like behaviors of restraint-stressed animals (Fig. 2 C and D). As shown in Fig. 2 E–G, optogenetic activation of TMN–NAc core histaminergic projections significantly increased both time in and entries into the open arm but did not influence the locomotor activity, indicating an anxiolytic effect of NAc core histaminergic afferent inputs in stress-induced anxiety-like rats. Moreover, photoactivation of NAc core histaminergic inputs in restraint-stressed rats remarkably decreased the time spent grooming (Fig. 2H), the number of digging bouts (Fig. 2I), and the number of buried marbles (Fig. 2J), suggesting an alleviation effect of activation of NAc core histaminergic afferents on obsessive-compulsive-like behaviors.
Fig. 2.
Optogenetic activation of hypothalamic TMN–NAc core histaminergic projections improves the anxiogenic and obsessive-compulsive-like behaviors induced by restraint stress. (A) Schematic of generation of HDC-Cre::tdTomato rats. (B) Confocal image of tdTomato (red) and immunofluorescent labeling for histamine (green) in the TMN in HDC-Cre::tdTomato rats. (C) The selective expression of ChR2-mCherry (red) in TMN histaminergic neurons (green) in HDC-ChR2 rats. (D) Scheme of the experimental paradigm showing the restraint stress-induced anxiety- and obsessive-compulsive-like behaviors in HDC-ChR2 rats that underwent optogenetic activation. (E–G) The time in and probability of entry into the open arm and locomotor activity of control (n = 7) or restraint-stressed (RS, n = 7) HDC-mCherry rats with bilateral microinjection of saline, as well as stressed HDC-ChR2 rats with bilateral microinjection of saline (n = 8) or IPP (n = 8). (H–J) The time spent grooming and the number of digging bouts and buried marbles of control (n = 8) or stressed (n = 8) HDC-mCherry rats with bilateral microinjection of saline, as well as stressed HDC-ChR2 rats with bilateral microinjection of saline (n = 9) or IPP (n = 9). Data are shown as means ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, n.s., no statistical difference.
We further assessed the contribution of the histamine H3 receptor to the behavioral effect of activation of NAc core histaminergic afferents. As shown in SI Appendix, Fig. S2, microinjection of RAMH or histamine into the NAc core significantly reduced the stress-induced anxiety‐like and obsessive-compulsive-like behaviors, which is consistent with the effect of optogenetic activation of NAc core histaminergic afferents. Moreover, microinjection of IPP into the NAc core totally blocked the amelioration effect of photoactivation of TMN–NAc core histaminergic projections on anxiogenic (Fig. 2 E–G) and obsessive-compulsive-like behaviors (Fig. 2 H–J) in restraint-stressed animals. Taken together, these results reveal that selective activation of the hypothalamic TMN–NAc core histaminergic pathway improves the anxiogenic and obsessive-compulsive-like behaviors induced by restraint stress via the H3 receptor.
Histamine H3 Heteroreceptor Activation Selectively Inhibits Glutamatergic Rather Than GABAergic Synaptic Transmission in the NAc Core.
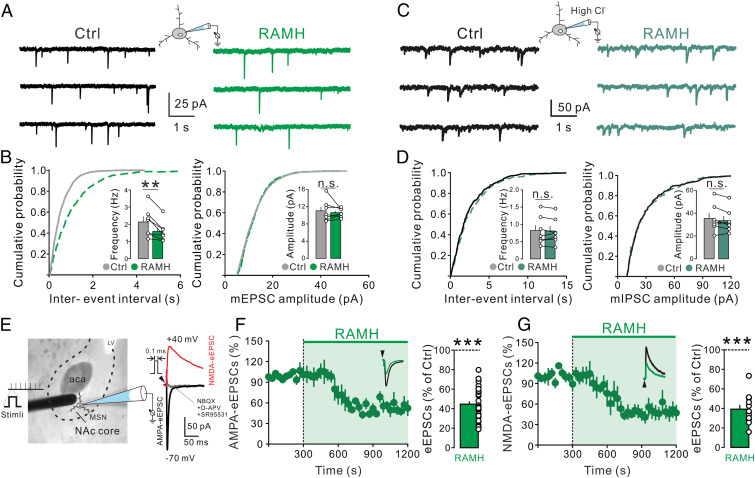
Since glutamate signaling in NAc is strongly involved in the pathogenesis and treatment of anxiety (29, 30) and OCD (31, 32), we further investigated the effect of H3 receptor activation on glutamatergic synaptic transmission on the GABAergic medium spiny neurons, the principle neurons of the NAc core, by whole-cell patch clamp recording. Bath application of RAMH (3 μM) significantly decreased the frequency of the glutamatergic miniature excitatory postsynaptic currents (mEPSCs) (Fig. 3 A and B, Left), which were totally blocked by NBQX and d-APV, antagonists for AMPA and NMDA receptors, respectively. In contrast, no significant difference was detected in the amplitude distributions of mEPSCs (Fig. 3 A and B, Right). We also observed that RAMH (3 µM) had no significant effect on the frequency and amplitude of miniature inhibitory postsynaptic currents (mIPSCs) (Fig. 3 C and D), indicating a selective inhibition of glutamatergic instead of GABAergic transmission in the NAc core by activation of H3 receptor.
Fig. 3.
H3 receptor activation inhibits the glutamatergic synaptic transmission in the NAc core neurons. (A) The mEPSCs recorded in a NAc core neuron before and during the application of H3 selective agonist RAMH (3 μM) in the presence of TTX and SR95531. (B) Plots of the cumulative distribution of the interevent interval and amplitude for the neuron illustrated in A showing RAMH decreased the frequency of mEPSC rather than the amplitude. Inset summary graphs show the average mEPSC frequency and amplitude in the absence and presence of RAMH (n = 7). (C) Raw current traces showing mIPSCs recorded in a NAc core neuron before and during the application of H3 agonist RAMH (3 µM) in the presence of TTX, NBQX, and d-APV. (D) Interevent interval and amplitude distribution for the neuron illustrated in C showing RAMH did not affect the mIPSC frequency or amplitude. Inset summary graphs show the average mIPSC frequency and amplitude in the absence and presence of RAMH (n = 7). (E) The placement of a stimulating electrode and a recorded NAc core neuron. Raw traces show AMPA or NMDA receptor-mediated eEPSCs recorded from the NAc core neurons. (F and G) Bath application of RAMH (3 μM) decreased the amplitude of AMPA (F, n = 29) and NMDA eEPSC (G, n = 12). Scatterplots and bar graphs show the effect of RAMH on AMPA and NMDA eEPSCs in the NAc core neurons. Data are shown as means ± SEM; **P < 0.01, ***P < 0.001, n.s., no statistical difference.
Furthermore, we evoked glutamatergic EPSCs (eEPSCs) in the NAc core neurons by local electrical stimulation and found that bath application of RAMH (3 μM) consistently inhibited the amplitude of AMPA eEPSCs and NMDA eEPSCs (Fig. 3 E–G). These results indicate that H3 receptor activation may suppress both AMPA- and NMDA-mediated glutamatergic synaptic transmission in NAc core neurons.
Next, we determined the global effect of histamine on glutamatergic synaptic transmission and the underlying receptor mechanism. In the 46 tested NAc core neurons in this study, 3 to 30 µM histamine, 100 μM 2-PyEA (a selective agonist for the H1 receptor), 100 μM dimaprit (a selective agonist for the H2 receptor), and 100 µM 4-methylhistamine (a selective agonist for the H4 receptor) did not induce any direct inward/outward currents (SI Appendix, Fig. S4), indicating that histamine has no direct postsynaptic action on the NAc core neurons through the mediation of H1, H2, and H4 receptors. Similar to RAMH, bath application of histamine (3 µM) significantly decreased the average frequency of mEPSCs (SI Appendix, Fig. S5, Left), but not the average amplitude (SI Appendix, Fig. S5, Right). In addition, histamine did not affect the average frequency and amplitude of mIPSCs (SI Appendix, Fig. S6). These results suggest that histamine may selectively inhibit glutamatergic transmission in the NAc core via the presynaptic H3 receptor.
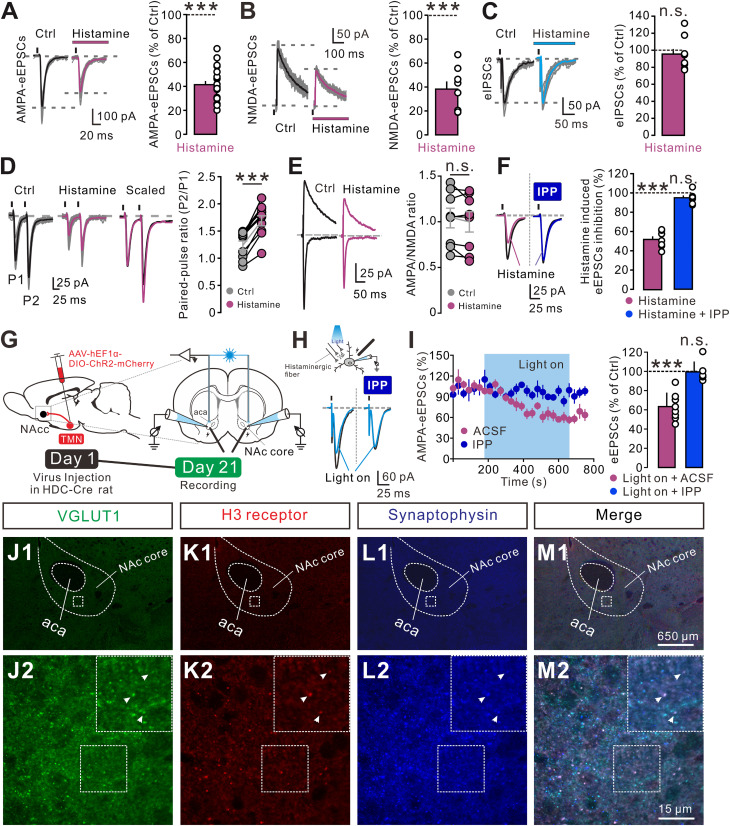
Moreover, histamine (3 µM) significantly decreased the AMPA eEPSCs and NMDA eEPSCs (Fig. 4 A and B), rather than the eIPSCs (Fig. 4C). We further examined the effect of histamine on the paired pulse ratio (PPR), which reflects the changes in neurotransmitter release at central synapses (13). As shown in Fig. 4D, histamine (3 µM) decreased the amplitudes of both eEPSCs. However, histamine significantly enhanced the paired pulse facilitation (PPF) of eEPSCs (Fig. 4D), indicating a reduction in the probability of presynaptic glutamate release (33). To further exclude the possibility of a histamine-induced reduction in the sensitivity and/or the number of postsynaptic ionotropic glutamate receptors, we measured the AMPA/NMDA ratio, a measure of the relative expression of the AMPA receptor and the NMDA receptor at central synapses (34). Although histamine (3 µM) reduced the amplitude of both AMPA and NMDA eEPSCs, it did not affect the AMPA/NMDA ratio (Fig. 4E). The unchanged AMPA/NMDA ratio, together with the increase in PPF, confirms that histamine inhibits the glutamatergic transmission in the NAc core by a presynaptic mechanism. Furthermore, bath application of IPP (10 µM) totally blocked the histamine-induced inhibition on eEPSCs (Fig. 4F), indicating the mediation of the H3 receptor. Finally, HDC-ChR2 rats were used to confirm the regulation of NAc core histaminergic afferent inputs on glutamatergic transmission and the underlying receptor mechanism (Fig. 4G). As shown in Fig. 4 H and I, optogenetic activation of histaminergic terminals in the NAc core inhibited eEPSCs, and IPP (10 µM) totally blocked the inhibition. Taken together, these results demonstrate that histamine and histaminergic afferent fibers selectively inhibit the glutamatergic synaptic transmission on NAc core neurons through the presynaptic H3 receptor.
Fig. 4.
Histamine selectively inhibits glutamatergic transmission by the presynaptic H3 receptor. (A–C) Histamine (3 µM) decreased the amplitude of AMPA (A, n = 21) and NMDA eEPSCs (B, n = 8), rather than eIPSCs (C, n = 9) in the NAc core neurons. (D) Histamine-induced decrease in amplitude of the eEPSCs is associated with an increase in the PPR (n = 10). (Left) Raw traces showing eEPSCs (average of 30 consecutive trials) evoked by paired stimuli (50 ms interval) in the absence and presence of histamine (3 µM). Superimposed and scaled eEPSC traces obtained in the absence (black) and presence (magenta) of histamine, in which the first eEPSC during histamine application is scaled to the amplitude of the first eEPSC collected in the control condition. (Right) The plots of PPR for each of the experiments in the absence and presence of histamine. (E) AMPA/NMDA ratio recorded at −70 and +40 mV in the absence and presence of histamine (3 µM) as a function of synaptic inputs in the NAc core neurons. The plots of the averaged AMPA/NMDA ratio for each of the experiments in the absence and presence of histamine (n = 8). (F) The inhibitory effect of histamine (n = 7) on eEPSCs was blocked by the selective H3 receptor antagonist IPP (n = 7). (G) Surgical manipulation and experimental schematic for slice optogenetic stimulation of histaminergic fibers in the NAc core. (H) The inhibitory effect of optogenetic activation of histaminergic afferents on the eEPSCs was blocked by the selective H3 receptor antagonist IPP. (I) Group data. (J–M) Triple immunofluorescent labeling of VGLUT1 (green), the H3 receptor (red), and synaptophysin (a presynaptic vesicle protein, blue) in the NAc core, indicating that the H3 receptor and VGLUT1 are colocalized in the presynaptic terminals of the NAc core. Arrows indicate the apposition of the H3 receptor, synaptophysin, and VGLUT1. Data shown are means ± SEM; ***P < 0.001, n.s., no statistical difference.
To confirm the above electrophysiological results, we employed immunostainings to examine the expression of the H3 receptor on glutamatergic presynaptic terminals in the NAc core. The NAc core is well known to receive excitatory glutamatergic inputs primarily from the PrL, BLA, vHipp, and thalamus (14). Since glutamatergic terminals from the prefrontal cortex, amygdala, and hippocampus have been reported to express VGLUT1 exclusively while terminals from the thalamus express VGLUT2 or both VGLUT2 and VGLUT1 (35), we assessed the coexpression of the H3 receptor and VGLUT1/VGLUT2. We found that the H3 receptor was colocalized with VGLUT1 and synaptophysin (a presynaptic vesicle protein) (Fig. 4 J–M) but was not coexpressed with VGLUT2 (SI Appendix, Fig. S7), indicating that the H3 receptor may be localized in the glutamatergic presynaptic terminals from the cortex, amygdala, and hippocampus rather than thalamus as a heteroreceptor in the NAc core.
Histamine H3 Receptor Activation Alleviates the Anxiogenic and Obsessive-Compulsive-Like Behaviors Induced by Optogenetic Activation of the PrL–NAc Glutamatergic Pathway.
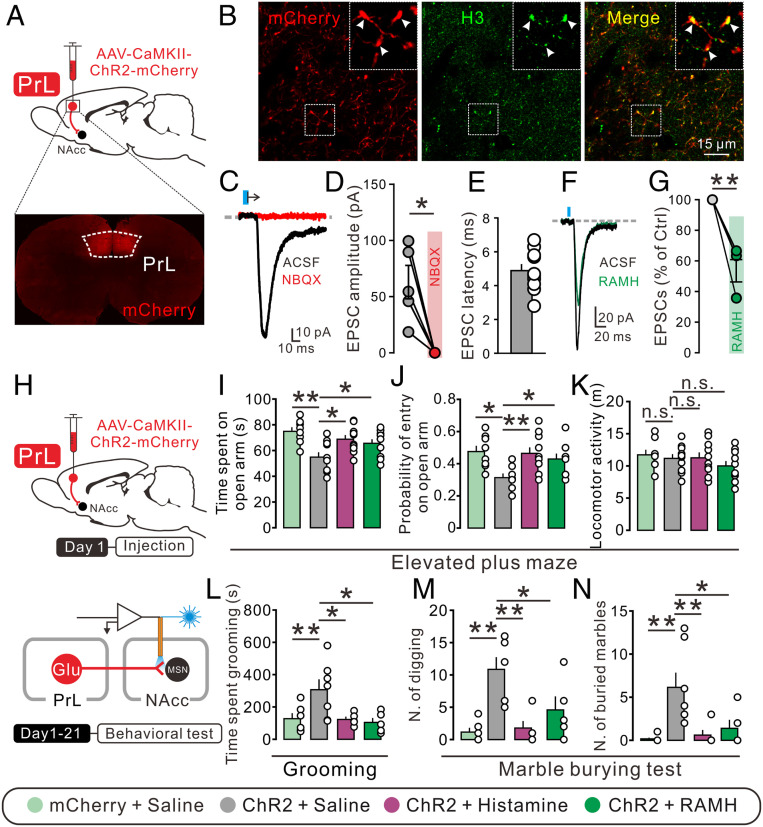
To determine the functional role of glutamatergic inputs from different sources in the NAc core on anxiety and obsessive-compulsive-like behaviors, we optogenetically manipulated the PrL–NAc, BLA–NAc, and vHipp–NAc glutamatergic pathways. The bilateral PrL, BLA, and vHipp were separately transduced with ChR2-mCherry under the control of the CaMKIIα promoter in order to target glutamatergic neurons in these brain regions (Fig. 5A and SI Appendix, Figs. S8A and S9A). It can be seen that the H3 receptor was expressed and localized on the mCherry-positive glutamatergic afferent boutons from the PrL, BLA, and vHipp (Fig. 5B and SI Appendix, Figs. S8B and S9B), and the light-evoked EPSCs on NAc core neurons were totally blocked by the AMPA receptor antagonist NBQX with a latency of 4.89 ± 0.38, 4.62 ± 0.34, and 4.43 ± 0.28 ms for the inputs from PrL, BLA, and vHipp, respectively (Fig. 5 C–E and SI Appendix, Figs. S8 C–E and S9 C–E), all of which is in the range of monosynaptic events. Moreover, we examined the effect of H3 receptor activation on the PrL–, BLA–, and vHipp–NAc core glutamatergic synaptic transmission and found that bath application of RAMH (3 μM) inhibited the amplitude of light-evoked EPSCs at all of the recorded PrL–, BLA–, and vHipp–NAc core glutamatergic synapses (Fig. 5 F and G and SI Appendix, Figs. S8 F and G and S9 F and G).
Fig. 5.
Histamine alleviates the anxiogenic and obsessive-compulsive-like behaviors induced by optogenetic activation of the PrL–NAc pathway. (A) Schematics of the optogenetic manipulation of the PrL–NAc glutamatergic pathway and confocal image of ChR2-mcherry expression in the PrL. (B) ChR2-mCherry (red) and H3 receptor (green) expression in the NAc core. (C and D) Bath application of NBQX totally blocked light-evoked EPSCs (n = 5). (E) The latency between light and EPSC onset of the NAc core neurons recorded (n = 10). (F and G) Bath application of RAMH (3 μM) decreased the amplitude of light-evoked EPSCs. (H) Experimental procedure for behavioral test with photoactivation of PrL–NAc glutamatergic terminals. (I–K) The time spent in the open arm, probability of entry into open arms, and locomotor activity of mCherry rats with bilateral microinjection of saline (n = 8), as well as ChR2 rats with bilateral microinjection of saline (n = 10), histamine (n = 10), and RAMH (n = 10). (L–N) The time spent grooming and the number of digging bouts and buried marbles of mCherry rats with bilateral microinjection of saline (n = 6), as well as ChR2 rats with bilateral microinjection of saline (n = 7), histamine (n = 5), and RAMH (n = 5). Data are shown as means ± SEM; *P < 0.05, **P < 0.01, n.s., no statistical difference.
We further observed the behavioral consequences of optogenetic excitation and chemogenetic inhibition of PrL–, BLA–, and vHipp–NAc core glutamatergic pathways. As shown in Fig. 5 I–K, photostimulation of PrL glutamatergic terminals within the NAc core decreased the open-arm time and entries in the elevated plus maze and increased the grooming (Fig. 5L), digging (Fig. 5M), and marble-burying behaviors (Fig. 5N). Moreover, chemogenetic inhibition of the PrL–NAc glutamatergic pathway significantly prevented the anxiogenic and obsessive-compulsive-like behaviors induced by acute restraint stress (SI Appendix, Fig. S10). These results suggest that the activation of the PrL–NAc glutamatergic pathway may contribute to the development of both anxiety- and obsessive-compulsive-like behaviors. Notably, microinjection of histamine or RAMH into the NAc core reversed the optogenetic excitation-induced anxiogenic-like behaviors (Fig. 5 I–K). Furthermore, histamine or RAMH also rescued the enhancement of grooming, digging, and marble burying behaviors induced by optogenetic excitation (Fig. 5 L–N). However, optogenetic activation of BLA–NAc core glutamatergic terminals did not alter anxiety-like behaviors (SI Appendix, Fig. S8 H–J) but increased obsessive-compulsive-like behaviors, which was rescued by RAMH (SI Appendix, Fig. S8 K–M). Chemogenetic suppression of the BLA–NAc core glutamatergic pathway alleviated obsessive-compulsive-like phenotypes in restraint-stressed animals (SI Appendix, Fig. S11). Photostimulation of vHipp-NAc core glutamatergic projections had no effect on both anxiety-like (SI Appendix, Fig. S9 H–J) and obsessive-compulsive-like behaviors (SI Appendix, Fig. S9 K–M). Taken together, all these results demonstrate that the PrL–NAc core rather than BLA– and vHipp–NAc core glutamatergic pathway may be responsible for the co-occurrence of anxiety and OCD, and histamine and histaminergic inputs may relieve both anxiety- and obsessive-compulsive-like behaviors by suppressing the common PrL–NAc core glutamatergic pathway via the H3 heteroreceptor.
Discussion
Anxiety is a common experience in our daily life and the most frequent comorbid condition with OCD (3, 4). Both of them are known to be stress responsive. Stressful life events often precede the onset of anxiety and OCD (25, 27), and anxiety and obsessive-compulsive symptoms increase at times of stress (26, 28). In the present study, we find that activation of hypothalamic TMN-NAc core histaminergic projections not only produces an anxiolytic effect on normal rats but also improves the anxiety- and obsessive-compulsive-like behaviors induced by acute restraint stress, which is mediated by its inhibition of glutamatergic synaptic transmission of the common PrL–NAc circuitry via the presynaptic H3 heteroreceptor.
The central histaminergic system originating from the hypothalamic TMN has been implicated in emotional and affective disorders. HDC-deficient mice that lack the ability to synthesize histamine show increased measures of anxiety (36), and central administration of histamine or intraperitoneal administration of the histamine precursor, l-histidine, significantly attenuates obsessive-compulsive-like behavior and obliterates the persistent behavior (37), supporting a preventive role for histamine against anxiety and OCD. Intriguingly, combining anterograde tracing with immunostaining, we reveal a direct projection from histaminergic neurons in the hypothalamic TMN to the NAc core. Optogenetic activation of histaminergic afferent inputs in the NAc core in HDC-ChR2 rats remarkably improved anxiety as well as repetitive, compulsive-like grooming, digging, and marble-burying behaviors produced by restraint stress. Together with the clinical reports that several histaminergic agents have been used as antipsychotics (22), we thus suggest that NAc core histaminergic modulation may be implicated in the psychopathology and treatment of comorbidity of anxiety and OCD.
Four histamine receptors, H1 to H4, mediate the physiological and pathological functions of histamine (18, 19). Therefore, we dissected the effect of histamine on NAc neurons and found that histamine had no direct postsynaptic effect on NAc neurons through the mediation of H1, H2, or H4 receptors but selectively inhibited glutamatergic mEPSCs rather than GABAergic mIPSCs by activation of the presynaptic H3 receptor. This result is comparable with the previous finding in the dorsal striatum and hippocampus (38, 39). In fact, a growing body of experimental and clinical evidence has indicated aberrantly elevated glutamatergic signaling in animal models and drug-naïve patients with anxiety and OCD (29, 31). We thus hypothesize that the H3 receptor in the NAc core may serve as a heteroreceptor to modulate glutamatergic transmission and consequently regulate anxiety and obsessive-compulsive-like behaviors. As expected, the suppression effect of excitation of TMN histaminergic afferents on glutamatergic eEPSCs in the NAc core and the consequent improvement of restraint stress-induced anxiety and OCD behaviors were mimicked and totally blocked, respectively, by pharmacological activation and antagonization of H3 receptors in the NAc core, suggesting that the suppression of NAc core glutamatergic transmission via H3 receptors may underlie the therapeutic effects of histamine on anxiety and OCD.
However, the exact glutamatergic circuits contributing to the pathophysiology and treatment of anxiety and OCD remain largely unknown. The NAc core receives dense excitatory glutamatergic afferents from various brain regions (14), including the prefrontal cortex, amygdala, hippocampus, and thalamus, among which the prefrontal cortex has been reported to be involved in the anxiety and checking symptoms of OCD (40–42). The PrL, a subregion in the prefrontal cortex mediating susceptibility to stress (43), sends direct glutamatergic afferent inputs and conveys cognitive and affective information to the NAc core to help it instigate approach behavior toward intense motivations of desire (14) and thus actively participate in strategy abandoning (13), cocaine seeking after extinction (44), and compulsive-like self-administration behaviors (45). Notably, in the present study, although we revealed that the histamine H3 receptor is expressed and localized in the glutamatergic terminals in the NAc core from PrL, BLA, and vHipp, rather than the thalamus, the PrL–NAc core pathway is the only circuitry among the glutamatergic afferent inputs in the NAc core which mediates the co-occurrence of anxiety- and obsessive-compulsive-like behaviors. Although activation of BLA–NAc core glutamatergic projections evoked obsessive-compulsive-like behaviors as previously reported (42), the pathway does not participate in regulating anxiety, and neither does the vHipp–NAc core glutamatergic projections, which may be involved in depressive regulation (46, 47). In this study, microinjection of histamine or RAMH into the NAc core alleviated both anxiety- and obsessive-compulsive-like phenotypes induced by optogenetic activation of the PrL–NAc glutamatergic pathway, and the anti-obsessive-compulsive effect of histamine also involved reducing BLA–NAc glutamatergic transmission. We thus indicate that the histamine H3 receptor in the NAc core may be a common therapeutic target for amelioration of anxiety and obsessive-compulsive-like behaviors.
Effective pharmacological treatment interventions for the comorbidity of anxiety and OCD are still lacking. Here we reveal the PrL–NAc core glutamatergic circuitry as a common pathway for the comorbidity of anxiety disorders and OCD. By activating the presynaptic H3 heteroreceptor localized in the NAc core glutamatergic terminals from the PrL, histaminergic afferent inputs may actively modulate the encoding information by glutamatergic inputs, especially the affective and cognitive signals from the PrL, and effectively regulate anxiogenic and obsessive-compulsive-like behaviors. Intriguingly, the NAc core is one of the most effective DBS targets in the clinic for treatment of anxiety and OCD (11, 12). Considering that our recent study on the mechanism underlying DBS of the subthalamic nucleus revealed that DBS induces an increase in histamine release in the subthalamic nucleus to alleviate Parkinsonian motor deficits (20), we speculate that the DBS–NAc core may also induce histamine release in the NAc core to activate the presynaptic H3 receptor, inhibit glutamatergic afferent inputs from the prefrontal cortex, and subsequently ameliorate anxiety- and obsessive-compulsive-like behaviors. Unlike presynaptic GABA (48, 49), adenosine (48), or serotonin (50) receptors functioning to modulate both excitatory and inhibitory synaptic transmission in NAc, the presynaptic histamine H3 receptor, selectively acting on glutamatergic neurotransmission, may be a better target for the treatment of glutamatergic dysfunction in NAc. Notably, several agonists for the H3 receptor, including RAMH and its prodrugs, have entered clinical trials and proved safe (51, 52). Therefore, developing strategies for targeting the H3 receptor to the NAc, such as ultrasound-/magnetic-responsive nanoparticle-based delivery systems (53, 54) for H3 receptor agonists, may pave a path for clinical treatment of anxiety disorders and OCD.
Materials and Methods
Experimental Animals.
HDC-Cre and wild-type Sprague-Dawley rats were individually housed on a 12 h light/dark cycle with water available ad libitum. All experiments, approved by the Animal Ethical and Welfare Committee of Nanjing University, were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (55). Details of the generation of the HDC-Cre rat line are given in SI Appendix.
Anterograde Tracing.
Anterograde tracer biotinylated dextran amine (BDA) was microelectrophoresed in TMN (A -4.5, L 1.2, and H 9.6) following our previous reports (20, 21, 56). The immunohistochemical experiment was performed 3 wk later to determine the injection site and location of anterograde labeled histaminergic fibers. Details are given in SI Appendix.
Immunohistochemistry.
Details are given in SI Appendix.
Western Blot.
Details are given in SI Appendix.
Stereotaxic Implantation and Microinjection.
Rats were submitted to stereotaxic surgery and bilateral implantation of a guide cannula 2 mm above the NAc core (anteroposterior 1.3, mediolateral 1.5, and dorsoventral 6.5) for microinjection of histamine (0.5 µg), RAMH (a selective H3 receptor agonist, 0.5 µg), IPP (a selective H3 receptor antagonist, 1.5 µg), and saline (0.9% NaCl) before behavioral tests. Details are given in SI Appendix.
Optogenetic and Chemogenetic Manipulation.
For virus injections, 0.5 µL of AAV2/9-hEF1α-DIO-ChR2-mCherry/AAV2/9-hEF1α-DIO-mCherry was bilaterally infused to the hypothalamic TMN (anteroposterior −4.5, mediolateral 1.2, and dorsoventral 9.6); pAAV-CaMKIIα-ChR2-mCherry/pAAV-CaMKIIα-mCherry/AAV2/9-hSyn-DIO-hM4Di-mCherry was infused to the PrL (anteroposterior +3.0, mediolateral 0.7, and dorsoventral 3.3), BLA (A −2.1, L 5.0, and H 8.6), or vHipp (anteroposterior −6.0, L 5.0, and dorsoventral 8.0); and AAV2/2 Retro Plus-hSyn-Cre-EGFP was microinjected into the NAc core (anteroposterior 1.3, mediolateral 1.5, and dorsoventral 6.5). For photostimulation of TMN–, PrL–, BLA–, and vHipp–NAc core afferent terminals, rats received chronically implantable optical fibers (200 µm core; 0.37 numerical aperture) aimed over the NAc core. For photostimulation of the TMN–NAc core histaminergic afferent terminals before the exposure to acute restraint stress, blue light was applied at 10 ms pulse width and a frequency of 10 Hz for 8 min. For photostimulation of the PrL–, BLA–, and vHipp–NAc core glutamatergic afferent terminals during the behavioral tests, blue light with a 5 ms pulse width and a frequency of 20, 20, and 4 Hz, respectively, was delivered. For chemogenetic inhibition, clozapine N-oxide (2 mg/kg) was administered by i.p. injection 5 min before exposure to restraint stress.
Behavioral Tests.
Anxiety-like behaviors were assessed by the elevated plus maze test, light/dark box test, and open field test, whereas obsessive-compulsive-like behaviors were evaluated by the number of marbles buried as well as grooming and digging behaviors in the marble-burying test. Moreover, to determine the impact of stress on anxiety and obsessive-compulsive-like behaviors, rats were exposed to acute restraint stress for 30 min or 1 h before anxiety- and obsessive-compulsive-like behavioral tests. Details are given in SI Appendix.
Patch Clamp Recordings in Brain Slices.
Patch clamp recordings in brain slices were applied to assess the effect of histamine on the GABAergic and glutamatergic neurotransmission in the NAc core neurons and the underlying receptor mechanisms. To record eEPSCs/eIPSCs, a concentric bipolar stimulating electrode was placed about 100 μm rostral to the recording electrode and at the same depth as the recorded neuron. For optogenetic stimulation, 470 nm light pulses were applied with a CoolLED system attached to the upright microscope. The photostimulation protocol was 10 ms pulses, three pulses in 3 s, repeated for 60 trials every 5 s and 5 ms pulses, one pulse in 30 s, repeated for 10 trials for photostimulation of the NAc core histaminergic and glutamatergic terminals, respectively. Details are given in SI Appendix.
Statistical Analysis.
All data were analyzed with SPSS 17.0 and are presented as mean ± SEM. Student’s t test and one-way ANOVA were employed for statistical analysis, and Newman–Keuls post hoc testing was used to further determine the differences between groups. P values < 0.05 were considered to be significant. The detailed statistical results for each experiment are summarized in SI Appendix, Table S1.
Supplementary Material
Acknowledgments
This work was supported by Grants 32030044, 31961160724, 81671107, 81971263, 31600834, and 31500848 from the National Natural Science Foundation of China and Grants BK20190008 and BK20180057 from the Natural Science Foundation of Jiangsu Province, China.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2008456117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Grant J. E., Clinical practice: Obsessive-compulsive disorder. N. Engl. J. Med. 371, 646–653 (2014). [DOI] [PubMed] [Google Scholar]
- 2.de Vries F. E., et al. , Compensatory frontoparietal activity during working memory: An endophenotype of obsessive-compulsive disorder. Biol. Psychiatry 76, 878–887 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Nemeroff C. B., et al. , DSM-5: A collection of psychiatrist views on the changes, controversies, and future directions. BMC Med. 11, 202 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruscio A. M., Stein D. J., Chiu W. T., Kessler R. C., The epidemiology of obsessive-compulsive disorder in the national comorbidity survey replication. Mol. Psychiatry 15, 53–63 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunaydin L. A., Kreitzer A. C., Cortico-basal ganglia circuit function in psychiatric disease. Annu. Rev. Physiol. 78, 327–350 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Corbit V. L., Gittis A. H., Ahmari S. E., A model of restraint: Nucleus accumbens fast-spiking interneurons inhibit unwanted actions. Biol. Psychiatry 86, 804–806 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Kühn S., Schubert F., Gallinat J., Structural correlates of trait anxiety: Reduced thickness in medial orbitofrontal cortex accompanied by volume increase in nucleus accumbens. J. Affect. Disord. 134, 315–319 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Hibar D. P. et al.; Enhancing Neuro Imaging Genetics through Meta Analysis (ENIGMA) Consortium and International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) , Significant concordance of genetic variation that increases both the risk for obsessive-compulsive disorder and the volumes of the nucleus accumbens and putamen. Br. J. Psychiatry 213, 430–436 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lázaro L., et al. , Cerebral activation in children and adolescents with obsessive-compulsive disorder before and after treatment: A functional MRI study. J. Psychiatr. Res. 42, 1051–1059 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Levita L., Hoskin R., Champi S., Avoidance of harm and anxiety: A role for the nucleus accumbens. Neuroimage 62, 189–198 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Bewernick B. H., et al. , Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol. Psychiatry 67, 110–116 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Figee M., et al. , Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat. Neurosci. 16, 386–387 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Cui Q., et al. , Dopamine receptors mediate strategy abandoning via modulation of a specific prelimbic cortex-nucleus accumbens pathway in mice. Proc. Natl. Acad. Sci. U.S.A. 115, E4890–E4899 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floresco S. B., The nucleus accumbens: An interface between cognition, emotion, and action. Annu. Rev. Psychol. 66, 25–52 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Dölen G., Darvishzadeh A., Huang K. W., Malenka R. C., Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellenbroek B. A., Ghiabi B., The other side of the histamine H3 receptor. Trends Neurosci. 37, 191–199 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Nevárez N., de Lecea L., Recent advances in understanding the roles of hypocretin/orexin in arousal, affect, and motivation. F1000Res. 7, F1000 Faculty Rev 1421 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panula P., Nuutinen S., The histaminergic network in the brain: Basic organization and role in disease. Nat. Rev. Neurosci. 14, 472–487 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Obara I., Telezhkin V., Alrashdi I., Chazot P. L., Histamine, histamine receptors, and neuropathic pain relief. Br. J. Pharmacol. 177, 580–599 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang Q. X., et al. , Regularizing firing patterns of rat subthalamic neurons ameliorates Parkinsonian motor deficits. J. Clin. Invest. 128, 5413–5427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z. P., et al. , Histamine H1 receptor contributes to vestibular compensation. J. Neurosci. 39, 420–433 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passani M. B., Blandina P., Histamine receptors in the CNS as targets for therapeutic intervention. Trends Pharmacol. Sci. 32, 242–249 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Lethbridge N. L., Chazot P. L., Ligand autoradiographical quantification of histamine H3 receptor in human dementia with Lewy bodies. Pharmacol. Res. 113, 245–256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillot C., et al. , A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience 114, 173–193 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Peral P., et al. , Risk factors for the onset of panic and generalised anxiety disorders in the general adult population: A systematic review of cohort studies. J. Affect. Disord. 168, 337–348 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Platt R., Williams S. R., Ginsburg G. S., Stressful life events and child anxiety: Examining parent and child mediators. Child Psychiatry Hum. Dev. 47, 23–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lochner C., et al. , Childhood trauma in obsessive-compulsive disorder, trichotillomania, and controls. Depress. Anxiety 15, 66–68 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Findley D. B., et al. , Development of the Yale Children’s Global Stress Index (YCGSI) and its application in children and adolescents with Tourette’s syndrome and obsessive-compulsive disorder. J. Am. Acad. Child Adolesc. Psychiatry 42, 450–457 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Filiou M. D., et al. , Proteomics and metabolomics analysis of a trait anxiety mouse model reveals divergent mitochondrial pathways. Biol. Psychiatry 70, 1074–1082 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Esterlis I., Holmes S. E., Sharma P., Krystal J. H., DeLorenzo C., Metabotropic glutamatergic receptor 5 and stress disorders: Knowledge gained from receptor imaging studies. Biol. Psychiatry 84, 95–105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakrabarty K., Bhattacharyya S., Christopher R., Khanna S., Glutamatergic dysfunction in OCD. Neuropsychopharmacology 30, 1735–1740 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Pittenger C., Bloch M. H., Williams K., Glutamate abnormalities in obsessive compulsive disorder: Neurobiology, pathophysiology, and treatment. Pharmacol. Ther. 132, 314–332 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurent A., et al. , Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. J. Neurosci. 22, 886–900 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kauer J. A., Malenka R. C., Synaptic plasticity and addiction. Nat. Rev. Neurosci. 8, 844–858 (2007). [DOI] [PubMed] [Google Scholar]
- 35.El Mestikawy S., Wallén-Mackenzie A., Fortin G. M., Descarries L., Trudeau L. E., From glutamate co-release to vesicular synergy: Vesicular glutamate transporters. Nat. Rev. Neurosci. 12, 204–216 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Schneider E. H., Neumann D., Seifert R., Modulation of behavior by the histaminergic system: Lessons from HDC-, H3R- and H4R-deficient mice. Neurosci. Biobehav. Rev. 47, 101–121 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Verma L., Agrawal D., Jain N. S., Enhanced central histaminergic transmission attenuates compulsive-like behavior in mice. Neuropharmacology 138, 106–117 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Ellender T. J., Huerta-Ocampo I., Deisseroth K., Capogna M., Bolam J. P., Differential modulation of excitatory and inhibitory striatal synaptic transmission by histamine. J. Neurosci. 31, 15340–15351 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown R. E., Reymann K. G., Histamine H3 receptor-mediated depression of synaptic transmission in the dentate gyrus of the rat in vitro. J. Physiol. 496, 175–184 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuesta S., Funes A., Pacchioni A. M., Social isolation in male rats during adolescence inhibits the Wnt/β-catenin pathway in the prefrontal cortex and enhances anxiety and cocaine-induced plasticity in adulthood. Neurosci. Bull. 36, 611–624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padilla-Coreano N., et al. , Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron 89, 857–866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun T., et al. , Basolateral amygdala input to the medial prefrontal cortex controls obsessive-compulsive disorder-like checking behavior. Proc. Natl. Acad. Sci. U.S.A. 116, 3799–3804 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tovote P., Fadok J. P., Lüthi A., Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Ma Y. Y., et al. , Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83, 1453–1467 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu Y., et al. , Compulsive drug use is associated with imbalance of orbitofrontal- and prelimbic-striatal circuits in punishment-resistant individuals. Proc. Natl. Acad. Sci. U.S.A. 116, 9066–9071 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bagot R. C., et al. , Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun. 6, 7062 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen C. J., et al. , Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat. Med. 25, 337–349 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Uchimura N., North R. A., Baclofen and adenosine inhibit synaptic potentials mediated by gamma-aminobutyric acid and glutamate release in rat nucleus accumbens. J. Pharmacol. Exp. Ther. 258, 663–668 (1991). [PubMed] [Google Scholar]
- 49.Manz K. M., Baxley A. G., Zurawski Z., Hamm H. E., Grueter B. A., Heterosynaptic GABAB receptor function within feedforward microcircuits gates glutamatergic transmission in the nucleus accumbens core. J. Neurosci. 39, 9277–9293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mocci G., Jiménez-Sánchez L., Adell A., Cortés R., Artigas F., Expression of 5-HT2A receptors in prefrontal cortex pyramidal neurons projecting to nucleus accumbens. Potential relevance for atypical antipsychotic action. Neuropharmacology 79, 49–58 (2014). [DOI] [PubMed] [Google Scholar]
- 51.O’Connor B. J., Lecomte J. M., Barnes P. J., Effect of an inhaled histamine H3-receptor agonist on airway responses to sodium metabisulphite in asthma. Br. J. Clin. Pharmacol. 35, 55–57 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leurs R., Bakker R. A., Timmerman H., de Esch I. J., The histamine H3 receptor: From gene cloning to H3 receptor drugs. Nat. Rev. Drug Discov. 4, 107–120 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Mura S., Nicolas J., Couvreur P., Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991–1003 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Airan R., Neuromodulation with nanoparticles. Science 357, 465 (2017). [DOI] [PubMed] [Google Scholar]
- 55.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
- 56.Ji M. J., Zhang X. Y., Chen Z., Wang J. J., Zhu J. N., Orexin prevents depressive-like behavior by promoting stress resilience. Mol. Psychiatry 24, 282–293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.