Highlights
-
•
High expression of extracellular heat shock proteins (HSPs) indicates highly aggressive tumors.
-
•
HSP profiling of extracellular vesicles (EVs) derived from various biological fluids and released by immune cells may open new perspectives for an identification of diagnostic, prognostic and predictive biomarkers of cancer.
-
•
Identification of specific microRNAs targeting HSPs in EVs may be a promising strategy for the discovery of novel biomarkers of cancer.
Keywords: heat shock proteins, Extracellular vesicles, Extracellular HSPs, miRNA, Biomarker, cancer
Abbreviations: HSP, heat shock proteins; miRNA, microRNA; MS, mass spectrometry; NSCLC, non-small cell lung cancer; AML, acute myeloid leukemia; ESCC, esophageal squamous cell carcinoma; CTC, circulating tumor cells; SCCNH, squamous cell carcinoma of the head and neck; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; DICS, ductal carcinoma in situ; CUSA, cavitron ultrasonic surgical aspirator; CHIP, C-terminus of HSP70 interacting protein; HOP, HSP70-HSP90 organizing protein; BAG, BCL2-associated athanogene; ccRCC, clear cell renal cell carcinoma; HL, Hodgkin lymphoma; DLBCL, Diffuse large B-cell lymphoma
Abstract
Heat shock proteins (HSPs) are a large family of molecular chaperones aberrantly expressed in cancer. The expression of HSPs in tumor cells has been shown to be implicated in the regulation of apoptosis, immune responses, angiogenesis and metastasis. Given that extracellular vesicles (EVs) can serve as potential source for the discovery of clinically useful biomarkers and therapeutic targets, it is of particular interest to study proteomic profiling of HSPs in EVs derived from various biological fluids of cancer patients. Furthermore, a divergent expression of circulating microRNAs (miRNAs) in patient samples has opened new opportunities in exploiting miRNAs as diagnostic tools. Herein, we address the current literature on the expression of extracellular HSPs with particular interest in HSPs in EVs derived from various biological fluids of cancer patients and different types of immune cells as promising targets for identification of clinical biomarkers of cancer. We also discuss the emerging role of miRNAs in HSP regulation for the discovery of blood-based biomarkers of cancer. We outline the importance of understanding relationships between various HSP networks and co-chaperones and propose the model for identification of HSP signatures in cancer. Elucidating the role of HSPs in EVs from the proteomic and miRNAs perspectives may provide new opportunities for the discovery of novel biomarkers of cancer.
Introduction
Heat shock proteins (HSPs) are evolutionally conserved and ubiquitously expressed molecular chaperones abundantly present in cancer [1], [2], [3]. Mammalian HSPs are divided into families, namely HSP70/ HSPA, HSP90/HSPC, HSP40/DNAJ, HSPB, HSP110/HSPH and chaperonins [4]. Several research groups showed that naturally occurring antitumor agents such as geldanamycin and radicicol exert their action by inhibiting ATPase activity of HSP90 chaperone [5], [6], [7], [8]. This finding has led to the development of various HSP90 inhibitors (Table 1). Subsequently, the ability of HSP70 to promote immune responses stimulated the development of HSP-based cancer vaccines [9,10]. Currently, various HSP-based therapies and several HSP-based biomarkers are assessed in clinical trials (Table 1).
Table 1.
HSPs in cancer clinical trials.
| Intervention | HSPs | Type of cancer | Study | Refs. |
|---|---|---|---|---|
| HSP inhibitors | ||||
| AUY922 (Luminespib) | HSP90 | • Advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction | Phase Ib | [23] |
| • Myeloproliferative Neoplasms | Phase II | [24] | ||
| • Advanced solid tumors | Phase I | [25] | ||
| • Lymphoma | Phase II | [26] | ||
| • Non-small cell lung cancer (NSCLC) | PhaseI/II | [27], [28] | ||
| • Gastrointestinal Stromal Tumor | Phase II | [29] | ||
| • Advanced Anaplastic lymphoma kinase (ALK)-positive NSCLC | Phase II | [30] | ||
| • NSCLC | Phase II | [31] | ||
| • Breast cancer | Phase I/II | [32] | ||
| • Multiple myeloma | Phase I/II | [33] | ||
| • Metastatic colorectal cancer | Phase I | [34] | ||
| HS-201 | HSP90 | Solid tumor | Phase I | [35] |
| HS-196 | HSP90 | Solid tumor | Phase I | [36] |
| STA-9090 (Ganetespib) | HSP90 | • SCLC | Phase II | [37] |
| • Acute Myeloid Leukemia, Acute Lymphoblastic Leukemia and Blast-phase Chronic Myelogenous Leukemia | Phase I | [38] | ||
| • Acute Myeloid Leukemia; High Risk Myelodysplastic syndrome | Phase I/II | [39] | ||
| • Ocular melanoma | Phase II | [40] | ||
| • Prostate cancer | Phase II | [41] | ||
| • NSCLC | Phase II | [42] | ||
| • Solid tumors | Phase I | [43], [44] | ||
| • Hematologic malignancies | Phase I | [45] | ||
| • Ovarian cancer | Phase II | [46] | ||
| • Platinum-resistant ovarian cancer | Phase I/II | [47] | ||
| • Melanoma | Phase II | [48] | ||
| • Metastatic human epidermal growth factor receptor 2-positive breast cancer | Phase I | [49] | ||
| • Breast cancer | Phase II | [50] | ||
| • Advanced Gastrointestinal Carcinomas; Non-Squamous NSCLC; Urothelial Carcinomas; Sarcomas | Phase I | [51] | ||
| • Malignant Peripheral Nerve Sheath Tumors | Phase I/II | [52] | ||
| HSP990 | HSP90 | Advanced solid tumors | Phase I | [53], [54] |
| TAS-116 | HSP90 | Advanced solid tumors | Phase I | [55] |
| KOS-953 (Tanespimycin) | HSP90 | Multiple myeloma | Phase III | [56] |
| CNF2024 (BIIB021) | HSP90 | B-cell Chronic Lymphocytic Leukemia | Phase I | [57] |
| NVP-BEP800 | HSP90 | Acute T Lymphoblastic Leukemia; Acute B Lymphoblastic Leukemia |
Pilot | [58] |
| AT13387 (Onalespib) | HSP90 | • Prostate cancer | Phase I/II | [59] |
| • Advanced solid tumor; Recurrent ovarian, fallopian tube, primary peritoneal or triple negative breast cancer | Phase I | [60] | ||
| • Solid tumors | Phase I | [61] | ||
| • Non-small cell lung cancer | Phase I/II | [62] | ||
| • Advanced triple negative breast cancer | Phase I | [63] | ||
| • Solid tumors | Phase I | [64] | ||
| IPI-504 (Retaspimycin) | HSP90 | • Non-small cell lung cancer • Metastatic melanoma • Advanced breast cancer |
Phase II Phase II Phase I/II |
[65] [66] [67] |
| Alvespimycin | HSP90 | • Relapsed chronic lymphocytic leukemia; small lymphocytic lymphoma; B-cell prolymphocytic leukemia | Phase I | [68] |
| • Lymphoma; Small Intestine Cancer; Solid tumors | Phase I | [69] | ||
| Debio 0932 | HSP90 | • Advanced solid tumors; Lymphoma • Non-small cell lung cancer |
Phase I Phase I |
[70] [71] |
| IPI-493 | HSP90 | Hematologic malignancies | Phase I | [72] |
| PU-H71 | HSP90 | Solid tumors; Non-Hodgkin's Lymphoma | Phase I | [73] |
| DS-2248 | HSP90 | Advanced solid tumors | Phase I | [74] |
| MPC-3100 | HSP90 | Refractory or relapsed cancer | Phase I | [75] |
| CNF1010 | HSP90 | B-cell positive chronic lymphocytic leukemia | Phase I | [76] |
| SNX-5422 | HSP90 | • Refractory solid tumor; Non-Hodgkin's lymphoma | Phase I | [77] |
| • Hematologic malignancies | Phase I | [78] | ||
| • Neuroendocrine tumors | Phase I | [79] | ||
| • Lung adenocarcinoma | Phase I | [80] | ||
| • Chronic lymphocytic leukemia | Phase I | [81], [82] | ||
| Minnelide | HSP70 | • Acute Myeloid Leukemia | Phase I | [83] |
| OGX-427 | HSP27 | • Prostate cancer; Ovarian cancer; Breast cancer; bladder cancer; NSCLC | Phase I | [84], [85] |
| • Bladder cancer | Phase I | [86] | ||
| • Castration resistant prostate cancer | Phase II | [87] | ||
| HSP-based vaccines | ||||
| HSP70 vaccine | HSP70 | Chronic Myelogenous Leukemia | Phase I | [88] |
| HSP70 vaccine | HSP70 | Breast Neoplasms | Phase I/II | [89] |
| HSPPC-96 vaccine (Vitespen) | HSP90B1 (gp96) | • High-grade glioma | Phase I | [90] |
| • Recurrent or progressive high-grade glioma | Phase I/II | [91] | ||
| • Malignant melanoma | Phase III | [92] | ||
| • Recurrent glioblastoma | Phase II | [93] | ||
| • Lymphoma | Phase II | [94] | ||
| • Pancreatic cancer | Phase I | [95] | ||
| • Recurrent soft tissue sarcoma | Phase II | [96] | ||
| • Liver cancer | Phase II/III | [97] | ||
| • Glioblastoma | Phase II | [98], [101] | ||
| • Liver cancer; Pancreatic adenocarcinoma | Phase I/II | [99] | ||
| • Gastric carcinoma | Phase I | [100] | ||
| HSP110-gp100 chaperone complex vaccine | HSP110 | Advanced melanoma | Phase I | [102] |
| HspE7 (SGN-00101) | HSP65* | Cervical intraepithelial neoplasia | Phase II | [103] |
| pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine | HSP70 | Cervical cancer | Phase I/II | [104] |
| Gp100 fused with OVA BiP and HSP70 | HSP70 | Melanoma | Phase I | [105] |
| HSP-based prognostic biomarkers | ||||
| HSP110DE9 | HSP110 | Colorectal cancer | Pilot | [106] |
| HSP-based predictive biomarkers | ||||
| HSP27 | HSP27 | Androgen-independent prostate cancer | Pilot | [107] |
| HSP90 | HSP90 | Peritoneal carcinomatosis | Pilot | [108] |
| HSP70 | HSP70 | Melanoma | Phase I | [109] |
derived from Mycobacterium bovis Bacillus Calmette-Guerin.
Researchers studying the role of HSPs in cancer proposed that malignant cells are “addicted to chaperones”, particularly emphasizing the importance of three well-studied HSPs such as HSP70, HSP27 and HSP90 in tumor development [11,12]. Indeed, overexpression of HSPs provides selective advantage to malignant cells by inhibiting apoptosis, promoting tumor metastasis and regulating immune responses [1,[13], [14], [15], [16]]. Considering critical role of HSPs in cancer, several studies have proposed HSPs as potential biomarkers for cancer diagnosis [17,18]. Controversially, researchers studying the HSP expression in various types of cancers have observed that HSPs are overexpressed in various types of tumors [1]. Thus, they concluded that HSPs might not be informative as tissue- restricted molecular markers, but rather can be used to identify the degree of differentiation and aggressiveness in some cancers [1]. Recent studies using gene expression have shown that HSPs can be used as prognostic markers to predict clinical outcome of breast cancer patients [19]. Furthermore, the discovery of the role of extracellular vesicles (EVs) in transferring proteomic and genetic information (mRNAs, microRNAs and DNA) and identification of HSPs in EVs have opened new opportunities and challenges for determining clinical biomarkers of cancer [20].
EVs are phospholipid bilayer- encapsulated particles released by many types of cells [21]. Advances in mass spectrometry (MS) provided the opportunity to identify and quantify thousands of EV cargo proteins, allowing for the search of potential biomarkers in different types of cancer (reviewed in [22]).
The main focus of this review is extracellular HSPs and microRNAs regulating HSPs in EVs as potential biomarkers for cancer diagnosis. We discuss expression of various HSP members in EVs derived from different biological fluids and patient samples and their use as biomarkers for cancer diagnosis. We will also provide novel perspective of using HSP profiling of EVs released by immune cells as clinical biomarkers and potential targets for investigational therapies. This review may help to better understand the role of HSP chaperones in EVs and their clinical importance for the identification of new cancer biomarkers and therapeutic targets.
Membrane-bound and secreted forms of HSPs in cancer
Plasma membrane plays an important role in HSP expression and localization [110]. Several studies demonstrated that heat stress or membrane fluidizers such as benzyl alcohol and heptanol induce changes in membrane fluidity and microdomain reorganization leading to subsequent activation of heat shock genes [111], [112], [113]. Noticeably, heat stress resulted in membrane hyperfluidization and membrane rearrangements in leukemia cells [114]. Dempsey and co-workers showed that membrane fluidizers affect the HSPs localization causing decrease in intracellular and increase in surface HSP60 and HSP70 [115]. In addition, HSP70-membrane positive tumors showed to contain globotriaoslyceramide allowing for the anchorage of HSP70 in plasma membrane [116]. HSP70-membrane interaction is also showed to be mediated by HSP70 binding to phosphatidylserine [117]. Since HSP70 released into extracellular space via endolysosomal system, elevated level HSP70 in lysosomes showed to stabilize lysosomal membranes of cancer cells by regulating sphingolipid catabolism [110,[118], [119], [120], [121], [122]].
Surface expression of HSPs often indicates highly aggressive tumors [14,110,123]. In this regard, many members of HSP family have been found in extracellular space or on the cell membranes- collectively called extracellular HSPs [124], [125], [126], [127], [128], [129], [130], [131]. High expression of extracellular HSPs showed to correlate with increased cell proliferation, cancer stage and poor clinical outcome suggesting potential use of HSP expression in cancer diagnosis [110,123,132]. Studies assessing extracellular HSPs as cancer biomarkers are summarized in Table 2. A clinical prospective pilot study conducted by Gobbo and colleagues showed that concentration of HSP70-positive exosomes in plasma of lung and breast cancer patients was significantly higher compared to healthy volunteers [133]. Moreover, plasma-derived HSP70-positive exosomes showed to be better in discriminating metastatic from non-metastatic patients than circulating tumor cells (CTC) [133]. Campanella and co-workers showed that HSP60 level in plasma-derived exosomes was significantly higher in patients with colorectal adenocarcinoma than in healthy controls[134]. Elevated expression of HSP70 was found in plasma-derived exosomes of stage IV melanoma patients [135]. Several studies showed that HSPs expression in blood could discriminate between patients with early and late stage of cancer, suggesting the potential use of HSPs in early detection of cancer (Table 2) [136], [137], [138], [139].
Table 2.
Selection of studies assessing extracellular HSPs in patient samples.
| HSPs | Cancer type | Sample | Findings | Refs |
|---|---|---|---|---|
| HSP70 | NSCLC |
Serum | Significantly higher expression of serum HSP70 compared to healthy volunteers. Positive correlation between serum HSP70 expression and tumor volume | [131] |
| Elevated level of antibodies to HSP70 in cancer patients than in healthy controls | [140] | |||
| SCLC |
Serum HSP70 was significantly higher in SCLC patients than in healthy individuals and correlated with poor clinical outcome | [141] | ||
| High level of serum HSP70 compared to controls. Serum HSP70 showed to correlate with stage of the disease | [142] | |||
| Lung cancer | High expression of HSP70 in serum increased the risk of developing lung cancer in Japanese males | [143] | ||
| Colorectal cancer |
High level of soluble HSP70 associated with poor survival | [144] | ||
| Significant increase in serum HSP70 with the stage of the disease. High baseline serum HSP70 associated with poor survival | [145] | |||
| Breast cancer | sHSP70 was significantly higher in breast cancer patients than in healthy individuals. HSP70 level of >2.41 ng/ml cutoff was used to predict breast cancer with >90% specificity and sensitivity | [146] | ||
| Breast, colon, colorectal cancers; GIST | Significantly higher serum HSP70 level than in normal samples | [147] | ||
|
Pancreatic cancer |
sHSP70 level was significantly higher in patients with pancreatic cancer compared to patients with chronic pancreatitis and healthy volunteers. The sensitivity and specificity for discriminating cancer patients from healthy controls were 74% and 90%, respectively | [148] | ||
| HCC | Significantly higher level of serum HSP70 in HCC patients than in control groups. High level of HSP70 was found in cohorts of patients with liver cirrhosis, who eventually developed HCC | [149] | ||
| ESCC | Significantly higher level of serum HSP70 autoantibody in patients with ESCC than in colon, gastric cancer patients and healthy controls | [150] | ||
| Head and neck cancer; Lung cancer; Colorectal cancer; Pancreatic cancer; Glioblastoma; Hematologic malignancies |
Significantly higher serum HSP70 in cancer patients compared to healthy volunteers | |||
| NSCLC | Plasma | High level of post-therapeutic circulating HSP70 associated with improved response to radiochemotherapy. | [151] | |
| AML; MDS; ALL | High level of circulating HSP70 associated with significantly shorter overall survival | [152] | ||
| Breast cancer; NSCLC |
Plasma- derived exosomes |
HSP70-positive tumor-derived exosomes showed to be better at predicting cancer dissemination than CTC and showed to correlate with the disease status and response to therapy. | [133] | |
| Melanoma | Statistically higher level of HSP70 in exosomes isolated from melanoma subjects with stage IV melanoma compared to healthy controls | [135] | ||
| Colon cancer; Lower rectal cancer; Gastric cancer; LSCC |
Patient tissue | mHSP70 expression showed to be associated with poor outcome in patients with lower rectal and squamous cell carcinoma of the lungs and positive clinical outcome in patients with colon and gastric cancers | [132] | |
| Colorectal cancer; AML; Lung cancer; Neuronal tumors; Pancreatic cancer |
mHSP70 expression was found in leukemic blasts of AML patients and freshly isolated biopsies from patients with colorectal, lung, neuronal and pancreatic tumors. No surface HSP70 expression was found on normal tissues and bone marrow of healthy donors. | [153] | ||
| Melanoma | HSP70-membrane positive phenotype was found in melanoma metastases |
[154] |
||
| SCCHN | Tumor biopsy and serum |
Significantly higher expression of mHSP70 in tumor biopsy than in reference tissue. Significantly higher expression of soluble HSP70 in SCCHN than in healthy controls. Elevated level of mHSP70 on tumors associated with high soluble HSP70 in patients’ serum. Prior therapy, soluble HSP70 showed to correlate with tumor volume. | [155] | |
| AML | Aspirated bone marrow cells | Elevated HSP70-membrane expression associated with poor patient prognosis | [156] | |
| Breast and pulmonary cancers | Urine- derived exosomes |
Elevated concentration of HSP70-positive exosomes in cancer patients compared to healthy volunteers | [157] | |
| HSP90 | NSCLC |
Serum |
No significant difference was found between serum antibodies to HSP90 of cancer patients compared to healthy controls | [140] |
| SCLC; LAC; LSCC | Serum HSP90AB1/HSP90β was significantly increased in patients compared to healthy controls and significantly associated with grade and stage of cancer | [158, 159] | ||
| Melanoma | Serum HSP90 significantly higher than in healthy controls. No correlation was found between the level of serum HSP90, patient survival and response to chemotherapy | [160] | ||
| HCC | Significantly elevated level of serum HSP90 in cancer patients compared to healthy individuals | [161] | ||
| Colorectal cancer | Serum HSP90AA1/HSP90α expression significantly higher in cancer patients compared to healthy volunteers | [162] | ||
| AML | Significantly higher serum HSP90AA1/HSP90α expression compared to healthy controls. | [163] | ||
| Ductal and lobular breast tumors | No significant association was found between the level of serum HSP90 and the severity of the lesion in patients with ductal and lobular breast tumors |
[164] |
||
| NSCLC; SCLC |
Plasma | Plasma HSP90AA1/HSP90α level was significantly higher in cancer patients than in healthy volunteers. Patients with advanced stages had higher plasma HSP90 expression than patients in early stages. Significant difference was found in the level of plasma HSP90 before and after surgery. | [138] | |
| Liver cancer | Level of plasma HSP90AA1/HSP90α could discriminate between patients with liver cancer and control groups, diagnose early-stage liver cancer with >90% sensitivity and specificity | [137] | ||
| Colorectal cancer | Significantly elevated level of plasma HSP90AA1/HSP90α in patients with colorectal cancer compared to healthy volunteers. Significantly higher level of plasma HSP90α in late stage than in early stage of cancer | [139] | ||
| Melanoma | Plasma-derived exosomes | HSP90 isoform was found in exosomes of 70% patients with stage IV melanoma | [135] | |
| HSPB | Gynecologic cancers | Serum | Significantly higher number of patients had serum antibodies to HSPB1/HSP27 compared to patients with benign lesions | [165] |
| Ovarian cancer | Significantly elevated level of anti-HSP27/HSPB1 antibodies in patients than in healthy women. Higher level of anti-HSP27 antibodies associated with less advanced stage of cancer | [166] | ||
| Breast cancer | HSPB1/HSP27 level was up-regulated in breast cancer patients | [167] | ||
| Significantly higher level of serum HSPB1/HSP27 in breast cancer patients than in healthy volunteers | [168] | |||
| Epithelial ovarian cancer | Elevated sHSP27 level in patients with peritoneal metastases. The serum level of HSP27 significantly decreased following chemotherapy in patients with peritoneal metastases. | [169] | ||
| Gynecologic cancers | Serum and serum-derived exosomes | Significantly higher level of sHSPB5/CRYAB in endometrial cancer patients compared to endometriosis patients. No significant difference in HSPB5, HSPB6/HSP20, HSPB8/HSP22 expression was found in serum-derived exosomes | [170] | |
| HSP40 | SCLC | Serum | Significantly higher level of autoantibodies to HSP40 in serum was found in lung cancer patients than in healthy controls | [171] |
| Chaperonins | Ovarian cancer | Serum | No significant difference in the level of antibodies to HSPD1/HSP60 was found between patients with ovarian cancer and healthy women. Significantly higher concentration of antibodies to HSPD1/HSP60 was found in early stages of cancer than in control groups | [172] |
| Colorectal cancer | Serum | Elevated level of sHSP60 in colorectal cancer patients compared to controls. HSP60 level in serum showed to be more specific for late-stage colorectal cancer | [136] | |
| Breast cancer; DCIS | Serum | Autoantibodies to HSP60 were found in patients with early breast cancer and DCIS compared to healthy individuals. | [173] | |
| Colorectal adenocarcinoma | Plasma-derived exosomes | The level of HSP60 in exosomes was significantly higher in patients before surgery than in patients after surgery and in healthy controls | [134] |
NSCLC, non-small cell lung cancer; mHSP70, membrane HSP70; sHSP70, serum HSP70; AML, acute myeloid leukemia; MSD, Myelodysplastic syndrome; ALL, Acute lymphoblastic leukemia; SCLC, small cell lung cancer; ESCC, esophageal squamous cell carcinoma; CTC, circulating tumor cells; SCCHN, squamous cell carcinoma of the head and neck; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; DCIS, ductal carcinoma in situ; LSCC, Lung squamous cell carcinoma; LAC, adenocarcinoma of the lung
HSPs in extracellular vesicles of cancer patients
EVs have been shown to be implicated in various stages of cancer progression including immune suppression, epithelial-mesenchymal transition, angiogenesis, proliferation and metastasis [21]. Several researchers have used MS proteomic analysis to determine and characterize EV proteome in various cancer tissues and biofluids [174], [175], [176], [177], [178], [179]. However, no previous study, has analyzed the HSP profile in EVs derived from clinical samples of cancer patients. For this review, we closely looked at the expression of HSPs in EVs from different biological fluids obtained from various MS studies, which have been summarized in Table 3. We have also included in the analysis co-chaperones involved in HSP70 and HSP90 functional cycles. HSP profile in EVs seems to vary in different types of cancer and biological fluids. Urine-derived EVs showed to contain members from different HSP families as well as various HSP70 and HSP90 co-chaperones and might be a promising liquid biopsy for the identification of noninvasive HSP-based biomarkers. However, it is important to note that data generated by MS has a lot of missing values and, therefore, the absence of the protein expression in a sample may not reflect the actual HSP proteome composition in EVs and may occur due to the challenges in analyzing different types of liquid biopsies by MS-based proteomics and differences in EV isolation methods discussed later in this section (reviewed in [180])[181].
Table 3.
Heat shock proteins in extracellular vesicles of cancer patients.
| Cancer type | HSP family | Heat Shock Proteins | Sample | Isolation method | Refs. |
|---|---|---|---|---|---|
| Melanoma | HSP70 network |
HSPA5;HSPA9;HSPA8;HSPA2;HSPA1A;HSPA12A;HSPA12B; HSPA14; HSPA13; Co-chaperones:DNAJC10;DNAJB11;DNAJC3;DNAJA1;DNAJC7;DNAJA2; DNAJC16;DNAJC11;DNAJB1;DNAJC9;DNAJC19;DNAJA3;DNAJB14;DNAJA4; DNAJB4;DNAJB2;DNAJC5; DNAJB6; DNAJB12; DNAJC25; DNAJB9; DNAJC30; DNAJC8; DNAJC14; DNAJC15; DNAJC13; HSPA4L; HSPA4; HSPH1; HSPBP1; SIL1; GRPEL1;BAG1;BAG2,BAG6;BAG3; BAG5; STIP1; STUB1; ST13 |
Tissue |
UC |
[176] |
| HSP90 network |
HSP90B1; HSP90AB1;HSP90AA1; TRAP1; Co-chaperones:Cdc37;TSC1;AHSA1; FKBP4;FKBP5; FKBP8; PPP5C; PPID; PPIF; STIP1 |
||||
| HSPB family | HSPB1;HSPB5 | ||||
| Chaperonins | HSPD1;HSPE1;CCT3;CCT4;CCT2; CCT6A; CCT5;CCT8; CCT7; CCT1 | ||||
| Melanoma | HSP70 network | HSPA1A/HSPA1B; HSPA8;HSPA5; Co-chaperones: ST13; HSPA4 |
Plasma |
UC |
[174] |
| HSP90 network | HSP90AA1; HSP90AB1;HSP90B1; | ||||
| HSPB family | HSPB1 | ||||
| Chaperonins | HSPD1;HSPE1;CCT2;CCT7;CCT4; CCT6A; CCT1 | ||||
| HSP70 network | HSPA1A/HSPA1B;HSPA5;HSPA8;HSPA2; HSPA6/HSPA7; Co-chaperones:DNAJA4;DNAJC5; ST13;DNAJB11;DNAJA2;DNAJC13; DNAJB2; STIP1; HSPA4 |
Seroma | |||
| HSP90 network | HSP90AA1; HSP90AB1; HSP90B1 Co-chaperones: STIP1 |
||||
| HSPB family | HSPB1; HSPB6; | ||||
| Chaperonins | HSPD1;CCT5;CCT3;CCT8;CCT4;CCT2;CCT7; CCT6A; CCT1 | ||||
| Glioblastoma | HSP70 network | HSPA12A;HSPA13;HSPA2;HSPA5;HSPA8; HSPA9; Co-chaperones:HSPH1;STIP1;STUB1;ST13;BAG2;BAG6;GRPEL1;DNAJA1; DNAJA2;DNAJA3;DNAJB1;DNAJB11; DNAJB2; DNAJC10; DNAJC11; DNAJC13; DNAJC19; DNAJC3; DNAJC5; HSPA4 |
CUSA fluid |
UC |
[175] |
| HSP90 network |
HSP90AA1; TRAP1; HSP90AB1;HSP90B1; Co-chaperones:AHSA1;STIP1;FKBP4;FKBP5; FKBP8; PPIF; |
||||
| HSPB family | HSPB1; HSPB6; HSPB5 | ||||
| Chaperonins | HSPD1;HSPE1;CCT2;CCT3;CCT4;CCT5;CCT6A;CCT7;CCT8; CCT1 | ||||
|
Pancreatic cancer |
HSP70 network | HSPA8; HSPA5; HSPA1A/HSPA1B; Co-chaperones: DNAJB11; ST13; |
Serum |
UC |
[179] |
| HSP90 network | HSP90AA1; HSP90AB1;HSP90B1; | ||||
| HSPB family | HSPB1 | ||||
| Chaperonins | – | ||||
| Prostate cancer |
HSP70 network | HSPA12A; HSPA2; HSPA5; HSPA8; Co-chaperones:HSPH1;DNAJA1;HSPA4;DNAJA2;DNAJB1;DNAJB2;DNAJB6; ST13; DNAJC13; DNAJC5; STIP1; STUB1; BAG2; BAG5; |
Urine |
DGC |
[177] |
| HSP90 network | HSP90AA1;HSP90B1;HSP90AB1;HSP90AB2P; Co-chaperones:Cdc37;STIP1;AHSA1; FKBP4; PPP5C |
||||
| HSPB family | HSPB5; HSPB1; | ||||
| Chaperonins | CCT2; CCT3; CCT4; CCT5; CCT6A; CCT7; CCT8; CCT1 | ||||
| HSP70 network | HSPA1A; HSPA5;HSPA8; Co-chaperones:DNAJA2;DNAJB6;DNAJB1;DNAJB2;DNAJA1;DNAJC5; ST13; HSPH1; STIP1; BAG2; HSPA4; |
Tissue | |||
| HSP90 network | HSP90AB1; HSP90AA1; HSP90B1; Co-chaperones: Cdc37; STIP1; FKBP4; FKBP5; |
||||
| HSPB family | HSPB1; HSPB5 | ||||
| Chaperonins | CCT1;CCT5; CCT8; CCT2; CCT7; CCT6A; CCT4;CCT3 | ||||
| Bladder cancer | HSP70 network | HSPA1A; HSPA8; HSPA2; HSPA5; Co-chaperones: DNAJA1; ST13; DNAJB1; DNAJA2;STIP1 |
Urine |
UC |
[208] |
| HSP90 network | HSP90AB1; HSP90AA1; Co-chaperones: STIP1; FKBP4; |
||||
| HSPB family | HSPB1 | ||||
| Chaperonins | CCT7; CCT2;CCT3;CCT6A;CCT4;CCT8; CCT1; | ||||
| Lung cancer | HSP70 network | Co-chaperones: DNAJC3;HSPA4; | Saliva |
UC |
[209] |
| HSP90 network | HSP90AB1;HSP90AA1; Co-chaperones: SIL1 |
||||
| HSPB family | HSPB1; | ||||
| Chaperonins | CCT4; | ||||
| Papillary thyroid cancer | HSP70 network | HSPA8; HSPA5;HSPA1B; Co-chaperones: STIP1; HSPA4;DNAJC5;DNAJA2; DNAJA1;DNAJC7; STUB1; ST13 |
Serum | UC | [210] |
| HSP90 network | HSP90B1;HSP90AA1;HSP90AB1; Co-chaperones: STIP1; |
||||
| HSPB family | HSPB1; | ||||
| Chaperonins | HSPD1; CCT2; CCT5; CCT6A;CCT3;CCT4;CCT7;CCT8; CCT1 |
CUSA, Cavitron ultrasonic surgical aspirator; UC, ultracentrifugation; DGC, density gradient centrifugation
In search of HSP-based biomarkers in EVs of cancer patients, it is important to understand relationships between different HSP networks as HSP families seem to be closely linked with each other. For example, DNA vaccines coding for HSP70 or HSP90 showed to affect HSP60-specific T cell response [182]. Along this line, HSP90 inhibitors showed to upregulate expression of HSP27 and HSP70 [183], [184], [185]. Furthermore, HSP70 and HSP90 networks interact via HSP70-HSP90 organizing protein (HOP/STIP1) while members of HSP40/DNAJ and HSP110/HSPH families act as co-chaperones for HSP70 [186,187]. In addition, HSP90 was found to be constitutively associated with chaperonin member CCT2/CCT-β [188]. These data strongly suggest that there is a cross-talk between various HSP networks.
HSP co-chaperone profile varies between EVs derived from different sample sources (Table 3). EV cargos derived from melanoma tissue contain major HSP90 co-chaperones and all five types of molecular co-chaperones critical for regulation of HSP70 functional cycle: DNAJ/HSP40 molecules deliver client proteins to HSP70, nucleotide-exchange factors (HSPH1, HSPBP1, SIL1, GRPEL1, BAG) stabilize open conformation of HSP70, HSP70 interacting protein (HIP/ST13) delays peptide release from HSP70, STIP1/ HOP co-chaperone transfers client proteins from HSP70 to HSP90 while C-terminus of HSP70 interacting protein (CHIP/STUB1) co-chaperone mediates degradation of HSP70 client proteins (Table 3) [189], [190], [191], [192], [193], [194], [195], [196]. Interestingly, EVs isolated from plasma of melanoma patients contained two HSP70 co-chaperones HSPA4/HSPH2 and ST13/HIP, and no HSP90 co-chaperones were observed, whereas EVs derived from lymphatic drainage of the matched patients showed to carry more members of HSP70 network (Table 3) [174]. It is important to note that the relationships of HSPs with its co-chaperones define the fate of the client peptides and functional state of HSPs. For example, low concentration of DNAJ is required to stimulate HSP70 folding function while increase in DNAJ concentration results in reduced refolding of client proteins [189,191]. Therefore, understanding relationships between HSP families as well as understanding inter-relationships of chaperones with co-chaperone networks in EVs may form the basis for the identification of multiple biomarkers.
Even though analysis of EV proteome may open new perspectives for HSP-based biomarker discovery, however, it is important to point out that technical challenges exist in isolation of EVs from cell culture conditioned media and various biological fluids [197]. Separation of EVs from non-EVs soluble proteins and lipid particles is critical step for further assessment of EV content for biomarker discovery and validation [181] There are several methods for EV isolation and the most commonly used is differential ultracentrifugation (UC), while other methods include density gradients (DG), immunoaffinity, precipitation, size-exclusion chromatography (SEC), ultrafiltration and microfluidics [197].In order to achieve better specificity, combination of different methods (ultrafiltration, DG and SEC) can be used following primary step of EV isolation [197,198]. UC showed to be widely accepted and cost-efficient method as the first step in purification of EVs [199]. However, the selection of EV isolations methods largely depends on the sample type and starting volume [199]. For example, SEC showed to be better at removing plasma proteins than UC and effective in isolating intact and functional active EVs when combined with ultrafiltration [200], [201], [202], [203]. A recent study of Brennan and colleagues have compared the most commonly used isolation techniques such as UC, ExoQuick kit, SEC using IZON qEV columns and UC coupled with iodixanol density gradient for isolation of EVs from 200 µl of human serum [204]. All of these methods showed to successfully isolate EVs with the size ranging from 61 nm to 150 nm, though particle size, the yield of EVs and protein content showed to differ based on isolation methods used [204]. Furthermore, SEC, DG centrifugation and UC showed the greatest depletion of lipoproteins while at the same time decreased EV yield [204]. Moreover, several studies showed that miRNA profile in EVs also vary depending on isolation methods [205,206]. International Society for Extracellular Vesicles (ISEV) formulated guidelines for Minimal Information for Studies of Extracellular Vesicles (MISEV) highlighting importance for standardization of sample collection, EVs isolation, characterization and storage [198]. Moreover, EV-TRACK database that has been launched recently involves reporting of experimental parameters related to EV isolation and characterization, which may further improve transparency and reproducibility of results [207]. Nevertheless, EVs derived from various biofluids carry specific molecular messages and, despite the challenges posed by isolation methods, EVs hold promise for prediction, diagnosis and therapy response for clinical cancer research [197].
HSPs in extracellular vesicles released by immune cells
Increasing evidence has shown that extracellular HSPs play an important role in tumor immunity. The ability of HSP70 to bind antigenic peptides and elicit CD8+ T cell response led to the entrance of HSP70-based anti-tumor vaccines into clinical trial timeline [211], [212], [213], [214], [215]. Furthermore, extracellular HSP70 activates T regulatory cells and myeloid-derived suppressor cells (MDSC), leading to the production of anti-inflammatory cytokines [216,217]. These suppressive effects of HSP70 on tumor microenvironment (TME) further results in decreased T cell response and reduced stimulatory capacity of myeloid-derived dendritic cells [218,219]. Additionally, HSPs showed to play important role in innate and adaptive immune responses (reviewed in [220]) [221,222]. Considering critical role of HSPs in tumor immunity, we were particularly interested in the HSP profiling of EVs released by immune cells. Table 4 summarizes the data obtained from MS experiments on the expression of HSPs in EVs released by different types of immune cells from healthy donors.
Table 4.
HSP profiling of extracellular vesicles secreted by immune cells of healthy donors.
| Immune cells | HSP family | Heat shock proteins | Isolation method | Refs. |
|---|---|---|---|---|
| NK cells | HSP70 network | HSPA13;HSPA14;HSPA9;HSPA1A/HSPA1B; HSPA1L;HSPA2;HSPA5;HSPA8; Co-chaperones: DNAJA1; DNAJA2; DNAJA4; DNAJB1; DNAJB11; DNAJB4; DNAJB6; DNAJC13;DNAJC3;DNAJC5; DNAJC7; DNAJC9; HSPA4L;HSPBP1; HSPH1; STIP1; STUB1; ST13; BAG2; BAG6; BAG5; HSPA4; |
UC | [224] |
| HSP90 network | HSP90AA1;HSP90AA4P;HSP90AB1;HSP90AB4P; HSP90B1; TRAP1 Co-chaperones: Cdc37;AHSA1; STIP1; FKBP4; FKBP5; PPP5C; PPID |
|||
| HSPB family | HSPB1;HSPB11; | |||
| Chaperonins | HSPD1; TCP1/CCT1; CCT2; CCT3; CCT4; CCT5; CCT8; CCT6A; CCT6B; CCT7 | |||
| T cells | HSP70 network | HSPA8; HSPA1L; HSPA2; HSPA5; Co-chaperones: HSPH1; DNAJA1; DNAJA2; ST13 |
UC | [255] |
| HSP90 network | HSP90AA1;HSP90AB1;HSP90AB3P; HSP90AB2P; HSP90AA2P; HSP90B1; Co-chaperones: Cdc37;FKBP4; |
|||
| HSPB family | – | |||
| Chaperonins | CCT5;CCT3; CCT4; CCT2;CCT6A; | |||
| mDC cells | HSP70 network | HSPA1L; HSPA1A/HSPA1B; HSPA6;HSPA8; Co-chaperones: HSPH1; HSPA4L; DNAJC13; DNAJA1; DNAJA2; DNAJC5; DNAJC7;STIP1; BAG6; HSPA4; |
UC | [256] |
| HSP90 network | HSP90AA1; HSP90AB1; TRAP1; HSP90B1 Co-chaperones: STIP1; Cdc37; FKBP4; FKBP5; |
|||
| HSPB family | HSPB1; | |||
| Chaperonins | HSPD1; | |||
| Platelets | HSP70 network | HSPA8; HSPA5; HSPA6; HSPA1L; HSPA1B; HSPA9; HSPA2; Co-chaperones: STIP1; DNAJB11; ST13; HSPA4 |
UC | [257] |
| HSP90 network | HSP90AA1; HSP90AB1; HSP90B1; TRAP1 Co-chaperones: STIP1; |
|||
| HSPB family | HSPB1; | |||
| Chaperonins | HSPD1; CCT1; CCT2; CCT3; CCT7;CCT5;CCT6A; CCT8 | |||
| Neutrophils | HSP70 network | HSPA1A; HSPA8; Co-chaperones: ST13; |
UC | [258] |
| HSP90 network | HSP90AA1; HSP90AB1; HSP90B1 Co-chaperones: - |
|||
| HSPB family | – | |||
| Chaperonins | HSPD1; CCT8; |
HSPs and natural killer cells
Lugini and collegues have shown that natural killer (NK) cells secrete exosomes in both resting and activated states [223]. Moreover, NKEVs derived from activated NK cells undergo uptake by tumor cells and not by resting peripheral blood mononuclear cells (PBMCs) [223]. Federici and co-workers have demonstrated that NK cells of healthy individuals have higher proliferation rate compared to NK cells isolated from melanoma patients [224]. Furthermore, lower amount of NKEVs was detected in 1 ml of plasma of melanoma patients compared to healthy controls [224]. Cancer patients showed altered phenotype of peripheral blood NK cells represented by low degranulation and interferon-γ production [225]. Since cancer causes defects in NK cell activity, it will be of interest to study the role of HSPs in NKEVs which may further help in the discovery of specific biomarkers of cancer.
Multhoff and colleagues showed that stress-inducible member of HSP70 family (HSPA1A) serves on the surface of tumor cells as a recognition structure for NK cells [226]. Furthermore, NK cell proliferation and cytolytic activity are enhanced with an addition of stress-inducible member of HSP70 (HSPA1A) or HSP70-derived TKD peptide in combination with a low dose of interleukin-2 (IL-2) or IL-15, but not when incubated with its constitutive analogue HSC70 (HSPA8) [227,228]. Along this line, expression of HSP70 and BCL2-associated athanogene 4 (BAG4) on the surface of tumor-derived exosomes showed to stimulate migration of NK cells towards HSP70-positive tumors [229,230]. Interestingly, MS proteomic analysis of EVs of healthy donors showed that NKEVs contain HSPA1A and all other major HSP members and co-chaperones (Table 4). Notably, only NK cells and DCs derived EVs showed to contain BAG6 (Table 4). Exosomes released from DCs with BAG6-surface positive expression showed to activate NK cell receptor NKp30 [231]. Given that BAG6 is a co-chaperone for HSP70, it can be further speculated that expression of HSP70-BAG6 complex on the surface of DCs –derived exosomes promotes NK cell activation.
HSP90 inhibitor geldanamycin showed to downregulate cytotoxic activity of NK cells in cancer cell lines, suggesting important role of HSP90 in functional activity of NK cells [232,233]. NKEVs-derived from healthy donors contain all major members of HSP90 family, including its stress-inducible HSP90α (HSPAA1) and constitutive HSP90β (HSP90AB1) forms as well as its mitochondrial isoform TNF receptor associated protein 1 (TRAP1) (Table 4). Further studies should be performed to assess the differences in HSP90 profiling of NKEVs in cancer patients.
Overall, NK cell-derived EVs have the widest HSP profiling compared to other immune cell types (T cells, DC cells, neutrophils, platelets), suggesting that HSP profiling of NKEVs obtained from peripheral blood of cancer patients may be useful for detection of clinical biomarkers of cancer. More importantly, understanding the cross-talk between DCs and NK cells in the form of HSPs in EVs may lead to the development of more effective HSP-based immunotherapy. The important factor that should be considered when utilizing EVs in comparative studies is that amount of EVs should be normalized and this is particular true for NKEVs [198,224]. Amount of EVs can be normalized by either particle number or total EV protein or multiple normalization strategies can be applied [198,200]. Several studies have used number of donor-secreting cells for normalization of EV amount [234,235]. As an example, Tkach and colleagues when compared pellets derived from DC-secreting cells of different centrifugation speeds (2k,10k and 100 k pellets) observed that using either protein amount or particle numbers for normalization of EVs may lead to the biased results and,thus, they chose to normalize by amount of EV pellet obtained from a given number of secreting cells [234]. Therefore, the choice of normalization strategies largely depends on the scientific question [198].
HSP profiling of EVs secreted by antigen presenting cells and t lymphocytes
Weng and colleagues demonstrated that HSP70-peptide complex extracted from fusion of DCs and tumor cells resulted in enhanced cytotoxic T lymphocyte (CTL) response [212]. HSPs can present tumor-associated antigens to antigen presenting cells (APCs) through MHC class I and class II pathways, leading to CD8+ and CD4+ T cell response [211,[236], [237], [238]]. EVs released by monocyte-derived DCs (mDCs) and T lymphocytes of healthy donors contain different HSP members (Table 4). It is interesting to point out that HSPB family members were not detected in EVs derived from T cells, whereas mDCs showed to release one member of chaperonin family HSPD1/HSP60, which was also not detected in EVs derived from T cells (Table 4). Chaperonin member HSPD1/HSP60 showed to have dual effects on an immune system, depending on the HSP60 concentration, site of HSP60 action and HSP60 epitope (reviewed in [239]). Pro-inflammatory HSP60 resulted in maturation of monocytes for the production of pro-inflammatory cytokines and activation of B cells for the production of anti-HSP60-autoantibodies and IL-10 [239], [240], [241]. By contrast, anti-inflammatory HSP60 acted via anti-ergotypic regulatory T cells [239,242]. These data suggest that each type of immune cells has specific profile of HSP members released in EVs which may reflect its functional activity in immune responses. Further studies should be performed to analyze the abundance of HSP members exported by different types of immune cells in EVs in different types of cancer.
HSPs and other types of immune cells
Neutrophils of healthy individuals showed to export in their EV cargos only two members of HSP70 network, such as major stress-inducible (HSPA1A) and its constitutive isoform HSC70 (HSPA8) as well as ST13/HIP co-chaperone that stabilizes high-affinity state of HSP70 by slowing substrate release from HSP70 and, thus, preventing protein aggregation (Table 4) [187,194]. Two members of chaperonin family HSP60/HSPD1 and CCT8 and no HSPB family members were found in EV cargos derived from neutrophils (Table 4). Recent studies have shown that HSPD1/HSP60 modulates neutrophil activation, whereas HSPB1/HSP27 regulates apoptosis, chemotaxis and exocytosis in neutrophils [243], [244], [245]. Interestingly, no HSP90 co-chaperones were detected in EVs derived from neutrophils of healthy donors, although HSP90 co-chaperones are required for HSP90 functional cycle and HSP90-HSP70 interaction (Table 4) [196,246]. Moreover, mitochondrial HSP90 isoform TRAP1 has not been detected in EVs released by T cells and neutrophils (Table 4). Several studies demonstrated important role of TRAP1 in tumor cell metabolism, therefore, further studies should be performed to assess expression of TRAP1 and HSP90 co-chaperones in EVs derived from neutrophils of cancer patients [247,248].
EVs released by platelets constitute 70–90% of all EVs present in peripheral blood [249,250]. Several studies showed that activation of platelets by thrombin leads to HSP27 phosphorylation, whereas HSC70-HSP90 complex showed to be involved in platelet adhesion (reviewed in [251]) [252,253]. In addition, HSP70 inhibition showed to prevent platelet aggregate formation and granule secretion [254]. Strikingly, EVs derived from platelets of healthy donors contain all major members of HSP70 and HSP90 networks as well as HSPB1/HSP27, HSPD1/HSP60 and CCT chaperonins. However, not many HSP co-chaperones showed to be detected in platelet- derived EVs (Table 4).
HSPs and microRNAs
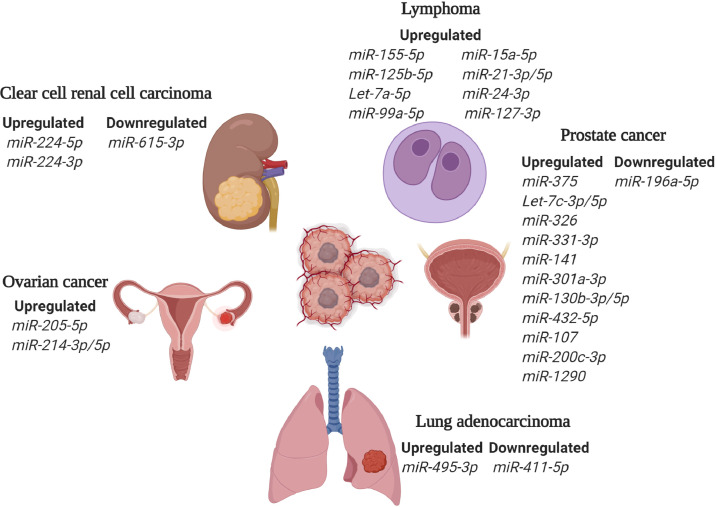
Another class of potential biomarkers abundantly present in circulation are small noncoding microRNAs (miRNAs) [259]. MiRNAs play important role in the regulation of gene expression [260,261]. Specifically, complementary base-pairing of miRNA with target messenger RNA (mRNA) or partial binding of miRNA to mRNA results in mRNA cleavage and translation repression, respectively [260,262,263]. Several studies reported altered expression of miRNAs in serum and plasma of cancer patients, making them potentially useful for identification of blood-based biomarkers [259,[264], [265], [266]]. Along this line, several studies have used specific miRNA signatures to identify tissue of origin of metastatic cancer and distinguish patients with different subtypes of cancer. For example, Feraccin and co-workers used tissue samples from primary and metastatic tumors to identify cancer-type specific 47-miRNA signature for predicting primary sites of metastatic cancers [267]. In another study, Yousseff and colleagues have used specific miRNA patterns to distinguish patients with different subtypes of renal cell carcinoma [268]. Furthermore, aberrant expression of certain miRNAs showed to indicate an early event in the development of pancreatic ductal adenocarcinoma (PDAC) precursor lesions, suggesting the use of miRNAs in early diagnosis [269]. In addition to the use of miRNAs in diagnosis, several studies used miRNAs as prognostic biomarkers of cancer. As an example, high expression of members of miR-183 family in tumor and serum showed to associate with poor outcome in patients with lung cancer [270]. Promisingly, it was also observed that miRNAs can be used to predict response to therapy in cancer patients [271]. As an example, highly expressed miR-21 associated with shorter overall survival in PDAC patients treated with gemcitabine [272]. Additionally, potential use of circulating miRNA as biomarkers showed to be particularly relevant for lymphoma patients (reviewed in [259]). Taking into account that miRNAs may deregulate HSPs and serve as potential predictive and prognostic biomarkers, it is important to identify miRNAs that target HSP networks [273]. Deregulated miRNAs in EVs obtained from clinical samples of cancer patients and their experimentally validated HSP targets are summarized in Table 5 (Fig. 1) [274].
Table 5.
Micro-RNAs and their HSP targets in EVs derived from cancer patients.
| MicroRNA | Target gene | Sample | Cancer | Refs. |
|---|---|---|---|---|
| ↑ miR-205–5p | HSPA8; DNAJA1 | Serum EV | Ovarian | [289] |
| ↑ miR-214–3p | HSP90AB1;HSPD1 | Serum EV | Ovarian | [289] |
| ↑ miR-214–5p | HSPA2;HSPA14; BAG2 | Serum EV | Ovarian | [289] |
| ↑ miR-495–3p | HSPA5; HSPA1B; HSP90AA1; DNAJC21 | Plasma EV | Lung | [290] |
| ↓ miR-411–5p | DNAJB9;DNAJC10;ST13 | Plasma EV | Lung | [290] |
| ↓ miR-615–3p | HSPA1B;HSPA4;CCT8;HSPA8;HSPA9;CCT1;HSP90AB1;DNAJA2;DNAJC9;DNAJC10; PPIF;CDC37;TRAP1;BAG6;BAG5;CCT7 | Plasma EV | ccRCC | [291] |
| ↑ miR-224–5p | HSP90AA1 | Serum EV | ccRCC | [292] |
| ↑ miR-224–3p | DNAJB4; DNAJC28 | Serum EV | ccRCC | [292] |
| ↑ miR-375 | HSP90AA1;DNAJC8; DNAJC2 | Urine EV | Prostate | [293, 294] |
| ↑ Let-7c-5p | HSPA4;DNAJC6;DNAJC16; DNAJC28 | Urine EV | Prostate | [294] |
| ↑ Let-7c-3p | DNAJB6; BAG4 | Urine EV | Prostate | [294] |
| ↓ miR-196a-5p | HSPA4L; TRAP1 | Urine EV | Prostate | [295] |
| ↑ miR-326 | HSPA1B | Plasma EV | Prostate | [296] |
| ↑ miR-331–3p | HSPA1B; HSPA8;CCT1; HSPD1;TRAP1;BAG6 | Plasma EV | Prostate | [296] |
| ↑ miR-141 |
HSPA4;DNAJC28;HSPA2;HSP90AA1; DNAJB2 |
Plasma EV; Serum EV |
Prostate | [296, 297] |
| ↑ miR-301a-3p | HSPA8;CCT6A;CCT8 | Plasma EV | Prostate | [296] |
| ↑ miR-130b-3p | HSPA8;HSP90B1;CCT6A | Plasma EV | Prostate | [296] |
| ↑ miR-130b-5p | HSPA1B; HSPA6; HSPA8;BAG2 | Plasma EV | Prostate | [296] |
| ↑ miR-1233–5p | HSP90AB1;DNAJC8;DNAJC24; DNAJC10; | Serum EV | ccRCC | [298] |
| ↑ miR-432–5p | DNAJB6 | Plasma EV | Prostate | [296] |
| ↑ miR-107 | DNAJA1;DNAJC10 | Plasma EV | Prostate | [296] |
| ↑ miR-200c-3p | DNAJB9;DNAJC3;DNAJB6;FKBP5;BAG4 | Plasma EV | Prostate | [299] |
| ↑ miR-1290 | DNAJB9 | Plasma EV | Prostate | [300] |
| ↑ miR-375 | HSP90AA1;DNAJC8; DNAJC2 | Plasma EV | Prostate | [299, 300] |
| ↑ miR-155–5p | HSPA4L; HSPB11; DNAJC2;DNAJC19; CCT2; DNAJB1; BAG5;CDC37 | Plasma EV | HL | [301] |
| ↑ miR-15a-5p | HSPA1A; HSPA1B; HSPA8; HSP90B1; DNAJA1; DNAJC9;DNAJC10;BAG4;CCT6B | Serum EV | DLBCL | [302] |
| ↑ miR-125b-5p | HSPA1B;HSPD1;HSPBP1 | Serum EV | DLBCL | [303] |
| ↑ miR-21–3p | DNAJC10;ST13 | Serum EV | DLBCL | [304] |
| ↑ miR-21–5p | DNAJC16;PPIF; FKBP5;STUB1 | Plasma EV | HL | [301] |
| ↑ Let-7a-5p | DNAJC6; DNAJC28; FKBP8 | Plasma EV | HL | [301] |
| ↑ miR-24–3p | DNAJC3; DNAJC10; DNAJB12 | Plasma EV | HL | [301] |
| ↑ miR-99a-5p | DNAJA3; FKBP5 | Serum EV | DLBCL | [303] |
| ↑ miR-127–3p | BAG5 | Plasma EV | HL | [301] |
ссRCC, Clear cell renal cell carcinoma; HL, Hodgkin lymphoma; DLBCL, Diffuse large B-cell lymphoma.
Fig. 1.
Deregulated miRNAs targeting HSPs in cancer. Graphical representation of deregulated miRNAs in various types of cancer. MicroRNAs regulate the expression of major members of HSP70 and HSP90 networks (Table 5).
MicroRNAs are protected from degradation by external RNAses when either encapsulated into EVs, bound to argonaute 2 (Ago2) or transported by high-density lipoproteins (HDL) [259,[275], [276], [277]]. Notably, during translational stress HSP90 together with its co-chaperones Cdc37, p23, Aha1 and STIP1/HOP showed to recruit Ago2 to stress granules [278]. Inhibition of HSP90 affects miRNA-gene silencing function of Argonaute [278]. Importantly, HSP90 are required for stable binding of Argonaute to Dicer, enzyme responsible for processing of small interfering RNAs and miRNAs in the cytoplasm for their further loading on RNA-induced silencing complex (RISC) [279], [280], [281]. HSP70 also showed to be associated with Ago2 following T-cell receptor (TCR) activation for the regulation of T-helper 17 (Th17) cells via miRNA expression in EL-4 murine lymphoma cells [282].
miR-21 showed to modulate resistance of cholangiocarcinoma cells to HSP90 inhibitors via DNAJB5 [283]. Along this line, miR-361 binds and regulates expression of HSP90α/HSP90AA1, leading to the suppression of epithelial-mesenchymal transition in cervical cancer lines [283,284]. miR-27a downregulates the expression of HSP90, resulting in further degradation of HSP90 client proteins in esophageal squamous cell carcinoma cell lines [285]. Stope and co-workers showed that HSP27/HSPB1 inhibits miR-1 in prostate cancer cells [286]. Interestingly, HSP70 inhibitor triptolide showed to upregulate expression of miR-142–3p, consequently inhibiting proliferation of pancreatic ductal adenocarcinoma cells [287]. Additionally, inhibition of miR-29a induces apoptosis by increasing expression of HSP60 and decreasing HSP90, HSP70, HSP27 and HSP40 levels in breast cancer cells [288]. Conclusively, miRNAs play important role in HSP regulation, therefore, elucidating the effects of miRNAs on HSPs may open new perspectives for diagnosis and treatment of cancer patients.
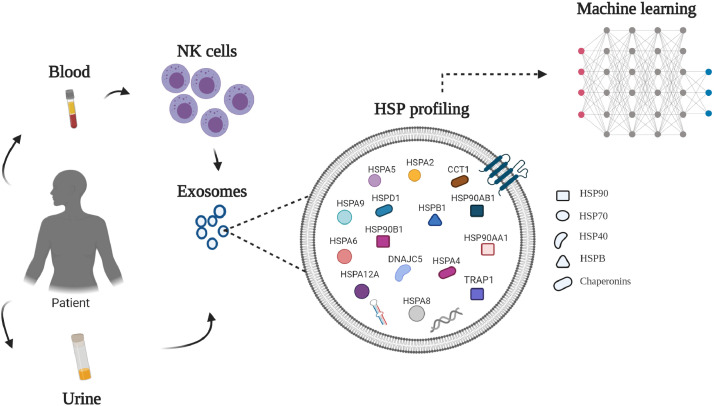
HSP profiling of EVs: future perspectives
Quantitative MS-based proteomics coupled with machine learning (ML) statistics is a novel and promising approach for identification of suitable biomarkers in EVs derived from biological fluids of cancer patients [22,305]. Unlike classical statistics, which identifies only differentially expressed proteins, ML performs simultaneous analysis of all proteins in a sample as well as analyzes relationships of these proteins across different patients, capturing all changes in a protein level. Based on our analysis of HSP profiling, we propose the following scheme for the identification of potential HSP candidates (Fig. 2).Extracellular vesicles derived from urine and NK cells showed to be the most abundant source of HSPs and co-chaperones and, therefore, can be a good source for identifying HSP signatures. Regarding a class of EVs, exosomes can be the most useful source for identification of cancer-specific biomarkers as they are formed by inward budding of endosomal membranes and, thus, reflect the protein composition of plasma membranes of the cells they were derived from [229].
Fig. 2.
HSP profiling of exosomes for identification of potential HSP-based biomarker candidates. The quantitative MS-based proteomics coupled with machine learning might be used to detect all changes in the level of expression in a sample and between cohorts of patients for identification of clinically useful HSP- based biomarkers in urine and/or NK- derived exosomes of cancer patients.
ML algorithms can be used to classify patients by the type of cancer in which specific proteins may contribute more or less to the cancer prediction model. Various types of cancers may have specific patterns of upregulated and downregulated HSPs that may distinguish them from healthy individuals and from patients with other non-cancerous conditions or even discriminate one type of cancer from another. Since proteins that constitute HSP networks may behave differently in different types of cancer and control groups, ML may be used to identify critical relationships between HSP networks and whether one network contribute more to the prediction of cancer than the other network. Furthermore, monitoring the changes in the level of HSPs in response to therapies may provide a further clues on how different types of therapies affect HSP release and how this is related to the outcome of the patient, which may provide further improvement towards the more personalized approach in treating cancer patients. It will be also of interest to determine specific threshold level of specific protein so that elevated or reduced level of one or multiple proteins is better at predicting cancer. The use of clinical data is advantageous as it may provide a great insight into the understanding of the role of HSPs in cancer. Use of ML that can analyze big data and find certain patterns opens new possibilities for the discovery of specific signatures of cancer and development of more efficient anti-cancer therapies.
Conclusion
Heat shock protein family constitutes a large network of molecular chaperones classified into several families with aberrant expression in cancer. Profiling of HSPs in EVs derived from various biological fluids may serve useful for diagnosis, prediction and treatment of cancer patients. More importantly, understanding relationships between different HSP networks and co-chaperones may form the basis for identification of multiple HSP-based biomarkers. In this review, we summarize mass spectrometry studies of EVs derived from various liquid biopsies and different types of immune cells with particular interest in HSP profiling. In addition, we have emphasized emerging role of circulating miRNAs in regulation of HSPs in the context of cancer. Further elucidating role of HSPs in EVs from proteomic and miRNAs perspectives may provide new opportunities for the discovery of clinically useful biomarkers and novel targets for cancer therapies.
Authors contributions
Zarema Albakova: Conceptualization, Formal analysis, Investigation, Data Curation, Writing – Original draft preparation, Writing - Reviewing & Editing, Visualization. Mohammad Kawsar Sharif Siam & Pradeep Kumar Sacitharan: Writing - Reviewing & Editing. Rustam H. Ziganshin, Dmitriy Y. Ryazantsev, Alexander M. Sapozhnikov: Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The figures were created with BioRender.com.
Funding
This work was funded by RFBR, project number 20–315–90081
References
- 1.Ciocca D.R., Calderwood S.K. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10(2):86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calderwood S.K. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem. Sci. 2006;31(3):164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Lindquist S., Craig E.A. The heat-shock proteins. Ann. Rev. Genet. 1988;22(1):631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 4.Kampinga H.H. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitesell L. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. 1994;91(18):8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulte T.W. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3(2):100–108. doi: 10.1379/1466-1268(1998)003<0100:arbttn>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S.V., Agatsuma T., Nakano H. Targeting of the protein chaperone, HSP90, by the transformation suppressing agent, radicicol. Oncogene. 1998;16(20):2639–2645. doi: 10.1038/sj.onc.1201790. [DOI] [PubMed] [Google Scholar]
- 8.Schulte T.W. Interaction of radicicol with members of the heat shock protein 90 family of molecular chaperones. Mol. Endocrinol. 1999;13(9):1435–1448. doi: 10.1210/mend.13.9.0339. [DOI] [PubMed] [Google Scholar]
- 9.Blachere N.E. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J. Exp. Med. 1997;186(8):1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Ann. Rev. Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 11.Calderwood S.K., Murshid A. Molecular chaperone accumulation in cancer and decrease in alzheimer's disease: the potential roles of HSF1. Front Neurosci. 2017;11:192. doi: 10.3389/fnins.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderwood S.K., Gong J. Heat shock proteins promote cancer: it's a protection racket. Trends Biochem. Sci. 2016;41(4):311–323. doi: 10.1016/j.tibs.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothammer A. Increased heat shock protein 70 (Hsp70) serum levels and low NK cell counts after radiotherapy - potential markers for predicting breast cancer recurrence? Radiat. Oncol. 2019;14(1):78. doi: 10.1186/s13014-019-1286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluger H.M. Using a xenograft model of human breast cancer metastasis to find genes associated with clinically aggressive disease. Cancer Res. 2005;65(13):5578–5587. doi: 10.1158/0008-5472.CAN-05-0108. [DOI] [PubMed] [Google Scholar]
- 15.Teng Y. Inactivation of the WASF3 gene in prostate cancer cells leads to suppression of tumorigenicity and metastases. Br. J. Cancer. 2010;103(7):1066–1075. doi: 10.1038/sj.bjc.6605850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosser D.D. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 1997;17(9):5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittal S., Rajala M.S. Heat shock proteins as biomarkers of lung cancer. Cancer Biol. Ther. 2020;21(6):477–485. doi: 10.1080/15384047.2020.1736482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seigneuric R. Heat shock proteins as danger signals for cancer detection. Front. Oncol. 2011;1:37. doi: 10.3389/fonc.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klimczak M. Heat shock proteins create a signature to predict the clinical outcome in breast cancer. Sci. Rep. 2019;9(1):7507. doi: 10.1038/s41598-019-43556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu R. Extracellular vesicles in cancer — implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018;15(10):617–638. doi: 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- 22.Bandu R., Oh J.W., Kim K.P. Mass spectrometry-based proteome profiling of extracellular vesicles and their roles in cancer biology. Exp. Mol. Med. 2019;51(3):1–10. doi: 10.1038/s12276-019-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ClinicalTrials.gov . National Library of Medicine (US). PI3K Inhibitor BYL719 in Combination With the HSP90 Inhibitor AUY922 in Patients With Advanced or Metastatic Gastric Cancer. 2012. https://clinicaltrials.gov/ct2/show/NCT01613950 Identifier: NCT01613950. Available online. (accessed 08 November 2020) [Google Scholar]
- 24.ClinicalTrials.gov . National Library of Medicine (US). HSP90 Inhibitor, AUY922, in Patients With Primary Myelofibrosis (PMF), Post-Polycythemia Vera Myelofibrosis (Post-PV MF), Post-Essential Thrombocythemia Myelofibrosis (Post-ET MF), and Refractory PV/ET. 2012. https://clinicaltrials.gov/ct2/show/NCT01668173 Identifier: NCT01668173.Available online. (accessed 08 November 2020) [Google Scholar]
- 25.ClinicalTrials.gov . National Library of Medicine (US). A Study of the Hsp90 Inhibitor AUY922 Plus Capecitabine for the Treatment of Patients With Advanced Solid Tumors. 2010. https://clinicaltrials.gov/ct2/show/NCT01226732 Identifier: NCT01226732. (accessed 08 November 2020) [Google Scholar]
- 26.ClinicalTrials.gov . National Library of Medicine (US). A Study of the HSP90 Inhibitor AUY922. 2011. https://clinicaltrials.gov/ct2/show/NCT01485536 Identifier: NCT01485536. Available online: (accessed 08 November 2020) [Google Scholar]
- 27.ClinicalTrials.gov . National Library of Medicine (US). Hsp90 Inhibitor AUY922 and Erlotinib Hydrochloride in Treating Patients With Stage IIIB-IV Non-Small Cell Lung Cancer. 2010. https://clinicaltrials.gov/ct2/show/NCT01259089 Identifier: NCT01259089. Available online. (accessed 08 November 2020) [Google Scholar]
- 28.ClinicalTrials.gov . National Library of Medicine (US). Pemetrexed Disodium and Hsp90 Inhibitor AUY922 in Treating Patients With Previously Treated Stage IV Non-Small Cell Lung Cancer. 2013. https://clinicaltrials.gov/ct2/show/NCT01784640 Identifier: NCT01784640. Available online: (accessed 08 November 2020) [Google Scholar]
- 29.ClinicalTrials.gov . National Library of Medicine (US). Study of Hsp90 Inhibitor AUY922 for the Treatment of Patients With Refractory Gastrointestinal Stromal Tumor. 2011. https://clinicaltrials.gov/ct2/show/NCT01404650 Identifier: NCT01404650. Available online: (accessed 08 November 2020) [Google Scholar]
- 30.ClinicalTrials.gov . National Library of Medicine (US). AUY922 for Advanced ALK-positive NSCLC. 2012. https://clinicaltrials.gov/ct2/show/NCT01752400 Identifier: NCT01752400. Available online: (accessed 08 November 2020) [Google Scholar]
- 31.ClinicalTrials.gov . National Library of Medicine (US). A Study of AUY922 in Non-small-cell Lung Cancer Patients Who Have Received Previous Two Lines of Chemotherapy. 2010. https://clinicaltrials.gov/ct2/show/NCT01124864 Identifier: NCT01124864. Availabe online. (accessed 08 November 2020) [Google Scholar]
- 32.ClinicalTrials.gov . National Library of Medicine (US). Combination of AUY922 With Trastuzumab in HER2+ Advanced Breast Cancer Patients Previously Treated With Trastuzumab. 2011. https://clinicaltrials.gov/ct2/show/NCT01271920 Identifier: NCT01271920. Available online: (accessed 08 November 2020) [Google Scholar]
- 33.ClinicalTrials.gov . National Library of Medicine (US). A Phase I-Ib/II Study to Determine the Maximum Tolerated Dose (MTD) of AUY922 Alone and in Combination With Bortezomib, With or Without Dexamethasone, in Patients With Relapsed or Refractory Multiple Myeloma. 2008. https://clinicaltrials.gov/ct2/show/NCT00708292 Identifier: NCT00708292. Available online. (accessed 08 November 2020) [Google Scholar]
- 34.ClinicalTrials.gov . National Library of Medicine (US). Study of AUY922 and Cetuximab in Patients With KRAS Wild-Type Metastatic Colorectal Cancer. 2011. https://clinicaltrials.gov/ct2/show/NCT01294826 Identifier: NCT01294826. Available online: (accessed 08 November 2020) [Google Scholar]
- 35.ClinicalTrials.gov . National Library of Medicine (US). HS-201, an HSP90 Inhibitor-linked Verteporfin for Detection of Solid Malignancies. 2019. https://clinicaltrials.gov/ct2/show/NCT03906643 Identifier: NCT03906643. Available online: (accessed 08 November 2020) [Google Scholar]
- 36.ClinicalTrials.gov . National Library of Medicine (US). A Study of HS-196, an HSP90 Inhibitor-linked NIR Probe for Solid Malignancies. 2017. https://clinicaltrials.gov/ct2/show/NCT03333031 Identifier: NCT03333031. Available online: (accessed 08 November 2020) [Google Scholar]
- 37.ClinicalTrials.gov . National Library of Medicine (US). Study of Hsp90 Inhibitor, STA-9090 for Relapsed or Refractory Small Cell Lung Cancer. 2010. https://clinicaltrials.gov/ct2/show/NCT01173523 Identifier: NCT01173523. Available online: (accessed 08 November 2020) [Google Scholar]
- 38.ClinicalTrials.gov . National Library of Medicine (US).A Phase 1 Study of the HSP90 Inhibitor, STA-9090 in Subjects With Acute Myeloid Leukemia, Acute Lymphoblastic Leukemia and Blast-phase Chronic Myelogenous Leukemia. 2009. https://clinicaltrials.gov/ct2/show/NCT00964873 Identifier: NCT00964873. Available online: (accessed 08 November 2020) [Google Scholar]
- 39.ClinicalTrials.gov . National Library of Medicine (US).A Trial to Establish the Feasibility of Combining Either the Tyrosine Kinase Inhibitor AC220,CXCR4 Inhibitor Plerixafor or HSP90 Inhibitor Ganetespib With Chemotherapy in Older Patients With Acute Myeloid Leukaemia and High Risk Myelodysplastic Syndrome. 2010. https://clinicaltrials.gov/ct2/show/results/NCT01236144 Identifier: NCT01236144. Available online: (accessed 08 November 2020) [Google Scholar]
- 40.ClinicalTrials.gov . National Library of Medicine (US). STA-9090(Ganetespib) in Metastatic Ocular Melanoma. 2010. https://clinicaltrials.gov/ct2/show/NCT01200238 Identifier: NCT01200238 . Available online: (accessed 08 November 2020) [Google Scholar]
- 41.ClinicalTrials.gov . National Library of Medicine (US).Hsp90 Inhibitor STA-9090 in Treating Patients With Metastatic Hormone-Resistant Prostate Cancer Previously Treated With Docetaxel-Based Chemotherapy. 2011. https://clinicaltrials.gov/ct2/show/NCT01270880 Identifier: NCT01270880. Available online: (accessed 08 November 2020) [Google Scholar]
- 42.ClinicalTrials.gov . National Library of Medicine (US). A Study of the HSP90 Inhibitor, STA-9090 in Subjects With Stage IIIB or IV Non-Small Cell Lung Cancer (NSCLC) 2009. https://clinicaltrials.gov/ct2/show/NCT01031225 Identifier: NCT01031225 . Available online. (accessed 08 November 2020) [Google Scholar]
- 43.ClinicalTrials.gov . National Library of Medicine (US). Study of STA-9090, Administered Once-Weekly in Patients With Solid Tumors. 2008. https://clinicaltrials.gov/ct2/show/NCT00687934 Identifier: NCT00687934. Available online: (accessed 08 November 2020) [Google Scholar]
- 44.ClinicalTrials.gov . National Library of Medicine (US). Study of STA-9090, Administered Twice-Weekly in Patients With Solid Tumors. 2008. https://clinicaltrials.gov/ct2/show/NCT00688116 Identifier: NCT00688116. Available online: (accessed 08 November 2020) [Google Scholar]
- 45.ClinicalTrials.gov . National Library of Medicine (US). STA-9090 for Treatment of AML, CML, MDS and Myeloproliferative Disorders. 2009. https://clinicaltrials.gov/ct2/show/NCT00858572 Identifier: NCT00858572. Available online. (accessed 08 November 2020) [Google Scholar]
- 46.ClinicalTrials.gov . National Library of Medicine (US).European Trial on Enhanced DNA Repair Inhibition in Ovarian Cancer. 2018. https://clinicaltrials.gov/ct2/show/NCT03783949 Identifier: NCT03783949. Available online: (accessed 08 November 2020) [Google Scholar]
- 47.ClinicalTrials.gov . National Library of Medicine (US).GANNET53: Ganetespib in Metastatic, p53-mutant, Platinum-resistant Ovarian Cancer. 2013. https://clinicaltrials.gov/ct2/show/NCT02012192 Identifier: NCT02012192. Available online: (accessed 08 November 2020) [Google Scholar]
- 48.ClinicalTrials.gov . National Library of Medicine (US). STA-9090(Ganetespib) in Patients With Unresectable Stage III or Stage IV Melanoma. 2012. https://clinicaltrials.gov/ct2/show/NCT01551693 Identifier: NCT01551693. Available online: (accessed 08 November 2020) [Google Scholar]
- 49.ClinicalTrials.gov . National Library of Medicine (US). Ganetespib, Paclitaxel, Trastuzumab and Pertuzumab for Metastatic Human Epidermal Growth Factor Receptor 2 Positive Breast Cancer. 2014. https://clinicaltrials.gov/ct2/show/NCT02060253 Identifier: NCT02060253. Available online. (accessed 08 November 2020) [Google Scholar]
- 50.ClinicalTrials.gov . National Library of Medicine (US).An Open-Label Multicenter Phase 2 Window of Opportunity Study Evaluating Ganetespib in Women With Breast Cancer. 2012. https://clinicaltrials.gov/ct2/show/NCT01677455 Identifier: NCT01677455. Available online: (accessed 08 November 2020) [Google Scholar]
- 51.ClinicalTrials.gov . National Library of Medicine (US).Ganetespib and Ziv-Aflibercept in Refractory Gastrointestinal Carcinomas, Non-Squamous Non-Small Cell Lung Carcinomas, Urothelial Carcinomas, and Sarcomas. 2014. https://clinicaltrials.gov/ct2/show/NCT02192541 Identifier: NCT02192541. Available online: (accessed 08 November 2020) [Google Scholar]
- 52.ClinicalTrials.gov. National Library of Medicine (US).SARC023: Ganetespib and Sirolimus in Patients With MPNST (Malignant Peripheral Nerve Sheath Tumors), 2013. Identifier:NCT02008877. Available online: https://clinicaltrials.gov/ct2/show/NCT02008877 (accessed 08 November 2020).
- 53.ClinicalTrials.gov . National Library of Medicine (US). ClinicalTrials.gov. National Library of Medicine (US).Dose Escalation of HSP990 in Japan/Korea. 2010. https://clinicaltrials.gov/ct2/show/NCT01064089 Identifier: NCT01064089.Available online: (accessed 08 November 2020) [Google Scholar]
- 54.ClinicalTrials.gov . National Library of Medicine (US).A Study of HSP990 Administered by Mouth in Adult Patients With Advanced Solid Tumors. 2009. https://clinicaltrials.gov/ct2/show/NCT00879905 Identifier: NCT00879905.Available online: (accessed 08 November 2020) [Google Scholar]
- 55.ClinicalTrials.gov . National Library of Medicine (US).A Study of TAS-116 in Patients With Solid Tumors. 2016. https://clinicaltrials.gov/ct2/show/NCT02965885 Identifier: NCT02965885. Available online: (accessed 08 November 2020) [Google Scholar]
- 56.ClinicalTrials.gov . National Library of Medicine (US).A Study of Tanespimycin (KOS-953) in Patients With Multiple Myeloma in First Relapse. 2007. https://clinicaltrials.gov/ct2/show/NCT00546780 Identifier: NCT00546780.Available online: (accessed 08 November 2020) [Google Scholar]
- 57.ClinicalTrials.gov . National Library of Medicine (US).Phase 1, Dose-Escalation Study of Oral CNF2024(BIIB021) in CLL. 2006. https://clinicaltrials.gov/ct2/show/NCT00344786 Identifier: NCT00344786. Available online: (accessed 08 November 2020) [Google Scholar]
- 58.ClinicalTrials.gov . National Library of Medicine (US).Preclinical Project on the Traitment of Acute Lymphoblastique Leukemia With NVP-BEP800, an Inhibitor of the Heat Shock Protein HSP90. 2020. https://clinicaltrials.gov/ct2/show/NCT04437420 Identifier: NCT04437420. Available online: (accessed 08 November 2020) [Google Scholar]
- 59.ClinicalTrials.gov . National Library of Medicine (US).A Study of HSP90 Inhibitor AT13387 Alone or in Combination With Abiraterone Acetate. 2012. https://clinicaltrials.gov/ct2/show/NCT01685268 Identifier: NCT01685268.Available online: (accessed 08 November 2020) [Google Scholar]
- 60.ClinicalTrials.gov . National Library of Medicine (US).Talazoparib and HSP90 Inhibitor AT13387 in Treating Patients With Metastatic Advanced Solid Tumor or Recurrent Ovarian, Fallopian Tube, Primary Peritoneal, or Triple Negative Breast Cancer. 2015. https://clinicaltrials.gov/ct2/show/NCT02627430 Identifier: NCT02627430. Available online: (accessed 08 November 2020) [Google Scholar]
- 61.ClinicalTrials.gov . National Library of Medicine (US).AT13387 in Adults With Refractory Solid Tumors. 2010. https://clinicaltrials.gov/ct2/show/NCT01246102 Identifier: NCT01246102. Available online: (accessed 08 November 2020) [Google Scholar]
- 62.ClinicalTrials.gov . National Library of Medicine (US).A Study of AT13387 in Patients With Non-Small Cell Lung Cancer (NSCLC) Alone and in Combination With Crizotinib. 2012. https://clinicaltrials.gov/ct2/show/NCT01712217 Identifier: NCT01712217. Available online: (accessed 08 November 2020) [Google Scholar]
- 63.ClinicalTrials.gov . National Library of Medicine (US).Onalespib and Paclitaxel in Treating Patients With Advanced Triple Negative Breast Cancer. 2015. https://clinicaltrials.gov/ct2/show/NCT02474173 Identifier: NCT02474173. Available online: (accessed 08 November 2020) [Google Scholar]
- 64.ClinicalTrials.gov . National Library of Medicine (US). Onalespib and CDKI AT7519 in Treating Patients With Solid Tumors That Are Metastatic or Cannot Be Removed by Surgery. 2015. https://clinicaltrials.gov/ct2/show/NCT02503709 Identifier: NCT02503709. Available online: (accessed 08 November 2020) [Google Scholar]
- 65.ClinicalTrials.gov . National Library of Medicine (US).IPI-504 in NSCLC Patients With ALK Translocations. 2010. https://clinicaltrials.gov/ct2/show/NCT01228435 Identifier: NCT01228435. Available online: (accessed 08 November 2020) [Google Scholar]
- 66.ClinicalTrials.gov . National Library of Medicine (US).A Study Evaluating the Safety and Antitumor Activity of IPI-504, in Patients With Metastatic Melanoma. 2008. https://www.clinicaltrials.gov/ct2/show/NCT00627419 Identifier: NCT00627419. Available online: (accessed 08 November 2020) [Google Scholar]
- 67.ClinicalTrials.gov . National Library of Medicine (US).A Study to Evaluate the Antitumor Activity and Safety of IPI-504 in Patients With Advanced Breast Cancer. 2008. https://clinicaltrials.gov/ct2/show/NCT00627627 Identifier: NCT00627627. Available online. (accessed 08 November 2020) [Google Scholar]
- 68.ClinicalTrials.gov . National Library of Medicine (US).Alvespimycin Hydrochloride in Treating Patients With Relapsed Chronic Lymphocytic Leukemia, Small Lymphocytic Lymphoma, or B-Cell Prolymphocytic Leukemia. 2010. https://clinicaltrials.gov/ct2/show/NCT01126502 Identifier: NCT01126502. Available online: (accessed 08 November 2020) [Google Scholar]
- 69.ClinicalTrials.gov . National Library of Medicine (US).17-Dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) in Treating Patients With an Advanced Solid Tumor or Lymphoma. 2004. https://www.clinicaltrials.gov/ct2/show/NCT00088868 Identifier: NCT00088868. Available online. (accessed 08 November 2020) [Google Scholar]
- 70.ClinicalTrials.gov . National Library of Medicine (US).Study of Debio 0932 in Patients With Advanced Solid Tumours or Lymphoma. 2010. https://clinicaltrials.gov/ct2/show/NCT01168752 Identifier: NCT01168752. Available online: (accessed 08 November 2020) [Google Scholar]
- 71.ClinicalTrials.gov . National Library of Medicine (US).A Clinical Study on the Safety and Efficacy of Debio 0932 in Combination With Standard of Care in Patients With Non-small Cell Lung Cancer NSCLC. 2012. https://clinicaltrials.gov/ct2/show/NCT01714037 Identifier: NCT01714037 . Available online: (accessed 08 November 2020) [Google Scholar]
- 72.ClinicalTrials.gov . National Library of Medicine (US).Dose Escalation of IPI-493 in Hematologic Malignancies. 2010. https://clinicaltrials.gov/ct2/show/NCT01193491 Identifier: NCT01193491. Available online: (accessed 08 November 2020) [Google Scholar]
- 73.ClinicalTrials.gov . National Library of Medicine (US).PU-H71 in Patients With Solid Tumors and Low-Grade Non-Hodgkin's Lymphoma That Have Not Responded to Standard Treatment. 2012. https://clinicaltrials.gov/ct2/show/NCT01581541 Identifier: NCT01581541. Available online. (accessed 08 November 2020) [Google Scholar]
- 74.ClinicalTrials.gov . National Library of Medicine (US).A Study of DS-2248 in Participants With Advanced Solid Tumors. 2011. https://clinicaltrials.gov/ct2/show/NCT01288430 Identifier: NCT01288430. Available online: (accessed 08 November 2020) [Google Scholar]
- 75.ClinicalTrials.gov . National Library of Medicine (US).Safety Study of MPC-3100 in Cancer Patients Who Have Failed Other Treatments. 2009. https://clinicaltrials.gov/ct2/show/NCT00920205 Identifier: NCT00920205. Available online: (accessed 08 November 2020) [Google Scholar]
- 76.ClinicalTrials.gov . National Library of Medicine (US).Phase 1, Dose-Escalation, Pharmacodynamic Study of IV CNF1010 in ZAP-70 Positive CLL. 2006. https://clinicaltrials.gov/ct2/show/NCT00319930 Identifier: NCT00319930. Available online: (accessed 08 November 2020) [Google Scholar]
- 77.ClinicalTrials.gov . National Library of Medicine (US).Safety And Pharmacology Study Of SNX-5422 Mesylate In Subjects With Refractory Solid Tumor Malignancies. 2007. https://clinicaltrials.gov/ct2/show/NCT00506805 Identifier: NCT00506805. Available online: (accessed 08 November 2020) [Google Scholar]
- 78.ClinicalTrials.gov . National Library of Medicine (US).Safety And Pharmacology Of SNX-5422 Mesylate In Subjects With Refractory Hematological Malignancies. 2008. https://clinicaltrials.gov/ct2/show/NCT00595686 Identifier: NCT00595686. Available online: (accessed 08 November 2020) [Google Scholar]
- 79.ClinicalTrials.gov . National Library of Medicine (US).Safety and Pharmacology of SNX-5422 Plus Everolimus in Subjects With Neuroendocrine Tumors. 2014. https://clinicaltrials.gov/ct2/show/NCT02063958 Identifier: NCT02063958. Available online: (accessed 08 November 2020) [Google Scholar]
- 80.ClinicalTrials.gov . National Library of Medicine (US).Safety and Pharmacology Study of SNX-5422 in Subjects With Resistant Lung Adenocarcinoma. 2013. https://clinicaltrials.gov/ct2/show/NCT01851096 Identifier: NCT01851096. Available online: (accessed 08 November 2020) [Google Scholar]
- 81.ClinicalTrials.gov . National Library of Medicine (US).Efficacy and Safety of SNX-5422 Added to an Established Dose of Ibrutinib in CLL. 2016. https://clinicaltrials.gov/ct2/show/NCT02973399 Identifier: NCT02973399. Available online: (accessed 08 November 2020) [Google Scholar]
- 82.ClinicalTrials.gov . National Library of Medicine (US).Safety and Activity of SNX-5422 Plus Ibrutinib in CLL. 2016. https://clinicaltrials.gov/ct2/show/NCT02914327 Identifer: NCT02914327. Available online: (accessed 08 November 2020) [Google Scholar]
- 83.ClinicalTrials.gov . National Library of Medicine (US).Minnelide in Adult Patients With Relapsed or Refractory Acute Myeloid Leukemia. 2017. https://clinicaltrials.gov/ct2/show/NCT03347994 Identifier: NCT03347994. Available online: (accessed 08 November 2020) [Google Scholar]
- 84.ClinicalTrials.gov . National Library of Medicine (US).Safety Study of an Antisense Product in Prostate, Ovarian, NSCL, Breast or Bladder Cancer. 2007. https://clinicaltrials.gov/ct2/show/NCT00487786 Identifier: NCT00487786. Available online: (accessed 08 November 2020) [Google Scholar]
- 85.Chi K.N. A phase I dose-escalation study of apatorsen (OGX-427), an antisense inhibitor targeting heat shock protein 27 (Hsp27), in patients with castration-resistant prostate cancer and other advanced cancers. Ann. Oncol. 2016;27(6):1116–1122. doi: 10.1093/annonc/mdw068. [DOI] [PubMed] [Google Scholar]
- 86.ClinicalTrials.gov . National Library of Medicine (US).A Study for Treatment of Superficial Bladder Cancer Using OGX-427. 2009. https://clinicaltrials.gov/ct2/show/NCT00959868 Identifier: NCT00959868. Available online: (accessed 08 November 2020) [Google Scholar]
- 87.ClinicalTrials.gov . National Library of Medicine (US).OGX-427 in Castration Resistant Prostate Cancer Patients. 2010. Identifier: NCT01120470. Available online: https://clinicaltrials.gov/ct2/show/NCT01120470, (accessed 08 November 2020) [Google Scholar]
- 88.ClinicalTrials.gov. National Library of Medicine (US).Study Using Vaccination With Heat Shock Protein 70 (HSP70) for the Treatment of CML in Chronic Phase, 2001. Identifier:NCT00027144. Available online: https://clinicaltrials.gov/ct2/show/NCT00027144 (accessed 08 November 2020).
- 89.ClinicalTrials.gov . National Library of Medicine (US).Vaccination Against High Risk Breast Cancer Using Tumor Derived Heat Shock Protein 70. 2001. https://clinicaltrials.gov/ct2/show/NCT00027131 Identifier: NCT00027131. Available online: (accessed 08 November 2020) [Google Scholar]
- 90.ClinicalTrials.gov . National Library of Medicine (US).Trial of Heat Shock Protein Peptide Complex-96 (HSPPC-96) Vaccine. 2016. https://clinicaltrials.gov/ct2/show/NCT02722512 Identifier: NCT02722512. Available online: (accessed 08 November 2020) [Google Scholar]
- 91.ClinicalTrials.gov . National Library of Medicine (US).GP96 Heat Shock Protein-Peptide Complex Vaccine in Treating Patients With Recurrent or Progressive Glioma. 2006. https://clinicaltrials.gov/ct2/show/NCT00293423 Identifier: NCT00293423. Available online: (accessed 08 November 2020) [Google Scholar]
- 92.ClinicalTrials.gov . National Library of Medicine (US).Study of Heat Shock Protein-Peptide Complex (HSPPC-96) Versus IL-2/DTIC for Stage IV Melanoma. 2002. https://clinicaltrials.gov/ct2/show/NCT00039000 Identifier: NCT00039000. Available online: (accessed 08 November 2020) [Google Scholar]
- 93.ClinicalTrials.gov . National Library of Medicine (US).Vaccine Therapy With Bevacizumab Versus Bevacizumab Alone in Treating Patients With Recurrent Glioblastoma Multiforme That Can Be Removed by Surgery. 2013. https://clinicaltrials.gov/ct2/show/NCT01814813 Identifier: NCT01814813. Available online: (accessed 08 November 2020) [Google Scholar]
- 94.ClinicalTrials.gov . National Library of Medicine (US).A Safety and Effectiveness Study of Vaccine Therapy in Patients With Indolent Lymphoma. 2004. https://clinicaltrials.gov/ct2/show/NCT00081809 Identifier: NCT00081809. Available online: (accessed 08 November 2020) [Google Scholar]
- 95.ClinicalTrials.gov . National Library of Medicine (US).Vaccine Therapy in Treating Patients With Stage I or Stage II Pancreatic Cancer. 2004. https://clinicaltrials.gov/ct2/show/NCT00003025 Identifier: NCT00003025. Available online: (accessed 08 November 2020) [Google Scholar]
- 96.ClinicalTrials.gov . National Library of Medicine (US).Vaccine Therapy in Treating Patients With Recurrent Soft Tissue Sarcoma. 2004. https://clinicaltrials.gov/ct2/show/NCT00005628 Identifier: NCT00005628. Available online: (accessed 08 November 2020) [Google Scholar]
- 97.ClinicalTrials.gov . National Library of Medicine (US).GP96 Heat Shock Protein-Peptide Complex Vaccine in Treating Patients With Liver Cancer. 2019. https://clinicaltrials.gov/ct2/show/study/NCT04206254 Identifier: NCT04206254. Available online: (accessed 08 November 2020) [Google Scholar]
- 98.ClinicalTrials.gov . National Library of Medicine (US).A Large-scale Research for Immunotherapy of Glioblastoma With Autologous Heat Shock Protein gp96. 2018. https://clinicaltrials.gov/ct2/show/NCT03650257 Identifier: NCT03650257. Available online: (accessed 08 November 2020) [Google Scholar]
- 99.ClinicalTrials.gov . National Library of Medicine (US).Immunotherapy of Tumor With Autologous Tumor Derived Heat Shock Protein gp96. 2014. https://clinicaltrials.gov/ct2/show/NCT02133079 Identifier: NCT02133079. Available online: (accessed 08 November 2020) [Google Scholar]
- 100.ClinicalTrials.gov . National Library of Medicine (US).Immunotherapy of Gastric Cancer With Autologous Tumor Derived Heat Shock Protein gp96. 2014. https://clinicaltrials.gov/ct2/show/NCT02317471 Identifier: NCT02317471. Available online: (accessed 08 November 2020) [Google Scholar]
- 101.ClinicalTrials.gov . National Library of Medicine (US).Research for Immunotherapy of Glioblastoma With Autologous Heat Shock Protein gp96. 2014. https://clinicaltrials.gov/ct2/show/NCT02122822 Identifier: NCT02122822. Available online: (accessed 08 November 2020) [Google Scholar]
- 102.ClinicalTrials.gov . National Library of Medicine (US).Vaccine Therapy in Treating Patients With Advanced Stage III-IV Melanoma. 2012. https://clinicaltrials.gov/ct2/show/NCT01744171 Identifier: NCT01744171. Available online: (accessed 08 November 2020) [Google Scholar]
- 103.ClinicalTrials.gov . National Library of Medicine (US).SGN-00101 in Treating Patients With Cervical Intraepithelial Neoplasia. 2003. https://www.clinicaltrials.gov/ct2/show/NCT00060099 Identifier: NCT00060099. Available online: (accessed 08 November 2020) [Google Scholar]
- 104.ClinicalTrials.gov . National Library of Medicine (US).Vaccine Therapy in Preventing Cervical Cancer in Patients With Cervical Intraepithelial Neoplasia. 2003. https://clinicaltrials.gov/ct2/show/NCT00054041 Identifier: NCT00054041. Available online: (accessed 08 November 2020) [Google Scholar]
- 105.ClinicalTrials.gov . National Library of Medicine (US).Vaccine Therapy in Treating Patients With Stage III or Stage IV Melanoma. 2004. https://clinicaltrials.gov/ct2/show/NCT00085397 Identifier: NCT00085397. Available online. (accessed 08 November 2020) [Google Scholar]
- 106.ClinicalTrials.gov . National Library of Medicine (US).BASE HSP110 : A New Therapeutic Target and a New Marker for Prognosis of Type MSI Colorectal Cancer. 2015. https://clinicaltrials.gov/ct2/show/NCT02458664 Identifier: NCT02458664. Available online: (accessed 08 November 2020) [Google Scholar]
- 107.ClinicalTrials.gov . National Library of Medicine (US).Analysis of the Mechanisms of Actions of Heat Shock Protein (Hsp27) Responsible of the Androgen-independent Evolution in Prostate Cancer. 2014. https://clinicaltrials.gov/ct2/show/NCT02055846 Identifier: NCT02055846 . Available online. (accessed 08 November 2020) [Google Scholar]
- 108.ClinicalTrials.gov . National Library of Medicine (US).Hyperthermic Intraperitoneal Chemotherapy (HIPEC) : Predictive Marker and Mechanism. 2016. https://clinicaltrials.gov/ct2/show/NCT02803515 Identifier: NCT02803515. Available online: (accessed 08 November 2020) [Google Scholar]
- 109.ClinicalTrials.gov . National Library of Medicine (US).Radiofrequency Therapy-Induced Endogenous Heat-Shock Proteins With or Without Radiofrequency Ablation or Cryotherapy in Treating Patients With Stage IV Melanoma. 2007. https://clinicaltrials.gov/ct2/show/NCT00568763 Identifier: NCT00568763. Available online: (accessed 08 November 2020) [Google Scholar]
- 110.Balogi Z. Hsp70 interactions with membrane lipids regulate cellular functions in health and disease. Prog. Lipid Res. 2019;74:18–30. doi: 10.1016/j.plipres.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 111.Balogh G. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005;272(23):6077–6086. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- 112.Nagy E. Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. Proc. Natl. Acad. Sci. 2007;104(19):7945–7950. doi: 10.1073/pnas.0702557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Török Z. Plasma membranes as heat stress sensors: From lipid-controlled molecular switches to therapeutic applications. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2014;1838(6):1594–1618. doi: 10.1016/j.bbamem.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 114.Balogh G. Heat Stress Causes Spatially-Distinct Membrane Re-Modelling in K562 Leukemia Cells. PLoS One. 2011;6(6):e21182. doi: 10.1371/journal.pone.0021182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dempsey N.C. Heat Shock Protein translocation induced by membrane fluidization increases tumor-cell sensitivity to chemotherapeutic drugs. Cancer Lett. 2010;296(2):257–267. doi: 10.1016/j.canlet.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 116.Gehrmann M. Tumor-specific Hsp70 plasma membrane localization is enabled by the glycosphingolipid Gb3. PLoS One. 2008;3(4):e1925. doi: 10.1371/journal.pone.0001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arispe N. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J. 2004;18(14):1636–1645. doi: 10.1096/fj.04-2088com. [DOI] [PubMed] [Google Scholar]
- 118.Mambula S.S. Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods. 2007;43(3):168–175. doi: 10.1016/j.ymeth.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Juhász K. Lysosomal rerouting of Hsp70 trafficking as a potential immune activating tool for targeting melanoma. Curr. Pharm. Des. 2013;19(3):430–440. doi: 10.2174/138161213804143644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lancaster G.I., Febbraio M.A. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J. Biol. Chem. 2005;280(24):23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 121.Nylandsted J. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 2004;200(4):425–435. doi: 10.1084/jem.20040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mambula S.S., Calderwood S.K. Heat Shock Protein 70 Is Secreted from Tumor Cells by a Nonclassical Pathway Involving Lysosomal Endosomes. J. Immunol. 2006;177(11):7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- 123.Juhasz K. The Complex Function of Hsp70 in Metastatic Cancer. Cancers. 2014;6(1):42–66. doi: 10.3390/cancers6010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tang X. Tumour-Secreted Hsp90α on External Surface of Exosomes Mediates Tumour - Stromal Cell Communication via Autocrine and Paracrine Mechanisms. Sci. Rep. 2019;9(1):15108. doi: 10.1038/s41598-019-51704-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ono K. HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J. Cell. Biochem. 2018;119(9):7350–7362. doi: 10.1002/jcb.27039. [DOI] [PubMed] [Google Scholar]
- 126.Noonan E.J. Cell number-dependent regulation of Hsp70B′ expression: Evidence of an extracellular regulator. J. Cell. Physiol. 2007;210(1):201–211. doi: 10.1002/jcp.20875. [DOI] [PubMed] [Google Scholar]
- 127.Eguchi T. Organoids with cancer stem cell-like properties secrete exosomes and HSP90 in a 3D nanoenvironment. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0191109. -e0191109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caruso Bavisotto C. Exosomal HSP60: a potentially useful biomarker for diagnosis, assessing prognosis, and monitoring response to treatment. Expert Rev. Mol. Diagn. 2017;17(9):815–822. doi: 10.1080/14737159.2017.1356230. [DOI] [PubMed] [Google Scholar]
- 129.Multhoff G. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int. J. Cancer. 1995;61(2):272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- 130.Fais S. Exosomal Hsp60: A Tumor Biomarker? In: Asea A.A.A., Kaur P., editors. Heat Shock Protein 60 in Human Diseases and Disorders. Springer International Publishing; Cham: 2019. pp. 107–116. [Google Scholar]
- 131.Gunther S. Correlation of Hsp70 serum levels with gross tumor volume and composition of lymphocyte subpopulations in patients with squamous cell and adeno non-small cell lung cancer. Front. Immunol. 2015;6:556. doi: 10.3389/fimmu.2015.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pfister K. Patient survival by Hsp70 membrane phenotype: association with different routes of metastasis. Cancer. 2007;110(4):926–935. doi: 10.1002/cncr.22864. [DOI] [PubMed] [Google Scholar]
- 133.Chanteloup G. Monitoring HSP70 exosomes in cancer patients' follow up: a clinical prospective pilot study. J. Extracell. Vesicles. 2020;9(1) doi: 10.1080/20013078.2020.1766192. -1766192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Campanella C. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer. 2015;121(18):3230–3239. doi: 10.1002/cncr.29499. [DOI] [PubMed] [Google Scholar]
- 135.Peinado H. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hamelin C. Identification and verification of heat shock protein 60 as a potential serum marker for colorectal cancer. FEBS J. 2011;278(24):4845–4859. doi: 10.1111/j.1742-4658.2011.08385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fu Y. Plasma Heat Shock Protein 90alpha as a Biomarker for the Diagnosis of Liver Cancer: An Official, Large-scale, and Multicenter Clinical Trial. EBioMedicine. 2017;24:56–63. doi: 10.1016/j.ebiom.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shi Y. Plasma levels of heat shock protein 90 alpha associated with lung cancer development and treatment responses. Clin. Cancer Res. 2014;20(23):6016. doi: 10.1158/1078-0432.CCR-14-0174. [DOI] [PubMed] [Google Scholar]
- 139.Kasanga M. Plasma heat shock protein 90-alpha have an advantage in diagnosis of colorectal cancer at early stage. Biomark. Med. 2018;12(8):881–890. doi: 10.2217/bmm-2018-0155. [DOI] [PubMed] [Google Scholar]
- 140.Zhong L. Antibodies to HSP70 and HSP90 in serum in non-small cell lung cancer patients. Cancer Detect. Prev. 2003;27(4):285–290. doi: 10.1016/s0361-090x(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 141.Balázs M. Serum heat shock protein 70, as a potential biomarker for small cell lung cancer. Pathol. Oncol. Res. 2017;23(2):377–383. doi: 10.1007/s12253-016-0118-x. [DOI] [PubMed] [Google Scholar]
- 142.Seiwert T.Y. Heat shock protein (HSP) overexpression in lung cancer and potential as a therapeutic target. Cancer Res. 2005;65(9 Supplement):559. [Google Scholar]
- 143.Suzuki K. Serum heat shock protein 70 levels and lung cancer risk: a case-control study nested in a large cohort study. Cancer Epidemiol. Biomark. Amp; Prev. 2006;15(9):1733. doi: 10.1158/1055-9965.EPI-06-0005. [DOI] [PubMed] [Google Scholar]
- 144.Kocsis J. Serum level of soluble 70-kD heat shock protein is associated with high mortality in patients with colorectal cancer without distant metastasis. Cell Stress Chaperones. 2010;15(2):143–151. doi: 10.1007/s12192-009-0128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gráf L. High serum Hsp70 level predicts poor survival in colorectal cancer: results obtained in an independent validation cohort. Cancer Biomark. 2018;23:539–547. doi: 10.3233/CBM-181683. [DOI] [PubMed] [Google Scholar]
- 146.Gunaldi M. Elevated serum levels of heat shock protein 70 are associated with breast cancer. Tohoku J. Exp. Med. 2015;236(2):97–102. doi: 10.1620/tjem.236.97. [DOI] [PubMed] [Google Scholar]
- 147.Dakappagari N. An investigation into the potential use of serum Hsp70 as a novel tumour biomarker for Hsp90 inhibitors. Biomarkers. 2010;15(1):31–38. doi: 10.3109/13547500903261347. [DOI] [PubMed] [Google Scholar]
- 148.Dutta S.K. Serum HSP70: a novel biomarker for early detection of pancreatic cancer. Pancreas. 2012;41(4):530–534. doi: 10.1097/MPA.0b013e3182374ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gehrmann M. Heat shock protein 70 serum levels differ significantly in patients with chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Front. Immunol. 2014;5(307) doi: 10.3389/fimmu.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fujita Y. Proteomics-based identification of autoantibody against heat shock protein 70 as a diagnostic marker in esophageal squamous cell carcinoma. Cancer Lett. 2008;263(2):280–290. doi: 10.1016/j.canlet.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 151.Ostheimer C. Dynamics of heat shock protein 70 serum levels as a predictor of clinical response in non-small-cell lung cancer and correlation with the hypoxia-related marker osteopontin. Front. Immunol. 2017;8:1305. doi: 10.3389/fimmu.2017.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yeh C.-H. Clinical correlation of circulating heat shock protein 70 in acute leukemia. Leuk. Res. 2010;34(5):605–609. doi: 10.1016/j.leukres.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hantschel M. Hsp70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones. 2000;5(5):438–442. doi: 10.1379/1466-1268(2000)005<0438:hpmeop>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Farkas B. Heat shock protein 70 membrane expression and melanoma-associated marker phenotype in primary and metastatic melanoma. Melanoma Res. 2003;13(2) doi: 10.1097/00008390-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 155.Gehrmann M. Hsp70–a biomarker for tumor detection and monitoring of outcome of radiation therapy in patients with squamous cell carcinoma of the head and neck. Radiat. Oncol. 2014;9:131. doi: 10.1186/1748-717X-9-131. -131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Steiner K. High HSP70-membrane expression on leukemic cells from patients with acute myeloid leukemia is associated with a worse prognosis. Leukemia. 2006;20(11):2076–2079. doi: 10.1038/sj.leu.2404391. [DOI] [PubMed] [Google Scholar]
- 157.Gobbo J. Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J. Natl. Cancer Inst. 2016;108(3) doi: 10.1093/jnci/djv330. [DOI] [PubMed] [Google Scholar]
- 158.Rong B. Erratum: Identification and verification of Hsp90-beta as a potential serum biomarker for lung cancer. Am. J. Cancer Res. 2016;6(6):1460. -1460. [PMC free article] [PubMed] [Google Scholar]
- 159.Rong B. Identification and verification of Hsp90-beta as a potential serum biomarker for lung cancer. Am. J. Cancer Res. 2014;4(6):874–885. [PMC free article] [PubMed] [Google Scholar]
- 160.Tas F. Clinical Significance of Circulating Serum Cellular Heat Shock Protein 90 (HSP90) level in patients with cutaneous malignant melanoma. Asian Pac. J. Cancer Prev. 2017;18(3):599–601. doi: 10.22034/APJCP.2017.18.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun Y. Differential proteomics identification of HSP90 as potential serum biomarker in hepatocellular carcinoma by two-dimensional electrophoresis and mass spectrometry. Int. J. Mol. Sci. 2010;11(4):1423–1433. doi: 10.3390/ijms11041423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen J.-S. Secreted heat shock protein 90α induces colorectal cancer cell invasion through CD91/LRP-1 and NF-κB-mediated Integrin αV Expression. J. Biol. Chem. 2010;285(33):25458–25466. doi: 10.1074/jbc.M110.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fredly H. Disease-stabilizing treatment with all-trans retinoic acid and valproic acid in acute myeloid leukemia: Serum hsp70 and hsp90 levels and serum cytokine profiles are determined by the disease, patient age, and anti-leukemic treatment. Am. J. Hematol. 2012;87(4):368–376. doi: 10.1002/ajh.23116. [DOI] [PubMed] [Google Scholar]
- 164.ZAGOURI F. Serum levels of HSP90 in the continuum of breast ductal and lobular lesions. In Vivo. 2011;25(4):669–672. [PubMed] [Google Scholar]
- 165.Korneeva I. Serum antibodies to the 27-kd heat shock protein in women with gynecologic cancers. Am. J. Obstet. Gynecol. 2000;183(1):18–21. doi: 10.1067/mob.2000.105431. [DOI] [PubMed] [Google Scholar]
- 166.Olejek A. Concentrations of antibodies against heat shock protein 27 in the sera of women with ovarian carcinoma. Int. J. Gynecol. Cancer. 2009;19:1516–1520. doi: 10.1111/IGC.0b013e3181bf425b. [DOI] [PubMed] [Google Scholar]
- 167.Rui Z. Use of serological proteomic methods to find biomarkers associated with breast cancer. Proteomics. 2003;3(4):433–439. doi: 10.1002/pmic.200390058. [DOI] [PubMed] [Google Scholar]
- 168.Banerjee S. Heat shock protein 27 differentiates tolerogenic macrophages that may support human breast cancer progression. Cancer Res. 2011;71(2):318–327. doi: 10.1158/0008-5472.CAN-10-1778. [DOI] [PubMed] [Google Scholar]
- 169.Zhao M. Heat shock protein 27: a potential biomarker of peritoneal metastasis in epithelial ovarian cancer? Tumor Biol. 2014;35(2):1051–1056. doi: 10.1007/s13277-013-1139-7. [DOI] [PubMed] [Google Scholar]
- 170.Wyciszkiewicz A. Expression of small heat shock proteins in exosomes from patients with gynecologic cancers. Sci. Rep. 2019;9(1):9817. doi: 10.1038/s41598-019-46221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oka M. Autoantibody to heat shock protein Hsp40 in sera of lung cancer patients. Jpn. J. Cancer Res.: Gann. 2001;92(3):316–320. doi: 10.1111/j.1349-7006.2001.tb01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bodzek P., Partyka R., Damasiewicz-Bodzek A. Antibodies against Hsp60 and Hsp65 in the sera of women with ovarian cancer. J. Ovarian Res. 2014;7:30. doi: 10.1186/1757-2215-7-30. -30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Desmetz C. Proteomics-based identification of HSP60 as a tumor-associated antigen in early stage breast cancer and ductal carcinoma in situ. J. Proteome Res. 2008;7(9):3830–3837. doi: 10.1021/pr800130d. [DOI] [PubMed] [Google Scholar]
- 174.García-Silva S. Use of extracellular vesicles from lymphatic drainage as surrogate markers of melanoma progression and BRAF (V600E) mutation. J. Exp. Med. 2019;216(5):1061–1070. doi: 10.1084/jem.20181522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hallal S. Extracellular vesicles from neurosurgical aspirates identifies chaperonin containing TCP1 Subunit 6A as a potential glioblastoma biomarker with prognostic significance. Proteomics. 2019;19(1-2) doi: 10.1002/pmic.201800157. [DOI] [PubMed] [Google Scholar]
- 176.Crescitelli R. Subpopulations of extracellular vesicles from human metastatic melanoma tissue identified by quantitative proteomics after optimized isolation. J. Extracell. Vesicles. 2020;9(1) doi: 10.1080/20013078.2020.1722433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dhondt B. Unravelling the proteomic landscape of extracellular vesicles in prostate cancer by density-based fractionation of urine. Journal of Extracellular Vesicles. 2020;9(1) doi: 10.1080/20013078.2020.1736935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen Y. Protein content and functional characteristics of serum-purified exosomes from patients with colorectal cancer revealed by quantitative proteomics. Int. J. Cancer. 2017;140(4):900–913. doi: 10.1002/ijc.30496. [DOI] [PubMed] [Google Scholar]
- 179.An M. Quantitative proteomic analysis of serum exosomes from patients with locally advanced pancreatic cancer undergoing chemoradiotherapy. J. Proteome Res. 2017;16(4):1763–1772. doi: 10.1021/acs.jproteome.7b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pietrowska M. MS-based proteomic analysis of serum and plasma: problem of high abundant components and lights and shadows of albumin removal. In: Capelo-Martínez J.-L., editor. Emerging Sample Treatments in Proteomics. Springer International Publishing; Cham: 2019. pp. 57–76. Editor. [DOI] [PubMed] [Google Scholar]
- 181.Patel G.K. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019;9(1):5335. doi: 10.1038/s41598-019-41800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Quintana F.J. Inhibition of adjuvant-induced arthritis by DNA vaccination with the 70-kd or the 90-kd human heat-shock protein:immune cross-regulation with the 60-kd heat-shock protein. Arthr. Rheumat. 2004;50(11):3712–3720. doi: 10.1002/art.20635. [DOI] [PubMed] [Google Scholar]
- 183.Lee C.-H. Inhibition of heat shock protein (Hsp) 27 potentiates the suppressive effect of Hsp90 inhibitors in targeting breast cancer stem-like cells. Biochimie. 2012;94(6):1382–1389. doi: 10.1016/j.biochi.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 184.Do K. Phase I study of the heat shock protein 90 (Hsp90) inhibitor onalespib (AT13387) administered on a daily for 2 consecutive days per week dosing schedule in patients with advanced solid tumors. Invest. New Drugs. 2015;33(4):921–930. doi: 10.1007/s10637-015-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shapiro G.I. First-in-human phase I Dose Escalation study of a second-generation non-ansamycin HSP90 Inhibitor, AT13387, in patients with advanced solid tumors. Clin. Cancer Res. 2015;21(1):87. doi: 10.1158/1078-0432.CCR-14-0979. [DOI] [PubMed] [Google Scholar]
- 186.Smith D.F. Identification of a 60-kilodalton stress-related protein, p60, which interacts with HSP90 and HSP70. Mol. Cell. Biol. 1993;13(2):869–876. doi: 10.1128/mcb.13.2.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mayer M.P., Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005;62(6):670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lin Y.F., Lee Y.F., Liang P.H. Targeting β-tubulin:CCT-β complexes incurs Hsp90- and VCP-related protein degradation and induces ER stress-associated apoptosis by triggering capacitative Ca2+ entry, mitochondrial perturbation and caspase overactivation. Cell Death. Dis. 2012;3(11):e434. doi: 10.1038/cddis.2012.173. -e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kampinga H.H., Craig E.A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 2010;11(8):579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jiang Y., Rossi P., Kalodimos C.G. Structural basis for client recognition and activity of Hsp40 chaperones. Science. 2019;365(6459):1313. doi: 10.1126/science.aax1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Diamant S., Goloubinoff P. Temperature-controlled activity of DnaK-DnaJ-GrpE chaperones: protein-folding arrest and recovery during and after heat shock depends on the substrate protein and the GrpE concentration. Biochemistry. 1998;37(27):9688–9694. doi: 10.1021/bi980338u. [DOI] [PubMed] [Google Scholar]
- 192.Albakova Z. HSP70 multi-functionality in cancer. Cells. 2020;9(3):1–26. doi: 10.3390/cells9030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bracher A., Verghese J. The nucleotide exchange factors of HSP70 molecular chaperones. Front. Mol. Biosci. 2015;2:10. doi: 10.3389/fmolb.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li Z., Hartl F.U., Bracher A. Structure and function of Hip, an attenuator of the HSP70 chaperone cycle. Nat. Struct. Mol. Biol. 2013;20(8):929–935. doi: 10.1038/nsmb.2608. [DOI] [PubMed] [Google Scholar]
- 195.Ballinger C.A. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 1999;19(6):4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rosenzweig R. The HSP70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019;20(11):665–680. doi: 10.1038/s41580-019-0133-3. [DOI] [PubMed] [Google Scholar]
- 197.Ramirez M.I. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10(3):881–906. doi: 10.1039/c7nr08360b. [DOI] [PubMed] [Google Scholar]
- 198.Théry C. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7(1) doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gardiner C. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J. Extracell. Vesicles. 2016;5:32945. doi: 10.3402/jev.v5.32945. -32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mol E.A. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomed. Nanotechnol. Biol. Med. 2017;13(6):2061–2065. doi: 10.1016/j.nano.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 201.Hong C.-S. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J. Extracellular Vesicles. 2016;5:29289. doi: 10.3402/jev.v5.29289. -29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nordin J.Z. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed. Nanotechnol. Biol. Med. 2015;11(4):879–883. doi: 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 203.Baranyai T. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Brennan K. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020;10(1):1039. doi: 10.1038/s41598-020-57497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rekker K. Comparison of serum exosome isolation methods for microRNA profiling. Clin. Biochem. 2014;47(1):135–138. doi: 10.1016/j.clinbiochem.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 206.Ding M. Comparison of commercial exosome isolation kits for circulating exosomal microRNA profiling. Anal. Bioanal. Chem. 2018;410(16):3805–3814. doi: 10.1007/s00216-018-1052-4. [DOI] [PubMed] [Google Scholar]
- 207.Van Deun J. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods. 2017;14(3):228–232. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- 208.Hiltbrunner S. Urinary exosomes from bladder cancer patients show a residual cancer phenotype despite complete pathological downstaging. Sci. Rep. 2020;10(1):5960. doi: 10.1038/s41598-020-62753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sun Y. Comparative proteomic analysis of exosomes and microvesicles in human saliva for lung cancer. J. Proteome Res. 2018;17(3):1101–1107. doi: 10.1021/acs.jproteome.7b00770. [DOI] [PubMed] [Google Scholar]
- 210.Dan L. Proteomics study of serum exosomes from papillary thyroid cancer patients. Endocr. Relat. Cancer. 2018;25(10):879–891. doi: 10.1530/ERC-17-0547. [DOI] [PubMed] [Google Scholar]
- 211.Udono H., Srivastava P.K. Comparison of tumor-specific immunogenicities of stress-induced proteins GP96, HSP90, and HSP70. J. Immunol. 1994;152(11):5398. [PubMed] [Google Scholar]
- 212.Weng D., Calderwood S.K., Gong J. Preparation of a heat-shock protein 70-based vaccine from DC-tumor fusion cells. Methods Mol. Biol. 2011;787:255–265. doi: 10.1007/978-1-61779-295-3_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li Z. Combination of imatinib mesylate with autologous leukocyte-derived heat shock protein and chronic myelogenous leukemia. Clin. Cancer Res. 2005;11(12):4460–4468. doi: 10.1158/1078-0432.CCR-05-0250. [DOI] [PubMed] [Google Scholar]
- 214.Krause S.W. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase i trial. Clin. Cancer Res. 2004;10(11):3699–3707. doi: 10.1158/1078-0432.CCR-03-0683. [DOI] [PubMed] [Google Scholar]
- 215.Specht H.M. Heat Shock Protein 70 (Hsp70) Peptide Activated Natural Killer (NK) cells for the treatment of patients with non-small cell lung cancer (NSCLC) after Radiochemotherapy (RCTx) - from preclinical studies to a clinical phase II trial. Front. Immunol. 2015;6:162. doi: 10.3389/fimmu.2015.00162. -162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wachstein J. HSP70 enhances immunosuppressive function of CD4(+)CD25(+)FoxP3(+) T regulatory cells and cytotoxicity in CD4(+)CD25(-) T cells. PLoS One. 2012;7(12):e51747. doi: 10.1371/journal.pone.0051747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chalmin F. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest. 2010;120(2):457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stocki P., Wang X.N., Dickinson A.M. Inducible heat shock protein 70 reduces T cell responses and stimulatory capacity of monocyte-derived dendritic cells. J. Biol. Chem. 2012;287(15):12387–12394. doi: 10.1074/jbc.M111.307579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Juhasz K. The complex function of hsp70 in metastatic cancer. Cancers. 2013;6(1):42–66. doi: 10.3390/cancers6010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Calderwood S.K., Gong J., Murshid A. Extracellular HSPs: the complicated roles of extracellular HSPs in immunity. Front. Immunol. 2016;7:159. doi: 10.3389/fimmu.2016.00159. -159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.van Eden W. The enigma of heat shock proteins in immune tolerance. Front. Immunol. 2017;8:1599. doi: 10.3389/fimmu.2017.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.van Eden W. The immunomodulatory potential of tolDCs loaded with heat shock proteins. Front. Immunol. 2017;8:1690. doi: 10.3389/fimmu.2017.01690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lugini L. Immune surveillance properties of human NK cell-derived exosomes. J. Immunol. 2012;189(6):2833. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- 224.Federici C. Natural-killer-derived extracellular vesicles: immune sensors and interactors. Front. Immunol. 2020;11:262. doi: 10.3389/fimmu.2020.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rocca Y.S. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun. 2012;19(1):76–85. doi: 10.1177/1753425912453187. [DOI] [PubMed] [Google Scholar]
- 226.Multhoff G. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J. Immunol. 1997;158(9):4341. [PubMed] [Google Scholar]
- 227.Multhoff G. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp. Hematol. 1999;27(11):1627–1636. doi: 10.1016/s0301-472x(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 228.Multhoff G. A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones. 2001;6(4):337–344. doi: 10.1379/1466-1268(2001)006<0337:amhpsn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gastpar R. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65(12):5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Stangl S. Influence of Hsp70 and HLA-E on the killing of leukemic blasts by cytokine/Hsp70 peptide-activated human natural killer (NK) cells. Cell Stress Chaperones. 2008;13(2):221–230. doi: 10.1007/s12192-007-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Simhadri V.R. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One. 2008;3(10):e3377. doi: 10.1371/journal.pone.0003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bae J. Heat shock protein 90 is critical for regulation of phenotype and functional activity of human T lymphocytes and NK cells. J. Immunol. 2013;190(3):1360–1371. doi: 10.4049/jimmunol.1200593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Huyan T. Heat shock protein 90 inhibitors induce functional inhibition of human natural killer cells in a dose-dependent manner. Immunopharmacol. Immunotoxicol. 2016;38(2):77–86. doi: 10.3109/08923973.2015.1119159. [DOI] [PubMed] [Google Scholar]
- 234.Tkach M. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017;36(20):3012–3028. doi: 10.15252/embj.201696003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cicero A.L. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 2015;6(1):7506. doi: 10.1038/ncomms8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ciocca D.R. Heat Shock Proteins (HSPs) based anti-cancer vaccines. Curr. Mol. Med. 2012;12(9):1183–1197. doi: 10.2174/156652412803306684. [DOI] [PubMed] [Google Scholar]
- 237.Murshid A., Gong J., Calderwood S.K. Heat shock protein 90 mediates efficient antigen cross presentation through the scavenger receptor expressed by endothelial cells-I. J. Immunol. 2010;185(5):2903–2917. doi: 10.4049/jimmunol.0903635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Murshid A., Gong J., Calderwood S.K. Hsp90-peptide complexes stimulate antigen presentation through the class II pathway after binding scavenger receptor SREC-I. Immunobiology. 2014;219(12):924–931. doi: 10.1016/j.imbio.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Quintana F.J., Cohen I.R. The HSP60 immune system network. Trends Immunol. 2011;32(2):89–95. doi: 10.1016/j.it.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 240.Flohé S.B. Human heat shock protein 60 induces maturation of dendritic cells versus a Th1-promoting phenotype. J. Immunol. 2003;170(5):2340. doi: 10.4049/jimmunol.170.5.2340. [DOI] [PubMed] [Google Scholar]
- 241.Cohen-Sfady M. Heat shock protein 60 activates B Cells via the TLR4-MyD88 pathway. J. Immunol. 2005;175(6):3594. doi: 10.4049/jimmunol.175.6.3594. [DOI] [PubMed] [Google Scholar]
- 242.Quintana F.J. HSP60 as a target of anti-ergotypic regulatory T cells. PLoS One. 2008;3(12):e4026. doi: 10.1371/journal.pone.0004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Osterloh A. Heat shock protein 60 (HSP60) stimulates neutrophil effector functions. J. Leukoc. Biol. 2009;86(2):423–434. doi: 10.1189/jlb.0109011. [DOI] [PubMed] [Google Scholar]
- 244.Jog N.R. Heat shock protein 27 regulates neutrophil chemotaxis and exocytosis through two independent mechanisms. J. Immunol. 2007;178(4):2421. doi: 10.4049/jimmunol.178.4.2421. [DOI] [PubMed] [Google Scholar]
- 245.Rane M.J. Heat shock protein 27 controls apoptosis by regulating Akt activation. J. Biol. Chem. 2003;278(30):27828–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- 246.Hoter A., El-Sabban M.E., Naim H.Y. The HSP90 family: structure, regulation, function, and implications in health and disease. Int. J. Mol. Sci. 2018;19(9):2560. doi: 10.3390/ijms19092560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chae Y.C. Control of tumor bioenergetics and survival stress signaling by mitochondrial HSP90s. Cancer Cell. 2012;22(3):331–344. doi: 10.1016/j.ccr.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Masgras I. The Chaperone TRAP1 as a modulator of the mitochondrial adaptations in cancer cells. Front. Oncol. 2017;7:58. doi: 10.3389/fonc.2017.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Italiano J.E., Jr., Mairuhu A.T.A., Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr. Opin. Hematol. 2010;17(6):578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Tao S.-C., Guo S.-C., Zhang C.-Q. Platelet-derived extracellular vesicles: an emerging therapeutic approach. Int. J. Biol. Sci. 2017;13(7):828–834. doi: 10.7150/ijbs.19776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Polanowska-Grabowska A.R.L.G.R. Heat-shock proteins and platelet function. Platelets. 2000;11(1):6–22. doi: 10.1080/09537100075742. [DOI] [PubMed] [Google Scholar]
- 252.Mendelsohn M.E., Zhu Y., O'Neill S. The 29-kDa proteins phosphorylated in thrombin-activated human platelets are forms of the estrogen receptor-related 27-kDa heat shock protein. PNAS. 1991;88(24):11212–11216. doi: 10.1073/pnas.88.24.11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Polanowska-Grabowska R. Platelet adhesion to collagen under flow causes dissociation of a phosphoprotein complex of heat-shock proteins and protein phosphatase 1. Blood. 1997;90(4):1516–1526. [PubMed] [Google Scholar]
- 254.Rigg R.A. Heat shock protein 70 regulates platelet integrin activation, granule secretion and aggregation. Am. J. Physiol. Cell Physiol. 2016;310(7):C568–C575. doi: 10.1152/ajpcell.00362.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Perez-Hernandez D. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J. Biol. Chem. 2013;288(17):11649–11661. doi: 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kowal J. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. PNAS. 2016;113(8):E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Garcia B.A. The platelet microparticle proteome. J. Proteome Res. 2005;4(5):1516–1521. doi: 10.1021/pr0500760. [DOI] [PubMed] [Google Scholar]
- 258.Dalli J. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol. Cell. Proteomics. 2013;12(8):2205–2219. doi: 10.1074/mcp.M113.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Drees E.E.E., Pegtel D.M. Circulating miRNAs as Biomarkers in aggressive B cell lymphomas. Trends Cancer. 2020;6(11):1–14. doi: 10.1016/j.trecan.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 260.Valencia-Sanchez M.A. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20(5):515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 261.Faruq O., Vecchione A. microRNA: diagnostic perspective. Front. Med. 2015;2:51. doi: 10.3389/fmed.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zeng Y., Yi R., Cullen B.R. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. PNAS. 2003;100(17):9779–9784. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Doench J.G., Petersen C.P., Sharp P.A. siRNAs can function as miRNAs. Genes Dev. 2003;17(4):438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Mitchell P.S. Circulating microRNAs as stable blood-based markers for cancer detection. PNAS. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Chen X. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 266.Heneghan H.M. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann. Surg. 2010;251(3) doi: 10.1097/SLA.0b013e3181cc939f. [DOI] [PubMed] [Google Scholar]
- 267.Ferracin M. MicroRNA profiling for the identification of cancers with unknown primary tissue-of-origin. J. Pathol. 2011;225(1):43–53. doi: 10.1002/path.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Youssef Y.M. Accurate molecular classification of kidney cancer subtypes using microRNA signature. Eur. Urol. 2011;59(5):721–730. doi: 10.1016/j.eururo.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 269.du Rieu M.l.C. MicroRNA-21 is induced early in pancreatic ductal adenocarcinoma precursor lesions. Clin. Chem. 2010;56(4):603–612. doi: 10.1373/clinchem.2009.137364. [DOI] [PubMed] [Google Scholar]
- 270.Zhu W. Overexpression of members of the microRNA-183 family is a risk factor for lung cancer: a case control study. BMC Cancer. 2011;11:393. doi: 10.1186/1471-2407-11-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lan H. MicroRNAs as potential biomarkers in cancer: opportunities and challenges. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Giovannetti E. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70(11):4528. doi: 10.1158/0008-5472.CAN-09-4467. [DOI] [PubMed] [Google Scholar]
- 273.Mulcahy L.A., Pink R.C., Carter D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracellular Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Huang H.-Y. miRTarBase 2020: updates to the experimentally validated microRNA–target interaction database. Nucl. Acids Res. 2019;48(D1):D148–D154. doi: 10.1093/nar/gkz896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Arroyo J.D. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. PNAS. 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Vickers K.C. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011;13(4):423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Cheng L. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracellular Vesicles. 2014;3(1):23743. doi: 10.3402/jev.v3.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Pare J.M. Hsp90 regulates the function of argonaute 2 and its recruitment to stress granules and P-bodies. Mol. Biol. Cell. 2009;20(14):3273–3284. doi: 10.1091/mbc.E09-01-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tahbaz N. Characterization of the interactions between mammalian PAZ PIWI domain proteins and Dicer. EMBO Rep. 2004;5(2):189–194. doi: 10.1038/sj.embor.7400070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Müller M., Fazi F., Ciaudo C. Argonaute proteins: from structure to function in development and pathological cell fate determination. Front. Cell Dev. Biol. 2020;7:360. doi: 10.3389/fcell.2019.00360. -360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tahbaz N., Carmichael J.B., Hobman T.C. GERp95 belongs to a family of signal-transducing proteins and requires Hsp90 activity for stability and golgi localization. J. Biol. Chem. 2001;276(46):43294–43299. doi: 10.1074/jbc.M107808200. [DOI] [PubMed] [Google Scholar]
- 282.Cwiklinska H. The heat shock protein HSP70 promotes Th17 Genes' expression via specific regulation of microRNA. Int. J. Mol. Sci. 2020;21(8):2823. doi: 10.3390/ijms21082823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lampis A. MIR21 drives resistance to heat shock protein 90 inhibition in cholangiocarcinoma. Gastroenterology. 2018;154(4):1066–1079.e5. doi: 10.1053/j.gastro.2017.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Xu D. MicroRNA-361-mediated inhibition of HSP90 expression and EMT in cervical cancer is counteracted by oncogenic lncRNA NEAT1. Cells. 2020;9(3):632. doi: 10.3390/cells9030632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wang X. MicroRNA-27a downregulates the expression of Hsp90 and enhances the radiosensitivity in esophageal squamous cell carcinoma. OncoTargets and therapy. 2019;12:5967–5977. doi: 10.2147/OTT.S197456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.STOPE M.B. Heat-shock protein HSPB1 attenuates MicroRNA miR-1 expression thereby restoring oncogenic pathways in prostate cancer cells. Anticancer Res. 2014;34(7):3475–3480. [PubMed] [Google Scholar]
- 287.MacKenzie T.N. Triptolide induces the expression of miR-142-3p: a negative regulator of heat shock protein 70 and pancreatic cancer cell proliferation. Mol. Cancer Ther. 2013;12(7):1266–1275. doi: 10.1158/1535-7163.MCT-12-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Choghaei E. Knockdown of microRNA-29a changes the expression of heat shock proteins in breast carcinoma MCF-7 Cells. Oncol. Res. Featur. Preclin. Clinical Cancer Therap. 2016;23(1-2):69–78. doi: 10.3727/096504015X14478843952906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Taylor D.D., Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 290.Yao B. A panel of miRNAs derived from plasma extracellular vesicles as novel diagnostic biomarkers of lung adenocarcinoma. FEBS open bio. 2019;9(12):2149–2158. doi: 10.1002/2211-5463.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Du M. Plasma exosomal miRNAs-based prognosis in metastatic kidney cancer. Oncotarget. 2017;8(38):63703–63714. doi: 10.18632/oncotarget.19476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Fujii N. Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma. Oncotarget. 2017;8(66):109877–109888. doi: 10.18632/oncotarget.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Koppers-Lalic D. Non-invasive prostate cancer detection by measuring miRNA variants (isomiRs) in urine extracellular vesicles. Oncotarget. 2016;7(16):22566–22578. doi: 10.18632/oncotarget.8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Foj L. Exosomal and non-exosomal urinary miRNAs in prostate cancer detection and prognosis. Prostate. 2017;77(6):573–583. doi: 10.1002/pros.23295. [DOI] [PubMed] [Google Scholar]
- 295.Rodríguez M. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol. Cancer. 2017;16(1):156. doi: 10.1186/s12943-017-0726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bryant R.J. Changes in circulating microRNA levels associated with prostate cancer. Br. J. Cancer. 2012;106(4):768–774. doi: 10.1038/bjc.2011.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Li Z. Exosomal microRNA-141 is upregulated in the serum of prostate cancer patients. OncoTargets Therapy. 2015;9:139–148. doi: 10.2147/OTT.S95565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhang W. MicroRNAs in serum exosomes as potential biomarkers in clear-cell renal cell carcinoma. Eur. Urol. Focus. 2018;4(3):412–419. doi: 10.1016/j.euf.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 299.Endzeliņš E. Detection of circulating miRNAs: comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer. 2017;17(1):730. doi: 10.1186/s12885-017-3737-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Huang X. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur. Urol. 2015;67(1):33–41. doi: 10.1016/j.eururo.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.van Eijndhoven M.A. Plasma vesicle miRNAs for therapy response monitoring in Hodgkin lymphoma patients. JCI insight. 2016;1(19):e89631. doi: 10.1172/jci.insight.89631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Fang C. Serum microRNAs are promising novel biomarkers for diffuse large B cell lymphoma. Ann. Hematol. 2012;91(4):553–559. doi: 10.1007/s00277-011-1350-9. [DOI] [PubMed] [Google Scholar]
- 303.Feng Y. Exosome-derived miRNAs as predictive biomarkers for diffuse large B-cell lymphoma chemotherapy resistance. Epigenomics. 2019;11(1):35–51. doi: 10.2217/epi-2018-0123. [DOI] [PubMed] [Google Scholar]
- 304.Inada K. Availability of circulating microRNAs as a biomarker for the early diagnosis of diffuse large B-cell lymphoma. Blood. 2014;124(21):2988. -2988. [Google Scholar]
- 305.Zhang C. Urine Proteome profiling predicts lung cancer from control cases and other tumors. EBioMedicine. 2018;30:120–128. doi: 10.1016/j.ebiom.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]