Abstract
The dopaminergic system controls several vital central nervous system functions, including the control of movement, reward behaviors and cognition. Alterations of dopaminergic signaling are involved in the pathogenesis of neurodegenerative and psychiatric disorders, in particular Parkinson’s disease, which are associated with a subtle and chronic inflammatory response. A substantial body of evidence has demonstrated the non-neuronal expression of dopamine, its receptors and of the machinery that governs synthesis, secretion and storage of dopamine across several immune cell types. This review aims to summarize current knowledge on the role and expression of dopamine in immune cells. One of the goals is to decipher the complex mechanisms through which these cell types respond to dopamine, in order to address the impact this has on neurodegenerative and psychiatric pathologies such as Parkinson’s disease. A further aim is to illustrate the gaps in our understanding of the physiological roles of dopamine to encourage more targeted research focused on understanding the consequences of aberrant dopamine production on immune regulation. These highlights may prompt scientists in the field to consider alternative functions of this important neurotransmitter when targeting neuroinflammatory/neurodegenerative pathologies.
Keywords: astrocyte, autoimmune disease, dopamine, dopamine receptors, D3R, immune transmitter, microglia, multiple sclerosis, neuroinflammation, Parkinson's disease
Introduction
Dopamine (DA) is one of the most well-known and well-studied neurotransmitters in the brain. This is because DA controls several vital functions, including the control of movement, and reward-related behaviors (Pinoli et al., 2017). It is also involved in regulating several aspects of cognition by modulating the expression of several plasticity-associated molecular substrates (Castorina et al., 2013; D’Amico et al., 2013; Marzagalli et al., 2016). DA exerts its functions through distinct neural pathways, with abnormalities of DA and DA signaling in these pathways leading to a range of neurodegenerative, psychiatric and autoimmune disorders (Rangel-Barajas et al., 2015). Many of these pathologies are accompanied by ongoing neuroinflammation, which is the localised inflammatory response of the central nervous system (CNS). Recently, a key role for DA in the regulation of immunity has been proposed. It has been shown that peripheral immune cells as well as microglia and astrocytes express all the elements of the machinery to synthesise (Mastroeni et al., 2009; Gaskill et al., 2012; Ronnberg et al., 2012; Mackie et al., 2018), metabolise and store DA (Matt and Gaskill, 2019), as well as expressing functional dopamine receptors (DRs) to control immune cell functions (McKenna et al., 2002).
The emerging evidence confirming DA as an immune transmitter adds another layer of complexity to CNS physiology and disease pathology. As DA is involved in the control of several vital functions, it is conceivable that abnormal DA signaling may cause important neurological dysfunctions. For example, Parkinson’s disease (PD) is characterized by a hypo-DAergic environment and is also linked to a chronic state of neuroinflammation (Wang et al., 2015). As will be shown in this review, all inflammatory cell types found to be abnormally expressed in PD patients and post-mortem brains express dopamine receptors (DRs) (McKenna et al., 2002). Since the mainstay treatment for PD is to replace the lost DA, it is important to consider how this restoration of DA affects the immune response associated with PD.
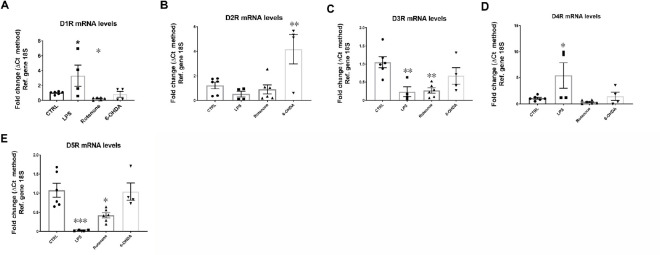
Despite a large body of evidence that suggests a role of DA in regulating inflammation, there are conflicting results and viewpoints on how DA might function as an immune regulator. These differences are due to differences in experimental models and the biological samples investigated (cell lines, animal tissue, and human samples), as well as differences in methodologies and experimental design. We will highlight some examples that illustrate DA’s regulatory activity of inflammation, which seems to depend on the immune cell subtype, activation state of the target cell and DA-releasing cell subtype, abundance of DRs and ligand availability. Since comparing these studies is challenging due to the significant impact that each of these factors have on DA utilization, research in this area has stagnated. This is detrimental as this dual role for DA has implications in how we study and manage DAergic and inflammatory pathologies. To illustrate the complexity of DA’s actions, we conducted a pilot study in which we analyzed the mRNA expression of all five DRs in BV2 microglial cells in response to a well-known inflammatory mediator (lipopolysaccharide, LPS) or to the PD mimetics, rotenone and 6-hydroxydopamine (6-OHDA) (Figure 1). We show that, compared with control cells, acute exposure (24 hours) to inflammation (LPS) or PD-like conditions (rotenone and 6-OHDA) triggers a differential and abnormal regulation of DR mRNA expression. Given the modulatory activities of DRs in these cells, the results suggest that dysregulated expression of DRs on microglial cells could participate, either directly or indirectly, in PD pathogenesis and the establishment of neuroinflammation. Furthermore, these results highlight the complex adaptive responses of the DA system, as the transcriptional regulation of each receptor subtype appears distinct for each insult, stressing the importance of dissecting the specific role/s for each receptor in different pathological contexts.
Figure 1.
Dysregulated DRs mRNA levels in BV-2 microglial cells exposed to PD mimetics (LPS, protenone and 6-OHDA) after 24 hours.
BV-2 cells at low passage (< 25) were plated at a density of 1.5 × 105 cells in 6-well plates with 10% FGM until 80% confluent and then incubated for further 24 hours in the presence of either LPS (1 μg/μL), rotenone (0.01 μM) and 6-OHDA (25 μM) in 1% FGM. mRNA was quantified using the ΔCt method and normalized using the 18S gene. Data are expressed as the mean ± SEM, obtained from two independent determinations, each run in duplicate or triplicate (n = 4–6). Bar graphs depicting mRNA levels of (A) the dopamine D1 receptor, (B) the dopamine D2 receptor, (C) the dopamine D3 receptor, (D) the dopamine D4 receptor and (E) the dopamine D5 receptor. *P < 0.05, **P < 0.01 vs. control (one-way ANOVA followed by a Sidak post hoc test). 18S: Ribosomal protein 18S (housekeeping gene); 6-OHDA: 6-hydroxydopamine; DR: dopamine receptor; LPS: lipopolysaccharide; PD: Parkinson's disease; Ref: reference.
Evidence of the expression of DA has been reviewed previously (Miyazaki et al., 2004; Levite, 2016). In this review, our goal is to introduce new important insights into DA’s biological role as an immune transmitter. We aim to prompt further investigation into this area of research and to highlight novel, non-neuronal activities of DA with regards to several DA- and neuroinflammation-related pathologies.
The current scientific literature was searched using PubMed with the following search terms: DA, dopamine receptors AND immune cell, receptor expression, glia, inflammation, neuroinflammation, CNS immune response. Papers were screened and assessed for inclusion according to their significance for the field of dopamine as an immune transmitter (Figure 1).
Dopamine Metabolism and Transport in the Immune System
DA is derived from the hydroxylation of L-tyrosine by the rate limiting enzyme, tyrosine hydroxylase (TH), forming l-3,4-dihydroxyphenylalanine (L-DOPA), which is then de-carboxylated by the aromatic amino acid decarboxylase to form DA (Figure 2). This process has been well-described in immune cells, specifically T lymphocytes (Qiu et al., 2005) and dendritic cells (Pacheco et al., 2009). In a prior study, Musso et al. (1996) showed that the addition of L-tyrosine and L-DOPA to lymphocyte cultures increased catecholamine levels in a dose dependent manner.
Figure 2.
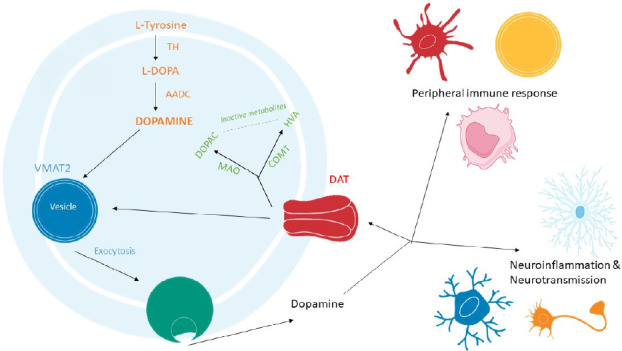
Dopamine biosynthesis, storage and metabolism pathways.
Dopamine is derived from the hydroxylation of L-tyrosine by the rate limiting enzyme, tyrosine hydroxylase (TH), forming l-3,4-dihydroxyphenylalanine (L-DOPA), which is then de-carboxylated by the aromatic amino acid decarboxylase (AADC) to form dopamine (orange). Dopamine is stored in vesicles through vesicular monoamine transporter 2 (VMAT2) and in neurons upon stimulation dopamine is released via exocytosis (blue). Dopamine is taken up by cells via the dopamine transporter (DAT) (yellow). Once dopamine re-enters the cell it can be incorporated back into vesicles by VMAT2 or dopamine can be inactivated through several metabolic pathways including oxidative deamination by monoamine oxidase (MAO) or by O-methylation by catechol-O-methyltransferase (COMT), leading to the formation of two catabolic products, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) respectively (green).
Several independent research groups have shown that astrocytes and microglia are able to synthesize and metabolize DA (Redell and Dash, 2007; Mastroeni et al., 2009; Bisaglia et al., 2013; Asanuma and Miyazaki, 2016; Morales et al., 2017; Schain and Kreisl, 2017; Winner et al., 2017; Petrelli et al., 2018; Matt and Gaskill, 2019). This is particularly important as these cells are in direct contact with DAergic neurons. It is therefore possible that glial cells could play a role in sustaining DA levels in the brain, in both normal homeostatic and pathological states. This suggestion has been raised by Asanuma and Miyazaki (2016), who showed that striatal astrocytes can act as a reservoir for L-DOPA and that, in turn, L-DOPA increased the neurotrophic action of astrocytes thereby protecting neurons from neurotoxic insult. This attribute of astrocytes could be exploited to enhance current PD therapies, as L-DOPA is the gold standard treatment for PD. For example, Asanuma et al. (2014) showed that L-DOPA immunoreactivity was increased on the side of a 6-OHDA lesion compared to the unlesioned, control side suggesting that when DAergic neurons are damaged excess L-DOPA is taken up by astrocytes. However, this study also demonstrated that despite expressing the enzymes required for metabolism, astrocytes were unable to convert L-DOPA to DA. This step could be a therapeutic target to restore DA lost in PD pathogenesis, it also suggests a cell-specific ability to synthesize DA. It would be interesting to determine how the rapid metabolism of dopamine and inability of astrocytes to convert L-DOPA to DA affects L-DOPA therapy in PD patients as targeting astrocytes themselves may not have a therapeutic benefit but could aid in current and future PD therapies.
In neurons, DA is typically stored in synaptic vesicles through vesicular monoamine transporter 2 (VMAT2) (Lohr et al., 2016). It has been shown that VMAT2 is expressed in immune cells as treatment of cultured cells with reserpine, a drug that typically inhibits the re-uptake and storage of DA into vesicles, reduced the intracellular concentration of DA while increasing DA accumulation in the cell culture supernatant of reserpine-treated immune cells (Cosentino et al., 2000).
In the brain, reuptake of excess DA by neurons occurs via the DA transporter (DAT). DAT mRNA and protein, as well as VMAT2 immunoreactivity have been detected on cell membranes and in vesicle-like structures of human lymphocytes (Amenta et al., 2001), suggesting that immune cells are also able to store DA (Figure 2). In lymphocytes, intracellular DA levels increase following an increase in extracellular DA, supporting the idea that cells of the adaptive immune system exhibit a functional cellular uptake mechanism (Mackie et al., 2018). This is important during the progression of PD when peripheral immune cells are recruited to the site of DAergic degeneration as these immune cells could further reduce available DA levels via VMAT2 activity.
When DA stores are at capacity, DA can be inactivated through several metabolic pathways, including oxidative deamination by monoamine oxidase (MAO) or by O-methylation by catechol-O-methyltransferase (COMT), leading to the formation of two catabolic products, 3,4-dihydroxyphenylacetic acid and homovanillic acid, respectively (Meiser et al., 2013). It should be noted that COMT is predominantly expressed by glial cells, particularly in microglia (Meiser et al., 2013). In neurons, COMT is either missing or found at very low levels. MAO-B is predominantly found in astrocytes (Tong et al., 2017). MAO-B activity in astrocytes has been proposed as a potential biomarker for chronic neuroinflammation, as increased astrocytic MAO-B expression is associated with cellular ageing and age-related neurodegeneration (Schain and Kreisl, 2017). In humans, Bidart et al. (1983) discovered that both T and B lymphocytes exhibit COMT immunoreactivity, and Balsa and collaborators later confirmed MAO activity in human lymphocytes and granulocytes (Bidart et al., 1983; Balsa et al., 1989).
These results indicate that both glial and immune cells are capable of producing, inactivating and transporting DA, but that they do this independently of the neuronal system (Additional Table 1). Despite evidence demonstrating that most immune cells possess each of the elements required to synthesize, metabolize and store DA, further investigations are warranted (Cosentino et al., 1999; Anlauf et al., 2006; Wang et al., 2016; Gopinath et al., 2020). This is partly due to the fact that, for peripheral immune cells, most gene expression studies show transcript profiles in peripheral blood leukocytes or peripheral blood mononuclear cells and do not specify the exact cell type(s) that express these components. Also, within the CNS itself, these components are usually only examined in relation to DAergic neurons. Furthermore, studies are needed to unveil how these cells can be manipulated to promote the recovery of lost DA, which characterizes PD, or perhaps more generally to rescue normal DA signaling, as seen in mood and other psychiatric disorders (Boyd and Mailman, 2012; Felger, 2017; Figure 2).
Additional Table 1.
Non-neuronal expression of the machinery required for dopamine synthesis, storage, uptake and metabolism
| Cell type | Synthesis | Storage | Uptake | Metabolism |
|---|---|---|---|---|
| Microglia | Mastroeni et al., 2009; Morales et al., 2017 |
n/a | Mastroeni et al., 2009 | Redell et al., 2007; Meiser et al., 2013 |
| Astrocytes | Asanuma and Miyazaki, 2016; Morales et al., 2017 |
Petrelli et al., 2018; Matt and Gaskill, 2019 |
Morales et al., 2017; Matt and Gaskill, 2019 |
Bisaglia et al., 2013; Schain et al., 2017; Winner et al., 2017; Petrelli et al., 2018 |
| Monocyte | Gaskill et al., 2012; Mackie et a., 2018; Gopinath et al., 2020 |
Gaskill et al., 2012; Gopinath et al., 2020 |
Gaskill et al., 2012; Mackie et a., 2018; Gopinath et al., 2020 |
n/a |
| Macrophages | (Gaskill et al., 2012) | Gaskill et al., 2012 | Gaskill et al., 2012; Mackie, et al., 2018 |
n/a |
| T-lymphocytes | Musso et al., 1996; Cosentino, et al., 2006; Wang et al., 2016 |
Cosentino, et al., 2006; Amenta et al., 2001 |
Amenta et al., 2001 | Balsa et al., 1989 |
| B-lymphocytes | Talhada et al., 2018 | Pacheco et al., 2014 | n/a | Balsa et al., 1989 |
| Dendritic cells | Nakano et al., 2009; Prado et al., 2012; Pacheco et al., 2014 |
Nakano et al., 2009; Prado et al., 2012 |
Prado et al., 2012 | Prado et al., 2012 |
| Neutrophils | Cosentino et al., 1999 | Cosentino et al., 1999 | Cosentino et al., 1999 | Bidart et al., 1983 |
| Eosinophils | n/a | n/a | n/a | Bidart et al., 1983 |
| Mast cells | Ronneberg et al., 2012 | Anlauf et al., 2006 | n/a | n/a |
(COMT and/or MAO) of dopamine. Several non-neuronal cell types are able to produce, transport and metabolise dopamine in a manner independent of dopamine neurotransmission. COMT: Catechol-O-methyltransferase; DAT: DA transporter; MAO: monoamine oxidase; TH: tyrosine hydroxylase; VMAT2: vesicular monoamine transporter 2.
Dopamine Receptors in the Immune System
DA mediates its functions via interactions with one or more of the five DRs expressed on the membrane of target cells. A large body of evidence confirms the presence of DRs on specific immune cell types (Miyazaki et al., 2004; Rosin et al., 2005; Nakano et al., 2008; Mastroeni et al., 2009; Arce-Sillas et al., 2019; Matt and Gaskill, 2019). McKenna et al. (2002) conducted the first study aimed at identifying the expression of all five DR subtypes across multiple cell types. Peripheral blood leukocytes from healthy volunteers were investigated using flow cytometry and a panel of DR subtype-specific antibodies. They found that T-lymphocytes and monocytes exhibited low levels of DR expression, whereas neutrophils and eosinophils had moderate expression levels while B-lymphocytes and natural killer (NK) cells had higher and more consistent expression of DRs (McKenna et al., 2002). The cell-specific expression profile of each DR is summarized in Additional Table 2.
Additional Table 2.
Dopamine receptor expression in non-neuronal cells
| Cell type | DA receptors | Reference | ||||
|---|---|---|---|---|---|---|
| D1-like | D2-like | |||||
| D1R | D5R | D2R | D3R | D4R | ||
| Microglia | + | + | + | + | Mastroeni et al., 2009 | |
| # | # | # | # | |||
| Astrocytes | + | + | + | + | + | Miyazaki et al., 2004 |
| # | # | # | # | # | ||
| Oligodendrocytes | + | + | Rosin et al., 2005 | |||
| # | # | |||||
| Monocyte | + | + | + | + | + | Matt and Gaskill, 2019 |
| # | # | # | # | # | ||
| Macrophages | + | + | + | + | + | Gaskill et al., 2012 |
| T-lymphocytes | + | + | + | + | + | (Arce-Sillas et al., 2019) |
| # | # | # | # | # | ||
| B-lymphocytes | + | + | + | + | + | Arce-Sillas et al., 2019 |
| # | # | # | # | # | ||
| Dendritic cells | + | + | + | + | + | Nakano et al., 2008 |
| Neutrophils | + | + | + | + | + | McKenna et al., 2002 |
| # | # | # | # | # | ||
| Eosinophils | + | + | + | + | + | McKenna et al., 2002 |
| # | # | # | # | # | ||
| Natural killer (NK) cells | + | + | + | + | + | McKenna et al., 2002 |
| # | # | # | # | # | ||
Summary of evidence showing either protein (+) and/or mRNA (#) expression of each dopamine receptor subtype in non-neuronal cells.
DRs are also present on resident glia (microglia and astrocytes) and other non-neuronal CNS cell types, like oligodendrocytes and macrophages (Additional Table 2). DRs belong to the G protein-coupled receptor superfamily and have been divided into two main subclasses, D1-like and D2-like based on their ability to regulate cyclic adenosine monophosphate (cAMP) levels, with D1-like receptors increasing cAMP production and D2-like receptors inhibiting the formation of cAMP (Matt and Gaskill, 2019). D1-like receptors are coupled to Gαs protein that increases the intracellular concentration of the second messenger cAMP through the activation of adenylyl cyclase (AC) (Klein et al., 2019) (Figure 3). AC activates protein kinase A (PKA) and protein kinase C, which both promote the transcription of cAMP response element binding protein (CREB), favoring an anti-inflammatory environment (Wang et al., 2018). D2-like receptors, via coupling to Gαi/o decrease the levels of cAMP (Boyd and Mailman, 2012). D2-like receptors activate β-arrestin pathway which downstream stimulates Akt signaling which regulates inflammation through phosphoinositide 3-kinases-Akt and glycogen synthase kinase 3 beta targets.
Figure 3.
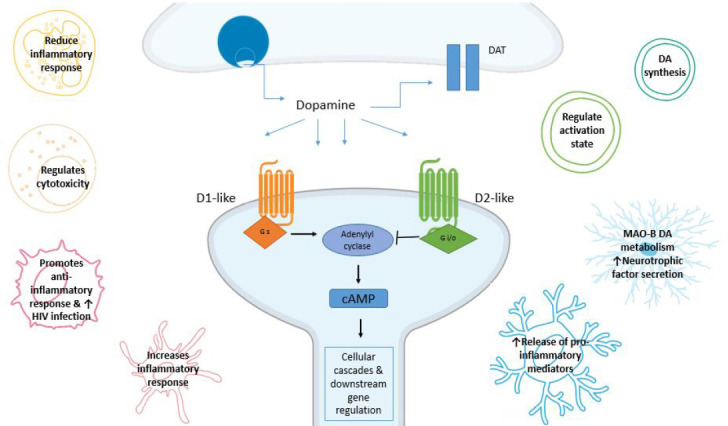
Dopamine receptor (DR) signaling pathways.
DRs belong to the G protein-coupled receptor (GPCR) superfamily and have been divided into two main subclasses, D1-like and D2- like based on their ability to regulate cAMP levels. This figure illustrates the beginning of the neuronal canonical signalling pathway with D1-like receptors activating adenylyl cyclase (AC) and increasing 3',5'-cyclic adenosine monophosphate (cAMP) concentration, while D2-like receptors inhibit AC and consequently reduce cAMP production. We also show how immune cells types have been shown to respond to dopamine (DA). However, limited research is available on how these responses occur. Cells represented include astrocytes (light blue), microglia (dark blue), T lymphocytes (cyan), B lymphocytes (green), neutrophils (yellow), macrophages (pink), dendritic cells (red) and natural killer cells (orange).
Both classes of DRs control the activity of classical pro-inflammatory pathways, including the nuclear translocation of the transcription factor-B (NF-κB) (Levite, 2016), with D2-like receptors promoting the transcription of CREB regulated proteins that promote neuronal survival and increase anti-inflammatory cytokines. The signalling of DRs is influenced by their different affinities for DA (Yang et al., 2020) and is further complicated by evidence showing the occurrence of an heterodimerization process, which recapitulates in a different pharmacological profile if compared with that of the monomers that constitute them (Maggio et al., 2009).
There is evidence suggesting that the expression of DRs on resident glial cells may be context specific, with an increase in DR expression during pathological stimuli (Rangel-Barajas et al., 2015; Talhada et al., 2018; Elgueta et al., 2019). As such, it is of no surprise that the abnormal DA signaling observed in PD and schizophrenia could be contributing not only to altered DA neurotransmission, but also to an altered immune response (Brito-Melo et al., 2012; Rangel-Barajas et al., 2015). Rangel-Barajas et al. (2015) showed that DRs display different affinities for DA. Studies of the effects of DA on the immune response have shown that both the bioavailability of DA and the expression of specific DR subtypes determines whether DA will trigger pro- or anti-inflammatory responses (Figure 3).
Furthermore, Pacheco (2017) deduced that low affinity DRs (i.e., D1R and D2R, respectively) are coupled to anti-ilammatory mechanisms, thereby DA binding to these receptors can dampen inflammation (Figure 4). Conversely, signaling generated by high-affinity DRs (D3R and D5R) have been found to promote inflammation (Pacheco, 2017) (Figure 4).
Figure 4.
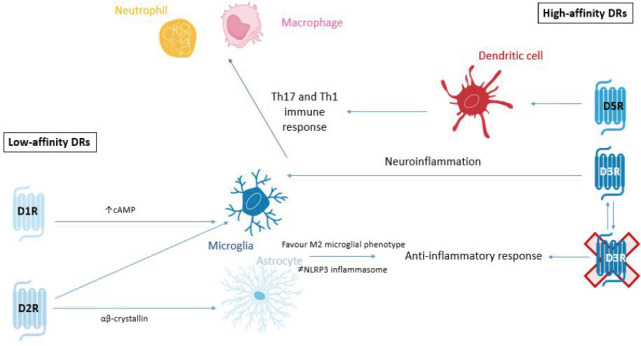
Summary of low-affinity and high-affinity actions of DRs.
In Parkinson's disease and PD models pro-inflammatory signaling has been shown to be mediated by high affinity D3 and D5 receptors. The RHS of the image shows the signaling of high-affinity receptors D3R and D5R. D3Rs on glial cells promote neuroinflammation but also secrete pro-inflammatory cytokines that activate Th17 and Th1 immune responses in CD4+ T cells which promotes the recruitment of neutrophils and macrophages to the inflammatory site. Furthermore, dopamine acting on D5R stimulates dendritic cells to acquire the Th17 pro-inflammatory phenotype. Moreover, in PD, it has been shown that D1 agonism promotes anti-inflammatory response through increase of cAMP and favoring of M2 microglia phenotype and inhibition of NLRP3 inflammasome. Also, D2 agonists acting on astrocytes and microglia inhibits NLRP3 inflammasome (via ab crystallin signaling in astrocytes). The inhibition of D3R has been shown to attenuate inflammation and subsequent degeneration in MPTP mouse models of PD. cAMP: Cyclic adenosine monophosphate; DR: dopamine receptor; MPTP: 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine; NLRP3: Nod-like receptor containing a pyrin domain 3; PD: Parkinson's disease.
Using PD as an example, we can better validate this concept. In a recent study, Elgueta et al. (2017) showed that D3R signalling promoted the development of PD by favouring neuroinflammation and the pathogenic CD4+ T cell response (Elgueta et al., 2017). Contreras and colleagues subsequently confirmed these findings demonstrating that D3Rs expressed on CD4+ T cells promote Th1 and Th17 mediated immunity. Th1 and Th17 mediated immunity stimulates the activation of pro-inflammatory pathways through the recruitment of neutrophils and macrophages to the inflammation site (Contreras et al., 2016). However, it was also shown that D2R-signalling in astrocytes promotes an anti-inflammatory response mediated by αβ-crystallin (Shao et al., 2013). In this study, they demonstrated that astrocytic D2R-deficiency exacerbated susceptibility to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurodegeneration and that wild-type mice treated with a D2R agonist displayed attenuated neurodegeneration of the nigrostriatal pathway (Shao et al., 2013). Additionally, in the MPTP model of PD in the mouse, D1R-signalling in microglial cells and astrocytes was shown to promote an anti-inflammatory effect, which was mediated by an increase in cAMP levels, ultimately promoting the degradation of the NLRP3 inflammasome (Yan et al., 2015).
Taken together, these studies suggest that high DA levels, such as those found in the nigrostriatal pathway under homeostatic conditions, would promote the stimulation of low-affinity DRs (D1R and D2R), thus inducing anti-inflammatory effects. Conversely, pathological conditions that cause depletion of DA levels, such as PD, would induce a selective stimulation of high-affinity DRs, specifically D3Rs, thereby triggering pro-inflammatory responses, establishing CNS inflammation and consequently, neurodegeneration (Vidal and Pacheco, 2019; Figure 4).
Dopamine-Mediated Neuroinflammation
DA is involved in the CNS-immune system interplay and in the autocrine/paracrine communication that exists between different immune cells. The effects of DA have been extensively studied in relation to the adaptive immune response and, in particular, how DA modulates T-lymphocyte activity (Sarkar et al., 2010; Levite, 2016). Comparatively little is known however, about how DA signaling regulates neuroinflammation and innate immunity. This is particularly important as evidence has shown that inflammatory responses from non-neuronal cells alone are sufficient to cause loss of DAergic neurons, as shown in studies of LPS-mediated neurotoxicity (Glass et al., 2010). Injection of LPS into the rodent brain results in increased levels of inflammatory mediators, including cyclooxygenase-2 and inducible nitric oxide synthetase (iNOS), prior to the loss of DAergic neurons (Hunter et al., 2007). This suggests that inflammation precedes neurodegeneration that ultimately results in PD.
We will take a closer look at evidence for each non-neuronal cell type to illustrate the complexity of DA’s immune regulation and highlight the significance of the role DA has on the immune response.
Dopamine and Innate Immunity
The innate immune response is the first line of defence and involves a fast, non-specific response. Innate immunity involves the coordinated responses of macrophages, natural killer (NK) cells, dendritic cells (DCs), neutrophils and more. We will focus on these four innate immune cell types as they are commonly found in the CNS following injury or in disease states.
Neutrophils
Neutrophils produce reactive oxygen species (ROS) and cytokines in response to pathogenic insult (Vermeren et al., 2018). As described above, McKenna et al. (2002) revealed the presence of all five DRs on neutrophils with D5R being the most abundantly expressed. In a mouse model of inflammation, DA agonists acting on neutrophils reduced their Th17-induced (pro-inflammatory) response (Nagatomo et al., 2017). It was also recently reported that L-DOPA treatment led to neutropenia in a patient with PD. This patient was shown to have reduced mRNA levels of all D2-like DRs and increased mRNA levels of TH and D5R (Mackie et al., 2018).
Macrophages
Macrophages have been described as the vacuum cleaners of the body due to their ability to engulf foreign and toxic substances. They clean up the inflammatory site removing anything that might be detrimental to the resident cells. DA suppresses the production of the pro-inflammatory interleukin-12 (IL-12) and promotes the secretion of IL-10, a key anti-inflammatory cytokine in macrophages in mice (Pinoli et al., 2017). In addition, it has been consistently shown that D2R promotes an increase in viral replication in human immunodeficiency virus (HIV)-infected macrophages (Gaskill et al., 2014). Recent studies have suggested a significant role of DA in regulating HIV-associated neurological disorders (HAND) (Gaskill et al., 2013).
Dendritic cells
Studies of DCs revealed they express the whole machinery to synthesise and store DA, which may act in an autocrine manner to stimulate DRs (Pacheco et al., 2014). A preclinical mouse model of multiple sclerosis (MS) has shown that stimulation of D5R on DCs exacerbates Th-17 driven experimental autoimmune encephalomyelitis (EAE) (Prado et al., 2012). A DA-mediated paracrine loop has been established between DCs and T cells (Nakano et al., 2011). DA stored in human DCs following its release acts on D1Rs present on naïve T cells to promote Th2 driven immunity. However, in the absence of DA, T cell differentiation shifts towards Th1 immunity (Sarkar et al., 2010). Nakano also suggested that since stimulation of cAMP increases DA concentration in DCs and because DA by acting through its D1Rs can increase cAMP concentration, it is possible that the released DA auto-regulates its synthesis in these cells by acting through D1Rs present in these cells (Nakano et al., 2008).
Natural killer cells
NK cells play a crucial role in the host-rejection of both tumors and virally infected cells. They act like cytotoxic T cells, destroying any cell that does not contain the “self” label. NK cells respond differently to DA depending on the specific DRs expressed on their membrane (Talhada et al., 2018). For example, activation of D1-like receptors with the agonist SFK-38393 enhanced NK cell cytotoxicity but activation of D2-like receptors with the agonist quinpirole attenuated NK cells cytotoxic functions (Talhada et al., 2018).
Dopamine and Adaptive Immunity
Data demonstrating the influence of DA on innate immunity, strongly supports the emerging view that the CNS and immune systems share signaling pathways previously thought to be system-specific. The adaptive immune response is usually activated when the innate immune response is insufficient to eliminate the pathogen and there is a slower, more targeted, antigen specific response involving T and B lymphocytes. T lymphocytes (T cells) are involved in the destruction and elimination of pathogens while B lymphocytes (B cells) provide antigen memory through the production of antibodies. Considerable work has been done by Talhada et al. (2018) and Pacheco et al. (2014) to dissect how dopamine regulates T-cell function, driven by the observations that T cells are altered in several disease pathologies including autoimmune diseases, several cancers and inflammatory disorders. This work highlights the importance of determining how DA might contribute to T-cell abnormalities and whether DA could itself be harnessed as a therapeutic target to reduce disease burden and severity.
T lymphocytes
The majority of studies investigating DA control of immunity has focused on T-cells. The complexity and context specificity of DA signaling is illustrated by the fact that the ultimate response of a T cell depends on the combined effects of DA concentration, DR sub-type expression, T-cell subtypes and T-cell activation state. For example, DA at physiological concentrations is able to evoke opposite functional responses in T cells. DA activates resting effector T cells (Teffs) resulting in their proliferation and cytokine production (Levite, 2016). However, if Teffs are already activated, DA will inhibit their function. Furthermore, D3R activation on human naïve T cells promotes inflammation through the selective secretion of tumour necrosis factor alpha (TNF-α), whereas, within the same population of cells, D2R activation selectively stimulates the release of IL-10, an anti-inflammatory cytokine (Talhada et al., 2018). These examples highlight the duplicity of DA’s actions in immune responses. Regulatory T cells are also capable of releasing high amounts of DA that acts in an autocrine DR-mediated manner to inhibit their suppressive activity (Pacheco et al., 2014).
B lymphocytes
B cells have been shown to produce DA which is upregulated upon mitogen induced activation, as shown by increased TH mRNA expression (Talhada et al., 2018). Additionally, intracellular vesicles containing DA in B cells are released in a calcium (Ca2+) dependent manner (Pacheco et al., 2014).
Studies investigating the role of the peripheral immune system on CNS disorders are becoming more predominant because the infiltration of peripheral immune cells is usually present at a more severe stage of the disease (Kempuraj et al., 2017). Since peripheral immune cells are recruited by neuroinflammatory processes that originate in the brain, it is important to determine whether DA secreted by resident microglia and astrocytes interacts with immune cells and contributes to the altered blood brain barrier and recruitment of the innate and adaptive immune systems.
Neuroinflammation
Central neuroinflammation refers to the inflammatory processes occurring within neural tissues of the CNS which are mediated by the production of cytokines, chemokines, reactive oxygen species and secondary messengers by microglia and astrocytes (DiSabato et al., 2016). Neuroinflammation has been repeatedly linked to most CNS pathologies characterized by abnormal DA signaling, including PD, schizophrenia and mood disorders (Teismann and Schulz, 2004; Fernandez-Egea et al., 2016; Felger, 2017). The chronic or sustained neuroinflammation seen in these diseases promotes the infiltration of peripheral immune cells, from both the adaptive and innate immune systems to the inflammatory site. It is critical to understand the role of DA in both glial and peripheral immune cells, as DA’s critical role both as a neurotransmitter and immune transmitter in these pathologies requires a careful balancing act when developing drug targets to ensure they have a beneficial action on both functions.
Microglia
Microglia are specialised scavengers of the CNS. When chronically activated, these cells become the main contributors to neuroinflammation, which in turn is responsible for the progression of neurodegeneration (Janda et al., 2018). It has been shown that human microglia are immunoreactive for all five DRs (McKenna et al., 2002). Microglia exist in four main phenotypic states in addition to the resting state, seen under homeostatic conditions which is referred to as the M0 phenotype. Under “activating” conditions, microglia can present with distinct phenotypes: M1 (classical activated, neurotoxic, pro-inflammatory) and M2a (alternatively activated, neuroprotective, anti-inflammatory) alternative type II activation (M2b), and acquired deactivation (M2c) (Zhang et al., 2018) (Figure 5). Importantly, it has been suggested that specific DR subtypes can influence whether microglia are either M1 or M2 activated, thereby controlling whether a pro- or anti-inflammatory response occurs. Strong support for this idea arises from several studies including data from Wang et al, (2019a) which showed that DA alters LPS-induced NO production in microglia via D1-like mediated AC/cAMP-PKA-ERL1/2-NF-κB-iNOS axis. Additionally, it has been shown that microglia activation was prevented in D3R deficient mice in an MPTP model of PD (Elgueta et al., 2017). Furthermore, the anti-PD drug rasagiline, a brain selective MAO inhibitor significantly reduced both ROS and NO secretion, as well as cytokine release in DA stimulated microglia. Collectively, these results suggest that DA can affect the ability of microglia to secrete cytokines, and importantly DR activation directs the shift towards specific microglial phenotypes. Since inflammation is associated with several DAergic pathologies, the ability of DA to determine the phenotype of microglia is very important to consider in attempting to understand the pathology of disease. Microglia are the key drivers of neuroinflammation and as such determining how DA can regulate these cells is crucial. For example, in PD it is well accepted that neuroinflammation drives progressive neurodegeneration. In fact, neuronal damage and uncontrolled inflammation amplify each other and induce a feed-forward cycle driving chronic progression of PD and other neurodegenerative diseases (Gao and Hong, 2008). These observations underpin the substantial experimental efforts in exploring the potential utility of traditional anti-inflammatories in slowing, reversing and preventing PD. However, due to the peripheral effects of long-term anti-inflammatory drugs use, novel targets are needed specifically to counteract CNS inflammation (Figure 5).
Figure 5.
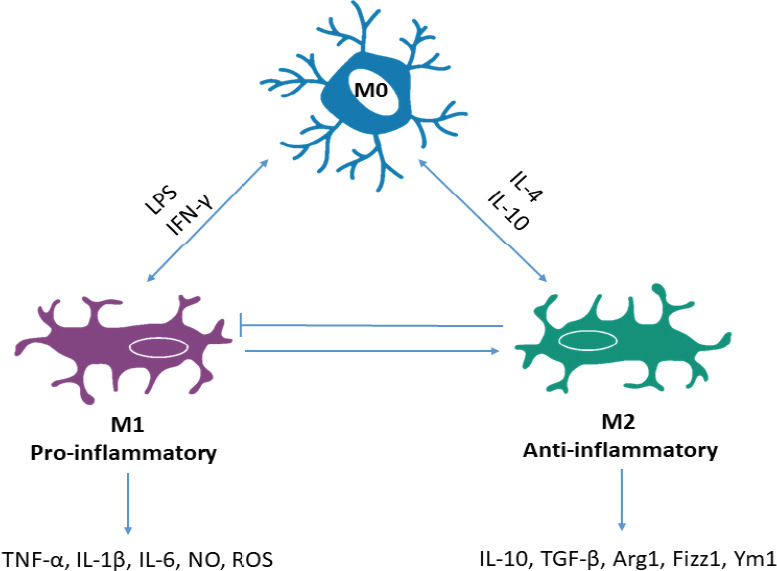
Microglia phenotypes.
Microglia exist in three main phenotypic states; resting microglia, seen under homeostatic conditions represent the M0 phenotype. Depending on the stimulus M0 microglia can be classically activated by LPS or IFN-γ and become M1 phenotype or M2 phenotype via alternate activation from IL-4 or IL-10. M1 represents a pro-inflammatory phenotype due to the production and secretion of inflammatory cytokines and mediators like TNF-α, IL-1β, IL-6, NO, ROS; whereas M2 are anti-inflammatory due to the production of anti-inflammatory cytokines and neuroprotective agents including IL-10, TGF-β, Arg1, Fizz1, Ym1. Furthermore, under homeostatic conditions, M1 microglia can promote M2 activity to maintain physiological levels of inflammation and M2 are able to block excess neurotoxic activity of M1. In chronic inflammation, this balance and autocrine control is lost and the M1 phenotype dominates creating a neurotoxic and pro-inflammatory environment. IFN-γ: Interferon- γ; IL: interleukin; LPS: lipopolysaccharide; ROS: reactive oxygen species; TGF-β: transforming growth factor-β; TNF-α: tumor necrosis factor-α.
Astrocytes
Astrocytes constitute the majority of the brain’s glial cells. Astrocytes are involved in the maintenance of homeostasis and promote neuronal survival by regulating metabolites, secreting neurotrophic factors and regulating blood flow (Guttenplan and Liddelow, 2019). The roles of astrocytes depends largely on the exchange of molecules from the extracellular space (Jennings et al., 2017). Cortical astrocytes stimulated with DA show a D1/5R mediated intracellular NADH increase, via PKA (Jennings et al., 2017). Stimulation with the selective D1 agonist SKF39383 promoted the release of nerve growth factor and glial cell-derived neurotrophic factor (GDNF), leading to the suggestion that DA activates astrocytes to promote the secretion of neuroprotective mediators. Parallel studies by Shao and colleagues showed that D2R-signalling in astrocytes promotes anti-inflammatory responses that appear to be mediated by αβ-crystallin (Shao et al., 2013). Activation of D2-like receptors has been linked to increases in brain-derived neurotrophic factor, GDNF mRNA expression and protein synthesis as well as suppression of CRYAB mediated neuroinflammation in vivo (Zhang et al., 2015). The role of astrocytes in neuroinflammation is often overlooked, as instead of producing neurotoxic factors like microglia, an important role of these cells is to provide neurotrophic factors that promote neuronal survival. As most strategies to reduce neuroinflammation focus on reducing microglia activity, the neurotrophic support from astrocytes is still required to help repair and promote the recovery of damaged neurons. On the basis of the data summarized here, we show that DA has the ability to increase the secretion of these neurotrophic factors, including GDNF and brain-derived neurotrophic factor. GDNF has long been considered a potential therapy for PD, and experimental studies suggest that GDNF has the ability to protect degenerating DA-neurons in PD as well as promote the regeneration of the nigrostriatal DA system (Hong et al., 2008). The ability to innately stimulate astrocyte release of GDNF could prove a useful therapeutic approach to PD as the fact that GDNF cannot cross the blood-brain barrier, is hindering current clinical trials with this agent. With this idea, broadening the approach to DA and neuroinflammation could provide new targets and approaches to several pathologies that are currently being overlooked.
Oligodendrocytes
Although not traditionally involved in inflammation, oligodendrocytes are another non-neuronal cell type that plays a major role in several CNS diseases like MS. Interestingly, D2R and D3R mRNA and immune-reactivities have been detected in cortical oligodendrocytes (Rosin et al., 2005). Evidence also suggests that D2R and D3R agonists are capable of protecting cultured rat cortical oligodendrocytes from oxidative glutamate toxicity and combined oxygen/glucose deprivation injury (Rosin et al., 2005), suggesting a protective role of these receptors. Although little is known about the role of oligodendrocytes in inflammation, recent studies have indicated key roles of these cells in several inflammatory processes, which are clearly seen in models of MS. Despite being an autoimmune disorder, the role of DA in the pathogenesis of MS has been revealed, highlighting the broad function of DA as an immune transmitter in its ability to regulate inflammation both within the brain and in the periphery.
Dopamine and Autoimmunity
DA levels are altered in the brain of mouse models recapitulating two well-known autoimmune conditions: MS and systemic lupus erythematosus (SLE). The altered expression of DRs in peripheral lymphocytes of MS and SLE patients also supports the importance of DAergic regulation in autoimmunity.
MS is a disease characterised by CD4+ T cell mediated progressive loss of neurological function, likely due to the destruction of axonal myelin sheath (Ferreira et al., 2014). T cells and DCs have consistently been shown to promote disease pathogenesis in both animal models and MS patients. DCs are capable of promoting the differentiation of CD4+ T cells towards the inflammatory Th17 phenotype, corresponding to evidence previously stated showing DA derived from DCs regulating T cell phenotype (Prado et al., 2012). Using an EAE model of MS, combined with MPTP administration, it was shown that MPTP-driven DA depletion in the CNS prior to MS pathology induction exacerbates the MS presentation (Balkowiec-Iskra et al., 2007). In fact, at both the onset and peak of EAE disease, an increase in striatal DA was observed, which was accompanied by a similar increase in striatal IL-1β and TNF-α mRNA expression (Pacheco et al., 2014). Although these findings require further assessment, the results obtained point to a critical role for DA in regulating the autoimmune response. In other studies, the D1R-like antagonist SCH-23390 prevented the development of EAE (Nakano et al., 2008). Patients with MS treated with interferon-β show reduced levels of D5Rs and TH expressed on Tregs and abolished DA-mediated inhibition of the suppressive activity of Tregs, which promotes disease progression (Pacheco et al., 2014). These observations link the DAergic system with the development and progression of MS. The role of DA in MS also suggests that DA could play a role in other autoimmune diseases.
SLE is an autoimmune disease of variable and unpredictable disease pathology (Jafari et al., 2013). SLE is characterized by several abnormalities of the cellular immune system, including a loss of B cell tolerance and the production of pathogenic autoantibodies (Ikenouchi-Sugita et al., 2008). Neurologic manifestations appear in between 18% and 67% of patients with SLE and are associated with inflammatory features in the brain (Ikenouchi-Sugita et al., 2008). Neurological manifestations include a range of CNS disorders like anxiety, depression and cognitive dysfunction (Muscal and Brey, 2010). Jafari et al. (2013) have shown the abnormal expression of D2R and D4R in T cells of SLE patients. They have suggested that these abnormalities contribute to SLE pathogenesis through the regulation of T cell function. They have proposed that T cell expression of D4R could provide a therapeutic target for SLE as it could be utilized to trigger T cell quiescence. The role of DA in SLE is demonstrated further in the spontaneous development of lupus-like disease in MRL-lpr mice which is accompanied by impaired DA catabolism and the degeneration of DAergic axon terminals in the midbrain (Jara et al., 2017). Notably, DA analogues have shown therapeutic effects in animal models and patients with lupus, for example, treatment with bromocriptine, a D2R/D3R agonist, resulted in a significant reduction of mean flares/patient and serum prolactin levels in SLE patients (Jara et al., 2017).
The evidence that DA plays an important role in modulating the pathogenesis of autoimmune disorders such as MS and lupus supports its role as an immune transmitter in addition to its canonical neurotransmitter role. In addition, it exemplifies the broad functions of DA in immunity that could be utilised as novel therapeutics for a wide range of diseases, extending beyond the traditional DAergic pathologies.
Dopamine and Parkinson’s Disease
The most well-known DAergic pathology is PD. PD is also the most common neurodegenerative movement disorder, characterized by the degeneration of neurons in the SnPC, resulting in a loss of DA availability in the striatum and associated pathways (i.e., the degeneration of the nigrostriatal pathway) (Glass et al., 2010). The nigrostriatal pathway is responsible for the control of movement, leading to the cardinal motor symptoms of PD, which include bradykinesia, resting tremor, rigidity and postural instability (Glass et al., 2010). PD is also associated with a myriad of non-motor symptoms such as olfactory deficits, autonomic dysfunction, pain, depression, cognitive deficits and sleep disorders, associated with the alteration of other DAergic pathways and non-DAergic signaling (Glass et al., 2010). The aetiology of PD remains unknown; however, several factors are thought to contribute to the pathogenesis of PD including genetics, environmental factors, oxidative stress and neuroinflammation.
The traditional PD therapeutics aim to replenish DA in the striatum either through L-DOPA (the precursor to DA) or via DA agonists (mainly D2 and D3). Since both the expression of DRs on the surface on microglia, astrocytes and lymphocytes have been shown (Arce-Sillas et al., 2019) along with their altered expression in post-mortem brains of PD patients, current evidence suggests that both glial cells and lymphocytes could respond to DA-based PD therapeutics as well (Gelders et al., 2018).
It has been suggested that D2Rs are the dominant receptors found in the checkpoint stations of the nigrostriatal pathway (ventral tegmental area and striatum). These receptors are thought to promote an anti-inflammatory phenotype in immune cells and glia (microglia and astrocytes) (Rangel-Barajas et al., 2015). As DAergic signaling is lost during PD, the reduction of activity at D2Rs likely favours a pro-inflammatory environment, a suggestion supported by the observation that quinpirole, a D2R specific agonist, suppressed neuroinflammation and protected against brain injury in a mouse model of PD (Zhang et al., 2015).
Conversely, it has been suggested that D3Rs promote a pro-inflammatory environment, as was discussed above, pharmacologic D3R antagonism was shown to be protective against MPTP-induced neurodegeneration and motor impairments (Elgueta et al., 2017). These observations are important to consider as PD therapeutics aim primarily to replace DA in the brain via administration of L-DOPA, the precursor to DA in the biosynthetic pathway (Figure 2). It is therefore possible that the increased availability of DA will re-activate D2R signalling and restore the balance to DA induced inflammation. However, L-DOPA induced dyskinesia, a disabling side effect of long-term L-DOPA treatment, is often associated with a dramatic increase in striatal neuroinflammation when compared to L-DOPA treated non-dyskinetic PD patients and controls (Carta et al., 2017).
Furthermore, DA agonists commonly used in PD are D2-like agonists that act on both D2R and D3Rs. For example, common DA agonists used in PD management include:
Pramipexole, a D3R-preferring agonist, with high selectivity for D2R (Collo et al., 2018);
Ropinirole, a D2R, D3R and D4R agonist (Cuevas et al., 2013);
Apomorphine, another D2R agonist (Borovac, 2016).
As these agonists are capable of activating both D2R and D3Rs, and given that in the presence of sufficient DA levels D2R abundance is by far higher than D3R, it is possible that the mechanism of action of these drugs might primarily involve the activation of D2R-driven anti-inflammatory activities and not D3Rs.
Furthermore, since DRs are ubiquitously expressed throughout the brain, it is important to target specific areas or cell types to minimize the damage to other neuronal functions. For example, blockade of D3R can reduce sensitivity to anxiety medication, a common co-morbidity for DAergic pathologies, including PD, but also reduce ethanol related reward-associated voluntary consumption (Leggio et al., 2014, 2015, 2019). Therefore, it is important to determine how DRs are regulated not only in different cell types but throughout different brain regions.
Adding another layer of complexity are the conflicting reports on the role of DA and each DA receptor subtype in immunity. This is partly due to the lack of highly selective drugs that are able to target specific receptor subtypes. However, Yang and colleagues have discussed the inconsistencies in studies on the D3R alone with evidence revealing that activation of the D3R can both promote and attenuate neuroinflammation (Yang et al., 2020). As we have highlighted in this review, the cross-talk between the DAergic and immune system is complex, as several factors contribute to regulate cells’ responsiveness to DA, including the abundance of DA in the extracellular space, DR expression, target cell type and the activation state of the target cell. It is important to control for these factors when investigating the role of DA as the neurotransmitter, its related receptors and/or metabolism components could be used as clinical biomarkers and/or therapeutic targets. For example, there is an association between CD4+ T cell DR expression and the degree of motor dysfunction, as assessed by Unified Parkinson’s Disease Rating Scale score. In fact, within the total CD4+ T cell population D1R expression decreased while in T memory cells D2R expression increased with increasing disease score (Kustrimovic et al., 2016). This suggests the DR expression levels in T cells could be used as a useful prognostic biomarker for PD patients. The ability to monitor PD through a routine blood test would present an enormous advantage as currently clinicians are restricted to qualitative clinical scoring, neuroimaging and a comprehensive medical history.
Dopamine, Parkinson’s disease and oxidative stress
It is well known that oxidative stress contributes to the pathogenesis of neurodegenerative disease, particularly PD. DAergic neurons are more susceptible to oxidative stress due to the fact that they are long-projecting neurons with poorly myelinated axons that provide extensive synaptic innervations. In addition, DAergic neurons have autonomous pacemaker activity, resulting in high energy requirements, and DA and its metabolites containing 2-hydroxyl residues generate highly reactive DA and DOPA quinones (Miyazaki and Asanuma, 2008). Consequently, DA neurons require efficient mechanisms to maintain neuron functioning and prevent the accumulation of ROS, damaged/misfolded proteins and regenerate essential survival components (Krashia et al., 2019). Additionally, DA quinones have been linked to mitochondrial dysfunction, inflammation and oxidative stress. This inherent highly oxidative environment means that, when there is excess DA or ineffective DA metabolism, ROS can accumulate creating oxidative stress in neurons. This in turn activates local immune cells, microglia and astrocytes, to secrete neurotrophic factors and cytokines to help reduce oxidative damage. However, in a disease like PD, we know that this sequence of events creates a vicious cycle whereby DA neurons produce more ROS, and glial cells keep secreting cytokines, leading to chronic neuroinflammation and the establishment of a self-propagating cycle that is ultimately detrimental to DAergic neurons. This cycle is also seen in other neurodegenerative diseases like Alzheimer’s disease (AD).
Dopamine and Alzheimer’s Disease
AD is the most common neurodegenerative disorder that is characterized by the presence of extracellular amyloid plaques, intracellular neurofibrillary tangles and hyper phosphorylated tau, neuronal loss, oxidative stress and neuroinflammation, primarily in the cortex and hippocampus (Serrano-Pozo et al., 2011).
The DA system has for long been associated with aging, with a decline in motor function associated with DA changes in the substantia nigra and striatum being a robust hallmark of aging (Rollo, 2009). One study revealed a marked increase in microglial reactivity in the SNpc in elderly humans (~81 years), which was reflected by high degree of neuronal death in these areas (Rollo, 2009). This is due to the higher susceptibility of DA neurons to oxidative stress, which inherently tends to increase with age.
As such, it is not surprising that there is some degree of overlap between the pathophysiology of AD and PD, as both diseases have been shown to correlate with increased levels of oxidative stress, abnormal DA regulation and evidence of neuroinflammation. This higher susceptibility to both oxidative stress and neuroinflammation may also be a contributing factor in the pathogenesis of AD, with abnormal DA signaling and expression seen in the mesolimbic pathway of AD patients, however the role of DA in AD pathogenesis remains to be elucidated (Nobili et al., 2017). The mesolimbic DAergic pathway consists of neurons arising from the ventral tegmental area which project predominantly to the prefrontal cortex, hippocampus and nucleus accumbens (Adinoff, 2004). The degeneration of DA neurons in this pathway correlates with impairments in memory and reward performance (Nobili et al., 2017). Consistently, pharmacological treatment to increase DAergic transmission improved cognitive impairment and memory deficits in AD patients and experimental models, emphasizing the critical role of DA in AD (Krashia et al., 2019).
For example, it has been shown that D2R in the hippocampus correlates with memory functioning in AD (Donthamsetti et al., 2018). Koch et al. (2014) also demonstrated that D2R/D3R agonists may restore cortical plasticity in AD patients, which is consistent with findings in post-mortem brains of AD patients showing that there is a significant decrease in D2-like receptors in the hippocampus and prefrontal cortex.
Moreover, additional ex vivo studies in AD patients have demonstrated that lymphocytes had a low density of D2-like receptors compared to controls (Arreola et al., 2016). These results suggest that as in PD, DA modulates the immune response in AD as well. This could contribute to the neuroinflammation and abnormal DA neurotransmission observed in AD patients.
Dopamine and Drug Abuse
DA plays a critical role in movement, memory, cognition and emotion (Yang et al., 2020). Due to its role in controlling operant reinforcement, incentive salience and its ability to trigger positively-valenced emotions via the reward system, DA has been implicated in drug abuse and addiction.
As we have seen in PD and AD, DA availability influences how immune cells respond to DA, which is evident not only in neurodegenerative diseases associated with reductions in DA, but also in situations where there is an excess of DA, as seen in drug abuse. One of the most commonly abused drugs is methamphetamine (METH), which remains a significant public health concern worldwide (Krasnova et al., 2016). METH is a known powerful modulator of DA and has several effects that influence DA release, including disrupting DA reuptake and packaging through DAT and VMAT2 (Hedges et al., 2018). The mechanism of METH ultimately results in excess extracellular release of DA.
Interestingly, METH has been shown to promote neuroinflammation through DRs. Wang et al. (2019b) have shown that METH exposure increased LPS induced pro-inflammatory cytokine production in the hippocampus, promoted microglia activation and increased the expression of D1-like receptors. These effects were inhibited after treatment with SCH-23390 (a D1-like receptor antagonist) and minocycline (microglial inhibitor) (Wang et al., 2019b), suggesting that this response was specific to DRs expressed by microglia. Furthermore, this study illustrated that D1-like receptor mediated increase in inflammation triggered by METH, involved ERK1/2 phosphorylation and activation of the CREB signalling cascade (Wang et al., 2019b). Another study that utilised a rat model of METH self-administration, revealed changes in the brain consistent with clinical findings in METH addicts (Krasnova et al., 2016). Analysis of striatal brain tissue showed increased expression of genes involved in CREB signalling and the activation of neuroinflammatory responses (Krasnova et al., 2016). Kitamura et al. (2010) and colleagues provided further support for the suggestion that METH neurotoxicity is mediated by microglia by showing that the activation of microglia accompanies METH neurotoxicity and that inhibition of microglial activation reduced dopamine depletion and restored DAT levels.
The consistency of the conclusions of these studies suggests a role of DA in the pathology of drug abuse and addictive behaviour. In addition, a number of researchers have suggested that neuroinflammation in the striatum underlies the cognitive deficits, depression and parkinsonian symptoms reported in METH addicts. This hypothesis could form the basis of a unifying theory of DA as a major contributor to several CNS pathologies.
Dopamine and Human Immunodeficiency Virus-1-Associated Neurological Disorders
HIV remains a major global health concern with an estimated 35 million people living with HIV (Zhu et al., 2018). Despite the widespread use of efficacious antiretroviral therapies to control peripheral infection more that 50% of HIV-positive individuals will suffer from neurological complications, otherwise known as HAND (McArthur and Smith, 2013). In HIV infected individuals, substance abuse may accelerate the development and/or increase the severity of HAND as studies have shown that altered DA signaling is a risk factor for HAND with most patients presenting with cognitive, memory, motor and behavioral deficits (Zhu et al., 2018).
Typically, HIV is unable to cross the blood-brain barrier alone. However, it has been proposed that CD14+ CD16+ monocytes are able to mediate the entry of HIV into the brain and that the migration of both infected and uninfected monocytes across the blood-brain barrier contributes to the establishment and propagation of CNS HIV viral reservoirs and chronic neuroinflammation, which are essential in the development of HAND. Since drug use elevates DA, and the fact that HIV-positive people tend to fall into drug use more frequently than the healthy population, Calderon and colleagues examined the effects of DA on monocytes and HIV infection. They found that the CNS migration of the CD14+ CD16+ monocyte subpopulation is increased by DA and D1-like receptor agonist SKF-38393, suggesting that the heightened migratory capacity of monocytes is mediated by D1-like receptors (Calderon et al., 2017).
Additionally, Nickoloff-Bybel et al. (2019) showed that DA increases HIV entry into macrophages via a calcium-dependent mechanism, revealing that D1-like receptor activation on infected macrophages stimulated calcium and protein kinase C activation. Since we know that other immune cells are also activated by DA, we can assume that high concentrations of DA can prime the CNS for further HIV viral infection, which not only increases viral reservoirs within the brain, but also contributes to HAND pathologies.
Conclusion
Despite strong evidence showing the expression of DA, its binding receptors and the components necessary to synthesize, store and metabolize DA in non-neuronal cells, the exact significance of these findings in the context of PD pathogenesis remains to be fully determined. Several studies have highlighted the importance of DA in regulating autoimmune diseases, such as MS and SLE, and many others have examined how DA contributes to the control of T lymphocyte functionality in disease states. Furthermore, studies in AD, drug abuse, HIV infection and HAND emphasize the myriad of roles DA has within the brain. The evidence presented in this review calls attention to the robust and extensive ways in which DA can control immunity and the important need for a greater understanding of this novel function of DA (Figure 6).
Figure 6.
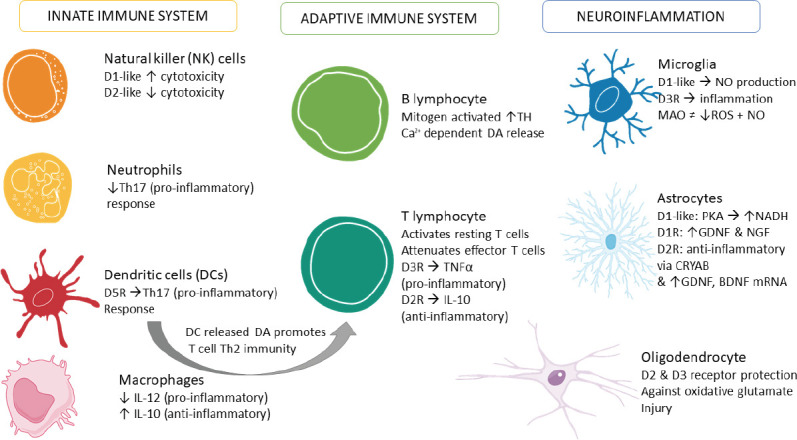
Summary of immune cells responses to dopamine.
This figure summarizes the evidence presented in this review. BDNF: Brain-derived neurotrophic factor; CRYAB: crystallin Alpha B; D2R: dopamine D2 receptor; D3R: dopamine D3 receptor; GDNF: glial cell-derived neurotrophic factor; IL: interleukin; MAO: monoamine oxidase inhibitor; NADH: nicotinamide adenine dinucleotide (NAD) + hydrogen (H); NGF: nerve growth factor; NO: nitric oxide; PKA: protein kinase A; ROS: reactive oxygen species; Th2: T helper 2; Th17: T helper 17.
It is well-accepted that microglia and, to a greater extent, astrocytes, are responsible for regulating the blood brain barrier function to either promote or reduce the permeability to peripheral leukocytes. Further studies are necessary to unveil DA’s regulatory activity on non-neuronal cells, especially microglia and astrocytes, as these cells are commonly altered in several CNS disease pathologies, including PD.
Furthermore, the emergence of DA as an immune transmitter may have future implications for our understanding of brain physiology, but also in the clinical management of neurological diseases, as it presents a wide array of new therapeutic and prognostic targets for CNS pathologies (Figure 6).
Additional files:
Additional Table 1: Non-neuronal expression of the machinery required for dopamine synthesis, storage, uptake and metabolism.
Additional Table 2: Dopamine receptor expression in non-neuronal cells.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was partially supported by a Research Development Fund (UTS Start-Up Grant 2018) from the University of Technology Sydney to AC.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was partially supported by a Research Development Fund (UTS Start-Up Grant 2018) from the University of Technology Sydney to AC.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amenta F, Bronzetti E, Cantalamessa F, El-Assouad D, Felici L, Ricci A, Tayebati SK. Identification of dopamine plasma membrane and vesicular transporters in human peripheral blood lymphocytes. J Neuroimmunol. 2001;117:133–142. doi: 10.1016/s0165-5728(01)00317-4. [DOI] [PubMed] [Google Scholar]
- 3.Anlauf M, Schafer MK, Schwark T, von Wurmb-Schwark N, Brand V, Sipos B, Horny HP, Parwaresch R, Hartschuh W, Eiden LE, Kloppel G, Weihe E. Vesicular monoamine transporter 2 (VMAT2) expression in hematopoietic cells and in patients with systemic mastocytosis. J Histochem Cytochem. 2006;54:201–213. doi: 10.1369/jhc.5A6739.2005. [DOI] [PubMed] [Google Scholar]
- 4.Arce-Sillas A, Sevilla-Reyes E, Alvarez-Luquin DD, Guevara-Salinas A, Boll MC, Perez-Correa CA, Vivas-Almazan AV, Rodriguez-Ortiz U, Castellanos Barba C, Hernandez M, Fragoso G, Sciutto E, Cardenas G, Adalid-Peralta LV. Expression of dopamine receptors in immune regulatory cells. Neuroimmunomodulation. 2019;26:159–166. doi: 10.1159/000501187. [DOI] [PubMed] [Google Scholar]
- 5.Arreola R, Alvarez-Herrera S, Perez-Sanchez G, Becerril-Villanueva E, Cruz-Fuentes C, Flores-Gutierrez EO, Garces-Alvarez ME, de la Cruz-Aguilera DL, Medina-Rivero E, Hurtado-Alvarado G, Quintero-Fabian S, Pavon L. Immunomodulatory effects mediated by dopamine. J Immunol Res. 2016;2016:3160486. doi: 10.1155/2016/3160486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asanuma M, Miyazaki I. 3-O-Methyldopa inhibits astrocyte-mediated dopaminergic neuroprotective effects of L-DOPA. BMC Neurosci. 2016;17:52. doi: 10.1186/s12868-016-0289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asanuma M, Miyazaki I, Murakami S, Diaz-Corrales FJ, Ogawa N. Striatal astrocytes act as a reservoir for L-DOPA. PLoS One. 2014;9:e106362. doi: 10.1371/journal.pone.0106362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balkowiec-Iskra E, Kurkowska-Jastrzebska I, Joniec I, Ciesielska A, Muszynska A, Przybylkowski A, Czlonkowska A, Czlonkowski A. MPTP-induced central dopamine depletion exacerbates experimental autoimmune encephalomyelitis (EAE) in C57BL mice. Inflamm Res. 2007;56:311–317. doi: 10.1007/s00011-007-6128-0. [DOI] [PubMed] [Google Scholar]
- 9.Balsa MD, Gómez N, Unzeta M. Characterization of monoamine oxidase activity present in human granulocytes and lymphocytes. Biochim Biophys Acta. 1989;992:140–144. doi: 10.1016/0304-4165(89)90002-0. [DOI] [PubMed] [Google Scholar]
- 10.Bidart JM, Motte P, Assicot M, Bohuon C, Bellet D. Catechol-O-methyltransferase activity and aminergic binding sites distribution in human peripheral blood lymphocyte subpopulations. Clin Immunol Immunopathol. 1983;26:1–9. doi: 10.1016/0090-1229(83)90167-8. [DOI] [PubMed] [Google Scholar]
- 11.Bisaglia M, Greggio E, Beltramini M, Bubacco L. Dysfunction of dopamine homeostasis: clues in the hunt for novel Parkinson’s disease therapies. FASEB J. 2013;27:2101–2110. doi: 10.1096/fj.12-226852. [DOI] [PubMed] [Google Scholar]
- 12.Borovac J. Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016;89:37–47. [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd KN, Mailman RB. Dopamine receptor signaling and current and future antipsychotic drugs. Handb Exp Pharmacol. 2012;212:53–86. doi: 10.1007/978-3-642-25761-2_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brito-Melo GE, Nicolato R, de Oliveira AC, Menezes GB, Lelis FJ, Avelar RS, Sa J, Bauer ME, Souza BR, Teixeira AL, Reis HJ. Increase in dopaminergic, but not serotoninergic, receptors in T-cells as a marker for schizophrenia severity. J Psychiatr Res. 2012;46:738–742. doi: 10.1016/j.jpsychires.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Calderon TM, Williams DW, Lopez L, Eugenin EA, Cheney L, Gaskill PJ, Veenstra M, Anastos K, Morgello S, Berman JW. Dopamine increases CD14(+)CD16(+) monocyte transmigration across the blood brain barrier: implications for substance abuse and HIV neuropathogenesis. J Neuroimmune Pharmacol. 2017;12:353–370. doi: 10.1007/s11481-017-9726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carta AR, Mulas G, Bortolanza M, Duarte T, Pillai E, Fisone G, Vozari RR, Del-Bel E. l-DOPA-induced dyskinesia and neuroinflammation: do microglia and astrocytes play a role. Eur J Neurosci. 2017;45:73–91. doi: 10.1111/ejn.13482. [DOI] [PubMed] [Google Scholar]
- 17.Castorina A, D’Amico AG, Scuderi S, Leggio GM, Drago F, D’Agata V. Dopamine D3 receptor deletion increases tissue plasminogen activator (tPA) activity in prefrontal cortex and hippocampus. Neuroscience. 2013;250:546–556. doi: 10.1016/j.neuroscience.2013.07.053. [DOI] [PubMed] [Google Scholar]
- 18.Collo G, Cavalleri L, Bono F, Mora C, Fedele S, Invernizzi RW, Gennarelli M, Piovani G, Kunath T, Millan MJ, Merlo Pich E, Spano P. Ropinirole and pramipexole promote structural plasticity in human iPSC-derived dopaminergic neurons via BDNF and mTOR signaling. Neural Plast. 2018;2018:4196961. doi: 10.1155/2018/4196961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Contreras F, Prado C, Gonzalez H, Franz D, Osorio-Barrios F, Osorio F, Ugalde V, Lopez E, Elgueta D, Figueroa A, Lladser A, Pacheco R. Dopamine receptor D3 signaling on CD4+ T cells favors Th1- and Th17-mediated immunity. J Immunol. 2016;196:4143–4149. doi: 10.4049/jimmunol.1502420. [DOI] [PubMed] [Google Scholar]
- 20.Cosentino M, Marino F, Bombelli R, Ferrari M, Lecchini S, Frigo G. Endogenous catecholamine synthesis, metabolism, storage and uptake in human neutrophils. Life Sci. 1999;64:975–981. doi: 10.1016/s0024-3205(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 21.Cosentino M, Bombelli R, Ferrari M, Marino F, Rasini E, Maestroni GJM, Conti A, Boveri M, Lecchini S, Frigo G. HPLC-ED measurement of endogenous catecholamines in human immune cells and hematopoietic cell lines. Life Sci. 2000;68:283–295. doi: 10.1016/s0024-3205(00)00937-1. [DOI] [PubMed] [Google Scholar]
- 22.Cuevas S, Villar VA, Jose PA, Armando I. Renal dopamine receptors, oxidative stress, and hypertension. Int J Mol Sci. 2013;14:17553–17572. doi: 10.3390/ijms140917553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Amico AG, Castorina A, Leggio GM, Drago F, D’Agata V. Hippocampal neurofibromin and amyloid precursor protein expression in dopamine D3 receptor knock-out mice following passive avoidance conditioning. Neurochem Res. 2013;38:564–572. doi: 10.1007/s11064-012-0949-0. [DOI] [PubMed] [Google Scholar]
- 24.DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139:136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donthamsetti P, Gallo EF, Buck DC, Stahl EL, Zhu Y, Lane JR, Bohn LM, Neve KA, Kellendonk C, Javitch JA. Arrestin recruitment to dopamine D2 receptor mediates locomotion but not incentive motivation. Mol Psychiatry. 2018 doi: 10.1038/s41380-018-0212-4. doi: 101038/s41380-018-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elgueta D, Aymerich MS, Contreras F, Montoya A, Celorrio M, Rojo-Bustamante E, Riquelme E, Gonzalez H, Vasquez M, Franco R, Pacheco R. Pharmacologic antagonism of dopamine receptor D3 attenuates neurodegeneration and motor impairment in a mouse model of Parkinson’s disease. Neuropharmacology. 2017;113:110–123. doi: 10.1016/j.neuropharm.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 27.Elgueta D, Contreras F, Prado C, Montoya A, Ugalde V, Chovar O, Villagra R, Henriquez C, Abellanas MA, Aymerich MS, Franco R, Pacheco R. Dopamine receptor D3 expression is altered in CD4(+) T-cells from Parkinson’s disease patients and its pharmacologic inhibition attenuates the motor impairment in a mouse model. Front Immunol. 2019;10:981. doi: 10.3389/fimmu.2019.00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felger JC. The role of dopamine in inflammation-associated depression: mechanisms and therapeutic implications. Curr Top Behav Neurosci. 2017;31:199–219. doi: 10.1007/7854_2016_13. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Egea E, Vertes PE, Flint SM, Turner L, Mustafa S, Hatton A, Smith KG, Lyons PA, Bullmore ET. Peripheral immune cell populations associated with cognitive deficits and negative symptoms of treatment-resistant schizophrenia. PLoS One. 2016;11:e0155631. doi: 10.1371/journal.pone.0155631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira TB, Barros PO, Teixeira B, Cassano T, Centuriao N, Kasahara TM, Hygino J, Vasconcelos CC, Filho HA, Alvarenga R, Wing AC, Andrade RM, Andrade AF, Bento CA. Dopamine favors expansion of glucocorticoid-resistant IL-17-producing T cells in multiple sclerosis. Brain Behav Immun. 2014;41:182–190. doi: 10.1016/j.bbi.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaskill PJ, Carvallo L, Eugenin EA, Berman JW. Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. J Neuroinflammation. 2012;9:203. doi: 10.1186/1742-2094-9-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaskill PJ, Calderon TM, Coley JS, Berman JW. Drug induced increases in CNS dopamine alter monocyte, macrophage and T cell functions: implications for HAND. J Neuroimmune Pharmacol. 2013;8:621–642. doi: 10.1007/s11481-013-9443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaskill PJ, Yano HH, Kalpana GV, Javitch JA, Berman JW. Dopamine receptor activation increases HIV entry into primary human macrophages. 2014 doi: 10.1371/journal.pone.0108232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelders G, Baekelandt V, Van der Perren A. Linking neuroinflammation and neurodegeneration in Parkinson’s disease. J Immunol Res. 2018;2018:4784268. doi: 10.1155/2018/4784268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gopinath A, Doty A, Mackie PM, Hashimi B, Francis M, Saadatpour L, Saha K, Shaw G, Ramirez-Zamora A, Okun MS, Streit WJ, Khoshbouei H. A novel approach to study markers of dopamine signaling in peripheral immune cells. J Immunol Methods. 2020;476:112686. doi: 10.1016/j.jim.2019.112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guttenplan KA, Liddelow SA. Astrocytes and microglia: Models and tools. J Exp Med. 2019;216:71–83. doi: 10.1084/jem.20180200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedges DM, Obray JD, Yorgason JT, Jang EY, Weerasekara VK, Uys JD, Bellinger FP, Steffensen SC. Methamphetamine induces dopamine release in the nucleus accumbens through a sigma receptor-mediated pathway. Neuropsychopharmacology. 2018;43:1405–1414. doi: 10.1038/npp.2017.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong M, Mukhida K, Mendez I. GDNF therapy for Parkinson’s disease. Expert Rev Neurother. 2008;8:1125–1139. doi: 10.1586/14737175.8.7.1125. [DOI] [PubMed] [Google Scholar]
- 41.Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem. 2007;100:1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- 42.Ikenouchi-Sugita A, Yoshimura R, Ueda N, Kodama Y, Umene-Nakano W, Nakamura J. Continuous decrease in serum brain-derived neurotrophic factor (BDNF) levels in a neuropsychiatric syndrome of systemic lupus erythematosus patient with organic brain changes. Neuropsychiatr Dis Treat. 2008;4:1277–1281. doi: 10.2147/ndt.s4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jafari M, Ahangari G, Saberi M, Samangoui S, Torabi R, Zouali M. Distorted expression of dopamine receptor genes in systemic lupus erythematosus. Immunobiology. 2013;218:979–983. doi: 10.1016/j.imbio.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Janda E, Boi L, Carta AR. Microglial phagocytosis and its regulation: a therapeutic target in Parkinson’s disease. Front Mol Neurosci. 2018;11:144. doi: 10.3389/fnmol.2018.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jara LJ, Medina G, Saavedra MA, Vera-Lastra O, Torres-Aguilar H, Navarro C, Vazquez del Mercado M, Espinoza LRJIR. Prolactin has a pathogenic role in systemic lupus erythematosus. Immunol Res. 2017;65:512–523. doi: 10.1007/s12026-016-8891-x. [DOI] [PubMed] [Google Scholar]
- 46.Jennings A, Tyurikova O, Bard L, Zheng K, Semyanov A, Henneberger C, Rusakov DA. Dopamine elevates and lowers astroglial Ca2+ through distinct pathways depending on local synaptic circuitry. Glia. 2017;65:447–459. doi: 10.1002/glia.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, Raikwar SP, Zahoor H, Saeed D, Natteru PA, Iyer S, Zaheer A. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front Cell Neurosci. 2017;11:216. doi: 10.3389/fncel.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitamura O, Takeichi T, Wang EL, Tokunaga I, Ishigami A, Kubo S. Microglial and astrocytic changes in the striatum of methamphetamine abusers. Leg Med (Tokyo) 2010;12:57–62. doi: 10.1016/j.legalmed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG. Dopamine: functions, signaling, and association with neurological diseases. Cell Mol Neurobiol. 2019;39:31–59. doi: 10.1007/s10571-018-0632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koch G, Di Lorenzo F, Bonni S, Giacobbe V, Bozzali M, Caltagirone C, Martorana A. Dopaminergic modulation of cortical plasticity in Alzheimer’s disease patients. Neuropsychopharmacology. 2014;39:2654–2661. doi: 10.1038/npp.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krashia P, Nobili A, D’Amelio M. Unifying hypothesis of dopamine neuron loss in neurodegenerative diseases: focusing on Alzheimer’s disease. Front Mol Neurosci. 2019;12:123. doi: 10.3389/fnmol.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krasnova IN, Justinova Z, Cadet JL. Methamphetamine addiction: involvement of CREB and neuroinflammatory signaling pathways. Psychopharmacology (Berl) 2016;233:1945–1962. doi: 10.1007/s00213-016-4235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kustrimovic N, Rasini E, Legnaro M, Bombelli R, Aleksic I, Blandini F, Comi C, Mauri M, Minafra B, Riboldazzi G, Sanchez-Guajardo V, Marino F, Cosentino M. Dopaminergic receptors on CD4+ T naive and memory lymphocytes correlate with motor impairment in patients with Parkinson’s disease. Sci Rep. 2016;6:33738. doi: 10.1038/srep33738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leggio GM, Torrisi SA, Castorina A, Platania CB, Impellizzeri AA, Fidilio A, Caraci F, Bucolo C, Drago F, Salomone S. Dopamine D3 receptor-dependent changes in alpha6 GABAA subunit expression in striatum modulate anxiety-like behaviour: Responsiveness and tolerance to diazepam. Eur Neuropsychopharmacol. 2015;25:1427–1436. doi: 10.1016/j.euroneuro.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Leggio GM, Camillieri G, Platania CB, Castorina A, Marrazzo G, Torrisi SA, Nona CN, D’Agata V, Nobrega J, Stark H, Bucolo C, Le Foll B, Drago F, Salomone S. Dopamine D3 receptor is necessary for ethanol consumption: an approach with buspirone. Neuropsychopharmacology. 2014;39:2017–2028. doi: 10.1038/npp.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leggio GM, Di Marco R, Gulisano W, D’Ascenzo M, Torrisi SA, Geraci F, Lavanco G, Dahl K, Giurdanella G, Castorina A, Aitta-Aho T, Aceto G, Bucolo C, Puzzo D, Grassi C, Korpi ER, Drago F, Salomone S. Dopaminergic-GABAergic interplay and alcohol binge drinking. Pharmacol Res. 2019;141:384–391. doi: 10.1016/j.phrs.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 57.Levite M. Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol (Oxf) 2016;216:42–89. doi: 10.1111/apha.12476. [DOI] [PubMed] [Google Scholar]
- 58.Lohr KM, Chen M, Hoffman CA, McDaniel MJ, Stout KA, Dunn AR, Wang M, Bernstein AI, Miller GW. Vesicular monoamine transporter 2 (VMAT2) level regulates MPTP vulnerability and clearance of excess dopamine in mouse striatal terminals. Toxicol Sci. 2016;153:79–88. doi: 10.1093/toxsci/kfw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mackie P, Lebowitz J, Saadatpour L, Nickoloff E, Gaskill P, Khoshbouei H. The dopamine transporter: An unrecognized nexus for dysfunctional peripheral immunity and signaling in Parkinson’s Disease. Brain Behav Immun. 2018;70:21–35. doi: 10.1016/j.bbi.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maggio R, Aloisi G, Silvano E, Rossi M, Millan M. Heterodimerization of dopamine receptors: new insights into functional and therapeutic significance. Parkinsonism Relat Disord. 2009;15:2–7. doi: 10.1016/S1353-8020(09)70826-0. [DOI] [PubMed] [Google Scholar]
- 61.Marzagalli R, Leggio GM, Bucolo C, Pricoco E, Keay KA, Cardile V, Castorina S, Salomone S, Drago F, Castorina A. Genetic blockade of the dopamine D3 receptor enhances hippocampal expression of PACAP and receptors and alters their cortical distribution. Neuroscience. 2016;316:279–295. doi: 10.1016/j.neuroscience.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 62.Mastroeni D, Grover A, Leonard B, Joyce JN, Coleman PD, Kozik B, Bellinger DL, Rogers J. Microglial responses to dopamine in a cell culture model of Parkinson’s disease. Neurobiol Aging. 2009;30:1805–1817. doi: 10.1016/j.neurobiolaging.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matt SM, Gaskill PJ. Where is dopamine and how do immune cells see it: dopamine-mediated immune cell function in health and disease. J Neuroimmune Pharmacol. 2019 doi: 10.1007/s11481-019-09851-4. doi: 101007/s11481-019-09851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McArthur J, Smith B. Neurologic complications and considerations in HIV-infected persons. Curr Infect Dis Rep. 2013;15:61–66. doi: 10.1007/s11908-012-0312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ. Dopamine receptor expression on human Tand B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol. 2002;132:34–40. doi: 10.1016/s0165-5728(02)00280-1. [DOI] [PubMed] [Google Scholar]
- 66.Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal. 2013;11:34. doi: 10.1186/1478-811X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyazaki I, Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta Med Okayama. 2008;62:141–150. doi: 10.18926/AMO/30942. [DOI] [PubMed] [Google Scholar]
- 68.Miyazaki I, Asanuma M, Diaz-Corrales FJ, Miyoshi K, Ogawa N. Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res. 2004;1029:120–123. doi: 10.1016/j.brainres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 69.Morales I, Sanchez A, Rodriguez-Sabate C, Rodriguez M. Striatal astrocytes engulf dopaminergic debris in Parkinson’s disease: A study in an animal model. PLoS One. 2017;12:e0185989. doi: 10.1371/journal.pone.0185989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muscal E, Brey RL. Neurologic manifestations of systemic lupus erythematosus in children and adults. Neurol Clin. 2010;28:61–73. doi: 10.1016/j.ncl.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Musso NR, Brenci S, Setti M, Indiveri F, Lotti G. Catecholamine content and in vitro catecholamine synthesis in peripheral human lymphocytes. J Clin Endocrinol Metab. 1996;81:3553–3557. doi: 10.1210/jcem.81.10.8855800. [DOI] [PubMed] [Google Scholar]
- 72.Nagatomo K, Suga S, Saitoh M, Kogawa M, Kobayashi K, Yamamoto Y, Yamada K. Dopamine D1 receptor immunoreactivity on fine processes of GFAP-positive astrocytes in the substantia nigra pars reticulata of adult mouse. Front Neuroanat. 2017;11:3. doi: 10.3389/fnana.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakano K, Higashi T, Hashimoto K, Takagi R, Tanaka Y, Matsushita S. Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun. 2008;373:286–291. doi: 10.1016/j.bbrc.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 74.Nakano K, Yamaoka K, Hanami K, Saito K, Sasaguri Y, Yanagihara N, Tanaka S, Katsuki I, Matsushita S, Tanaka Y. Dopamine induces IL-6-dependent IL-17 production via D1-like receptor on CD4 naive T cells and D1-like receptor antagonist SCH-23390 inhibits cartilage destruction in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol. 2011;186:3745–3752. doi: 10.4049/jimmunol.1002475. [DOI] [PubMed] [Google Scholar]
- 75.Nickoloff-Bybel EA, Mackie P, Runner K, Matt SM, Khoshbouei H, Gaskill PJ. Dopamine increases HIV entry into macrophages by increasing calcium release via an alternative signaling pathway. Brain Behav Immun. 2019;82:239–252. doi: 10.1016/j.bbi.2019.08.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nobili A, Latagliata EC, Viscomi MT, Cavallucci V, Cutuli D, Giacovazzo G, Krashia P, Rizzo FR, Marino R, Federici M, De Bartolo P, Aversa D, Dell’Acqua MC, Cordella A, Sancandi M, Keller F, Petrosini L, Puglisi-Allegra S, Mercuri NB, Coccurello R, et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat Commun. 2017;8:14727–7. doi: 10.1038/ncomms14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pacheco R. Targeting dopamine receptor D3 signalling in inflammation. Oncotarget. 2017;8:7224–7225. doi: 10.18632/oncotarget.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pacheco R, Contreras F, Zouali M. The dopaminergic system in autoimmune diseases. Front Immunol. 2014;5:117. doi: 10.3389/fimmu.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pacheco R, Prado CE, Barrientos MJ, Bernales S. Role of dopamine in the physiology of T-cells and dendritic cells. J Neuroimmunol. 2009;216:8–19. doi: 10.1016/j.jneuroim.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 80.Petrelli F, Dallérac G, Pucci L, Calì C, Zehnder T, Sultan S, Lecca S, Chicca A, Ivanov A, Asensio CS, Gundersen V, Toni N, Knott GW, Magara F, Gertsch J, Kirchhoff F, Déglon N, Giros B, Edwards RH, Mothet JP, et al. Dysfunction of homeostatic control of dopamine by astrocytes in the developing prefrontal cortex leads to cognitive impairments. Mol Psychiatry. 2018 doi: 10.1038/s41380-018-0226-y. doi: 101038/s41380-018-0226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pinoli M, Marino F, Cosentino M. Dopaminergic regulation of innate immunity: a review. J Neuroimmune Pharmacol. 2017;12:602–623. doi: 10.1007/s11481-017-9749-2. [DOI] [PubMed] [Google Scholar]
- 82.Prado C, Contreras F, Gonzalez H, Diaz P, Elgueta D, Barrientos M, Herrada AA, Lladser A, Bernales S, Pacheco R. Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity. J Immunol. 2012;188:3062–3070. doi: 10.4049/jimmunol.1103096. [DOI] [PubMed] [Google Scholar]
- 83.Qiu YH, Cheng C, Dai L, Peng YP. Effect of endogenous catecholamines in lymphocytes on lymphocyte function. J Neuroimmunol. 2005;167:45–52. doi: 10.1016/j.jneuroim.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 84.Rangel-Barajas C, Coronel I, Floran B. Dopamine receptors and neurodegeneration. Aging Dis. 2015;6:349–368. doi: 10.14336/AD.2015.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Redell JB, Dash PK. Traumatic brain injury stimulates hippocampal catechol-O-methyl transferase expression in microglia. Neurosci Lett. 2007;413:36–41. doi: 10.1016/j.neulet.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rollo CD. Dopamine and aging: intersecting facets. Neurochem Res. 2009;34:601–629. doi: 10.1007/s11064-008-9858-7. [DOI] [PubMed] [Google Scholar]
- 87.Ronnberg E, Calounova G, Pejler G. Mast cells express tyrosine hydroxylase and store dopamine in a serglycin-dependent manner. Biol Chem. 2012;393:107–112. doi: 10.1515/BC-2011-220. [DOI] [PubMed] [Google Scholar]
- 88.Rosin C, Colombo S, Calver AA, Bates TE, Skaper SD. Dopamine D2 and D3 receptor agonists limit oligodendrocyte injury caused by glutamate oxidative stress and oxygen/glucose deprivation. Glia. 2005;52:336–343. doi: 10.1002/glia.20250. [DOI] [PubMed] [Google Scholar]
- 89.Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. The immunoregulatory role of dopamine: an update. Brain Behav Immun. 2010;24:525–528. doi: 10.1016/j.bbi.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schain M, Kreisl WC. Neuroinflammation in neurodegenerative disorders-a review. Curr Neurol Neurosci Rep. 2017;17:25. doi: 10.1007/s11910-017-0733-2. [DOI] [PubMed] [Google Scholar]
- 91.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek E, Chen T, Li SB, Xu M, Zhou JN, Hu G, Zhou JW. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature. 2013;494:90–94. doi: 10.1038/nature11748. [DOI] [PubMed] [Google Scholar]
- 93.Talhada D, Rabenstein M, Ruscher K. The role of dopaminergic immune cell signalling in poststroke inflammation. Ther Adv Neurol Disord. 2018;11:1756286418774225. doi: 10.1177/1756286418774225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teismann P, Schulz JB. Cellular pathology of Parkinson’s disease: astrocytes, microglia and inflammation. Cell Tissue Res. 2004;318:149–161. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- 95.Tong J, Rathitharan G, Meyer JH, Furukawa Y, Ang LC, Boileau I, Guttman M, Hornykiewicz O, Kish SJ. Brain monoamine oxidase B and A in human parkinsonian dopamine deficiency disorders. Brain. 2017;140:2460–2474. doi: 10.1093/brain/awx172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vermeren S, Karmakar U, Rossi AG. Immune complex-induced neutrophil functions: A focus on cell death. Eur J Clin Invest. 2018;48:e12948. doi: 10.1111/eci.12948. [DOI] [PubMed] [Google Scholar]
- 97.Vidal PM, Pacheco R. Targeting the dopaminergic system in autoimmunity. J Neuroimmune Pharmacol. 2019 doi: 10.1007/s11481-019-09834-5. doi: 101007/s11481-019-09834-5. [DOI] [PubMed] [Google Scholar]
- 98.Wang B, Chen T, Li G, Jia Y, Wang J, Xue L, Chen Y. Dopamine alters lipopolysaccharide-induced nitric oxide production in microglial cells via activation of D1-like receptors. Neurochem Res. 2019a;44:947–958. doi: 10.1007/s11064-019-02730-7. [DOI] [PubMed] [Google Scholar]
- 99.Wang B, Chen T, Xue L, Wang J, Jia Y, Li G, Ren H, Wu F, Wu M, Chen Y. Methamphetamine exacerbates neuroinflammatory response to lipopolysaccharide by activating dopamine D1-like receptors. Int Immunopharmacol. 2019b;73:1–9. doi: 10.1016/j.intimp.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 100.Wang H, Xu J, Lazarovici P, Quirion R, Zheng W. cAMP response element-binding protein (CREB): a possible signaling molecule link in the pathophysiology of schizophrenia. Front Mol Neurosci. 2018;11:255. doi: 10.3389/fnmol.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang XQ, Liu Y, Cai HH, Peng YP, Qiu YH. Expression of tyrosine hydroxylase in CD4(+) T cells contributes to alleviation of Th17/Treg imbalance in collagen-induced arthritis. Exp Biol Med (Maywood) 2016;241:2094–2103. doi: 10.1177/1535370216660635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Winner BM, Zhang H, Farthing MM, Karchalla LM, Lookingland KJ, Goudreau JL. Metabolism of dopamine in nucleus accumbens astrocytes is preserved in aged mice exposed to MPTP. Front Aging Neurosci. 2017;9:410. doi: 10.3389/fnagi.2017.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 105.Yang P, Perlmutter JS, Benzinger TLS, Morris JC, Xu J. Dopamine D3 receptor: A neglected participant in Parkinson Disease pathogenesis and treatment. Ageing Res Rev. 2020;57:100994. doi: 10.1016/j.arr.2019.100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang L, Zhang J, You Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front Cell Neurosci. 2018;12:306. doi: 10.3389/fncel.2018.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Y, Chen Y, Wu J, Manaenko A, Yang P, Tang J, Fu W, Zhang JH. Activation of dopamine D2 receptor suppresses neuroinflammation through alphaB-crystalline by inhibition of NF-kappaB nuclear translocation in experimental ICH mice model. Stroke. 2015;46:2637–2646. doi: 10.1161/STROKEAHA.115.009792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu J, Ananthan S, Zhan CG. The role of human dopamine transporter in NeuroAIDS. Pharmacol Ther. 2018;183:78–89. doi: 10.1016/j.pharmthera.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]