Abstract
Some patients in the community receive a high burden of antibiotics. We aimed at describing the characteristics of these patients, antibiotics used, and conditions for which they received antibiotics. We carried out a cross‐sectional study. Setting: Thirty Health Primary Care Areas from 12 regions in Spain, covering 5,960,191 inhabitants. Patients having at least 30 packages of antibacterials for systemic use dispensed in 2017 were considered. Main outcome measures: Prevalence of antibiotic use, conditions for which antibiotics were prescribed, clinical characteristics of patients, comorbidities, concomitant treatments, and microbiological isolates. Patient’s average age was 70 years; 52% were men; 60% smokers/ex‐smokers; 54% obese. Overall, 93% of patients had, at least, one chronic condition, and four comorbidities on average. Most common comorbidities were cardiovascular and/or hypertension (67%), respiratory diseases (62%), neurological/mental conditions (32%), diabetes (23%), and urological diseases (21%); 29% were immunosuppressed, 10% were dead at the time of data collection. Patients received three antibiotic treatments per year, mainly fluoroquinolones (28%), macrolides (21%), penicillins (19%), or cephalosporins (12%). Most frequently treated conditions were lower respiratory tract (infections or prophylaxis) (48%), urinary (27%), and skin/soft tissue infections (11%). Thirty‐five percent have been guided by a microbiological diagnosis, being Pseudomonas aeruginosa (30%) and Escherichia coli (16%) the most frequent isolates. In conclusion, high antibiotic consumers in the community were basically elder, with multimorbidity and polymedication. They frequently received broad‐spectrum antibiotics for long periods of time. The approach to infections in high consumers should be differentiated from healthy patients receiving antibiotics occasionally.
Keywords: ambulatory Care, antibiotic prescribing, antimicrobial stewardship programs, infection, outpatients
Patients receiving a high burden of antibiotics in the community in Spain.
Abbreviations
- ASP
antimicrobial stewardship programs
- UTIs
urinary tract infections
- COPD
chronic obstructive pulmonary disease
1. INTRODUCTION
Antimicrobial resistance is a growing problem, widely recognized as a major threat to public health. 1 , 2 , 3 , 4 In general practice, there is a major concern that some common infections are becoming increasingly difficult to be treated, and those infections caused by antibiotic‐resistant bacteria may take longer to be resolved. 5 Primary Care is responsible for most of the antibiotics prescriptions in human health. 6 , 7 , 8 , 9 There is a high prevalence of infectious diseases in this setting, 10 and two thirds of patients treated for infectious diseases receive antibiotic therapy. 2 , 6 , 7 This leads to 25‐30% of the population receiving antibiotics annually. 11 , 12
Not all patients in the community receive the same burden of antibiotics. 13 , 14 While some patients receive antibiotics occasionally, others receive antimicrobial treatments on a continuous or cyclic manner for prolonged periods of time, in the context of infectious diseases with indication of long‐term treatments, recurrent infections or for prophylactic purposes. 13 These patients are more susceptible to infections by multidrug resistant bacteria, 15 , 16 , 17 leading to more frequent use of broad‐spectrum antibiotics. This limits the possibility of treating future infections in the ambulatory setting. 18
Improving the appropriate use of antibiotics has become an increasing priority for Health Services. The establishment of powerful interventions, preferably integrated into antimicrobial stewardship programs (ASP), is essential. 19 , 20 It is a priority to identify which patients receive a high burden of antibiotics in the community, and which of them could benefit from interventions aimed to improving the use of antibiotic. At this time, when global attention is focused on the SARS‐CoV‐2 pandemic, it is necessary not to forget the importance of maintaining ASP interventions since optimizing the use of antibiotics should not cease to be a priority. 21 Furthermore, given the higher rates of inpatient utilization of broad‐spectrum antibiotics during the pandemic, a higher spread of bacterial resistance could be expected. 22
The objective of this work was to characterize the patients who receive a high burden of antibiotics in the community, the antibiotics used, and clinical conditions for which patients are treated. Our results will serve to design interventions aimed at optimizing antibiotic prescribing in these patients.
2. MATERIALS AND METHODS
2.1. Design
A descriptive cross‐sectional study was carried out in Primary Care, covering a total population of 5 960 191 inhabitants. The study setting comprised 30 Healthcare Areas from 12 of the 17 regions in Spain (Table S1). The Spanish National Healthcare System is organized in Health Areas. A Health Area is an administrative district that clusters a group of Primary Care centers and professionals under its organizational and functional dependency. Primary Care provides essential care for the entire population. Furthermore, each Health Area has one or more referral hospitals for hospital care.
The unit of analysis was the patient, identified by the use of his personal social insurance system card number. Inclusion criteria for high consumers were as follows: all patients assigned to Primary Care centers from the study areas, having at least 30 packages of antibacterial agents for systemic use (J01, according to the WHO Anatomical Therapeutic Chemical classification system 23 ) dispensed from January to December 2017.
2.2. Data sources and outcomes
Patients were identified through databases from computerized pharmacy records of reimbursed and dispensed drugs, from their Regional Health Care Services. Individual clinical data, diagnoses, and microbiological tests from patients were collected from the Electronic medical records of Public Health Services maintained for routine health‐care activities. Diagnoses and microbiological data were searched in consultation sheets, hospital admission or discharge records, laboratory data applications, etc. Diagnoses were classified into the following categories: upper respiratory tract, lower respiratory tract, urinary tract, skin and soft tissue, gastrointestinal, dental, and others. The missing data were counted as a lack of the value in some variables due to the lack of records in the Clinical History. Patients without any data available in the electronic medical records or those with private pharmacy were excluded. Population data were obtained from the Statistics National Institute. 24
Data were collected between 1 June and 31 October 2018 by Primary Care Pharmacists from the Public Health Services. An electronic form with restricted online access to researchers was designed ad hoc for the data collection. The anonymity of participants was guaranteed by their identification in the electronic form through a numerical code. Data were stored securely in a data center with perimeter security.
The following variables, defined at the population level, were calculated: prevalence of antibiotic use (that is the percentage of the population that consumes antibiotics during the year), the average number of antibiotic prescriptions per patient, and the average number of days with antibiotic per patient. Variables collected at the patient level were as follows: age, gender, smoking behavior (smoke now or former smoker), obesity, or high body mass index (BMI; obesity: BMI>30 kg/m2), residence in nursing home or in long‐term care facilities, urinary incontinence, bedridden, number and type of underlying chronic health conditions (Table S2), condition that caused antimicrobial treatment, hospital admissions, death, type of antibiotic prescribed, number of antibiotic treatments received, antibiotic days, concomitant treatments (Table S3), prescriber medical specialty, type of sample for microbiological testing, and microbiological isolates.
2.3. Statistical analysis
A descriptive analysis of the data was carried out to determine the prevalence and profile of high antibiotic consumers. Summary statistics were computed using frequencies and percentages for categorical variables and median (50th percentile), and interquartile range (25‐75th percentiles) for continuous variables with asymmetric distribution. Confidence intervals were calculated at 95% (95% CI). STATA Corp. V12 was used for statistical analysis.
2.4. Ethics statement
This research was conducted in accordance with the Declaration of Helsinki and national and institutional legislation in Spain regarding clinical research and personal data protection. It has been approved by Hospitals Virgen del Rocío and Virgen Macarena Ethics Committee on Health Research (Seville, Spain) (Code 0295‐N‐18). This project is registered in the clinical studies database of Spanish Medicines Agency and Health Products (EPA‐OD code GTI‐ANT‐2018‐01).
3. RESULTS
3.1. Study population
We have identified 1,876,927 patients with antibiotics prescribed during the study period. The prevalence of antibiotic use was 31%. Among these patients, 1,162 were high consumers according to the criteria established in this study, and 889 met the inclusion criteria. Characteristics of high consumers are described in Table 1. Median age was 70 (58‐80) years; 52% were men; around 60% were smokers or ex‐smokers; 54% were obese.
Table 1.
Descriptive analysis of patients
|
Number of patients (N, %) or median |
95% Confidence interval or interquartile range | |
|---|---|---|
| Total | 889 (100) | |
| Age (years), median | 70 | [58‐80] |
| Gender (% women) | 423 (48) | 44‐51 |
| Smokers or ex‐smokers a | 375 (59) | 55‐62 |
| Obesity b | 279 (54) | 49‐58 |
| Urinary incontinence | 129 (15) | 12‐17 |
| Bedridden c | 62 (8) | 6‐10 |
| Residence in nursing homes or long‐term care facilities d | 58 (7) | 6‐9 |
| Number of comorbidities, median | 4 | [2‐5] |
| Comorbid conditions: | ||
| Hypertension, heart disease, cerebrovascular disease | 593 (67) | 64‐70 |
| Arterial hypertension | 515 (58) | 55‐61 |
| Heart failure | 99 (11) | 9‐13 |
| Peripheral arterial disease | 51 (6) | 4‐8 |
| Stable coronary heart disease | 45 (5) | 4‐7 |
| Stroke | 43 (5) | 4‐7 |
| Acute myocardial infarction | 34 (4) | 3‐5 |
| Transient ischemic attack | 29 (3) | 2‐5 |
| Angina pectoris | 20 (2) | 1‐4 |
| Other | 144 (16) | 14‐19 |
| Respiratory tract chronic conditions | 553 (62) | 59‐65 |
| Asthma | 480 (54) | 51‐57 |
| Chronic Obstructive Pulmonary Disease | 302 (34) | 31‐37 |
| Bronchiectasis | 262 (30) | 27‐33 |
| Chronic bronchitis | 34 (4) | 3‐5 |
| Cystic fibrosis | 25 (3) | 2‐4 |
| Tuberculosis (previous or latent) | 22 (3) | 2‐4 |
| Chronic respiratory failure | 9 (1) | 1‐2 |
| Emphysema | 3 (0) | 0‐1 |
| Chronic neurological or mental diseases | 285 (32) | 29‐35 |
| Depression | 228 (26) | 23‐29 |
| Dementia | 79 (9) | 7‐11 |
| Alzheimer | 28 (3) | 2‐5 |
| Parkinson | 21 (2) | 2‐4 |
| Schizophrenia | 17 (2) | 1‐3 |
| Situation that leads to immunosuppression | 254 (29) | 26‐32 |
| Malignancies | 147 (17) | 14‐19 |
| Transplant | 53 (6) | 5‐8 |
| Prolonged use of corticoids | 26 (3) | 2‐4 |
| Human immunodeficiency virus infection (VIH) | 14 (2) | 1‐3 |
| Other | 53 (6) | 5‐8 |
| Urological diseases | 186 (21) | 18‐24 |
| Chronic renal failure | 109 (13) | 10‐15 |
| Benign prostate hyperplasia | 97 (11) | 9‐13 |
| Obstructive uropathy | 15 (2) | 1‐3 |
| Other conditions | ||
| Diabetes | 202 (23) | 20‐26 |
| Dermatitis | 52 (6) | 4‐8 |
| Liver failure | 31 (4) | 2‐5 |
| Psoriasis | 11 (1) | 1‐2 |
| Number of chronic treatments (median) | 5 | [3‐7] |
| Type of concomitant treatment | ||
| Proton pump inhibitors | 572 (64) | 61‐68 |
| Corticoids: | 533 (60) | 57‐63 |
| Inhaled | 307 (35) | 31‐38 |
| Systemic | 226 (25) | 23‐28 |
| Antihypertensives | 498 (56) | 53‐59 |
| Bronchodilators | 473 (53) | 50‐54 |
| Analgesics | 417 (47) | 44‐50 |
| Benzodiazepines | 342 (39) | 35‐42 |
| Lipid‐lowering agents | 297 (33) | 30‐37 |
| Antidepressants | 228 (26) | 23‐29 |
| Antidiabetics | 199 (22) | 20‐26 |
| NSAIDs | 192 (22) | 19‐25 |
| Antiplatelet drugs | 185 (21) | 18‐24 |
| Anticoagulants | 136 (15) | 13‐18 |
| Antihistamines | 91 (10) | 8‐12 |
| Other immunosuppressive agents | 72 (8) | 6‐10 |
| Antipsychotics | 71 (8) | 6‐10 |
| Mucolytics | 44 (5) | 4‐7 |
| Cough suppressants | 8 (1) | 0‐2 |
| Patients with hospital admissions e | 383 (53) | 50‐57 |
| Exitus f | 82 (10) | 8‐12 |
Data from 641 patients.
Data from 520 patients.
Data from 775 patients.
Data from 805 patients.
Data from 717 patients.
Data from 818 patients.
Overall, 93% of patients had at least one of the analyzed comorbidities while 92% were receiving concomitant chronic treatments (Table 1). Most common comorbid conditions were chronic respiratory disease, cardiovascular (60%; 67% considering those with hypertension), neurological/mental, diabetes, urological disease, and 29% were immunosuppressed. High consumers had four chronic conditions on average, 53% were admitted, at least once, during the year of the study, and 10% were dead at the time of data collection (Table 1).
For their pathologies, patients received a median of five concomitant chronic treatments (Table 1). Treatments most commonly prescribed were proton pump inhibitors (PPI), corticoids, antihypertensives, bronchodilators, analgesics, benzodiazepines, lipid‐lowering agents, antidepressants, and antidiabetics.
3.2. Antibiotic treatments and infectious diseases
Patients received a total of 3,226 antibiotic treatments during the study period. Half of the patients (51%) received antibiotics for the entire annual period: 24% received them in a single antibiotic course and 76% received several courses (18% received two courses; 17% received three courses; 13% received four courses, and 28% received ≥5 courses). On average, patients received three antibiotics treatments per year [p25‐p75: 2‐5].
Regarding the therapeutic group, fluoroquinolones was the most prescribed antibiotic group, followed by macrolides, penicillins, and cephalosporins (Table 2). When analyzed by antibiotic agents, azithromycin, amoxicillin‐clavulanate, levofloxacin, and ciprofloxacin were the most prescribed antibiotics (Table 2).
Table 2.
Frequency of antibiotic prescribing
| Total | Number of antibiotic treatments (N, %) | 95% Confidence interval |
|---|---|---|
| 3,226 (100) | ||
| Fluoroquinolones | 913 28) | 27‐30 |
| Levofloxacin | 398 (12) | 11‐14 |
| Ciprofloxacin | 373 (12) | 11‐13 |
| Moxifloxacin | 103 (3) | 3‐4 |
| Norfloxacin | 34 (1) | 1‐2 |
| Other | 5 (0) | 0‐0 |
| Macrolides and lincosamides | 674 (21) | 20‐22 |
| Azithromycin | 574 (18) | 17‐19 |
| Clindamycin | 55 (2) | 1‐2 |
| Clarithromycin | 40 (1) | 1‐2 |
| Other | 5 (0) | 0‐0 |
| Penicillins | 605 (19) | 18‐20 |
| Amoxicillin‐clavulanate | 401 (12) | 11‐14 |
| Amoxicillin | 112 (4) | 3‐4 |
| Beta‐lactamase sensitive penicillins | 32 (1) | 1‐1 |
| Cloxacillin | 32 (1) | 1‐1 |
| Ampicillin | 25 (1) | 0‐1 |
| Other | 3 (0) | 0‐0 |
| Cephalosporins | 377 (12) | 11‐13 |
| Cefuroxime | 192 (6) | 5‐7 |
| Cefditoren | 88 (3) | 2‐3 |
| Cefixime | 59 (2) | 1‐2 |
| Ceftriaxone | 15 (1) | 0‐1 |
| Other | 23 (1) | 1‐1 |
| Aminoglycosides | 130 (4) | 3‐5 |
| Gentamicin | 77 (2) | 2‐3 |
| Tobramycin | 40 (1) | 1‐2 |
| Amikacin | 13 (0) | 0‐1 |
| Tetracyclines | 49 (2) | 1‐2 |
| Doxycycline | 32 (01) | 1‐1 |
| Minocycline | 17 (1) | 0‐1 |
| Other antibiotics | ||
| Fosfomycin | 255 (8) | 7‐9 |
| Co‐trimoxazole | 176 (6) | 5‐6 |
| Nitrofurantoin | 49 (2) | 1‐2 |
The absence of a diagnosis in clinical records was detected for 4% of antibiotic prescriptions: 3,095 treatments were linked to some information about the type of infection or prophylaxis, and 2,786 treatments were linked to a specific diagnosis. The distribution by site of infection was as follows:
-
‐
48% of use for lower respiratory tract conditions (33% infections, 12% prophylaxis),
-
‐
27% of use for urinary tract conditions (23% infections, 3% prophylaxis),
-
‐
11% of use for skin and soft tissue infections,
-
‐
4% of use for upper respiratory tract infections.
Based on records, antibiotics were mainly used to treat infections, with 21% treatments used with prophylactic purposes. Prophylaxis of lower respiratory infections accounted for 69% of all established prophylaxis.
Specific diagnosis for which antibiotics were used can be observed in Table 3. Lower urinary tract infections (UTIs) were the most treated condition (21%), followed by chronic obstructive pulmonary disease (COPD) exacerbations (11%), and overinfected bronchiectasis (11%) (Table 3).
Table 3.
Conditions treated with antibiotics
| Total | Number of antibiotic treatments linked to specific diagnosis (N, %) | 95% Confidence interval |
|---|---|---|
| 3,095 (100) | ||
| Lower respiratory tract conditions | 1,494 (48) | 47‐50 |
| Exacerbation of COPD | 351 (11) | 10‐13 |
| Overinfected bronchiectasis | 326 (11) | 10‐12 |
| Overinfected bronchiectasis prophylaxis | 194 (6) | 5‐7 |
| Exacerbation of COPD prophylaxis | 182 (6) | 5‐7 |
| Acute bronchitis | 117 (4) | 3‐5 |
| Chronic bronchitis | 80 (3) | 2‐3 |
| Pneumonia | 56 (2) | 1‐2 |
| Other | 80 (3) | 2‐3 |
| Urinary tract conditions | 833 (27) | 25‐29 |
| Lower urinary tract infection | 661 (21) | 20‐23 |
| Lower urinary tract infection prophylaxis | 95 (3) | 3‐4 |
| Prostatitis | 17 (1) | 0‐1 |
| Pyelonephritis | 15 (1) | 0‐1 |
| Other | 8 (0) | 0‐1 |
| Skin and soft tissue conditions | 348 (11) | 10‐12 |
| Diabetic foot | 49 (2) | 1‐2 |
| Boil/abscess | 46 (2) | 1‐2 |
| Cellulitis | 35 (1) | 1‐2 |
| Pressure ulcer infection | 33 (1) | 1‐2 |
| Fistula | 20 (1) | 0‐1 |
| Other | 33 (1) | 1‐2 |
| Upper respiratory tract infections | 126 (4) | 3‐5 |
| Sore throat/pharyngotonsillitis | 44 (1) | 1‐2 |
| Recurrent pharyngotonsillitis | 33 (1) | 1‐2 |
| Acute otitis media | 10 (0) | 0‐1 |
| Other | 15 (1) | 0‐1 |
| Bone infection (osteomyelitis) | 39 (1) | 1‐2 |
| Periprosthetic infection | 37 (1) | 1‐2 |
| Gastrointestinal infection | 27 (1) | 1‐1 |
| Dental infection | 24 (1) | 0‐1 |
The distribution of antibiotics by condition is described in Figure 1.
Figure 1.
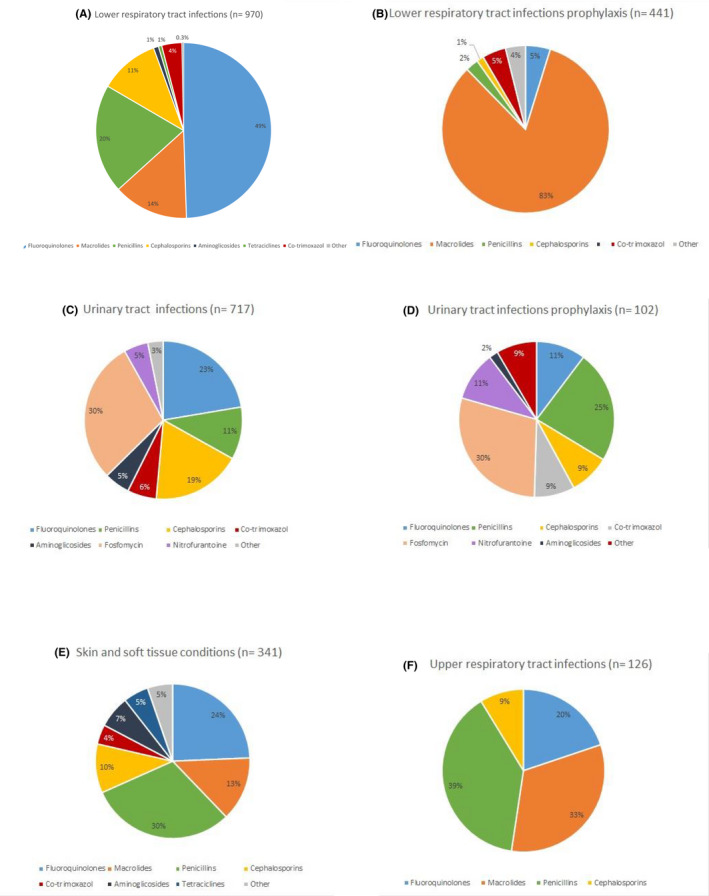
Distribution of antibiotics by condition: (A) lower respiratory tract infections, (B) lower respiratory tract prophylaxis, (C) urinary tract infections, (D) urinary tract infections prophylaxis, (E) skin and soft tissue conditions, and (F) upper respiratory tract infections
Regarding the most widely used antibiotics: fluoroquinolones were the most prescribed in lower respiratory tract infections. They were also frequently prescribed for the treatment of skin and soft tissue infections, upper respiratory tract infections, and UTIs. Macrolides accounted for the 83% of the of lower respiratory tract infection prophylaxis, and were also frequently used in the treatment of upper respiratory tract infections. Fosfomycin was the most widely used antibiotic in the treatment of UTIs and UTIs prophylaxis. Penicillins were mainly used in the treatment of upper respiratory tract infections, skin and soft tissue infections, UTIs, and UTI prophylaxis. Finally, cephalosporins have had a smaller use than previous groups, although we have stated a relative high use in the treatment of UTIs, lower respiratory tract infections, and skin and soft tissue infections.
Clinicians who made the prescriptions were specialists in Family and Community Medicine (50% of the total), followed by specialists in Pneumology (25%), Internal Medicine (8%), and Urology (6%). These four professional groups accounted for 88% of prescriptions.
3.3. Microbiological data
A total of 1,074 samples were recorded for microbiological assessment, that is, 35% of antibiotic treatments would have been guided by a microbiological diagnosis: 49% corresponded to bronchial exudates, followed by urine cultures (32%), and skin exudates (13%) (Table 4). In 84% of cases, the identification of one or several etiological agents was recorded. Pseudomonas aeruginosa (30%), Escherichia coli (16%), Staphylococcus aureus (6%), and Klebsiella pneumoniae (4%) were the most frequent isolates. According to the records, 29% of Staphylococcus aureus, 14% of Escherichia coli, and 11% Klebsiella pneumoniae were multidrug resistant. The identification of the ethiological agent by microbiological sample is detailed in Table 4.
Table 4.
Type of microbiological sample and isolates
|
Microbiological sample and isolates |
Number of samples (N, %) |
Number of isolates (N, %) |
|---|---|---|
| Total | 1,074 (100) | 906 (100) |
|
Bronchial exudate |
527 (49) |
436 (48) |
| Pseudomonas aeruginosa | 201 (46) | |
| Mycobacterium intracellulare , M. avium | 47 (11) | |
| Haemophilus influenzae | 27 (6) | |
| Staphylococcus aureus | 19 (4) | |
| Stenotrophomonas maltophilia | 17 (4) | |
| Serratia marcescens | 14 (3) | |
| Urine |
348 (32) |
264 (29) |
| Escherichia coli | 120 (46) | |
| Klebsiella pneumoniae | 34 (13) | |
| Pseudomonas aeruginosa | 34 (13) | |
| Enterococcus faecalis | 27 (10) | |
| Proteus mirabilis | 26 (10) | |
|
Skin exudate |
135 (13) |
120 (13) |
| Pseudomonas aeruginosa | 32 (27) | |
| Staphylococcus aureus | 26 (22) | |
| Escherichia coli | 16 (13) | |
| Proteus mirabilis | 11 (9) | |
| Other | 64 (6) | 86 (10) |
4. DISCUSSION
This observational study shows that patients who receive a high burden of antimicrobials in the community are, basically, older adults with multiple chronic conditions and polymedication. The studied population had a high prevalence of comorbidities, a high management from hospital care and hospital admissions (53% patients), as well as a high risk of dying (10% exitus).
In our study, prevalence of hypertension, asthma, CODP, neurological and mental disorders, diabetes, malignancies, or chronic renal failure were more frequent than that reported for general population receiving antibiotics in the community. 11 , 23 COPD and asthma were about 10 times more frequent in the study patients than in general population receiving antibiotics in the community in Spain, 11 and the prevalence of hypertension, heart diseases, and diabetes was three times higher. 11 It should be emphasized the high percentage of patients with chronic renal failure among high consumers (12%).
Other aspects besides comorbidities such as smoking behavior, overweight/obesity, or polypharmacy, common conditions in patients in our study, might be associated with antibiotic prescribing decisions. In relation to concomitant medication, PPI, NSAIDs, or corticosteroids which may contribute to increase susceptibility to infections were frequently used by the study patients. Around 60% of high antibiotic consumers in our study received corticosteroids. This has been previously shown to be a better predictor of antibiotic prescribing than comorbidities themselves. 13
Lower respiratory tract, urinary tract, and skin and soft tissue infections or prophylaxis justified most antibiotic treatments in high consumers. However, there are great differences between the conditions in these patients and those treated in general population: antibiotic use for the treatment of exacerbations of COPD and bronchiectasis, which represent a 2% of the use of antibiotics by general population in the community, 25 accounted for 34% of treatments in high consumers. By contrast, upper respiratory tract infections, which represent the 53% of infections treated with antibiotics in the ambulatory setting, 25 represented a 4% of infections treated in high consumers. In addition, prophylactic treatments, very scarce in healthy population, accounted for 21% of all treatments in high consumers, and expose these patients to antibiotics for prolonged periods of time, in a continuous or a cyclic manner.
Regarding the antibiotics prescribed, fluoroquinolones, macrolides, cephalosporins, or co‐trimoxazole which represent a low prescription in the community in Spain (10%, 6%, 3%, and 0.3%, respectively) 25 accounted for 28%, 21%, 12%, and 5%, respectively, in high consumers (Table 2). The profile of antibiotic use was very heterogeneous by condition (Figure 1). The most notorious result was the relatively high use of fluoroquinolones and cephalosporins, two groups of antibiotics associated with the selection for resistant bacteria, for the treatment of all kind of infectious diseases, and the wide use of macrolides for the prophylaxis of lower respiratory tract infections.
The high prevalence of Pseudomonas aeruginosa and multidrug‐resistant bacteria in these patients would justify these treatments, but at the same time, this makes the patients increasingly susceptible to infections by multidrug‐resistant microorganisms. 15 , 17 In addition, the use of long‐term macrolides for immunomodulatory purposes is a common practice, 26 although the appropriateness of both the indication and the duration of treatments should be tested.
Comorbidity is a main driver of prescribing in high consumers. The higher the comorbidity, the more antibiotic prescriptions patients received. 12 , 27 , 28 Rates of antibiotic prescribing to patients with asthma and COPD are 1.6‐ and 3‐fold, respectively, higher than rates in general population, and patients with heart failure, peripheral arterial disease, diabetes, or coronary artery disease are prescribed 47‐69% more antibiotics than individuals without these conditions. 12 Despite the great advances in the prevention, diagnosis, and treatment of infectious diseases, these continue to cause great morbidity and mortality in people with chronic conditions.
Guides on the management of infections in Primary Care recommend a restrictive use of antibiotics in this level of care, but justify the administration of prolonged or repeated course of antibiotics in some patients with chronic diseases such as diabetes, COPD, heart failure, or those with multiple pathologies. 12 , 27 , 28 , 29 , 30 , 31 The reason for this different management lies in the tendency of these patients to get more severe and recurrent infectious diseases, with worse prognosis, and with a higher probability of hospitalization and mortality than in healthy people. 12 , 28 , 31 , 32 , 33 , 34 Nonetheless, unnecessary antibiotic treatments, excessively long treatments, the unjustified use of broad‐spectrum antibiotics, or injustified prophylaxis should be avoided in patients with comorbidities.
This study has several strengths. First, it includes a large representative sample of the national territory, including patients of all ages cared by the Public Health Care Services for all possible diagnoses. The use of individual patients as the unit of analysis is a major strength compared with studies conducted with aggregated data. Full information about patient’s characteristics and underlying pathologies was collected. On the other hand, antibiotic prescriptions were linked to clinical information in 96% cases. The degree of underregistration of diagnoses in medical records was very low compared to other studies conducted in the Primary Care setting, which reported 30‐60% diagnoses unknown. 25 , 35 All this enhances the generalizability of the results.
Several limitations of the study should be pointed out. First, antibiotic prescribing data were exclusively collected from the computerized pharmacy records of dispensed drugs from Regional Public Health Care Services. Hospital inpatient antibiotic use, private medicine, outpatient parenteral antibiotic therapy, or over‐the‐counter sales were not measured. Second, we were unable detect patient adherence, only dispensations made. We assumed a good therapeutic compliance, and that the actual consumption of drugs corresponded to the dispensations made. It could be to an overestimation of the total amount of antibiotics taken by patients actually. Third, we did not accessed to hospital records. Information about other treatments or infections may have been lost for not being registered in Primary Care Digital Health History.
Our results have several implications for practice: (a) It is crucial that antimicrobial guides include clear indications for patients with chronic conditions, to avoid unnecessary treatments or prophylaxis, to adjust the duration of treatments to the minimum effective, and to reserve broad‐spectrum antibiotics to cases of failure or intolerance to narrow‐spectrum antibiotics. (b) ASP should consider specific interventions for patients with comorbidities and frequent infections, with a different approach than healthy patients. (c) Since professionals from both care settings (Primary Care and Hospital) are involved in care of high consumers, coordination among them should be encouraged in relation to the establishment of antibiotherapy, especially if it is indicated in the long‐term.
In conclusion, a high prevalence of aged patients with high‐risk comorbidities among practices could account for legitimate medical reasons of higher antibiotic prescribing rates, and higher prescription of broad‐spectrum antibiotics in the ambulatory setting. The approach to infections and antibiotic use by these patients should be carried out from both health‐care settings, since these patients are with high management from hospital care and frequent hospital admissions. Further studies should be addressed to determine whether high consumers are prescribed antibiotics appropriately or whether the excessive antibiotic use by these patients could be decreased or avoided.
DISCLOSURES
ON has been reimbursed by Novartis and Mundipharma for Congress attendance. VO has received funding for conference attendance from Novonordisk Lab, and Lilly has participated as an author of a book sponsored by Pfizer, and received training course enrollment by Amgen Lab. The other authors declare that they have no conflict of interests to disclose.
AUTHOR’S CONTRIBUTIONS
ON has received funding for conference attendance from Novartis and Mundipharma, and VO from Novonordisk and Lilly. VO has participated as an author of a book sponsored by Pfizer, and received training course enrollment by Amgen. The other authors declare that they have no conflict of interests to disclose.
ETHICS APPROVAL STATEMENT
This research has been approved by Hospitals Virgen del Rocío and Virgen Macarena Ethics Committee on Health Research (Seville, Spain) (Code 0295‐N‐18). This project is registered in the clinical studies database of Spanish Medicines Agency and Health Products (EPA‐OD code GTI‐ANT‐2018‐01).
Supporting information
Table S1. Participating region and number of patients provided to study
Table S2. Comorbid conditions analyzed
Table S3. Concomitant treatments analyzed
ACKNOWLEDGMENTS
The authors thank the members of the Infectious Diseases SEFAP group: Ana‐Aurelia Iglesias Iglesias, Reyes García Díaz‐Guerra, Virginia Arroyo Pineda, Mª Belén de la Hija Díaz, María Ana Prado Prieto, Pablo García Vázquez, Pablo March, Mónica Susana Mateu García, Lucía Jamart Sánchez, José Manuel Izquierdo Palomares, María Concepción Celaya Lecea, Natalia Rilla Villar, Antonia Candás Villar, Marisa Nicieza, Álvaro Fernández Ferreiro, Luis Sánchez Álvarez, Ane Goenaga Ansola, Carmen Pata Iglesias, Rafael Mª Torres García, Marta Rovira, Amaia Alcorta Lorenzo, Àngels Lladó.
Fernández‐Urrusuno R, Meseguer Barros CM, Anaya‐Ordóñez S, et al; the Infectious Diseases SEFAP team . Patients receiving a high burden of antibiotics in the community in Spain: a cross‐sectional study. Pharmacol Res Perspect.2021;9:e00692 10.1002/prp2.692
Funding Information
This work received a Research Prize of the Spanish Society of Primary Care Pharmacists (SEFAP), 2019.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Zhang Y, Lee BY, Donohue JM. Ambulatory antibiotic use and prescription drug coverage in older adults. Arch Intern Med. 2010;170:1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith RD, Coast J. Antimicrobial resistance: a global response. Bulletin World Health Organization 2002;80:126–133. [PMC free article] [PubMed] [Google Scholar]
- 3. WMA Statement on Resistance to Antimicrobial Drugs , 59th WMA General Assembly, Seoul, Korea, October 2008. https://www.wma.net/policies‐post/wma‐statement‐on‐resistance‐to‐antimicrobial‐drugs/. Accessed July 29, 2020.
- 4. MacKenzie FM, Struelens MJ, Towner KJ. et al on behalf of the ARPAC Steering Group and the ARPAC Consensus Conference Participants . Report of the consensus conference on antibiotic resistance; prevention and control (ARPAC). Clin Microbiol Infect 2005; 11:937–954. [DOI] [PubMed] [Google Scholar]
- 5. Butler CC, Hillier S, Roberts Z, et al. Antibiotic‐resistant infections in primary care are symptomatic for longer and increase workload: outcomes for patients with E coli UTIs. Br J Gen Pract. 2006;56:686–692. [PMC free article] [PubMed] [Google Scholar]
- 6. Petersen I, Hayward AC, SACAR Surveillance Subgroup . Antibacterial prescribing in primary care. J Antimicrob Chemother. 2007;60:i43–47. [DOI] [PubMed] [Google Scholar]
- 7. Grijalva CG, Pekka Nuorti J, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van der Velden AW, Kuyvenhoven MM, Verheij TJM. Improving antibiotic prescribing quality by an intervention embedded in the primary care practice accreditation: the ARTI4 randomized trial. J Antimicrob Chemother. 2016;71(1):257–263. [DOI] [PubMed] [Google Scholar]
- 9. Fleming‐Dutra KE, Bartoces M, Roberts RM, et al. Characteristics of primary care physicians associated with high outpatient antibiotic prescribing volume. Open Forum Infect Dis. 2018;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palin V, Mölter A, Belmonte M, et al. Antibiotic prescribing for common infections in UK general practice: variability and drivers. J Antimicrob Chemother. 2019;74:2440–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernández‐Urrusuno R, Flores Dorado M. ,Vilches Arenas A et al. Appropriateness of antibiotic prescribing in a primary care area: A cross‐sectional study. Enferm Infecc Microbiol Clin. 2014;32(5):285–292. (in Spanish). [DOI] [PubMed] [Google Scholar]
- 12. Shallcross L, Beckley N, Rait G, et al. Antibiotic prescribing frequency amongst patients in primary care: a cohort study using electronic health records. J Antimicrob Chemother. 2017;72:1818–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pouwels KB, Dolk FC, Smith DRM, et al. Explaining variation in antibiotic prescribing between general practices in the UK. J Antimicrob Chemother. 2018;73 Suppl 2:ii27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lallana‐Alvarez MJ, Feja‐Solana C, Arnesto‐Gómez J, et al. Outpatient antibiotic prescription in Aragon and the differences by gender and age. Enferm Infecc Microbiol Clin. 2012;30(10):591–596. (in Spanish). [DOI] [PubMed] [Google Scholar]
- 15. Marston HD, Dixon DM, Knisely JM, et al. Antimicrobial Resistance. JAMA. 2016;316(11):1193–1204. [DOI] [PubMed] [Google Scholar]
- 16. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta‐analysis. BMJ. 2010;340:c2096. [DOI] [PubMed] [Google Scholar]
- 17. Catry B, Latour K, Bruyndonckx B, et al. Characteristics of the antibiotic regimen that affect antimicrobial resistance in urinary pathogens. Antimicrobial Resistance and Infection Control. 2018;7:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. European Centre for Disease Prevention and Control . Last‐line antibiotics are failing: options to address this urgent threat to patients and healthcare systems. Stockholm: ECDC 2016. https://www.ecdc.europa.eu/en/publications‐data/last‐line‐antibiotics‐are‐failing‐options‐address‐urgent‐threat‐patients‐and. Accessed July 29, 2020.
- 19. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. CID. 2016;62(10):e51–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. King L, Fleming‐Dutra KE, Hicks LA. Advances in optimizing the prescription of antibiotics in outpatient settings. BMJ. 2018;363:k3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furukawa D, Graber CJ. Antimicrobial stewardship in a pandemic: picking up the pieces. Clin Infect Dis. 2020;. 10.1093/cid/ciaa1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thaden JT, Maskarinec SA. When two for the price of one isn’t a bargain: Estimating prevalence and microbiology of bacterial co‐infections in patients with COVID‐19. Clin Microbiol Infect. 2020;. 10.1016/j.cmi.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO Collaborating Centre for Drug Statistics Methodology . ATC/DDD Index 2010. http://www.whocc.no/atc_ddd_index/. Accessed January 28, 2020.
- 24. Instituto Nacional de Estadística [Internet] . https://www.ine.es/. Accessed January 28, 2020.
- 25. Fernández‐Urrusuno R, Flores Dorado M, Vilches Arenas A, et al. Improving the appropriateness of antimicrobial use in primary care after the implementation of a local antimicrobial guide in both levels of care. Eur J Clin Pharmacol. 2014;70:1011–1020. [DOI] [PubMed] [Google Scholar]
- 26. Kipourou M, Manika K, Papavasileiou A, et al. Immunomodulatory effect of macrolides: At what cost? Respir Med Case Rep. 2016;17:44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ternhag A, Grünewald M, Nauclér P, et al. Antibiotic consumption in relation to socio‐demographic factors, co‐morbidity, and accessibility of primary health care. Scand J Inf Dis. 2014;46:888–896. [DOI] [PubMed] [Google Scholar]
- 28. Bont J, Hak E, Birkhoff CE, et al. Is co‐morbidity taken into account in the antibiotic management of elderly patients with acute bronchitis and COPD exacerbations? Fam Practice. 2007;24:317–322. [DOI] [PubMed] [Google Scholar]
- 29. Gulliford MC, Dregan A, Moore MV, et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJOpen. 2014;4:e006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blommaert A, Coenen S, Gielen B, et al. Patient and prescriber determinants for the choice between amoxicillin and broader‐spectrum antibiotics: a nationwide prescription‐level analysis. J Antimicrob Chemother. 2013;68(2383):2392. [DOI] [PubMed] [Google Scholar]
- 31. Rothberg MB, Pekow PS, Lahti M, et al. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–2042. [DOI] [PubMed] [Google Scholar]
- 32. Bont J, Hak E., Hoes AW, et al. A prediction rule for elder primary care patients with lower respiratory tract infection. Eur Respir J. 2007;29:969–975. [DOI] [PubMed] [Google Scholar]
- 33. Gupta A, Mody P, Pandey A. Inappropriate antibiotic therapy in a patient with heart failure and prolonged QT interval. JAMA Int Med. 2015;175:1748–1749. [DOI] [PubMed] [Google Scholar]
- 34. Hope EC, Crump RE, Hollingsworth TD, et al. Identifying English practices that are high antibiotic prescribers accounting for comorbidities and other legitimate medical reasons for variation. EClinicalMedicine. 2018;6:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brauer R, Ruigómez A, Downey G, et al. Prevalence of antibiotic use: a comparison across various European health care data sources. Pharmacoepidemiol Drug Saf. 2016;25(Suppl 1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Participating region and number of patients provided to study
Table S2. Comorbid conditions analyzed
Table S3. Concomitant treatments analyzed
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


