Dementia is a progressive cognitive impairment that affects the activities of daily living. Alzheimer’s disease (AD) is the most common form of the dementia worldwide accounting for 60–80% of all dementia cases. With an estimated cost exceeding $290 billion in the USA, understanding and development of future therapeutic strategies is vital. In this perspective, we will be examining the current thinking of AD research and therapeutic strategies, while proposing a possible new direction for diagnosis, understanding, and treatment targets. Non-coding RNA accounts for the largest population of the human transcriptome. Long noncoding RNA (lncRNA) is a recent molecule of interest in the biomedical research which is non protein coding and is of length greater than 200 nucleotides. LncRNAs have been shown to play diverse roles within the cells such as posttranscriptional and posttranslational regulation, chromatin modulation, and protein complex organization. Given the flexible and diverse role in disease pathophysiology, lncRNAs may serve as novel therapeutic targets for diagnosis and treatment. Evidently, recent studies showed that dysregulation of lncRNA influences the clinical course of tumorigenesis, neurological disorders, cardiovascular disease, diabetes, and acquired immunodeficiency syndrome (Kazimierczyk et al., 2020). This indicates that lncRNA can provide a unique avenue of research and possible therapeutic targets in AD.
Current AD research: AD is the most common form of dementia, characterized by progressive neuronal death associated with neuropathological findings of neurofibrillary tangles and senile plaques. Despite considerable advances in the knowledge of AD pathogenesis, we know little about how to prevent or delay the ongoing neurodegenerative process. Therefore, a deeper understanding of the molecular mechanisms involved in AD pathogenesis is essential, which would contribute to the development of a novel therapeutic target. The vast majority of AD occurs on a seemingly sporadic basis. While both genetic and environmental factors may drive sporadic AD, the ε4 allele of apolipoprotein E gene is a major genetic risk factor for the AD development. However, it has been found that a familial form of AD is driven by mutations in amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2), which occurs much earlier than sporadic AD, between the ages of 30 and 50 years. Mutations in the genes APP/PSEN1/PSEN2 for familial form of AD or dysfunction in apolipoprotein E leads to an unfavorable accumulation of the amyloid beta (Aβ) peptide. This eventually leads to widespread neuronal/synaptic dysfunction and ultimately dementia (Selkoe and Hardy, 2016). Unfortunately, attempts to ameliorate the disease course by removing Aβ or reducing its production have been largely unsuccessful. The failure of drugs targeting Aβ deposition in the brain has fueled an increasing interest in alternative disease-causing mechanisms. The recent progress of deep-transcriptome sequencing and genome-wide analyses have identified several dysregulated lncRNAs in AD, but the expression patterns, interacting proteins, and biological functions of these lncRNAs in AD remain largely unexplored. Thus, understanding how lncRNAs regulate neuronal function in the brain may allow for the development of effective therapies for AD and related neurodegenerative diseases. Interestingly, lncRNA biology is the rapidly emerging area of focus in biomedical research field. It is evident from the exponential increase of published articles on lncRNA. As searched on PubMed, lncRNA and cancer showed 34 articles in 2010 and 3174 in 2019. Evidently, lncRNA in AD has gained some attention with 85 articles on PubMed in 2019. This shows lncRNA as a potential and novel target in AD, which needs to be explored for its mechanism and etiology.
LncRNAs in AD: Once considered transcriptional noise, lncRNAs are associated with specialized functions in specific cell types. They are predominantly located in the nucleus helping in one of their functions of epigenetic regulation (Luo and Chen, 2016). With diverse roles, cell specific functions, and dysregulation linked to cancer, epilepsy, cardiovascular, neurodegenerative, and genetic diseases, it stands to reason that lncRNA would confer promoting or inhibiting effects in the development of AD, similar to its contribution in other diseases (Luo and Chen, 2016). Although, the role of lncRNAs in AD are fairly studied over the past few years, the mechanisms of its involvement in neurodegeneration and cognitive impairment are unknown. Recently, several lncRNAs, BACE1-AS, 51A, 17A, NDM29, BC200, MALAT1, BDNF-AS and NAT-Rad18 have been shown to play a role in AD. These lncRNAs regulate synaptic plasticity, APP processing, tau phosphorylation and inflammation, major causal agents in AD (Figure 1; Cortini et al., 2019). In addition, it has been suggested that lncRNAs also interact with miRNAs and regulate a broad range of biological processes through their crosstalk with miRNAs. Besides that, a recently identified lncRNA EBF3-AS plays a role in promotion of neuronal apoptosis in a murine model of AD and was abnormally expressed in the brains of AD patients (Gu et al., 2018). The conserved anti-sense noncoding RNA β-secretase-1 (BACE1-AS) is highly expressed in the brains of AD patients and mouse models of AD and drives feed-forward regulation of BACE1 (Faghihi et al. 2008). BACE1-AS increases in response to cell stressors, suggesting its role in overall progression of AD. A study by Zhang and coworkers demonstrated that silencing of BACE-AS by short interfering RNA reduces the production of Aβ1–42 oligomers in cellular system and improves cognitive function through regulation of BACE1, and tau phosphorylation in in vivo (Zhang et al., 2018). Therefore, it is conceivable that BACE1-AS could serve as a potential biomarker for diagnosis and therapeutic target for the treatment of AD. Similarly, lncRNAs 51A, 17A, and NDM29 have been shown to increase Aβ abundance (Luo and Chen, 2016). The lncRNA 51A has been shown in a recent study to drive a splicing shift in variant A of SORL1. The decrease in SORL1 variant A leads to an impaired processing of APP leading to increase in the formation of Aβ. Neuroblastoma differentiation marker 29 (NDM29) drives neuroblastoma cell differentiation to nonmalignant neuron-like phenotypes. In cells dependent upon NDM29 maturation, there is an increased synthesis of APP and subsequent Aβ formation and secretion (Cortini et al., 2019). The lncRNA BC200 regulates cell viability and apoptosis via modulating the expression of BACE1 and its level is significantly upregulated in the AD patients (Mus et al., 2007). Increased expression of NAT-Rad18 leads to dysregulation of DNA Repair mechanism leading to an increase in neuron sensitivity to apoptosis and AD progression (Luo and Chen, 2016). LncRNA MALAT1 was originally identified in lung cancer and found to promote cell proliferation and metastasis in various cancers such as lung, ovarian, and pancreatic cancer. However, in a recent study inhibition of MALAT1 has been shown to inhibit neuron apoptosis and promote neurite outgrowth (Ma et al., 2019). This evidence suggests that MALAT1 can provide a new route in the understanding of the mechanism of AD disease progression and provide new therapeutic targets. LncRNA BDNF-AS plays an important role in the regulation of BDNF protein expression. Inhibition of BDNF-AS increases mRNA levels of BDNF, enhances protein levels of BDNF and triggers neuronal differentiation. Given the role of BDNF in AD and other neurological disorders, pharmacological inhibitors of BDNF-AS may have significant therapeutic potential in the treatment of AD. These findings provided proof-of-concept evidence and support for the potential roles of lncRNA in AD pathogenesis. Understanding the molecular mechanisms underlying lncRNA dysregulation in AD may provide new insights into AD pathophysiology and open new therapeutic avenues.
Figure 1.
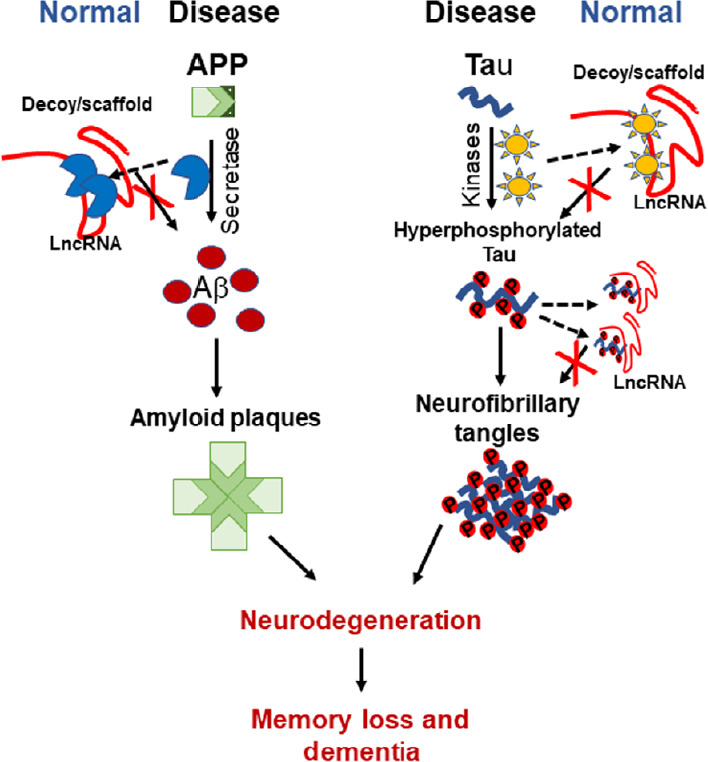
A schematic diagram representing the possible lncRNA neuroprotective function in Alzheimer's disease.
LncRNA might function as Decoy and/or Scaffold to sequester secretase enzyme and hence hinders enzyme function, decreasing amyloid beta (Aβ) aggregation. It can sequester kinases to decrease tau hyperphosphorylation. LncRNA can also decrease neurofibrillary tangles burden phosphorylated, by keeping hyperphosphorylated tau proteins apart. APP: Amyloid precusor protein; Aβ: amyloid beta; lncRNA: long non-coding RNA.
Novel biomarkers: Early diagnosis of any disease more often leads to better outcomes. The unique aspects of lncRNA make it a leading candidate as a diagnostic and/or prognostic biomarker. LncRNA has been mostly characterized in cancer and has also been shown to be a biomarker candidate in other diseases too. While recent studies on lncRNAs have shown them to be aberrantly expressed, and some appear to be cancer specific. The specific expression, stability in bodily fluids, and increased expression often correlating to the severity of the disease making lncRNAs an attractive candidate for non-invasive screening. LncRNAs are already in clinical trials as biomarkers. LncRNA PCA3 is one of these, due to its high levels and specificity to prostate cancer along with its detectable limits in urine (Bhan et al., 2017). HOTAIR circulation has been suggested as a possible diagnosis’s marker for breast cancer. In addition, a recent study by Tan and colleagues have demonstrated that HOTAIR is a peripheral biomarker for glioblastoma multiforme patients (Tan et al., 2018). The expression of BACE1-AS is upregulated in blood samples from AD patients, indicating BACE1-AS may serve as a plausible biomarker for AD (Zhang et al., 2018). Similarly, increased lncRNA 51A expression has been detected in the plasma of AD patients, suggesting lncRNA 51A in plasma could be used as a potential diagnostic and prognostic biomarker for AD. MALAT1 expression can be used as marker for lung cancer. Interestingly, a study by Yoa et al. (2016) reported decreased lncRNA MALAT1 levels in the cerebrospinal fluid of AD patients compared with the control group, suggesting lncRNA MALAT1 monitoring in cerebrospinal fluid could serves a diagnostic marker for AD. The flexibility demonstrated in cancer and other neural diseases along with evidence suggesting lncRNA playing a key role in the development of AD indicate, that lncRNA might provide effective candidates for non-invasive screening for AD.
Conclusion: The last 25 years of AD research and therapeutic development has been focused on understanding and preventing the accumulation of toxic Aβ. The amyloid cascade hypothesis has provided a strong backbone that has led to great leaps in knowledge when it comes to AD. This backbone has also led to stagnation in AD diagnosis and therapeutics. This is evident in the non-existence of disease modifying therapeutic options and earlier detection of AD. LncRNA provides a novel route of research for the development of new therapies and diagnostic markers for AD. Recent studies have provided important insights of the biological function and clinical relevance of lncRNAs in AD. Although, there is increasing recognition that lncRNAs have been implicated in AD pathogenesis, but it remains unknown how they influence AD development and progression. Understanding the regulatory function and molecular mechanisms of lncRNAs in AD pathophysiology will fuel the development of innovative diagnosis strategies and potential therapeutic targets. LncRNA is already beginning to be uncovered as a potential key player in the development of AD as shown in the studies mentioned above. However, it is not just a promotive agent in AD as demonstrated by MALAT1 expression, leading to inhibition of neuron apoptosis and neurite growth. Moreover, findings on the role of lncRNAs in cancers and glioblastoma may shed new light on understanding of molecular mechanisms of lncRNA in AD. With AD the most common form of dementia, and more than triple the current estimated 44 million people to live with dementia worldwide by 2050, there is an urgent need for new therapies and diagnostic tools for AD. Undoubtedly, activators or inhibitor of lncRNAs will provide a potential and novel strategy for the treatment of AD. Interestingly, antisense oligonucleotides (ASOs) targeting aberrantly expressed lncRNAs may represent a feasible treatment for AD. Development of specific ASOs downregulating BACE1-AS, 51A, 17A, NDM29 and BDNF-AS may represent novel and potential therapeutic strategy in order to regulate Aβ production, inflammation and tau phosphorylation in AD. However, the major challenge in using ASOs in neurodegenerative disorders is the poor bioavailability, susceptible nuclease degradation nature, and limited blood-brain barrier permeability. EVs, small nanovesicles, also frequently referred to as exosomes, recently been recognized as potential drug delivery systems for therapeutic interventions. It is desirable to load ASOs targeting lncRNAs in EVs and examine their therapeutic effects on AD-related neuropathology and cognitive function. Furthermore, lncRNAs may be used as potential biomarker for clinical assessment of AD stages, as their abnormal expression pattern can be easily detected in plasma or cerebrospinal fluid of AD patients. The use of lncRNAs as diagnostic and prognostic markers has already been exploited in the cancer research but more research is needed to establish lncRNAs as biomarker for AD patients. In conclusion, the role of lncRNAs in the AD pathogenesis is largely unexplored and understanding the molecular mechanisms of lncRNAs in AD will uncover more avenues to early diagnosis and effective therapies.
Our work on lncRNA in AD was supported by the Division of Rehabilitation Sciences, College of Health Professions, University of Tennessee Health Science Center.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Isaac G. Onyango, Gencia Biotechnology, Charlottesville, USA.
P-Reviewer: Onyango IG; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortini F, Roma F, Villa C. Emerging roles of long non-coding RNAs in the pathogenesis of Alzheimer’s disease. Ageing Res Rev. 2019;50:19–26. doi: 10.1016/j.arr.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, Finch CE, St Laurent G, 3rd, Kenny PJ, Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu C, Chen C, Wu R, Dong T, Hu X, Yao Y, Zhang Y. Long noncoding RNA EBF3-AS promotes neuron apoptosis in Alzheimer’s disease. DNA Cell Biol. 2018;37:220–226. doi: 10.1089/dna.2017.4012. [DOI] [PubMed] [Google Scholar]
- 5.Kazimierczyk M, Kasprowicz MK, Kasprzyk ME, Wrzesinski J. Human long noncoding RNA interactome: detection, characterization and function. Int J Mol Sci. 2020 doi: 10.3390/ijms21031027. doi: 103390/ijms21031027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo Q, Chen Y. Long noncoding RNAs and Alzheimer’s disease. Clin Interv Aging. 2016;11:867–872. doi: 10.2147/CIA.S107037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma P, Li Y, Zhang W, Fang F, Sun J, Liu M, Li K, Dong L. Long non-coding RNA MALAT1 inhibits neuron apoptosis and neuroinflammation while stimulates neurite outgrowth and its correlation with miR-125b mediates PTGS2, CDK5 and FOXQ1 in Alzheimer’s disease. Curr Alzheimer Res. 2019;16:596–612. doi: 10.2174/1567205016666190725130134. [DOI] [PubMed] [Google Scholar]
- 8.Mus E, Hof PR, Tiedge H. Dendritic BC200 RNA in aging and in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:10679–10684. doi: 10.1073/pnas.0701532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan SK, Pastori C, Penas C, Komotar RJ, Ivan ME, Wahlestedt C, Ayad NG. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol Cancer. 2018;17:74. doi: 10.1186/s12943-018-0822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao J, Wang XQ, Li YJ, Shan K, Yang H, Wang YN, Yao MD, Liu C, Li XM, Shen Y, Liu JY, Cheng H, Yuan J, Zhang YY, Jiang Q, Yan B. Long non-coding RNA MALAT1 regulates retinal neurodegeneration through CREB signaling. EMBO Mol Med. 2016;8:346–362. doi: 10.15252/emmm.201505725. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Zhang W, Zhao H, Wu Q, Xu W, Xia M. Knockdown of BACE1-AS by siRNA improves memory and learning behaviors in Alzheimer’s disease animal model. Exp Ther Med. 2018;16:2080–2086. doi: 10.3892/etm.2018.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]


