Traumatic spinal cord injury (SCI) leads to chronic locomotor impairment and disability. Unfortunately, there are no effective treatments currently available for SCI patients (Bradbury and Burnside, 2019). Developing novel repair interventions to mitigate the devastating nature of SCI and translating them clinically are urgent medical needs to improve the quality of life of patients with SCI. The lumbar spinal motoneurons (MNs) are the final common pathway for hindlimb locomotion since all neural activities that influence hindlimb movement converging upon these neurons. With above-level (cervical and thoracic) SCIs, the lumbar MNs are not directly injured by the initial mechanical impact, but they undergo profound degeneration with dendritic atrophy and synaptic stripping due to a trauma-induced decrease of supraspinal and propriospinal innervations, leading to impaired locomotor function (Figure 1A; Wang et al., 2018). While most SCI studies have been focused on the neuroregeneration or neuroprotection of injured spinal cord at the lesion site, few studies have explored the potential benefit of modulating lumbar motor circuitry for locomotor recovery after an above-level SCI. Filling this gap is an important task for developing the care and treatment of SCI.
Figure 1.
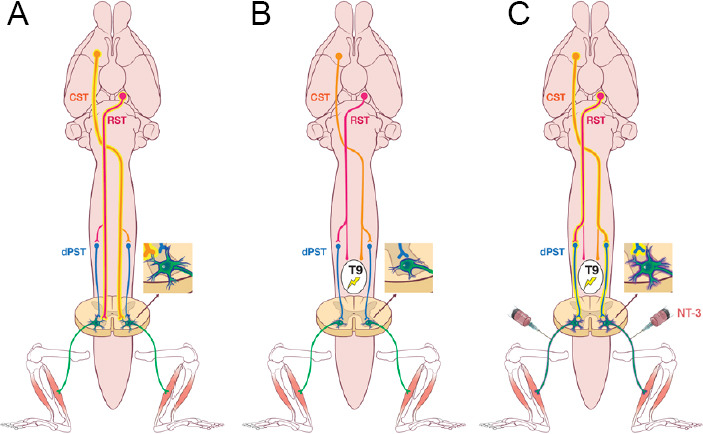
NT-3-mediated propriospino-motoneuron circuit reorganization improves locomotor recovery following spinal cord injury.
(A) Both lumbar motoneurons (MNs) and higher motor centers, including the corticospinal (CST), rubrospinal (RST) and descending propriospinal (dPST) tracts, are required to initiate and maintain locomotor function in normal conditions. (B) Despite the spared dPST, a moderate thoracic (T9) contusion abolishes the CST and RST innervation and causes lumbar MN dendritic atrophy. (C) Remodeling of propriospino-MN circuit by neurotrophin-3 (NT-3) gene therapy through a peripheral delivery route promotes MN dendritic regrowth and relays the supraspinal commands from the CST and RST down to the lumbar cord that enables locomotor recovery.
Neurotrophin-3 (NT-3), a member of the neurotrophin family of proteins, was discovered in 1990 by two independent groups (Hohn et al., 1990; Maisonpierre et al., 1990). A broad distribution of this factor was found in the kidney, lung, cerebellum, medulla, and hippocampus, suggesting its role as a trophic factor for growing sympathetic and sensory neurons and survival of proprioceptive neurons. Later published evidence shows that NT-3 is essential for the development and maturation of the nervous system, including the regulation of neuronal survival, neurite outgrowth, synaptic plasticity, and neurotransmission (Alto et al., 2009). Among them, NT-3 mRNA is highly expressed in the developing spinal cord in MNs but decrease in the adult spinal cord. NT-3 is essential for MN survival, target finding, innervation, and synapse formation, mainly during development and early postnatal maturation (Keefe et al., 2017).
We hypothesize that adeno-associated viral vector (AAV)-mediated NT-3 overexpression may be the potential approach to reverse SCI-induced MN degeneration by mimicking the role of NT-3 played during MN development. By using a clinically approved serotype 2 of AAV (AAV2) mediated transfection of human NT-3 (encoded by NTF3), we first found that NT-3 overexpression induced neurite outgrowth in cultured spinal cord neurons (Wang et al., 2018). Importantly, we found that retrograde transport of NT-3 to the lumbar MNs by injection of AAV2-NT-3 into transiently demyelinated sciatic nerve significantly ameliorated SCI-induced lumbar MN dendritic atrophy and synaptic stripping by remodeling of lumbar motor circuits, thus leading to improvement of locomotor function after thoracic spinal cord contusion (Wang et al., 2018). Combined with the findings of Petruska et al. (2010) that AAV-NT-3 delivery altered MN synaptic transmission and Ruitenberg et al. (2005)work that AAV-NT-3 overexpression modified the local lumbar neural circuitry, NT-3 gene therapy may promise tremendous potential for translation research on preventing spinal MN from degeneration and modifying spinal MN function in amyotrophic lateral sclerosis and SCI. Interestingly, Wang et al. (2018) previously revealed that expression of NT-3 within the spinal cord promotes regeneration of sensory axons. Now, we also observed high NT-3 expression in DRG via a peripheral delivery route. These findings suggested that it would be worth testing whether NT-3 gene therapy have functional role in aiding the repair of sensory function and chronic pain in SCI and CNS disorder.
Literature shows that the expression of tropomyosin receptor kinase C, the highest affinity receptor of NT-3, was not limited to MNs, but was seen on many other neurons throughout the grey matter of the spinal cord, suggesting that other populations of neurons may also respond to the NT-3 expression (Keefe et al., 2017). Additionally, Duricki et al. (2016) demonstrate that intramuscular delivery of NT-3 improves recovery by facilitating the sprouting of corticospinal tract after stroke, indicating NT-3 is secreted after transport to the spinal cord. Based on these findings, we assume that MN-overexpressed NT-3 secretes outside of lumbar MNs and plays an important role in the remolding of surrounding spinal neurons or spared descending axons, which may be accounted for NT-3-mediated locomotor recovery after SCI. This hypothesis was proved in our recent study (Han et al., 2019. In this study, we revealed the importance of descending pathways in locomotor performance; and demonstrated that the propriospino-MN relay circuits that bypass the injury site are the key contributor to AAV2-NT-3 (the same vector as we used before (Wang et al., 2018)) mediated locomotor recovery following SCI (Figure 1). The identification of these underlying mechanisms would not only be a conceptual breakthrough, but also suggest that therapeutic strategies that simultaneously target both descending propriospinal pathways and lumbar MNs would enable a better functional recovery for patients with SCI. As CNS axons that damaged after SCI have limited regeneration capacity, this finding also called for developing therapeutic treatments that enable to reactivate the remaining superspinal and/or intraspinal motor pathways for functional restoration (Figure 1). Wagner et al. (2018) has proved a similar concept with numerous efforts by developing an implantable stimulation system that targets the lumbosacral spinal cord to improve locomotor performance following SCI. The promising results with this therapy have been obtained in early-phase clinical trials.
Given the diversity of disease targets, it has become clear that there can be no single vector that is suitable for all applications. AAV vectors are one of the most promising vector systems for efficient gene transfer and long-term gene expression for CNS disorders. CNS-directed AAV-NT-3 gene therapies were shown to be safe and well-tolerated in phase I and II clinical trials for CNS disorders (Lykken et al., 2018). However, no clinical studies have been initiated to investigate the effectiveness of NT-3 for promoting motor functional recovery after SCI. In our animal studies, we took advantage of the human NT-3 transgene which could effectively be translated into clinical therapy. Thus, our preclinical study paves the way for AAV-NT-3 as a gene therapy for SCI. However, when it comes to clinical translation, we need to contemplate some limitations in our studies and beyond. Since we utilized a peripheral nerve delivery route that involves induced transient demyelination of the sciatic nerve to achieve high NT-3 expression in the spinal cord, an alternative virus delivery approach such as injection into hindlimb muscles would be considered for future operation, which may require the generation of higher titer viral preparations. In addition, although AAVs display low immunogenicity and apparent lack of pathogenicity, the observation of host cell-mediated immunity targeting antigens of the AAV has been reported in AAV-based gene therapies during clinical trials (Manno et al., 2006). It is therefore critical to first determine vector–host interactions in patients that underpin the future success of AAV-NT-3 gene therapy.
As the pathology of the lesioned spinal cord is enormously complex, it is almost a certainty in the SCI science community that there is no single “magic bullet” that can address all medical problems, and that the coming treatments will involve combinations of other improved therapies. For instance, electrical/epidural stimulation has promised huge gains for people with paralysis. However, continuous stimulation is poorly effective in facilitating locomotion overground (Wagner et al., 2018) possibly due to the atrophy in the neuromuscular system that follows chronic paralysis. NT-3 gene therapy would be the potential for elevating the neuromuscular plasticity to counteract their deteriorations. Recent research has also shown that neurotrophins related signaling pathways are involved in aerobic exercise after SCI. Perhaps AAV-NT-3 gene therapy will be more effective for improving motor function when combined with rehabilitation post-SCI. Continuing research will include the improvement of the efficiency of NT-3 expression in certain cell types in CNS, and the incorporation of established therapeutic treatments to extend the range of applications of NT-3 gene therapy.
We apologize for authors whose works were listed but could not be cited due to reference limitations.
This work was supported in part by NIH 1R01 100531, 1R01 NS103481, Merit Review Award I01 BX002356, I01 BX003705, I01 RX002687 from the USA Department of Veterans Affairs, Mari Hulman George Endowment Funds (to XMX).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Alto LT, Havton LA, Conner JM, Hollis ER, Blesch A, Tuszynski MH. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradbury EJ, Burnside ER. Moving beyond the glial scar for spinal cord repair. Nat Commun. 2019;10:3879. doi: 10.1038/s41467-019-11707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duricki DA, Hutson TH, Kathe C, Soleman S, Gonzalez-Carter D, Petruska JC, Shine HD, Chen Q, Wood TC, Bernanos M, Cash D, Williams SC, Gage FH, Moon LD. Delayed intramuscular human neurotrophin-3 improves recovery in adult and elderly rats after stroke. Brain. 2016;139:259–275. doi: 10.1093/brain/awv341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Q, Ordaz JD, Liu NK, Richardson Z, Wu W, Xia Y, Qu W, Wang Y, Dai H, Zhang YP, Shields CB, Smith GM, Xu XM. Descending motor circuitry required for NT-3 mediated locomotor recovery after spinal cord injury in mice. Nat Commun. 2019;10:5815. doi: 10.1038/s41467-019-13854-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hohn A, Leibrock J, Bailey K, Barde YA. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990;344:339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- 6.Keefe KM, Sheikh IS, Smith GM. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int J Mol Sci. 2017 doi: 10.3390/ijms18030548. doi: 103390/ijms18030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lykken EA, Shyng C, Edwards RJ, Rozenberg A, Gray SJ. Recent progress and considerations for AAV gene therapies targeting the central nervous system. J Neurodev Disord. 2018;10:16. doi: 10.1186/s11689-018-9234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990;247:1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- 9.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 10.Petruska JC, Kitay B, Boyce VS, Kaspar BK, Pearse DD, Gage FH, Mendell LM. Intramuscular AAV delivery of NT-3 alters synaptic transmission to motoneurons in adult rats. Eur J Neurosci. 2010;32:997–1005. doi: 10.1111/j.1460-9568.2010.07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruitenberg MJ, Levison DB, Lee SV, Verhaagen J, Harvey AR, Plant GW. NT-3 expression from engineered olfactory ensheathing glia promotes spinal sparing and regeneration. Brain. 2005;128:839–853. doi: 10.1093/brain/awh424. [DOI] [PubMed] [Google Scholar]
- 12.Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, Rowald A, Seáñez I, Caban M, Pirondini E, Vat M, McCracken LA, Heimgartner R, Fodor I, Watrin A, Seguin P, Paoles E, Van Den Keybus K, Eberle G, Schurch B, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71. doi: 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Wu W, Wu X, Sun Y, Zhang YP, Deng LX, Walker MJ, Qu W, Chen C, Liu NK, Han Q, Dai H, Shields LB, Shields CB, Sengelaub DR, Jones KJ, Smith GM, Xu XM. Remodeling of lumbar motor circuitry remote to a thoracic spinal cord injury promotes locomotor recovery. 2018 doi: 10.7554/eLife.39016. Elife. doi: 10.7554/eLife.39016. [DOI] [PMC free article] [PubMed] [Google Scholar]


