Significance
Postoperative peritoneal adhesions are frequent complications for almost any types of abdominal and pelvic surgery and lead to numerous medical problems. Current antiadhesion solutions include pharmacological treatment and physical barriers but are underperformed and can only alleviate or reduce adhesions to some extent. Here we show that a zwitterionic polymer solution after a convenient topical application onto the traumatized surface can reliably and completely prevent the postoperative adhesions, as demonstrated in three clinically relevant but increasingly challenging rat models. The overall success rate of complete prevention is above 93%—a major leap of antiadhesion performance.
Keywords: zwitterionic polymer, postoperative adhesion, antiadhesion, adhesiolysis, hepatectomy
Abstract
Postoperative adhesions are most common issues for almost any types of abdominal and pelvic surgery, leading to adverse consequences. Pharmacological treatments and physical barrier devices are two main approaches to address postoperative adhesions but can only alleviate or reduce adhesions to some extent. There is an urgent need for a reliable approach to completely prevent postoperative adhesions and to significantly improve the clinical outcomes, which, however, is unmet with current technologies. Here we report that by applying a viscous, cream-like yet injectable zwitterionic polymer solution to the traumatized surface, postoperative adhesion was completely and reliably prevented in three clinically relevant but increasingly challenging models in rats. The success rate of full prevention is over 93% among 42 animals tested, which is a major leap in antiadhesion performance. Clinically used Interceed film can hardly prevent the adhesion in any of these models. Unlike current antiadhesion materials serving solely as physical barriers, the “nonfouling” zwitterionic polymer functioned as a protective layer for antiadhesion applications with the inherent benefit of resisting protein/cell adhesions. The nonfouling nature of the polymer prevented the absorption of fibronectins and fibroblasts, which contribute to the initial and late-stage development of the adhesion, respectively. This is the key working mechanism that differentiated our “complete prevention” approach from current underperforming antiadhesion materials. This work implies a safe, effective, and convenient way to fully prevent postoperative adhesions suffered by current surgical patients.
Postoperative peritoneal adhesions are frequent complications for almost any types of abdominal and pelvic surgery and are found in up to 93% of the patients (1, 2). Postoperative adhesions are severe issues leading to many adverse consequences including chronic pain, female infertility, intestinal obstruction, and even death (3, 4). This significantly increases the suffering and economic burden to the patients (5–7). To address the adhesion-related complications, further surgical interventions (e.g., adhesiolysis) are always indispensable in clinical practice (8, 9). Nevertheless, the established tissue adhesions from previous surgery can result in difficult surgical procedures and longer operation times during the reoperation (10). In addition, patients may suffer a high risk of recurrent adhesion following the surgical lysis of preexisting adhesions (11–14).
Pharmacological treatments and physical barrier-based devices are two main approaches having been evaluated to prevent or reduce the formation of postoperative adhesions (4, 5, 15–18). Local or systemic administration of antiinflammatory drugs and anticoagulants, including aspirin, dexamethasone, and heparin, have been tested for postoperative adhesion prevention, but the rapid clearance of drugs in the abdominal cavity greatly limits their therapeutic effects (19, 20). So far none of the drug treatments was able to completely prevent adhesion in clinics.
The physical barrier-based systems used to prevent postoperative adhesions include solid sheets, polymer solutions, and hydrogels. The most widely used and Food and Drug Administration (FDA)–approved products in the United States are solid antiadhesion films, Interceed (oxidized regenerated cellulose; Johnson & Johnson) and Seprafilm (sodium hyaluronate-carboxymethyl cellulose; Genzyme). Nevertheless, all these film products can hardly be placed to cover the entire injured tissues with irregular shapes (20, 21). Furthermore, Interceed requires meticulous hemostasis during the application (22), which is impractical during a surgery. Seprafilm can easily adhere to any moist surface, including the surgeon’s gloves, during the placement, causing inconvenience, reposition issues, and even failure (23). Infusing polymer solutions such as liters of Adept (icodextrin 4% solution) were found to overcome the disadvantages of film products to some extent, but their applications are in general very limited due to the short dwelling time in the abdominal cavity (24, 25). Adept obtained FDA approval for a marginal reduction of adhesion in patients undergoing gynecological laparoscopic adhesiolysis (26). Injectable hydrogels are easy to handle, can completely cover the injured site, and have been tested for preventing or alleviating postoperative adhesions (27–30). Few of them have proven to be consistently effective in the subsequent clinical trials, and to the best of our knowledge none of them has been approved by the FDA for antiadhesion applications in the United States.
Overall, it remained a major challenge to develop a safe, effective, and convenient approach to fully prevent postoperative adhesions given a number of barrier systems developed for postoperative adhesion prevention (27–31). From a clinical perspective, a complete prevention of postoperative adhesions is highly needed to significantly improve patient outcomes such as reoperation rates, chronic abdominal pain, or infertility (32).
The mechanism for adhesion development has been debatable (1, 32–35), but in general it is believed to be triggered by a mass of serosanguinous exudates on the traumatized surface within a few hours after surgery. The exudate contains platelets and extracellular matrices and activated coagulation cascade and fibrin deposition at the wound surface—a natural wound-healing process. The fibrin matrix serves as a weak, temporal adhesive and a tissue–tissue adherence can quickly form resulting from a physical contact. The matrix is next invaded by inflammatory cells which further recruit other cells, in particular fibroblasts, enriched by day 4 after the surgery. The gradually clusterized and aligned fibroblasts together with the collagen secretion replace the temporal fibrin matrix and form a mature and permanent adhesion by the first week.
Zwitterionic polymers are known for ultralow fouling property in resisting protein and cell adhesion (36, 37). Zwitterionic carboxybetaine polymers, in particular, have structures similar to glycine betaine that is present in human tissue and daily diet (28). They are generally considered biocompatible and nontoxic and have been safely applied in several in vitro and in vivo conditions, showing no observable cytotoxicity (38, 39), no stimulation of an immune response against the polymer (40), and no inflammatory signs or foreign body reaction to the polymer implant (38, 41, 42). Inspired by the protein and cell adhesion process prevailing during the adhesion development, we hypothesized that a full prevention of postoperative adhesions can be accomplished by applying a protective layer of “nonfouling” zwitterionic polymers on the traumatized surface, which can prevent the adhesion of protein containing exudate and fibroblasts that contributes to the initial and late-stage development of the adhesion, respectively. It should be noted that current antiadhesion materials mainly served as a physical barrier but without the “nonfouling” characteristics and typically resulted in limited antiadhesion performance.
Here we prepared a viscous, gelatinous yet injectable zwitterionic poly(carboxybetaine acrylamide) (PCBAA) solution and first evaluated its efficacy in resisting protein adsorption and fibroblasts adhesion in vivo. We demonstrated that fibronectin adsorption (a key extracellular matrix protein in the exudate) can be fully prevented on the zwitterionic polymer-protected injured surface of the rat abdominal wall wound in vivo within 24 h of surgery. In addition, applying PCBAA on the traumatized surface could remarkably reduce fibroblast invasion and adhesion on the abdominal wall wound in vivo by day 4 after surgery. With these promising results, we further evaluated the in vivo antiadhesion efficacy of the prepared zwitterionic polymers, employing three different but increasingly challenging adhesion models (Fig. 1A). The results showed that zwitterionic PCBAA polymer can completely and reliably prevent postoperative adhesion in all three models (abdominal wall defect–cecum abrasion adhesion model: 12 out of 12 animals; repeated-injury adhesion model: 11 out of 12, except 1 developed the lowest level of adhesion [score 1] surrounding sutured area; 70% hepatectomy adhesion model: 18 out of 18 in the diaphragm and hepatic hilum, 16 out of 18 on the cut surface, except 2 developed the lowest level of adhesion [score 1]), whereas Interceed film (most popular in the United States) can only slightly reduce but cannot fully prevent adhesion in all these models.
Fig. 1.
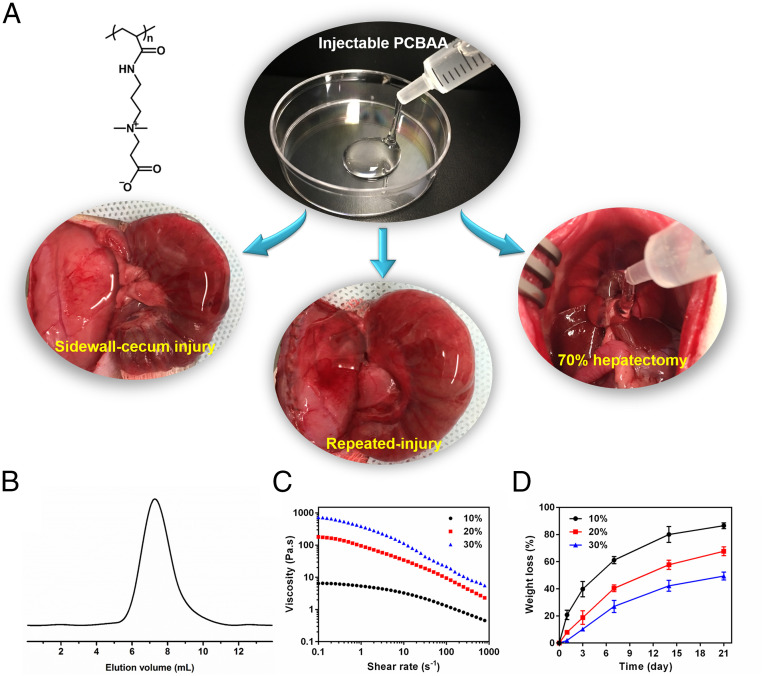
(A) The injectable cream-like zwitterionic PCBAA and its antiadhesion efficacy evaluation in rat sidewall defect–cecum abrasion adhesion model, repeated-injury recurrent adhesion model, and 70% hepatectomy-induced adhesion model, respectively. (B) GPC spectrum of the prepared PCBAA. (C) Steady-shear rheology of PCBAA solutions at 25 °C showing a shear-thinning behavior. (D) In vitro dissolution studies of PCBAA solutions with different concentrations. Data presented as mean ± SD (n = 3).
The zwitterionic PCBAA we report here is able to completely prevent postoperative adhesion as illustrated in these three models and is expected to efficiently resolve adhesion issues in clinical operative scenarios. The antiadhesion working mechanism of PCBAA has also been elucidated through in vivo studies—we found reduced fibronectin adhesion was associated with reduced fibroblast cell adhesion and high performance in preventing adhesion in the three animal models; similar mechanistic exploration, however, has rarely been conducted on current antiadhesion materials.
Characterization of Zwitterionic PCBAA
The molecular weight (MW) of the prepared zwitterionic PCBAA was 42 kDa (Fig. 1B) as characterized using gel permeation chromatography (GPC). The low MW is expected to enable the ultimate removal of the polymer from systemic circulation after in vivo application [below the MW cutoff for glomerular filtration, 70 kDa (43)]. In a shear rheology study, PCBAA solutions at different concentrations showed marked shear-thinning behavior with reducing viscosity by at least one order of magnitude over shear rates extending from 0.1 to 1,000/s (Fig. 1C). An in vitro dissolution test indicated that PCBAA solutions were gradually dissolved over time (Fig. 1D); 10 wt % PCBAA solution had more than 60% dissolved on day 7 and had almost totally dissolved on day 21. By contrast, a 30 wt % PCBAA solution showed no observable dissolution on day 7. We chose 20 wt % PCBAA solution for the in vivo adhesion prevention study, which showed a medium viscosity (cream-like yet injectable) and dissolution rate (Fig. 1 and SI Appendix, Fig. S1).
Zwitterionic PCBAA Resisted Fibronectin Adsorption on Rat Abdominal Wall Wound In Vivo
After surgical trauma to the peritoneal surface, deposited matrix (from exudate and body fluid) acts as a docking site for subsequent inflammatory cells and fibroblasts, facilitating the connection between damaged intraabdominal surfaces (32, 33). Among the various components of the deposited matrix, fibronectin is critical and can direct cellular adhesion and migration (mainly macrophages and fibroblasts) at the initial stage in the formation of postoperative adhesions (44, 45). It is reasonable to speculate that resisting fibronectin adsorption on the traumatized surface in vivo can reduce the fibroblast adhesion and subsequent permanent tissue adhesion formation.
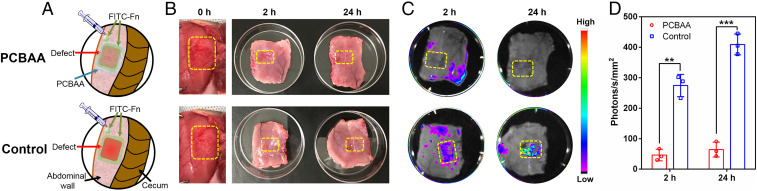
To evaluate the efficacy of zwitterionic PCBAA in resisting fibronectin adsorption on the injured rat abdominal wall in vivo, we created a peritoneal defect on the right lateral abdominal wall, followed by injecting 500 μL of the cream-like zwitterionic PCBAA solution (20 wt %) onto the injured abdominal wall. Then, 250 μL of prepared fluorescein isothiocyanate–labeled fibronectin (FITC-Fn) solution (1 mg/mL, mimicking the one from body fluid) was applied on the PCBAA-protected injured site (Fig. 2A). For the control group, the FITC-Fn solution was directly applied onto the defect. By 2 h and 24 h after the surgery, the FITC-Fn–treated abdominal walls were harvested and lightly washed three times with sterilized phosphate-buffered saline and then photographed with the Carestream In Vivo Xtreme Imaging System (Bruker). There was no significant difference in the appearance of the abdominal wall defects between the two groups on gross observation (Fig. 2B). Nevertheless, fluorescence photography clearly showed almost no fluorescence (no fibronectin absorption) on the PCBAA protected trauma at either 2 h or 24 h (Fig. 2C). By contrast, the untreated group showed significant signals (fibronectin absorption) on the defect, with fluorescent intensity increasing over time after the surgery. These results suggest that the zwitterionic PCBAA effectively resisted fibronectin adsorption on the surgical trauma after abdominal surgery.
Fig. 2.
In vivo study of FITC-Fn absorption on injured abdominal walls. (A) Schematic illustration showing FITC-Fn applied to abdominal wall defect (with or without PCBAA protection). (B and C) Gross observations (B) and representative fluorescence images (C) showing FITC-Fn adsorbed onto the untreated wounds but no adsorption on the PCBAA-treated defects at 2 h and 24 h after surgery. (D) Quantification of FITC-Fn fluorescent intensity in the region of abdominal wall defects as indicated in C. The results are presented as mean ± SD (n = 3). A two-tailed t test analysis was used for statistical analysis. **P < 0.01; ***P < 0.001.
Zwitterionic PCBAA Resisted Fibroblast Adhesion on Rat Abdominal Wall and Cecum Wound Model In Vivo
Fibroblast adhesion and proliferation at the deposited matrix play a key role in late-stage adhesion formation. Once invaded by fibroblasts, the initial fibrin matrix is gradually replaced by deposited collagen, leading to the formation of permanent adhesion (32, 33, 45). Therefore, prevention of fibroblast invasion and adhesion is a key step for antiadhesion and was evaluated.
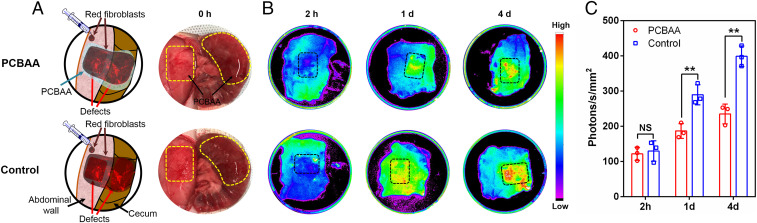
Sprague–Dawley (SD) rat dermal fibroblasts carrying red fluorescence (TurboFP602 red fluorescent protein) were purchased with their fluorescence verified and calibrated before the in vivo study, using both EVOS FL fluorescence microscope (AMG) and the Carestream In Vivo Xtreme Imaging System (Bruker) (SI Appendix, Fig. S2). The rat abdominal wall and cecum wound model was created by introducing two defects through abrasion of the cecum and partial abdominal wall excisions, followed by injecting 500 μL of PCBAA solution on each of the defects. Two hundred fifty microliters of fibroblasts (2 × 105 cell/mL) carrying red fluorescence were then seeded on the PCBAA-protected injured sites (Fig. 3A). For the control group, the fibroblasts were directly seeded onto the defect. Two hours after the surgery, no fluorescence (no fibroblasts adhesion) was observed on the PCBAA-protected and unprotected defects (Fig. 3B); 2 h might be too short for fibroblast adhesion to develop. One and 4 d after the surgery, significantly more and more intensive fluorescence (fibroblast adhesion) was shown on the unprotected defects, but limited increase in fluorescence was observed on the PCBAA-protected surface. These results suggest that applying zwitterionic PCBAA on the traumatized surface remarkably prevented fibroblast invasion and adhesion for at least 4 d in vivo after the surgery, and thereby could reduce collagen secretion and deposition and subsequent tissue adhesion formation. This result greatly implies the potential of PCBAA to prevent postoperative adhesions.
Fig. 3.
In vivo study of red fluorescence-labeled rat fibroblasts (rat dermal fibroblasts expressing TurboFP602 red fluorescent protein) adhesion on injured abdominal wall and cecum. (A) Schematic illustration showing red fluorescence-labeled fibroblasts applied to abdominal wall and cecum defects (with or without PCBAA protection). (B) Representative fluorescence images showing no significant red fluorescence-labeled fibroblast adhesion on the PCBAA-treated abdominal wall defects at 2 h, 1 d, and 4 d after the surgery, while abundant fibroblasts adhered onto the untreated wounds at 1 d and 4 d after surgery. (C) Quantification of fluorescent intensity in the region of abdominal wall defects as indicated in B. The results were presented as mean ± SD (n = 3). A two-tailed t test analysis was used for statistical analysis. **P < 0.01; NS, not significant.
It should be noted that fibronectins and fibroblasts typically come from the submesothelium during an injury, and meanwhile they can enter the body fluid in the abdominal cavity as well. The source of these adhesive proteins and cells contributing to an adhesion could be from both adjacent submesothelium and body fluid. In our in vivo assay, the added fluorescently labeled fibronectins and fibroblasts only mimicked those in the body fluid but still provided an evaluation of the protein/cell interaction with the treated and untreated traumatized surface.
Zwitterionic PCBAA Prevented Postoperative Adhesion in a Rat Abdominal Wall–Cecum Defect Model
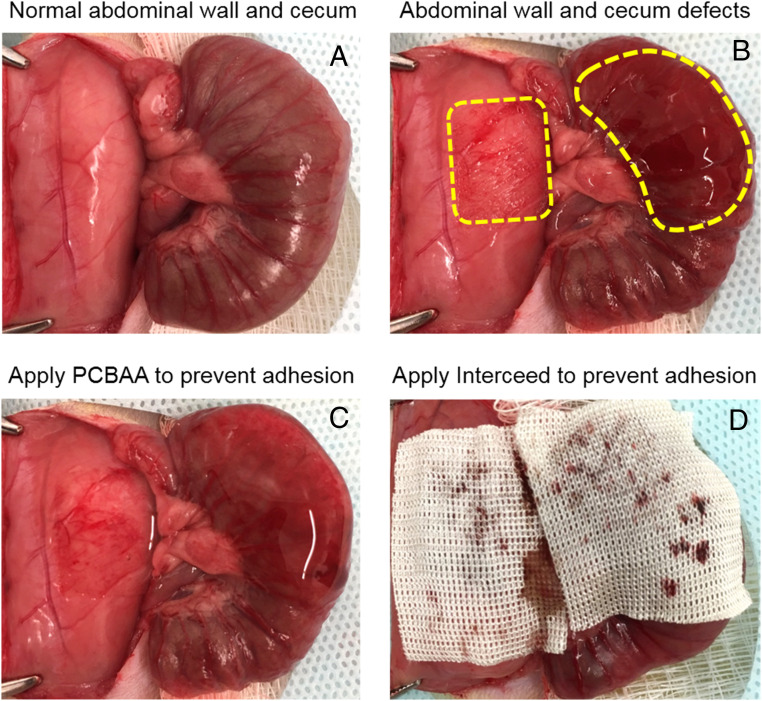
The in vivo antiadhesion efficacy of PCBAA was first evaluated in a rat model of sidewall defect–cecum abrasion, where the defects were created by abrasion of the cecum and partial abdominal wall excisions (Fig. 4 A and B). To apply the antiadhesion formulation, 1 mL of PCBAA solution was injected onto the injured abdominal wall and damaged cecum (Fig. 4C). As a positive control, the defects were covered by the commercial Interceed film (most popular in the United States) (Fig. 4D). For negative control, no antiadhesion material was applied to the injured area. Unlike commercial film-based antiadhesive products, injectable PCBAA has no hemostasis requirement or repositioning of issue during the application.
Fig. 4.
The establishment of a rat sidewall defect–cecum abrasion adhesion model and the application of PCBAA and commercial Interceed film onto the defects. (A) The normal abdominal sidewall and cecum. (B) The establishment of abdominal sidewall and cecum defect, as the dotted boxes indicate. (C and D) PCBAA (C) and commercial Interceed film (D) were applied on the injured abdominal wall and cecum.
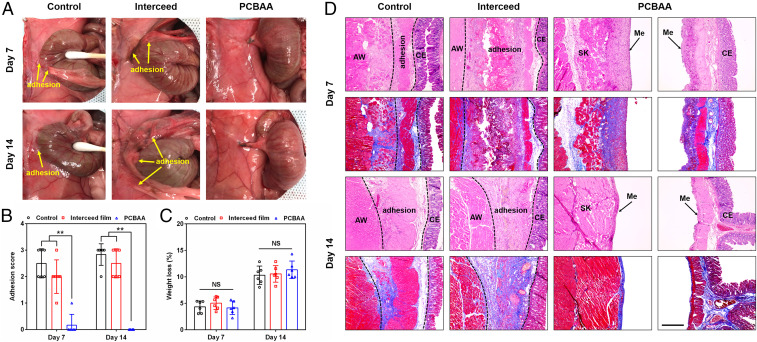
Seven and 14 d after the surgery, the peritoneum was opened and the extent of adhesion was evaluated (Fig. 5A) and scored (Fig. 5B). For the control group receiving no treatment, all rats (n = 6) suffered from severe abdominal adhesions scored at 2 and 3 at day 7, and most developed more serious and tenacious adhesions with score 3 at day 14. For the group treated with Interceed films, rats still suffered from peritoneal adhesions at day 7, but both the adhesion scores and adhesion area were significantly reduced (mostly score 2) compared with the untreated group, indicating the film could alleviate the postoperative adhesion to some extent. At day 14, adhesions in the film group became worse, which were not only visible in injured sites but also involved the circumjacent mesentery. It should be noted that the postoperative adhesions in the untreated and the film groups occurred not only between the injured cecum and abdominal wall but also between the abrased cecum and the proximal mesentery in some cases. By comparison, for PCBAA-treated group, nearly no adhesion was observed on day 7 and both the damaged abdominal wall and cecum were partly recovered. At day 14, 100% no adhesion was observed at all (score 0), and both the injured abdominal wall and cecum were completely recovered; this indicates that PCBAA could fully prevent postsurgical peritoneal adhesions in this model. In addition, no apparent PCBAA residue presented on the treated sites or abdominal cavity at day 14 postsurgery, suggesting that the material was able to be absorbed. The change of body weight after the surgery did not show significant difference among these groups (P > 0.05) (Fig. 5C).
Fig. 5.
Evaluation of antiadhesion efficacy at days 7 and 14 after surgery in a rat sidewall defect–cecum abrasion adhesion model. (A) Postoperative adhesions were observed in the untreated control and Interceed film groups while no adhesion was observed in rats treated with PCBAA on days 7 and 14 after the surgery. (B and C) Distribution of adhesion scores (B) and weight loss (C) in the untreated, film, and PCBAA groups on days 7 and 14 after the surgery. The results are presented as mean ± SD (n = 6). **P < 0.01; NS, not significant. (D) Representative histology images (H&E and Masson trichrome staining) of tissues from different groups on days 7 and 14 after surgery, respectively. AW: abdominal wall; CE: cecal mucosa; Me: mesothelial layer; SK: skeletal muscle of AW. The deposited collagen in the adhesion site was stained in blue while muscle showed red in Masson trichrome staining. (Scale bar, 400 μm.)
To further evaluate the retention of PCBAA presented on the treated sites, PCBAA was labeled with Cy7 and the applied PCBAA-Cy7 was examined both visually and through fluorescent quantification in the rat sidewall defect–cecum abrasion adhesion model (SI Appendix, Fig. S3). One and 3 d after the application, significant amount of polymer remained at the traumatized site. Seven days after the application only a minimum amount of the polymer remained. Fibroblast invasion and adhesion within the first 3 d after the surgical injury is a key step for tissue adhesion formation. Our data indicated that PCBAA can remain on the application site during the critical days and beyond.
We further collected tissue samples from different groups and conducted histological analysis through hematoxylin and eosin (H&E) and Masson trichrome staining (Fig. 5D). For the untreated group on day 7, the skeletal muscles of the injured abdominal wall and the smooth muscles of injured cecum were fused with connective tissues (adhesive region), which was composed of granulation tissue, collagen deposition, and fibroblasts and some inflammatory cells (SI Appendix, Fig. S4). The deposited collagen in the adhesion site can be easily observed in Masson trichrome staining with intense blue. The film group on day 7 presented an adhesive structure similar to the untreated control group with looser adhesion region and less collagen deposition. On day 14, both untreated and film-treated groups showed a more compacted adhesion region with increased thickness and more granulation tissue and collagen deposition. In addition, many new blood vessels could be observed in the adhesion site (SI Appendix, Fig. S4), indicating the formation of mature adhesions. For the PCBAA-treated group, histological observations of the injured abdominal wall and cecum were performed separately since no adhesion was developed at all. On day 7, it was clearly observed that the injured abdominal wall (skeletal muscle was shown) and cecum were recovered with a comparatively complete neomesothelial cell layer on top of the damaged sites. On day 14, the damaged abdominal wall and cecum had completely remesothelialized with a smooth and fully developed mesothelium layer similar to that in the normal tissue (SI Appendix, Fig. S5). These results indicate that the PCBAA completely and reliably prevented the formation of postoperative adhesion in the abdominal wall defect–cecum abrasion adhesion model without interfering with regular wound healing.
Zwitterionic PCBAA Prevented Recurrent Adhesion after Adhesiolysis in a Rat Repeated-Injury Adhesion Model
In clinical practice, adhesiolysis is an indispensable operation for patients to eliminate preexisting postoperative adhesions (8–10). Unfortunately, the new trauma resulting from the surgical lysis of the prior adhesions tends to induce recurrent adhesion (11–13). Although minimally invasive procedures such as laparoscopy have been used in adhesiolysis to decrease the peritoneal trauma and thus prevent new adhesion formation, there is still a high incidence (more than 55%) of recurrent adhesion regardless of adhesiolysis being performed by laparoscopy or laparotomy (12, 13, 44). As a matter of fact, the prevention of recurrent adhesion after adhesiolysis is more difficult because the trauma is more severe and the adhesion mechanism is more complicated when compared with the primary adhesion (12, 13, 46). Despite a number of barrier systems and pharmacological treatments developed for postoperative adhesion prevention (27–31, 47–50), most of these approaches aim for primary adhesion prevention while few of them target the recurrent adhesion prevention after adhesiolysis or showed a sufficient efficacy. Of note, a few antiadhesion materials under development showed efficacy in conventional sidewall defect–cecum abrasion adhesion models (49, 50) but failed to perform satisfactorily on recurrent adhesion prevention in more rigorous repeated-injury adhesion models that are much closer to clinical practice (51–53). Here, we further evaluated the efficacy of PCBAA on the prevention of recurrent adhesion after adhesiolysis with a more rigorous rat repeated-injury adhesion model.
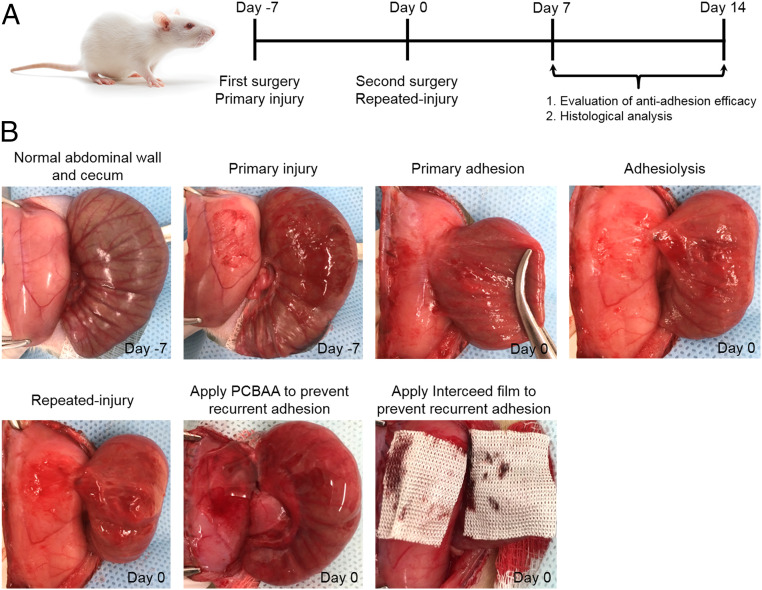
The repeated-injury adhesion model was established by creating a first abdominal wall and cecum injury with the peritoneum closed without any antiadhesion material treatment (on day −7), followed by a second surgery on day 0 to reopen the incision, lysing the adhesion site resulting from the first surgery with an appropriate dissection as needed, and abrading the separated abdominal wall and cecum monodirectionally with a sterilized brush until bleeding surfaces were produced (Fig. 6 A and B). For treatment groups, the repeated-injured sites were covered either by 1 mL PCBAA solution or Interceed film before the final closure.
Fig. 6.
Establishment of a rat repeated-injury model to evaluate the antiadhesion efficacy of PCBAA and commercial Interceed film. (A) Schematic of the experimental schedule. (B) Procedures to establish the rat repeated-injury model and apply antiadhesion materials onto the reinjured sites. Abdominal wall defect–cecum abrasion model was established in SD rats at the first surgery (day −7). The established adhesion was lysed at the second surgery and the repeated injury was performed by reabrading the detached abdominal wall and cecum (day 0). Then, PCBAA and Interceed film were applied on the reinjured abdominal wall and cecum.
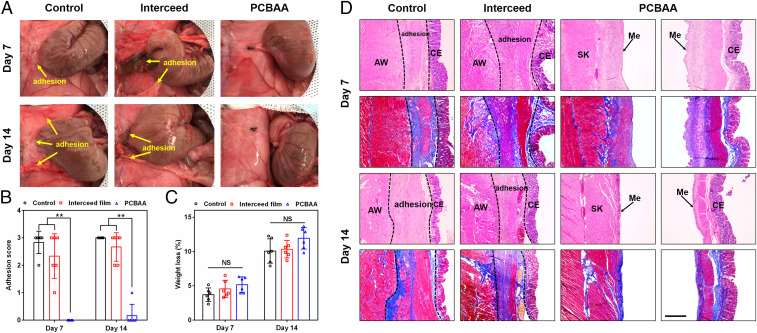
Seven and 14 d after the second surgery, the rats were killed and the extent of recurrent adhesions was evaluated (Fig. 7A) and scored (Fig. 7B). On day 7 for the control group receiving no treatment, five out of six rats suffered from severe recurrent adhesions scored at 3 and the remaining one scored at 2. The adhesion area was observed to be larger than the initial injured area, indicating the uninjured surface of the abdominal wall and cecum were also involved in the recurrent adhesion. On day 14, all six rats in the untreated group developed more serious vascularized adhesions scored at 3. The adhesions were not only visible between the injured sites but also involved the uninjured surface and circumambient mesentery. For the group treated with Interceed films, rats still suffered recurrent adhesions at day 7 and 14 similar to the untreated group, but both the adhesion scores and adhesion area were reduced. By contrast, 100% no adhesion was observed (score 0) in the PCBAA-treated animals on day 7 and the reinjured abdominal wall was mostly recovered (the wound area was still visible). On day 14, nearly no adhesion was observed and the damaged abdominal wall and cecum were completely healed with visible new blood vessels on the smooth surface. These results indicate that PCBAA could promisingly prevent recurrent postsurgical peritoneal adhesions as demonstrated in this model which mimics complications after adhesiolysis.
Fig. 7.
Evaluation of recurrent adhesion prevention on days 7 and 14 after the second surgery in a rat repeated-injury model. (A) Severe adhesions were observed in the control and Interceed film groups while no adhesion was observed in rats treated with PCBAA on days 7 and 14 after the second surgery. (B and C) Distribution of adhesion scores (B) and weight loss (as percentage of starting body mass) (C) in the untreated control, film, and PCBAA groups on days 7 and 14 after the second surgery. The results are presented as mean ± SD (n = 6). **P < 0.01; NS, not significant. (D) Representative histology images (H&E and Masson trichrome staining) of tissues from different groups on days 7 and 14 after the second surgery, respectively. AW: abdominal wall; CE: cecal mucosa; Me: mesothelial layer; SK: skeletal muscle of AW. The deposited collagen in the adhesion site was stained in blue while muscle showed red in Masson trichrome staining. (Scale bar, 400 μm.)
Additionally, on day 14 after the second surgery no apparent PCBAA residue presented on the treated sites or abdominal cavity, indicating that the material was able to be absorbed. The change of body weight after the surgery did not show significant difference among these groups (P > 0.05) (Fig. 7C). Rats treated with PCBAA were closely monitored and recorded after the second surgery to evaluate potential signs of toxicity. All animals displayed normal behavior without any adverse reactions such as slow movement, eye secretion, or abnormal food and water intake.
Tissue samples from different groups were collected to perform histological analysis by H&E and Masson trichrome staining (Fig. 7D). On day 7 after the second surgery, the tissues taken from the adhesion site in the control and film groups showed that the injured cecum was fused to the skeletal muscles of damaged abdominal wall, and the resulting adhesion contained a great deal of granulation tissue, fibroblasts, inflammatory cells, and deposited collagen (staining with intense blue in Masson trichrome) (SI Appendix, Fig. S6). For the film-treated group, some residual film was observed in the adhesion region. On day 14, both untreated and film-treated groups showed mature adhesion with neovascularization and more granulation tissue and collagen deposition (SI Appendix, Fig. S6). For the animals treated with PCBAA, both the injured abdominal wall and cecum were recovered with an integral neomesothelial cell layer despite some fibroblasts, inflammatory cells, and collagen populated at the damaged sites on day 7. Two weeks later, the surfaces of the healed abdominal wall and cecum were completely remesothelialized with decreasing collagen deposition. These results showed that PCBAA can fully prevent the recurrent adhesion in the rigorous rat repeated-injury adhesion model, implying a high potential to address this tough clinical complication.
Zwitterionic PCBAA Prevented Postoperative Adhesion in a Rat 70% Hepatectomy-Induced Adhesion Model
Liver cancer is the fifth-most-prevalent human malignancy and the second-most-frequent cause of cancer-related death (54–56). Despite the progress in cancer treatment over the last 50 y, liver resection remains the most available and effective therapy for patients with hepatocellular carcinoma and currently represents the only potentially curative therapy for liver metastases from colorectal cancers (57–59). Nevertheless, intraabdominal adhesions induced by hepatectomies posed significant clinical challenges; they not only brought suffering and postoperative complications to the patients but also created additional troubles in reoperative procedures, such as longer operation time and a higher risk of bleeding or injury to adhered organs (60, 61). In particular, numerous patients required repeated liver resections in clinical practice due to the frequent recurrent liver cancer after a first liver resection, whereas the presence of adhesions around the remaining liver from the previous hepatectomy significantly impeded the feasibility of repeat liver resection due to increased technical difficulties (62–65).
In this work, we adopted a classical rat partial hepatectomy model (∼70% of the total liver) (66) to study hepatectomy-induced peritoneal adhesion. This model presents several distinctive features compared with commonly reported adhesion models (67). 1) The median lobe (ML) and the left lateral lobe (LLL) of the liver (70% of the total liver) are resected, which is a more standardized injury than previous abrasion models. 2) This model creates a complex three-dimensional (3D) cut surface, which is harder to completely cover than sidewall defect models. 3) This model can steadily reproduce more severe adhesions compared with other animal models since the hepatectomy consistently causes severe trauma to the peritoneal cavity. 4) The locations of adhesion formation are uncertain because the injured surface is in contact with several surrounding tissues, including hepatic hilum, diaphragm, remnant liver, small bowel, omentum, and so on. Therefore, this standardized and reproducible model with severe adhesions can serve as a powerful tool to evaluate and compare the efficacy of various antiadhesive materials for adhesion prevention. So far tested materials including commercial films such as Interceed and other hydrogel-based formulations (68, 69) failed to prevent the adhesion under this model (67). Here we further evaluate the antiadhesion efficacy of the PCBAA using this rat 70% hepatectomy adhesion model.
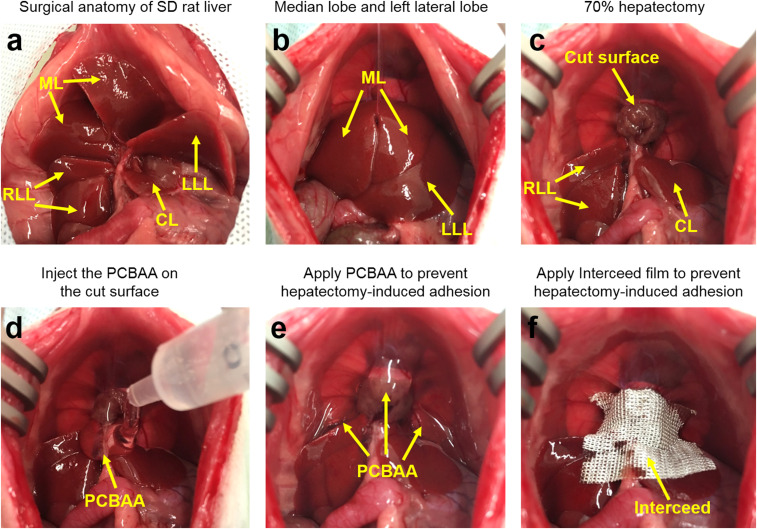
The SD rat liver is composed of four main lobes (Fig. 8A), including the LLL, ML, right liver lobe (RLL), and the caudate lobe (CL), among which ML and LLL account for ∼70% of the total liver. To establish the 70% hepatectomy model, the ML and LLL (Fig. 8B) were resected after ligating the hepatic pedicle through a midline incision (Fig. 8C). For the untreated control group, the peritoneal cavity was closed without any antiadhesion materials. For PCBAA group, the complex cut surface was completely covered with 2 mL of injected PCBAA (Fig. 8 D and E). For the film-treated group, the Interceed film was applied to cover the cut surfaces and the surface of remnant liver lobes. Nevertheless, full coverage of the cut surfaces with this film product was difficult since the cut surfaces presented highly complicated 3D structure (Fig. 8F).
Fig. 8.
Surgical anatomy of SD rat liver lobes and the establishment of a 70% hepatectomy adhesion model to evaluate the antiadhesion efficacy of Interceed film and PCBAA. (A) Surgical anatomy of SD rat liver lobes. (B and C) Establishment of a rat 70% hepatectomy adhesion model through ligation and resection the ML and the LLL. (D and E) PCBAA was injected on the cut surface and the liver surface of remnant liver lobes. (F) Interceed film was applied to cover the cut surface and the liver surface of remnant liver lobes.
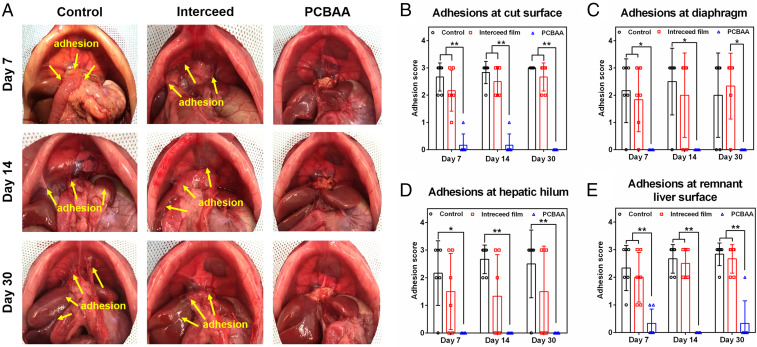
One week after the hepatectomy, six rats in each group were killed and the abdomen was opened to evaluate and score the adhesions. All of the rats in the untreated group developed severe adhesions (Fig. 9A). The location of the adhesions was not limited to the cut surface and adjacent organs (mainly small bowel or omentum) but also included the diaphragm, hepatic hilum, and remnant liver surface (adhesion-scored respectively in Fig. 9 B–E). The Interceed film-treated rats showed slightly lower adhesion scores compared to the untreated ones. Most rats developed adhesions between the cut surface of the liver and the omentum, rather than small bowel that presented in the control group. Meanwhile, the adhesions were also observed between the diaphragm and the omentum (Fig. 9A). Of note, the adhesions around the cut surface were relatively easier to separate than those in the untreated control group, indicating the film could alleviate the postoperative adhesion to some extent. For the PCBAA-treated group, hardly any adhesion was observed in this highly challenging adhesion model. No apparent PCBAA residue was observed in the abdominal cavity on day 7 after the hepatectomy and the cut surface with a small portion of the residual liver parenchyma could be clearly observed without any adhesion. On days 14 and 30 after the hepatectomy, all of the animals in control and film group developed more mature and vascularized adhesions that were hard to separate. The PCBAA group by contrast did not suffer from adhesion except for two rats developed slight adhesion between the superior right lobe and the inferior right lobe. In addition, it was clearly observed that the remnant RLL and posterior CL showed an obvious increase in volume than that on day 7—an expected compensatory liver regeneration after partial hepatectomy. In addition, histological examinations showed that the increscent remnant lobes were similar to that in the healthy rat (SI Appendix, Fig. S7) without any abnormality. This exciting result further demonstrates that PCBAA is a top-performing antiadhesion material effectively preventing postoperative adhesions after hepatectomy. To the best of our knowledge, other materials have rarely been reported to completely prevent hepatectomy-induced postoperative adhesions.
Fig. 9.
Evaluation of postoperative adhesions on days 7, 14, and 30 in a rat 70% hepatectomy model. (A) Severe adhesions were observed in the control and Interceed film groups while almost no adhesion was observed in rats treated with PCBAA after the hepatectomy. (B–E) Adhesions presented at the cut surface (B), diaphragm (C), hepatic hilum (D), and remnant liver surface (E) were scored respectively for the control, film, and PCBAA groups after the hepatectomy. The results are presented as mean ± SD (n = 6). *P < 0.05; **P < 0.01.
In summary, we have shown that the injectable zwitterionic PCBAA polymer can completely and reliably prevent postoperative adhesions under three clinically relevant but increasingly challenging models. The working mechanism for PCBAA is that it can fully prevent protein (fibronectin) adsorption on traumatized surface in vivo within 24 h of surgery (initial stage development of the adhesion) and remarkably reduce cell (fibroblasts) invasion and adhesion in vivo by day 4 after surgery (the late-stage development of the adhesion). We expect that zwitterionic PCBAA polymers with top-performing antiadhesion efficacy will be a safe, effective, and convenient approach to fully prevent postoperative adhesions suffered by current surgical patients.
Methods
Materials, polymer preparation and characterization, in vivo protein and cell adhesion study, the rat abdominal wall and cecum defect model, the rat repeated-injury adhesion model, and the rat 70% heptectomy-induced adhesion model are described in SI Appendix.
The animal experiments were approved by the Institutional Animal Care and Use Committee at Wayne State University and performed in compliance with the relevant laws and institutional guidelines.
Statistical Analysis.
All data were presented as mean ± SD and statistical analysis was performed using GraphPad Prism software (GraphPad Software). Adhesion scores did not always follow a normal distribution, so statistical analysis was performed using a nonparametric Mann–Whitney U test. The data on body weight were normally distributed and analyzed by one-way ANOVA followed by Tukey multicomparison tests. For all statistical analyses, significance was accepted at the 95% confidence level, and all analyses were two-tailed. Statistical differences were defined as *P < 0.05 and **P < 0.01, and differences with P < 0.05 were considered significant.
Supplementary Material
Acknowledgments
This work was supported by the faculty start-up fund at Wayne State University, the NSF (1410853 and 1809229), and the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH (DP2DK111910 and R01DK123293). We thank Jessica B. Back at Microscopy, Imaging, and Cytometry Resources Core of Wayne State University for support with fluorescence photography. We thank Prof. Weiping Ren of Wayne State University Biomedical Engineering for access to the rheometer.
Footnotes
Competing interest statement: Z.C. and E.Z. are inventors on a patent related to the reported technology.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2012491117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Okabayashi K., et al. , Adhesions after abdominal surgery: A systematic review of the incidence, distribution and severity. Surg. Today 44, 405–420 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Brochhausen C., et al. , Current strategies and future perspectives for intraperitoneal adhesion prevention. J. Gastrointest. Surg. 16, 1256–1274 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Ten Broek R. P. G., et al. , Benefits and harms of adhesion barriers for abdominal surgery: A systematic review and meta-analysis. Lancet 383, 48–59 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Diamond M. P., Reduction of postoperative adhesion development. Fertil. Steril. 106, 994–997.e1 (2016). [DOI] [PubMed] [Google Scholar]
- 5.van Goor H., Consequences and complications of peritoneal adhesions. Colorectal Dis. 9 (suppl. 2), 25–34 (2007). [DOI] [PubMed] [Google Scholar]
- 6.ten Broek R. P., et al. , Burden of adhesions in abdominal and pelvic surgery: Systematic review and met-analysis. BMJ 347, f5588 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman M. G., McLain A. D., Moran B. J., Impact of previous surgery on time taken for incision and division of adhesions during laparotomy. Dis. Colon Rectum 43, 1297–1299 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Swank D. J., et al. , Laparoscopic adhesiolysis in patients with chronic abdominal pain: A blinded randomised controlled multi-centre trial. Lancet 361, 1247–1251 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Robinson J. K., Colimon L. M. S., Isaacson K. B., Postoperative adhesiolysis therapy for intrauterine adhesions (Asherman’s syndrome). Fertil. Steril. 90, 409–414 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Zhang E., et al. , Thermoresponsive polysaccharide-based composite hydrogel with antibacterial and healing-promoting activities for preventing recurrent adhesion after adhesiolysis. Acta Biomater. 74, 439–453 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Vrijland W. W., Jeekel J., van Geldorp H. J., Swank D. J., Bonjer H. J., Abdominal adhesions: Intestinal obstruction, pain, and infertility. Surg. Endosc. 17, 1017–1022 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Tittel A., Treutner K. H., Titkova S., Öttinger A., Schumpelick V., Comparison of adhesion reformation after laparoscopic and conventional adhesiolysis in an animal model. Langenbecks Arch. Surg. 386, 141–145 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Tittel A., Treutner K. H., Titkova S., Öttinger A., Schumpelick V., New adhesion formation after laparoscopic and conventional adhesiolysis: A comparative study in the rabbit. Surg. Endosc. 15, 44–46 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Lin X. N., et al. , Randomized, controlled trial comparing the efficacy of intrauterine balloon and intrauterine contraceptive device in the prevention of adhesion reformation after hysteroscopic adhesiolysis. Fertil. Steril. 104, 235–240 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Ouaïssi M., et al. , Post-operative adhesions after digestive surgery: Their incidence and prevention: Review of the literature. J. Visc. Surg. 149, e104–e114 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Ten Broek R. P., Kok-Krant N., Bakkum E. A., Bleichrodt R. P., van Goor H., Different surgical techniques to reduce post-operative adhesion formation: A systematic review and meta-analysis. Hum. Reprod. Update 19, 12–25 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Arung W., Meurisse M., Detry O., Pathophysiology and prevention of postoperative peritoneal adhesions. World J. Gastroenterol. 17, 4545–4553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellebrekers B. W., Trimbos-Kemper T. C., Trimbos J. B. M., Emeis J. J., Kooistra T., Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil. Steril. 74, 203–212 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Kucukozkan T., Ersoy B., Uygur D., Gundogdu C., Prevention of adhesions by sodium chromoglycate, dexamethasone, saline and aprotinin after pelvic surgery. ANZ J. Surg. 74, 1111–1115 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Li J., et al. , Highly bioadhesive polymer membrane continuously releases cytostatic and anti-inflammatory drugs for peritoneal adhesion prevention. ACS Biomater. Sci. Eng. 4, 2026–2036 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Shahram E., et al. , Evaluation of chitosan-gelatin films for use as postoperative adhesion barrier in rat cecum model. Int. J. Surg. 11, 1097–1102 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Ethicon US, LLC , “GYNECARE INTERCEED Absorbable Adhesion Barrier. Instructions for use.” (Ethicon US, LLC, 2019).
- 23.Kamihira O., et al. , A new treatment for retroperitoneal fibrosis: Initial experiences of using Seprafilm® to wrap the ureter. BJU Int. 114, 563–567 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Verco S. J., et al. , Development of a novel glucose polymer solution (icodextrin) for adhesion prevention: Pre-clinical studies. Hum. Reprod. 15, 1764–1772 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Diamond M. P., Group T. S. A. S.; The Sepracoat Adhesion Study Group , Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: A prospective, randomized, blinded, placebo-controlled multicenter study. Fertil. Steril. 69, 1067–1074 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Trew G., et al. , Gynaecological endoscopic evaluation of 4% icodextrin solution: A European, multicentre, double-blind, randomized study of the efficacy and safety in the reduction of de novo adhesions after laparoscopic gynaecological surgery. Hum. Reprod. 26, 2015–2027 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Lin L. X., et al. , In situ cross-linking carbodiimide-modified chitosan hydrogel for postoperative adhesion prevention in a rat model. Mater. Sci. Eng. C 81, 380–385 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Chen C. H., et al. , Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr. Polym. 173, 721–731 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z., et al. , Biodegradable and thermoreversible PCLA-PEG-PCLA hydrogel as a barrier for prevention of post-operative adhesion. J. Biomat. 32, 4725–4736 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., et al. , Naproxen nanoparticles-loaded thermosensitive chitosan hydrogel for prevention of post-operative adhesions. ACS Biomater. Sci. Eng. 4, 1580–1588 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Stapleton L. M., et al. , Use of a supramolecular polymeric hydrogel as an effective post-operative pericardial adhesion barrier. Nat. Biomed. Eng. 3, 611–620 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Moris D., et al. , Postoperative abdominal adhesions: Clinical significance and advances in prevention and management. J. Gastrointest. Surg. 21, 1713–1722 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Boland G. M., Weigel R. J., Formation and prevention of postoperative abdominal adhesions. J. Surg. Res. 132, 3–12 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Maciver A. H., McCall M., James Shapiro A. M., Intra-abdominal adhesions: Cellular mechanisms and strategies for prevention. Int. J. Surg. 9, 589–594 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z., et al. , Encapsulation of cell-adhesive RGD peptides into a polymeric physical hydrogel to prevent postoperative tissue adhesion. J. Biomed. Mater. Res. B Appl. Biomater. 100, 1599–1609 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Jiang S., Cao Z., Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 22, 920–932 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Mi L., Jiang S., Integrated antimicrobial and nonfouling zwitterionic polymers. Angew. Chem. Int. Ed. Engl. 53, 1746–1754 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Zhang L., et al. , Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 31, 553–556 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Cao Z., Zhang L., Jiang S., Superhydrophilic zwitterionic polymers stabilize liposomes. Langmuir 28, 11625–11632 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Zhang P., et al. , Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. Proc. Natl. Acad. Sci. U.S.A. 112, 12046–12051 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie X., et al. , Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer. Nat. Biomed. Eng. 2, 894–906 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang E., Cao Z., Coated glucose sensors dodge recalibration. Nat. Biomed. Eng. 2, 881–882 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Bhavsar D., Subramanian K., Sethuraman S., Krishnan U. M., Translational siRNA therapeutics using liposomal carriers: Prospects & challenges. Curr. Gene Ther. 12, 315–332 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Rojo D., Conget P., Acellular derivatives of mesenchymal stem cells prevent peritoneal adhesions in an animal model. J. Surg. Res. 223, 198–206 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Alpay Z., Saed G. M., Diamond M. P., Postoperative adhesions: From formation to prevention. Semin. Reprod. Med. 26, 313–321 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Cubukçu A., Alponat A., Gönüllü N. N., Mitomycin-C prevents reformation of intra-abdominal adhesions after adhesiolysis. Surgery 131, 81–84 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Zhang E., et al. , Biodegradable and injectable thermoreversible xyloglucan based hydrogel for prevention of postoperative adhesion. Acta Biomater. 55, 420–433 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Li L., et al. , Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials 35, 3903–3917 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Yang B., et al. , Preventing postoperative abdominal adhesions in a rat model with PEG-PCL-PEG hydrogel. Int. J. Nanomedicine 7, 547–557 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeo Y., et al. , In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials 27, 4698–4705 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Wu Q., et al. , Thermosensitive hydrogel containing dexamethasone micelles for preventing postsurgical adhesion in a repeated-injury model. Sci. Rep. 5, 13553 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He T., et al. , Improving antiadhesion effect of thermosensitive hydrogel with sustained release of tissue-type plasminogen activator in a rat repeated-injury model. ACS Appl. Mater. Interfaces 8, 33514–33520 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Yeo Y., et al. , Prevention of peritoneal adhesions with an in situ cross-linkable hyaluronan hydrogel delivering budesonide. J. Control. Release 120, 178–185 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Jemal A., et al. , Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Ryerson A. B., et al. , Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 122, 1312–1337 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Affo S., Yu L. X., Schwabe R. F., The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu. Rev. Pathol. 12, 153–186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bruix J., Han K. H., Gores G., Llovet J. M., Mazzaferro V., Liver cancer: Approaching a personalized care. J. Hepatol. 62 (suppl. 1), S144–S156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belghiti J., Fuks D., Liver resection and transplantation in hepatocellular carcinoma. Liver Cancer 1, 71–82 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhong J. H., et al. , Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann. Surg. 260, 329–340 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Hu M., Zhao G., Xu D., Liu R., Laparoscopic repeat resection of recurrent hepatocellular carcinoma. World J. Surg. 35, 648–655 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Kanazawa A., et al. , Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J. Hepatobiliary Pancreat. Sci. 20, 512–517 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Battula N., et al. , Repeat liver resection for recurrent colorectal metastases: A single-centre, 13-year experience. HPB (Oxford) 16, 157–163 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shelat V. G., et al. , Outcomes of repeat laparoscopic liver resection compared to the primary resection. World J. Surg. 38, 3175–3180 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Chan A. C., et al. , Feasibility of laparoscopic re-resection for patients with recurrent hepatocellular carcinoma. World J. Surg. 38, 1141–1146 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Morise Z., Perspective of laparoscopic liver resection for hepatocellular carcinoma. World J. Gastrointest. Surg. 7, 102–106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Higgins G. M., Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. (Chic.) 12, 186–202 (1931). [Google Scholar]
- 67.Shimizu A., et al. , New hepatectomy-induced postoperative adhesion model in rats, and evaluation of the efficacy of anti-adhesion materials. Surg. Today 44, 314–323 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Yeo Y., et al. , In situ cross-linkable hyaluronan hydrogels containing polymeric nanoparticles for preventing postsurgical adhesions. Ann. Surg. 245, 819–824 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ito T., et al. , The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials 28, 975–983 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.