Processing and presentation of self-antigens and foreign antigens and their loading into class I and class II molecules of the major histocompatibility complex (MHC) constitute a major feature of the cellular immune response and induce CD8 and CD4 T cell immune responses (1, 2), respectively. The MHC complex, in human, called human leukocyte antigen complex (HLA), is the most polymorphic locus of the mammalian genome, with hundreds of allelic variations, with the most polymorphic domain represented by the peptide binding domain (3, 4). Different MHC alleles have different antigen-binding profiles which, in turn, can affect the susceptibility or resistance to infectious diseases (4). MHC alleles are believed to be maintained by pathogen-driven selection, mediated through either heterozygote advantage or frequency-dependent selection (5).
Although antigen presentation has been studied for decades (2, 6), the mechanisms that enable loading of specific peptides onto MHC molecules are not fully elucidated. In the context of MHC class I antigen presentation, the diverse antigenic peptides that originate from endogenous protein degradation or defective protein translation are loaded onto MHC class I heavy chains complexed with the light chain β2-microglobulin within the peptide loading complex (PLC) (7, 8) into the endoplasmic reticulum (ER) (Fig. 1). The PLC consists of the peptide transporter associated with antigen presentation, TAP, which pumps peptides from the cytosol into the ER and multiple chaperone molecules, including tapasin (9–12). Tapasin allows formation of peptide:MHC class I complexes and facilitates the loading of high-affinity antigenic peptides onto the MHC class I molecules by stabilizing a peptide-receptive conformation and editing the repertoire of bound peptides, removing those peptides which cannot meet a minimum affinity threshold (13–16). In contrast to MHC class I molecules, tapasin shows limited polymorphism, suggesting that the antigen processing and peptide loading machinery has evolved to present antigens by most MHC class I molecules (17). Of note is that tapasin mutant mice have defects in MHC class I cell surface expression, antigen presentation, CD8 T cell development, and immune responses (18).
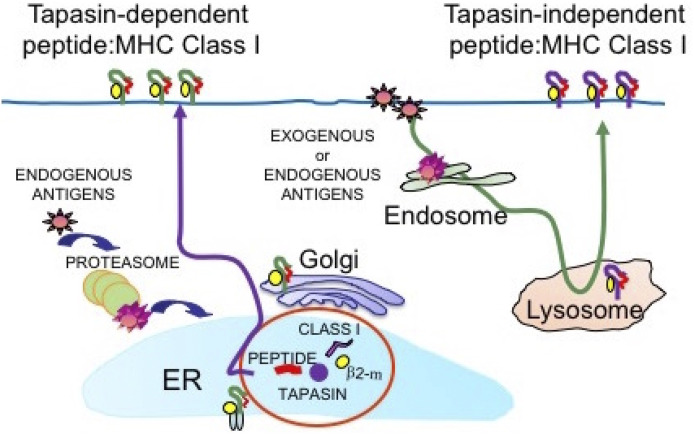
Fig. 1.
MHC class I molecules evolved to present antigens in a tapasin-dependent (Left) and tapasin-independent (Right) fashion. These two different mechanisms would occur in different cellular compartments and involve different molecular pathways. In the classical MHC class I antigen presentation (Left), a chaperone molecule such as tapasin facilitates and stabilizes loading of high-affinity peptides into class I molecules within the ER. However, certain MHC class I alleles may bind a broader range of peptides with lower affinity independently from tapasin in lysosome or lysosome-like structures and transfer the peptides:MHC class I complexes on the cell surface without ER/Golgi involvement (Right). Evolution of these diverse mechanisms would allow MHC class I alleles to present antigens even in the absence of tapasin, to ensure clearance or control of pathogen dissemination and tumor immune surveillance.
In PNAS, Bashirova et al. (19) characterize 97 HLA class I allotypes that are most represented within Europeans and African Americans, and their level of dependence on tapasin, and correlate this with the breadth of immune response to HIV and dengue viruses. First, the authors determine the impact of the two variants of tapasin, which differ by one amino acid at position 240 of the mature protein, on HLA class I expression, with each variant transduced separately into tapasin-deficient and -sufficient cell lines. They then analyze the tapasin dependence (TD) scores for each allele based on the ratio of expression level in tapasin-deficient cells over the sufficient cell lines measured by flow cytometer. A higher and lower TD score indicate the dependence on or independence of tapasin for HLA cell surface expression, respectively. Of note is that similar TD scores for 26 out of 27 HLA were further validated in a different transduced cell line. To test the hypothesis that antigen presentation on tapasin-independent fashion was favoring presentation of a broader antigen repertoire, thereby influencing the breadth of the peptide repertoire, the authors tested the CD8 T cell responses against a panel of 410 overlapping HIV peptides in a cohort of HLA genotyped HIV clade C-infected individuals and correlated the breadth with the TD. CD8 T cell responses to each peptide were measured by enzyme-linked immune absorbent spot (ELISpot) assays, and results for each HLA allele were correlated to the correspondent TD. These analyses show significant negative correlations between the TD score and the number of peptides associated with the HLA-B, HLA-A, and HLA-A/B/C combined but not with HLA-C. Importantly, the authors tested the association between TD scores of each individual and breadth of their corresponding responses, regardless of the HLA genotype. Next, for each individual, they calculated the sum of the TD scores for two alleles at each given locus, and a “global” TD score for the four or six alleles of their HLA-A/B and HLA-A/B/C, respectively. These analyses demonstrated a significant negative correlation of the TD score with the total number of HIV peptides that elicited CD8 T cell responses. Notably, a similar correlation was found in the dengue virus repertoire size for a set of 16 HLA-A and 11 HLA-B allotypes using computational prediction algorithms. Finally, Bashirova et al. correlated the TD score with the outcome of HIV disease progression. Statistical analyses of two independent HIV cohorts demonstrated that a higher global TD was associated with a rapid progression to AIDS. Of note is that these results were further validated using the influence of TD on viral load in an independent cohort of 4,306 individuals prior to initiation of antiretroviral therapy with longitudinal viral load measurements. More importantly, in a large cohort of HIV-infected individuals, the authors demonstrate that tapasin-independent HLA allotypes are associated with slower disease progression and lower viral load (19).
Studies exploring the differential dependence of alleles on tapasin have shown that tapasin plays a role in stabilizing the “peptide-receptive” state of certain alleles, whereas other alleles reach a “peptide-receptive” conformational form in the absence of tapasin (13, 20). In another set of studies, Park et al. (21) showed that HLA class I alleles with acidic amino acids at position 114 strongly associated with tapasin and were dependent on tapasin for high-affinity peptide loading and cell surface expression, whereas HLA class I alleles that do not require tapasin are capable of binding a broad spectrum of the peptide repertoire with efficient cell surface expression. This has been demonstrated even for alleles closely related, such as the HLA-B*4405 and HLA-B*4402 alleles which evolved to load antigens in dependent and independent tapasin fashion, respectively (22, 23).
MHC class I molecules can also present exogenous antigens by a process known as cross-presentation (6). Interestingly, Goodridge et al. (24) showed that, during the exogenous presentation of viral, tumor, and minor histocompatibility antigens, nonclassic HLA-F molecules may directly interact with HLA class I molecules to stabilize those HLA class I molecules not loaded with peptide or complexed with β2-microglobulin into an “open conformers” form on the cell membrane. The study also showed that antigens, classic HLA class I molecules in “open conformers” form, and possibly HLA-F are transferred from the surface into lysosomes or lysosome-like structures through the endosomal pathway. Therefore, antigen-specific peptides are produced by protein degradation and transferred to the cell membrane complexed to MHC class I molecules independently of TAP and tapasin, as described in the MHC class II antigen presentation pathway (24).
Given the importance of peptide loading and editing mechanisms in the antigen presentation process, HLA alleles which encode tapasin-independent molecules might have evolved in response to evolutionary pressures exerted by pathogens. For example, the adenovirus protein E19 targets tapasin to prevent antigen presentation and to evade CD8 T cell recognition (25). Thus, the evolution of tapasin-independent mechanisms would allow certain MHC class I molecules to present viral and tumor antigens even when tapasin functions are impaired, to ensure clearance or control of pathogen dissemination and tumor surveillance (26–28) (Fig. 1).
Importantly, findings from Bashirova et al. (19) suggest that HLA tapasin independence not only influences immune responses by broadening the repertoire of antigenic peptides, also documented by other groups, but can significantly affect infectious disease outcomes. Ultimately, the discovery of the HLA allotypes and their lack of TD for peptide editing will have important practical implications in designing novel successful vaccines and immunotherapies against infectious diseases and cancer.
Footnotes
The authors declare no competing interest.
See companion article, “HLA tapasin independence: Broader peptide repertoire and HIV control,” 10.1073/pnas.2013554117.
References
- 1.Germain R. N., Margulies D. H., The biochemistry and cell biology of antigen processing and presentation. Annu. Rev. Immunol. 11, 403–450 (1993). [DOI] [PubMed] [Google Scholar]
- 2.York I. A., Rock K. L., Antigen processing and presentation by the class I major histocompatibility complex. Annu. Rev. Immunol. 14, 369–396 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Jin P., Wang E., Polymorphism in clinical immunology—From HLA typing to immunogenetic profiling. J. Transl. Med. 1, 8−11 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trowsdale J., Knight J. C., Major histocompatibility complex genomics and human disease. Annu. Rev. Genomics Hum. Genet. 14, 301–323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radwan J., Babik W., Kaufman J., Lenz T. L., Winternitz J., Advances in the evolutionary understanding of MHC polymorphism. Trends Genet. 36, 298–311 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Embgenbroich M., Burgdorf S., Current concepts of antigen cross-presentation. Front. Immunol. 9, 1643 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulpke S., Tampé R., The MHC I loading complex: A multitasking machinery in adaptive immunity. Trends Biochem. Sci. 38, 412–420 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Natarajan K., Jiang J., Margulies D. H., Structural aspects of chaperone-mediated peptide loading in the MHC-I antigen presentation pathway. Crit. Rev. Biochem. Mol. Biol. 54, 164–173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouvier M., Accessory proteins and the assembly of human class I MHC molecules: A molecular and structural perspective. Mol. Immunol. 39, 697–706 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Ellgaard L., Helenius A., Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 4, 181–191 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Lehner P. J., Surman M. J., Cresswell P., Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line. 220. Immunity 8, 221–231 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Wright C. A., Kozik P., Zacharias M., Springer S., Tapasin and other chaperones: Models of the MHC class I loading complex. Biol. Chem. 385, 763–778 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Chen M., Bouvier M., Analysis of interactions in a tapasin/class I complex provides a mechanism for peptide selection. EMBO J. 26, 1681–1690 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howarth M., Williams A., Tolstrup A. B., Elliott T., Tapasin enhances MHC class I peptide presentation according to peptide half-life. Proc. Natl. Acad. Sci. U.S.A. 101, 11737–11742 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams A. P., Peh C. A., Purcell A. W., McCluskey J., Elliott T., Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity 16, 509–520 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Zarling A. L., et al. , Tapasin is a facilitator, not an editor, of class I MHC peptide binding. J. Immunol. 171, 5287–5295 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Kaufman J., Antigen processing and presentation: Evolution from a bird’s eye view. Mol. Immunol. 55, 159–161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandea A. G., 3rd, et al. , Impaired assembly yet normal trafficking of MHC class I molecules in Tapasin mutant mice. Immunity 13, 213–222 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Bashirova A. A., et al. , HLA tapasin independence: Broader peptide repertoire and HIV control. Proc. Natl. Acad. Sci. U.S.A. 117, 28232–28238 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sieker F., Straatsma T. P., Springer S., Zacharias M., Differential tapasin dependence of MHC class I molecules correlates with conformational changes upon peptide dissociation: A molecular dynamics simulation study. Mol. Immunol. 45, 3714–3722 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Park B., Lee S., Kim E., Ahn K., A single polymorphic residue within the peptide-binding cleft of MHC class I molecules determines spectrum of tapasin dependence. J. Immunol. 170, 961–968 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Zernich D., et al. , Natural HLA class I polymorphism controls the pathway of antigen presentation and susceptibility to viral evasion. J. Exp. Med. 200, 13–24 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badrinath S., et al. , Position 156 influences the peptide repertoire and tapasin dependency of human leukocyte antigen B*44 allotypes. Haematologica 97, 98–106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodridge J. P., et al. , HLA-F and MHC-I open conformers cooperate in a MHC-I antigen cross-presentation pathway. J Immunol. 191, 1567−1577 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett E. M., Bennink J. R., Yewdell J. W., Brodsky F. M., Cutting edge: Adenovirus E19 has two mechanisms for affecting class I MHC expression. J. Immunol. 162, 5049–5052 (1999). [PubMed] [Google Scholar]
- 26.Hansen T. H., Bouvier M.. MHC class I antigen presentation: Learning from viral evasion strategies. Nat. Rev. Immunol. 9, 503–513 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Harvey I. B., Wang X., Fremont D. H., Molluscum contagiosum virus MC80 sabotages MHC-I antigen presentation by targeting tapasin for ER-associated degradation. PLoS Pathog. 15, e1007711 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shionoya Y., et al. , Loss of tapasin in human lung and colon cancer cells and escape from tumor-associated antigen-specific CTL recognition. OncoImmunology 6, e1274476 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]