Abstract
Background
Eligibility criteria and endpoints for cancer cachexia trials—and whether weight loss should be included—remain controversial. Although most cachexia trials enrol patients after initial cancer diagnosis, few studies have addressed whether weight loss well after a cancer diagnosis is prognostic.
Methods
We pooled data from non‐small cell lung cancer patients from prospectively conducted trials within the Alliance for Clinical Trials in Oncology (1998–2008), a nationally funded infrastructure. We examined (i) weight data availability and weight changes and (ii) survival.
Results
A total of 822 patients were examined. Of these, 659 (80%) were on treatment at the beginning of Cycle 2 of chemotherapy; weight was available for 656 (80%). By Cycles 3 and 4, weight was available for 448 (55%) and 384 (47%), respectively. From baseline to immediately prior to Cycle 2, 208 (32%) gained weight; 225 (34%) lost <2% of baseline weight; and 223 (34% of 656) lost 2% or more. Median survival from the beginning of Cycle 2 was 13.0, 10.9, and 6.9 months for patients with weight gain, weight loss of <2%, and weight loss of 2% or more, respectively. In multivariate analyses, adjusted for age, sex, performance score, type of treatment, and body mass index, weight loss of 2% or more was associated with poor overall survival compared with weight gain [hazard ratio (HR) = 1.66; 95% confidence interval (CI): 1.33–2.07; P < 0.001] and compared with weight loss of <2% (HR = 1.57; 95% CI: 1.27–1.95; P < 0.001). Although weight loss of <2% was not associated with poorer overall survival compared with weight gain, it was associated with poorer progression‐free survival (HR = 1.24; 95% CI: 1.01–1.51; P = 0.036). Similar findings were observed in a separate 255‐patient validation cohort.
Conclusions
Weight should be integrated into cancer cachexia trials because of its ease of frequent measurement and sustained prognostic association.
Keywords: Weight loss, Landmark analysis, Prognosis, Survival, Lung cancer
Introduction
The selection of appropriate eligibility criteria and endpoints for cancer cachexia clinical trials is an area of avid interest and unresolved debate. Cachexia occurs in the majority of patients with advanced, incurable cancer. It is a syndrome characterized by weight loss, poor appetite, attrition of both lean tissue and fat but especially the former, an otherwise inexplicable increase in basal metabolic rate, a decline in patient functional status, and poor survival. Indeed, the multifaceted nature of this constellation of signs and symptoms only adds to the quandary of choosing the most appropriate clinical trial eligibility criteria and endpoints. 1 , 2 , 3 Not surprisingly, the recent unsuccessful cancer cachexia clinical trials have prompted only further discussion of clinical trial design and choice of appropriate eligibility criteria and endpoints. 4 , 5 , 6 , 7
Simple, unadorned, and inexpensive, weight has, at times, been incorporated into cancer cachexia trials. 8 , 9 , 10 Dewys and others have shown that among 3047 chemotherapy‐naïve patients with cancer, self‐reported weight loss over the preceding 6 months at clinical trial entry predicts an early demise. 11 Several other studies have confirmed this observation, showing that weight loss at the time of cancer diagnosis or at the time of another hallmark baseline appraisal predicts an early demise. In fact, a large, often‐cited, international consensus statement incorporates weight loss into its definition of cancer cachexia. 12
Despite the above, several investigators have questioned the role of weight in cancer cachexia trials. The complexity of body composition within the context of cancer is such that some investigators have suggested that measurements of specific body compartments with perhaps modalities such as computerized tomography should surpass the role of weight loss in assessing outcomes in cachexia trials. Additionally, although baseline weight loss is associated with a poor prognosis, few studies have addressed whether ongoing weight loss—or weight gain—after the initiation of chemotherapy carries any association with survival. Hence, the current study was undertaken with a twofold set of goals. First, it sought to characterize the weight trajectory of patients with non‐small cell lung cancer and to assess patient dropout rates over time in order to speculate on whether a tool such as computerized tomography, typically used to assess tumour response/body composition at 6–8 week intervals in contrast to weight at 3–4 week intervals, appears to generate comparable data for cachexia assessment. Second, the current study sought to determine whether weight change after initiation of chemotherapy—a so‐called landmark analysis, with the term ‘landmark’ used here as a statistical term—is a predictive marker, as opposed to only a baseline marker, of prognosis. Importantly, this study relied on prospectively gathered, multi‐institutional, United States' government‐funded data to address the foregoing goals.
Methods
Overview
The Mayo Clinic Institutional Review Board and the Alliance for Clinical Trials in Oncology approved the study protocol prior to initiation and reporting of analyses. The current study sought to understand patterns of weight loss during chemotherapy and justification—or lack thereof—for the incorporation of weight data in future cancer cachexia clinical trials as an eligibility criterion and early indicator of long‐term outcome.
Clinical trial selection
The current study focused on therapeutic trials in patients with non‐small cell lung cancer within the North Central Cancer Treatment Group (now integrated into and renamed the Alliance for Clinical Trials in Oncology), which enrolled patients from 1998 through 2008, with the exception of the following: (i) those that included surgery or concurrent radiation as part of the tested therapeutic intervention, as the inclusion of such studies would have introduced an unacceptable degree of patient and clinical outcome heterogeneity; (ii) those with irretrievable archival information about the study protocol or data set; and (iii) those that had been closed to enrolment prior to completion of planned accrual.
The current study relied on pooled individual patient data from non‐small cell lung cancer clinical trials (Table 1). This cancer type was chosen because non‐small cell lung cancer is the most common cause of cancer‐related death in the United States and because it has been the focus of at least two large, multisite cancer cachexia clinical trials. 4 , 5 , 6 , 7
Table 1.
Included trials
| Trial identifier | Dates of accrual | Sample size | Cancer therapy |
|---|---|---|---|
| N0528 | 08/08/2007–11/24/2008 | 96 | (A) Gemcitabine + carboplatin + cediranib vs. (B) gemcitabine + carboplatin |
| N0426 | 07/06/2006–01/25/2007 | 48 | Pemetrexed + bevacizumab |
| N0326 | 02/10/2005–06/09/2005 | 25 | Sorafenib |
| N0323 | 04/30/2004–10/19/2006 | 52 | Temsirolimus |
| N0222 | 12/03/2004–02/22/2006 | 62 | (A) Carboplatin + paclitaxel followed by gefitinib vs. (B) gefitinib |
| N0026 | 09/05/2001–05/23/2003 | 150 | (A, B, C) Pemetrexed + gemcitabine (administered via different schedules) |
| N0022 | 04/30/2001–03/06/2002 | 58 | Vinorelbine |
| N9921 | 02/02/2000–02/07/2001 | 49 | Carboplatin + paclitaxel |
| 982453 | 12/15/1999–03/30/2001 | 36 | Irinotecan + docetaxel |
| 982452 | 03/08/1998–06/12/2000 | 99 | (A, B) Docetaxel + gemcitabine (administered via different schedules) |
| 972451 | 06/01/1999–04/06/2004 | 147 | (A) Carboxy‐amino‐imidazole (CAI) vs. (B) placebo |
Statistical analyses
Patient demographics and baseline characteristics including age, sex, race, height, weight, body mass index (BMI), performance score (PS), and disease stage were summarized using mean (with standard deviation) or median (with range) values for continuous variables and frequencies (with percentage) for categorical variables. The percentage of patients with weight data and reasons for missing weight data at each treatment cycle were summarized. Percent weight loss at each treatment cycle was summarized using mean (standard deviation) and median values (range) and plotted by number of treatment cycles. Weight measurements were inspected for outliers and corrected in the event of an apparent data entry error (seven cases). Missing weight measurements were imputed by linear interpolation when weight was recorded for the immediately preceding and following cycles (four cases). A linear regression model was built to determine the association between baseline factors and percent weight change between the start of Cycles 1 and 2. Due to the large number of patients dropping off these trials due to death, disease progression, adverse events, and other reasons, a model for weight change beyond Cycle 2 was deemed meaningless and not considered.
Overall survival and progression‐free survival were estimated using the Kaplan–Meier estimator. Time to death and to disease progression were measured from the start of Cycle 2. Landmark Cox proportional hazards regression models were used to determine the effect of post‐treatment weight change on overall survival and progression‐free survival. The proportional hazards assumption was checked using scaled Schoenfeld residuals. All models reported in this study satisfied the proportional hazards assumption. All statistical tests were conducted at the two‐sided significance level of 0.05. All analyses were conducted using SAS version 9.4 and R version 3.4.2.
Results
Patient and trial characteristics
Eleven non‐small cell lung cancer trials were used for the current study's primary analyses, which encompassed a total of 822 patients (Table 1).
Table 2 shows the frequency and percentage of patients with complete weight data by chemotherapy treatment cycle and reasons why weight data were not available. At the beginning of Cycle 2, weight data were available for 656 (80%) patients. Reasons why weight data were not available included the following: 20 (2%) patients died on study, 75 (9%) went off study due to disease progression, and 68 (8%) went off study for other reasons that included adverse events or choice of alternative treatment (Table 2). By the beginning of Cycle 3, weight data were available for only 448 (55%) patients. By this time, the number of patients who went off study due to disease progression increased to 229 (28%). At the start of chemotherapy Cycle 4, weight data were available for 384 (47%) patients, while the number of patients who went off treatment due to disease progression increased to 267 (33%). The number of patients on study with missing weight data was negligible across all time points (four patients, 0.5%). Figure 1 shows the weight trajectory of patients by availability of weight data. Patients who went off treatment prior to the beginning of chemotherapy Cycle 2 or 3 experienced more weight loss than patients who remained on treatment at Cycle 4.
Table 2.
Reasons for availability and unavailability of patient data (percentages denote the percentage of 822 patients with Cycle 1 weight data)
| Status | Start of Cycle 2 (%) | Start of Cycle 3 (%) | Start of Cycle 4 (%) |
|---|---|---|---|
| Available patient data | 656 (80) | 448 (55) | 384 (47) |
| Unavailable patient data | |||
| Death on study | 20 (2.4) | 26 (3.2) | 31 (3.8) |
| Disease progression | 75 (9.1) | 229 (27.9) | 267 (32.5) |
| Adverse events or complications | 25 (3.0) | 53 (6.4) | 66 (8.0) |
| Other medical complication | 4 (0.5) | 4 (0.5) | 6 (0.7) |
| Patient withdrawal or refusal | 29 (3.5) | 43 (5.2) | 47 (5.7) |
| Alternate treatment | 2 (0.2) | 4 (0.5) | 6 (0.7) |
| Other reason | 8 (1.0) | 11 (1.3) | 11 (1.3) |
| Missing weight | 3 (0.4) | 4 (0.5) | 4 (0.5) |
Figure 1.
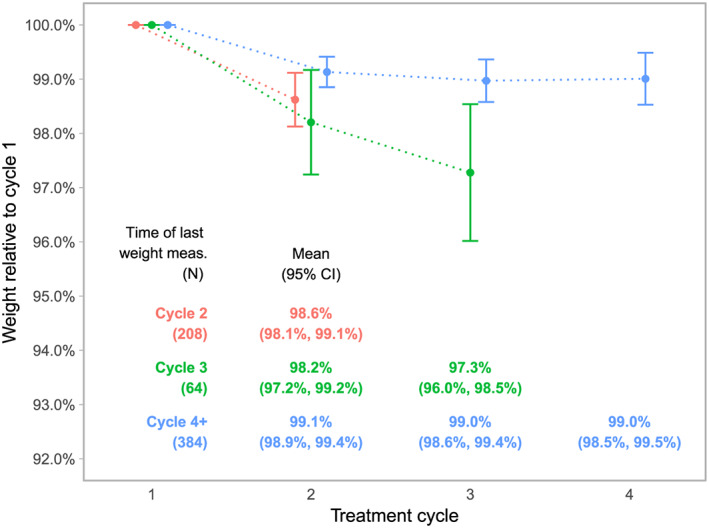
Weight change by treatment cycle. CI, confidence interval.
Weight loss between chemotherapy Cycles 1 and 2
The mean weight change between cycles 1 and 2 of cancer treatment was −1.1% (standard deviation: 3.2) with median −0.8% (range: −15.6% to 11.9%). From baseline to immediately prior to Cycle 2 of cancer treatment, 208 (32% of 656) patients gained weight, 225 (34% of 656) lost between 0 and 2%, and 223 (34% of 656 patients with Cycle 1 weight data) lost more than 2%. Table 3 shows patient characteristics by weight change. Linear regression models that examined the associations between weight loss and a variety of variables, specifically age (65 years of age or older versus younger); sex, PS, BMI (categorized as underweight at </=18.5, normal weight at 18.5 to 24.9, overweight at 25 to 29.9, or obese at 30 or greater), and trial and treatment arm (accounting for time effect and type and line of treatment) revealed that baseline BMI classified as overweight was associated with more weight loss [0.7%; 95% confidence interval (CI): 0.14–1.26%; P = 0.015] compared with normal BMI. The pattern of weight loss varied according to treatment trial/arm (overall P < 0.001).
Table 3.
Patient demographics based on weight change from Cycle 1 to 2
| Weight gain (N = 208) | Weight loss <2.0% (N = 225) | Weight loss >2.0% (N = 223) | P value | |
|---|---|---|---|---|
| Age | 0.9199 a | |||
| Mean (SD) | 66.2 (10.15) | 66.2 (10.12) | 65.7 (10.38) | |
| Median (range) | 67 (38, 91) | 67 (33, 91) | 67 (37, 90) | |
| Sex, n (%) | 0.5782 b | |||
| Female | 87 (41.8) | 85 (37.8) | 94 (42.2) | |
| Male | 121 (58.2) | 140 (62.2) | 129 (57.8) | |
| Race, n (%) | 0.8360 b | |||
| White | 185 (96.9) | 182 (94.3) | 177 (95.2) | |
| Other | 6 (3.1) | 11 (5.7) | 9 (4.8) | |
| Missing | 17 | 32 | 37 | |
| Baseline BMI | 0.1543 a | |||
| Mean (SD) | 25.9 (4.86) | 27.0 (5.95) | 26.8 (5.15) | |
| Median (range) | 25.4 (15.1, 41.6) | 26.2 (15.8, 47.0) | 26.5 (15.5, 48.0) | |
| Baseline BMI category, n (%) | 0.1149 b | |||
| Normal weight | 84 (41.2) | 82 (37.4) | 66 (32.0) | |
| Obese | 34 (16.7) | 56 (25.6) | 45 (21.8) | |
| Overweight | 77 (37.7) | 74 (33.8) | 90 (43.7) | |
| Underweight | 9 (4.4) | 7 (3.2) | 5 (2.4) | |
| Missing | 4 | 6 | 17 | |
| Performance score, n (%) | 0.2782 b | |||
| 0 | 95 (45.7) | 97 (43.1) | 83 (37.2) | |
| 1 | 103 (49.5) | 120 (53.3) | 125 (56.1) | |
| 2 | 10 (4.8) | 8 (3.6) | 15 (6.7) | |
| Disease stage, n (%) | 0.1730 b | |||
| IIIA | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| IIIB | 32 (16.1) | 41 (19.2) | 29 (13.6) | |
| IV | 164 (82.4) | 171 (80.3) | 184 (86.4) | |
| Other | 3 (1.5) | 1 (0.5) | 0 (0.0) | |
| Missing | 9 | 12 | 10 | |
BMI, body mass index; SD, standard deviation.
Kruskal–Wallis P value.
χ2 P value.
Weight loss and survival
Weight change in the first cycle of cancer treatment was classified into three categories: any amount of weight gain (n = 208), weight loss less than 2% (n = 225), and weight loss of 2% or more (n = 223). The median survival time from the beginning of Cycle 2 was 13.0, 10.9, and 6.9 months for patients with any weight gain, with weight loss of <2%, and with loss of 2% or more, respectively. Compared with weight gain and to weight loss of <2%, weight loss of 2% or more showed a statistically significant association with poor survival (Figure 2). In the multivariate Cox model, risk of overall mortality was higher with weight loss ≥2% compared with weight gain [hazard ratio (HR) = 1.66; 95% CI: 1.33–2.07; P < 0.001] and compared with loss of <2% (HR = 1.57; 95% CI: 1.27–1.95; P < 0.001) (Table 4). There was no statistically significant survival difference between weight gain and weight loss of <2%. Other variables adjusted for in the multivariate Cox model included age (no association with survival); sex (male patients had higher risk of mortality; HR = 1.46; 95% CI: 1.23–1.73; P < 0.001 compared with female patients); PS (poorer scores were associated with higher mortality; HR = 1.27; 95% CI: 1.06–1.53; P = 0.01; HR = 2.24; 95% CI: 1.60–3.12; P < 0.001; for PS of 1 or 2 compared with 0, respectively); baseline BMI (higher BMI was associated with lower mortality; HR = 0.80; 95% CI: 0.66–0.96; P = 0.02; HR = 0.73; 95% CI: 0.58–0.93; P = 0.01; for overweight or obese compared with normal, respectively).
Figure 2.
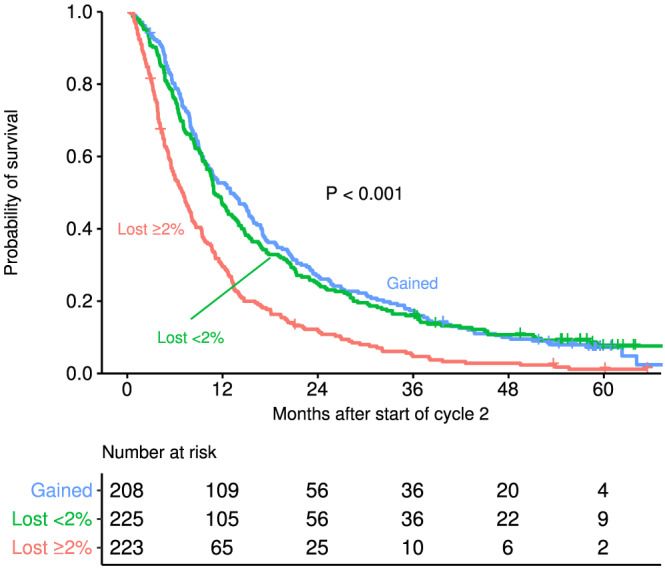
Overall survival curves from beginning of Cycle 2 by weight loss between Cycle 1 and immediately prior to Cycle 2.
Table 4.
Results from multivariate cox models
| Variables | Overall mortality (inverse of OS) | Treatment failure (inverse of PFS) | ||
|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | P value | Hazard ratio (95% confidence interval) | P value | |
| Weight change c | <0.001 a | <0.001 a | ||
| Gained | 1 | 1 | ||
| Lost <2% | 1.05 (0.86–1.29) | 0.625 | 1.24 (1.01–1.51) | 0.036 |
| Lost ≥2% | 1.66 (1.33–2.07) | <0.001 | 1.54 (1.24–1.91) | <0.001 |
| Sex | ||||
| Female | 1 | 1 | ||
| Male | 1.46 (1.23–1.73) | <0.001 | 1.36 (1.49–1.60) | <0.001 |
| Age | ||||
| <65 years | 1 | 1 | ||
| ≥65 years | 0.89 (0.74–1.08) | 0.239 | 0.80 (0.66–097) | 0.020 |
| Baseline BMI | 0.033 a | 0.246 a | ||
| Normal | 1 | 1 | ||
| Underweight | 0.92 (0.58–1.44) | 0.702 | 1.14 (0.72–1.78) | 0.583 |
| Overweight | 0.80 (0.66–0.96) | 0.020 | 0.89 (0.74–1.08) | 0.242 |
| Obese | 0.73 (0.58–0.93) | 0.010 | 0.82 (0.65–1.02) | 0.079 |
| Performance score | 0.001 a | 0.059 a | ||
| 0 | 1 | 1 | ||
| 1 | 1.27 (1.06–1.53) | 0.011 | 1.20 (1.00–1.43) | 0.046 |
| ≥2 | 2.24 (1.60–3.12) | <0.001 | 1.37 (0.99–1.89) | 0.059 |
| Trial/arm b | 0.003 | 0.013 | ||
BMI, body mass index; OS, overall survival; PFS, progression‐free survival.
Overall P value.
Due to a large number of combinations, HRs (hazard ratios) by trial/arm combinations are not listed.
Weight lost ≥2% vs. lost <2%: Overall mortality HR (95% CI) = 1.57 (1.27–1.95), P < 0.001; Treatment failure HR (95% CI) = 1.24 (1.01–1.53), P = 0.037.
Similar analyses with progression‐free survival indicate that weight loss of 2% or more was associated with poorer outcomes compared with weight loss of <2% (HR = 1.24; 95% CI: 1.01–1.53; P = 0.037) and compared with weight gain (HR = 1.54; 95% CI: 1.24–1.91; P < 0.001) (Figure 3). In contrast to what was observed in overall survival, weight loss of <2% was associated with poorer progression‐free survival compared with weight gain of any amount (HR = 1.24; 95% CI: 1.01–1.51; P = 0.036) (Table 4). Male patients had poorer progression‐free survival (HR = 1.36; 95% CI: 1.49–1.60; P < 0.001). Patients 65 years of age or older had better progression‐free survival (HR = 0.80; 95% CI: 0.66–0.97; P = 0.02).
Figure 3.
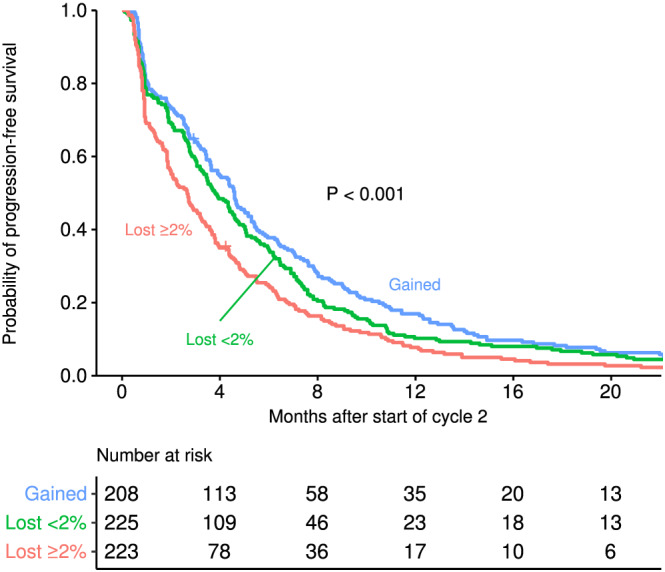
Progression‐free survival curves from beginning of Cycle 2 by weight loss between Cycle 1 and immediately prior to Cycle 2.
To validate the above observations, we applied these analyses to data from a separate 312‐patient trial. 13 Data for weight change after the initiation of cancer therapy were available for 255 (82%) patients enrolled in the trial. Of these 255 patients, 110 (43%) gained weight, 80 (31%) lost <2%, and 65 (26%) lost 2% or more. Kaplan–Meier survival curves were stratified by weight change categories in the same order observed in the analysis above (Figure 4). In multivariate Cox model adjusted for age, sex, PS, baseline BMI, and treatment, weight loss of 2% or more in the first cycle was associated with poorer survival compared with weight gain (HR = 1.46; 95% CI: 1.04–2.04; P = 0.03), but presumably because of small numbers and compromised power, no statistically significant associations with respect to survival were observed with the other weight comparisons.
Figure 4.
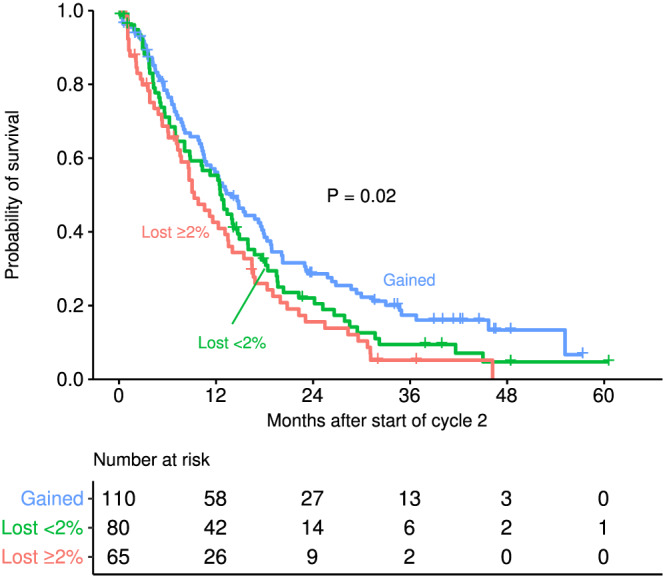
Overall survival curves from beginning of Cycle 2 by weight loss between Cycle 1 and immediately prior to Cycle 2 for the validation cohort.
Discussion
This study shows that weight is a dynamically changing, time‐dependent variable in cancer patients, that its decline is associated with poor clinical outcomes, and that enrolling weight‐losing patients on cachexia trials well after their initial cancer diagnosis is clinically and prognostically justified. Even a moderate weight loss of 2% or more early in the treatment course can be predictive of poorer overall survival. This association between weight loss of 2% or more and survival were highly significant (<0.001) and robust even when one adjusts for multiple comparisons (three pairwise between weight change categories) with conservative Bonferroni corrections. Furthermore, patients actually did gain weight with cancer therapy, a favourable, previously reported observation that prompted us to evaluate data based on weight gain as the referent. 14 Although computerized tomography scans are increasingly recognized for their ability to measure body compartment‐based tissue loss, the data presented here suggest that the simple measurement of weight over time offers advantages. 15 , 16 Patient dropout rates were extreme to the point of making analyses meaningless around the time that computerized tomography scans are typically obtained (6–8 weeks from start of cancer therapy) and thereby suggest the current practice of commandeering these scans to assess body composition results in high rates of missing data and potentially even flawed conclusions. Taken together, the data presented here show that weight is a reliably trackable endpoint for cancer cachexia trials and that its use carries important, sustained clinical prognostic relevance over time.
The use of a landmark analysis—with the term ‘landmark’ used here as a statistical term—is important but not unprecedented. Kimura and others used a similar approach when analysing data from 134 non‐small cell lung cancer patients. 17 The data presented here build on this important prior work by increasing the sample size by more than sixfold, by using prospectively gathered data (as opposed to retrospective data in the Kimura study) and by integrating a validation set into the study design. In effect, the study reported here serves not only to confirm the findings from Kimura and others but also to provide definitive evidence that weight loss over time is meaningful both from a clinical and research perspective. Weight loss should continue to be an eligibility criterion for cachexia trials post‐cancer diagnosis and should be measured in clinical trials aimed at testing new approaches to palliate cancer cachexia.
Interestingly, weight gain appears to be associated with improved progression‐free survival compared with even a negligible amount of weight loss (2%). The fact that similar findings were not observed for overall survival is likely reflective of the high likelihood that, over time, patients went on to receive a variety of other cancer treatment that favourably impacted overall survival but would not have impacted the more immediate progression‐free survival outcome. This association between weight gain and progression‐free survival only further underscores the value of using weight—including weight gain—as a clinically relevant endpoint in cancer cachexia trials.
This study has both strengths and weaknesses. The fact that this study used prospectively gathered data from a multi‐institutional setting is a major strength. To our knowledge, such data have been analysed in this manner for the first time. Similarly, although this study did not incorporate clinical trials that tested immunotherapy, it did incorporate a variety of agents that are currently a part of state‐of‐the‐art cancer care. Thus, the observations reported here are relevant to contemporary oncology. With respect to weaknesses, one might argue that a study that focuses on weight lacks the novelty that one might encounter in a study that focuses on a new molecular marker. However, we counter this so‐called potential weakness by underscoring the pragmatic nature of these findings. Nearly all patients who visit an oncology clinic are weighed frequently over time; thus, the findings reported here carry widespread clinical relevance. Additionally, the information reported here is critical to the conduct of future cancer cachexia studies and provides justification for enrolling patients who have already begun chemotherapy and continue to lose weight to such trials. These weight‐losing patients carry a poor prognosis, should remain candidates for future cancer cachexia trials, and should be monitored on these trials for changes in weight.
In summary, the findings from this 800+ patient cohort and a 200+ patient validation cohort have value from a clinical and research standpoint. Simple weight measurements should continue to be integrated into clinical care as well as into future cancer cachexia clinical trials.
Author contributions
The authors' responsibilities were as follows. J.L. and A.J. conceived and designed the study; J.L., C.L., and E.W. acquired the data; J.L., C.L., E.W., and A.J. wrote the manuscript; and J.L., C.L., E.W., N.R.F., S.J.M., X.W., R.K., A.A., and A.J. read and approved the final version.
Conflict of interest
None of the authors reported a conflict of interest related to the study.
Funding
This work was supported by the Fred C and Katherine B Andersen Foundation and by R01CA195473. A.J. is the Betty J. Foust and Parents' Professor of Oncology.
Ethical statement
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 18
Le‐Rademacher J., Lopez C., Wolfe E., Foster N. R., Mandrekar S. J., Wang X., Kumar R., Adjei A., and Jatoi A. (2020) Weight loss over time and survival: a landmark analysis of 1000+ prospectively treated and monitored lung cancer patients, Journal of Cachexia, Sarcopenia and Muscle, 11, 1501–1508, 10.1002/jcsm.12625
References
- 1. Crawford J. What are the criteria for response to cachexia treatment? Ann Palliat Med 2019;8:43–49. [DOI] [PubMed] [Google Scholar]
- 2. Laird BJA, Balstad TR, Solheim TS. Endpoints in clinical trials in cancer cachexia: where to start? Curr Opin Support Palliat Care 2018;12:445–452. [DOI] [PubMed] [Google Scholar]
- 3. Fearon K, Argiles JM, Baracos VE, Bernabei R, Coats A, Crawford J, et al. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle 2015;6:272–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crawford J, Prado CM, Johnston MA, Gralla RJ, Taylor RP, Hancock ML, et al. Study design and rationale for the phase 3 clinical development program of enobosarm, a selective androgen receptor modulator, for the prevention and treatment of muscle wasting in cancer patients (POWER trials). Curr Oncol Rep 2016;18:37, Review. PMID: 27138015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katakami N, Uchino J, Yokoyama T, Naito T, Kondo M, Yamada K, et al. Anamorelin (ONO‐7643) for the treatment of patients with non‐small cell lung cancer and cachexia: results from a randomized, double‐blind, placebo‐controlled, multicenter study of Japanese patients (ONO‐7643‐04). Cancer 2018;124:606–616, Epub 2017 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Currow D, Temel JS, Abernethy A, Milanowski J, Friend J, Fearon KC. ROMANA 3: a phase 3 safety extension study of anamorelin in advanced non‐small‐cell lung cancer (NSCLC) patients with cachexia. Ann Oncol 2017;28:1949–1956, PMID: 28472437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol 2016;17:519–531, Epub 2016 Feb 20. PMID:26906526. [DOI] [PubMed] [Google Scholar]
- 8. Jatoi A, Steen PD, Atherton PJ, Moore DF, Rowland KM, Le‐Lindqwister NA, et al. A double‐blind, placebo‐controlled randomized trial of creatine for the cancer anorexia/weight loss syndrome (N02C4): an Alliance trial. Ann Oncol 2017;28:1957–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jatoi A, Rowland K, Loprinzi CL, Sloan JA, Dakhil SR, MacDonald N, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer‐associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol 2004;22:2469–2476. [DOI] [PubMed] [Google Scholar]
- 10. Wiskemann J, Clauss D, Tjaden C, Hackert T, Schneider L, Ulrich CM, et al. Progressive resistance training to impact physical fitness and body weight in pancreatic cancer patients: a randomized controlled trial. Pancreas 2019;48:257–266. [DOI] [PubMed] [Google Scholar]
- 11. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–497. [DOI] [PubMed] [Google Scholar]
- 12. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495, Epub 2011 Feb 4. Review. PMID: 21296615. [DOI] [PubMed] [Google Scholar]
- 13. Edelman MJ, Wang X, Hodgson L, Cheney RT, Baggstrom MQ, Thomas SP, et al. Phase III randomized, placebo‐controlled, double‐blind trial of celecoxib in addition to standard chemotherapy for advanced non‐small cell lung cancer with cyclooxygenase overexpression: CALGB 30801 (Alliance). J Clin Oncol 2017;35:2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin L, Watanabe S, Fainsinger R, Lau F, Ghosh S, Quan H, et al. Prognostic factors in patients with advanced cancer: use of the patient‐generated subjective global assessment in survival prediction. J Clin Oncol 2010;28:4376–4383. [DOI] [PubMed] [Google Scholar]
- 15. Prado CM. Body composition in chemotherapy: the promising role of CT scans. Curr Opin Clin Nutr Metab Care 2013;16:525–533. [DOI] [PubMed] [Google Scholar]
- 16. Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr 2014;38:940–953, Epub 2014 Sep 19. Review. Erratum in: JPEN J Parenter Enteral Nutr. 2016 Jul;40(5):742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kimura M, Naito T, Kenmotsu H, Taira T, Wakuda K, Oyakawa T, et al. Prognostic impact of cancer cachexia in patients with advanced non‐small cell lung cancer. Support Care Cancer 2015;23:1699–1708, Epub 2014 Nov 29. [DOI] [PubMed] [Google Scholar]
- 18. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


