Abstract
The retina may suffer neurodegenerative damages, as other tissues of the central nervous system do, and serious eye diseases may develop. One of them is age-related macular degeneration, which causes progressive loss of vision due to retina degeneration. Treatment of age-related macular degeneration focuses on antioxidant agents and anti-vascular endothelial growth factor compounds, among others, that prevent/diminish oxidative stress and reduce neovascularisation respectively. The phytochemicals, medicinal plants and/or plant-diet supplements might be a useful adjunct in prevention or treatment of age-related macular degeneration owing to their antioxidant and anti-vascular endothelial growth factor properties. This review article presents the most investigated plants and natural products in relation to age-related macular degeneration, such as saffron, ginkgo, bilberry and blueberry, curcuma or turmeric, carotenoids, polyphenols, and vitamins C and E. This study provides up-to-date information on the effects, treatments, safety and efficiency of these phytotherapy products.
Keywords: age-related macular degeneration, bilberry, blueberry, curcuma, carotenoids, ginkgo1, polyphenols, saffron, vitamins
Introduction
Age-related macular degeneration (AMD) is a disease that causes progressive loss of central vision as a consequence of the macular region degeneration (Muangnoi et al. 2019). The development of this disease is related to histopathological features, which include pigmentary disturbances, drusen, Bruch’s membrane thickening and basal laminar deposits. Drusen are lipid material deposits that accumulate underneath the retinal pigment epithelium (RPE) and can be observed as pale yellow spots on the retina (Evans and Lawerenson, 2017). The RPE is formed by specialized cells that constitute the outer blood-retinal barrier. This epithelium lies on the interface between the neural retina and the choriocapillaris. The presence of drusen causes pigment epithelium disturbances and represents a significant risk factor for AMD. AMD could be assorted according to its severity, but is most frequently classified into early and late stages (Muangoi et al., 2019; Figure 1). The early stage, with slow progression, commonly causes mild vision distortion or is asymptomatic. Small- or medium-sized drusen are formed at the back of the eye, and no retinal pigmentary changes appear. Late-stage AMD includes the neovascular form that rapidly progress, and the atrophic form that more slowly develops. As its name suggests, the neovascular form implies the presence of the choroidal neovascularisation complex, which is associated with retinal lesions such as pigment epithelial detachments, presence of intraretinal and subretinal fluid, macular haemorrhage, hard exudates, or subretinal fibrous scars (Mitchell et al., 2018). It is also known as wet AMD. Athropic AMD, also termed dry AMD, is associated with outer retinal thinning that surrounds the central macula region. Advanced stages (geographic atrophy) are characterized by the degeneration of photoreceptors and their supporting tissues (RPE, Bruch’s membrane and choriocapillaris; Li et al., 2018). The RPE, which maintains retinal homeostasis, is particularly prone to oxidative stress, which contributes to AMD development (Li et al., 2019). Cells from this tissue possess great metabolic activity and, thus, high energy requirements, and therefore large numbers of mitochondria. Mitochondrial activity, mainly oxidative phosphorylation, leads to reactive oxygen species (ROS) generation. In addition, light exposure increases eyes’ vulnerability to oxidative stress. Superoxide dismutase, catalase or glutathione peroxidise, are antioxidant enzymes that neutralise these reactive species. An imbalance between ROS production and neutralization leads to oxidative stress, which affects macromolecules (proteins, lipids, DNA), and untimely tissue damage or cell apoptosis (Bungau et al., 2019). Furthermore as humans become older, there is an increasing risk of the oxidation of cellular constituents and, as a consequence, malfunctions and tissue degeneration. The RPE and retinal macula are tissues affected by oxidative stress (Datta et al., 2017). Other risk factors for AMD related to oxidative stress are alcohol consumption, cigarette smoking or dietary factors (Kim et al., 2017). Treatment of wet AMD focuses on antioxidants that prevent or diminish oxidative stress, and also anti-vascular endothelial growth factor (VEGF) compounds to reduce neovascularization by lowering VEGF release and/or expression (Alshamani et al., 2019). Beneficial effects of plant extracts are related to bioactive phytochemicals, such as carotenoids and polyphenols, and their synergistic combinations. Such phytochemicals are able to reduce oxidative stress, and modulate angiogenesis and inflammation, by protecting the RPE from damage (Peddada et al., 2019). Furthermore, the keen interest shown in them is also related to their good tolerability (few secondary effects) and efficacy. Nutritional dietary supplements of a botanical origin can affect several pathways at the same time by reducing inflammation, oxidative stress or neurodegeneration (Heitmar et al., 2019). They can also improve ocular blood flow and signal transduction, or reduce apoptosis in the RPE (Xu et al., 2017). Even anti-VEGF and antioxidant treatments have had positive results, but there is no cure to date for wet AMD and no treatment for dry AMD, being this disease the leading cause of blindness and visual impairment in the elderly population (older than 70 years of age) in industrialized countries (Hanus et al., 2015). Wong et al. (2014) have estimated that around 196 million of people in 2020, which will increase to 288 million people in 2040, will develop this disease worldwide. Nowadays 8.7% of the world’s adult population has AMD. Thus implementing eye care programs to prevent or treat AMD has become a global health priority.
Figure 1.
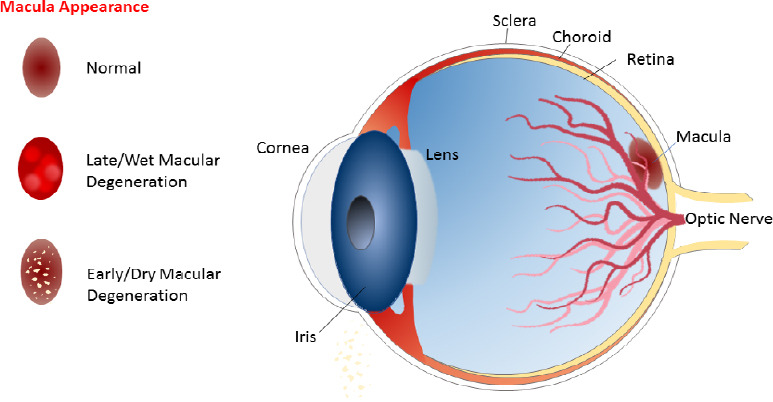
Age-related macular degeneration types and macular appearance.
This review focuses on the effect of phytochemicals, medicinal plants or plant-diet supplementation on treating or preventing AMD. The aim of our article is to integrate and analyse up-to-date information on the use of natural products and plants to safely and effectively prevent and treat this disease. A systematic and standardized review was conducted using scientific databases such as PubMed, MEDLINE, and SciFinder. Academic Google was also consulted. Articles from 2010 to 2019 were included. The search terms were ‘AMD’, ‘oxidative processes’, ‘antioxidant substances’, ‘AMD AND/OR saffron’, ‘AMD AND/OR Ginkgo biloba’, ‘medicinal plants AND AMD’, ‘polyphenols AND AMD’, ‘polyphenols AND eye health’, ‘flavonoids and AMD’, ‘flavonoids AND eye health’, ‘anthocyanins AND AMD’, ‘anthocyanins AND eye health’, ‘resveratrol AND AMD’, ‘resveratrol AND eye health’, ‘age related macular degeneration AND carotenoids OR xanthophyllls OR lutein OR zeaxanthin OR vitamin A. Papers published before 2010 were used whenever necessary to explain concepts and processes.
Medical Plants
Saffron
Crocus sativus L. (Iridaceae), commonly known as saffron has been used as a herbal medicine, and as a coloring and flavoring spice, since ancient times (Evans, 2009). In the last 10 years, growing evidence has highlighted the pharmacological profile of saffron and its constituents, including potential therapeutic applications on the central nervous system (WHO, 2007; Melnyk et al., 2010; Poma et al., 2012; Christodoulou et al., 2015; Nassiri-Asl and Hosseinzadeh, 2015; Bagur et al., 2018; Bukhari et al., 2018; Leone et al., 2018; Hatziagapiou et al., 2019; Heitmar et al., 2019; Moratalla-López et al., 2019; Mykhailenko et al., 2019). The neuroprotective activity of saffron has also been investigated in AMD. Thus, pharmacological studies on saffron supplementation therapy provide important evidence about its neuroprotective actions, and several research works attribute saffron’s therapeutic properties to its main components crocins, crocetin, picrocrocin, and safranal (Figure 2). Most studies indicate that saffron has a potent antioxidant activity, mainly due to saffron carotenoids (Christodoulou et al., 2015; Rahaiee et al., 2015; Broadhead et al., 2016; Di Marco et al., 2019). Furthermore, the binding capacity of saffron metabolites to biomolecules protects them from free radicals (Kanakis et al., 2009; Christodoulou et al., 2015). Although, saffron’s antioxidant activity is attributed mainly to crocin, the synergistic effect of all bioactive constituents should be considered (Di Marco et al., 2019). It is interesting to note that many authors have reported that saffron components possess antinflammatory and antiapoptotic effects, possibly by the inhibition of caspase-mediated apoptosis after retinal damage (Nam et al., 2010; Yamauchi et al., 2011; Ohno et al., 2012; Tamaddonfard et al., 2013). It is also known that crocin and crocetin increase oxygen diffusion and improve ocular blood flow in the retina and choroid, factors that play an important role in the disease (Xuan et al., 1999; Giaccio, 2004). Later Corso et al. (2016) described a novel mechanism responsible for saffron’s neuroprotective effect through the regulation of P2X7 receptors, which are affected in AMD. Nevertheless, further research is required to clarify the exact mechanism as peculiar characteristics of saffron components support the hypothesis that saffron does not act as a simple antioxidant, but has complex mechanisms of action that range from antioxidant activity to direct gene expression control (Natoli et al., 2010). In addition to animal models that have demonstrated the protective effects of saffron and its components (Fernández-Sánchez et al., 2012; Di Marco et al., 2013; Bisti et al., 2014), promising from clinical trials results have been reported (Table 1). Seven published clinical studies have assessed the impact of oral saffron supplementation (daily dose range: 20–50 mg) on vision-related parameters in AMD patients, of which four were randomized controlled trials (Falsini et al., 2010; Lashay et al., 2016; Riazi et al., 2017; Broadhead et al., 2019), and three were longitudinal interventional studies which reported pre- versus post-intervention comparisons with no control (placebo) group (Piccardi et al., 2012; Marangoni et al., 2013; Di Marco et al., 2019). Both objective and subjective vision-related measures were evaluated. Short-term studies and longer-term follow up demonstrated that saffron supplementation improved visual functions. However, a direct quantitative comparison is not possible because formulation, dose, intervention duration, test methods and outcome measures varied in the cited studies. Toxicology research currently considers saffron safe for human consumption as the dose of 30 mg/day seems efficacious and toxic effects have been reported with 5 g and more, with a lethal dose of approximately 20 g (Mohamadpour et al., 2013; Christodoulou et al., 2015). However, long-term and large-scale research works are required to elucidate the effect on human health.
Figure 2.
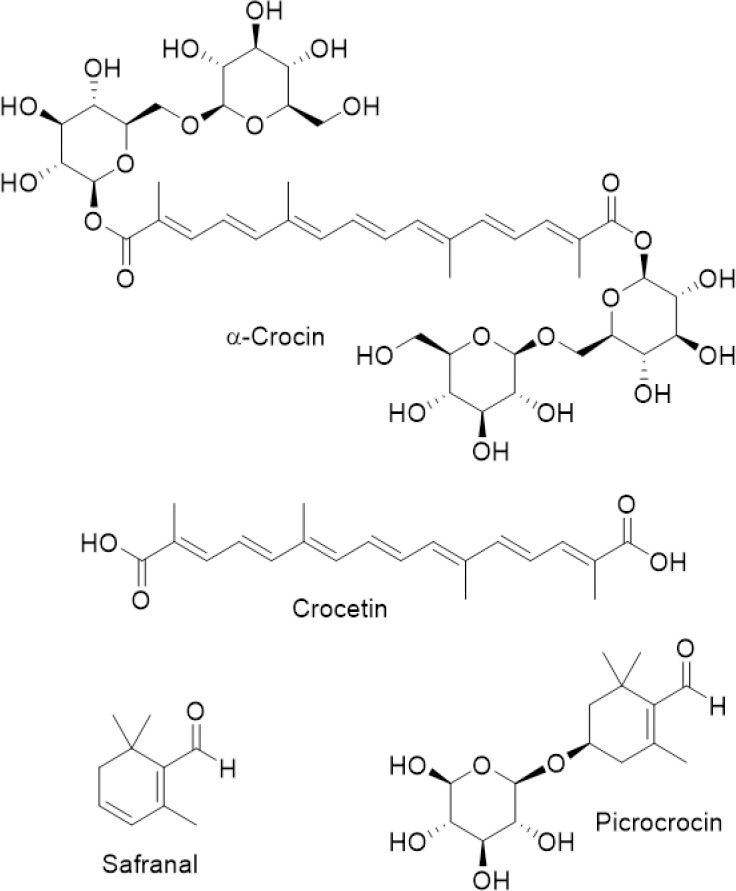
Chemical structures of saffron components of much pharmacological interest.
Table 1.
Summary of data in clinical trials relating to the use of saffron and ginkgo EGb761
| Subjects (n = eyes) | Treatment | Type of study | Visual parameters and results | Reference |
|---|---|---|---|---|
| Early AMD (n = 25) | 20 mg/d | Double-blind, placebo controlled, crossover, RCT 3-month period with cross over for another 3 months | fERG: ↑ in amplitude in patients, but not in placebo group | Falsini et al., 2010 |
| Early AMD (n = 29) | 20 mg/d | Longitudinal interventional open-label study 3 monthly follow-ups over a 15-month period of treatment | Mean VA: ↑ 2 lines fERG: ↑ 0.3 log units | Piccardi et al., 2012 |
| Early AMD (n = 33) Presence of known AMD risk genotypes | 20 mg/d | Longitudinal 3 monthly follow-ups over a 12-month period | fERG: ↑ amplitude and sensitivity amplitude that stabilized after 3 months independent of genotype | Marangoni et al., 2013 |
| Dry and wet AMD (n = 40) | 15 mg, twice a day | Placebo-controlled, RCT 6-month period with follow-ups at 3 and 6 months | CMT: ↓ in saffron and placebo groups in wet AMD, but not in dry AMD ERG: ↑ amplitude in the saffron group (dry and wet AMD) compared to placebo after 3 months, but not 6 months | Lashay et al., 2016 |
| Mild/moderate dry AMD (n = 54) | 50 mg/d | Placebo-controlled, RCT three months | CMT: unchanged BCVA: ↑ in saffron, but not in placebo group CS: ↑ in saffron, but not in placebo group | Riazi et al., 2017 |
| Mild/moderate AMD (n = 96) 73.2% consuming AREDS supplements | 20 mg/d | Double-blind, placebo-controlled, crossover, RCT 3 months followed by crossover for 3 months | BCVA: ↑ in saffron group and AREDS + saffron, but not in placebo mfERG response density: ↑ in AREDS+saffron, but not in the saffron or placebo group mfERG latency: ↓ in saffron group, but not in placebo group | Broadhead et al., 2019 |
| Intermediate AMD (n = 42) Two groups (n = 19) lutein/zeaxanthin n = 23 saffron | – | Longitudinal open-label study 8 monthly follow-ups over a 29 (±5)-month period | fERG Saffron treated AMD patients: Visual function remains stable Lutein/zeaxanthin treated patients: Deterioration of retinal functions | Di Marco et al., 2019 |
AREDS: Age-related eye disease study; BCVA: best-corrected visual acuity; CMT: central macular thickness; CS: contrast sensitivity; ERG: electroretinography; fERG: focal electroretinography; mfERG: multifocal electroretinography; RCT: randomized clinical trial.
Ginkgo
The therapeutic benefits of ginkgo (Ginkgo biloba L., Ginkgoaceae) extracts have long since been known, with a long-standing history of use in traditional Chinese medicine (Chassagne et al., 2019). More recently, experimental and clinical studies have revealed the potential benefits of ginkgo for a wide range of pathological conditions, including neuroprotective activities (Singh et al., 2012; Mohanta et al., 2014; Montes et al., 2015; Datta et al., 2017; Mojaverrostami et al., 2018; Shu et al., 2019). Regarding its chemical composition, two main groups of bioactive metabolites are considered responsible for ginkgo medicinal effects: terpene lactones (Ginkgolides A, B, C and J and bilobalide) and ginkgo flavones (quercetin, kaempferol and iso-rhamnetin glycosides as the main flavonoids, together with biflavonoids ginkgetin/isoginkgetin; Figure 3) (Wilkinson and Fraunfelder, 2011; Ude et al., 2013). The pharmacologically active ginkgo leaf extracts are available as standardised preparations EGb 761 and LI1370 (Evans, 2013).Free radical quenching properties, reduction of platelet aggregation and improved blood flow of ginkgo extracts are attributed to the antioxidant and radical scavenging properties of ginkgo flavonoids and terpenoids, along with the potent platelet activating factor inhibition of ginkgolides (Wilkinson and Fraunfelder, 2011; Singh et al., 2012; Sin et al., 2013). In addition, flavonoids probably reduce oxidative damage to lipid membranes (Singh et al., 2012). Consequently, due to vascular factors and oxidative damage are hypothesized as potential mechanisms in AMD pathology, an interest in using ginkgo extract has aroused for AMD treatment. Currently, ginkgo extract is considered safe within the daily dose range (Sin et al., 2013). It is worth mentioning, however, the increased risk of bleeding because ginkgolides are potent platelet activating factor antagonists (Evans, 2013). In vitro and in vivo experiments have shown the beneficial effects of EGb 761 on functional retinal impairment, retinal microcirculation and retinal tissue protection from oxidative stress (Spadiene et al., 2013; Martinez-Solis et al., 2019). Preliminary clinical studies have investigated the efficacy of ginkgo extracts (Evans, 2013), including two randomized controlled trials with positive effects on vision in patients with AMD (Table 1). According to Evans (2013) and Sin et al. (2013), deeper research on ginkgo clinical efficacy should be carried out.
Figure 3.
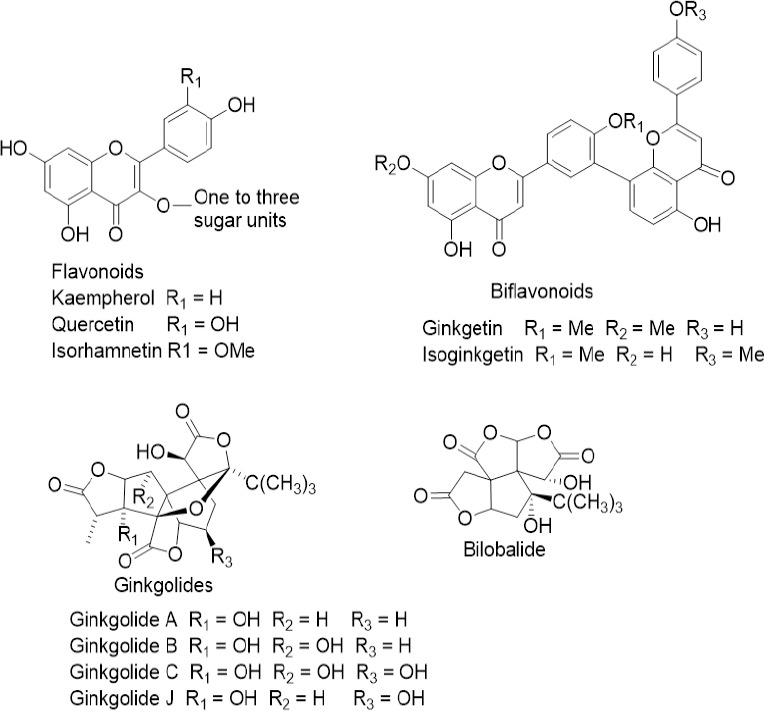
Structures of bilobalide and main Ginkgo biloba flavonoids, biflavonoids, and ginkgolides.
Bilberry and blueberry
Berry extracts containing anthocyanins have become quite popular in recent years among patients with AMD. Bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) from Ericaceae contain a variety of phenolic compounds, including flavonols (quercetin, catechins), tannins, ellagitannins and phenolic acids. However, anthocyanins make by far the largest contribution to their phytochemical mix. Anthocyanins are used for manye age-related ocular disorders, but human data on bilberry for eye disorders are lacking (Gerding, 2009). The mechanisms of action behind berries’ beneficial effect on the eye are not completely understood. The ability to improve oxygen and blood delivery to the eye, and their potent antioxidant and free radical scavenging properties, suggest a potential benefit for AMD. In addition, as proven a long time ago, anthocyanosides have an affinity for the pigmented epithelium area of the retina, the portion of the retina responsible for vision and adjustments to light and dark (Levy and Glovinsky, 1998). Evidence (Milbury et al., 2007) has also shown that bilberry anthocyanins modulate oxidative stress defence enzymes heme oxygenase-1 (HO-1) and glutathione S-transferase-pi in human retinal pigment epithelial cells. Recently, Huang et al. (2018) demonstrated that the blueberry anthocyanin extract, and some of its components such as malvidin, malvidin-3-glucoside, and malvidin-3-galactoside, are able to reduce H2O2-induced oxidative stress by lowering the levels of ROS and malondialdehyde, and by increasing levels of superoxide dismutase, catalase and glutathione peroxidase in human retinal pigment epithelial cells. One cause of AMD is the accumulation of lipofuscin A2E (a pyridinium bisretinoid) and damage mediated by blue light illumination. A2E absorbs blue light and forms singlet oxygen through excitation, which can either cause damage to the retinal pigment epithelial cells or the epoxidation of A2E. A2E cannot be enzymatically degraded and accumulates in the RPE with age. Indeed the study of Jang et al. (2007) has shown that anthocyanins from blueberries are capable of reducing A2E-epoxidation by quenching singlet oxygen and protecting RPE cells from A2E cytotoxicity. In turn, treatment time appears to be an important factor in preventing macular degeneration. Fursova et al. (2005) observed that long-term (1.5 to 3 months) supplementation with bilberry extract to OXYS rats, affected by accelerated aging, which is associated with high sensitivity to oxidative stress, was effective in preventing degeneration. After 3 months of treatment, more than 70% of the control OXYS rats had macular degeneration, while bilberry supplementation completely prevented impairments in lenses and the retina. More recently, Osada et al. (2017) demonstrated that bilberry extract attenuates photo-induced apoptosis and visual dysfunction. This is most likely due, at least in part, to ROS reduction, and to subsequent endoplasmic reticulum stress attenuation in a murine model of photo-stressed retina (750 mg/kg body weight). It is important to highlight that the main complication for the clinical diffusion of anthocyanins is the potential slight absorption in cells or the circulatory system as anthocyanins are large highly water-soluble molecules. Kalt et al. (2008) revealed that pigs fed with diets supplemented with 0%, 1%, 2% or 4% (w/w) blueberries for 4 weeks accumulated anthocyanins in tissues beyond the blood-brain barrier (liver, eye, cortex and cerebellum).
Curcuma or turmeric
Curcumin (diferuloylmethane) is the main active curcuminoid and a water-insoluble pigment isolated from turmeric (curcuma), the dried rhizome of Curcuma longa L, (Zingiberaceae). It possesses multiple therapeutic properties and is considered a potential candidate for treating eye diseases (Radomska-Leśniewska et al., 2019). Several researchers (Wang et al., 2013; Pescosolido et al., 2014) have demonstrated the beneficial effects of curcumin on ocular diseases, such as chronic anterior uveitis, diabetic retinopathy, glaucoma, AMD, and dry eye syndrome, which make curcumin a potent therapeutic drug candidate for inflammatory and degenerative retinal eye diseases. In vitro and in vivo studies suggest that curcumin has various mechanisms, which supports the concept of it interacting with multiple cellular signalling pathways and modulating numerous molecular targets. It exerts anti-inflammatory, anti-tumour, antioxidant and antiangiogenic properties through the modulation of numerous biochemical mediators (Hewlings et al., 2017; Peddada et al., 2019). Peroxisome proliferator-activated receptor-γ (PPAR-γ) ligands play an antiangiogenic role in AMD. Curcumin, a PPAR-γ agonist, may therefore play a role in mitigating AMD progression through the down-regulation of the proinflammatory functions of microglia. Recently, Saberi et al. (2019) reported that curcumin activates PPAR-γ that, in turn, down-regulates matrix metalloproteinases production. It is well-known that matrix metalloproteinase-9 causes extracellular matrix degradation and stimulates RPE cell migration to Bruch’s membrane, and thus contributes to AMD pathogenesis. It has also been shown that curcuma extract and its curcuminoids provide significant protection against photooxidative damage and apoptosis in human retinal pigment epithelial cells laded with A2E in a dose-dependent manner (Park et al., 2017). Other curcuminoids, such as 7-(3,4 dihydroxyphenyl)-5-hydroxy-1-phenyl-(1E)-1-heptene isolated from Curcuma comosa, also protect human retinal pigment epithelial cells against oxidative stress, (H2O2)-induced partly by enhancing several anti-oxidant defence mechanisms. The anti-oxidant activity (IC50) of this compound is similar to that of vitamin C (Jitsanong et al., 2011). Previously, Mandal et al. (2009) observed significant retinal neuroprotection in rats fed with diets supplemented with curcumin, which involved the inhibition of nuclear factor-κB activation and the down-regulation of cellular inflammatory genes. When tested on retina-derived cell lines (661W and ARPE-19), curcumin pre-treatment protected these cells from H2O2-induced cell death by up-regulating cellular protective enzymes, such as HO-1, thioredoxin. Studies into curcumin’s efficacy have been limited mostly to animal studies. Moreover, the biomedical potential of curcumin is not easy to develop given its low solubility and oral bioavailability. Improving the pharmacological profile by reconstituting curcumin with non-curcuminoid components of turmeric has been found to substantially increase bioavailability (Antony et al., 2008). Curcumin is well tolerated and does not show any dose-limiting toxicity when administered at doses of up to 8 g/day for 3 months (Basnet and Skalko-Basnet, 2011).
Natural Products
Carotenoids
Carotenoids are relatively complex molecules included in the group of orange, yellow and red lipo-soluble pigments synthesised by plants, algae, bacteria and fungi. More than 750 different structures have been described, some of which (α and β-carotenes, β-cryptoxanthin) are considered provitamin A as they can be transformed into retinol in the intestine or liver (vitamin A). To act as provitamin A, a carotenoid must contain a β-ionone structure. Carotenoids are included in the most prominent lipophilic antioxidants group. They protect organisms from photosensitised formation and 1O2 accumulation through the absorption of excitation energy and its dissipation as heat. For this reason, they play an important role in ultraviolet- and light-stress damage. Their efficiency as antioxidant and scavengers of ROS, especially single molecular oxygen and peroxyl radicals, means they play a key role in the prevention and treatment of diseases related to oxidative stress and ageing, including AMD (Khoo et al., 2019; Sandmann, 2019). Humans cannot synthesise carotenoids, which must be obtained from diet or using supplements. Although they are widespread in nature, only 40 are present in the human diet and only half can be found in tissues and biological fluids. The major carotenoids present in human blood and tissues are α- and β-carotene, lycopene, lutein, zeaxanthin and β-cryptoxanthin,, of which, lutein, zeaxanthin and their stereoisomer meso-zeaxanthin selectively accumulate in the retina of the eye to enrich the macular pigment of the central macula area. In the central fovea of the human retina, lutein and zeaxanthin reach concentrations between 0.1 and 1 mM, which is about 1000-fold higher than in other tissues. The inner plexiform layer, Henle’s fiber layer and Muller cells are the places where maximal accumulation occurs (Jia et al., 2017; Buscemi et al., 2018). The double bound position in one of the end rings creates differences in their activities and functions, which are correlated with their retinal spatial distribution. In the inner macula, the concentration of zeaxanthin, which is a much more effective antioxidant, is about twice that of lutein. Conversely, lutein is the dominant component in the peripheral retina (Jia et al., 2017). Meso-zeaxanthin, a substance that is much better capable of quenching oxygen radicals, is considered a metabolite of lutein and its presence in ocular tissues indicates its conversion at the eye level (Bungau et al., 2019). Lutein has a greater filtering efficiency and zeaxanthin is better at preventing UV-induced light lipid peroxidation (Jia et al., 2017). Macular carotenoids are considered the most potent antioxidants to protect against the development of AMD and also other eye disorders. These substances are believed to protect the retina in several ways: filtering high energy and short wave-length blue light; protecting the underlying photoreceptor cell layer from light-induced damage; neutralizing oxidation reactions in photoreceptor cells (Berstein, 2016). It has been reported that lutein, zeaxanthin and astaxanthin can also modulate other signaling cellular pathways in retinal pigment epithelium cells. In ARPE-19 cells, these substances can activate the nuclear factor erythroid 2-related factor 2 pathway, thereby protecting the cells against oxidative damage by upregulation of the antioxidant system. The nuclear factor erythroid 2-related factor 2 pathway regulates the transcription of genes (NQO1, GCLm, and HO-1) with antioxidant effects and related to antioxidant active enzymes expression such as superoxide dismutase, NQO1, HO-1 and catalase, or to glutathione or thioredoxin metabolism. Some evidence exists that lutein can reduce inflammation in these cells by protecting the proteosoma against photo-oxidative damage and the modulation of the expression of inflammation-related genes like IL-8 (Frede, 2017). Moreover, carotenoids can preserve optimal visual performance by reducing age-related lipofuscin formation in the retinal pigment epithelium. Lipofuscin is a protein-lipid mixture integrated by metabolites from vitamin A and products from lipid peroxidation. This compound induces mitochondria damage and apoptosis in retinal pigment epithelium cell cultures. It has been found that lutein and zeaxanthin diminish the amount of lipofuscin formed in vivo and in culture cells (Berstein, 2016). These substances can also abolish the elevated expression of matrix metalloproteinase-2 (MMP-2) and tissue inhibitor metalloproteinase-2 (TIMP-2), observed in H2O2 treated ARPE-19 cells, and are able to contribute beneficially to matrix homeostasis altered in AMD involved in oxidative stress (Jia et al., 2017). While most research works seem to have confirmed a relation between xanthophylls intake and an increase in their serum and macular concentrations, not all of them have observed a benefit in preventing and treating AMD. Human nutritional studies indicate that lutein and zeaxanthin administration may improve visual performance by enhancing contrast sensitivity, and by reducing glare disability and photo stress recovery (Khoo et al., 2019). Although several studies have shown the protective effects of these carotenoids in AMD, in others the results have been negative because these substances did not delayed the progression of AMD (Evans and Lawrenson, 2017; Khoo et al. 2019, Bungau et al., 2019). Age-Related Eye Disease Study 2 (AREDS 2) is a multicentre, randomized placebo-controlled study designed to demonstrate whether supplementation with lutein and zeaxanthin can slow the AMD progression rate. This study shows that lutein (10 mg/d) and zeaxathin (2 mg/d) supplementation intake are inversely associated with the prevalence of neovascular AMD, geographical atrophy, and large or extensive intermediate drusen. However, a comparison with placebo demonstrates no statistically significant reduction in progression to advanced AMD (AREDS, 2014). The significant dose range of lutein intervention to improve visual function in the AMD population is 2.5 to 20 mg/d, with 2.5 mg/d being the lowest effective dose. The recommended dietary intake of lutein supplements does not exceed 6 mg/d in discontinuous treatment (Jia et al., 2017). It is noteworthy that although AREDS formulation initially contained β-carotene as an antioxidant substance, it had to be replaced with lutein and zeaxanthin because of a potentially increased incidence of lung cancers, mostly in participants who previously smoked. For this reason, lutein and zeaxanthin should be considered a better option than β-carotene in preventing the development of AMD (AREDS, 2014). Another study concludes that lutein-rich and zeaxanthin-rich diets may protect against intermediate AMD in female patients under the age of 75 years. The Blue Mountain Eye study reports that higher dietary lutein and zeaxanthin intake reduce the risk of incident early or neovascular AMD over 5 and 10 years (Sin, 2013). A systematic Cochrane review, published in 2017 and included six studies, indicates that lutein intake (with or without zeaxanthin) induces only a slight effect in reducing progression to late AMD, neovascular AMD and geographic atrophy compared to a placebo (Evans and Lawrenson, 2017). It is also worth mentioning the possible benefit of the synergic association of these carotenoids, mainly lutein, with other antioxidants such as polyphenols and ω-3 LCPUFAs (Yanai et al., 2018). In a cross-sectional case-controlled study, the authors find that lower circulatory levels of carotenoids and omega-3 PUFAs, and higher levels of omega-6 PUFAs, oleic acid and saturated fatty acids, increase the odds ratio of developing neovascular AMD (Ng, 2017). No adverse reactions to short-term or long-term lutein/zeaxanthin dietary and/or supplement normal intake are reported. However, researchers note that smokers who take high lutein supplement doses (>10 mg/d) are at increased risk of lung cancer. Yellow spots may also appear on skin during the long-term intake of higher doses (≥ 15 mg/d) of this substance (Jia et al., 2017). No in vitro mutagenic and cytotoxic activities are observed (Ravikrishnan, 2011). The approved Acceptable Daily Intake (ADI) values for lutein are 1 mg/kg per day (EFSA- European Food Safety Authority) and 2 mg/kg per day (JECFA- Joint FAO/WHO Expert Committee on Food Additives) (Jia et al., 2017). Vitamin A plays also an important role in retinal health because some carotenoids can be cleaved in vivo into vitamin A active compounds through beta-carotene oxygenase 1 (BCO1) pathway. Vitamin A is a fat-soluble substance found in three different oxidative states: alcohol (retinol), acid (retinoic) and aldehyde (retinal). It normally exists in the body as retinol (Barlett and Eperjesi, 2004). Epidemiological and interventional studies, but not all, show that a significant increase in the intake of fruit and vegetables rich in vitamin A lowers the risk for any AMD stage (Khoo et al., 2019). Excessive vitamin A intake is potentially toxic to the liver and other tissues, and is also associated with intracranial hypertension (Barlett and Eperjesi, 2004).
Polyphenols
Polyphenols represent a large group of phytochemicals present in many fruit and vegetables, and in some beverages prepared from plants, such as wine, tea and coffee. They have proven health benefits: antioxidant, anti-inflammatory, antiallergic, antimicrobial, and antiviral effects (Bungau et al., 2019). One of the best known health-enhancing properties of these compounds is their positive effect on vision (Pawlowska et al., 2019), which are listed in the Figure 4. The main polyphenols investigated for age-related eye diseases are listed below.
Figure 4.
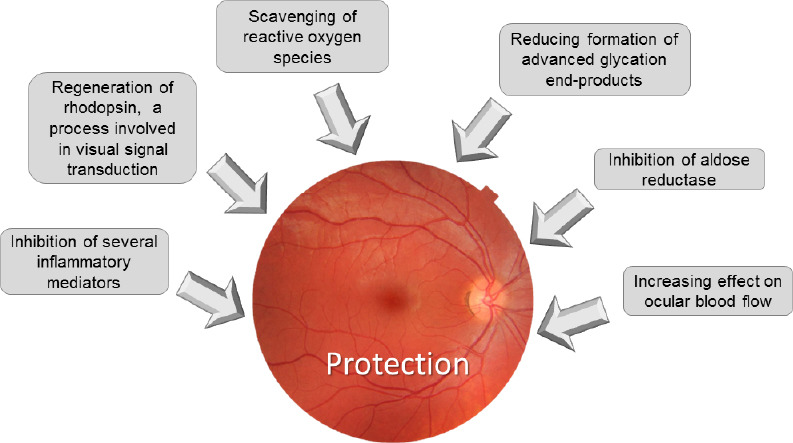
Beneficial effects of polyphenols on the eye lens.
Stilbenes: resveratrol
Resveratrol (3,5,41-trihydroxy-trans-stilbene) (RV) (Figures 4 and 5) is found in food sources such as, bilberries, blueberries, and others Vaccinum species, is also presents in cocoa, peanuts, wine, grape juice and other grape products. The study of the therapeutic use of this substance on vision is growing, specifically in disease prevention terms (Abu-Amero et al., 2016). Regarding the activity of RV, Richer et al. (2014) suggest that this compound might contribute to reestablish the retinal architecture, decrease lipofuscin accumulation, increase choroidal thickness (perfusion), cut the glare recovery time, enlarge the macular pigment volume, and improve both contrast sensitivity and visual function, which all apparently improve retinal-RPE-choroidal cellular health. These authors conclude that RV offers long-term efficacy against AMD. The review by Abu-Amero et al. (2016) indicates that RV prevents oxidative stress-induced and sodium iodate-induced apoptosis of human RPE cells in vitro. Moreover, the proliferation of RPE cells has been shown to lower via the inhibition of extracellular signal-regulated protein kinases one and two (ERK1/2) and the mitogen-activated protein kinase signaling cascade. RV has also been reported to protect RPE cells from autoimmune antibodies-induced apoptosis in vitro (down-regulated pro-apoptotic Bcl-2-associated X protein (BAX), which is highly relevant in autoimmune-associated retinopathies. Due to the anti-apoptotic activity RV could be considered a good candidate in the prevention of neurodegenerative diseases like AMD. Although there are many in vitro studies that show the antioxidant efficacy of VR, however in vivo studies show moderate efficacy, that could be important to AMD. RV is reported to exhibit a dose-dependent protective effect against hydrogen peroxide-induced cytoxicity in human retinal D407 RPE cells by rising glutathione peroxidase, catalase and superoxide dismutase activities that lower intracellular ROS levels. Pathogenic late-stage AMD is often characterised by choroidal angiogenesis. The anti-oxidative and anti inflammatory effects of RV are knowed to diminish the incidence of CNV. RV produces inhibitory actions on inflammatory cytokine by transforming growth factor-beta and hypoxia-induced VEGF secretion by human RPE cells this confirms RV can be useful as dietary supplement to manage CNV processes in AMD. In addition, RV down-regulates the expression of nuclear factor-kappaB and hypoxia-induced factor-1α transcription factors by thereby inhibiting VEGF secretion. RV can also activate the eukaryotic factor-2 kinase and to reduce endothelial cell proliferation, VEGF secretion and migration by a novel SIRT1 independent pathway to prevent pathologically aberrant injury-induced angiogenesis. RV acts favorably in mitochondrial biogenesis and has demonstrated to protect RPE cells against acrolein-induced oxidative cytotoxicity by increasing mitochondrial bioenergetics. It has a significant protective effect against hydrogen peroxide-induced cytotoxicity in the RPE. RV has been effective in reducing intracellular ROS accumulation induced by hydrogen peroxide in epithelial crystalline cells in humans. It can also be effective for the eye’s microcirculation because of its vascular improvement properties. RV avoids retinal damage produced by light, and in addition, usually prevents damage, dysfunction and apoptosis in eyes with AMD (Fernández-Araque et al., 2017).
Figure 5.
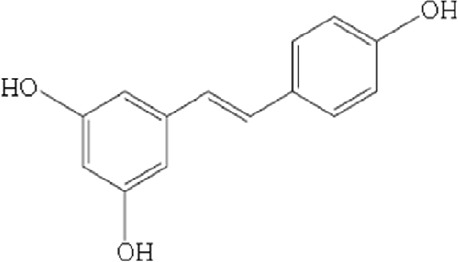
Chemical structure of resveratrol.
Flavonoids
Flavonoids are a group of polyphenolic compounds that confer colour to fruis and vegetables. The classification of flavonoids is shown in Table 2.
Table 2.
Subtypes of flavonoids, modified from Srinivasan, 2014
| Subtypes of flavonoids | Example |
|---|---|
| Flavones | Apigenin, luteolin |
| Flavonols | Quercentin*, kaempherol |
| Flavanones | Naringenin, hesperidin |
| Flavanonols | Dihydroquercentin, dihydrokaempherol |
| Flavan-3-ols Flavan-4-ols Flavan-3,4-diols | Catechin, gallocatechin, epicatechin |
| Anthocyanidins | Cyanidin*, delphindin, pelargonidin |
| Isoflavones | Genistein, daidzein |
*Flavonoids with more useful effects in eye health.
Flavonols: quercetin
Quercetin is a flavonol-type flavonoid ubiquitously found in plant-based foods, such as onions, apples and tea, and is probably the most prominent dietary antioxidant (Wang et al., 2017). Like other polyphenols, it is able to decrease oxidative stress caused by exposure to light; this is confirmed by a reduction in 4-hydroxynonenal, malondialdehyde, 3-nitrotyrosine, and 8-oxo-2′-deoxyguanosine, a marker of oxidative DNA damage levels, and up-regulated HO-1. Quercetin is able to lower levels of proapoptotic (BAX) proteins and to increase those of antiapoptotic (BCL2) proteins. In addition, like all polyphenols, this compound reduces the expression of angiogenic factors VEGF and hypoxia-induced factor-1α (hypoxia-inducible factor 1α), as Pawlowska et al. (2019) demonstrated. Quercetin protects against the light-induced reduction of the outer nuclear layer. Wang et al. (2013, 2017) suggests a mechanism for quercentin protective action in the retina that reduces oxidative stress produced by light exposure. Generally, that protective action may be accentuated by a direct scavenging of ROS and anti-inflammatory, antiapoptotic and antiangiogenic effects, and by an indirect decrease in A2E photooxidation, a lowering of methylglyoxal levels, and the inhibition of the receptor of advanced end glycation product up-egulation. Some polyphenols as quercentin regulate the activity of antioxidant enzymes, including phase II enzymes (Pawlowska et al., 2019). In addition, this flavonoid appears to inhibit CNV induced by laser irradiation in rabbit eyes both in vivo and in vitro, and increases choroidal blood flow. All those actions suggest that quercentin can be a promising candidate for treating AMD. In consequence, quercentin is considered to improve anti VEGF therapy, and this is the only effective treatment in wet AMD (Zhuang, 2011; Pawlowska et al., 2019). Several experimental reports demonstrate that quercentin reduces oxidative stress, which causes apoptosis in RPE cells. This compound protects retinas subjected to oxidative stress and inflammation, induced by light, which can provide new strategies to impede AMD occurrence and progression (Bungau et al., 2019).
Anthocyanidins
Anthocyanidins are red–purple pigments found in plants and come in glycosylated forms. These substances are known to have antioxidants proprieties and they are found in the blue, purple and red fruits, vegetables and flowers. Cell culture studies, animal models, and human clinical trials show that anthocyanidins and anthocyanins possess antioxidative and antimicrobial activities, and improve visual and neurological health (Khoo et al., 2019). Specifically in vision health, the effects of anthocyanins are to: 1) improve the visual function in patients with normal tension glaucoma; 2) prevent the impairment of photoreceptor cell function during retinal inflammation; 3) decrease lens opacity, together with a lower MDA level, by suppressing the cell death of HLE-B3 (lens epithelial cell line) under H2O2-induced oxidative stress; 4) prevent retinal degeneration induced by N-methyl-N-nitrosourea; 5) increase ocular blood flow, but no significant changes in intraocular pressure (Khoo et al., 2017). Particularly, cyanidin-3-O-glucoside (C3g) proves an effective ROS scavenger. In addition, C3g down-regulates VEGF and inhibits senescence induced by light exposure. C3g is shown to inhibit the activation of the G-protein transducing by metarhodopsin II. In addition, C3g stimulates the rhodopsin regeneration, as other anthocyanins present in blackcurrants (Pawlowska et al., 2019).
Vitamin C and vitamin E
Vitamins C and E are extensively studied nutrients for preventing and treating macular degeneration and eye-related diseases. They are known to act as antioxidants. Vitamin C, or ascorbic acid, is an effective antioxidant agent that protects proteins, lipids, carbohydrates and nucleic acids from free radicals and ROS damage. However, no significant association between vitamin C intake and AMD has been observed. The same applies for vitamin E. There is a need to fully explore the potential of these vitamins to prevent AMD (Khoo et al., 2019).
Reflection and Conclusions
AMD treatment focuses on antioxidant substances to prevent or reduce oxidative stress effects, and anti-VEGF substances to reduce neovascularisation, among others. In view of the results of this review, several medicinal plants and natural products might be a beneficial supplementary treatment or an alternative treatment, depending on the AMD type. On the one hand, and according to background knowledge (Datta et al., 2017; Bungau et al., 2019), retinal diseases linked to ageing, such as AMD, are associated with oxidative stress. On the other hand, the late AMD form includes the chorioidal neovascularisation complex, which is associated with serious retina problems (Mitchell et al., 2018). Among medicinal plants and according to the most up-to-date scientific background, saffron, ginkgo, bilberry and blueberry, and curcuma or turmeric, are the most reported possibilities. Regarding natural products, carotenoids and polyphenols are the most commonly used with positive results on AMD prevention or treatment. Among medicinal plants, saffron improves visual function at safe doses far below the lethal dose, as clinical trials show (Falsini et al., 2010; Piccatdi et al., 2012; Marango et al., 2013; Lashay et al., 2016; Riazi et al., 2017; Broadhead et al., 2019; Di Marco et al., 2019). These positive effects appear to be mainly related to antioxidant ability, although the mechanism of action seems complex by ranging from antioxidant activity to gene expression control (Natoli et al., 2010). The same occurs with berry extracts from bilberry and blueberry, whose beneficial effects on AMD are due mainly to the antioxidant ability, together with their capability to improve eye blood flow (Osada et al., 2017; Huang et al., 2018). Although the effects of both species are similar, the main active phytochemicals differ, with carotenoids for saffron and polyphenols (flavonoids) for bilberry and blueberry. The medicinal plants that possess antioxidant and antiangiogenic properties are curcuma and ginkgo. These species are useful in late AMD when neovascularisation exists. However, curcuma, and its main active principle, curcumin, possess low solubility and oral bioavailability that must be compensated for by the addition or presence of non-curcuminoid components to increase their bioavailability (Antony et al., 2008). Regarding isolated compounds, carotenoids (including A vitamin) and polyphenols are the most reported natural products used for AMD treatment with positive results. The former are antioxidant substances responsible for saffron activity. Among these substances, lutein and zeaxanthin are more recommended for reducing the AMD risk than carotene (AREDS, 2014). Furthermore, no clinically significant adverse reactions attributable to lutein and zeaxanthin are reported. However, anti-VEGF activity is not described and would not, therefore, be effective in reducing neovascularisation. Carotenois have different effects, according to the studies consulted. Most of them show positive results in the prevention and protection of AMD. However, there are clinical trials and epidemiological studies that show that these substances do not have a clear beneficial effect on AMD, because they could not delay their progression. Perhaps the heterogenization of researches is one of the causes that produce these differences. Among polyphenols, it is worth highlighting two substance types, stilbenes and flavonoids, more specifically, RV as stilbene, and quercetin and anthocyanidins as flavonoids. The main action of RV is antioxidant (Abu-Amero et al., 2016). Thus, in principle, it could be a good substance for preventing or reducing AMD without neovascularisation, but its anti-inflammatory and anti-oxidant properties combined can be used against AMD with neovascularisation (Fernandez-Araque et al., 2017). Finally, and according to the information found in consulted articles, vitamins C and E show no clear relation with AMD improvement. Despite positive results being reported, in all cases the authors agree that more research, including clinical trials, are necessary to understand the mechanism of action of certain substances, and to know doses and dose regimens to ensure that these treatments are safe and effective. In order to fill in all review information, we would like to make a final reflection and a proposal. According to AREDS 2 (Age-Related Eye Disease Study 2), taking vitamin and mineral supplements daily improves AMD, particularly in the case of dry AMD, when there are many drusen (AREDS2 et al., 2012). This study proposes taking nutritional supplements every day to reduce the risk of suffering from late or wet AMD. The components of this supplement include vitamin C, vitamin E, lutein, zeaxanthin, zinc and copper (Chew et al., 2015). In addition, Khoo et al. (2019) commented that several antioxidants, such as anthocyanins, carotenoids, flavonoids and vitamins reduce the risk of getting eye-related diseases. They stressed that the combined antioxidants and vitamins have a synergistic effect that improves prevention and reduces the risk of macular degeneration. Based on these reflections and all the information in our review work, we can make a proposal to use medicinal plants with anti-VEGF properties and natural antioxidant products to treat and improve AMD. Therefore, Curcuma or Ginkgo plus lutein, zeaxanthin and vitamins may be a good option, but clinical trials are necessary.
Additional file: Open peer review report 1 (84.7KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Vikas Mishra, Basanaheb Bhirao Ambedkar University, India.
P-Reviewer: Mishra V; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Abu-Amero KK, Kondkar AA, Chalam KV. Resveratrol and ophthalmic diseases. Nutrients. 2016;8:200. doi: 10.3390/nu8040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Age-Related Eye Disease Study 2 (AREDS2) Research Group. Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL, 3rd, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Chandra SR, Blodi BA, Domalpally A, Friberg T, Wong WT, Rosenfeld PJ, Agrón E, Toth CA, Bernstein PS, Sperduto RD. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No.3. JAMA Ophthalmol. 2014;132:142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alshamrani M, Sikder S, Coulibaly F, Mandal A, Pal D, Mitra AK. Self-assembling topical nanomicellar formulation to improve curcumin absorption across ocular tissues. AAPS Pharm Sci Tech. 2019;20:254. doi: 10.1208/s12249-019-1404-1. [DOI] [PubMed] [Google Scholar]
- 4.Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A pilot cross-over study to evaluate human oral bioavailability of BCM-95® CG (Biocurcumax™), a novel bioenhanced preparation of curcumin. Indian J Pharm Sci. 2008;70:445–449. doi: 10.4103/0250-474X.44591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AREDS2 Research Group1. Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, Sperduto R, Ferris FL. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1) Ophthalmology. 2012;119:2282–2289. doi: 10.1016/j.ophtha.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagur MJ, Alonso Salinas GL, Jiménez-Monreal AM, Chaouqi S, Llorens S, Martínez-Tomé M, Alonso GL. Saffron: An old medicinal plant and a potential novel functional food. Molecules. 2018;23:30. doi: 10.3390/molecules23010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartlett H, Eperjesi F. An ideal ocular nutritional supplement. Ophthalmic Physiol. 2004;24:339–349. doi: 10.1111/j.1475-1313.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 8.Basnet P, Skalko-Basnet N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;6:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisti S, Maccarone R, Falsini B. Saffron and retina: neuroprotection and pharmacokinetics. Vis Neurosci. 2014;31:355–361. doi: 10.1017/S0952523814000108. [DOI] [PubMed] [Google Scholar]
- 11.Broadhead GK, Chang A, Grigg JR, Mccluskey P. Efficacy and safety of saffron supplementation: current clinical findings critical reviews. Crit Rev Food Sci Nutr. 2016;56:2767–2776. doi: 10.1080/10408398.2013.879467. [DOI] [PubMed] [Google Scholar]
- 12.Broadhead GK, Grigg JR, McCluskey P, Hong T, Schlub TE, Chang AA. Saffron Therapy for the treatment of mild/ moderate age-related macular degeneration: A randomized clinical trial. Graefe’s Arch Clin Exp Ophthalmol. 2019;257:31–40. doi: 10.1007/s00417-018-4163-x. [DOI] [PubMed] [Google Scholar]
- 13.Bukhari SI, Manzoor M, Dhar MK. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed Pharmacother. 2018;98:733–745. doi: 10.1016/j.biopha.2017.12.090. [DOI] [PubMed] [Google Scholar]
- 14.Bungau S, Abdel-Daim MM, Tit DM, Ghanem E, Sato S, Maruyama-Inoue M, Yamane S, Kadonosono K. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxid Med Cell Longev. 2019;2019:9783429. doi: 10.1155/2019/9783429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buscemi S, Corleo D, Di Pace F, Petroni ML, Satriano A, Marchesini G. Thee effect of lutein on eye and extra-eye health. Nutrients. 2018 doi: 10.3390/nu10091321. doi: 103390/nu10091321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chassagne F, Huang X, Lyles JT, Quave CL. Validation of a 16th Century traditional chinese medicine use of Ginkgo biloba as a topical antimicrobial. Front Microbiol. 2019;10:775. doi: 10.3389/fmicb.2019.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chew EY, Clemons TE, Agrón E, Launer LJ, Grodstein F, Bernstein PS. Age-Related Eye Disease Study 2 (AREDS2) Research Group (2015) Effect of omega-3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: the AREDS2 randomized clinical trial. JAMA. 314:791–801. doi: 10.1001/jama.2015.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christodoulou E, Kadoglou NP, Kostomitsopoulos N, Valsami G. Saffron: A natural product with potential pharmaceutical applications. J Pharm Pharmacol. 2015;67:1634–1649. doi: 10.1111/jphp.12456. [DOI] [PubMed] [Google Scholar]
- 19.Corso L, Cavallero A, Baroni D, Garbati P, Prestipino G, Bisti S, Nobile M, Picco C. Saffron reduces ATP-induced retinal cytotoxicity by targeting P2X7 receptors. Purinergic Signal. 2016;12:161–174. doi: 10.1007/s11302-015-9490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Marco F, Romeo S, Nandasena C, Purushothuman S, Adams C, Bisti S, Stone J. The time course of action of two neuroprotectants, dietary saffron and photobiomodulation, assessed in the rat retina. Am J Neurodegener Dis. 2013;2:208–220. [PMC free article] [PubMed] [Google Scholar]
- 22.Di Marco S, Carnicelli V, Franceschini N, Di Paolo M, Piccardi M, Bisti S, Falsini B. Saffron: A multitask neuroprotective agent for retinal degenerative diseases. Antioxidants. 2019;8:224. doi: 10.3390/antiox8070224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans JR. Ginkgo biloba extract for age-related macular degeneration. Cochrane Database Syst Rev. 2013;1:CD001775. doi: 10.1002/14651858.CD001775.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2017;7:CD000254. doi: 10.1002/14651858.CD000254.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans WC. Trease and Evans’ Pharmacognosy. 16th ed. Edimburg: Saunders; 2009. [Google Scholar]
- 26.Falsini B, Piccardi M, Minnella A, Savastano C, Capoluongo E, Fadda A, Balestrazzi E, Maccarone R, Bisti S. Influence of saffron supplementation on retinal flicker sensitivity in early age related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:6118–6124. doi: 10.1167/iovs.09-4995. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Araque A, Giaquinta A, Laudo C, Rojo AA. Los antioxidantes en el proceso de patologías oculares. Nutr Hosp. 2017;34:469–478. doi: 10.20960/nh.420. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Sánchez L, Lax P, Esquiva G, Martín-Nieto J, Pinilla I, Cuenca N. Safranal, a saffron constituent, attenuates retinal degeneration in P23H rats. PLoS One. 2012;7:e43074. doi: 10.1371/journal.pone.0043074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frede K, Ebert F, Kipp AP, Schwerdtle T, Baldermann S. Lutein activates the transcription factor Nrf2 in human retinal pigment epithelial cells. J Agric Food Chem. 2017;65:5944–5952. doi: 10.1021/acs.jafc.7b01929. [DOI] [PubMed] [Google Scholar]
- 30.Fursova AZh, Gesarevich OG, Gonchar AM, Trofimova NA, Kolosova NG. Dietary supplementation with bilberry extract prevents macular degeneration and cataracts in senesce-accelerated OXYS rats. Adv Gerontol. 2005;16:76–79. [PubMed] [Google Scholar]
- 31.Gerding H. Primary or secondary prophylaxis of AMD with anthocyanins. Klin Monbl Augenheilkd. 2009;226:216–219. doi: 10.1055/s-0028-1109326. [DOI] [PubMed] [Google Scholar]
- 32.Giaccio M. Crocetin from saffron: an active component of an ancient spice. Crit Rev Food Sci Nutr. 2004;44:155–172. doi: 10.1080/10408690490441433. [DOI] [PubMed] [Google Scholar]
- 33.Hanus J, Anderson C, Wang S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res Rev. 2015;24:286–298. doi: 10.1016/j.arr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatziagapiou K, Kakouri E, Lambrou GI, Bethanis K, Tarantilis PA. Antioxidant properties of Crocus sativus L. and Its constituents and relevance to neurodegenerative diseases; focus on Alzheimer’s and Parkinson’s disease. Curr Neuropharmacol. 2019;17:377–402. doi: 10.2174/1570159X16666180321095705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heitmar R, Brown J, Kyrou I. Saffron (Crocus sativus L) in ocular diseases: A narrative review of the existing evidence from clinical studies. Nutrients. 2019 doi: 10.3390/nu11030649. doi: 103390/nu11030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hewlings S, Kalman D. 3. Vol. 10. Foods doi; 2017. Curcumin: A review of its’ effects on human health; p. foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang WY, Wu H, Li DJ, Song JF, Xiao YD, Liu CQ, Zhou JZ, Sui ZQ. Protective effects of blueberry anthocyanins against H2O2-induced oxidative injuries in human retinal pigment epithelial cells. J Agric Food Chem. 2018;66:1638–1648. doi: 10.1021/acs.jafc.7b06135. [DOI] [PubMed] [Google Scholar]
- 38.Jang YP, Zhou J, Nakanishi K, Sparrow JR. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem Photobiol. 2007;81:529–536. doi: 10.1562/2004-12-14-RA-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia YP, Sun L, Yu HS, Liang LP, Li W, Ding H, Song XB, Zhang LJ. The pharmacological effects of lutein and zeaxanthin on visual disorders and cognition diseases. Molecules. 2017 doi: 10.3390/molecules22040610. doi: 103390/molecules22040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jitsanong T, Khanobdee K, Piyachaturawat P, Wongprasert K. Diarylheptanoid 7-(3,4 dihydroxyphenyl)-5-hydroxy-1-phenyl-(1E)-1-heptene from Curcuma comosa Roxb. protects retinal pigment epithelial cells against oxidative stress-induced cell death. Toxicol In Vitro. 2011;25:167–176. doi: 10.1016/j.tiv.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Kalt W, Blumberg JB, McDonald JE, Vinqvist-Tymchuk MR, Fillmore SA, Graf BA, O’Leary JM, Milbury PE. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J Agric Food Chem. 2008;56:705–712. doi: 10.1021/jf071998l. [DOI] [PubMed] [Google Scholar]
- 42.Kanakis CD, Tarantilis PA, Pappas C, Bariyanga J, Tajmir-Riahi HA, Polissiou MG. An overview of structural features of DNA and RNA complexes with saffron compounds: models and antioxidant activity. J Phytochem Photobiol B. 2009;95:204–212. doi: 10.1016/j.jphotobiol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khoo HE, Ng HS, Yap WS, Goh H, Yim HS. Nutrients for prevention of macular degeneration and eye-related diseases. Antioxidants. 2019;8:85. doi: 10.3390/antiox8040085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim EK, Kim H, Vijayakumar A, Kwon O, Chang N. Associations between fruit and vegetable, and antioxidant nutrient intake and age-related macular degeneration by smoking status in elderly Korean men. Nutr J. 2017;16:77. doi: 10.1186/s12937-017-0301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lashay A, Sadough G, Ashrafi E, Lashay M, Movassat M, Akhondzadeh S. Short-term outcomes of saffron supplementation in patients with age-related macular degeneration: A double-blind, Placebo-controlled, randomized trial. Med Hypothesis Discov Innov Ophthalmol. 2016;5:32–38. [PMC free article] [PubMed] [Google Scholar]
- 47.Leone S, Recinella L, Chiavaroli A, Orlando G, Ferrante C, Leporini L, Brunetti L, Menghini L. Phytotherapic use of the Crocus sativus L. (Saffron) and its potential applications: A brief overview. Phytother Res. 2018;32:2364–2375. doi: 10.1002/ptr.6181. [DOI] [PubMed] [Google Scholar]
- 48.Levy Y, Glovinsky Y. The effect of anthocyanoside on night vision. Eye. 1998;12:967–9. doi: 10.1038/eye.1998.250. [DOI] [PubMed] [Google Scholar]
- 49.Li M, Huisingh C, Messinger J, Dolz-Marco R, Ferrara D, Freund KB, Curcio CA. Histology of geographic atrophy secondary to age-related macular degeneration: A multilayer approach. Retina. 2018;38:1937–1953. doi: 10.1097/IAE.0000000000002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S, Chaudhary SC, Zhao X, Gaur U, Fang J, Yan F, Zheng W. Artemisinin protects human retinal pigmented epithelial cells against hydrogen peroxide-induced oxidative damage by enhancing the activation of AMP-active protein kinase. Int J Biol Sci. 2019;15:2016–2028. doi: 10.7150/ijbs.30536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandal MN, 1, Patlolla JM, Zheng L, Agbaga MP, Tran JT, Wicker L, Kasus-Jacobi A, Elliott MH, Rao CV, Anderson RE. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Med. 2009;46:672–679. doi: 10.1016/j.freeradbiomed.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marangoni D, Falsini B, Piccardi M, Ambrosio L, Minnella AM, Savastano MC, Bisti S, Maccarone R, Fadda A, Mello E, Concolino P, Capoluongo E. Functional effect of saffron supplementation and risk genotypes in early age-related macular degeneration: A preliminary report. J Transl Med. 2013;11:228. doi: 10.1186/1479-5876-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martínez-Solís I, Acero N, Bosch-Morell F, Castillo E, González-Rosende ME, Muñoz-Mingarro D, Ortega T, Sanahuja MA, Villagrasa V. Neuroprotective potential of Ginkgo biloba in retinal diseases. Planta Med. 2019 doi: 10.1055/a-0947-5712. doi: 101055/a-0947-5712. [DOI] [PubMed] [Google Scholar]
- 54.Melnyk JP, Wang S, Marcone MF. Chemical and biological properties of the world’s most expensive spice: Saffron. Food Res Int. 2010;43:1981–1989. [Google Scholar]
- 55.Milbury PE, Graf B, Curran-Celentano JM, Blumberg JB. Bilberry (Vaccinium myrtillus) anthocyanins modulate heme oxygenase-1 and glutathione S-transferase-pi expression in ARPE-19 cells. Invest Ophthalmol Vis Sci. 2007;48:2343–2349. doi: 10.1167/iovs.06-0452. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 57.Mohamadpour AH, Ayati Z, Parizadeh MR, Rajbai O, Hosseinzadeh H. Safety evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iran J Basic Med Sci. 2013;16:39–46. [PMC free article] [PubMed] [Google Scholar]
- 58.Mohanta TK, Tamboli Y, Zubaidha PK. Phytochemical and medicinal importance of Ginkgo biloba L. Nat Prod Res. 2014;28:746–752. doi: 10.1080/14786419.2013.879303. [DOI] [PubMed] [Google Scholar]
- 59.Mojaverrostami S, Bojnordi MN, Ghasemi-Kasman M, Ebrahimzadeh MA, Hamidabadi HG. A review of herbal therapy in multiple sclerosis. 2018 doi: 10.15171/apb.2018.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montes P, Ruiz-Sánchez E, Rojas C, Rojas P. Ginkgo biloba extract 761: a review of basic studies and potential clinical use in psychiatric disorders. CNS Neurol Disord Drug Targets. 2015;14:132–149. doi: 10.2174/1871527314666150202151440. [DOI] [PubMed] [Google Scholar]
- 61.Moratalla-López N, Bagur MJ, Lorenzo C, Martínez-Navarro ME, Salinas MR, Alonso Gonzalo L. Bioactivity and bioavailability of the major metabolites of Crocus sativus L. flower. Molecules. 2019;24:2827. doi: 10.3390/molecules24152827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muangnoi C, Sharif U, Ratnatilaka Na Bhuket P, Rojsitthisak P, Paraoan L. Protective effects of curcumin ester prodrug, curcumin diethyl disuccinate against H2O2-induced oxidative stress in human retinal pigment epithelial cells: potential therapeutic avenues for age-related macular degeneration. Int J Mol Sci. 2019 doi: 10.3390/ijms20133367. doi: 103390/ijms20133367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mykhailenko O, Kovalyov V, Goryacha O, Ivanauskas L, Georgiyants V. Biologically active compounds and pharmacological activities of species of the genus Crocus: A review. Phytochemistry. 2019;162:56–89. doi: 10.1016/j.phytochem.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 64.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, Jung WS, Cho KH, Park JH, Kang I, Hong JW, Lee EH. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Nassiri-Asl M, Hosseinzadeh H. Neuropharmacology effects of saffron (Crocus sativus) and its active constituents. In: Watson RR, Preedy VR, editors. Bioactive nutraceuticals and dietary supplements in neurological and brain disease: Prevention and therapy. Amsterdam: Academic Press; 2015. pp. 29–39. [Google Scholar]
- 66.Natoli R, Zhu Y, Valter K, Bisti S, Eells J, Stone J. Gene and noncoding RNA regulation underlying photoreceptor protection: Microarray study of dietary antioxidant saffron and photobiomodulation in rat retina. Mol Vis. 2010;16:1801–1822. [PMC free article] [PubMed] [Google Scholar]
- 67.Ng AL, Leung HH, Kawasaki R, Ho WL, Chow LL, Chow SS, Lee JC, Wong IY. dietary habits, fatty acids and carotenoid levels are associated with neovascular age-related macular degeneration in chinese. Nutrients. 2019 doi: 10.3390/nu11081720. doi: 103390/nu11081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohno Y, Nakanishi T, Umigai N, Tsuruma K, Shimazawa M, Hara H. Oral administration of crocetin prevents inner retinal damage induced by N-methyl-D-aspartate in mice. Eur J Pharmacol. 2012;690:84–89. doi: 10.1016/j.ejphar.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 69.Osada H, Okamoto T, Kawashima H, Toda E, Miyake S, Nagai N, Kobayashi S, Tsubota K, Ozawa Y. Neuroprotective effect of bilberry extract in a murine model of photo-stressed retina. PLoS One. 2017;12:e0178627. doi: 10.1371/journal.pone.0178627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park SI, Lee EH, Kim SR, Jang YP. Anti-apoptotic effects of Curcuma longa L. extract and its curcuminoids against blue light-induced cytotoxicity in A2E-laden human retinal pigment epithelial cells. J Pharm Pharmacol. 2017;69:334, 340. doi: 10.1111/jphp.12691. [DOI] [PubMed] [Google Scholar]
- 71.Pawlowska E, Szczepanska J, Koskela K, Kaarniranta K, and Blasiak J. Dietary polyphenols in age-related macular degeneration: protection against oxidative stress and beyond. Oxid Med Cell Longev. 2019;2019:9682318. doi: 10.1155/2019/9682318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peddada KV, Brown A, Verma V, Nebbioso M. Therapeutic potential of curcumin in major retinal pathologies. Int Ophthalmol. 2019;39:725–734. doi: 10.1007/s10792-018-0845-y. [DOI] [PubMed] [Google Scholar]
- 73.Pescosolido N, Giannotti R, Plateroti AM, Pascarella A, Nebbioso M. Curcumin: therapeutical potential in ophthalmology. Planta Med. 2014;80:249–254. doi: 10.1055/s-0033-1351074. [DOI] [PubMed] [Google Scholar]
- 74.Piccardi M, Marangoni D, Minnella AM, Savastano MC, Valentini P, Ambrosio L, Capoluongo E, Maccarone R, Bisti S, Falsini B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: Sustained benefits to central retinal function. Evid Based Complement Alternat Med. 2012;2012:429124. doi: 10.1155/2012/429124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poma A, Fontecchio G, Carlucci G, Chichiriccò G. Anti-inflammatory properties of drugs from saffron crocus. Antiinflamm Anti-Allergy Agents Med Chem. 2012;11:37–51. doi: 10.2174/187152312803476282. [DOI] [PubMed] [Google Scholar]
- 76.Radomska-Leśniewska DM, Osiecka-Iwan A, Hyc A, Góźdź A, Dąbrowska AM, Skopiński P. Therapeutic potential of curcumin in eye diseases. Cent Eur J Immunol. 2019;44:181–189. doi: 10.5114/ceji.2019.87070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rahaiee S, Moini S, Hashemi M, Shojaosadati SA. Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L): a review. J Food Sci Technol. 2015;52:1881–1888. doi: 10.1007/s13197-013-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ravikrishnan R, Rusia S, Ilamurugan G, Salunkhe U, Deshpande J, Shankaranarayanan J, Shankaranarayana ML, Soni MG. Safety assessment of lutein and zeaxanthin (Lutemax™ 2020): Subchronic toxicity and mutagenicity studies. Food Chem Toxicol. 2011;49:2841–2848. doi: 10.1016/j.fct.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Reinisalo M, Kårlund A, Koskela A, Kaarniranta K, Karjalainen RO. Polyphenol stilbenes: molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxid Med Cell Longev. 2015;2015:340520. doi: 10.1155/2015/340520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riazi A, Panahi Y, Alishiri AA, Hosseini MA, Zarchi AAK, Sahebkar A. The impact of saffron (Crocus sativus) supplementation on visual function in patients with dry age-related macular degeneration. Ital J Med. 2017;11:196–201. [Google Scholar]
- 81.Richer S, Patel S, Sockanathan S, Ulanski LJ, Miller L, Podella C. Long-term beneficial effects on structure and visual function in human patients. Nutrients. 2014;6:4404–4420. doi: 10.3390/nu6104404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saberi-Karimian M, Katsiki N, Caraglia M, Boccellino M, Majeed M, Sahebkar A. Vascular endothelial growth factor: An important molecular target of curcumin. Crit Rev Food Sci Nutr. 2019;59:299–312. doi: 10.1080/10408398.2017.1366892. [DOI] [PubMed] [Google Scholar]
- 83.Sandmann G. Antioxidant protection from UV- and light-stress related to carotenoid structures. Antioxidants (Basel) 2019 doi: 10.3390/antiox8070219. doi:103390/antiox8070219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shu Z, Shar AH, Shahen M, Wang H, Alagawany M, Abd El-Hack ME, Kalhoro SA, Rashid M, Shar PA. Pharmacological uses of Ginkgo biloba extracts for cardiovascular disease and coronary heart diseases. Int J Pharmacol. 2019;15:1–9. [Google Scholar]
- 85.Sin HP, Liu DT, Lam DS. Lifestyle modification, nutritional and vitamins supplements for age-related macular degeneration. Acta Ophthalmol. 2013;91:6–11. doi: 10.1111/j.1755-3768.2011.02357.x. [DOI] [PubMed] [Google Scholar]
- 86.Singh M, Mathur G, Jain CK, Mathur A. Phyto-pharmacological potential of Ginkgo biloba: A review. J Pharm Res. 2012;5:5028–5030. [Google Scholar]
- 87.Spadiene A, Savickiene N, Jurgeviciene N, Zalinkevicius R, Norkus A, Ostrauskas R, Skesters A, Silova A, Rodovicius H, Francaite-Daugeliene M. Effect of ginkgo extract on eye microcirculation in patients with diabetes. Cent Eur J Med. 2013;8:736–741. [Google Scholar]
- 88.Srinivasan K. Polyphenols in Vision and Eye Health. In: Preedy VR, editor. Handbook of Nutrition, Diet and the Eye. San Diego: Academic Press; 2014. pp. 413–421. [Google Scholar]
- 89.Tamaddonfard E, Farshid AA, Eghdami K, Samadi F, Erfanparast A. Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responses induced by carrageenan in rats. Pharmacol Rep. 2013;65:1272–1280. doi: 10.1016/s1734-1140(13)71485-3. [DOI] [PubMed] [Google Scholar]
- 90.Ude C, Schubert-Zsilavecz M, Wurglics M. Ginkgo biloba extracts: a review of the pharmacokinetics of the active ingredients. Clin Pharmacokinet. 2013;52:727–749. doi: 10.1007/s40262-013-0074-5. [DOI] [PubMed] [Google Scholar]
- 91.Wang LL, Sun Y, Huang K, Zheng L. Curcumin, a potential therapeutic candidate for retinal diseases. Mol Nutr Food Res. 2013;57:1557–1568. doi: 10.1002/mnfr.201200718. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y, Zhao L, Wang C, Hu J, Guo X, Zhang D, Wu W, Zhou F, Ji B. Protective effect of quercetin and chlorogenic acid, two polyphenols widely present in edible plant varieties, on visible light-induced retinal degeneration in vivo. J Funct Foods. 2017;33:103–111. [Google Scholar]
- 93.WHO. Monographs on Selected Medicinal Plants. Vol. 3. Geneva, Switzerland: World Health Organization; 2007. [Accessed 14 September 2019]. Available online: http://appswhoint/medicinedocs/en/m/abstract/Js14213e/ [Google Scholar]
- 94.Wilkinson JT, Fraunfelder FW. Use of herbal medicines and nutritional supplements in ocular disorders: an evidence-based review. 2011 doi: 10.2165/11596840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 95.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 96.Xu Z, Sun T, Li W, Sun X. Inhibiting effects of dietary polyphenols on chronic eye diseases. J Funct Foods. 2017;39:186–197. [Google Scholar]
- 97.Xuan B, Zhou YH, Li N, Min ZD, Chiou GC. Effects of crocin analogs on ocular blood flow and retinal function. J Ocul Pharmacol Ther. 1999;15:143–152. doi: 10.1089/jop.1999.15.143. [DOI] [PubMed] [Google Scholar]
- 98.Yamauchi M, Tsuruma K, Imai S, Nakanishi T, Umigai N, Shimazawa M, Hara H. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur J Pharmacol. 2011;650:110–119. doi: 10.1016/j.ejphar.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 99.Yanai R, Chen S, Uchi SH, Nanri T, Connor KM, Kimura K. Attenuation of choroidal neovascularization by dietary intake of ω-3 long-chain polyunsaturated fatty acids and lutein in mice. PLoS One. 2018;13:e0196037. doi: 10.1371/journal.pone.0196037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhuang P, Shen Y, Lin BQ, Zhang WY, Chiou GC. Effect of quercetin on formation of choroidal neovascularization (CNV) in age-related macular degeneration (AMD) Eye Sci. 2011;26:23–29. doi: 10.3969/j.issn.1000-4432.2011.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


