Abstract
BACKGROUND:
Persistent use of prescription opioids beyond the period of surgical recovery is a large part of a public health problem linked to the current opioid crisis in the U.S. However, few studies have been conducted to examine if morphine reward is influenced by acute pain and injury.
METHODS:
In a mouse model of incisional injury and minor trauma, animals underwent conditioning, extinction and drug primed reinstatement with morphine, to examine the rewarding properties of morphine in the presence of acute incisional injury and drug induced relapse, respectively. In addition, we sought to determine whether these behaviors were influenced by kappa opioid receptor signaling, and measured expression of prodynorphin mRNA in the nucleus accumbens and medial prefrontal cortex after conditioning and prior to reinstatement with morphine and incisional injury.
RESULTS:
In the presence of incisional injury, we observed enhancement of morphine reward with morphine conditioned place preference but attenuated morphine primed reinstatement to reward. This adaptation was not present in animals conditioned 12 days after incisional injury when nociceptive sensitization had resolved, however they showed enhancement of morphine primed reinstatement. Prodynorphin expression was greatly enhanced in the nucleus accumbens and medial prefrontal cortex of mice with incisional injury and morphine conditioning, and remained elevated up to drug-primed reinstatement. These changes were not observed in mice conditioned 12 days after incisional injury. Further, kappa opioid receptor (KOR) blockade with nor-binaltorphimine (norBNI) prior to reinstatement reversed the attenuation induced by injury.
CONCLUSIONS:
These findings suggest enhancement of morphine reward as a result of incisional injury, but paradoxically a protective adaptation with incisional injury from drug induced relapse resulting from KOR activation in the reward circuitry. Remote injury conferred no such protection, and appeared to enhance reinstatement.
Keywords: incisional injury, morphine conditioned place preference, reinstatement, kappa opioid receptor
INTRODUCTION
It is estimated that about 10% of surgical patients use prescribed opioids longer than 90 days after major or minor surgery 1. Persistent opioid use becomes harmful for the patient because it may impede functional recovery, lead to opioid induced hyperalgesia and support the development of opioid use disorder 2-4. Moreover, the concurrence of pain and opioid use disorder is common 5. Despite the importance of these issues, few studies have been conducted studying the interaction of injury, pain and opioid reward. For example, while the presence of inflammatory pain was observed to enhance morphine reward 6, neuropathic pain attenuated morphine reward 7. Therefore, it remains inconclusive whether the presence of pain can modulate opioid reward, particularly in models of surgical pain.
Akin to natural rewarding stimuli, most drugs of abuse are understood to exert their action by increasing dopamine release in the nucleus accumbens, a vital brain substrate of the reward circuity, 8. Interestingly, similar effects in the nucleus accumbens induced by pain have also been characterized. Relief of acute pain also increases dopamine release in the nucleus accumbens 9. Conversely, the presence of chronic pain causes modification in the reward circuitry such that behavioral effects of natural rewarding stimuli is diminished 9,10. In addition, the medial prefrontal cortex directly influences activity of the nucleus accumbens, and is also thought to have a crucial role in drug relapse11. This region also is thought to undergo anatomical and functional remodeling in the presence of chronic pain.12 However the functional role of the medial prefrontal cortex pertaining to drug relapse and injury remains poorly characterized.
The dynorphin-kappa opioid receptor (KOR) system may be involved in opioid-pain interactions affecting reward. Dynorphin is the selective neuropeptide that binds to and acts on KOR 13,14. The expression of dynorphin and KOR is differentially distributed in the brain, especially co-expressed in the reward circuity especially the medial prefrontal cortex and the nucleus accumbens15,16. Functionally, KOR activation and blockade has been shown to modulate drug reward, and reinforcement17. Besides its role in drug reward, dynorphin-KOR signaling also has a facilitatory role in pain transmission within the CNS 18,19, and pain enhances prodynorphin expression through epigenetic mechanisms 18,20,21. Therefore, dynorphin-KOR signaling, known to be involved in both pain and reward, may serve as a common molecular mediator affecting injury and morphine reward.
The purpose of the study was to identify possible interplay between incisional injury and morphine reward using the conditioned place preference paradigm, extinction and drug primed reinstatement. We also sought to elucidate possible neuroadaptations in dynorphin and KOR signaling in the nucleus accumbens and medial prefrontal cortex associated with this behavioral phenotype.
MATERIALS AND METHODS
Study Design
An overview of the study design for the behavioral and molecular studies is illustrated in a flowchart (Figure 1a,b) and timeline (Figure 1c,d). The flowchart also highlights the sample size changes in each experimental phase, with larger sizes observed in the initial preference testing than in the terminal assays (Figure 1a, b).
Figure 1:
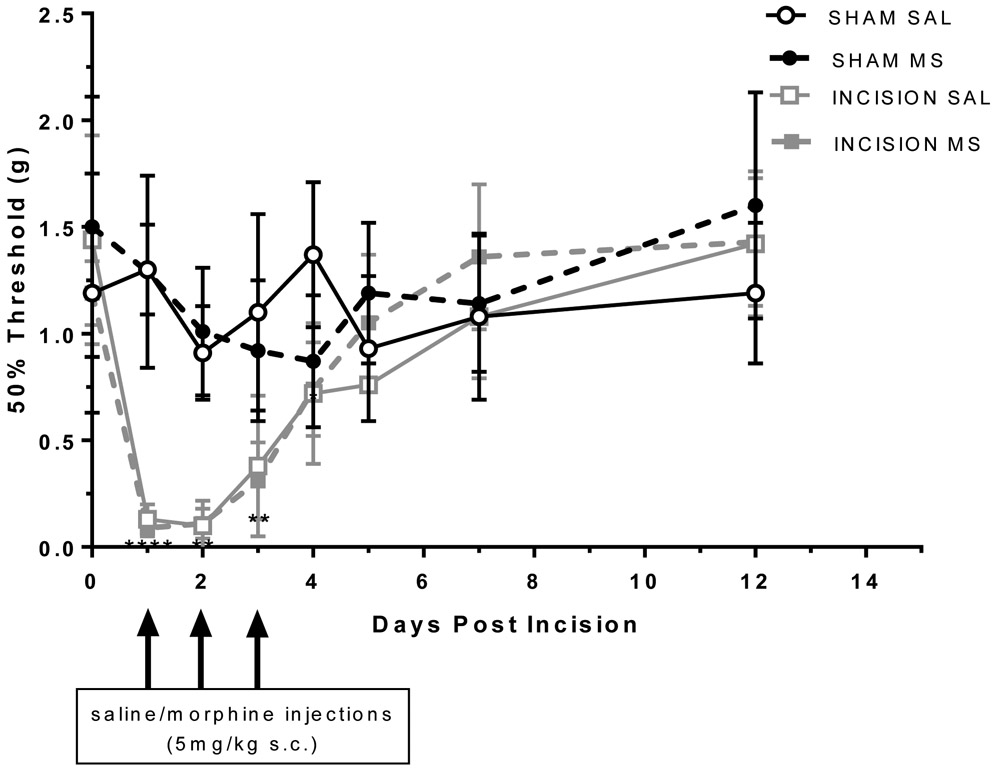
Mechanical allodynia with acute incisional injury and morphine administration.
Animals
All experimental protocols were reviewed and approved by the Veterans Affairs Palo Alto healthcare system Institutional Animal Care and Use committee prior to initiating these studies. All protocols were performed in concordance with the guidelines for the study of pain in awake animals as established by the International Association for the Study of Pain. Male C57BL/6J mice aged 10-12 weeks were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were housed four per cage and maintained on a 12hr light-dark cycle and ambient temperature of 22°C with ad lib access to food and water. Assignment to treatment groups was done in random fashion.
Drug Administration
Morphine sulfate was obtained from Sigma Chemical (St. Louis, MO) and dissolved in a sterile 0.9% saline solution. Saline or morphine (5mg/kg) was injected subcutaneously (s.c.) in a volume of 2mL/kg. Nor-binaltorphimine (norBNI), a selective KOR antagonist, was purchased from Sigma Chemical (St. Louis, MO) and dissolved in 0.9% saline. 10mg/kg s.c. of norBNI was then administered prior to drug- primed reinstatement in one study. The doses of morphine and norBNI selected for our studies were based on our pilot studies showing robust and reproducible conditioned place preference and reinstatement, as well as on prior publications 22.
Hindpaw incisional injury model
The hind paw incision model was performed as described in prior published studies 23-25. Briefly, mice were induced under anesthesia with 5% isoflurane and maintained at 2% isoflurane that was delivered through a nose cone. After aseptic preparation with povidone and alcohol, a 5mm longitudinal incision was made with a No. 11 scalpel on the superolateral aspect of the plantar surface of the right hind paw. The incision was made deep enough to expose the plantaris muscle and flexor tendons to the hind paw. The plantaris muscle was incised longitudinally, and after controlling bleeding, a 6-0 silk suture was placed to close the incision. Afterwards, triple antibiotic ointment containing bacitracin, neomycin and polymyxin was placed on the closed incision to minimize infection. The mice were monitored briefly for complete recovery after anesthesia and placed back in their home cages.
Behavioral testing
Nociceptive testing for mechanical allodynia- Mechanical nociceptive thresholds were assessed using von Frey filaments according to a modified “up-down” algorithm described by Chaplan et. al 26 and as used in previous studies 21,23,25. Mice were placed on elevated wire mesh platforms in clear cylindrical plastic enclosures that were 10cm in diameter and 30cm high. After 20mins of acclimation, fibers of increasing stiffness with initial bending force of 0.2g were applied to the plantar surface of the hindpaw adjacent to the incision, left for 3-5secs with enough force to bend the fiber up to 50% of its initial length. Withdrawal of the hind paw and/or licking of the paw was scored as a response. If no response was elicited, the next stiffer fiber was used in the same manner. If a response was obtained the next less stiff fiber was used. Testing was concluded when four fibers were used after the first one provoking a response, which allowed for estimation of the withdrawal threshold using a curve-fitting algorithm described by Poree. et. al 27.
Conditioned place preference- Morphine conditioned place preference (CPP) was conducted using an unbiased, counterbalanced design in a three-chamber apparatus (MED associates Inc., St. Albans VT). The apparatus consisted of three compartments; two outer compartments with different visual, and tactile cues for contextual conditioning and a central neutral compartment. The apparatus was equipped with photosensors and camera with automatic data collection to a computer. Before conditioning, the mice were placed in the apparatus with free access to all three chambers for 20mins and the time spent in each chamber was recorded. Mice that spent >80% of total time in one chamber were excluded from the study during this pretest day. For three days with two conditioning session per day. During a conditioning day, mice were given saline in the morning and placed in their designated conditioned chamber for 30mins. 4hours later they were given morphine in the afternoon and confined to their designed conditioned chamber for 40mins in an unbiased, counterbalanced fashion. This was repeated for a total of three days with 6 conditioning sessions. After conditioning, in the posttest day, the mice were placed in the middle chamber and allowed free access to all three chambers for 20mins, and time spent in the chambers were recorded. Preference scoring was determined by subtracting the time spent in the morphine paired chamber from time spent in the saline paired chamber. Prior pilot studies conducted demonstrated this protocol to produced robust, reproducible morphine CPP.
Extinction- Animals that have established morphine induced place preference underwent active extinction sessions. They were placed in the three-chamber apparatus and allowed free access to all chambers for 30mins. Time spent in each chamber was recorded during the first 20mins during this session. 2 sessions per day were conducted. Animals were judged to have demonstrated extinction if their preference score was <50sec. Afterwards the animal’s extinction sessions were terminated.
Drug-primed reinstatement of morphine conditioned place preference- Animals that had established morphine induced place preference and subsequent extinction of place preference, underwent morphine priming induced reinstatement in the following fashion. Animals were injected with morphine (5mg/kg), and 5mins later they were placed in the three-chamber apparatus and allowed free access to all chambers for 20mins. Time spent in each chamber was recorded and the preference scores were determined.
RNA isolation and real time quantitative PCR
Mice were euthanized by CO2 asphyxiation and brain tissues of nucleus accumbens and medial prefrontal were rapidly dissected on wet ice. Tissue samples were quickly frozen on dry ice and stored at −80° C until read for analysis. For real-time quantitative PCR, total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to manufacture instructions. The purity and concentration were determined using spectrophotometry. The total RNA sample were reversed transcribed into cDNA using a First strand cDNA synthesis kit (Invitrogen, Carlsbad, CA). Real time PCR was performed in an ABI prism 7900HT system (Applied Biosystems, Foster City, CA). All PCR experiments were performed using the SYSBR green I master kit (Applied Biosystem). The primer sets for prodynorphin were CCATCCCAGAATCCAGAGAA (Forward) and CCAGGGTAGGGTGCATAAGA (Reverse) as previously described 24. The prodynorphin primers were purchased from SABiosciences (SABiosciences, Valencia, CA). As negative controls, RNA samples of beta actin, GADPH were also run as an internal control. Relative fold of gene expression of samples was determined by the delta-delta CT method as previously described 24, and treatment groups were normalized to the control group.
Statistical analysis
Data on behavioral and molecular studies are presented as means ± SD. Behavioral data related to morphine conditioning were analyzed by one-way ANOVA with Sidak’s post hoc tests. Data on von Frey testing were analyzed by two-way repeated measures ANOVA with Tukey’s multiple comparison testing. Molecular data were analyzed by one-way ANOVA with Dunnett’s post hoc tests. Statistical significance was determined at the alpha level of 0.05 and a power level of 0.8. (Prism 7 GraphPad software, La Jolla, CA). Group sizes were determined based on pilot studies and power analyses for at least 6 animals per group was needed in most behavioral tests. However, due to the variability in behavioral responses, sample size reduction between preference testing and drug primed reinstatement was expected in treatment groups (Figure 1a,b). This manuscript adheres to the EQUATOR guidelines as appropriate.
RESULTS
Development of mechanical allodynia to incisional injury
Animals with hindpaw incision showed decreased mechanical thresholds compared to sham group (Figure 2). There was a significant interaction with incision versus sham groups that was time dependent with effects lasting up to 4 days administration (time x injury F(21,140 =5.61; p<0.0001). However, there was no significant effect of treatment with morphine administration on mechanical thresholds suggesting a lack of opioid-induced hyperalgesia.
Figure 2:
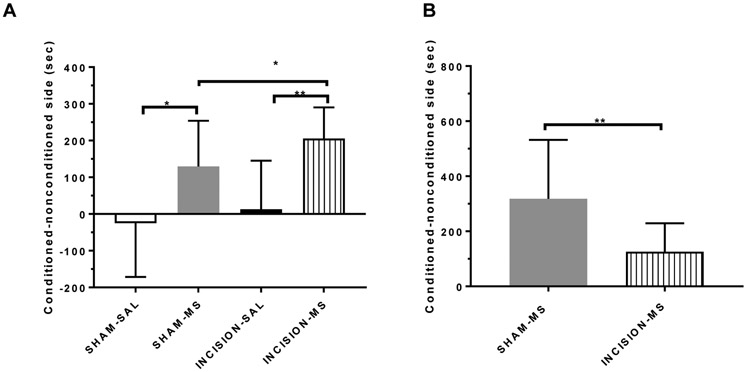
Morphine induced conditioned place preference with acute incisional injury (A) and subsequent morphine primed reinstatement (B).
Enhancement of morphine conditioned place preference in the presence of incisional injury and attenuation of drug-primed reinstatement in injured animals conditioned with morphine.
Animals conditioned with morphine spent more time in the drug paired side as compared to saline controls demonstrating conditioned place preference (Figure 3A), t(25)=2.74;p=0.0112, n=9, n=18 respectively). Additionally, animals conditioned with morphine that also had hindpaw incision showed conditioned place preference to the drug paired side compared to animals conditioned with saline (n=16, n=7 respectively), and it was significantly higher than animals without injury (Figure 3A), F(3,40)= 8.63; p<0.001; t(32)=2.06; p=0.0477). Animals in treatment groups that showed conditioned place preference to morphine underwent extinction and were re-exposed to morphine (Sham n=10; Incision n=12). While animals without injury reinstated preference to the drug-paired side after re-exposure to morphine (Figure 3b), animals with injury showed an attenuated response (t(20)=2.76; p=0.0121) to drug primed reinstatement.
Figure 3:
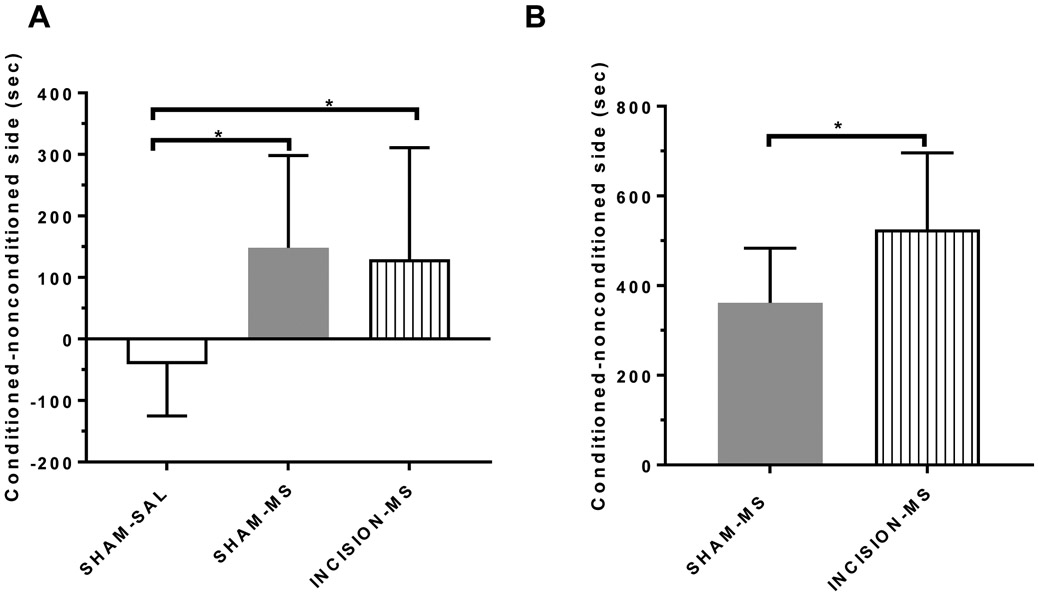
Morphine induced conditioned place preference after remote prior incisional injury (A) and subsequent morphine primed reinstatement (B).
Enhancement of drug primed reinstatement in animals recovered from prior incisional injury.
Twelve days after hindpaw incision, animals underwent conditioned place preference with either saline or morphine (sham saline n=5; sham morphine n=16, incision morphine n=18). There were no differences between time spent in the drug-paired side in all morphine treatment groups in this study (Figure 4), (F(2,39)= 4.08; p=0.0247; Post hoc test incision morphine x sham morphine t (32)= 0.329; p=0.744). Animals that showed conditioned place preference to morphine underwent extinction and were re-exposed to morphine. While animals without injury (sham n=9) reinstated preference to the drug-paired side after re-exposure to morphine (Figure 4b), animals recovered from prior injury (incision n=8) showed an enhanced response to reinstatement of place preference (t (15)=2.30; p=0.0361).
Figure 4:
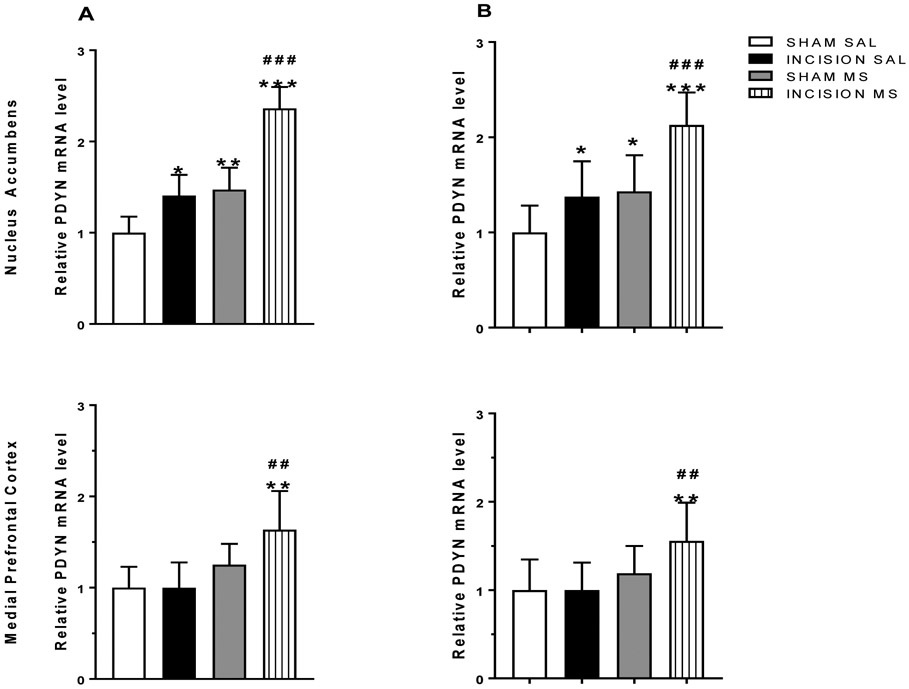
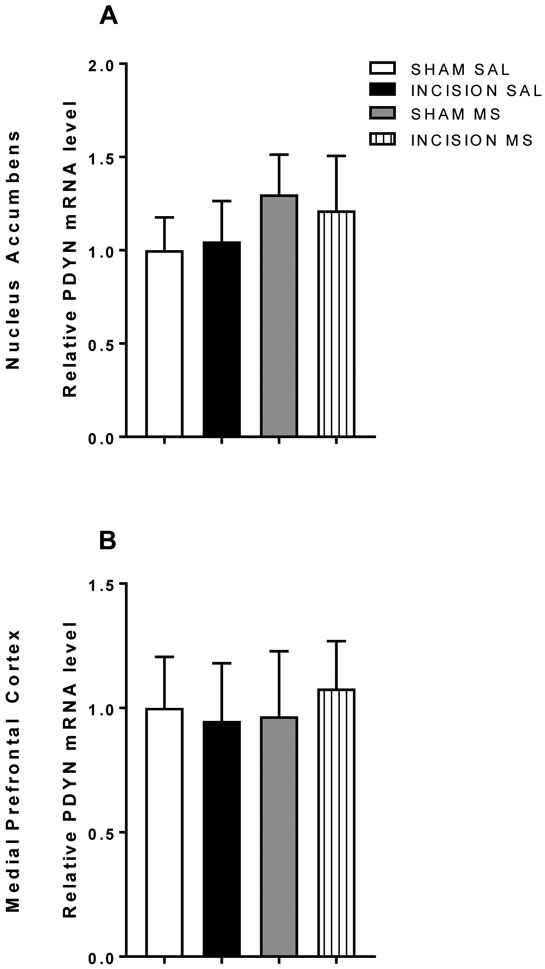
Increased prodynorphin expression with incisional injury and conditioning with morphine remains persistently elevated prior to reinstatement.
Elevated expression of prodynorphin mRNA in the nucleus accumbens and medial prefrontal cortex induced by incisional injury during expression of conditioned place preference, and remains persistent prior to reinstatement
Brain tissues from the nucleus accumbens and medial prefrontal cortex were taken from injured animals previously conditioned with morphine and other treatment groups (n=6/group). The relative ratios of dynorphin mRNA were elevated after morphine conditioning and even higher in the presence of incisional injury (Figure 5a) in tissues from the nucleus accumbens. In the medial prefrontal cortex, elevated levels of dynorphin mRNA were present with morphine conditioning and incisional injury (Figure 5a). Persistent changes in dynorphin mRNA were observed when brain tissues were extracted prior to drug-primed reinstatement testing (Figure 5b; n=12/group).
Figure 5:
Prodynorphin expression with conditioning with morphine in the presence of prior remote incisional injury.
No differences in dynorphin mRNA expression in the nucleus accumbens or medial prefrontal cortex after recovery from incisional injury prior to drug-primed reinstatement
Brain tissues from the nucleus accumbens and medial prefrontal cortex were taken from animals well recovered from injury and previously conditioned with morphine and other treatment groups. The relative ratios of dynorphin mRNA were similar in all treatment groups (Figure 6) in tissues from the nucleus accumbens and the medial prefrontal cortex.
Figure 6:
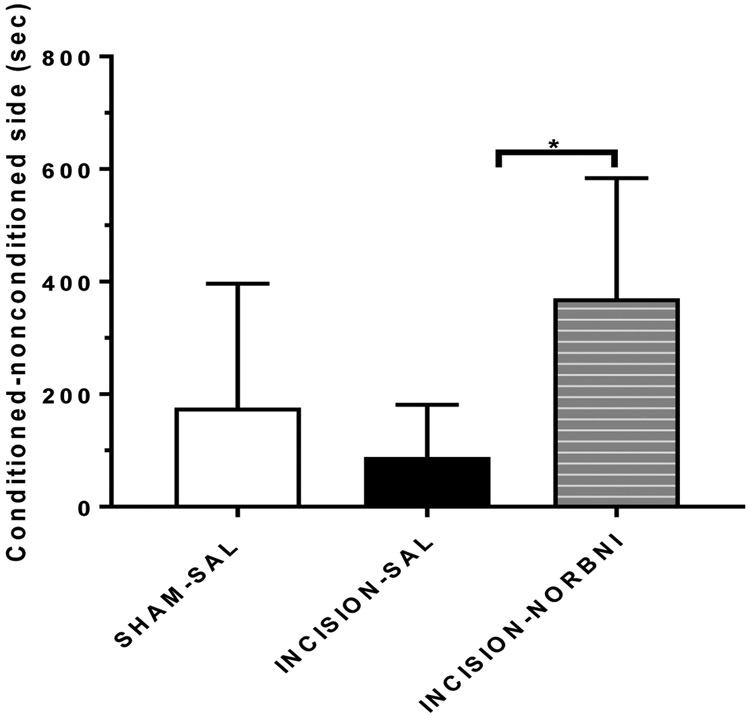
Kappa opioid receptor blockade with systemic administration of norBNI (10mg/kg s.c.) reverses attenuated responses to morphine primed reinstatement from incisional injury.
KOR blockade reversed attenuated drug-primed re-instatement in injured animals conditioned with morphine.
Injured animals that showed conditioned place preference to morphine and re-exposed to morphine. While injured animals (n=6) showed a trend towards an attenuated response by spending less time in the drug-paired side after re-exposure to morphine (Figure 7), animals with injury that were administered norBNI (n=6) prior to reinstatement showed preference to the drug paired side (Figure 7; (F (3,39)= 2.99; p=0.0544).
DISCUSSION
There is concern that exposure to prescription opioids during the perioperative period and after recovery presents a potential pathway towards opioid use disorders. Our present study demonstrates that the presence of incisional injury enhances morphine reward but paradoxically attenuates subsequent morphine primed reinstatement suggesting a protective adaptation from drug primed reinstatement and relapse. This protective adaptation appears to involve persistent over-expression of prodynorphin and KOR activation localized to the nucleus accumbens and medial prefrontal cortex. However, a remote history of injury confers no such protection from drug primed reinstatement. It enhances drug-primed reinstatement, which appears to be independent of modulation in the dynorphin- KOR system in the nucleus accumbens and medial prefrontal cortex.
We observed the enhancement of morphine reward in the presence of incisional injury, which is thought to be a clinically relevant model of minor trauma 28. Other pain models have also demonstrated similar enhancement of morphine reward including inflammatory pain 29 and osteoarthritis30. The initial enhancement of morphine-induced place preference may be due to pain relief, morphine reward or a combination of both 29,31. On the contrary in a chronic neuropathic pain model, sciatic nerve injury, there is attenuation of morphine reward 7. Collectively, these studies suggest a modulatory effect of injury on morphine reward that is dependent on the type of pain elicited and perhaps the duration of pain.
The attenuation of drug primed reinstatement as a result of incisional injury and the enhancement of drug primed reinstatement by remote injury are novel findings that will require additional mechanistic evaluation. Drug primed reinstatement is a well described behavioral model used to study relapse to reward and/or drug seeking behaviors 32. Our findings suggest a decreased propensity to relapse to morphine reward that is dependent on whether the drug was initially administered during or outside of injury. Our findings also suggest that a remote history of injury confers increased propensity to drug primed relapse, which highlights other unidentified factors that may influence drug relapse. Some known factors impacting reinstatement include stress, environmental cues associated with initial use and psychological distress 11. These factors may interact with injury in modulating reinstatement, but this possibility remains to be examined in a systematic fashion. It should be recognized that we administered morphine to injured animals for three days after injury, and it is possible that the protective effect of injury wanes if opioids were to continue to be administered.
The observed enhancement of morphine reward with incisional injury could perhaps be explained by the presence of opioid induced hyperalgesia (OIH) if this form of hyperalgesia were to result from morphine administration during the conditioning process. In theory, OIH presenting during conditioning might strengthen place preference due to increased relief from this pain sensitized state. Specifically, incision-induced pain might be exacerbated by the effects of OIH as we have shown in previous studies20,24. We have established that OIH can prolong incision-related mechanical and thermal pain sensitization in mice for several days, although the severity of OIH is related to the dose and duration of opioid administration 23,25. In the present experiments, the morphine administration protocol was not of sufficient intensity to cause measurable OIH. However, in a clinical setting where patients had more substantial opioid use histories, the temporary relief of OIH by opioid administration might help drive more opioid consumption.
Our findings demonstrated a greatly increased prodynorphin expression in the nucleus accumbens and prefrontal cortex with incisional injury, changes that were not present with remote injury prior to reinstatement. This suggests a temporal element of KOR activation in these neural substrates resulting from injury. To assess if the activation of KOR had functional consequences with drug-primed reinstatement, KOR blockade with norBNI was used to observe behavior change drug primed reinstatement behavior after injury. The administration of systemic KOR antagonist resulted in reversal and enhancement of drug-primed reinstatement, which would have been attenuated with injury. Of note, norBNI, while a well-studied long acting kappa antagonist, its selectivity for KOR in certain in vivo assays has been questioned33. Therefore it is not possible to completely rule out other effects mediated by systemic norBNI that are independent of KOR.
Available compounds such as buprenorphine and dezocine, two drugs with mixed partial mu receptor agonist and KOR antagonist activity with clinical utility, can be further examined for their potential role in opioid reward, dependence and reinstatement behaviors34. Studies are shown so far both dezocine and buprenorphine administered after extinction of morphine CPP attenuates morphine primed reinstatement, and administration of dezocine attenuates morphine withdrawal scores, suggesting that both effects are mediated independent of KOR involvement34. Our findings suggest that with the use of these compounds, the protective adaptation from drug relapse as a result of KOR activation from injury may be lost, which can lead up to intriguing investigations in the future for their potential positive or negative therapeutic values on opioid reward and addictive behaviors.
The role of KOR signaling within the reward circuitry is known to influence reinstatement to drug reward and drug seeking behaviors, in particular stress induced drug relapse 17,35,36. Indeed, in studies on forced stress reinstatement with drug reward, KOR blockade attenuates while KOR activation enhances forced stress induced reinstatement to drug seeking and reward 17,35. In the case of drug primed reinstatement using a different drug, the influence of KOR also appears to be a stress-mediated mechanism. In this study, both stress thru HPA axis activation and elevated prodynorphin expression in the nucleus accumbens and amygdala were involved in drug-primed reinstatement to heroin seeking by yohimbine. Furthermore, the drug-primed reinstatement was attenuated KOR blockade 37.
In our study, KOR blockade resulted in reversal and enhancement of drug-primed reinstatement with injury, which has not been previously described. This suggests the factors and/or neural substrates mediating drug-primed reinstatement versus stress-induced reinstatement may be distinct. The differences can perhaps be explained by discrete systems in the reward circuitry mediating drug primed reinstatement and stress induced reinstatement respectively 38. Future investigations can focus on other triggers of reinstatement to drug reward such as stress in the presence of injury, and can examine if KOR signaling is similarly involved as with prior studies.
Studies have reported possible risk factors associated with continued persistent use of prescription opioids beyond surgical recovery to include co-morbid psychological distress such as depression, anxiety, pain catastrophizing, and presence of opioid use prior to surgery 39,40 From our study, we speculate that another factor may include whether there was a history of remote trauma/ surgery prior to the use of prescription opioids, but not if patients were only administered opioids at the time of surgery or trauma. However, determining the circumstances in which the patients receives the prescription opioids and identifying what are prominent risk factors becomes complicated. For instance, some patients that have had trauma and injuries requiring surgery may also have a preexisting history of substance abuse31,32, which they would be already at risk for continued use if prescribed opioids. Still, it seems reasonable to suggest that opioid administration be limited to the acute postoperative period where the drugs are not only most needed but also safest to use with respect to vulnerability for developing an opioid use disorder. Although our studies have only helped initiate the study of the interaction of opioid use and surgical injury, the importance of generating more comprehensive work in this field is crucial.
Supplementary Material
KEY POINTS SUMMARY:
Question: In an animal model, does the presence and history of minor injury affect morphine reward and relapse to reward?
Findings: The presence of minor injury enhances morphine reward but attenuates relapse to morphine reward.
Meaning: The use of opioids in a state of acute injury, although initially more rewarding, may confer a neuroadaptive protection against drug relapse.
ACKNOWLEDGEMENTS
We would like to thank Peyman Sahbaie, MD for editing recommendations. The authors do not have any financial or relationships to disclose leading to a conflict of interest. This work was supported by NIDA T32 DA035165 and I01 BX000881 06.
Footnotes
CONFLICTS OF INTEREST: None
REFERENCES
- 1.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349(20):1943–1953. [DOI] [PubMed] [Google Scholar]
- 3.Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain. 2006;7(1):43–48. [DOI] [PubMed] [Google Scholar]
- 4.Martell BA, O'Connor PG, Kerns RD, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116–127. [DOI] [PubMed] [Google Scholar]
- 5.Ilgen MA, Perron B, Czyz EK, McCammon RJ, Trafton J. The timing of onset of pain and substance use disorders. Am J Addict. 2010;19(5):409–415. [DOI] [PubMed] [Google Scholar]
- 6.Hipolito L, Wilson-Poe A, Campos-Jurado Y, et al. Inflammatory Pain Promotes Increased Opioid Self-Administration: Role of Dysregulated Ventral Tegmental Area mu Opioid Receptors. J Neurosci. 2015;35(35):12217–12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narita M, Shibasaki M, Mizuo K, Suzuki T. Changes in G-protein activity mediated through the stimulation of dopamine and GABA(B) receptors in the mesolimbic dopaminergic system of morphine-sensitized mice. Addict Biol. 2003;8(3):319–325. [DOI] [PubMed] [Google Scholar]
- 8.Gaetano Di Chiara AI. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences. 1988;85(14):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navratilova E, Morimura K, Xie JY, Atcherley CW, Ossipov MH, Porreca F. Positive emotions and brain reward circuits in chronic pain. J Comp Neurol. 2016;524(8):1646–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci. 2014;17(10):1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106(7):2423–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215(4531):413–415. [DOI] [PubMed] [Google Scholar]
- 14.James IF, Chavkin C, Goldstein A. Selectivity of dynorphin for kappa opioid receptors. Life Sci. 1982;31(12-13):1331–1334. [DOI] [PubMed] [Google Scholar]
- 15.Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol. 1986;249(3):293–336. [DOI] [PubMed] [Google Scholar]
- 16.Mansour A, Fox CA, Burke S, et al. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350(3):412–438. [DOI] [PubMed] [Google Scholar]
- 17.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens CW, Lacey CB, Miller KE, Elde RP, Seybold VS. Biochemical characterization and regional quantification of mu, delta and kappa opioid binding sites in rat spinal cord. Brain Res. 1991;550(1):77–85. [DOI] [PubMed] [Google Scholar]
- 19.Hylden JL, Nahin RL, Traub RJ, Dubner R. Effects of spinal kappa-opioid receptor agonists on the responsiveness of nociceptive superficial dorsal horn neurons. Pain. 1991;44(2):187–193. [DOI] [PubMed] [Google Scholar]
- 20.Liang DY, Li X, Clark JD. Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice. J Pain. 2013;14(1):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang DY, Sun Y, Shi XY, Sahbaie P, Clark JD. Epigenetic regulation of spinal cord gene expression controls opioid-induced hyperalgesia. Mol Pain. 2014;10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takemori AE, Ho BY, Naeseth JS, Portoghese PS. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther. 1988;246(1):255–258. [PubMed] [Google Scholar]
- 23.Sun Y, Sahbaie P, Liang DY, et al. Epigenetic regulation of spinal CXCR2 signaling in incisional hypersensitivity in mice. Anesthesiology. 2013;119(5):1198–1208. [DOI] [PubMed] [Google Scholar]
- 24.Sahbaie P, Liang DY, Shi XY, Sun Y, Clark JD. Epigenetic regulation of spinal cord gene expression contributes to enhanced postoperative pain and analgesic tolerance subsequent to continuous opioid exposure. Mol Pain. 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahbaie P, Shi X, Guo TZ, et al. Role of substance P signaling in enhanced nociceptive sensitization and local cytokine production after incision. Pain. 2009;145(3):341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- 27.Poree LR, Guo TZ, Kingery WS, Maze M. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesth Analg. 1998;87(4):941–948. [DOI] [PubMed] [Google Scholar]
- 28.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570–587. [DOI] [PubMed] [Google Scholar]
- 29.Lim G, Kim H, McCabe MF, et al. A leptin-mediated central mechanism in analgesia-enhanced opioid reward in rats. J Neurosci. 2014;34(29):9779–9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, Tao W, Hou YY, Wang W, Kenny PJ, Pan ZZ. MeCP2 repression of G9a in regulation of pain and morphine reward. J Neurosci. 2014;34(27):9076–9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z, Tao W, Hou YY, Wang W, Lu YG, Pan ZZ. Persistent pain facilitates response to morphine reward by downregulation of central amygdala GABAergic function. Neuropsychopharmacology. 2014;39(9):2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller DP D; Stewart J Persistence and drug induced reinstatement of a morphine induced conditioned place preference. Behavioral Pain Research. 2002;13:8. [DOI] [PubMed] [Google Scholar]
- 33.Birch PJ, Hayes AG, Sheehan MJ, Tyers MB. Norbinaltorphimine: antagonist profile at kappa opioid receptors. Eur J Pharmacol. 1987;144(3):405–408. [DOI] [PubMed] [Google Scholar]
- 34.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron. 2013;77(5):942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polter AM, Bishop RA, Briand LA, Graziane NM, Pierce RC, Kauer JA. Poststress block of kappa opioid receptors rescues long-term potentiation of inhibitory synapses and prevents reinstatement of cocaine seeking. Biol Psychiatry. 2014;76(10):785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Leri F, Grella SL, Aldrich JV, Kreek MJ. Involvement of dynorphin and kappa opioid receptor in yohimbine-induced reinstatement of heroin seeking in rats. Synapse. 2013;67(6):358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalivas PW, Pierce RC, Cornish J, Sorg BA. A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol. 1998;12(1):49–53. [DOI] [PubMed] [Google Scholar]
- 39.Carroll I, Barelka P, Wang CK, et al. A pilot cohort study of the determinants of longitudinal opioid use after surgery. Anesth Analg. 2012;115(3):694–702. [DOI] [PubMed] [Google Scholar]
- 40.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.