Abstract
Purpose
To report the findings of unilateral cystoid macular edema (CME) associated with bilateral sub-foveal hyperreflectivity in a patient previously diagnosed with breast cancer and treated with docetaxel and cyclophosphamide.
Observations
A 69-year-old female on docetaxel and cyclophosphamide chemotherapy treatment for breast cancer developed blurry vision 20 days after initiation of therapy. Ophthalmic examination revealed reduced visual acuity with bilateral retinal pigment epithelium mottling and annular sub-foveal thickening in the left eye. Optical coherence tomography (OCT) showed cystoid macular changes in the right eye and central sub-macular hyperreflectivity of outer layers in both eyes. Six-months after discontinuation of therapy, OCT findings remained unchanged and visual acuity did not improve.
Conclusionsand importance
CME may occur in patients taking taxanes, but this finding associated with sub-macular hyperreflectivity of the outer layers in diagnostic testing has never been reported before. Sub-macular deposits found in this patient may be responsible for decreased vision and did not respond to cessation of therapy.
Keywords: Docetaxel, Cystoid macular edema, Subfoveal hyperreflectivity, Ocular toxicity
1. Introduction
Docetaxel is an antineoplastic agent of the taxane class of drugs used for the treatment of several types of tumors.1, 2, 3 Cystoid macular edema (CME) is a well-known complication of therapy and to our knowledge there are no reports of bilateral outer layer hyperreflectivity on Spectral Domain Optical Coherence Tomography (SDOCT) associated with this chemotherapeutic agent. We present a patient with breast cancer who developed unilateral CME and bilateral outer retinal layer hyperreflectivity during combination chemotherapy with docetaxel and cyclophosphamide.
1.1. Case report
A 69-year-old female was referred to our clinic with a two-month history of blurry vision in both eyes. The patient was diagnosed with recurrent breast cancer seven months prior. She was treated with excisional surgery and started on chemotherapy with docetaxel and cyclophosphamide 20 days before the development of symptoms.
Her past medical history is significant for breast carcinoma diagnosed 19 years ago treated with radical mastectomy and reconstruction, chemotherapy and radiotherapy, hypothyroidism and hypercholesterolemia. Past ocular history is significant for cataract surgery in the right eye followed by vitrectomy for macular hole repair at an outside facility. Family history is non-contributory.
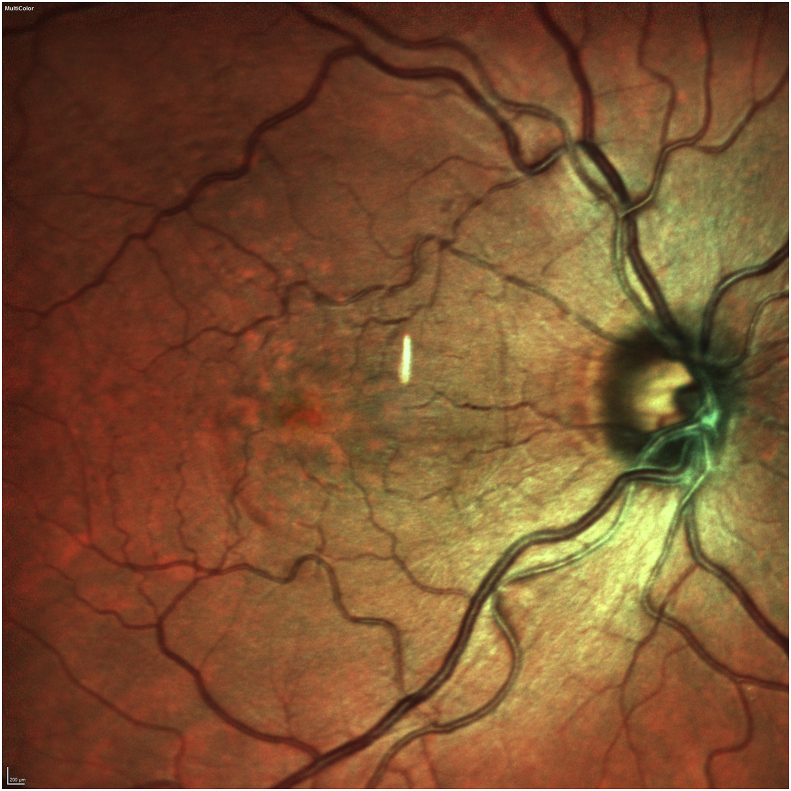
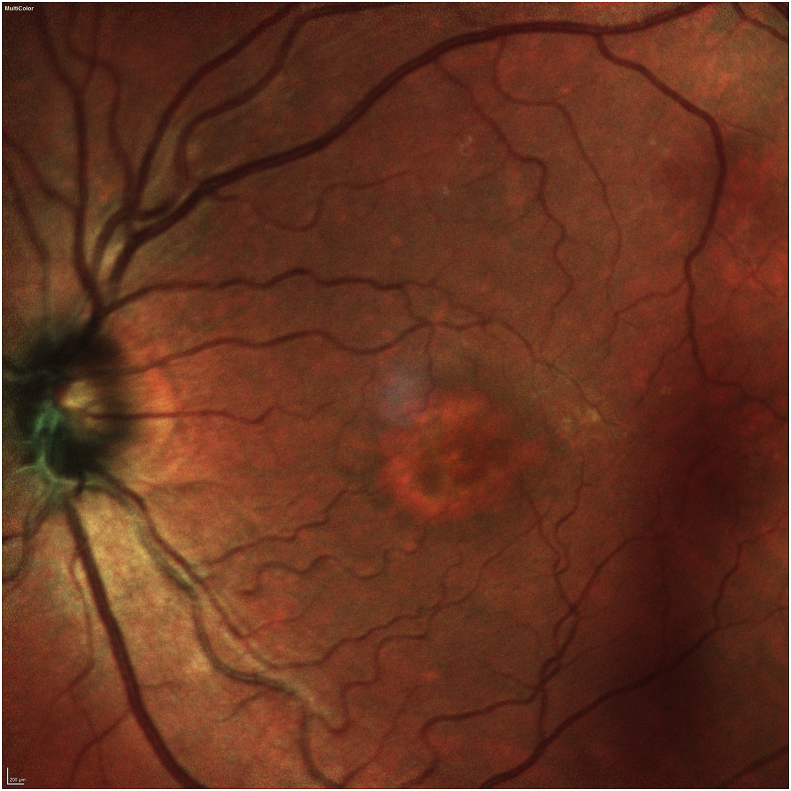
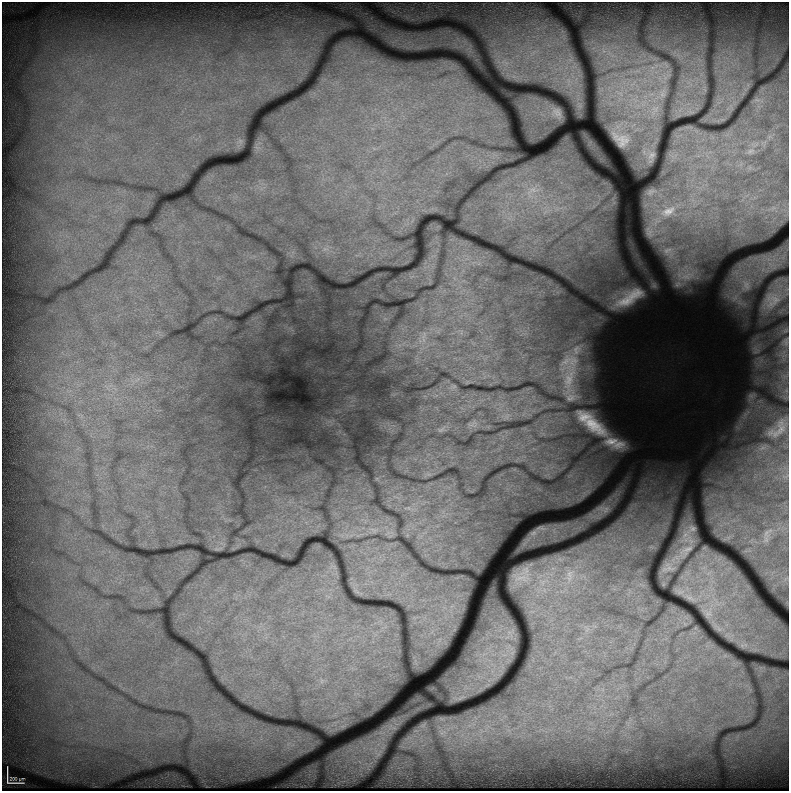
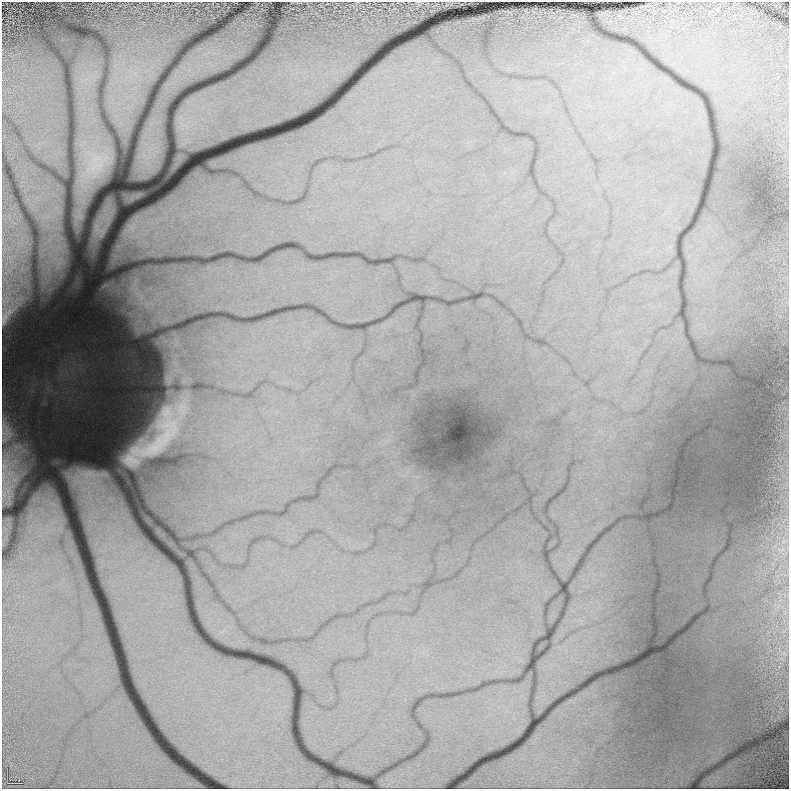
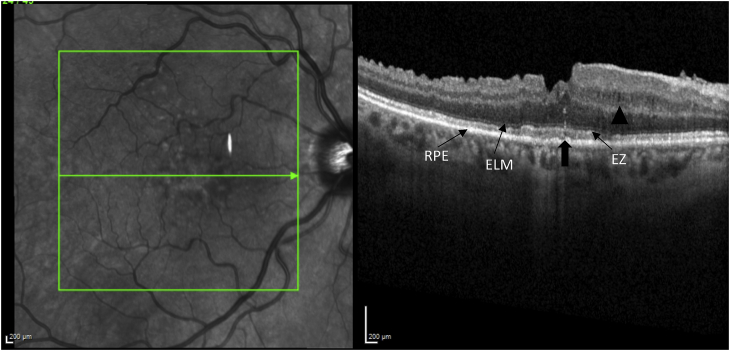
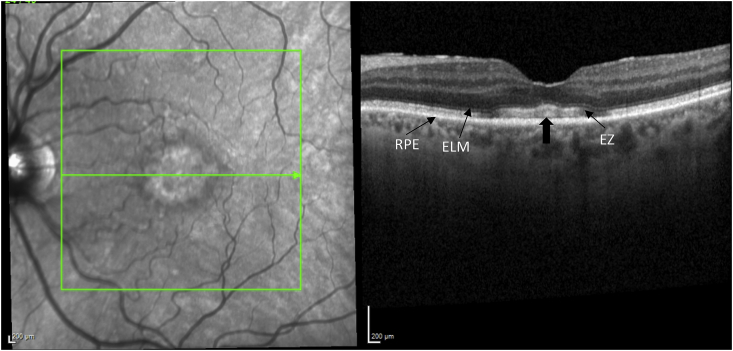
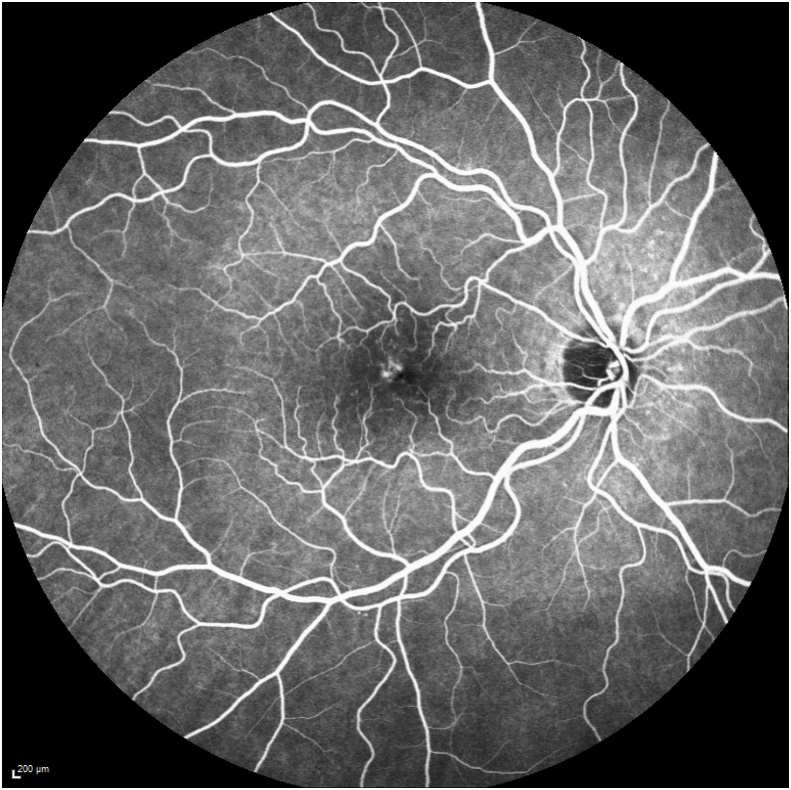
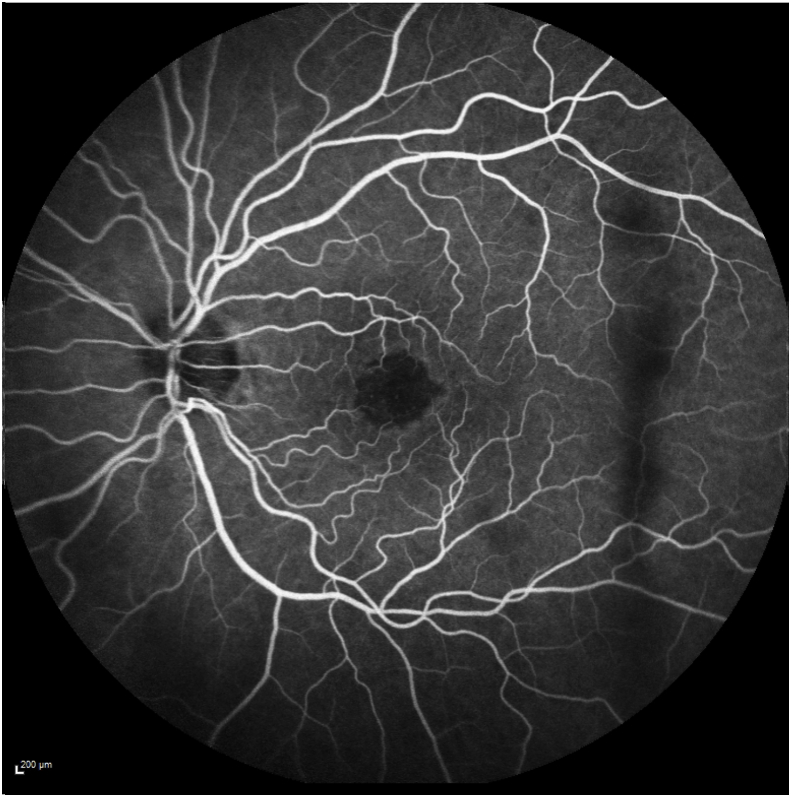
Her exam revealed a best corrected visual acuity (BCVA) of 20/50 in the right eye and 20/40 in the left eye. Intraocular pressures were 19 mmHg in the right eye and 17 mmHg in the left eye. Her external ocular exam, anterior segment, and vitreous were normal bilaterally. The fundus exam was notable for macular drusen and retinal pigment epithelial (RPE) mottling centrally in both eyes and annular sub-foveal RPE thickening in the left eye (Fig. 1A, Fig. 1B-A and 1-B), with slight hyper autofluorescence surrounding the fovea (Fig. 2A, Fig. 2B-A and 2-B) in fundus autofluorescence (FAF). Spectral Domain Optical Coherence Tomography (SDOCT) showed central sub-macular hyperreflectivity of the outer layers (between the ellipsoid zone and Retinal Pigment epithelium) with disruption of the ellipsoid band in both eyes and fine CME in the right eye (Fig. 3A, Fig. 3B-A and 3-B). Fluorescein Angiography (FA) revealed a couple of focal non-leaking hyperfluorescent spots temporal to the fovea in the right eye, probably due to residual defects from prior macular hole surgery eyes, and a nummular central hyperfluorescent area in the left eye (Fig. 4A, Fig. 4B-A and 4-B).
Fig. 1A.
Fundus photo of the right eye showing macular drusen and RPE mottling centrally.
Fig. 1B.
Fundus photo of the left eye showing macular drusen and annular sub-foveal retinal pigment epithelium thickening.
Fig. 2A.
Autofluorescence photo of the right eye showing mild hyper autofluorescence on the edge of soft drusen surrounding the fovea.
Fig. 2B.
Autofluorescence photo of the left eye showing mild hyper autofluorescence on the edge of soft drusen surrounding the fovea.
Fig. 3A.
SD-OCT of the right eye, showing blunted foveal contour, drusen, retinal nerve fiber layer dissociation following vitrectomy and internal limiting membrane stripping for macular hole repair, fine nasal cystoid macular edema (arrowhead) and central sub-macular hyperreflectivity between the RPE and ellipsoid zone. (arrow).
Fig. 3B.
SD-OCT of the left eye, showing presence of foveal contour and central sub-macular hyperreflectivity of the outer layers (arrow).
Fig. 4A.
FA of the right eye showing a couple of hyperfluorescent spots superotemporal to the fovea and stippling in central macula with no leakage in late stages, due to residual defect from macular hole surgery.
Fig. 4B.
FA of the left eye showing nummular central hypofluorescent area with no leakage.
The patient was started on oral acetazolamide for taxane-induced CME in the right eye and scheduled for follow-up. One month later, the patient's symptoms subjectively improved but no change was noted on SDOCT or BCVA. Her oncologist was contacted and since she had only one more cycle of chemotherapy remaining, the decision was made to continue with the current treatment and to follow-up after completion. Six-months after completion of chemotherapy no improvement in BCVA or SD-OCT was seen.
2. Discussion
Docetaxel-induced CME is a known but rare complication of therapy first reported by Teitelbaum et al., in 2003.2 Since then, 7 case reports can be found in the literature, some of them associated with taxane use alone but others associated with the use of other medications such as gemcitabine and hydroxicloroquine. 4, 5, 6, 7, 8. And a couple of cases associated with retinitis pigmentosa and fluid retention syndrome.1,9
Patients present with a non-leaking form of CME, characterized by macular cystoid changes on exam and normal filling of the choroidal and retinal vessels without evidence of leakage on FA.3, 6, 10 The pathophysiology remains unclear, but a couple of theories being postulated include1 dysfunction in the cytoskeleton of the RPE with an intact choroid-pigment epithelium border and2 toxicity to Muller cells with subsequent fluid accumulation and subclinical leakage of extracellular fluid.1,6
In all case reports, regardless of concurrent chemotherapy, complete resolution of edema has been accomplished within 4–10 weeks of taxane cessation.3 Although most cases resolve spontaneously, several authors have used systemic or topical carbonic anhydrase inhibitors, topical anti-inflammatory agents, or intravitreal bevacizumab to accelerate resolution or when taxane therapy cannot be discontinued.3,5
Our patient presented with CME in the right eye associated with bilateral hyperreflective changes on SDOCT that didn't respond to cessation of therapy. Even though the presence of CME can be related to the history of previous macular surgery due to its subtle presentation and it's absence in the fellow eye, its association with bilateral hyperreflective deposits raises the question if the use of both medications in a patient with prior history of macular surgery increases the risk of retinal toxicity in patients undergoing chemotherapy.
The presence of subretinal deposits have been previously associated with a wide spectrum of clinical entities such as vitreomacular traction, adult-onset foveomacular dystrophies and cuticular and reticular drusen among others; and have been referred as acquired vitelliform lesions (AVL) 11. AVL can assume variable configurations and its etiology is likely disturbance of outer retinal-RPE structures and metabolism leading to accumulation of subretinal material 11. However, these lesions are rarely associated with soft drusen and usually show in FAF marked hyper autofluorescence, finding not seen in our patient. Nonetheless, outer layer hyperreflectivity associated with taxane and cyclophosphamide intake may be considered another clinical entity that may induce formation of AVL.
The use of combination chemotherapy and introduction of novel cancer agents has resulted in an increase of cases with chemotherapy-induced ocular toxicity. 4 When evaluating a patient, it is important to evaluate systemic disease, ocular history and concurrent treatment that may accelerate development of retinal toxicities. 4 It also seems reasonable to consider the length of treatment, dose of medication as well as the duration of CME in the final visual prognosis. 2
Early detection, and close ophthalmologic follow-up may allow prevention of chemotherapy-induced permanent ocular side effects. 4 Ophthalmologists and oncologists should work together and weigh risks and benefits of continuing chemotherapeutic treatment when ocular pathology or toxicity develop. 1
3. Conclusion
Outer retinal layer disruption and increased macular thickness has been a common finding on OCT in patients developing CME induced by taxanes, but no outer layer hyperreflectivity has ever been reported in the literature. Moreover, visual acuity returns to baseline in previous reports. Our patient presented with vision loss associated with unilateral CME and bilateral outer layer changes evidenced by hyper reflecivity on SDOCT. The origin of this finding is unknown, but it most likely related to drug toxicity or deposits in the retina. As well, there is possibly a synergistic effect exacerbated by cyclophosphamide use. In this case the CME cleared but the outer layer hyperreflectivity has been unchanged for 6 months following taxane cessation. Most importantly, the BCVA has not improved to baseline unlike previous reports.
3.1. Patient consent
Consent to publish the case report was verbally given by the patient. This report does not contain any personal information that could identify the patient.
Funding
No funding or grant support.
Disclosures
The authors have no financial or conflicting interests to disclose.
Acknowledgments
None.
References
- 1.Enzsoly A., Kammerer K., Nemeth J. Bilateral cystoid macular edema following docetaxel chemotherapy in a patient with retinitis pigmentosa: a case report. BMC Ophthalmol. 2015;15:32. doi: 10.1186/s12886-015-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teitelbaum B.A., Tresley D.J. Cystic maculopathy with normal capillary permeability secondary to docetaxel. Optom Vis Sci. 2003;80:277–279. doi: 10.1097/00006324-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Hassall M.M., Andrew N.H. Single-eye trial of a topical carbonic anhydrase inhibitor versus intravitreal bevacizumab for the treatment of taxane drug-induced cystoid macula edema. BMJ Case Rep. 2016:10. doi: 10.1136/bcr-2015-212733. 2016 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shih C.H., Lee Y.C. Impaired retinal pigment epithelium in paclitaxel-induced macular edema: a case report. Medicine (Baltim) 2018 Jun;97(26) doi: 10.1097/MD.0000000000011229. Medicine (Baltimore). 2018. PMID: 29952984 Free PMC article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoe T., Fukada I., Kobayashi K. Cystoid macular edema during treatment with paclitaxel and bevacizumab in a patient with metastatic breast cancer: a case report and literature review. Case Rep Oncol. 2017;10(2):605–612. doi: 10.1159/000477897. 2017 Jul 11. eCollection 2017 May-Aug. PMID: 28868019 Free PMC article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassi E., Loizzi V., Furino C. Cystoid macular edema secondary to paclitaxel therapy for ovarian cancer: a case report. Mol Clin Oncol. 2017 Aug;7(2):285–287. doi: 10.3892/mco.2017.1296. Epub 2017 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valeshabad A.K., Mieler W.F., Setlur V. Posterior segment toxicity following gemcitabine and docetaxel chemotherapy. Optom Vis Sci. 2015;92(5):e110–e113. doi: 10.1097/OPX.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elhusseiny A.M., Relhan N., Smiddy W.E. Docetaxel-induced maculopathy possibly potentiated by concurrent hydroxychloroquine use. Am J Ophthalmol Case Rep. 2019 Sep 26;16 doi: 10.1016/j.ajoc.2019.100560. eCollection 2019 Dec. PMID: 31650088 Free PMC article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Telander D.G., Sarraf D. Cystoid macular edema with docetaxel chemotherapy and the fluid retention syndrome. Semin Ophthalmol. 2007;22(3):151–153. doi: 10.1080/08820530701457373. [DOI] [PubMed] [Google Scholar]
- 10.Joshi M.M., Garretson B.R. Paclitaxel maculopathy. Arch Ophthalmol. 2007;125(5):709–710. doi: 10.1001/archopht.125.5.709. [DOI] [PubMed] [Google Scholar]
- 11.Freund K.B., Laud K., Lima L.H., Spaide R.F., Zweifel S., Yannuzzi L.A. Acquired Vitelliform Lesions: correlation of clinical findings and multiple imaging analyses. Retina. 2011;31:13–25. doi: 10.1097/IAE.0b013e3181ea48ba. [DOI] [PubMed] [Google Scholar]