Parkinson’s disease (PD) is a neurodegenerative disorder belonging to a group of human pathologies known as synucleinopathies, which includes multiple system atrophy or dementia with Lewy bodies (Spillantini et al., 1998). These diseases share a common neuropathological feature, the presence of α-synuclein (α-Syn) deposits, although they differ in the cellular and anatomical compartment in which α-Syn inclusions accumulate. PD affects more than 1% of people over 60 years of age, thus being the second most prevalent neurodegenerative disease in the world and the most common synucleinopathy. The loss of dopaminergic neurons in the substantia nigra pars compacta during PD progression induces a pronounced dopamine concentration decrease in the synaptic area, which translates into motor symptoms such as bradykinesia, rigidity or resting tremor (Martí et al., 2003). Damaged neurons were reported to have large proteinaceous inclusions, named Lewy’s bodies and neurites, which constitute the major histopathological hallmark in PD. Amyloid fibrils of α-Syn were identified as the main component of these inclusions (Spillantini et al., 1997). The detection of genetic mutations (Polymeropoulos et al., 1997) and multiplications in the SNCA gene (Singleton et al., 2003), which encodes for α-Syn, linked to the early onset and higher penetrance of PD, provide evidence for the connection between the aggregation of this particular protein and PD.
α-Syn is an intrinsically disordered protein involved in presynaptic vesicle trafficking. The sequence of this protein can be dissected into three different regions (Fusco et al., 2014): an N-terminal domain, containing imperfect KTKGEV repeats and responsible for membrane binding due to its amphipathic character; a central and highly hydrophobic region known as the non-amyloid component, which nucleates amyloid aggregation; and a C-terminal domain whose amino acidic composition seems to modulate α-Syn aggregation propensity. In normal conditions, this protein is found as a soluble and disordered monomer that can adopt a helical structure upon binding to membranes. Recent studies suggest that α-Syn might also form helical tetramers under native conditions. In pathological conditions, α-Syn shifts its conformation to assemble into fibrils displaying the characteristic cross-β amyloid fold. Mutations in the α-Syn N-terminal domain and truncations in the C-terminal region significantly impact its aggregation and associated toxicity.
Intracellular α-Syn aggregation can be recapitulated in vitro with the purified recombinant protein. As for many other amyloidogenic proteins, the in vitro aggregation kinetics of α-Syn follow a sigmoidal curve. Fibrillar structures observed at the plateau phase are preceded by the progressive assembly of monomeric α-Syn into oligomeric species and protofibrils (Figure 1A). These metastable oligomeric structures have been described as the most toxic species and can be transmitted from damaged to neighboring healthy neurons in a prion-like manner, thus disseminating α-Syn aggregation within the brain (Hansen et al., 2011).
Figure 1.
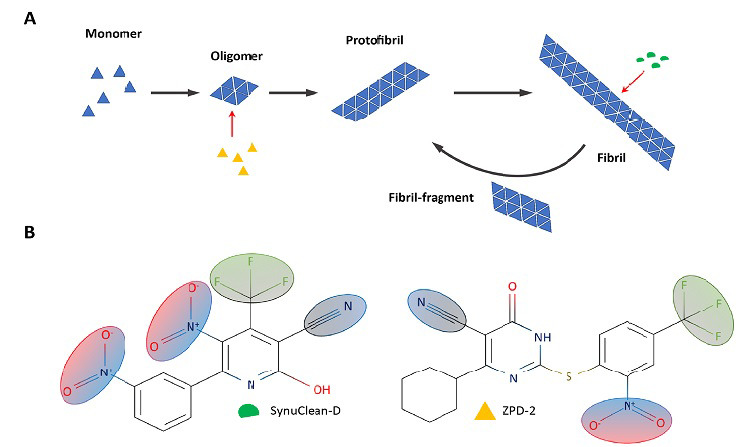
Schematic representation of α-Synuclein sequence and aggregation.
(A) Simplified aggregation scheme of α-Synuclein indicating the suggested interaction of ZPD-2 (yellow) and SynuClean-D (green). (B) SynuClean-D (green) and ZPD-2 (yellow) structures. Shared functional groups are shaded.
Altogether, the aggregation of α-Syn appears as a reliable target to develop disease-modifying therapies for PD. Multiple strategies have been proposed to target this process, either by accelerating α-Syn intracellular clearance with autophagy promoters; by clearing extracellular aggregates with specific antibodies; by reducing α-Syn in-cell concentration with gene silencing techniques; or by blocking its aggregation with small molecules (Fields et al., 2019). Several small compounds, including EGCG, NPT200-11, CLR01, or mannitol-based structures, have demonstrated their efficacy against α-Syn deposition in vitro and/or in animal models of PD. However, drug discovery programs aimed to target α-Syn are characterized by high attrition rates, and many molecules fail as they progress to trials in humans, finding difficulties in translating their in vitro/in vivo inhibitory activity into patients’ amelioration.
The lack of well-defined three-dimensional structures for monomeric and oligomeric α-Syn precludes both the rational optimization of leading molecules and the discovery of de novo drugs able to bind these species at specific pockets, which constitutes a significant bottleneck in the development of disease-modifying therapies for PD. In this context, the high-throughput screening of large chemical libraries emerges as one of the few strategies amenable to identify novel and efficient modulators of α-Syn aggregation (Pujols et al., 2017). However, screening for aggregation inhibitors is inherently tricky, because this reaction strongly depends on the protein quality and the particular assay conditions, usually displaying low reproducibility between different experiments, something that becomes critical when a large number of compounds should be tested. Pujols et al. (2017) optimized a robust high-throughput screening protocol that allowed to screen more than 14,400 chemically diverse molecules. This protocol exploits the increase in Thioflavin-T fluorescence upon binding to amyloids to derive accurate aggregation kinetics for each tested compound but also implements orthogonal techniques, such as transmission electron microscopy, nanoparticle tracking, and static light-scattering. This combined analysis allows discarding false positives resulting from either the quenching of the fluorescence signal or the interference of Thioflavin-T binding to fibrils. This information is further completed with an analysis of the dose- and time-dependent activity of the molecules. Using this approach, the authors have recently reported the discovery of two compounds displaying significant inhibitory capacity: SynuClean-D (SC-D) (Pujols et al., 2018) and ZPD-2 (Peña-Díaz et al., 2019; Figure 1B).
Under close to physiological conditions, SC-D and ZPD-2 displayed a 50 and 80% of aggregation inhibition at a 0.7:1 α-Syn: compound molar ratio, respectively. The protocol seems to be biased towards the detection of molecules that do not interact with monomeric α-Syn since NMR studies with15 N labeled α-Syn indicated that none of the two compounds bind to this soluble form. Indeed, SC-D and ZPD-2 were also able to inhibit α-Syn aggregation at substoichiometric concentrations (7:1 α-Syn: compound molar ratio), which indicates that they target aggregated α-Syn structures. This behavior represents a clear advantage for its potential therapeutic use since they would only target pathological α-Syn assemblies, without interfering with the activity of the soluble and functional protein. Both compounds significantly modified the kinetic constants of the reaction. However, while SC-D diminished the autocatalytic rate constant, ZPD-2 exerted a greater effect on the homogenous nucleation rate constant. These data suggested that ZPD-2 and SC-D target different aggregated α-Syn species, being more active at the early and late stages of the aggregation kinetics, respectively. The characterization of the time-depended activity of ZPD-2 confirmed that this molecule was more effective when added before the reaction begins or at early times after its initiation. In contrast, SC-D displayed a time-independent activity, and similar inhibition levels were reached at different addition points. These results allowed the authors to conclude that ZPD-2 acted mainly as an anti-oligomeric compound, whereas SC-D target larger structures, including mature fibrils (Figure 1A). Effectively, when SC-D was added to mature aged fibrils, these structures were significantly dismantled, in good agreement with a bioinformatic study suggesting that SC-D can be accommodated within the fibrils, adjacent to the non-amyloid component domain. Despite their different structures, SC-D and ZPD-2, share several common chemical groups and possess structural similarities with previously described inhibitors of aggregation: hydrophobic scaffolds, built of aromatic rings, that project polar ramifications to solvent (Figure 1B). This structural configuration might allow the interaction with hydrophobic clusters, exposed in aggregated species, as well as the destabilization of the ensemble by the difficult to accommodate polar moieties. It can be speculated that those shared groups would account for a generic anti-aggregational activity, whereas the specific nature of the rings and substituents would endorse the molecules with a particular target selectivity.
Remarkably, SC-D and ZPD-2 also prevented the aggregation of two α-Syn familiar mutants, H50Q and A30P, causing early onset of PD. These α-Syn variants facilitate the formation of early oligomers, thus accelerating the overall aggregation reaction (Marvian et al., 2019). Thus, it was not surprising to observe that ZPD-2 inhibits more efficiently than SC-D the aggregation of these variants when added at the beginning of the reaction. The molecules were also assayed in Protein Misfolding Cyclic Amplification assays, which constitute an in vitro approach to assess the ability of the compounds to abrogate the transmission of preformed aggregates or seeds. Both molecules exhibited and unprecedented capacity to avoid α-Syn seeded polymerization.
To test the activity of SC-D and ZPD-2 in vivo, the authors used two different Caenorhabditis elegans models of PD, where the expression of human α-Syn in either muscular or neuronal cells induced the formation of protein inclusions, whose accumulation impacted the animal mobility or induced the loss of dopaminergic neurons, respectively. When administrated to animals at L4 larval stage, SC-D and ZPD-2 decreased the number of α-Syn inclusions in muscular cells by 42% and 20% compared to untreated worms. Both compounds exerted an intense neuroprotective activity in the neuronal model, again with SC-D being the most active. Treatment with SC-D promoted an impressive 3-fold increase in the proportion of animals that keep all the head dopaminergic neurons intact, despite they express human α-Syn at high levels, a property not reported previously for any other anti-aggregation compound. The higher activity of SC-D in both the muscular and neuronal PD models likely owes to the fact that the L4 larval stage is intended to mimic aged PD patients, where mature fibrils, rather than early oligomeric assemblies, are expected to be the predominant species.
Overall, SC-D and ZP2-D illustrate the virtues of high-throughput screening in the search for modulators of protein aggregation. Even though these two hits do not possess drug-like properties yet, they constitute important tool compounds to inform the further discovery of disease-modifying therapies for PD.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Fields CR, Bengoa-Vergniory N, Wade-Martins R. Targeting alpha-synuclein as a therapy for Parkinson’s disease. Front Mol Neurosci. 2019;12:299. doi: 10.3389/fnmol.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fusco G, De Simone A, Gopinath T, Vostrikov V, Vendruscolo M, Dobson CM, Veglia G. Direct observation of the three regions in alpha-synuclein that determine its membrane-bound behaviour. Nat Commun. 2014;5:3827. doi: 10.1038/ncomms4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen C, Angot E, Bergström AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martí MJ, Tolosa E, Campdelacreu J. Clinical overview of the synucleinopathies. Mov Disord. 2003;18:S21–27. doi: 10.1002/mds.10559. [DOI] [PubMed] [Google Scholar]
- 5.Marvian AT, Koss DJ, Aliakbari F, Morshedi D, Outeiro TF. In vitro models of synucleinopathies: informing on molecular mechanisms and protective strategies. J Neurochem. 2019;150:535–565. doi: 10.1111/jnc.14707. [DOI] [PubMed] [Google Scholar]
- 6.Peña-Díaz S, Pujols J, Conde-Giménez M, Čarija A, Dalfo E, García J, Navarro S, Pinheiro F, Santos J, Salvatella X, Sancho J, Ventura S. ZPD-2, a small compound that inhibits alpha-synuclein amyloid aggregation and its seeded polymerization. Front Mol Neurosci. 2019;12:306. doi: 10.3389/fnmol.2019.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 8.Pujols J, Peña-Díaz S, Conde-Giménez M, Pinheiro F, Navarro S, Sancho J, Ventura S. High-throughput screening methodology to identify alpha-synuclein aggregation inhibitors. Int J Mol Sci. 2017 doi: 10.3390/ijms18030478. doi: 103390/ijms18030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pujols J, Peña-Díaz S, Lázaro DF, Peccati F, Pinheiro F, González D, Carija A, Navarro S, Conde-Giménez M, García J, Guardiola S, Giralt E, Salvatella X, Sancho J, Sodupe M, Outeiro TF, Dalfó E, Ventura S. Small molecule inhibits alpha-synuclein aggregation, disrupts amyloid fibrils, and prevents degeneration of dopaminergic neurons. Proc Natl Acad Sci U S A. 2018;115:10481–10486. doi: 10.1073/pnas.1804198115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 11.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]


