Abstract
Aloe vera L. is widely cultivated in many countries due to its importance as an all-purpose herbal or medicinal plant. The growth and yield of this plant can be enhanced by application of fertilizer. It is expected that a higher and balanced nutrient supply will result in higher crop production maintaining soil health, which is possible when the applied fertilizers are done in way that is efficient. So, there is a need to understand the amount of applied and type of fertilizer that will give the best output for farmers and to formulate economical market products. This study was conducted to investigate the effect of N fertilizer on leaf yield, its uptake and requirement, critical concentration, use efficiency and economics of Aloe vera L. Plants were grown at six levels of N: 0, 40, 80, 100, 150 and 200 kg ha−1 from urea and diammonium phosphate (DAP) following completely randomized design with three replicates under field condition. The highest values of yield and yield attributes and profit based on benefit cost ratio (3.81 for urea and 2.91 for DAP) were obtained with 150 kg N ha−1 (urea) and 100 kg N ha−1 (DAP). Leaf biomass yield increased by 18–128 % in urea-N and 30–139 % in DAP-N fertilized plant over control while DAP > urea by 7.59 %. Sucker production (mean number) was urea-N (4.95 Plant−1) > DAP-N (2.28 Plant−1). Both gel and leaf N concentration and uptake was highest at 200 kg ha−1 for both sources. For 80 % leaf biomass yield, minimum requirement of N was ca 74.90 (urea) and 89.60 kg ha−1 (DAP). Growth and yield parameters to N application exhibited significant and positive correlations. Critical leaf N concentration was ca 0.88% (DAP) and 0.90% (urea) while mean and maximum NUE was 34% and 64 % (urea) and 43% and 69% (DAP), respectively. Farmers can be advised to apply N at the rate of 150 kg ha−1 from urea for producing economically higher yield and better-quality A. vera leaves.
Keywords: Aloe vera L., Leaf yield, NUE, Critical N concentration, Urea, DAP, Chemistry, Food science, Agricultural science, Environmental science, Biological sciences
Aloe vera L.; Leaf yield; NUE; Critical N concentration; Urea; DAP; Chemistry; Food science; Agricultural science; Environmental science; Biological sciences.
1. Introduction
Aloe vera L. is an important member of Liliaceae family and genus Aloe (Nie et al., 2018; Cock, 2015; Hasanuzzaman et al., 2008; Reynolds, 2004) widely cultivated in many countries due to its importance as an all-purpose herbal or medicinal plant. Due to this, the plant is sometimes referred as a “miraculous plant”, ‘the wonder plant’, ‘plant of immortality’ and ‘nature powder’ (Lanka, 2018; Akev et al., 2015). It leaves of this plant contain fat compounds, carbohydrates, proteins, lipids, and 18 essential amino acids, vitamins (e.g., A, C, E, vitamin B12, folic acid), minerals, glycoprotein, C-glucosylchromone, anthraquinones, emodin, salicylic acid and various kinds of enzymes (Lanka, 2018; Hamman, 2008; Surjushe et al., 2008). Also contained in the plant are secondary metabolites including alkaloids, aloins, lectins, lignin, saponins, tannins, phenolic and glukomannan are also present in the plant (Boudreau and Beland, 2006; Darini et al., 2013). A. vera is familiar for using as functional food supplement and preservative of foods for the presence of antioxidant molecules, high amount of carbohydrates, and vitamins as its constituents (Gupta et al., 2020a). The plant has wide use in cosmetology and medicine (Lanka, 2018; Cock, 2015; Akev et al., 2015; Eshun and He, 2004; Hamman, 2008). A. vera extract can also be used for the perturbation of enzymatic and nonenzymatic antioxidative indices levels in rats (Gupta et al. 2019, 2020b).
As the nutrient uptake and crop yields are the principal factors that determine optimal fertilization practices (Ju and Christie, 2011), it is expected that a higher and balanced nutrient supply will result in higher crop production maintaining soil health. This is possible when the applied fertilizers are done in way that is efficient way. Thereby minimizing loss of nutrient and improving its efficiency (Li et al., 2009). Nitrogen (N) is a key nutrient for plant growth and playing vital role in plant biochemical processes associated with amino acid, protein, enzymes, and chlorophyll molecule (Abbas and Fares, 2009). In soil, nitrogen enhancing plant growth will result in increase in leaf area and number of leaves and having direct impact on vegetative and reproductive phases of plants (Zhang et al., 2014; Ibrahim et al., 2011; Acquaah, 2005; Fageria and Baligar, 2005). In the atmosphere, by composition N is the largest and yet remains the most limiting in most of the plants. The mechanism by which N is imputed in soil from the atmosphere includes non-symbiotic and symbiotic fixation, and rainfall in addition (Sullivan et al., 2014). The natural soil N input mechanism does not significantly support food production globally as there is a continuous population growth (Crews and Peoples, 2004), therefore, the use of N from synthetic fertilizer sources is highly necessary. Nitrogen generally determines crop yield and when applied in excess, will lead to low N use efficiency (NUE). Researchers such as Fageria and Baligar (2005), Hirel et al. (2007) and Garnett et al. (2009) have developed methods in improving the low NUE beyond the environmental, agronomic, and breeding perspectives. These methods involved genetic variability and quantitative genetics as well as using specific root phenotypes such as root morphology; root to shoot ratios; root vigour, root length density; and root N transport and metabolism. Two widely used source of N include Urea and DAP. They are preferable by farmers for their crop cultivation. These fertilizers when applied in soil are hydrolyzed to produce NH4+. Though DAP is known to give poor yields in calcareous soils where a greater portion of insoluble reaction products may be prevalent (Tisdale and Nelson, 1970), soil released substantial amounts of NH+-N for relatively longer period from DAP than urea amended soil (Mohiuddin et al., 2006). The ammonium ion present in DAP enhances phosphorus (P) uptake by altering soil pH and P solubility near the plant root (Soon and Miller, 1971). Albeit the huge potential of commercial cultivation of A. vera in Bangladesh for both domestic and international market, efforts is still very limited, due to information gap regarding fertilizer requirement, nutritional values, and marketing. Critical leaf N concentration, NUE and N requirement for the cultivation of A. vera is yet to be reported in Bangladesh. The comparative performance of urea and DAP as a source of N for A. vera cultivation with respect to leaf biomass yield, its content and use efficiency have also not been reported. The objectives of the study were to determine N requirement, NUE and critical leaf N concentration and economics for the growth and yield of A. vera in urea and DAP amended soil.
2. Materials and methods
2.1. Experimental site
The pot experiment was carried out with A. vera in the farmer's nursery, Kashiganj, Tarakanda, Mymensingh, Bangladesh during September 2018 to May 2019. Geographically the experimental site was located at 24°75′26.5″ N latitude and 90°50′12.2″ E longitude at an elevation of 18 m above the sea level. The site belonged to the Non-calcareous Dark Grey Floodplain soil under the Agro-Ecological Zone of Old Brahmaputra Floodplain and classified as Cambisols according to World Reference Base (AEZ-9) (IUSS WG WRB, 2015). The climate of the experimental area is under the sub-tropical climatic zone, which is characterized by moderate to high temperature, heavy rainfall, high humidity and relatively long day during kharif (April to September) and scanty rainfall, low humidity, low temperature and short-day period during rabi season (October to March).
2.2. Experimental set up
Non-calcareous soil was collected from 0-15 cm depth of the selected area, made free from plant residues and other extraneous materials, air dried, grinded, and sieved through a 2 mm sieve, 500 g was separated and preserved. Soil pH was measured using a glass-electrode pH meter, the soil water ratio being 1: 2.5 as described by Saha et al. (2018). Organic C was determined following wet digestion method (Nelson and Sommers, 1982). The amount of organic matter was calculated by multiplying the per cent organic carbon with the van Bemmelen factor 1.73 (Piper, 1950). Micro-Kjeldahl method (Bremner and Mulvany, 1982) was used to measure soil total N. Soil available P was extracted by NaHCO3 (pH 8.51) solution and determined colorimetrically using molybdate blue ascorbic acid method (Olsen and Sommers, 1982). Exchangeable K, Ca and Mg was extracted by ammonium acetate extraction method (Coleman et al., 1959) and determined by flame photometer as outlined by Knudsen et al. (1982). Calcium and Mg concentration was determined by complexometric method of titration using Na2EDTA as a complexing agent (Page et al., 1982). Available S was extracted by CaCl2 solution and determined turbidimetrically using BaCl2 crystals (Fox et al., 1964). Available B was extracted following a hot water extraction method (Page et al., 1982) and determined by spectrophotometer using azomethine-H (Keren, 1996). Available Cu, Fe, Mn and Zn was extracted following DTPA extraction method and measured by atomic absorption spectrophotometer (Model UNICAM 969, England) (Lindsay and Norvell, 1978). The soil was silty loam in texture, bulk density, particle density and field capacity were 1.46, 2.59 g cm−3 and 27.24%, respectively. Organic matter, pH, total N, exchangeable K, Ca and Mg, were 1.05%, 5.90, 0.06%, 0.13, 3.32 0.78, meq 100g−1, respectively. Available P, S, Zn and B were 3.00, 4.00, 1.81, 0.06 μg g−1 soil, respectively. Ten kg processed soil was taken in each plastic pot of 30 cm in height with 24.50 cm diameter at the top and 20 cm diameter at the bottom. The pot was filled by soil leaving 2 cm from the top and labeled with proper tagging. Six level of N was applied at the rate of 0 (N0), 40 (N40), 80 (N80), 100 (N100), 150 (N150) and 200 (N200) kg ha−1 from urea and DAP. Urea was applied in 3 installments (one-third during land preparation and rest at 60 days after transplanting (DAT) and 120 DAT and DAP in 2 installments (half during soil and pot preparation and half at 60 DAT). Other nutrients P, K, S, Zn and B were applied as basal dose at the rate of 80, 120, 40, 3 and 1 kg ha−1 from TSP, MoP, gypsum, zinc sulphate and boric acid, respectively as prescribed by Biswas (2010). For DAP fertilizer, rest amount of P was adjusted from TSP. Experiment was set up following completely randomized design (CRD) with three replications. The test crop used in the experiment was Aloe vera L. belonging to the family Liliaceae and sub-family Asphodeloideae. Eighteen-month-old A. vera seedlings were collected from Oshudhi village, Natore Sadar, Natore and one seedling per pot was transplanted. Intercultural operations were done as and when necessary.
2.3. Harvesting and cleaning
A. vera leaf was harvested at 14 days interval up to 178 DAT. Leaves were collected carefully and cleaned with tap water followed by distilled water to remove soil and other foreign materials. Paper towel was used to remove adhering water.
2.4. Growth and yield parameters
To understand the growth and development of A. vera plant, specific growth and yield parameters were studied. These have been briefly described under the following heads.
2.5. Plant height
Height of the individual leaf of each plant was measured in centimeter (cm) from ground to the apex of leaf. The average plant height was recorded at 14, 28, 58, 88, 118, 148 and 178 DAT.
2.6. Leaves plant−1
Total number of leaves was counted and recorded at 14, 28, 58, 88, 118, 148 and 178 DAT.
2.7. Leaf area plant−1
After harvest, 3 leaves were randomly selected from each pot during harvest and leaf area was obtained by multiplying leaf length and breadth (expressed in cm2).
2.8. Leaf biomass yield and dry leaf weight
Fresh leaf biomass yield and weight of dried leaf after air drying, sun drying and oven drying at 60 °C for 48 h were recorded and expressed in g plant−1.
2.9. Fresh and dry gel weight
Fresh and dry gel weight after air drying, sun drying and oven drying at 60 °C for 48 h were recorded and expressed in g plant−1.
Preparation of A. vera leaf for chemical analyses: For preparing the extraction, the fresh leaf was chopped, washed, and cut from the middle. The gel was separated by scraping it with a spoon. Then the gel and chopped leaves were sun dried for 2 days. Sun dried leaf and gel was oven dried at 70 °C for 48 h and ground, preserved in polythene bag and kept in desiccators.
N determination: Total N was determined by Kjeldahl method (Page et al., 1982). Powdered leaf and gel samples were digested with conc. H2SO4 in presence of K2SO4 catalyst mixture (K2SO4: CuSO4.5H2O: Se = 10:1:0.1). Nitrogen in the digest was collected by distillation with NaOH followed by titration of the distillate trapped in H3BO3 indicator solution with standard H2SO4.
N uptake: Uptake was calculated from N concentration using the following formula (1) (Maniruzzaman et al., 2017)
| (1) |
N use efficiency (NUE): NUE was calculated using the following formula (2) (Daradjat et al., 1991)
| (2) |
Relative yield: Relative yield was calculated using the following formula (3) (Fageria et al., 2010)
| (3) |
Critical N concentration and N requirement: The critical N concentration in A. vera leaf was estimated from the relative amount of leaf biomass to achieve 80% of the maximum production of leaf biomass yield (Kouno and Ogata, 1988). The relative leaf biomass yield was plotted on the ordinate (Y axis) against the respective N concentration of leaf on the abscissa (X axis). For N requirement estimation, the applied N was plotted on the X axis against the relative leaf biomass yield on the Y axis.
Economic analysis: The cost of production was analyzed per hectare basis to find out the most economic dose of N fertilizer. All input cost included the cost for lease of land and interests at the rate of 8% for one year on running capital. The market price of A. vera leaf and sucker were considered for estimating the cost and return. The benefit cost ratio (BCR) was calculated as follows in Eq. (4) (Tarafder et al., 2020)
| BCR = Gross return per ha−1 (Tk.)/Total cost of production ha−1 (Tk.) | (4) |
Statistical analyses: Data were tabulated and analyzed using statistical software Minitab 2017 Version 17.0 (Minitab Inc, USA). The means for all the treatments were calculated and analysis of variance (ANOVA) for all the characters under consideration was performed by Tukey's range test to determine the significant difference between groups. Overall statistical analysis of the present study was done following Gomez and Gomez (1984).
3. Results
3.1. Plant height
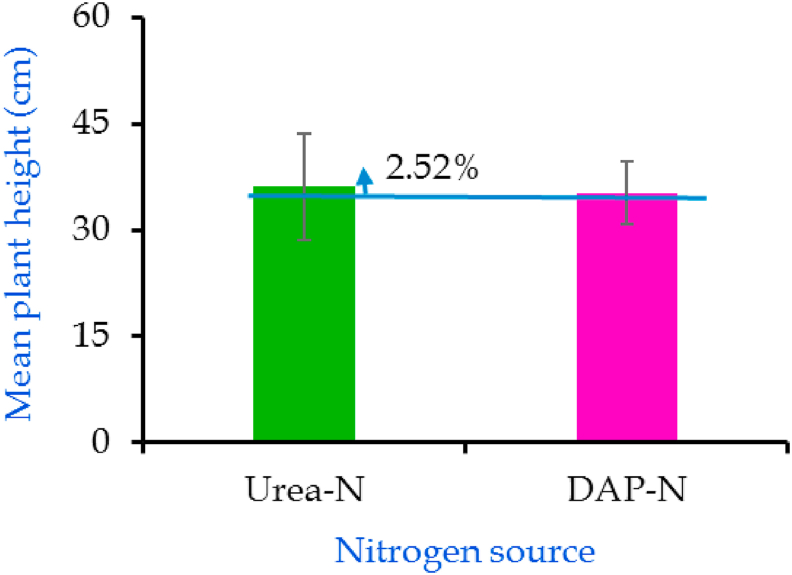
Different N rates had significant effect on plant height of A. vera (Figure 1). An increase in plant height was observed from planting stage to harvesting irrespective of treatments. Nitrogen application at all levels increased plant height by 4.91–11.13 cm and 2.56–9.91 cm in case of urea-N and DAP-N, respectively. Tallest plant was obtained when N was applied at the rate of 150 kg ha−1 from urea and 100 kg ha−1 from DAP and the shortest plant was from control. The mean plant height at harvest was 36 cm in case of urea-N and 35 cm in case of DAP-N (Figure 2). However, there were no significant differences (p > 0.05) between them.
Figure 1.
Effects of different levels of N from urea and DAP on the plant height of A. vera. Bars indicate standard error (±SE) at 0.05.
Figure 2.
Comparative performance of urea-N and DAP-N on the plant height of A. vera. Bars indicate standard error (±SE) at 0.05.
3.2. Number of leaves plant−1
Leaf number responded significantly due to the application of different levels of N (Figure 3). The result revealed that number of leaves plant−1 progressively increased with the increasing levels of N application up to 150 kg ha−1 in case of urea-N and 100 kg ha−1 in case of DAP and then declined. The application of N influenced the number of leaves plant−1 variably from 15 to 178 DAT irrespective of fertilizers and treatments. Rapid increase in leaf number was observed between 28 and 178 DAT in both fertilizers. The highest number of leaves plant−1 (16.67 in urea and 18.00 in DAP) at 178 DAT was counted from the plant receiving 150 kg N ha−1 from urea and 100 kg N ha−1 from DAP. On the other hand, lowest plant height was obtained from the control treatment which might be due to no N application. Average 14.83 leaves were obtained from urea-N treated plant and 15.91 leaves were obtained from DAP-N treated plant (Figure 4).
Figure 3.
Effects of different levels of N from urea and DAP on the number of leaves of A. vera. Bars indicate standard error (±SE) at 0.05.
Figure 4.
Comparative performance of urea-N and DAP-N on the number of leaves of A. vera. Bars indicate standard error (±SE) at 0.05.
3.3. Leaf biomass yield
The leaf biomass yield of A. vera varied significantly due to the application of different levels of N fertilizer (Figure 5). The highest leaf biomass yield pot−1 was measured from the plant receiving 150 kg N ha−1 from urea (1952 g) and 100 kg N ha−1 from DAP (2015 g) that were significantly higher than other levels of N. The lowest values were obtained from the control. Nitrogen application at all levels increased leaf biomass yield at harvest by 18–128% in urea-N and 30–139% in DAP-N, respectively over control. All doses showed significant differences (p < 0.05) for both urea-N and DAP-N and from the controls.
Figure 5.
Effects of different levels of N from urea and DAP on the leaf biomass yield of A. vera. Bars indicate standard error (±SE) at 0.05.
Comparatively 7.59% higher mean leaf biomass yield was obtained by the application of DAP-N than urea-N (Figure 6). Nitrogen application at all levels increased leaf biomass yield on average 92% in urea-N and 89% in DAP-N, respectively over control.
Figure 6.
Comparative performance of urea and DAP on the leaf biomass yield of A. vera. Bars indicate standard error (±SE) at 0.05.
3.4. Total leaf area and number of suckers
The data pertaining to the total leaf area and number of suckers plant−1 at harvest as influenced by different levels of N have been presented in Table 1. Leaf area plant−1 responded significantly (p < 0.05) due to the application of different levels of N. Results revealed that the highest total leaf area plant−1 at harvest was measured from the plant receiving 150 kg ha−1 from urea-N (3714 cm2) and 100 kg ha−1 from DAP-N (3994 cm2) which was significantly (p < 0.05) higher than other levels of N. The lowest leaf area was found from the control treatment. The number of suckers also significantly and progressively increased with the increased levels of N application up to 200 kg ha−1 in urea (9.67) and 150 kg ha−1 in DAP (4.00) and then declined with further addition. The lowest number of suckers was found from the control. Mean number of sucker production was more than double in urea-N fertilized soil (4.95 Plant−1) than DAP-N (2.28 Plant−1). Comparatively 117% higher number of suckers was obtained by the application of urea-N than DAP-N (Figure 7).
Table 1.
Effects of different levels of N from urea and DAP on total leaf area, number of suckers, fresh gel weight, dry gel weight and leaf biomass yield increase over control of Aloe vera L.
| N level (kg ha−1) | Total leaf area plant−1 (cm2) |
Suckers pot−1 (No) |
Fresh gel weight (g pot−1) |
Dry gel weight (g pot−1) |
∗LBY increase over control (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Urea | DAP | Urea | DAP | Urea | DAP | Urea | DAP | Urea | DAP | |
| N0 | 1844e | 1664e | 1.33d | 0.33c | 484e | 534e | 7.44c | 8.68f | _ | _ |
| N40 | 2348d | 2252d | 2.67cd | 1.00bc | 671d | 695d | 9.14c | 10.17e | 18 | 30 |
| N80 | 2659cd | 2614c | 3.67cd | 1.67b | 973c | 941c | 14.80b | 15.05d | 94 | 63 |
| N100 | 3084b | 3995a | 6.33ab | 3.67a | 1088b | 1335a | 16.81b | 21.98a | 105 | 139 |
| N150 | 3714a | 3182b | 6.00c | 4.00a | 1252a | 1256a | 19.85a | 19.73b | 128 | 121 |
| N200 | 2784bc | 2858c | 9.67a | 3.00a | 1161b | 1062b | 17.41ab | 17.41c | 116 | 92 |
| SE | 83.89 | 63.93 | 0.70 | 0.17 | 13.45 | 17.87 | 0.45 | 0.24 | - | - |
| CV (%) | 5.42 | 4.18 | 33.09 | 37.27 | 3.53 | 4.87 | 7.40 | 3.22 | - | - |
LBY = Leaf biomass yield; CV = Coefficient of variance, SE± = Standard error of means. Values with the same alphabet in column are not significantly different at 5% level of probability.
Figure 7.
Comparative performance of urea and DAP on the mean sucker number of A. vera. Bars indicate standard error (±SE) at 0.05.
3.5. Fresh and dry gel weight
The fresh gel weight of A. vera plant−1 at harvest varied significantly due to the application of different levels of N fertilizer (Table 1). The highest fresh gel weight plant−1 (1252 g in urea-N and 1335 g in DAP-N) at harvest was found from the plant receiving 150 kg N ha−1 from urea-N and 100 kg N ha−1 from DAP-N which was significantly higher than other levels of N. The lowest fresh gel weight was found from the control treatment. The fresh gel weight was higher in case DAP-N applied pot than urea-N. Dry gel weight of A. vera plant−1 at harvest varied significantly (Table 1). The highest dry weight plant−1 (19.85 g in urea-N and 21.98 g in DAP-N) at harvest was measured from the plant receiving 150 kg N ha−1 from urea-N and 100 kg N ha−1 from DAP-N and the lowest values were obtained from the control treatment. The dry gel weight was higher in case DAP-N applied pot than urea-N.
3.6. Gel N concentration
There was a significant effect (p < 0.05) of different levels of N on its concentration in A. vera gel (Table 2). Nitrogen concentration of the gel was increased with the increased levels of N irrespective of fertilizers used. The highest N concentration (0.62% in urea-N and 0.68% in DAP-N) was obtained when N was applied at the rate of 200 kg ha−1 which was significantly higher than other levels of N. The lowest N concentration was obtained from the plants receiving no N in case of both fertilizers. The gel N concentration of the plants grown in the pot fertilized with urea was lower the N concentration of the plants grown in the pot fertilized with DAP.
Table 2.
Effects of different levels of N from urea and DAP on the gel and leaf N concentration and its uptake by Aloe vera leaf.
| N level (kg ha−1) | Gel N conc. (%) |
Leaf N conc. (%) |
Leaf N uptake (mg pot−1) |
|||
|---|---|---|---|---|---|---|
| Urea | DAP | Urea | DAP | Urea | DAP | |
| N0 | 0.17c | 0.21e | 0.35e | 0.46d | 159.41d | 142.63b |
| N40 | 0.38b | 0.27de | 0.73d | 0.58cd | 273.78cd | 237.61b |
| N80 | 0.46ab | 0.36cd | 0.88cd | 0.74c | 469.02bc | 321.05b |
| N100 | 0.52ab | 0.49bc | 1.09bc | 1.11b | 683.37ab | 792.92a |
| N150 | 0.55ab | 0.57ab | 1.15ab | 1.20a | 739.69a | 739.49a |
| N200 |
0.62a |
0.68a |
1.37a |
1.44ab |
893.64a |
828.90a |
| SE | 0.04 | 0.03 | 0.04 | 0.05 | 43.48 | 46.70 |
| CV (%) | 15.61 | 14.59 | 8.19 | 10.19 | 15.11 | 15.18 |
CV = Coefficient of variance, SE± = Standard error of means. Values with the same alphabet in column are not significantly different at 5% level of probability.
3.7. Leaf N concentration and uptake
Different levels of N exerted significant influence on its concentration and uptake by A. vera leaf (Table 2). Nitrogen concentration of the leaf was increased with the increased levels of N irrespective of fertilizers used. The highest N concentration (1.17% in urea-N and 1.44% in DAP-N) was obtained when N was applied at the rate of 200 kg ha−1 which was significantly higher than other levels of N. The lowest N concentration was obtained from the plants receiving no N in case of both fertilizers. The leaf N concentration of the plants grown in the pot fertilized with urea was lower than N concentration of the plants grown in the pot fertilized with DAP. The effects of different levels of N on its uptake were significant (Table 2). The trend was similar like N concentration of A. vera leaf. Mean leaf N concentration was more than double (0.93%) compared to gel-N concentration (0.44%) irrespective of the fertilizer sources (Figure 8). Comparatively 110% higher N concentration was obtained in the leaf compared to gel of A. vera.
Figure 8.
Mean N concentration of A. vera gel and leaf as influenced by urea and DAP. Bars indicate standard error (±SE) at 0.05.
The N uptake was maximum (893.64 mg pot−1 in urea-N200 and 828.90 mg pot−1 in DAP-N200) which showed no significant difference (p > 0.05). Similarly, when N was applied at the rate of 150 kg ha−1 from both urea and DAP, there were also no significant difference (p > 0.05). Nitrogen uptake as expected was increased with N levels up to 200 kg ha−1. In contrast, the lowest content and uptake of N was obtained from control treatments.
3.8. Correlation studies among yield and yield attributes of Aloe vera L
Statistical relationships between leaf biomass yield (LBY) with plant height and leaf area, LBY and fresh gel weight of A. vera were studied (Figure 9). The results revealed that plant height and total leaf area were significantly and positively correlated with leaf biomass yield having the correlation coefficients (r) of 0.91∗∗ and 0.90∗∗, respectively. Fresh gel weight was also significantly and positively correlated (r = 0.98) with LBY.
Figure 9.
Correlations and regression equations between leaf biomass yield (LBY) with plant height and leaf number, fresh gel weight (FGW) with LBY as influenced by different levels of N from urea and DAP.
3.9. Critical leaf N concentration of A. vera
From Figure 10, the N concentration corresponding to the arbitrary point at 80% to achieve the maximum leaf biomass production was estimated by the fitted curve to be ca 0.90 and 0.88% in A. vera leaf grown in urea-N and DAP-N treated pot, respectively.
Figure 10.
Correlation between leaf N concentration and relative leaf biomass yield of A. vera.
3.10. Nitrogen requirement for Aloe vera L
From the fitted curve used in determining the N requirement, it was revealed that the corresponding estimated minimum amount of N for 80% leaf biomass production in the plant grown in urea-N and DAP-N treated pot was estimated to be ca 74.90 and 89.60 kg ha−1, respectively (Figure 11).
Figure 11.
Correlation between applied N and relative leaf biomass yield of A. vera L.
3.11. Nitrogen use efficiency
The nitrogen use efficiency of A. vera as influenced by different levels of N is shown in Figure 12. The result revealed that, in case of DAP-N, the highest value of NUE (69%) was recorded in N100 and the lowest in N80. But in case of urea-N, the highest NUE (55%) was obtained from 80 kg N ha−1 and lowest at the rate of 200 kg N ha−1. Mean NUE was higher in DAP (42.47%) fertilized soil than urea (33.88%) and that was almost 25.35% higher than urea (Figure 13).
Figure 12.
Effect of different levels of N from urea and DAP on NUE ofA. vera. Bars indicate standard error (±SE) at 0.05.
Figure 13.
Comparative performance of urea and DAP on mean NUE of A. vera. Bars indicate standard error (±SE) at 0.05.
3.12. Economic analysis
Net return of different treatments of N from urea and DAP (Table 3) showed that net return varied significantly among N levels and source. The highest net return (Tk. 14,48,060 or USD 17,076 in Urea-N and Tk. 9,91,866 or USD 11697 in DAP-N) was found from the plant receiving 150 kg N ha−1 from urea and 100 kg N ha−1 from DAP which was significantly higher than other levels of N. The net return showed significant differences (P < 0.05) for different urea-N treatment except for N0 to N40 while DAP showed only significant differences (p < 0.05) for N0, N100, N150 and N200. In contrast, the lowest net return was obtained from the control. Mean net income of (Tk. 8,58,650) was generated from urea-N fertilized plant compared to (Tk 6,89,638) from DAP-N fertilization from one hectare of land. Comparatively 24.51% higher net return was obtained by the application of urea than DAP (Figure 14).
Table 3.
Comparative economic per hectare profitability of Aloe vera L. as influenced by different levels of N from urea and DAP.
| N level (kg ha−1) | Urea |
DAP |
BCR |
|||
|---|---|---|---|---|---|---|
| Net return (Tk.) | Net return (USD) | Net return (Tk.) | Net return (USD) | Urea | DAP | |
| N0 | 351247d | 4142d | 361747d | 4266d | 1.69d | 1.71d |
| N40 | 601730d | 7096d | 504594c | 5950c | 2.18d | 1.98c |
| N80 | 663214cd | 7821cd | 605442c | 7140c | 2.29cd | 2.17c |
| N100 | 1050955b | 12393b | 991866a | 11697a | 3.05b | 2.91a |
| N150 | 1448060a | 17076a | 892175ab | 10521ab | 3.81a | 2.70ab |
| N200 | 1036664bc | 12225bc | 781985b | 9222b | 3.00bc | 2.47b |
| SE | 71642 | 24508 | 0.14 | 0.05 | ||
| CV (%) | 16.95 | 7.39 | 9.25 | 3.79 | ||
CV = Coefficient of variance, SE± = Standard error of means. Values with the same superscript are not significantly different at 5% level of probability.
The Tk was converted to USD according to the Bangladesh bank exchange rate accessed on 29th November 2020 (https://www.bb.org.bd/econdata/exchangerate.php).
Figure 14.
Comparative performance of urea-N and DAP-N on mean net return of A. vera. Bars indicate standard error (±SE) at 0.05.
Data in Table 3 revealed that benefit cost ratio of A. vera significantly influenced by the different levels of N. In case of urea treatment, N150indicated the highest benefit cost ratio (3.81). But in case of DAP treatment, N100 showed highest benefit cost ratio (2.91).
4. Discussion
Plants that are efficient in absorption and utilization of the absorbed nutrients greatly enhance the efficiency of applied fertilizers. It is expected that a higher and balanced nutrient supply will result in higher yield of A. vera. So, there is a need to understand the amount of applied and type of fertilizer that will give the best output for farmers and to formulate economical market products.
The plant height was generally higher than control (Figure 1), while urea-N > DAP-N (Figure 2). The reasons of obtaining higher plant height from urea-N than DAP-N might be due to maximum uptake of N from urea for its quick availability in these levels. This result was supported by previous studies by Jasso-Chaverria et al. (2005), Waseem et al. (2008) and (Barandozi et al., 2011), they found that the improvement of vegetative growth with increased N fertilizer rate attributed to increased uptake of N and its associated role in photosynthesis and carbon dioxide assimilation. Further, Maniruzzaman et al. (2016) reported the influence of the highest N level (N300) to produce the tallest stevia plant (88 cm) in acid soil whereas N250 (94 cm) in non-calcareous soil. Hejazi et al. (2013) reported that 250 kg ha−1 N had the highest values of leaf length of Artichoke (Cynarascolymus L.). Egbuchua and Enujeke (2015) showed that at 50 and 75 kg N ha−1 application rates, leaf length of A. vera increased from 17.21 cm to 18.82 cm as compared to the control. However, contrasting results was obtained by Dastagir and Hussain (2015), when they found better plant height with the application of DAP than that of urea.
The treatments showed increasing number of leaves plant−1 due to the applied fertilizers, which could possibly be ascribed to the fact that N often increases plant growth and subsequently more production of leaves. In a related study, Egbuchua and Enujeke (2015) showed a significant influence of N application for the number of leaves of A. vera and the highest number of leaves (10.30) was found with highest application rate (75 kg N ha−1). Almost similar result was reported by Allahdadi and Farzane (2018) who found the maximum number of leaf plant−1 of Artichoke plant (Cynarascolymus L.) was recorded by 200 kg N ha−1. Maniruzzaman et al. (2016) also recorded maximum number of leaves of stevia plant−1 with N250 which was significantly higher than all other N levels. This could be due to the slow nutrient releasing capacity of DAP than urea. Succinctly, the splitting of N applications can improve N use efficiency because the greatest need is in the phase of fastest mass growth, i.e. typically well into the vegetative phase. Ideally the timing and proportion of the N split(s) matches the demand. This also limits the risk of denitrification and leaching. The plant height and leaf number were better in the application of 150 kg N ha−1 compared to other N application rate. This might be due to better synchronization of N supply according to plant demand which is crucial for better growth and development of plants. Moreover, the optimum supply of N from 150 kg N ha−1 might also play a synergistic role with other nutrients and ensure balanced nutrients supply whereas this might not be the case for lower and excess application of N.
The higher leaf number and the leaf area could be the reason for obtaining higher biomass yield compared to the control (Figure 5) while DAP had higher yield than urea. Previously, similar result was found by Dastagiret al. (2015) who observed that the average leaf fresh biomass yield of A. vera were significantly increased with the increased doses of N from urea and DAP and better result was obtained from different doses of DAP than the same doses of urea. This result is in accordance with the findings of Khan et al. (2012), Egbuchua and Enujeke (2015) and Goussous and Mohammad (2009). Khan et al. (2012) reported that availability of nutrients increased the biomass of plants. The study is also in concomitant with the findings of Egbuchua and Enujeke (2015) who reported highest fresh biomass weight of A. vera using highest N application rate. Goussous and Mohammad (2009) reported an increase of leaves fresh weight of Allium cepa due to N and P fertilizers.
The significant response of leaf area to comparatively higher rates of N-levels for both fertilizer might be considered as an indication that N was taken up by the plant and subsequently utilized in cell multiplication, amino acid synthesis and energy formation that acts as structural compound of the chloroplast which carries out photosynthesis (Ng'etich et al., 2013). Dastagiret al. (2015) also observed similar result that the average leaf area of A. vera were significantly increased with the increased doses of N from urea and DAP and they found better result from the higher dose of DAP than urea. Maniruzzaman et al. (2016) found significantly highest total leaf area plant−1 in acid soil and in non-calcareous soil at 60 DAT from the plant receiving 250 kg N ha−1. Ng'etich et al. (2013) also found a general trend with increase in leaf area as the N-fertilizer was increased with that of 160 kg N ha−1 yielded the highest leaf area of 209 % at 59 DAS when evaluated against the control. Enhanced leaf parameters with increased levels of fertilizers were also reported previously (Khanom et al., 2008). The significant increase in sucker number was highest at 200 kg ha−1 in urea (9.67) and 150 kg ha−1 in DAP (4.00) and then declined with further addition. We further observed that the mean number of sucker production was more than double in urea-N fertilized soil (4.95 Plant−1) than DAP-N (2.28 Plant−1) (Figure 8). Higher number of sucker production in urea-N fertilized soil could be related to fastest release of N from urea compared to DAP during the early growth period of the mother plant. Interestingly leaf area and number of suckers inversely responded to N application from urea and DAP irrespective of N levels.
The fresh and dry gel weight of A. vera plant−1 at harvest varied significantly with DAP-N treatment having higher weight than urea-N (Table 1). Dastagir et al. (2015) also observed similar result for dry weight of A. vera which was significantly increased with the increased doses of N from urea and DAP. Chowdhury et al. (2020) found the highest gel weight plant−1 (2956 g) of A. vera at harvest measured from the plant receiving 25% inorganic fertilizer and 75% poultry manure. Allahdadi and Farzane (2018) found the highest fresh weight of Artichoke (Cynarascolymus L.) plant by application of 200 kg N ha−1. The experiments conducted by Saha et al. (2005) and Nematian et al. (2010) confirmed that the nutrient minerals, such as N and K, increase leaf growth and lead to a substantial amount of gel in A. vera. Previously, many reporters found increased leaf fresh weight from the increased N application (Sheikh and Ishak, 2016; Khaghani et al., 2012; Alizadeh et al., 2010). Hossain et al. (2007) observed that N fertilizers significantly increased the dry leaf weight of A. indica. Allahdadi and Farzane (2018) also reported that the consumption of 200 kg N ha−1 had the highest dry leaf weight of Artichoke (Cynarascolymus L.) plant.
There was a significant and increasing trend on different levels of soil applied N on its concentration in A. vera gel and leaf (Table 2). Control treatments generally showed lowest content and uptake of nutrients. Nitrogen concentration of the gel and leaf increased with the increased levels of N irrespective of fertilizers used; although leaf N uptake of the plants grown in the pot fertilized with urea was lower than the N uptake of the plants grown in the pot fertilized with DAP. Higher nutrient concentration might be due to the higher rate of N application and higher nutrient uptake may be related to higher biomass yield obtained from those rates. Plants generally have the ability to uptake organic chemicals such as N from soil (Isiuku and Enyoh, 2019). Mean leaf N concentration was more than double (0.93%) compared to gel-N concentration (0.44%) irrespective of the fertilizer sources (Figure 9). It could be due to the presence of peel in leaf which is likely to contain more N than gel. Between the gel and leaf, higher concentration was observed in the later. This could be due to the highest dry leaf yield harvested from that treatment and N concentration because nutrient uptake was calculated from their concentrations and corresponding dry leaf yield. These results are in conformity with those of Angkapradipta et al. (1986) who reported that N concentration in stevia plant increased due to its increased doses. Saha al. (2019) concluded that increased N uptake by silver beet resulted from increased application of N fertilizer.
Correlation analysis revealed that plant height and total leaf area were significantly and positively correlated. Similarly, observation was shown by fresh gel weight and LBY (Figure 9). These results suggest that the applied fertilizers (urea and DAP) in the soils conveyed similar effects on the plants vegetative growth and biomass yield. Similar results were reported by Chowdhury et al. (2020) who found significant and positive correlation of plant height, number of leaves and fresh leaf weight with fresh gel weight of A. vera.
The critical nutrition concentration which estimates the critical N concentration is the minimum N concentration necessary to achieve maximum aboveground biomass at any time during the growing season. The obtained minimum concentration of N corresponding to the arbitrary point at 80% to achieve the maximum leaf biomass production, which was estimated to be ca 0.90 and 0.88 % in A. vera leaf grown in urea-N and DAP-N treated pot, respectively (Figure 10). Maniruzzaman et al. (2016) reported the critical N concentration of 1.43 and 1.50 % in the leaves of stevia plants grown in acid and non-calcareous soils, respectively under different levels of N.
Specific nutrient requirement of a crop is called “the minimum content of that nutrient associated with the maximum yield” or “the minimum rate of intake of the nutrient associated with the maximum growth rate” (Loneragan, 1968). Nitrogen requirement are crop specific. From the fitted curve, it was revealed that the corresponding estimated minimum amount of N for 80% leaf biomass production in the plant grown in urea-N and DAP-N treated pot was estimated to be ca 74.90 and 89.60 kg ha−1, respectively (Figure 11). Higher values were obtained Maniruzzamanet al. (2016) estimated for stevia grown in acid and non-calcareous soils to be 273 and 257 kg ha−1, respectively.
The nitrogen use efficiency (NUE) of A. vera informs on the ability of the plant to utilize the applied N in soil effectively. The efficiency can be considered moderate with maximum value of 69 % for DAP-N and 55 % for urea-N (Figure 13). This suggests that A. vera utilizes DAP-N in soil than urea-N for better growth. This could be due to the slow release of N from DAP than urea and urea showed a faster and higher rate of mineralization while DAP had a steady state and relatively slower rate of mineralization (Mohiuddin et al., 2006). On the other hand, more urea-N may not be used by the plants due to loss by leaching and volatilization than DAP-N. This result is supported by the previous reporters (Cao et al., 2018) who suggested considering the N sources to improve NUE in crop production system and reducing N loss from urea application as a first and important step. Because N response is independent of yield level and N availability is strongly dependent on the environment, optimum fertilizer N rates are often unpredictable (Dhital and Raun, 2016). However, improved N economy from reduced N fertilizer inputs must operate within acceptable crop yield levels (Hirel et al., 2007). This result is in line with a review study which indicated that NUE of cereal in 2015 was 35, 41, 30, and 21% for the world, the United States, China, and India, respectively (Omara et al., 2019).
The economic analysis for the application of DAP and urea was studied. The results presented in Table 3, showed that net return varied significantly among N levels and source but higher than control. Higher mean net income of BDT (Bangladesh Taka) was obtained from urea-N compared to DAP-N, which indicate that the better profit (24.51%) will be obtained by applying urea-N in the planting of A. vera (Figure 14). This could be to the high sucker production (Figure 7) in the urea-N treated plant than DAP-N which ultimately added more profit.
The results for the benefit cost ratio of A. vera significantly influenced by the different levels of N (Table 3), which was obtained for N150 and N100 applied urea and DAP treatment, respectively. Chowdhury et al. (2020) found the highest BCR (1.72) of A. vera applying 25% inorganic fertilizer along with 75% poultry manure and the lowest (1.11) from control. According to Kelly and Murekezi (2000), treatments with BCR values lower than 2 are not worthy in farmers’ perspectives. The farmer cannot shift from one crop cultivation to another unless benefits are sure. According to CIMMYT (1988), marginal benefits need to be 1.18 times the marginal costs to be attractive to farmers. All fertilizer treatments did not meet this requirement. This is consistent with the result reported by Celestin (2009) in Rwanda that application of FYM and ½ DAP +½ FYM were more profitable in maize but not in common bean and soybean. A previous report (Rajkhowa et al., 2003) at Jorhat observed that application of 100% fertilizer significantly increased economics of mungbean over control. Tarafder et al. (2020) found that the application of 3 t ha−1 poultry manures along with 70% inorganic fertilizers showed economically better results indicating the highest BCR (BARI Mung-6: 2.47, BINA Mung-8: 2.13).
5. Conclusions
Application of different levels of N exerted significant influence on the growth, leaf yield and nutrient uptake by A. vera. The highest values of plant height, leaf number, leaf biomass yield and profit based on benefit cost ratio, leaf area, number of suckers, fresh and dry gel weight of A. vera were obtained from the plant fertilized with N at the rate of 150 kg ha−1 from urea and at the rate of 100 kg ha−1 from diammonium phosphate. Comparatively 24.50% higher net return was obtained by the application of urea than diammonium phosphate. Nitrogen application at all levels increased leaf biomass yield at harvest over control. About 7.59% higher leaf biomass yield was obtained from fertilization with diammonium phosphate than urea. Mean number of sucker production was more than double in urea fertilized pot than diammonium phosphate. Nitrogen concentration of A. vera gel and leaf and its uptake by leaf were also significantly influenced by their additions. Highest values were obtained from the plant receiving the highest doses of N. The applied N was more concentrated in the leaf than gel. Significant and positive correlations were found among the growth and yield parameters of Aloe vera L. due to N application. The minimum requirements of N to produce 80% leaf biomass yield were 74.90 and 89.60 kg ha−1 from urea and diammonium phosphate, respectively. Critical leaf N concentration of A. vera was estimated to be ca 0.90 and 0.88 % for urea and diammonium phosphate. The highest NUE was estimated to be ca 64 and 69 % for urea and diammonium phosphate, respectively. The results suggest that farmers can be advised to apply N at the rate of 150 kg ha−1 from urea for producing higher yield and better-quality A. vera. leaf. Of course, more research work is required to validate this results in various soil and climatic conditions for better production of this important industrial crops.
Declarations
Author contribution statement
Md. Akhter Hossain Chowdhury: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Taslima Sultana, Md. Arifur Rahman, Biplob Kumar Saha: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Tanzin Chowdhury: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Christian Ebere Enyoh: Analyzed and interpreted the data; Wrote the paper.
Wang Qingyue: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Bangladesh Agricultural University Research System.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Supplying A. vera seedlings and help during experimental set up by Mr. Ahmed Ali of Kashiganj, Tarakanda, Mymensingh are thankfully acknowledged. The authors are grateful to Dr Karen Little, Post-doctoral research fellow, Monash University, Clayton, Australia for english and scientific editing of the manuscript.
Contributor Information
Md. Akhter Hossain Chowdhury, Email: m.a.h.chowdhury@bau.edu.bd.
Christian Ebere Enyoh, Email: Cenyoh@gmail.com.
References
- Abbas F., Fares A. Best management practices in citrus production. Tree and Forestry SciBiotech. 2009;3:1–11. [Google Scholar]
- Akev N., Can A., Sütlüpınar N., Çandöken E., Özsoy N., Özden T.Y., Yanardağ R., Üzen E. Twenty years of research on Aloe vera. J. Fac. Pharm. Istanbul. 2015;45(2):191–215. [Google Scholar]
- Alizadeh A., Khoshkhui M., Javidnia K., Firuzi O., Tafazoli E., Khalighi A. Effects of fertilizer on yield, essential oil composition, total phenolic content and antioxidant activity in Saturejahortensis L. (Lamiaceae) cultivated in Iran. J. Med. Plants Res. 2010;4(1):33–40. [Google Scholar]
- Allahdadi M., Farzane P. Influence of different levels of nitrogen fertilizer on some phytochemical characteristics of artichoke (Cynarascolymus L.) leaves. J. Med. Plants. 2018;6:109–115. [Google Scholar]
- Angkapradipta P., Tuti-Watsite, Faturachim P. The N, P and K fertilizer requirements of Stevia rebaudiana Bert. onlatosolic soil Menaraperkebaunan, Horti. Abstracts. 1986;56:9217. [Google Scholar]
- Acquaah G. Prentice Hall of India Private Limited; New Delhi, India 460p: 2005. Principles of Crop Production Theory, Techniques and Technology. [Google Scholar]
- Barandozi F.N., Enferadi S.T., Naghavi M.R., Hassani M.E., Mostofi Y., Mousavi A. Effects of fertilizer on morphological traits in Aloe vera. J. Med. Plants Res. 2011;5(18):4537–4541. [Google Scholar]
- Biswas B.C. Cultivation of medicinal plant. Indian Fertilizer Marketing News. 2010;41:1–4. [Google Scholar]
- Boudreau M.D., Beland F.A. An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera. J. Environ. Sci. Health. 2006;24:103–154. doi: 10.1080/10590500600614303. [DOI] [PubMed] [Google Scholar]
- Bremner J.M., Mulvaney C.S. Nitrogen-total. In: Page A.L., Miller R.H., Keeney D.R., editors. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties. American Society of Agronomy, Soil Science Society of America; Madison, Wisconsin: 1982. pp. 595–624. [Google Scholar]
- Cao Y., SunH, Zhang J., Chen G., Zhu H., Zhou S., Xiao H. Effects of wheat straw addition on dynamics and fate of nitrogen applied to paddy soils. Soil Tillage Res. 2018;178:92–98. [Google Scholar]
- Celestin N.P. Department of Environmental Sciences, Kenyatta University; Kenya: 2009. Effects of Inorganic and Organic Fertilizers on Nutrient Uptake, Soil Chemical Properties and Crop Performance in maize Based Cropping Systems in Eastern Province of Rwanda. [Google Scholar]
- Chowdhury T., Chowdhury M.A.H., Rahman M.A., Nahar K., Chowdhury M.T.I., Khan M.S.I. Response of Aloe vera to inorganic and organic fertilization in relation to leaf biomass yield and post harvest fertility of soil. Bulg. J. Agric. Sci. 2020;26(2):346–354. [Google Scholar]
- Coleman N.T., Weed S.B., Macracken R.J. Cation exchange capacity and exchangeable cations in piedmont soils of north Caroline. Proc. Soil Sci. Am. 1959;23:146–149. [Google Scholar]
- Crews T.E., Peoples M.B. Legume versus fertilizer sources of nitrogen: ecological tradeoffs and human needs. Agric. Ecosyst. Environ. 2004;102:279–297. [Google Scholar]
- CIMMYT . CIMMYT; Mexico D. F.: 1988. From Agronomic Data to Farmer Recommendations: an Economic Training Manual. Completely Revised Edition. [Google Scholar]
- Cock I.E. Novel Natural Products: Therapeutic Effects in Pain, Arthritis and Gastro-Intestinal Diseases. Springer; Basel: 2015. The genus aloe: phytochemistry and therapeutic uses including treatments for gastrointestinal conditions and chronic inflammation; pp. 179–235. [DOI] [PubMed] [Google Scholar]
- Daradjat A.A., Tejasarwana R., Danakusuma M.T., Fagi A.M. Three-quadrant analysis of nitrogen in the soil-rice system on two lalosol soils in West Java, Indonesia. In: Vries P de, Laar van FWT., Kropff M.J., editors. Simulation and Systems Analysis for Rice Production (SARP) Pudoc; Wageningen, Netherlands: 1991. pp. 155–161. [Google Scholar]
- Darini M.T.H., Indradewa D., Shiddieq D., Purwantoro A. Response growth and Aloe vera contains aloin explant plantlets from different sources. J. Sci. 2013;4:5–12. [Google Scholar]
- Dastagir G., Hussain S. Effect of fertilizer treatments on the growth of Aloe vera L. Pakistan J. Bot. 2015;47:147–155. [Google Scholar]
- Dhital S., Raun W.R. Variability in optimum nitrogen rates for maize. Agron. J. 2016;108:2165–2173. [Google Scholar]
- Egbuchua C.N., Enujeke E.C. Growth indices of aloe vera as influenced by nitrogen and phosphorus fertilizers in oxisols of rain forest zone, Nigeria. Global J. Biochem. Biotechn. 2015;4:45–49. [Google Scholar]
- Eshun K., He Q. Aloe vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries-a review. Crit. Rev. Food Sci. Nutr. 2004;44:91–96. doi: 10.1080/10408690490424694. [DOI] [PubMed] [Google Scholar]
- Fageria N.K., Baligar V.C., Jones C.A. CRC Press; 2010. Growth and Mineral Nutrition of Field Crops. [Google Scholar]
- Fageria N.K., Baligar V.C. Enhancing N use efficiency in crop plants. Adv. Agron. 2005;88:97–185. [Google Scholar]
- Fox R.L., Olsen R.A., Rhoades H.F. Evaluating the sulphur status of soil by plant and soil test. Proc. Soil Sci. Soc. Am. 1964;28:243–246. [Google Scholar]
- Garnett T., Conn V., Kaiser B.N. Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ. 2009;32:1272–1283. doi: 10.1111/j.1365-3040.2009.02011.x. [DOI] [PubMed] [Google Scholar]
- Gomez K.A., Gomez A.A. John Wiley & Sons; 1984. Statistical Procedures for Agricultural Research. [Google Scholar]
- Goussous S.J., Mohammad M.J. Comparative effect of two arbuscularmycorrhizae and N and P fertilizers on growth and nutrient uptake of onions. Int. J. Agric. Biol. 2009;11(4):463–467. [Google Scholar]
- Gupta V.K., Yarla N.S., Pereira M. de L., Siddiqui N.J., Sharma B. Recent advances in ethnopharmacological and toxicological properties of bioactive compounds from Aloe barbadensis (miller), Aloe vera. Curr. Bioact. Compd. 2020;16(1):1–23. [Google Scholar]
- Gupta V.K., Kumar A., Pereira M.D., Siddiqi N.J., Sharma B. Anti-inflammatory and antioxidative potential of Aloe vera on the cartap and malathion mediated toxicity in wistar rats. Int. J. Environ. Res. Publ. Health. 2020;17(14):5177. doi: 10.3390/ijerph17145177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V.K., Siddiqi N.J., Ojha A.K., Sharma B. Hepatoprotective effect of Aloe vera against cartap- and malathion-induced toxicity in Wistar rats. J. Cell. Physiol. 2019;234(10):18329–18343. doi: 10.1002/jcp.28466. [DOI] [PubMed] [Google Scholar]
- Hamman J. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13:1599–1616. doi: 10.3390/molecules13081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M., Ahamed K.U., Khalequzzaman K.M., Shamsuzzaman A.M.M., Nahar K. Plant characteristics, growth and leaf yield of (Aloe vera L.) as affected by organic manure in pot culture. Aust. J. Crop. Sci. 2008;2:1–13. [Google Scholar]
- Hejazi S., Mirhadi M.J., Nourmohammadi G.H., DehghanzadehH The effect of planting date, organic and nitrogen manures on morphological traits and chlorogenic acid of Artichoke (Cynarascolymus L.) Int. J. Agron. Plant Prod. 2013;4(1):45–49. [Google Scholar]
- Hirel B.J., Le Gouis B.N., Gallais A. The challenge of improving N use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 2007;58:2369–2387. doi: 10.1093/jxb/erm097. [DOI] [PubMed] [Google Scholar]
- Hossain K.L., Wadud M.A., Kashem M.A., Santosa E., Ali M.S. Effect of different nitrogen and potassium rates on agronomic characters of Aloe indica. Bul. Agron. 2007;35(1):58–62. [Google Scholar]
- Ibrahim M.H., Jaafar H.Z.E., Rahmat A., Abdul Rahman Z. Involvement of nitrogen on flavonoids, glutathione, anthocyanin, ascorbic acid and antioxidant activities of Malaysian medicinal plant LabisiapumilaBlume (kacip fatimah) Intl. J. Molecular Sci. 2011;13(1):393–408. doi: 10.3390/ijms13010393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isiuku B.O., Enyoh C.E. Water pollution by heavy metal and organic pollutants: brief review of sources, effects and progress on remediation with aquatic plants. Anal Methods in Environ. Chem. J. 2019;2(3):5–38. [Google Scholar]
- IUSS Working Group WRB . FAO; Roma IT EU: 2015. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106; p. 192. [Google Scholar]
- Jasso-Chaverria C., Hochmuth G.J., Hochmuth R.C., Sargent S.A. Fruit yield, size, and colour responses of two greenhouse cucumber types to nitrogen fertilization in perlite soilless culture. Horti. Techno. 2005;15:565. [Google Scholar]
- Ju X., Christie P. Calculation of theoretical nitrogen rate for simple nitrogen recommendations in intensive cropping systems: a case study on the North China Plain. Field Crop. Res. 2011;124:450–458. [Google Scholar]
- Kelly V., Murekezi A. Ministry of Agriculture, Animal Resources, and Forestry; Kigali, Rwanda: 2000. Fertilizer Response and Profitability in Rwanda -A Synthesis Offindings from MINAGRI Studies Conducted by the Food Security Research Project (FSRP) and the FAO Soil Fertility Initiative. [Google Scholar]
- Keren R. Boron. In: Sparks D.L., editor. Methods of Soil Analysis. Part 3. American Society of Agronomy; Madison, Wisconsis, United States of America: 1996. pp. 603–623. [Google Scholar]
- Khaghani S., Shakouri M.J., Mafakheri S., Aslanpour M. Effect of different chemical fertilizers on chicory (Cichoriumintybus L.) Indian J. Sci. Technol. 2012;5(1):974–6846. [Google Scholar]
- Khan M.A., MarwatKB AminA., Nawaz A., Khan H. Soil solarization: an organic weed management approach in cauliflower. Commun. Soil Sci. Plant Anal. 2012;43(13):1847–1860. [Google Scholar]
- Khanom S., Chowdhury M.A.H., Islam M.T., Saha B.K. Influence of organic and inorganic fertilizers on mineral nutrition of Stevia in different types of soil. J. Bangladesh Agric. Univ. 2008;6:27–32. [Google Scholar]
- Knudsen D., Peterson G.A., Pratt P.F. Chemical and Microbiological Properties, (methods of soil an2); 1982. Lithium, Sodium, and Potassium. Methods of Soil Analysis. Part 2; pp. 225–246. [Google Scholar]
- Kouno K., Ogata S. Sulphur supplying capacity of soils and critical S values of forage crops. Soil Sci. Plant Nutr. 1988;34:327–339. [Google Scholar]
- Lanka S. A review on Aloe vera-The wonder medicinal plant. J. Drug Deliv. Therapeut. 2018;8(5-s):94–99. [Google Scholar]
- Li X., Lu J., Wu L., Chen F. The difference of potassium dynamics between yellowish red soil and yellow cinnamon soil under rapeseed (Brassica napus L.)–rice (Oryza sativa L.) rotation. Plant Soil. 2009;320:141–151. [Google Scholar]
- Lindsay W.L., Norvell W.A. Development of a DTPA soil test for Zn, Fe, Mn and Cu. SSSA (Soil Sci. Soc. Am.) J. 1978;42:421–428. [Google Scholar]
- Loneragan J.F. Nutrient requirements of plants. Nature. 1968;220:1307–1308. doi: 10.1038/2201307a0. [DOI] [PubMed] [Google Scholar]
- Maniruzzaman M., Chowdhury M.A., Mohiuddin K.M., Chowdhury T. Nitrogen requirement and critical N content of stevia grown in two contrasting soils of Bangladesh. Res. Agric. Livestock and Fisheries. 2016;3:87–97. [Google Scholar]
- Maniruzzaman M., Chowdhury T., Rahman M.A., Chowdhury M.A.H. Phosphorus use efficiency and critical P content of stevia grown in acid and non-calcareous soils of Bangladesh. Res. Agric. Livest., Fish. 2017;4(2):55–68. [Google Scholar]
- Mohiuddin K.M., Ali M.S., Chowdhury M.A.H. Transformation of nitrogen in urea and DAP amended soils. J. Bangladesh Agric. Univ. 2006;4(2):201–210. [Google Scholar]
- Nelson D.W., Sommers L.E. Total Carbon, organic carbon and organic matter. In: Page A.L., Miller R.H., Keeney D.R., Amer, editors. Methods of Soil Analysis, Part 2. second ed. Soc. Agron. Inc.; Madison, Wisconsis, United States of America: 1982. pp. 539–580. [Google Scholar]
- Nematian A., Ghoushchi F., Farnia A., Ariapour A., Mashhadi A.B.M. The effect of planting density and nitrogen fertilizer on active substances in medicinal plant Aloe vera L. Iranian J. Medici. Aromat. Plants. 2010;10:85–89. [Google Scholar]
- Ng’etich O.K., Niyokuri A.N., Rono J.J., Fashaho A., Ogweno J.O. Effect of different rates of nitrogen fertilizer on the growth and yield of zucchini (Cucurbitapepo cv. Diamant L.) Hybrid F1 in Rwandan high altitude zone. Int. J. Agril. Crop Sci. 2013;5(1):54–62. [Google Scholar]
- Nie S., Cui S.W., Xie M. Glucomannans from Dendrobium officinale and aloe. Bioactive Polysaccharides. 2018:295–347. [Google Scholar]
- Olsen S.R., Sommers L.E. Phosphorus. In: Page A.L., Miller R.H., Keeney D.R., editors. Methods of Soil Analysis, Part 2. American Society of Agronomy. Inc. Madison; Wisconsis, United States of America: 1982. pp. 403–427. [Google Scholar]
- Omara P., Aula L., Oyebiyi F., Raun W.R. World cereal nitrogen use efficiency trends: review and current knowledge. Agrosystems, Geosci. Environ. 2019;2:180045. [Google Scholar]
- Page R.E., Jr., Laidlaw H.H., Jr. Closed population honeybee breeding. 1. Population genetics of sex determination. J. Apicult. Res. 1982;21:30–37. [Google Scholar]
- Piper C.S. Adelaide University. Hassel press Australia; 1950. Soil and Plant Analysis. [Google Scholar]
- Reynolds T. Aloe chemistry. In: Reynolds T., editor. Aloes: the Genus Aloe. CRC Press; Boca Raton. Florida, USA: 2004. pp. 39–74. [Google Scholar]
- Rajkhowa D.J., Saikia M., Rajkhowa K.M. Effect of vermicompost and levels of fertilizer on green gram. Legume Res. 2003;26(1):63–65. [Google Scholar]
- Saha B.K., Rose M.T., Wong V.N.L., Cavagnaro T.R., Patti A.F. Nitrogen dynamics in soil fertilized with slow release brown coal-urea fertilizers. Sci. Rep. 2018;8:14577. doi: 10.1038/s41598-018-32787-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B.K., Rose M.T., Wong V.N.L., Cavagnaro T.R., Patti A.F. A slow release brown coal-urea fertilizer reduced gaseous N loss from soil and increased silver beet yield and N uptake. Sci. Total Environ. 2019;649:793–800. doi: 10.1016/j.scitotenv.2018.08.145. [DOI] [PubMed] [Google Scholar]
- Saha R., Palit S., Ghosh B.C., Mittra B.N. Performance of Aloe vera as influenced by organic and inorganic sources of fertilizer supplied through fertigation. Acta Hortic. 2005;676:171–175. [Google Scholar]
- Sheikh S., Ishak C.F. Effect of nitrogen fertilization on antioxidant activity of Mas cotek (Ficusdeltoidea Jack) J. Med. Plants Stud. 2016;4(4):208–214. [Google Scholar]
- Soon Y.K., Miller M.H. Changes in the rhizosphere duel to NH4+ and NO; fertilization and phosphorus uptake by corn seedlings (Zea mays L.) Soil Sci. Soc. Amer. J. 1971;4l:77–80. [Google Scholar]
- Sullivan B.W., Smith W.K., Townsend A.R., Nasto M.K., Reed S.C., Chazdon R.L., Cleveland C.C. Spatially robust estimates of biological N(N) fixation imply substantial human alteration of the tropical N cycle. Proc. Natl. Acad. Sci. USA. 2014;111:8101–8106. doi: 10.1073/pnas.1320646111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surjushe A., Vasani R., Saple D.G. Aloe vera: a short review. Indian J. Dermatol. 2008;53:163–166. doi: 10.4103/0019-5154.44785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarafder S., Rahman M.A., Hossain M.A., Chowdhury M.A.H. Yield of vignaradiata L. And post-harvest soil fertility in response to integrated nutrient management. Agricul.Bio.Sci. J. 2020;6(1):32–43. [Google Scholar]
- Tisdale S.L., Nelson W.L. second ed. The Macmillan Co.; New York, N.Y: 1970. Soil Fertility and Fertilizer. [Google Scholar]
- Waseem K., Kamran Q.M., Jilani M.S. Effect of different levels of nitrogen on the growth and yield of cucumber (Cucumissativus L.) J. Agric. Res. 2008;46:259–266. [Google Scholar]
- Zhang F., Wan X., Zheng Y., Sun L., Chen Q., Zhu X., Guo Effects of nitrogen on the activity of antioxidant enzymes and gene expression in leaves of Populus plants subjected to cadmium stress. J. Plant Interact. 2014;9(1):599–609. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.