Abstract
Patient: Male, 23-year-old
Final Diagnosis: Cytokine release syndrome • disseminated intravascular coagulation • multiple organ failure
Symptoms: Auditory hallucinations • fever • headache • restlessness • suicidal thoughts
Medication: —
Clinical Procedure: —
Specialty: Infectious Diseases • Neurology
Objective:
Unusual clinical course
Background:
Cytotoxic lesions of the corpus callosum (CLOCC) is a rare clinical and radiological syndrome that has been associated with various infectious etiologies. CLOCC are among the recently described neurological associations with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with coronavirus disease 2019 (COVID-19). We report a case of CLOCC in a man with SARS-CoV-2 infection who presented with auditory hallucinations and rapidly developed systemic inflammatory response syndrome (SIRS).
Case Report:
A 23-year-old man with no past medical and psychiatric history presented with auditory hallucinations, restlessness, and suicidal ideations. A nasopharyngeal swab specimen tested using real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay was positive for SARS-CoV-2. A brain MRI revealed an isolated oval-shaped lesion in the splenium of the corpus callosum, with hyperintense signal on diffusion-weighted imaging (DWI) and hypointense on apparent diffusion coefficient (ADC) maps, suggestive of CLOCC. After a dramatic hospital course associated with multiple organ dysfunction syndrome (MODS) and severe intra-abdominal and cerebral bleeding, he developed cardiac arrest and died on hospital day 15.
Conclusions:
This case highlights the need for increased vigilance for the atypical manifestations of SARS-CoV-2 infection. In addition, it suggests that CLOCC can be considered as a differential diagnosis by clinicians in patients with SARS-CoV-2 infection who present with unexplained neurological and neuropsychiatric symptoms, leading to poor outcome.
MeSH Keywords: Coronavirus, Corpus Callosum, COVID-19, Neurotoxicity Syndromes
Background
Since its declaration as a global pandemic on March 11 2020, SARS-CoV-2 infection, the cause of COVID-19, has been associated with a spectrum of pathologies which continues to widen as the world is battling the outbreak [1]. Atypical manifestations of the disease are increasingly being reported [2]. CLOCC, previously known as mild encephalopathy with reversible splenial lesion (MERS), is a rare clinical and radiological syndrome which was first reported by Tada et al. in 2004 [3]. Typically, it is marked by neurological and neuropsychiatric symptoms coupled with a brain MRI finding of an ovoid reversible lesions in the central portion of the splenium of the corpus callosum (SCC) without any accompanying lesions [4].
The following diagnostic criteria for MERS/CLOCC were proposed by Hoshino et al. in 2012: onset with neuropsychiatric symptoms, such as abnormal speech and/or behavior, and impaired consciousness and convulsion, within 1 week after the onset of fever; complete recovery without sequelae, mostly within 10 days after the onset of neuropsychiatric symptoms; high-signal-intensity T1 and T2 lesions in the splenium of corpus callosum, in the acute stage; lesions may involve the entire corpus callosum and the cerebral white matter in a symmetric fashion; and lesions disappear within 1 week, with no residual signal changes or atrophy [5].
CLOCC can be triggered by viral or bacterial infections as well as non-infectious causes, such as anti-epileptic drug withdrawal, epilepsy, metabolic disturbances, cerebrovascular diseases, and traumatic brain injuries [6]. The association of COVID-19 and CLOCC has seldom been reported in the literature. In August 2020, Forestier et al. reported the first case of CLOCC as a presenting neuroradiological manifestation of COVID-19, in a 55-year-old man [7]. We report a case of CLOCC in a 23-year-old man presenting with auditory hallucinations and development of systemic inflammatory response in the context of SARS-CoV-2 infection. To the best of our knowledge, the present case is the most severe to be reported in the literature.
Case Report
A 23-year-old man, with no history of prior illnesses, presented to the emergency department with a 2-day history of auditory hallucinations, restlessness, and suicidal ideations. Fever, malaise, headache, dizziness, and vomiting were also reported. He was not taking any medications, and had no significant travel history. He was a life-long nonsmoker and never consumed alcohol or used illicit drugs. Upon presentation, he was febrile (39.5°C) with sinus tachycardia (174 beats/min) and tachypnea (36 breaths/min). Blood pressure was 122/70 mmHg and oxygen saturation was 98% on room air. A neurological examination revealed altered sensorium with disorientation and delayed verbal responses; otherwise, there were no focal neurologic deficits or meningeal signs. The rest of the physical examination was normal.
Initial blood investigations, as charted in Table 1, revealed leukocytosis, thrombocytopenia and acute kidney injury. Elevated pro-inflammatory markers were observed, including C-reactive protein, ferritin, interleukin-2, interleukin-6, D-dimer, and procalcitonin. Liver enzymes were elevated, whereas serum electrolytes were normal. Cerebrospinal fluid (CSF) examination revealed normal chemistry and cell count. CSF viral panel including enterovirus, herpes simplex virus type 1 and 2, varicella virus, cytomegalovirus, Epstein-Barr virus, mumps, adenovirus, and parechovirus was negative. CSF bacterial and fungal cultures were all negative. Infection with SARS-CoV-2 was confirmed based on testing of a nasopharyngeal swab using the Cobas SARS-CoV-2 Test (Roche Diagnostics, Rotkreuz, Switzerland), which is a reverse transcriptase-polymerase chain reaction (RT-PCR) assay that has received Emergency Use Authorization from the US Food and Drug Administration. Two nasopharyngeal aspirates were positive for the virus during the course of illness, as shown in Table 1. RT-PCR testing was conducted in an authorized laboratory, the Hamad Medical Corporation (HMC) central laboratory.
Table 1.
Pertinent laboratory investigations on admission (hospital day 1), mid-hospitalization (hospital day 9), and at time of death (hospital day 15).
| Laboratory variables | Day 1 | Day 9 | Day 15 (death) | Normal range |
|---|---|---|---|---|
| White blood cell (×103/uL) | 14.0 | 22.1 | 6.7 | 4.0–10.0 |
| Hemoglobin (gm/dL) | 14.0 | 2.7 | 9.6 | 13.0–17.0 |
| Platelet (×103/uL) | 100 | 46 | 90 | 150–400 |
| Alanine aminotransferase (U/L) | 87 | 144 | 31 | 0–41 |
| Aspartate aminotransferase (U/L) | 308 | 42 | 23 | 0–40 |
| Creatinine (umol/L) | 193 | 778 | 1000 | 62–106 |
| Sodium (mmol/L) | 137 | 148 | 146 | 136–145 |
| Potassium (mmol/L) | 3.7 | 3.8 | 5.9 | 3.5–5.1 |
| C-reactive protein (mg/L) | 379.8 | 33.7 | 0.0–5.0 | |
| Procalcitonin (ng/mL) | 54.40 | 4.04 | <0.5 | |
| Ferritin (ug/L) | 100000 | 1328 | 38.0–270.0 | |
| Interleukin-2R (ng/mL) | 31.78 | N/A | 1.20–8.80 | |
| Interleukin-6 (pg/mL) | 41 | 95 | ≤7 | |
| D-Dimer (mg/L) | 3.76 | 10.42 | 0.00–0.44 | |
| Fibrinogen (gm/L) | 8.12 | 1.3 | 1.70–4.20 | |
| Prothrombin time (seconds) | 16.1 | 23.9 | 13.2 | 9.4–12.5 |
| Activated partial thromboplastin time (seconds) | 29.6 | 47.6 | 35.9 | 25.1–36.5 |
| CT value COVID-19 PCR | 34.50 | 31.9 |
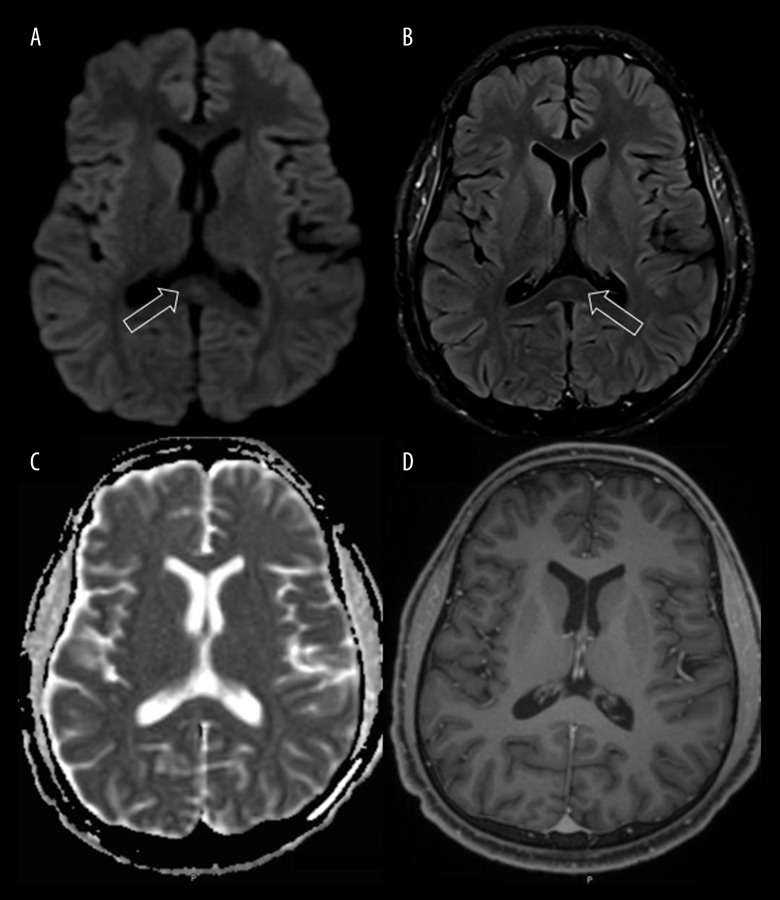
Initial computed tomography (CT) of the brain was unremarkable. Brain MRI revealed an isolated oval-shaped lesion in the splenium of corpus callosum, characterized by increased signal intensity on both diffusion-weighted and fluid-attenuated inversion recovery images (DWI and FLAIR), low signal intensity on apparent diffusion coefficient (ADC) maps, and an isoin-tense signal without contrast enhancement on T1-weighted images. These findings were suggestive of cytotoxic lesion of the corpus callosum (Figure 1). Accordingly, a diagnosis of CLOCC in association with COVID-19 was made.
Figure 1.
Brain Magnetic resonance imaging (MRI) on admission. Diffusion-weighted (A) and fluid-attenuated inversion recovery (B) imaging demonstrates a hyperintense signal in the splenium of corpus callosum, with associated loss of signal on apparent diffusion coefficient maps (C) corresponding to restricted diffusion. T1-weighted images with contrast (D) showed an isointense signal without contrast enhancement. These findings were suggestive of cytotoxic lesion of corpus callosum. Arrows indicate the splenium of the corpus callosum.
The patient was treated with dexamethasone, favipiravir, piperacillin tazobactam, and azithromycin as per our hospital protocol for COVID-19 pneumonia. On hospital day 2, the patient’s condition worsened. He developed signs of MODS, including acute respiratory distress syndrome (ARDS) requiring mechanical ventilator, worsening of his acute kidney injury requiring renal replacement therapy, fulminant liver failure, and severe myocarditis marked by global left ventricular hypokinesia and drop in ejection fraction to 25%. Favipiravir was stopped in view of the fulminant hepatitis, and dexamethasone was continued.
On hospital day 7, the patient was extubated and maintained oxygen saturation on 1 liter of oxygen via nasal cannula. The inflammatory markers and liver enzymes were trending down.
It was difficult to assess his neuropsychiatric status after extubating him, as the patient was drowsy with a Glasgow coma scale of 10 (E 4, V2, M 4).
On hospital day 9, his condition suddenly deteriorated, with severe hemodynamic instability and drop in Glasgow coma scale down to 3, so he was re-intubated. Urgent laboratory investigations, as shown in Table 1, revealed a severe drop in hemoglobin, severe thrombocytopenia, elevated prothrombin time and activated partial thromboplastin time, raised D-dimer, and low fibrinogen. Urgent CT of the abdomen revealed massive intraperitoneal hemorrhage (Figure 2), with unclear source, for which he underwent urgent exploratory laparotomy to control the bleeding. Head CT was also repeated, which showed massive intracranial hemorrhage (Figure 3) with diffuse brain edema along with subfalcine and transtentorial herniation. The intraperitoneal and intracranial hemorrhage were attributed to his low platelet count and deranged coagulation from disseminated intravascular coagulation (DIC), which was assumed to be secondary to COVID-19-related cytokine storm. Unfortunately, the patient had a cardiac arrest and died on hospital day 15.
Figure 2.
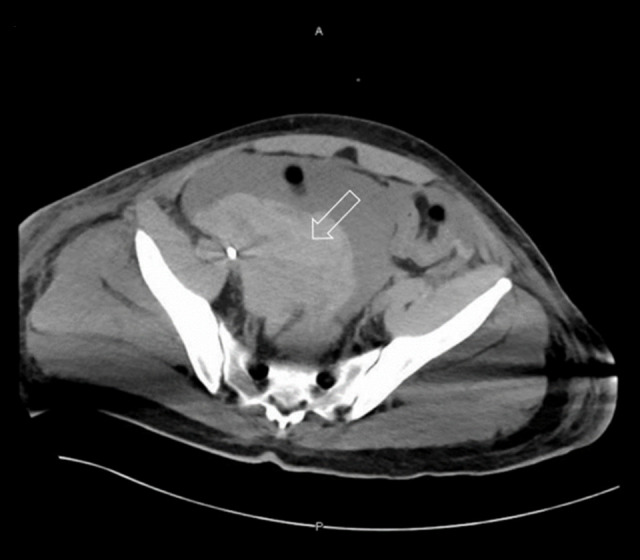
Abdominal Computed tomography (CT) scan on hospital day 9. Hypodense elements suggestive of intraperitoneal hemorrhage (seen in non-contrast). Main bulk of the hemorrhage is seen in the right side of the pelvis (arrow). Source of active bleeding cannot be determined.
Figure 3.
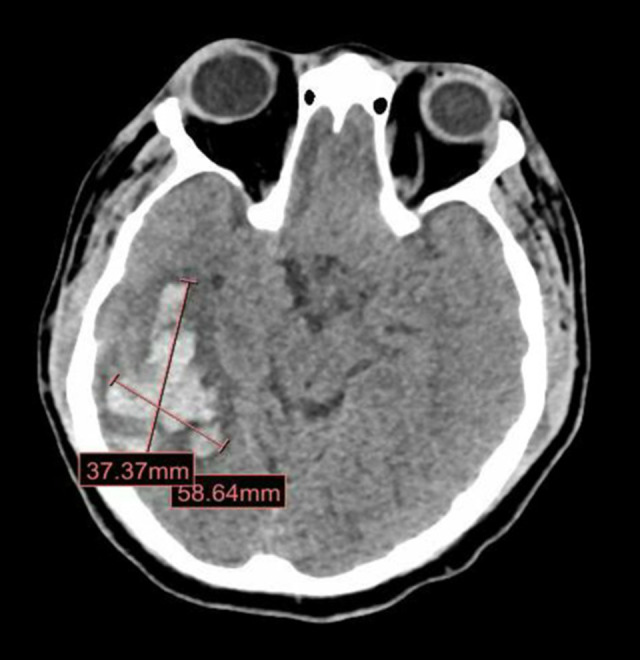
Brain Computed tomography (CT) scan on hospital day 9. Large hyperdense intracranial bleed in the right parietotemporal region with surrounding edema, measuring 5.8×3.7 cm.
Discussion
Amidst the COVID-19 pandemic, multiple neurological manifestations are increasingly being reported, including, ischemic stroke, intracranial hemorrhage, diffuse leukoencephalopathy, acute demyelination, and transient cytotoxic edema [8]. A cross-sectional study conducted between March 23 and May 7, 2020 evaluated the neuroimaging findings in 73 patients with COVID-19 who had acute de novo neurological manifestations: 43 had abnormal MRI findings, including mainly cerebrovascular thrombotic events, in addition to restricted diffusion foci within the corpus callosum, which was consistent with CLOCC [9]. Typically, as noted in this case, CLOCC appear on imaging as a well-circumscribed, small, oval lesion in the midline within the substance of the splenium. On MRI, the lesion has the following features: transient high signal intensity on T2-weighted images (T2-WI), FLAIR, and DWI, decreased intensity on apparent diffusion coefficient (ADC) maps, and no contrast enhancement [10]. In Table 2, we charted individual cases of CLOCC secondary to SARS-CoV-2 infection, as reported in the literature.
Table 2.
A Summary of individual cases reporting CLOCC in context of SARS-CoV-2 infection.
| Author(s) | Age/Sex | Clinical presentation | Confirmed SARS-CoV-2 serology/RT-PCR | MRI findings | Outcome |
|---|---|---|---|---|---|
| Kakadia Do et al. [15] | 69/M | Disorientation, inattention, bradyphrenia, fever | Elevated SARS-CoV-2 IgM and IgG antibodies | Hyperintensity in SCC | Complete resolution of neurological symptoms and corpus callosum lesion after 2 weeks |
| Agarwa et al. [16] | 73/M | Altered consciousness, fever, and respiratory distress | PCR positive for SARS-CoV-2 | Isolated lesion in SCC | Improved and stepped down from ICU after 4 weeks. No documentation about resolution of corpus callosum lesion or neurological status follow-up |
| Hayashi et al. [17] | 75/M | Altered sensorium, tremors, ataxia, and urinary incontinence | PCR positive for SARS-CoV-2 | Abnormal hyperintensity in SCC | Neurological symptoms resolved after 3 days. Patient died after 12 days secondary to respiratory failure |
| Moreau et al. [18] | 26/M | Acute confusion, agitation, inappropriate speech, fever, dry cough | Positive SARS-CoV-2 IgG | Hyperintense round lesion in SCC | Neurological status improved within 48 h and his cardiac dysfunction resolved within 1 week. Follow-up MRI showed resolution of the corpus callosum lesion |
| Forestier et al. [7] | 55/M | Headache, high-grade fever, dizziness and impaired consciousness | PCR positive for SARS-Cov-2 | Increased diffusion-weighted signal in SCC | Improved and extubated 17 days later, follow up MRI showed complete regression of the corpus callosum lesion |
CLOCC – cytotoxic lesions of corpus callosum; SCC – splenium of corpus callosum; RT-PCR – real-time reverse transcription polymerase chain reaction test; SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2.
The pathogenesis of CLOCC remains unclear. It has been proposed that inflammatory cytokines interleukin 1 (IL-1) and 6 (IL-6) trigger a cascade, resulting in markedly increased levels of glutamate, which accumulates in the extracellular space of neuronal tissues, eliciting an excitotoxic effect which triggers the sodium-potassium pump, allowing sodium ions to enter cells and potassium ions to leave cells, hence resulting in an influx of water into the callosal neurons, which manifests as cytotoxic edema and diffusion restriction, which is observed on the MRI findings described in these patients. Moreover, it has been suggested that the corpus collosum, and particularly the splenium, contain higher density of both cytokine and glutamate receptors, as opposed to other brain areas, thus making them more vulnerable for cytotoxic edema [11].
Clinically, neurological symptoms preceded or accompanied by fever is the most common manifestation in CLOCC. However, it can present with a spectrum of nonspecific symptoms: cognitive impairment, seizures, behavioral changes, confusion, acute urinary retention, and delirium are among the common neurological symptoms [12]. In our case, the CLOCC might have contributed to the acute encephalopathy, causing delirium, which presented with prominent neuropsychiatric symptoms, auditory hallucinations, suicidal ideations, and restlessness.
Most of the reported neurological complications in COVID-19 are recognized as a consequence to the cytokine storm triggered by the virus [13]. This systemic inflammatory response leads to excessive activation of immune cells and the generation of pro-inflammatory cytokines, leading to exacerbation of symptoms and life-threatening complications [14]. In this case, we assumed that MODS, DIC, and CLOCC were secondary to an autoimmune injury from a cytokine storm triggered by SARS-CoV-2. This is supported by the elevated pro-inflammatory cytokines (IL-2 and IL-6), raised pro-inflammatory markers, and absence of etiological factors in the initial stage other than SARS-CoV-2.
Conclusions
Although no causal relationship has been established and the exact pathophysiology of CLOCC remains obscure, the emerging studies indicate an association between COVID-19 and CLOCC which might nominate SARS-CoV-2 to the list of causative pathogens. This case highlights the need for increased vigilance for the atypical manifestations of SARS-CoV-2 and might as well serve as a reminder for clinicians to add CLOCC to their list of differentials in patients who present with unexplained neurological and neuro-psychiatric manifestations.
Acknowledgments
The authors are indebted to Dr. Muna Al-maslamani for her continuous support during the preparation of this manuscript.
References:
- 1.Pryce-Roberts A, Talaei M, Robertson NP. Neurological complications of COVID-19: A preliminary review. J Neurol. 2020;267(6):1870–73. doi: 10.1007/s00415-020-09941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Small CN, Beatty NL. Atypical features of COVID-19: A literature review. J Clin Outcomes Manag. 2020;27(3):131–34. [Google Scholar]
- 3.Tada H, Takanashi JI, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004;63(10):1854–58. doi: 10.1212/01.wnl.0000144274.12174.cb. [DOI] [PubMed] [Google Scholar]
- 4.Yuan J, Yang S, Wang S, et al. Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) in adults – a case report and literature review. BMC Neurol. 2017;17(1):103. doi: 10.1186/s12883-017-0875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoshino A, Saitoh M, Oka A, et al. Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev. 2012;34(5):337–43. doi: 10.1016/j.braindev.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Monco JC, Cortina IE, Ferreira E, et al. Reversible splenial lesion syndrome (RESLES): What’s in a name? J Neuroimaging. 2011;21(2):e1–14. doi: 10.1111/j.1552-6569.2008.00279.x. [DOI] [PubMed] [Google Scholar]
- 7.Forestier G, de Beaurepaire I, Bornet G, Boulouis G. Cytotoxic lesion of the corpus callosum as presenting neuroradiological manifestation of COVID-2019 infection. J Neurol. 2020 doi: 10.1007/s00415-020-10166-1. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal A, Pinho M, Raj K, et al. Neurological emergencies associated with COVID-19: stroke and beyond. Emerg Radiol. 2020;11:1–8. doi: 10.1007/s10140-020-01837-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chougar L, Shor N, Weiss N, et al. Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS-CoV-2 infection and neurological manifestations. Radiology. 2020;17:202422. doi: 10.1148/radiol.2020202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SE, Choi DS, Shin HS, et al. Splenial lesions of the corpus callosum: Disease spectrum and MRI findings. Korean J Radiol. 2017;18(4):710–21. doi: 10.3348/kjr.2017.18.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starkey J, Kobayashi N, Numaguchi Y, Moritani T. Cytotoxic lesions of the corpus callosum that show restricted diffusion: Mechanisms, causes, and manifestations. Radiographics. 2017;37(2):562–76. doi: 10.1148/rg.2017160085. [DOI] [PubMed] [Google Scholar]
- 12.Tetsuka S. Reversible lesion in the splenium of the corpus callosum. Brain Behav. 2019;9(11):e01440. doi: 10.1002/brb3.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: A literature review. J Clin Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Y, Liu J, Zhang D, et al. Cytokine storm in COVID-19: The current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakadia Do B, Ahmed J, Siegal T, et al. Mild encephalopathy with reversible splenium lesion (MERS) in a patient with COVID-19. J Clin Neurosci. 2020;79:272–74. doi: 10.1016/j.jocn.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal N, Martini R, Pedrotti G, Della Sala SW. Unusual lesion in the splenium of the corpus callosum and coronavirus infectious disease-19. BJR Case Rep. 2020;6(3):20200068. doi: 10.1259/bjrcr.20200068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi M, Sahashi Y, Baba Y, et al. COVID-19-associated mild encephalitis/ encephalopathy with a reversible splenial lesion. J Neurol Sci. 2020;415:116941. doi: 10.1016/j.jns.2020.116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreau A, Ego A, Vandergheynst F, et al. Cytotoxic lesions of the corpus callosum (CLOCCs) associated with SARS-CoV-2 infection. 2020 doi: 10.1007/s00415-020-10164-3. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]