Abstract
Linalool and 1,8-cineole are plant-derived isoprenoids with anticancer activities in lung cancer cells, nevertheless, the cellular and molecular mechanisms of action remain poorly understood. The purpose of this study was to determine the anticancer mechanisms of action of linalool and 1,8-cineole in lung adenocarcinoma A549 cells.
Linalool (0–2.0 mM) and 1,8-cineole (0–8.0 mM) inhibited cell proliferation by inducing G0/G1 and/or G2/M cell cycle arrest without affecting cell viability of normal lung WI-38 cells. None of the two monoterpenes were able to induce apoptosis, as observed by the lack of caspase-3 and caspase-9 activation, PARP cleavage, and DNA fragmentation. Linalool, but not 1,8-cineole, increased reactive oxygen species production and mitochondrial membrane potential depolarization. Reactive oxygen species were involved in cell growth inhibition and mitochondrial depolarization induced by linalool since the antioxidant N-acetyl-L-cysteine prevented both effects. Besides, linalool (2.0 mM) and 1,8-cineole (8.0 mM) inhibited A549 cell migration. The combination of each monoterpene with simvastatin increased the G0/G1 cell cycle arrest and sensitized cells to apoptosis compared with simvastatin alone. Our results showed that both monoterpenes might be promising anticancer agents with antiproliferative, anti-metastatic, and sensitizer properties for lung cancer therapy.
Keywords: Linalool; 1,8-Cineole; Non-small lung cancer cells; Cytostatic effects; Reactive oxygen species; Cell migration; Chemosensitizers; Cell biology; Cell culture; Cytotoxicity; Bioactive plant product; Pharmaceutical science; Natural product; Biochemistry; Oxidative stress; Cancer research; Chemotherapy
linalool; 1,8-cineole; non-small lung cancer cells; cytostatic effects; reactive oxygen species; cell migration; chemosensitizers; Cell biology; Cell Culture; Cytotoxicity; Bioactive Plant Product; Pharmaceutical Science; Natural product; Biochemistry; Oxidative Stress; Cancer Research; Chemotherapy
1. Introduction
Lung cancer is the major cause of cancer-associated deaths worldwide. It has been estimated that lung cancer caused 1.76 million deaths in 2018, accounting for almost one in five (18.4%) of total cancer-related deaths [1]. Lung cancer is classified into two main types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC constitutes about 85% of all lung cancers [2]. Lung cancer presents high mortality/incidence rates (greater than 80%) and poor 5-year survival (10–20%) [3]. Such unfavorable prognosis arises from the highly metastatic behavior of lung cancer cells. Most patients are diagnosed only after they show clinical symptoms in advanced metastases. Moreover, lung cancer cells tend to become resistant to a wide variety of anticancer drugs [4].
Therapeutic strategies for lung cancer include chemotherapy, radiotherapy, surgery, immunotherapy, and targeted therapy. Chemotherapy is the main therapeutic choice, and it is employed both as a first-line option or as adjuvant/neoadjuvant in combined treatments [5, 6, 7, 8]. Conventional chemotherapeutics for NSCLC include etoposide, docetaxel, paclitaxel, cisplatin, irinotecan, and doxorubicin among others, which are generally administered in pairwise combinations [8]. The use of drug combinations is intended to enhance the individual effect of each one as well as to minimize or avoid potential tumor resistance to monotherapy, which is recurrent in NSCLC [9]. Also, conventional chemotherapy presents severe toxic side effects such as hair loss, nausea and vomiting, diarrhea, loss of appetite, leukocyte decrease, and tiredness, leading to considerable disruption of the treatments [10, 11]. These undesirable effects are caused by the poor selectivity of the antineoplastic drugs for malignant tissue over normal tissue. Consequently, antineoplastic agents inhibit not only the proliferation of cancer cells but also of normal cells with a high rate of division -hematopoietic precursors, epithelial cells, and hair cells- [10].
Looking for alternatives to replace or complement the current chemotherapeutics, phytochemicals arise as natural, abundant, low-cost, and low- or even non-toxic molecules with various pharmacological properties including anticancer activity [12, 13, 14]. Monoterpenes are aromatic 10-carbon isoprenoids isolated from essential oils present in plants. These molecules are widely used in the food industry and cosmetics and are recognized as safe (GRAS) by the Food and Drug Administration (FDA, USA). Many studies have shown the antitumor activity of monoterpenes in cancer cells in vitro and in vivo through promoting cell cycle arrest, apoptosis, autophagy, and/or senescence [15, 16, 17, 18]. The main anticancer mechanisms reported for these natural compounds include the inhibition of proteins involved in cell survival and proliferation (Ras, ERK, Akt), modulation of oxidative stress and endoplasmic reticulum stress, and depolarization of mitochondrial membrane potential [18, 19, 20]. In addition, it was reported increased anticancer activity of antineoplastic drugs combined with monoterpenes, suggesting their potential as novel anticancer molecules also employed as sensitizer compounds [21, 22, 23].
Linalool is a linear monoterpene present in over 200 species of plants like coriander, basil, laurel, cinnamon, grapevine, green and black teas [24]. 1,8-cineole (eucalyptol), is a cyclic-ether monoterpene found in several plants such as eucalyptus, rosemary, sage, bay, cinnamon, and tea [24]. Both monoterpenes showed pharmacological properties including analgesic, sedative, antioxidant, anti-inflammatory, bactericidal, fungicidal, hypolipidemic, and anticancer activities [25, 26, 27]. We have previously shown for the first time that linalool and 1,8-cineole inhibit the proliferation of NSCLC A549 cells [28]. Moreover, we demonstrated that the combination of each monoterpene with simvastatin - a lipid-lowering drug with high potential in drug repurposing for cancer treatment-synergistically inhibited A549 cell proliferation [28]. However, the mechanisms involved remained elusive.
The present study aimed to explore the anticancer mechanisms of linalool and 1,8-cineole, alone or each one in combination with simvastatin, on lung adenocarcinoma A549 cells.
2. Materials and methods
2.1. Reagents and antibodies
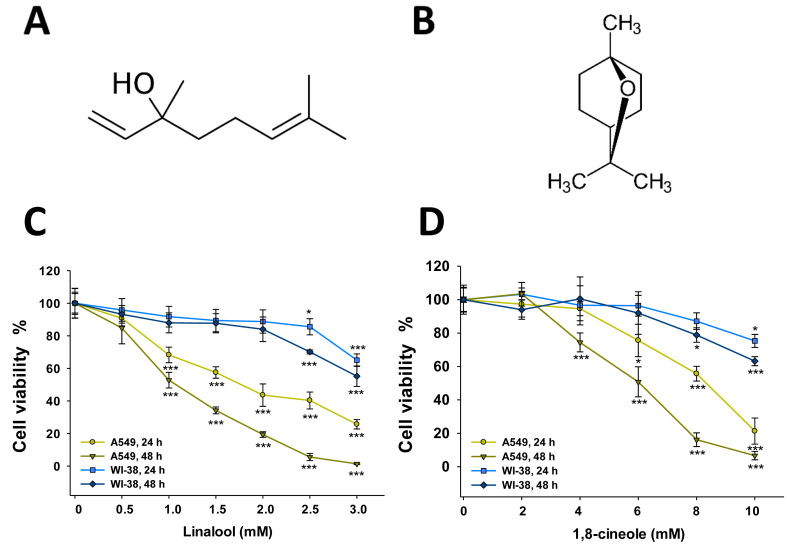
(-)-linalool (>95%, Figure 1A), 1,8-cineole (>99%, Figure 1B), simvastatin, 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA), N-acetyl-L-cysteine (NAC), rhodamine-123, and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Co. (St Louis, MO, USA). Dulbecco's Minimal Essential Medium (DMEM), and penicillin/streptomycin were obtained from Gibco (Invitrogen Corporation, USA). Protease inhibitors were from Thermo Scientific (Rockford, USA). Antibodies for PARP, caspase-3, and caspase-9 were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-GAPDH antibody was from Sigma Aldrich Co (St Louis, MO, USA).
Figure 1.
Effect of linalool (A) and 1,8-cineole (B) on A549 and WI-38 cell viability. Cells were exposed to increasing concentrations of linalool (C) or 1,8-cineole (D) for 24 and 48 h. Cell viability was determined by the MTT assay. Data are expressed as means ± SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
2.2. Cell culture
Human lung adenocarcinoma A549 cells were obtained from the American Type Culture Collection (ATCC CCL-185). WI-38 cells (normal human embryonic lung fibroblasts, ATCC CCL-75) were a kind gift from Dr. Natalia Scaglia (School of Medicine, National University of La Plata). Both cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (Natocor, Argentina) and 1% penicillin/streptomycin in 5% CO2 at 37 °C. The medium was replaced every 2–3 days until cells were passaged after reaching 80–90% confluence. In experiments, cells were cultured under standard conditions for 24 h before the beginning of the treatments. Then, the cells were exposed to DMEM supplemented with 0.2% ethanol (vehicle), 1,8-cineole, or linalool for up to 48 h. The medium was replaced every 24 h. Linalool and 1,8-cineole were dissolved in ethanol 0.2%, a concentration of vehicle that does not affect cellular viability or metabolic activity of A549 cells.
2.3. Cell viability
A549 (4 × 103) and WI-38 (8 × 103) cells seeded in 96-well plates were treated with DMEM supplemented with ethanol 0.2%, linalool (0–3 mM) or 1,8-cineole (0–10 mM) for 24 or 48 h. To evaluate the role of oxidative stress on cell viability, A549 cells were pre-incubated or not with the antioxidant NAC (5 mM) for 2 h prior to the addition of linalool (1.0 and 2.0 mM), 1,8-cineole (4.0 and 8.0 mM), or hydrogen peroxide 0.25 mM (positive control) for 24 h.
Cell viability was determined by the MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) [29]. After treatments, the cells were washed twice in PBS and incubated with MTT solution (0.5 mg/ml in PBS) for 3 h. Formazan was dissolved in 0.1 ml DMSO, the plates were shaken for 10 min, and the absorbance at 560 nm measured with a microplate reader (Beckman Coulter DTX 880).
2.4. Cell cycle analysis
Cells were plated in 6-well plates at a density of 1.5 × 105 and allowed to adhere 24 h. Then, the cells were exposed to ethanol 0.2%, linalool (1.0 and 2.0 mM), 1,8-cineole (4.0 and 8.0 mM), simvastatin 10 μM, or the combination of each monoterpene (linalool 1.0 mM or 1,8-cineole 4.0 mM) with simvastatin 10 μM for 24 h. After treatments, the cells were harvested, centrifuged at 500 × g for 5 min, resuspended in PBS (NaCl 137 mM; KCl 2.7 mM, 10.0 mM Na2HPO4, 2.0 mM KH2PO4, pH 7.4), and fixed in 70% (v/v) ice-cold ethanol at 4 °C overnight. After washing twice with cold PBS, the cells were incubated with RNase A (500 U/mL; Biodynamics, Argentina) at 37 °C for 20 min. Nuclei were stained with propidium iodide (0.025 mg/ml PI in PBS containing 0.1% (v/v) Triton X-100) in the dark for 30 min. The stained cells were detected with a FACSAria II flow cytometry cell sorter (Becton Dickinson, California, USA) and the cell cycle distribution was analyzed by Flow Jo 7.6.2 (Tree Star, Oregon, USA).
2.5. Cell death
A549 (4 × 103) cells were seeded in 96-well plates and treated with DMEM supplemented with ethanol 0.2%, linalool (1.0 and 2.0 mM), or 1,8-cineole (4.0 and 8.0 mM) for 24 or 48 h. Cell death was determined by trypan blue assay as previously described [28]. Live (trypan blue-excluding) and death (blue-stained) cells were counted in a Neubauer hemocytometer.
2.6. In situ detection of apoptosis
A549 cells (1.5 × 105) plated on sterile coverslips (24 × 24 mm) in 6- well plates were incubated for 48 h with ethanol 0.2%, DMSO 5% (positive control), linalool (1.0 and 2.0 mM), 1,8-cineole (4.0 and 8.0 mM), simvastatin (10, 25 and 50 μM), or the combination of each monoterpene (linalool 1.0 mM or 1,8-cineole 4.0 mM) with simvastatin 10 μM. DMSO 5% was chosen as a positive control for apoptosis in A549 cells based on the literature available [30]. Apoptosis was determined by dUTP deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) assay (In-Situ Cell Death Detection Kit, TMR Red, Roche, Mannheim, Germany) following the manufacturer's protocol. Then, the samples were mounted with ProLong® Gold Antifade Reagent containing 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Invitrogen, Life Technologies, Carlsbad, CA, USA). The TUNEL-positive cells were observed under an Olympus BX51 Fluorescence Microscope (Olympus, Tokyo, Japan) and images were examined by means of ImagePro Plus v. 5.1 software (Media Cybernetics, Silver Spring, MD).
2.7. Determination of reactive oxygen species (ROS)
Intracellular ROS were determined using DCFH-DA (Sigma Aldrich) as previously described [16]. A549 cells seeded in 24-well plates (2.5 × 104) were exposed for 24 h to ethanol 0.2%, linalool (1.0 and 2.0 mM), 1,8-cineole (4.0 and 8.0 mM), hydrogen peroxide 0.25 mM (positive control), or pre-incubated with 5 mM NAC for 2 h before the addition of linalool 2.0 mM or 1,8-cineole 8.0 mM. Following, the cells were stained with 10 μM DCFH-DA in serum-free DMEM in the dark at 37 °C for 30 min. After washing three times with PBS, the cells were analyzed under an Olympus LX71 Inverted Fluorescence Microscope (Olympus, Tokyo, Japan) at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
2.8. Evaluation of mitochondrial membrane potential (MMP)
MMP was measured using the fluorescent dye rhodamine-123, as previously described [16]. A549 cells were seeded in 24-well plates (2.5 × 104) and treated for 24 h with linalool (1.0 and 2.0 mM), 1,8-cineole (4.0 and 8.0 mM), hydrogen peroxide 0.25 mM, rotenone 2 μM (positive control), or pre-treated with NAC (5 mM, 2 h) before being exposed to linalool 2.0 mM or 1,8-cineole 8.0 mM. Then, the cells were washed three times with PBS, stained with 5 μM rhodamine-123 in free-serum DMEM for 30 min at 37 °C in the dark. After washing three times with PBS, cells were imaged under an Olympus LX71 Inverted Fluorescence Microscope (Tokyo, Japan) at 488/525 nm (ex/em). The fluorescence intensity was measured using ImageJ software 1.52 a.
2.9. Wound healing assay
A549 cells were seeded in 24-well plates at a density of 7.5 × 104 for 24 h. The monolayers were scratched with a sterile yellow pipette tip and washed with serum-free DMEM to remove the detached cells. Then, the cells were treated with ethanol 0.2% (control), linalool (1.0 and 2.0 mM) or 1,8-cineole (4.0 and 8.0 mM) dissolved in serum-free DMEM. The migration of A549 cells was observed under an Olympus LX71 Inverted Fluorescence Microscope (Tokyo, Japan) at 0 and 48 h after wounding. The wound healing area was measured by Image J 1.51k software (National Institute of Health, USA).
2.10. Western blot analysis
A549 cells (6 × 105) were seeded in 100 mm Petri dishes and allowed to attach 24 h before being treated with ethanol 0.2% (control), linalool 2.0 mM, 1.8-cineole 8.0 mM, or DMSO 5% for 48 h. Then, the cells were rinsed twice with PBS and lysed in RIPA lysis buffer [(50 mM Tris-HCl – pH 8.0, 150 mM NaCl, 1.0% (v/v) NP-40, 0.5% (w/v) sodium deoxycholate, 0.1% SDS, and 1 mM EDTA)] supplemented with protease inhibitors (Thermo Fisher Scientific, USA; Cat. #A32963) for 30 min on ice and centrifuged 15 min at 14,000 × g at 4 °C. The protein content was quantified by the method of Bradford [31]. Protein samples (50 μg) were subjected to Western Blot according to standard procedures previously reported [16]. GAPDH was employed as a loading control.
2.11. Statistics
Results are expressed as a relative change compared with ethanol 0.2% controls and presented as means ± SD. Statistical analysis was performed through the use of the one-way analysis of variance (ANOVA) and the Tukey–Kramer multiple-comparisons test or the unpaired t-test with the significance level set at p < 0.05 (GraphPad inStat program). The IC50 values for cell viability were calculated by nonlinear-regression curves (SigmaPlot software; Systat Software, Inc., Point Richmond, CA).
3. Results
3.1. Effect of linalool and 1,8-cineole on A549 and WI-38 cell viability
To investigate the effects of linalool and 1,8-cineole on cancer A549 and normal WI-38 lung cell growth inhibition, the MTT assay was performed. Both monoterpenes promoted a dose- and time-dependent inhibition on A549 cells. Linalool inhibited cell growth starting from 1.0 mM at 24 and 48 h (Figure 1C, p < 0.05) whereas 1,8-cineole significantly decreased cell viability from 8.0 mM after 24 h, and from 4.0 mM after 48 h, respectively (Figure 1D, p < 0.05 in both cases). On the other hand, linalool concentrations up to 2.0 mM incubated for 24 and 48 h were not cytotoxic for WI-38 cells (Figure 1C) meanwhile 1,8-cineole slightly decreased WI-38 cell viability only from 8.0 mM after 48 h incubation (Figure 1D). The IC50 values obtained are summarized in Table 1. Based on these results, linalool 1.0 and 2.0 mM and, 1,8-cineole 4.0 and 8.0 mM were selected for the following experiments since they inhibit the growth of A549 tumor cells without significant effects on normal WI-38 cells.
Table 1.
IC50 values of linalool and 1,8-cineole on A549 and WI-38 cells.
| Linalool |
1,8-cineole |
|||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| A549 | 1.79 ± 0.11 | 1.13 ± 0.09 | 8.30 ± 0.26 | 5.84 ± 0.21 |
| WI-38 | >3 | >3 | >10 | >10 |
3.2. Linalool and 1,8-cineole induced A549 cell cycle arrest
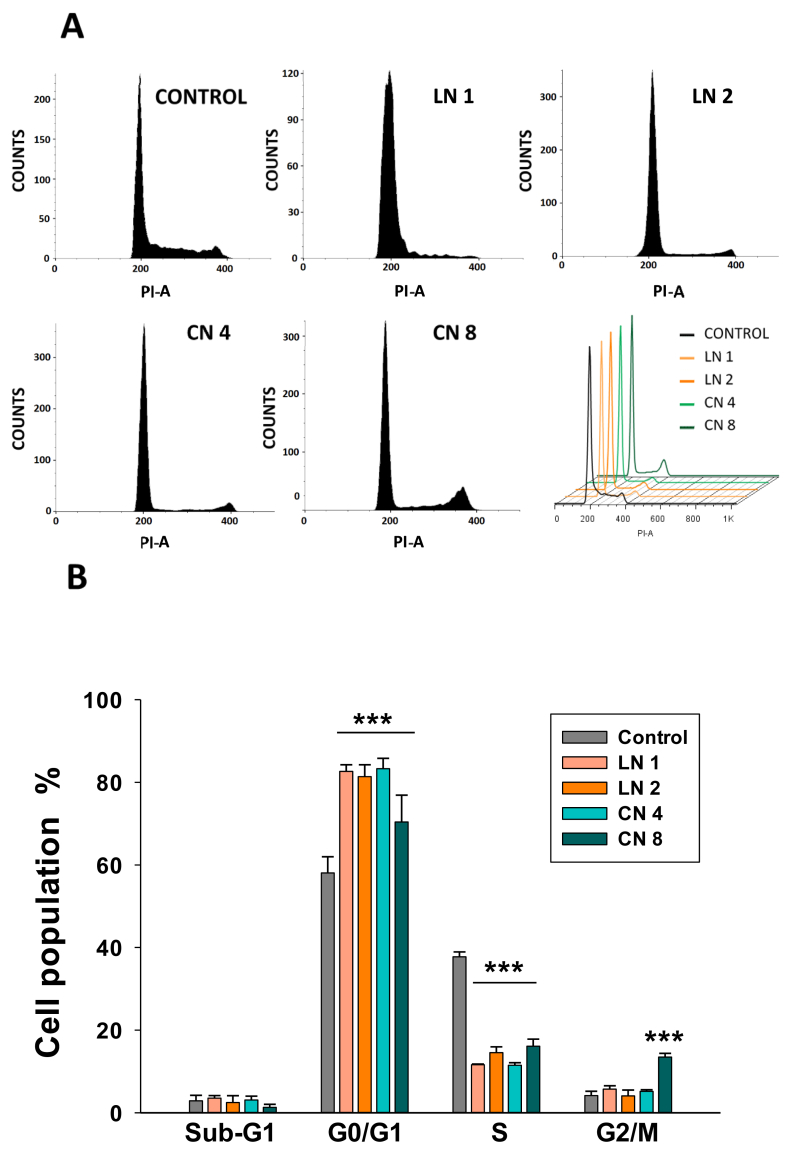
To gain some insight into the antiproliferative mechanisms of linalool and 1,8-cineole, cell cycle analysis was carried out by flow cytometry (Figure 2A). Both monoterpenes induced a strong G0/G1 arrest. Linalool increased G0/G1 population from 58.1% in control cells to 82.7 and 81.4% after incubated with linalool 1.0 and 2.0 mM, respectively (Figure 2B, p < 0.001). This effect was accompanied by a reduction in S-phase cells from 37.8% to 11.6 and 14.6%, respectively (p < 0.001). No significant changes were found in the G2/M phase and Sub-G1 cells. A similar profile was obtained in 1,8-cineole-treated cells, but in this case, the number of G2/M cells significantly augmented after 8.0 mM incubation (4.2% in control cells to 13.5% in treated cells; p < 0.05) (Figure 2B). These results show that linalool and 1,8-cineole present cytostatic effects in A549 cells and no apoptotic (Sub-G1) cells are detected after 24-h exposure.
Figure 2.
Linalool and 1,8-cineole induced A549 cell cycle arrest. A549 cells were exposed to ethanol 0.2% (Control), linalool 1.0 (LN 1) and 2.0 (LN 2) mM, or 1,8-cineole 4.0 (CN 4) and 8.0 (CN 8) mM for 24 h. The cell cycle was analyzed by flow cytometry (A) and quantified (B). Data are expressed as mean ± SD (n = 4). ∗∗∗p < 0.001.
3.3. Linalool and 1,8-cineole did not trigger apoptosis in A549 cells
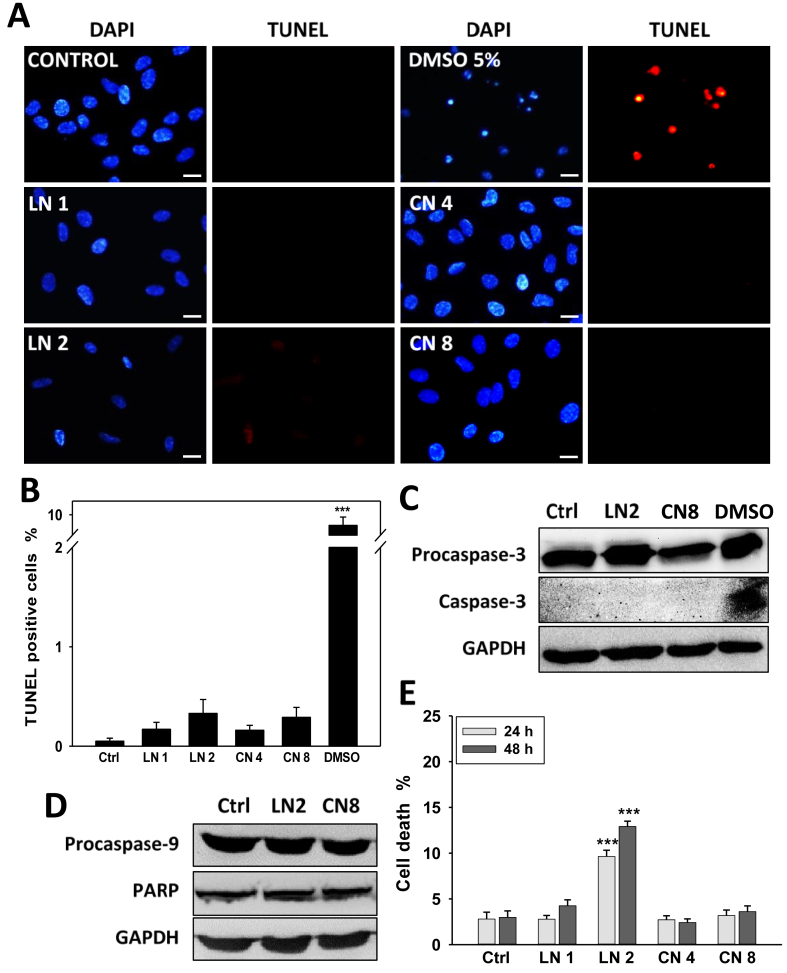
Given that none of each monoterpene increased Sub-G1 cell population (Figure 2B) or induced the activation of caspase-3 (data not shown) after 24 h, we next evaluated the capability of linalool and 1,8-cineole to induce apoptosis in A549 cells after 48 h treatments. First, we analyzed DNA fragmentation –a hallmark of late apoptosis-by TUNEL assay. As shown in Figure 3A-B, none of both monoterpenes was able to trigger apoptosis, even in conditions where cell viability was inhibited up to 80–85% (linalool 2.0 mM or 1,8-cineole 8.0 mM, 48 h). Then, to confirm these observations, we analyzed the cleavage (activation) of caspase-3 (an effector caspase induced by both intrinsic and extrinsic pathways), caspase-9 (the initiator caspase of the intrinsic pathway), and PARP (a substrate for effector caspases) by western blot. As observed in Figure 3, no cleaved caspase-3 (Figure 3C) or changes in procaspase-9 and PARP levels (Figure 3D) were found. These results show that none of both monoterpenes induced apoptosis.
Figure 3.
Effect of linalool and 1,8-cineole on A549 cell apoptosis. Cells were exposed to ethanol 0.2% (Control), linalool 1.0 (LN 1) and 2.0 (LN 2) mM, 1,8-cineole 4.0 (CN 4) and 8.0 (CN 8) mM, or DMSO 5% (DMSO, positive control) for 48 h, and different apoptotic markers were studied. (A) Representative images of TUNEL assay performed using a commercial kit. Scale bar, 10 μm. (B) Around a total of ten to fifteen fields (200–500 cells/field) from three independent experiments were evaluated and TUNEL-positive cells were quantified. Data are presented as the mean ± SD; ∗∗∗p < 0.001. (C–D) Representative western blot of procaspase-3 and caspase-3 (C) and, procaspase-9 and PARP (D). GAPDH was used as a loading control. (E) Cell death was analyzed by trypan blue staining. Data are presented as the mean ± SD. ∗∗∗p < 0.001.
To determine whether linalool or 1,8-cineole induce cell death in A549 cells, the trypan blue exclusion assay was performed (Figure 3E). Linalool 2.0 mM increased cell death from about 2.8 and 3.0% in control cells to 9.6 and 12.9% after 24 and 48 h, respectively (p < 0.001). On the other hand, 1,8-cineole did not induce cell death in any experimental condition. The increase in trypan blue-stained cells but not in TUNEL-positive cells suggests that linalool may induce cell death types distinct from apoptosis in A549 cells.
3.4. Effects of linalool and 1,8-cineole on oxidative stress and ROS-induced cell growth inhibition
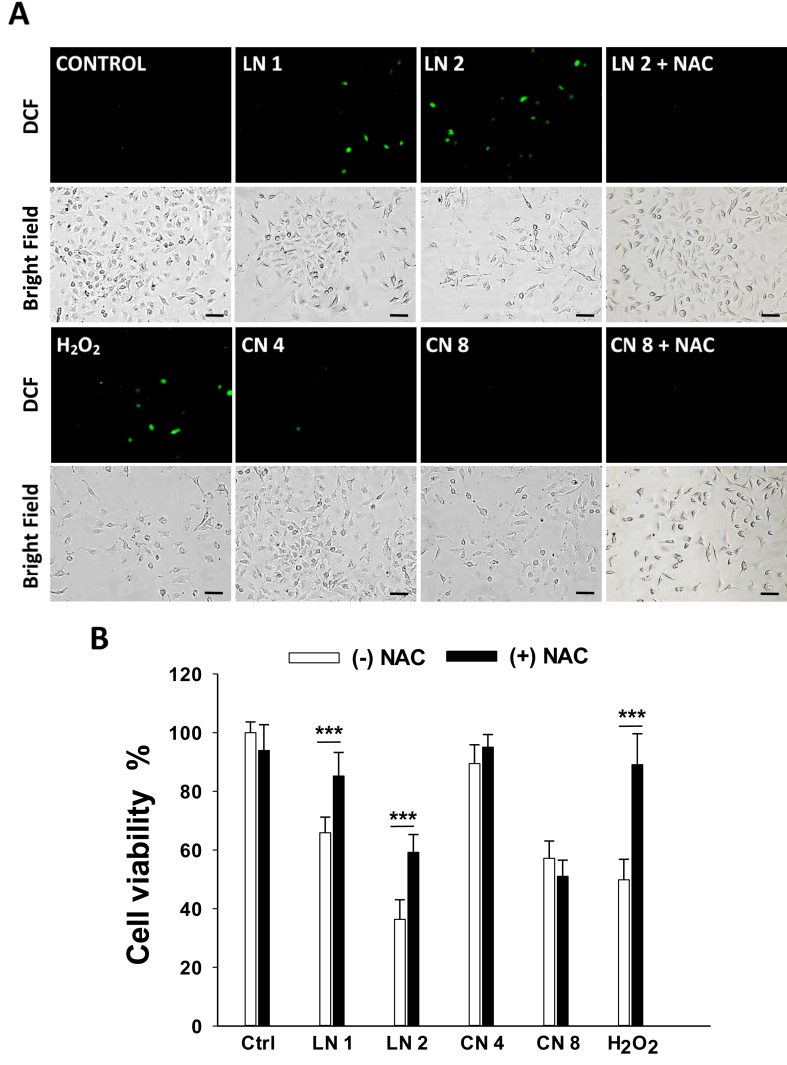
We then studied whether linalool and 1,8-cineole trigger ROS generation. Figure 4A shows that linalool, but not 1,8-cineole, was able to induce ROS production in A549 cells, even from low concentrations such as 1.0 mM linalool. Moreover, pre-treatment with the antioxidant NAC prevented such an increase in 2.0 mM linalool-treated cells and improved the number of A549 cells in comparison to non-pre-treated cells (Figure 4A). To gain understanding of whether ROS production was involved in the antiproliferative activity, A549 cells were exposed to linalool or 1,8-cineole in the absence or presence of NAC for 24 h and cell viability was assessed. As shown in Figure 4B, NAC prevented cell viability loss from 65.9 and 36.4% to 85.2 and 59.2% in linalool 1.0- and 2.0-mM treated cells, respectively (p < 0.001). As expected, NAC failed to prevent cell growth inhibition induced by 1,8-cineole (Figure 4B).
Figure 4.
Effects of linalool and 1,8-cineole on intracellular reactive oxygen species (ROS) in A549 cells. (A) Cells were incubated with ethanol 0.2% (Control), linalool 1.0 (LN 1) and 2.0 (LN 2) mM, 1,8-cineole 4.0 (CN 4) and 8.0 (CN 8) mM, hydrogen peroxide 0.25 mM (H2O2, positive control), or pre-incubated with NAC 5 mM 2 h prior to treatment with linalool 2.0 mM (LN 2 + NAC) or 1,8-cineole 8.0 mM (CN 8 + NAC) for 24 h. ROS production was analyzed under an Inverted Fluorescence Microscope. Scale bar, 50 μm. (B) Effect of the antioxidant NAC on A549 cell viability. Cells were pre-incubated or not for 2 h with NAC 5 mM before the addition of ethanol 0.2%, LN 1, LN 2, CN 4, CN 8, or H2O2 for 24 h. Cell viability was determined by the MTT assay. Data are presented as the mean ± SD (n = 12). ∗∗∗p < 0.001.
These results evidence that ROS partially contribute to the antiproliferative effects of linalool but are not involved in 1,8-cineole-induced cell growth inhibition.
3.5. Linalool, but not 1,8-cineole, induced depolarization of mitochondrial membrane potential (MMP)
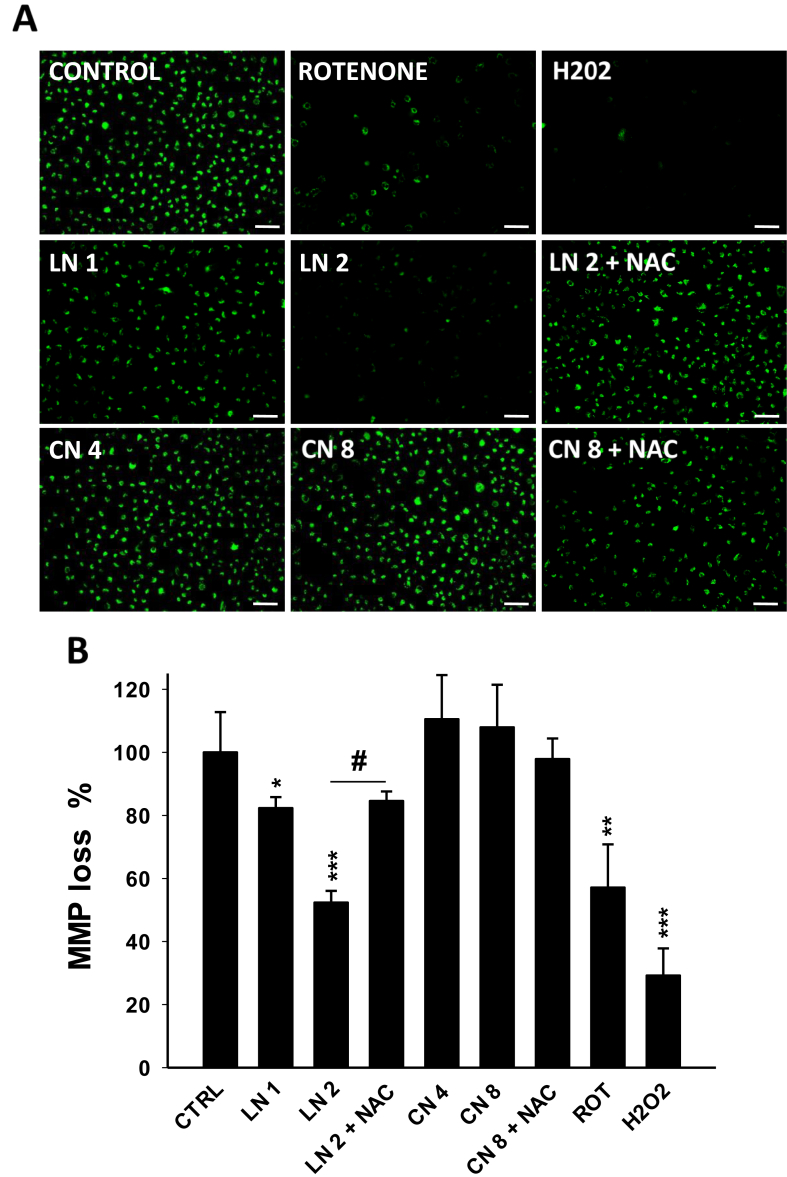
Mitochondria are the primary source of ROS in the cell and, at the same time, excessive ROS promote mitochondrial damage and MMP collapse. Both oxidative stress and mitochondrial dysfunction may contribute to antiproliferative processes other than apoptosis. Consequently, MMP was evaluated through the incorporation of rhodamine-123 (Figure 5A). We found that linalool 1.0 and 2.0 mM induced a significant decreased of 17.7 and 47.6% in MMP, respectively (Figure 5B). NAC prevented to some extent the MMP collapse promoted by linalool 2.0 mM (Figure 5B, p < 0.001), as observed by greater green fluorescence intensity of A549 cells when pre-incubated with the antioxidant molecule (Figure 5A). On the other hand, 1,8-cineole did not cause any significant effect on MMP (Figure 5 A-B).
Figure 5.
Effects of linalool and 1,8-cineole on mitochondrial membrane potential (MMP) in A549 cells. (A) Cells were incubated with ethanol 0.2% (Control), linalool 1.0 (LN 1) and 2.0 (LN 2) mM, 1,8-cineole 4.0 (CN 4) and 8.0 (CN 8) mM, hydrogen peroxide 0.25 mM (H2O2), or rotenone 2 μM (ROT, positive control) for 24 h and then stained with rhodamine-123. (A) Cells were observed and photographed under a fluorescence microscope. Scale bar, 50 μm. (B) Quantitative analysis of rhodamine-123 green fluorescence in A549 cells expressed as a percentage of MMP loss compared to control cells. Data are shown as mean ± SD (n = 6). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 vs. Control; #p < 0.001 vs. LN 2.
These findings show that linalool induced the depolarization of MMP in A549 cells, which may be involved in cell viability loss, and suggest that linalool-induced oxidative stress is responsible for MMP depolarization.
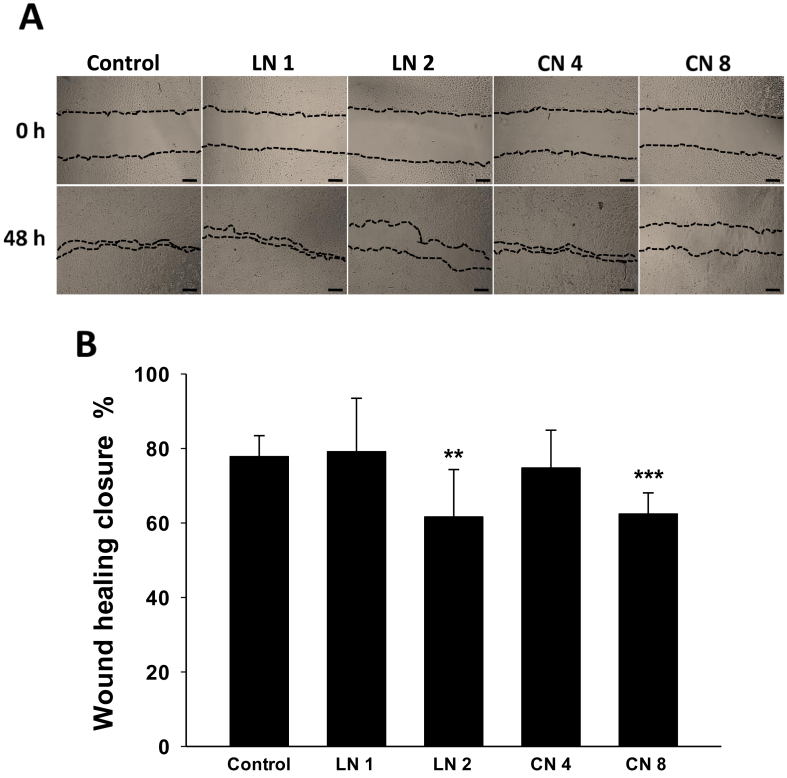
3.6. Linalool and 1,8-cineole inhibited A549 cell migration
To expand the knowledge on the anti-cancer potential of linalool and 1,8-cineole in A549 cells, the effect on cell migration was studied through the wound healing assay. We found that after 48 h, both monoterpenes significantly suppressed A549 cell migration at the higher concentrations employed (Figure 6A). Linalool 2.0 mM and 1,8-cineole 8.0 mM reduced the wound healing closure to 61.7 and 62.5%, respectively, in comparison to the 77.9% observed in control cells (Figure 6B, p < 0.01 in both cases). These results suggest that both isoprenoids also exert anti-metastatic effects on NSCLC A549 cells.
Figure 6.
Effects of linalool and 1,8-cineole on A549 cell migration. The wound-healing assay was performed to study the migration of A549 cells following linalool and 1,8-cineole exposure. (A) Representative images were captured at 0 and 48 h, Scale bar, 250 μm. (B) Quantitative analysis of wound healing closure (%). Data are presented as the mean ± SD (n = 8). ∗∗p < 0.01; ∗∗∗p < 0.001.
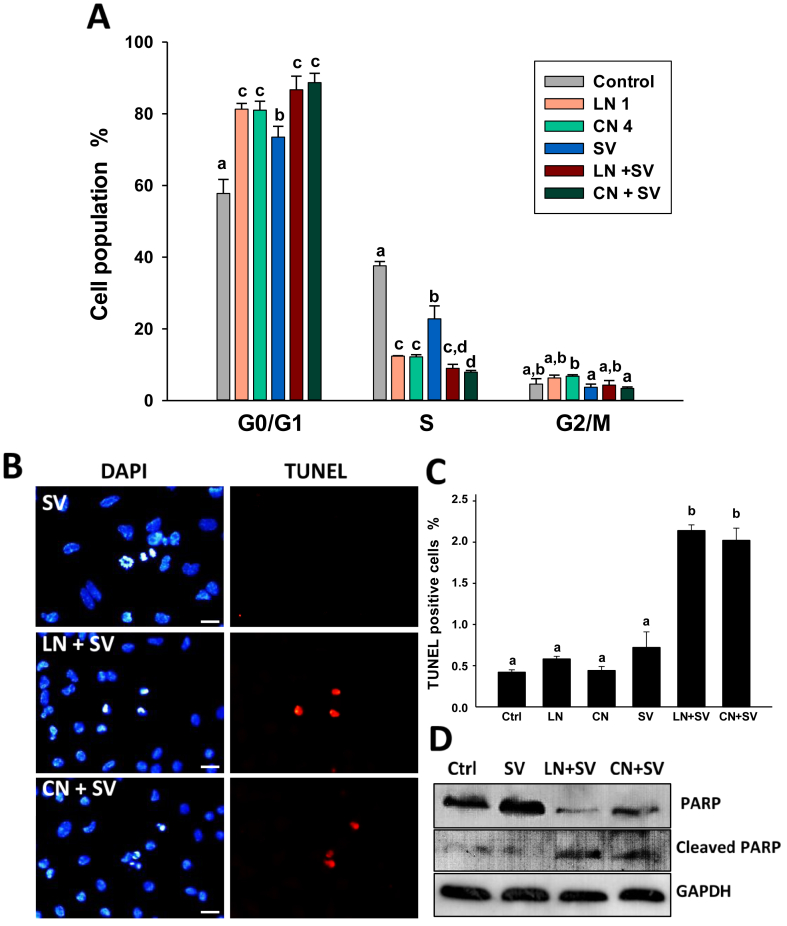
3.7. Linalool and 1,8-cineole sensitized A549 cells to simvastatin-induced cell cycle arrest and apoptosis
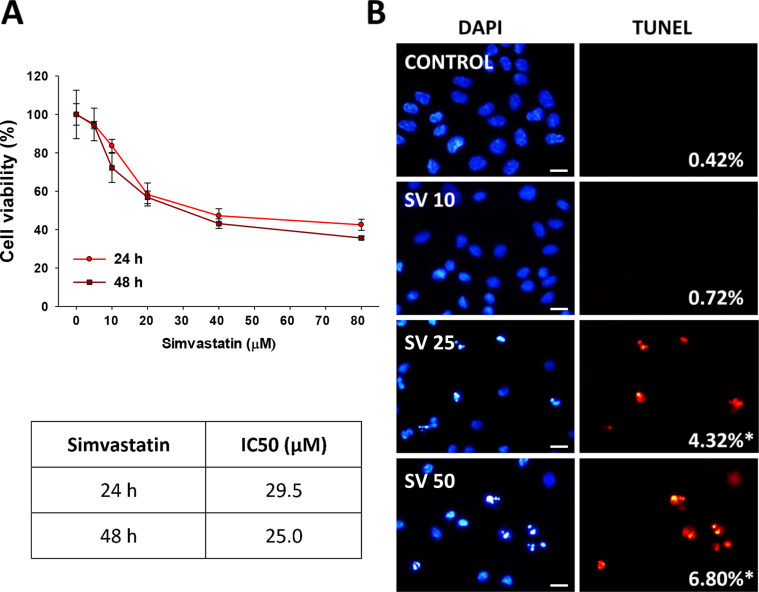
In a previous study, we demonstrated that pairwise combinations of linalool or 1,8-cineole with simvastatin synergistically inhibited A549 cell growth [28]. Here, we evaluated the mechanisms by which linalool and 1,8-cineole act as sensitizer molecules when combined with simvastatin. We analyzed the effect of the pairwise-combination of simvastatin and each monoterpene (linalool 1.0 mM or 1,8-cineole 4.0 mM) on cell cycle progression and apoptosis (Figure 7). We used simvastatin 10 μM, a sub-IC50 concentration that inhibited A549 cell viability by 16.4 and 27.8% after 24 and 48 h, respectively (Figure A1). This concentration had moderate effects on the cell cycle but did not induce apoptosis in A549 cells (Figure 7).
Figure 7.
Linalool and 1,8-cineole increased simvastatin-mediated cell cycle arrest and apoptosis in A549 cells. Cells were exposed to ethanol 0.2% (Control), linalool 1.0 mM (LN 1), 1,8-cineole 4.0 mM (CN 4) and simvastatin 10 μM (SV) alone or each monoterpene combined with simvastatin (LN + SV, CN + SV). (A) The cell cycle was analyzed following 24 h treatments. Scale bar, 10 μm. (B) Representative images of TUNEL assay performed after exposing A549 cells to the treatments for 48 h. (C) Around a total of ten to fifteen fields (200–500 cells/field) were evaluated and TUNEL-positive cells were quantified. Data are expressed as mean ± SD. Means without the same letter are different (p < 0.05). (D) Representative western blot of PARP and cleaved-PARP. GAPDH was used as a loading control.
As shown in Figure 7A, the combined treatments increased the G0/G1 cell population and decreased S-phase cells compared to individual treatments, resulting in both cases, statistically different from cells treated with simvastatin alone (p < 0.05). Thereafter, we investigated the effects of the combination of each monoterpene and simvastatin on apoptosis (Figure 7B). As observed for simvastatin 10 μM (Figure 7C), and linalool 1.0 mM and 1,8-cineole 4.0 mM (Figures 3 and 7C), none of the three compounds employed alone showed to induce apoptosis compared to control cells. However, the combination of each monoterpene and simvastatin showed a significant 3- to 5-fold increase in TUNEL-positive cells respect to control cells and individual treatments (Figure 7C, p < 0.05). These results were confirmed by the detection of cleaved PARP in the combined treatments (Figure 7D).
4. Discussion
Nature is an excellent source of molecules with antitumor activity. Approximately 60% of the anticancer drugs currently available for clinical use come from natural sources, including most of the antineoplastic drugs against NSCLC [32]. Linalool and 1,8-cineole have been evaluated as potential chemopreventive and chemotherapeutic molecules as mono-drug agents or combined with conventional drugs. Numerous in vitro and in vivo studies demonstrated the anticancer potential of these monoterpenes in lung [28], liver [16, 28, 33], breast [23], ovarian [34], skin [35], cervical [36, 37], colon [38, 39], sarcoma [40], glioma [41], leukemia [36, 42, 43], prostate [44, 45], oral [46] and gastric [47] cancer cells. Nevertheless, to our knowledge, the mechanisms by which linalool and 1,8-cineole impair the proliferation of NSCLC A549 cells remain unexplored.
One of the main and most difficult goals in cancer drug discovery is to obtain molecules with preferential activity toward cancer cells, with no or minimal toxicity against normal tissues. In this study, we show that linalool (1.0–2.0 mM) and 1,8-cineole (4.0–8.0 mM) significantly inhibited A549 cell viability without showing inhibitory effects on normal lung WI-38 cells. The effective concentrations of linalool and 1,8-cineole reported to inhibit in vitro cancer cell proliferation vary from micromolar (μM) [35, 36, 39, 42, 43, 45, 47] to millimolar (mM) [16, 33, 34, 37, 38, 40, 44, 46]. This heterogeneity in sensitivity depends on the cancer cell type but also each cell line evaluated. For example, leukemia cells are between the most sensitive cells to both monoterpenes [36, 42, 43]. Interestingly, Gu and collaborators [43] found that the activity of linalool strongly depended on the p53 status of a panel of leukemia cells, obtaining IC50 ranging from 80 μM (p53 wild-type) to greater than 640 μM (mutated p53). Several studies employing linalool [34, 40, 44], 1,8-cineole [38], or other monoterpenes [15, 48] at millimolar concentrations in vitro, showed strong antitumor activity in vivo at non-toxic doses (10–400 mg/Kg). Among them, Jana et al. [40] required ~2.0 mM linalool to inhibit 50% cell viability in S-180 sarcoma cells in vitro. When they orally administered 200 mg/kg linalool in mice bearing S-180 tumor, a concentration of 102 ng/ml (0.66 μM) linalool was measured in sera after 18 h, which is much lower than used in in vitro cultured cells. However, such treatment applied every 48 h for 3 weeks drastically reduced tumor growth by 50%.
Cell cycle arrest is considered one of the major causes of cell growth inhibition. Numerous monoterpenes, among them linalool and 1,8-cineole, showed cytostatic antiproliferative effects against cancer cells by causing G0/G1 [33,36] or G2/M [35, 49] cell growth arrest. In this study, 1,8-cineole appeared to affect cell cycle distribution in a different manner depending on the concentration. 1,8-cineole induced a robust G0/G1 arrest along with a considerable decrease of S-phase cells at both concentrations. However, at 8.0 mM, it also raised the proportion of cells in the G2/M phase and partially diminished G0/G1 cells respect to the lower concentration (4.0 mM). On the other hand, linalool induced a marked G0/G1 cell cycle arrest from 1.0 mM onwards, accompanied by a strong diminution of S-phase cells, as observed previously in liver cancer cells [33].
Apoptosis is one of the chief strategies for cancer therapy exerted by most chemotherapeutic agents. It has been reported the ability of linalool and 1,8-cineole to induce apoptosis in a wide variety of cell lines, including skin [35], oral [46], sarcoma [40], and liver [33] among others. In this study, several apoptotic markers such as sub-G1 cells, DNA fragmentation, and cleavage of caspase-3, caspase-9, or PARP were evaluated. Even exposing cells to concentrations higher than IC50, neither linalool nor 1,8-cineole induced apoptosis in A549 cells. Many natural compounds with anticancer effects showed similar results in this NSCLC cell line, suggesting that mechanisms of cell death other than apoptosis (autophagic cell death, necroptosis, ferroptosis, etc.) mediate their antitumor action [50, 51]. Here, we observed that linalool, but not 1,8-cineole, induced modest cell death. 1,8-cineole likely mediates only cytostatic activity in A549 cells, which could be indicative of developing senescence, a permanent cell cycle arrest [52]. Some compounds induce cytostatic autophagy before the establishment of the senescent phenotype, a process that may require several days [16, 52]. Indeed, we have previously shown that 1,8-cineole induced AMPK activity, an energy sensor protein that promotes autophagy [17], before developing senescence in hepatocarcinoma HepG2 cells [16]. 1,8-cineole induced AMPK in A549 cells, but no senescence markers like p21-or nuclear size increase were observed in our conditions (data not shown). Nevertheless, additional features and longer-time experiments are needed to confirm whether autophagy and/or senescence are involved.
ROS play a key role in multiple signaling pathways that are crucial to the fate of both healthy and cancer cells [53]. Mitochondria are the major endogenous source of ROS in human tissues and produce them at physiological levels as a natural byproduct of oxidative phosphorylation. Cancer cells show increased levels of ROS than their normal counterparts, mainly as a consequence of the augmented metabolic activity in mitochondria, alterations in the electronic transport chain, and oncogenic signaling in cancer cells [53, 54]. The high levels of ROS in cancer cells are associated with tumorigenesis, angiogenesis, metastasis, and tumor resistance [53, 54]. Increasing ROS levels to highly toxic amounts activates several ROS-dependent cell death pathways. A common strategy followed by several anticancer agents -including phytochemicals-is targeting mitochondria, leading to ROS generation and mitochondrial dysfunction [55]. Elevation of ROS in moderate quantities may induce cytostatic autophagy, cell cycle arrest, and senescence in cancer cells, whereas greater amounts of ROS promote cytotoxic cell death [52]. Moreover, oxidative stress and mitochondrial collapse may contribute to cell death independent from apoptosis through autophagic cell death, necroptosis, or ferroptosis, among others [52, 56, 57]. Linalool, 1,8-cineole, and a wide variety of other monoterpenes exerted their anticancer action through ROS production and MMP depolarization [16, 33, 34, 35, 39, 40, 47]. Here, we found that linalool, but not 1,8-cineole, induced ROS production in A549 cells. Furthermore, the presence of the antioxidant NAC reverted to some degree the linalool-induced cell growth inhibition in A549 cells but had no effect on the antiproliferative action of 1,8-cineole. Besides, only linalool promoted a significant loss in MMP and the preincubation with NAC rescued the cells from such depolarization. Linalool likely has some effect on mitochondrial components promoting the generation of mitochondrial ROS, which in turn favor the mitochondrial collapse, as previously described elsewhere [33, 41, 58].
NSCLC A549 cells have shown a marked resistance to apoptosis induction mediated by several anticancer agents. Such resistance was associated with the high expression of proteins of the antioxidant system in this cell line [59]. This fact may play a role in the inability of 1,8-cineole to induce oxidative stress and of both monoterpenes to trigger apoptosis in A549 cells, unlike what was observed in other cell lines [33, 35, 38].
Cell migration is a key feature of cancer metastasis. This is a complex biological process that allows cancer cells to invade the neighboring tissues and spread to other body parts, thus promoting metastases [60]. The anti-migratory effects of phytochemicals have been widely explored in highly metastatic cells, including NSCLC cells [18, 61]. Nonetheless, to our knowledge, no studies explored the ability of linalool and 1,8-cineole to inhibit cancer cell migration. Here, we observed that both compounds reduced A549 cell migration at the higher concentrations tested.
The need for new and more effective treatments against cancer encouraged researchers to study new uses for affordable drugs approved for other diseases, a strategy known as “drug repurposing”. Statins are hypolipidemic drugs with high potential as anticancer agents, particularly in cancer cells with high 3-hydroxy-3-methylglutaryl coenzyme A reductase -the target enzyme of statins- and Ras activity, as is the case of NSCLC A549 cells [62, 63]. Monoterpenes showed to potentiate the anticancer activity of statins and to reduce the statin-associated adverse effects, thus proposed as adjuvant molecules in statin-based chemotherapies [63]. We have previously shown the antiproliferative synergistic effects of the combination of linalool or 1,8-cineole with simvastatin [28]. Here, we unraveled the effects of these combinations on cell cycle progression and apoptosis in A549 cells. Linalool and 1,8-cineole significantly increased cells arrested in G0/G1 cells when combined with simvastatin respect to A549 cells exposed to the statin alone. Moreover, both monoterpenes sensitized cells to simvastatin-induced apoptosis. The sum of the effects on cell cycle and apoptosis may account for the antiproliferative synergism previously reported for the combinations of each monoterpene and simvastatin [28].
In conclusion, our study shows that linalool and 1,8-cineole are non-toxic monoterpenes that inhibit A549 cell growth through cell cycle arrest but not apoptosis. Oxidative stress-induced by linalool plays a role in the mitochondrial membrane potential depolarization and inhibition of cell proliferation. Both monoterpenes inhibited cell migration. Moreover, linalool and 1,8-cineole sensitized A549 cells to simvastatin-triggered cell cycle arrest and apoptosis. Further investigation is required to disclose whether alternative ways of non-apoptotic cancer cell death or senescence are involved, as well as to explore additional anti-metastatic mechanisms and signaling pathways implicated. Although in vivo experiments are needed to confirm the in vitro results, these findings suggest that linalool and 1,8-cineole have potential as cytostatic, anti-metastatic, and chemosensitizer compounds in NSCLC therapy.
Declarations
Author contribution statement
B. Rodenak-Kladniew: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
M. Agustina Castro and M. Galle: Performed the experiments; Analyzed and interpreted the data.
R. Crespo: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
M. García de Bravo: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), and the National University of La Plata (UNLP), Argentina.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Dr. B. Rodenak- Kladniew wants to thank the “Subsidio de Jóvenes Investigadores UNLP 2019” and “Programa de Retención de Doctores de la UNLP 2019”. The authors are grateful to Dr. R. Goya from INIBIOLP for his valuable contribution to this work.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Figure A1.
Effects of simvastatin on A549 cell viability and apoptosis. (A) A549 cells were treated with increasing concentrations of simvastatin (0–80 μM) for 24 and 48 h. Cell viability was assessed by the MTT assay and IC50 values were obtained as described in Materials and Methods (n = 8). (B) A549 cells were exposed ethanol 0.2% (Control) and simvastatin 10 μM (SV 10), 25 μM (SV 25) or 50 μM (SV 50) for 48 h and TUNEL assay performed. Representative images of cells observed under a direct fluorescence microscope. Images were analyzed as detailed in Figure 3A. Scale bar, 10 μm. Data are expressed as the mean ± SD. *p < 0.001.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Zappa C., Mousa S.A. Non-small cell lung cancer: current treatment and future advances. Transl. Lung Cancer Res. 2016;5:288. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allemani C., Weir H.K., Carreira H., Harewood R., Spika D., Wang X.-S., Bannon F., V Ahn J., Johnson C.J., Bonaventure A. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camidge D.R., Pao W., V Sequist L. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat. Rev. Clin. Oncol. 2014;11:473. doi: 10.1038/nrclinonc.2014.104. [DOI] [PubMed] [Google Scholar]
- 5.Lynch T.J., Bondarenko I., Luft A., Serwatowski P., Barlesi F., Chacko R., Sebastian M., Neal J., Lu H., Cuillerot J.-M. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non–small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 6.Gotwals P., Cameron S., Cipolletta D., Cremasco V., Crystal A., Hewes B., Mueller B., Quaratino S., Sabatos-Peyton C., Petruzzelli L. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch F.R., V Scagliotti G., Mulshine J.L., Kwon R., Curran W.J., Jr., Wu Y.-L., Paz-Ares L. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 8.American cancer society. 2020. https://www.cancer.org/cancer/lung-cancer/treating-small-cell/chemotherapy.html (accessed October 2020)
- 9.Rotow J., Bivona T.G. Understanding and targeting resistance mechanisms in NSCLC. Nat. Rev. Cancer. 2017;17:637. doi: 10.1038/nrc.2017.84. [DOI] [PubMed] [Google Scholar]
- 10.Oun R., Moussa Y.E., Wheate N.J. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. 2018;47:6645–6653. doi: 10.1039/c8dt00838h. [DOI] [PubMed] [Google Scholar]
- 11.Khanna R., Anger C. 1551P_Prpatterns of patients stopping their anti-cancer drug due to its associated side effects in France, Germany, Italy, Spain and UK (EU5) Ann. Oncol. 2014;25 [Google Scholar]
- 12.Choudhari A.S., Mandave P.C., Deshpande M., Ranjekar P., Prakash O. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front. Pharmacol. 2020;10:1614. doi: 10.3389/fphar.2019.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu R.H. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J. Nutr. 2004;134:3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 14.González-Vallinas M., González-Castejón M., Rodríguez-Casado A., Ramírez de Molina A. Dietary phytochemicals in cancer prevention and therapy: a complementary approach with promising perspectives. Nutr. Rev. 2013;71:585–599. doi: 10.1111/nure.12051. [DOI] [PubMed] [Google Scholar]
- 15.Yu X., Lin H., Wang Y., Lv W., Zhang S., Qian Y., Deng X., Feng N., Yu H., Qian B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther. 2018;11:1833. doi: 10.2147/OTT.S155716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodenak-Kladniew B., Castro A., Stärkel P., Galle M., Crespo R. 1, 8-Cineole promotes G0/G1 cell cycle arrest and oxidative stress-induced senescence in HepG2 cells and sensitizes cells to anti-senescence drugs. Life Sci. 2020:117271. doi: 10.1016/j.lfs.2020.117271. [DOI] [PubMed] [Google Scholar]
- 17.Ashrafizadeh M., Ahmadi Z., Mohammadinejad R., Kaviyani N., Tavakol S. Monoterpenes modulating autophagy: a review study. Basic Clin. Pharmacol. Toxicol. 2020;126:9–20. doi: 10.1111/bcpt.13282. [DOI] [PubMed] [Google Scholar]
- 18.Tomko A.M., Whynot E.G., Ellis L.D., Dupré D.J. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers (Basel) 2020;12:1985. doi: 10.3390/cancers12071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mączka W., Wińska K., Grabarczyk M. One hundred faces of geraniol. Molecules. 2020;25:3303. doi: 10.3390/molecules25143303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye Z., Liang Z., Mi Q., Guo Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. J. Buon Off. J. Balk. Union Oncol. 2020;25:280–285. [PubMed] [Google Scholar]
- 21.Rabi T., Bishayee A. d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: generation of reactive oxygen species and induction of apoptosis. J. Carcinog. 2009;8:9. doi: 10.4103/1477-3163.51368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carnesecchi S., Langley K., Exinger F., Gosse F., Raul F. Geraniol, a component of plant essential oils, sensitizes human colonic cancer cells to 5-fluorouracil treatment. J. Pharmacol. Exp. Ther. 2002;301:625–630. doi: 10.1124/jpet.301.2.625. [DOI] [PubMed] [Google Scholar]
- 23.Ravizza R., Gariboldi M.B., Molteni R., Monti E. Linalool, a plant-derived monoterpene alcohol, reverses doxorubicin resistance in human breast adenocarcinoma cells. Oncol. Rep. 2008;20:625–630. [PubMed] [Google Scholar]
- 24.Duke J.A., Beckstrom-Sternberg S.M. U.S. Department of Agriculture, Agricultural Research Service; 1992–2016. Dr. Duke's Phytochemical and Ethnobotanical Databases.https://phytochem.nal.usda.gov/phytochem/chemicals (accessed October 2020) [Google Scholar]
- 25.Aprotosoaie A.C., Hăncianu M., Costache I., Miron A. Linalool: a review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014;29:193–219. [Google Scholar]
- 26.Dhakad A.K., V Pandey V., Beg S., Rawat J.M., Singh A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: a review. J. Sci. Food Agric. 2018;98:833–848. doi: 10.1002/jsfa.8600. [DOI] [PubMed] [Google Scholar]
- 27.Seol G.H., Kim K.Y. Drug Discov. From Mother Nat. Springer; 2016. Eucalyptol and its role in chronic diseases; pp. 389–398. [Google Scholar]
- 28.Rodenak-Kladniew B., Polo M., Montero Villegas S., Galle M., Crespo R., García de Bravo M. Synergistic antiproliferative and anticholesterogenic effects of linalool, 1,8-cineole, and simvastatin on human cell lines. Chem. Biol. Interact. 2014;214:57–68. doi: 10.1016/j.cbi.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 30.Eskandani M., Hamishehkar H., Dolatabadi J.E.N. Cytotoxicity and DNA damage properties of tert-butylhydroquinone (TBHQ) food additive. Food Chem. 2014;153:315–320. doi: 10.1016/j.foodchem.2013.12.087. [DOI] [PubMed] [Google Scholar]
- 31.Castro M.A., Rodenak-Kladniew B., Massone A., Polo M., De Bravo M.G., Crespo R. Citrus reticulata peel oil inhibits non-small cell lung cancer cell proliferation in culture and implanted in nude mice. Food Funct. 2018;9:2290–2299. doi: 10.1039/C7FO01912B. [DOI] [PubMed] [Google Scholar]
- 32.Bhanot A., Sharma R., Noolvi M.N. Natural sources as potential anti-cancer agents: a review. Int. J. Phytomed. 2011;3:9. [Google Scholar]
- 33.Rodenak-Kladniew B., Castro A., Stärkel P., De Saeger C., García de Bravo M., Crespo R. Linalool induces cell cycle arrest and apoptosis in HepG2 cells through oxidative stress generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life Sci. 2018;199:48–59. doi: 10.1016/j.lfs.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Han H.D., Cho Y.-J., Cho S.K., Byeon Y., Jeon H.N., Kim H.-S., Kim B.-G., Bae D.-S., Lopez-Berestein G., Sood A.K. Linalool-incorporated nanoparticles as a novel anticancer agent for epithelial ovarian carcinoma. Mol. Cancer Ther. 2016;15:618–627. doi: 10.1158/1535-7163.MCT-15-0733-T. [DOI] [PubMed] [Google Scholar]
- 35.Sampath S., Subramani S., Janardhanam S., Subramani P., Yuvaraj A., Chellan R. Bioactive compound 1, 8-Cineole selectively induces G2/M arrest in A431 cells through the upregulation of the p53 signaling pathway and molecular docking studies. Phytomedicine. 2018;46:57–68. doi: 10.1016/j.phymed.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Chang M.-Y., Shieh D.-E., Chen C.-C., Yeh C.-S., Dong H.-P. Linalool induces cell cycle arrest and apoptosis in leukemia cells and cervical cancer cells through CDKIs. Int. J. Mol. Sci. 2015;16:28169–28179. doi: 10.3390/ijms161226089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Efferth T., Herrmann F., Tahrani A., Wink M. Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine. 2011;18:959–969. doi: 10.1016/j.phymed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Murata S., Shiragami R., Kosugi C., Tezuka T., Yamazaki M., Hirano A., Yoshimura Y., Suzuki M., Shuto K., Ohkohchi N. Antitumor effect of 1, 8-cineole against colon cancer. Oncol. Rep. 2013;30:2647–2652. doi: 10.3892/or.2013.2763. [DOI] [PubMed] [Google Scholar]
- 39.Iwasaki K., Zheng Y.W., Murata S., Ito H., Nakayama K., Kurokawa T., Sano N., Nowatari T., Villareal M.O., Nagano Y.N., Isoda H., Matsui H., Ohkohchi N. Anticancer effect of linalool via cancer-specific hydroxyl radical generation in human colon cancer. World J. Gastroenterol. 2016;22:9765. doi: 10.3748/wjg.v22.i44.9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jana S., Patra K., Sarkar S., Jana J., Mukherjee G., Bhattacharjee S., Mandal D.P. Antitumorigenic potential of linalool is accompanied by modulation of oxidative stress: an in vivo study in sarcoma-180 solid tumor model. Nutr. Cancer. 2014;66:835–848. doi: 10.1080/01635581.2014.904906. [DOI] [PubMed] [Google Scholar]
- 41.Cheng Y., Dai C., Zhang J. SIRT3-SOD2-ROS pathway is involved in linalool-induced glioma cell apoptotic death. Acta Biochim. Pol. 2017;64:343–350. doi: 10.18388/abp.2016_1438. [DOI] [PubMed] [Google Scholar]
- 42.Moteki H., Hibasami H., Yamada Y., Katsuzaki H., Imai K., Komiya T. Specific induction of apoptosis by 1, 8-cineole in two human leukemia cell lines, but not a in human stomach cancer cell line. Oncol. Rep. 2002;9:757–760. [PubMed] [Google Scholar]
- 43.Gu Y., Ting Z., Qiu X., Zhang X., Gan X., Fang Y., Xu X., Xu R. Linalool preferentially induces robust apoptosis of a variety of leukemia cells via upregulating p53 and cyclin-dependent kinase inhibitors. Toxicology. 2010;268:19–24. doi: 10.1016/j.tox.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y., Cheng X., Wang G., Liao Y., Qing C. Linalool inhibits 22Rv1 prostate cancer cell proliferation and induces apoptosis. Oncol. Lett. 2020;20:1. doi: 10.3892/ol.2020.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun X.-B., Wang S.-M., Li T., Yang Y.Q. Anticancer activity of linalool terpenoid: apoptosis induction and cell cycle arrest in prostate cancer cells. Trop. J. Pharm. Res. 2015;14:619–625. [Google Scholar]
- 46.Cha J.-D., Kim Y.-H., Kim J.-Y. Essential oil and 1, 8-cineole from Artemisia lavandulaefolia induces apoptosis in KB cells via mitochondrial stress and caspase activation. Food Sci. Biotechnol. 2010;19:185–191. [Google Scholar]
- 47.Ding H., Han Z., Cao J., Yang X. Linalool suppresses proliferation and promotes apoptosis in gastric cancer cells via activation of reactive oxygen species-mediated P53 pathway. Curr. Top. Nutraceutical Res. 2020;18:325–330. [Google Scholar]
- 48.Torres A., Vargas Y., Uribe D., Carrasco C., Torres C., Rocha R., Oyarzun C., San Martın R., Quezada C. Pro-apoptotic and anti-angiogenic properties of the α/β-thujone fraction from Thuja occidentalis on glioblastoma cells. JN Oncol. 2016;128:9–19. doi: 10.1007/s11060-016-2076-2. [DOI] [PubMed] [Google Scholar]
- 49.Li Y., Wen J., Du C., Hu S., Chen J., Zhang S., Zhang N., Gao F., Li S., Mao X. Thymol inhibits bladder cancer cell proliferation via inducing cell cycle arrest and apoptosis. Biochem. Biophys. Res. Commun. 2017;491:530–536. doi: 10.1016/j.bbrc.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Sun W., Yu J., Gao H., Wu X., Wang S., Hou Y., Lu J.-J., Chen X. Inhibition of lung cancer by 2-methoxy-6-acetyl-7-methyljuglone through induction of necroptosis by targeting receptor-interacting protein 1. Antioxid. Redox Signal. 2019;31:93–108. doi: 10.1089/ars.2017.7376. [DOI] [PubMed] [Google Scholar]
- 51.Wang X., Shao Q.-H., Zhou H., Wu J.-L., Quan W.-Q., Ji P., Yao Y.-W., Li D., Sun Z.-J. Ginkgolide B inhibits lung cancer cells promotion via beclin-1-dependent autophagy. BMC Complement. Med. Ther. 2020;20:1–11. doi: 10.1186/s12906-020-02980-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Lewinska A., Adamczyk-Grochala J., Kwasniewicz E., Deregowska A., Wnuk M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017;265:117–130. doi: 10.1016/j.toxlet.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 53.Tong L., Chuang C.-C., Wu S., Zuo L. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015;367:18–25. doi: 10.1016/j.canlet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan L.B., Chandel N.S. Mitochondrial reactive oxygen species and cancer. Canc. Metabol. 2014;2:17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fulda S., Galluzzi L., Kroemer G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 56.Su L.-J., Zhang J.-H., Gomez H., Murugan R., Hong X., Xu D., Jiang F., Peng Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019;2019:1–13. doi: 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.V Ziegler D., Wiley C.D., Velarde M.C. Mitochondrial effectors of cellular senescence: beyond the free radical theory of aging. Aging Cell. 2015;14:1–7. doi: 10.1111/acel.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Usta J., Kreydiyyeh S., Knio K., Barnabe P., Bou-Moughlabay Y., Dagher S. Linalool decreases HepG2 viability by inhibiting mitochondrial complexes I and II, increasing reactive oxygen species and decreasing ATP and GSH levels. Chem. Biol. Interact. 2009;180:39–46. doi: 10.1016/j.cbi.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 59.Kweon M.-H., Adhami V.M., Lee J.-S., Mukhtar H. Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J. Biol. Chem. 2006;281:33761–33772. doi: 10.1074/jbc.M604748200. [DOI] [PubMed] [Google Scholar]
- 60.Weigelt B., Peterse J.L., Van’t Veer L.J. Breast cancer metastasis: markers and models. Nat. Rev. Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 61.Singh T., Katiyar S.K. Honokiol inhibits non-small cell lung cancer cell migration by targeting PGE2-mediated activation of β-catenin signaling. PloS One. 2013;8 doi: 10.1371/journal.pone.0060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iannelli F., Lombardi R., Milone M.R., Pucci B., De Rienzo S., Budillon A., Bruzzese F. Targeting mevalonate pathway in cancer treatment: repurposing of statins. Recent Pat. Anti-Cancer Drug Discov. 2018;13:184–200. doi: 10.2174/1574892812666171129141211. [DOI] [PubMed] [Google Scholar]
- 63.Mo H., Jeter R., Bachmann A., Yount S.T., Shen C.-L., Yeganehjoo H. The potential of isoprenoids in adjuvant cancer therapy to reduce adverse effects of statins. Front. Pharmacol. 2019;9:1515. doi: 10.3389/fphar.2018.01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.