Abstract
Background
Muscle weakness is a key factor in the increase risk of falls and might also play a significant role in the increase of risk of hip fracture. Computed tomography‐measured muscle size and muscle density are well‐established imaging biomarkers used in studies of physical function, frailty or cancer, but limited to hip fracture. In particular, it is warranted to have a better understanding of the performance of muscle size and density in the discrimination of acute hip fractures. We also aim to determine age‐related differences of muscle size and density in healthy controls and hip fracture patients.
Methods
Four hundred thirty‐eight low‐energy acute hip fracture cases and 316 healthy controls from the China Action on Spine and Hip Status study were included in the study. Muscle cross‐sectional area and density were measured for the gluteus maximus and gluteus medius and minimus. Areal bone mineral density (aBMD) of the femoral neck and total hip was measured. Using propensity score matching, we generated three samples with cases and controls matched for age, body mass index, and gender: femoral neck fracture (FNF), intertrochanteric fracture (ITF), and any hip fracture vs. controls, respectively.
Results
Handgrip strength, gluteus muscle area and density, and bone parameters of the matched hip fracture groups were lower than those of the correspondence control groups, respectively (P < 0.05). The univariate analysis showed that associations of aBMD with FNF and with ITF were significantly weaker than associations between fracture and muscle parameters. Gluteus medius and minimus muscle density showed the highest areas under the curve (AUC) with FNF (0.88, 95% confidence interval, 0.85–0.92) and trochanteric fracture (0.95, 95% confidence interval, 0.92–0.97). The model including all muscle and bone parameters provided the highest AUC (FNF: AUC 0.912; ITF: AUC 0.958), and AUC results of another selected model without muscle density showed that association with fracture significantly dropped (FNF: AUC 0.755; ITF: AUC 0.858). Separate results for the two age groups younger and older than 70 years showed no age‐related significant differences in discriminate models.
Conclusions
Muscle density performs better than aBMD from hip computed tomography X‐ray absorptiometry and muscle size in discrimination of hip fracture. Combination of aBMD and muscle density provided the best discrimination. The integration of muscle assessments may trigger a paradigm shift in hip fracture prediction. Gluteus muscle density should also be evaluated as treatment outcome.
Keywords: Acute hip fracture, Muscle density, Muscle size, Bone mineral density
Introduction
Hip fractures (HFs) in the elderly are often referred to as ‘The Last Fracture of Life’, with a high degree of morbidity, mortality, and disability. 1 Therefore, it is important to identify subjects with a high HF risk to start preventive actions as soon as possible. Areal bone mineral density (aBMD) determined by dual‐energy X‐ray absorptiometry (DXA) is an established parameter for the diagnosis of osteoporosis and the prediction of HF risk. However, predictive capabilities of aBMD for HF are moderate. 2 , 3 , 4 Three‐dimensional (3D) distribution of bone mineral density (BMD) and geometrical parameters such as cortical thickness measured by quantitative computed tomography (CT) have not substantially improved HF prediction. 5 With the development of computed tomography X‐ray absorptiometry (CTXA), two‐dimensional aBMD of the proximal femur can be derived from a 3D CT dataset. CTXA aBMD values of the total femur and femoral neck (FN) are equivalent to DXA aBMD values and can be used to calculate T‐scores according to the World Health Organization definition of osteoporosis. 6
Apart from a decrease of bone strength, muscle weakness might also play a significant role in the increase of risk of HF. Falls are common in the elderly, and almost all osteoporotic HFs are associated with falls. The risk of falls is multifactorial, but muscle weakness is a key factor in the increase risk of falls. 7 , 8 , 9 , 10 Reduction in muscle performance is associated with the loss of skeletal muscle mass. 11 However, substantial decreases in skeletal muscle function with ageing can occur with only minimal loss of skeletal muscle mass. 12 , 13 The interdependence may be partially related to the presence of fatty infiltration, which is one aspect of muscle quality. The assessment of muscle quality may be more important than the quantification of muscle mass. One aspect of muscle quality, namely, muscle density measured by CT as mean attenuation of skeletal muscle in Hounsfield units (HU), has already been widely used in research studies to assess muscle quality. 14
Computed tomography‐measured muscle size [cross‐sectional area (CSA)] and muscle density (mean HU) of the abdomen and mid‐thigh muscle bundle are well‐established measurements parameters used in studies of physical function, frailty, or cancer. 14 , 15 , 16 However, only a few studies targeted the relationship of hip muscle characteristics with HF. 17 , 18 , 19 In particular, it is warranted to have a better understanding of the performance of muscle size and density in the discrimination of acute HFs.
The main aim of this study is to explore the value of muscle parameters for the discrimination of acute HFs. For this purpose, we used data from the China Action on Spine and Hip Status study including acute low‐energy HFs cases and healthy controls. We also aim to determine age‐related differences of muscle size and density in healthy controls and HF patients. We hypothesize that HF patients have lower muscle density than age‐matched controls, and muscle density performs better than bone variables and muscle size in discriminating HFs.
Methods
Study design
China Action on Spine and Hip Status is a study to determine prevalence of osteoporotic fracture, osteoporosis, and osteoarthritis in an elderly Chinese population using quantitative CT and/or DXA. 20 The present subanalysis used CT scans from subjects obtained immediately after fragility HF and CT scans obtained from number of matched subjects without HFs. In this cross‐sectional case–control analysis, we investigated and compared the gluteal muscle size and CT density for the discrimination of HF.
Participants
A total of 918 subjects with suspected HFs, admitted to the Beijing Jishuitan Hospital emergency department of orthopaedic trauma between January 2012 and May 2016, were recruited for this study. In this institution, CT scans are performed routinely for subjects with suspected or already confirmed HFs. Based on the CT images, fractures were categorized into FN fracture (FNF) or trochanteric fracture by an experienced musculoskeletal radiologist (Y. S.). A one‐page questionnaire inquiring demographic data (e.g. age, gender, height, and weight), details of the fall (when, how, and where), fracture history, and medical history was completed by the patients or their relatives after the CT examination. Three hundred and sixteen community‐dwelling subjects without HF of at least 60 years and in good health were recruited as controls from the neighbourhood of Beijing Jishuitan Hospital (Figure 1).
Figure 1.

Flow chart of participant selection for the study. *Subjects with diseases potentially affecting bone metabolism, or having received treatments with potential or known effects on bone metabolism. Details are as follows: malignant tumours (n = 30); paralysis due to cerebrovascular accident (n = 35); the poor healed lower extremity fracture, avascular necrosis, or hip dysplasia (n = 23); bone metastases (n = 3); proximal femur osteochondroma (n = 1); lumbar fractures or proximal humerus fractures occurred within the last 3 months (n = 6); recently staying in bed due to acute pancreatitis (n = 2); ovariectomy (n = 2); medical usage of glucocorticoid hormone or Cushing's syndrome (n = 6); elevated parathyroid hormone level (n = 1); rheumatoid arthritis (n = 22); psoriasis (n = 2); severe renal insufficiency (n = 2); and myasthenia gravis (n = 1). CASH, China Action on Spine and Hip Status.
Inclusion and exclusion criteria of the HF patients were similar to those described by Su et al. 21 In brief, only fully ambulatory, community‐dwelling Chinese Han adults with an HF resulting from low‐energy injury were included. Thus, only falls from standing or sitting height were considered as cause for HF. In addition, CT had to be taken within 48 h after injury in order to minimize changes in aBMD and body composition due to bed rest after fracture. Subjects with prior and with bilateral HFs or inability to stand or walk before the event of HF were excluded.
Exclusion criteria of the control subjects were inability to sit and stand independently or inability to walk with or without an assistive device. Further exclusion criteria for both groups were stroke, neurological disorders, metabolic diseases, rheumatic diseases, heart failure, severe chronic obstructive pulmonary disease and coagulation disorders, and other diseases that limited function.
The study was approved by the ethics committee of Beijing Jishuitan Hospital. Informed consent was obtained from each participant.
Computed tomography acquisition
Spiral CT imaging of the hip was performed for all study participants using two Toshiba Aquilion CT scanners (Toshiba Medical Systems Division, Tokyo, Japan). Scans were acquired in supine position from the top of the acetabulum to 3 cm below the lesser trochanter and included both legs. Scan parameters were 120 kVp, 125 mAs, 50 cm field of view, 512 × 512 matrix, 1 mm reconstructed slice thickness, and a standard reconstruction kernel with filtered back‐projection.
Muscle density assessments
Cross‐sectional area and density were measured of the gluteus maximus (G.max) at the level of the greater trochanter and of the gluteus medius and minimus (G.med/min) muscle at the level of the third sacral (S3) (Figure 2). In subjects with HF, the non‐fractured hip was analysed. In 97 participants, the CT scan did not cover the S3 level; therefore, G.med/min muscle density and area were measured at S4 or S5 levels, only. Supporting Information, Table S1 shows that there was no significant difference (0.8 HU) in muscle density between the S3 and non‐S3 levels. Difference in muscle area between the S3 and non‐S3 levels was not significant either; however, the absolute difference of about 4.1 cm2 accounted for a bias of up to 10% compared with the mean area value at the S3 level. For this reason, we did not include G.med/min muscle CSA in the analysis.
Figure 2.
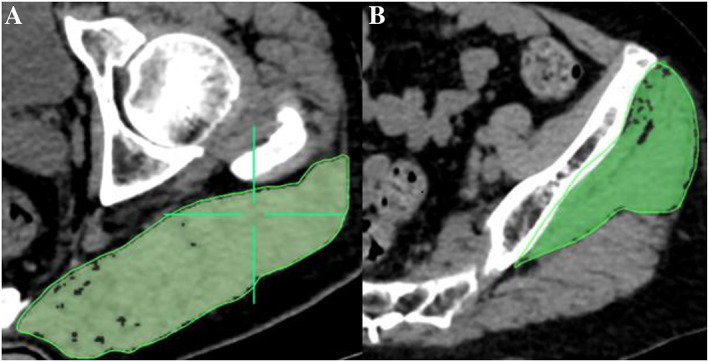
(A) Measurement of cross‐sectional area and mean computed tomography values of the gluteus maximus muscle at the level of the greater trochanter of the femur. (B) Measurement of the gluteus medius and minimus muscle at the third sacral level. Muscle region is represented by the area highlighted in green.
OsiriX software (Lite Version 10.0.2, Pixmeo, Geneva, Switzerland) was used for analysis. Muscle segmentation was performed manually using the ‘pencil’ tool to outline muscle contours. Within the resulting muscle regions of interest, a threshold of −29 HU was used to distinguish muscle tissue from fat. All muscle measurements were performed by the same investigator (Y. Z.) who had received training by an expert radiologist in CT muscle imaging prior to the analysis. For practice purposes, a sample of about 20 images had been analysed together with the expert prior to the beginning of the measurement study.
Muscle strength assessments
Handgrip strength of the dominant hand was measured using a Jamar dynamometer (Jamar®, Los Angeles, CA, USA). Details of measuring grip strength were reported previously. 22
Bone mineral density
Areal BMD (g/cm2) of the FN and total hip (TH) was calculated from the hip CT scans using the CTXA technique (Version 4.2.3, Mindways Inc). 23 The Medical Image Analysis Framework option Femur (MIAF Femur Version 7.1.0MRH) was used to measure 3D FN cortical thickness (FN CortThick). 20
Statistical analysis
Study participants were categorized as healthy controls, with an FNF, or with an intertrochanteric fracture (ITF). The two fracture groups were also combined as any HF group. Continuous variables are presented as mean ± standard deviation. Categorical variables are described as counts and corresponding percentages.
In order to minimize the between‐group selection bias and to control for potential confounding factors, including age, gender, and body mass index (BMI), propensity score matching (PSM) was applied to match subjects for the following analyses: (i) subjects with FNF vs. control subjects; (ii) subjects with ITF vs. control subjects; and (iii) subjects with any HF vs. control subjects.
Mean differences between two groups each were analysed using the Mann–Whitney U test. Three different multivariable subsets were used for independent binary logistic regression models to calculate odds ratios of fracture per standard deviation increase: (i) the muscle model included G.max muscle CSA and density and G.med/min muscle density; (ii) the BMD model included TH aBMD and FN aBMD; and (iii) the bone structure model included FN CortThick. Results of the muscle strength model, which included handgrip strength, are not shown because of the low number of only 66 HF patients. Receiver operating characteristic (ROC) curve analyses were applied, and area under the curve (AUC) values with 95% confidence intervals (CIs) were used as performance characteristics. The Hosmer–Lemeshow tests were used to evaluate model robustness by calculating the Pearson χ 2 statistic from the table of observed and expected frequencies. Results are not significant if models fit well. Four selected logistic regression models were used to determine best variable subsets for fracture discrimination: (i) G.max muscle CSA and density, G.med/min muscle density, and FN aBMD; (ii) G.max muscle density, G.med/min muscle density, and FN aBMD; (iii) G.med/min muscle density and FN aBMD; and (iv) G.max muscle CSA and FN aBMD. One‐degree‐of‐freedom tests were used to compare the latter three ROC curves with the first selected model. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Study sample characteristics
Figure 1 illustrates the recruitment of study participants. Fifteen participants (from 316) were excluded from the control group: eight because of invalid handgrip strength measurements and seven because of missing CT scans or inadequate image quality (i.e. motion artefacts). Of the 301 control participants, 107 were male and 194 female. After the review of the questionnaire, of medical records, and of the CT images, 480 cases of the 918 low trauma HF patients were excluded. After exclusion, the study cohort included 739 participants: 301 non‐fracture controls, 258 subjects with FNF, and 180 subjects with ITF. Characteristics of the study cohort are shown in Table 1. The HF patients were older and had higher systolic blood pressure, lower BMI, lower handgrip strength, lower gluteus muscle CSA and density, lower aBMD in TH and FN regions, and lower cortical thickness of FN.
Table 1.
Characteristics of study participants
| Characteristics | Unmatched population [mean ± SD or % (n)] | Mean difference a | P value b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HC (n = 301) (1) | FNF (n = 258) (2) | ITF (n = 180) (3) | HF (n = 438) (4) | (2) vs. (1) | (3) vs. (1) | (4) vs. (1) | (2) vs. (1) | (3) vs. (1) | (4) vs. (1) | |
| Male gender, % (n) | 35.6 (107) | 20.9 (54) | 25.6 (46) | 22.8 (100) | n/a | n/a | n/a | n/a | n/a | n/a |
| Age (years) | 68.4 ± 6.1 | 74.0 ± 10.0 | 78.5 ± 8.6 | 75.8 ± 9.7 | 5.6 (7.6) | 10.1 (12.9) | 7.5 (9.8) | <0.01 | <0.01 | <0.01 |
| Weight (kg) | 66.8 ± 10.0 | 60.2 ± 10.7 | 61.1 ± 12.0 | 60.6 ± 11.3 | −6.6 (−11.0) | −5.7 (−9.3) | −6.2 (−10.3) | <0.01 | <0.01 | <0.01 |
| Height (cm) | 162.5 ± 7.5 | 161.6 ± 7.4 | 160.9 ± 8.6 | 161.3 ± 7.9 | −0.9 (−0.5) | −1.5 (−0.9) | −1.1 (−0.7) | 0.14 | 0.01 | 0.02 |
| BMI (kg/cm2) | 25.2 ± 2.9 | 22.9 ± 3.4 | 23.6 ± 4.1 | 23.2 ± 3.7 | −2.3 (−10.0) | −1.7 (−7.1) | −2.0 (−8.8) | <0.01 | <0.01 | <0.01 |
| Handgrip (kg) | 25.6 ± 8.5 | 14.3 ± 9.6 | 11.9 ± 9.7 | 13.1 ± 9.7 | −11.3 (−79.0) | −13.7 (−114.4) | −12.5 (−95.5) | <0.01 | <0.01 | <0.01 |
| G.MaxM area (cm2) | 39.4 ± 7.4 | 31.8 ± 7.1 | 29.7 ± 7.4 | 31.0 ± 7.3 | −7.5 (−23.7) | −9.6 (−32.3) | −8.4 (−27.1) | <0.01 | <0.01 | <0.01 |
| G.MaxM density (HU) | 33.5 ± 6.6 | 25.1 ± 7.0 | 21.9 ± 7.3 | 23.8 ± 7.3 | −8.4 (−33.5) | −11.6 (−52.9) | −9.7 (−40.8) | <0.01 | <0.01 | <0.01 |
| G.Med/MinM density (HU) | 42.4 ± 4.4 | 31.9 ± 6.4 | 29.4 ± 5.8 | 30.9 ± 6.3 | −10.5 (−32.8) | −12.9 (−43.9) | −11.5 (−37.2) | <0.01 | <0.01 | <0.01 |
| SBP (mmHg) | 126.5 ± 8.6 | 144.2 ± 19.4 | 145.4 ± 22.8 | 144.8 ± 20.8 | 17.7 (12.3) | 18.9 (13.0) | 18.2 (12.6) | <0.01 | <0.01 | <0.01 |
| DBP (mmHg) | 74.1 ± 7.6 | 74.5 ± 18.2 | 75.6 ± 13.7 | 75.0 ± 16.4 | 0.4 (0.6) | 1.5 (2.0) | 0.9 (1.2) | 0.11 | 0.30 | 0.09 |
| TH aBMD (g/cm2) | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | −0.2 (−31.6) | −0.2 (−45.4) | −0.2 (−36.9) | <0.01 | <0.01 | <0.01 |
| FN aBMD (g/cm2) | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | −0.2 (−32.9) | −0.2 (−42.9) | −0.2 (−36.8) | <0.01 | <0.01 | <0.01 |
| FN CortThick (mm) | 1.8 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.3 | −0.2 (−11.0) | −0.2 (−9.9) | −0.2 (−10.5) | <0.01 | <0.01 | <0.01 |
BMI, body mass index; DBP, diastolic blood pressure; FN aBMD, femoral neck areal bone mineral density; FN CortThick, cortical thickness of femoral neck; FNF, femoral neck fracture; G.MaxM, gluteus maximus muscle; G.Med/MinM, gluteus medius and minimus muscle; HC, healthy control; HF, any hip fracture; HU, Hounsfield units; ITF, intertrochanteric fracture; SBP, systolic blood pressure; SD, standard deviation; TH aBMD, total hip areal bone mineral density.
Data in parentheses are percentage mean differences.
P values were obtained for fracture risk using two‐sample Wilcoxon tests for continuous variables.
After PSM using age, gender, and BMI, 159 FNF cases were matched with 159 controls and 101 ITF cases with 101 controls (Table 2). Two hundred three cases with any HF were matched with 203 controls. The HF group was further divided in a younger group (age <70 years) consisting of 100 HF cases matched with 115 controls and an older group (age ≥70 years) consisting of 103 HF cases and 88 controls (Table 3).
Table 2.
Characteristics of matched participants using propensity score in FNF, ITF, and HC groups
| Characteristics [mean ± SD or % (n)] | Matched population | Mean difference a | P value b | Matched population | Mean difference a | P value b | ||
|---|---|---|---|---|---|---|---|---|
| HC (n = 159) | FNF (n = 159) | HC (n = 101) | ITF (n = 101) | |||||
| Male gender, % (n) | 23.3 (37) | 26.4 (42) | n/a | n/a | 39.6 (40) | 28.7 (29) | n/a | n/a |
| Age (years) | 69.9 ± 6.3 | 69.8 ± 9.4 | −0.1 (−0.2) | 0.8 | 73.2 ± 6.8 | 73.6 ± 6.0 | 0.4 (0.5) | 0.8 |
| Weight (kg) | 64.4 ± 10.8 | 64.5 ± 9.5 | 0.1 (0.1) | 0.8 | 64.9 ± 8.8 | 64.4 ± 11.1 | −0.5 (−0.7) | 0.8 |
| Height (cm) | 161.0 ± 7.4 | 163.1 ± 7.5 | 2.2 (1.3) | 0.01 | 162.1 ± 7.7 | 162.2 ± 8.6 | 0.1 (0.1) | 0.8 |
| BMI (kg/cm2) | 24.8 ± 3.1 | 24.2 ± 3.0 | −0.6 (−2.3) | 0.1 | 24.7 ± 2.7 | 24.5 ± 4.05 | −0.2 (−0.6) | 0.7 |
| Handgrip (kg) | 23.7 ± 7.9 | 16.6 ± 9.6 | −7.1 (−42.8) | <0.01 | 24.9 ± 9.0 | 15.4 ± 10.3 | −9.6 (−62.2) | <0.01 |
| G.MaxM area (cm2) | 37.1 ± 6.9 | 34.0 ± 7.0 | −3.1 (−9.1) | <0.01 | 38.8 ± 7.4 | 31.8 ± 7.2 | −7.1 (−22.2) | <0.01 |
| G.MaxM density (HU) | 32.5 ± 6.4 | 26.0 ± 6.9 | −6.5 (−24.9) | <0.01 | 33.1 ± 6.3 | 22.7 ± 7.9 | −10.4 (−45.9) | <0.01 |
| G.Med/MinM density (HU) | 41.6 ± 4.5 | 33.0 ± 6.1 | −8.3 (−25.1) | <0.01 | 41.1 ± 4.6 | 30.5 ± 5.3 | −10.6 (−34.9) | <0.01 |
| SBP (mmHg) | 126.9 ± 8.9 | 143.1 ± 20.3 | 16.2 (11.4) | <0.01 | 128.0 ± 7.8 | 146.7 ± 21.5 | 18.7 (12.7) | <0.01 |
| DBP (mmHg) | 74.0 ± 8.6 | 72.7 ± 20.0 | −1.3 (−1.8) | 0.7 | 75.1 ± 7.1 | 79.7 ± 11.8 | 4.6 (5.8) | 0.03 |
| TH aBMD (g/cm2) | 0.8 ± 0.12 | 0.6 ± 0.1 | −0.1 (−19.8) | <0.01 | 0.8 ± 0.2 | 0.6 ± 0.1 | −0.2 (−34.5) | <0.01 |
| FN aBMD (g/cm2) | 0.7 ± 0.1 | 0.5 ± 0.1 | −0.1 (−21.5) | <0.01 | 0.7 ± 0.1 | 0.5 ± 0.09 | −0.2 (−33.1) | <0.01 |
| FN CortThick (mm) | 1.8 ± 0.3 | 1.7 ± 0.3 | −0.1 (−8.6) | <0.01 | 1.9 ± 0.3 | 1.7 ± 0.3 | −0.2 (−12.6) | <0.01 |
BMI, body mass index; DBP, diastolic blood pressure; FN aBMD, femoral neck areal bone mineral density; FN CortThick, cortical thickness of femoral neck; FNF, femoral neck fracture; G.MaxM, gluteus maximus muscle; G.Med/MinM, gluteus medius and minimus muscle; HC, healthy control; HU, Hounsfield units; ITF, intertrochanteric fracture; SBP, systolic blood pressure; SD, standard deviation; TH aBMD, total hip areal bone mineral density.
Data in parentheses are percentage mean differences.
P values were obtained for fracture risk using two‐sample Wilcoxon tests for continuous variables.
Table 3.
Characteristics of matched participants using propensity score in total fracture group and HC group
| Characteristics [mean ± SD or % (n)] | Total population | Mean difference a | P value b | <70 years old | Mean difference a | P value b | ≥70 years old | Mean difference a | P value b | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC (n = 203) | HF (n = 203) | HC (n = 115) | HF (n = 100) | HC (n = 88) | HF (n = 103) | |||||||
| Male gender, % (n) | 28.6 (58) | 28.6 (58) | n/a | n/a | 25.2 (29) | 22.0 (22) | n/a | n/a | 33.0 (29) | 35.0 (36) | n/a | n/a |
| Age (years) | 69.6 ± 6.7 | 69.3 ± 8.0 | −0.3 (−0.4) | 0.87 | 64.5 ± 2.7 | 62.5 ± 4.5 | −2.0 (−3.2) | <0.01 | 76.2 ± 4.1 | 75.9 ± 4.3 | −0.3 (−0.4) | 0.40 |
| Weight (kg) | 65.1 ± 9.9 | 65.1 ± 10.1 | 0.02 (0.03) | 0.90 | 66.5 ± 11.1 | 62 ± 9.8 | −4.5 (−7.3) | <0.01 | 63.2 ± 7.7 | 68.1 ± 9.6 | 4.9 (7.8) | <0.01 |
| Height (cm) | 161.3 ± 7.3 | 163.1 ± 7.8 | 1.8 (1.1) | 0.02 | 161.7 ± 7.6 | 163.1 ± 7.2 | 1.4 (0.8) | 0.13 | 160.8 ± 6.9 | 163.2 ± 8.3 | 2.4 (1.5) | 0.08 |
| BMI (kg/cm2) | 25.0 ± 3.0 | 24.5 ± 3.6 | −0.5 (−1.9) | 0.11 | 25.4 ± 3.3 | 23.3 ± 2.9 | −2.1 (−9.0) | <0.01 | 24.4 ± 2.5 | 25.7 ± 3.8 | 1.2 (5.0) | 0.02 |
| Handgrip (kg) | 24.3 ± 8.1 | 14.8 ± 10.2 | −9.5 (−39.2) | <0.01 | 25.6 ± 8.3 | 14.8 ± 10.2 | −10.8 (−72.7) | <0.01 | 22.6 ± 7.5 | 14.8 ± 10.4 | −7.9 (−34.8) | <0.01 |
| G.MaxM area (cm2) | 37.8 ± 6.6 | 34.1 ± 7.2 | −3.7 (−9.8) | <0.01 | 38.4 ± 6.5 | 35.5 ± 7.7 | −2.9 (−8.2) | <0.01 | 37 ± 6.7 | 32.7 ± 6.3 | −4.2 (−11.5) | <0.01 |
| G.MaxM density (HU) | 32.8 ± 6.5 | 25.8 ± 7.1 | −7.1 (−21.5) | <0.01 | 33.5 ± 6.4 | 28.3 ± 6 | −5.2 (−18.4) | <0.01 | 31.9 ± 6.6 | 23.3 ± 7.2 | −8.6 (−27.1) | <0.01 |
| G.Med/MinM density (HU) | 42.1 ± 4.5 | 32.8 ± 6.0 | −9.1 (−21.6) | <0.01 | 43.3 ± 4.5 | 34.1 ± 5.5 | −9.1 (−26.7) | <0.01 | 40.5 ± 4.1 | 31.9 ± 5.4 | −8.6 (−21.4) | <0.01 |
| SBP (mmHg) | 126.6 ± 8.5 | 143.7 ± 22.3 | 17.1 (13.5) | <0.01 | 125.2 ± 8.6 | 136.6 ± 20.5 | 11.5 (8.4) | <0.01 | 128.4 ± 8.1 | 150.2 ± 22.2 | 21.8 (17.0) | <0.01 |
| DBP (mmHg) | 74.2 ± 6.3 | 75.4 ± 18.7 | 1.2 (1.6) | 0.03 | 74.4 ± 5.9 | 70.3 ± 22.4 | −4.1 (−5.9) | 0.65 | 73.9 ± 6.8 | 80.1 ± 13.1 | 6.2 (8.4) | 0.01 |
| TH aBMD (g/cm2) | 0.8 ± 0.2 | 0.6 ± 0.1 | −0.2 (−19.3) | <0.01 | 0.8 ± 0.1 | 0.6 ± 0.1 | −0.1 (−21.8) | <0.01 | 0.8 ± 0.2 | 0.6 ± 0.1 | −0.2 (−20.6) | <0.01 |
| FN aBMD (g/cm2) | 0.7 ± 0.1 | 0.5 ± 0.09 | −0.1 (−20.5) | <0.01 | 0.7 ± 0.1 | 0.5 ± 0.1 | −0.1 (−23.8) | <0.01 | 0.7 ± 0.2 | 0.5 ± 0.1 | −0.1 (−21.6) | <0.01 |
| FN CortThick (mm) | 1.9 ± 0.3 | 1.7 ± 0.3 | −0.1 (−8.1) | <0.01 | 1.8 ± 0.3 | 1.7 ± 0.3 | −0.2 (−8.9) | 0.03 | 1.9 ± 0.3 | 1.7 ± 0.3 | −0.2 (−8.2) | <0.01 |
BMI, body mass index; DBP, diastolic blood pressure; FN aBMD, femoral neck areal bone mineral density; FN CortThick, cortical thickness of femoral neck; G.MaxM, gluteus maximus muscle; G.Med/MinM, gluteus medius and minimus muscle; HC, healthy control; HF, any hip fracture; HU, Hounsfield units; SBP, systolic blood pressure; SD, standard deviation; TH aBMD, total hip areal bone mineral density.
Data in parentheses are percentage mean differences.
P values were obtained for fracture risk using two‐sample Wilcoxon tests for continuous variables.
Handgrip strength, gluteus muscle area and density, and bone parameters of the matched HF groups were lower than those of the correspondence control groups, respectively (P < 0.05).
Relationship between muscle parameters and age
To explore the age‐related TH fracture risk discrimination, we therefore stratified the analyses by age (age cut point 70 years). Figure 3 shows of G.med/min muscle density and G.max muscle density and CSA stratified for the two age ranges below and above 70 years. For all three muscle parameters, variables were significantly different between the two age groups, with the exception of G.max muscle CSA in control group (P = 0.278). Interestingly in HF group, G.max muscle CSA differed by 13% between the two age groups. However, the age‐related difference in G.max muscle CSA was numerically lower than the corresponding difference of 18% of G.max muscle density.
Figure 3.
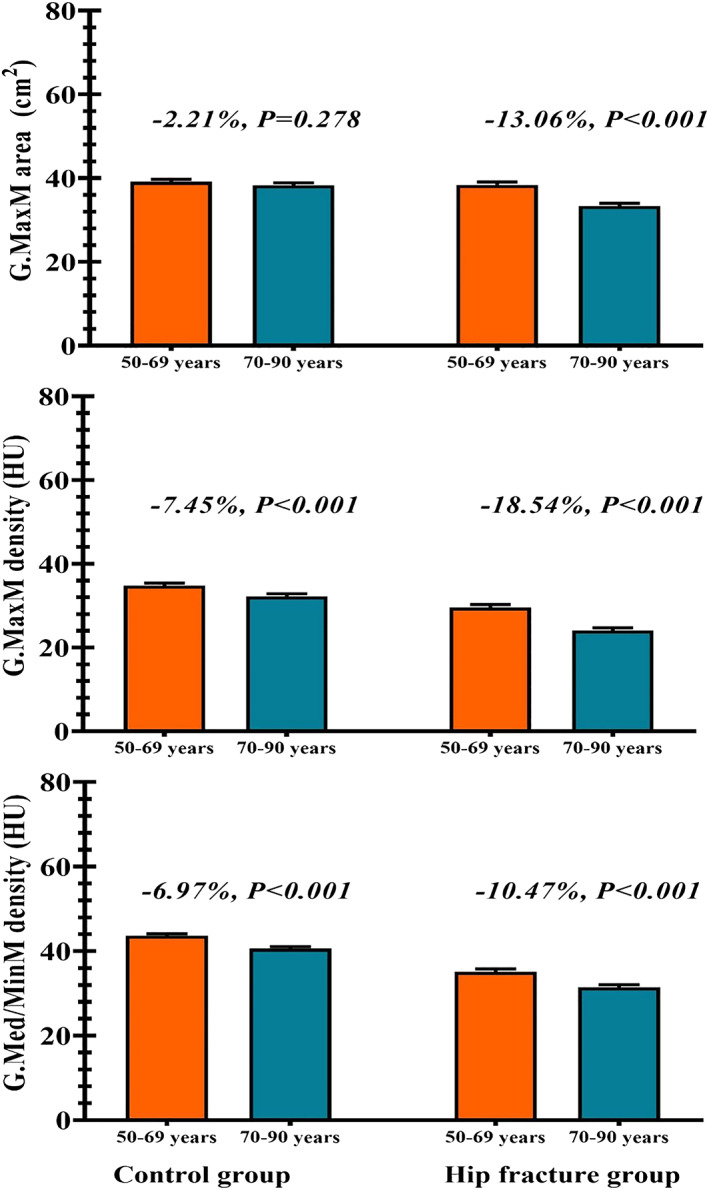
Gluteus muscle area and density by age groups in control and fracture groups. G.MaxM, gluteus maximus muscle; G.Med/MinM, gluteus medius and minimus muscle; HU, Hounsfield units.
Associations of muscle and bone parameters with hip fracture
Table 4 shows the associations of G.max muscle CSA and density, G.med/min muscle density, FN and TH aBMD, and FN CortThick with HF after matching for age, BMI, and gender by PSM. Figure 4 shows ROC curves for bone and muscle variables shown in Table 4. The univariate analysis showed that associations of aBMD with FN and with trochanteric fracture were significantly weaker than associations between fracture and muscle density parameters. G.med/min muscle density showed the highest AUC with FNF (0.88; 95% CI, 0.85–0.92) and trochanteric fracture (0.95; 95% CI, 0.92–0.97). Association with fracture was significantly higher than for G.max muscle area (FNF: P < 0.0001; ITF: P < 0.0001) and for FN aBMD (FNF: P = 0.008; IFT: P < 0.0001). AUC values of multivariate bone and muscle models (Table 3) were almost identical to those of FN aBMD and G.med/min muscle density, respectively. In contrast, the AUC value of FN cortical thickness (Table 3) was rather low (FNF: 0.62; ITF: 0.70).
Table 4.
ORs of fracture risk per SD increase in PS‐matched population by fracture type
| Model/variables | Femoral neck fracture risk (N = 318) | Intertrochanteric fracture risk (N = 202) | Any hip fracture risk (N = 406) | |||
|---|---|---|---|---|---|---|
| ORs (95% CIs) | P value | ORs (95% CIs) | P value | ORs (95% CIs) | P value | |
| Univariate muscle parameters | ||||||
| G.MaxM area (cm2) | 0.63 (0.50, 0.80) | <0.01 | 0.35 (0.24, 0.50) | <0.01 | 0.58 (0.47, 0.71) | <0.01 |
| G.MaxM density (HU) | 0.34 (0.25, 0.45) | <0.01 | 0.17 (0.11, 0.28) | <0.01 | 0.31 (0.24, 0.41) | <0.01 |
| G.Med/MinM density (HU) | 0.10 (0.06, 0.16) | <0.01 | 0.03 (0.01, 0.08) | <0.01 | 0.07 (0.04, 0.12) | <0.01 |
| Multivariate muscle model | ||||||
| G.MaxM area (cm2) | 1.06 (0.76, 1.49) | 0.72 | 0.65 (0.36, 1.16) | 0.15 | 1.01 (0.74, 1.38) | 0.96 |
| G.MaxM density (HU) | 1.76 (1.04, 2.98) | 0.03 | 1.97 (0.79, 4.90) | 0.15 | 1.64 (1.03, 2.62) | 0.04 |
| G.Med/MinM density (HU) | 0.06 (0.03, 0.12) | <0.01 | 0.02 (0.004, 0.08) | <0.01 | 0.05 (0.02, 0.09) | <0.01 |
| AUC (95% CIs), P value a | 0.887 (0.850, 0.923), 0.50 | 0.945 (0.916, 0.974), 0.55 | 0.904 (0.875, 0.933), 0.89 | |||
| Univariate BMD variables | ||||||
| TH aBMD (g/cm2) | 0.30 (0.22, 0.42) | <0.01 | 0.18 (0.11, 0.29) | <0.01 | 0.24 (0.17, 0.33) | <0.01 |
| FN aBMD (g/cm2) | 0.35 (0.26, 0.48) | <0.01 | 0.16 (0.09, 0.28) | <0.01 | 0.27 (0.20, 0.37) | <0.01 |
| Multivariate BMD model | ||||||
| TH aBMD (g/cm2) | 0.74 (0.44, 1.23) | 0.24 | 0.42 (0.19, 0.90) | 0.03 | 0.31 (0.19, 0.52) | <0.01 |
| FN aBMD (g/cm2) | 0.93 (0.23, 0.68) | <0.01 | 0.34 (0.15, 0.78) | 0.01 | 0.71 (0.43, 1.19) | 0.20 |
| AUC (95% CIs), P value a | 0.759 (0.706, 0.812), 0.46 | 0.851 (0.798, 0.904), 0.46 | 0.799 (0.756, 0.842), 0.54 | |||
| Bone structure model | ||||||
| FN CortThick (mm) | 0.57 (0.43, 0.75) | <0.01 | 0.41 (0.28, 0.62) | <0.01 | 0.57 (0.45, 0.73) | <0.01 |
| AUC (95% CIs), P value a | 0.618 (0.553, 0.682), 0.01 | 0.702 (0.626, 0.777), 0.18 | 0.617 (0.561, 0.674), <0.01 | |||
| Selected Model 1 | ||||||
| G.MaxM area (cm2) | 1.54 (1.03, 2.29) | 0.03 | 0.92 (0.47, 1.79) | 0.80 | 1.53 (1.05, 2.24) | 0.04 |
| G.MaxM density (HU) | 1.76 (1.01, 3.09) | 0.05 | 1.90 (0.73, 4.98) | 0.19 | 1.76 (1.05, 2.95) | 0.04 |
| G.Med/MinM density (HU) | 0.06 (0.03, 0.13) | <0.01 | 0.03 (0.01, 0.11) | <0.01 | 0.05 (0.02, 0.10) | <0.01 |
| FN aBMD (g/cm2) | 0.34 (0.21, 0.55) | <0.01 | 0.26 (0.11, 0.60) | <0.01 | 0.29 (0.18, 0.48) | <0.01 |
| AUC (95% CIs), P value a | 0.912 (0.88, 0.944), 0.71 | 0.958 (0.932, 0.984), 0.66 | 0.930 (0.905, 0.955), 0.28 | |||
| Selected Model 2 | ||||||
| G.MaxM density (HU) | 1.90 (1.07, 3.39) | 0.02 | 1.84 (0.73, 4.65) | 0.20 | 1.83 (1.10, 3.03) | 0.02 |
| G.Med/MinM density (HU) | 0.06 (0.03, 0.14) | <0.01 | 0.03 (0.01, 0.11) | <0.01 | 0.06 (0.03, 0.11) | <0.01 |
| FN aBMD (g/cm2) | 0.42 (0.27, 0.66) | <0.01 | 0.25 (0.11, 0.56) | <0.01 | 0.34 (0.23, 0.52) | <0.01 |
| AUC (95% CIs), P value a | 0.908 (0.877, 0.941), 0.40 | 0.957 (0.93, 0.983), 0.58 | 0.927 (0.903, 0.952), 0.71 | |||
| Selected Model 3 | ||||||
| G.Med/MinM density (HU) | 0.11 (0.07, 0.19) | <0.01 | 0.05 (0.02, 0.13) | <0.01 | 0.09 (0.05, 0.16) | <0.01 |
| FN aBMD (g/cm2) | 0.42 (0.28, 0.64) | <0.01 | 0.26 (0.12, 0.61) | <0.01 | 0.35 (0.23, 0.53) | <0.01 |
| AUC (95% CIs), P value a | 0.902 (0.869, 0.935), 0.74 | 0.956 (0.930, 0.982), 0.98 | 0.923 (0.898, 0.948), 0.89 | |||
| Selected Model 4 | ||||||
| G.MaxM area (cm2) | 1.04 (0.78, 1.39) | 0.81 | 0.61 (0.39, 0.94) | 0.02 | 0.94 (0.72, 1.21) | 0.62 |
| FN aBMD (g/cm2) | 0.3 (0.21, 0.43) | <0.01 | 0.20 (0.11, 0.36) | <0.01 | 0.25 (0.18, 0.35) | <0.01 |
| AUC (95% CIs), P value a | 0.755 (0.702, 0.808), 0.73 | 0.858 (0.806, 0.910), 0.69 | 0.797 (0.754, 0.840) | |||
AUC, area under curve; BMD, bone mineral density; CIs, confidence intervals; FN aBMD, femoral neck areal bone mineral density; FN CortThick, cortical thickness of femoral neck; G.MaxM, gluteus maximus muscle; G.Med/MinM, gluteus medius and minimus muscle; HU, Hounsfield units; ORs, odds ratios; PS, propensity score; SD, standard deviation; TH aBMD, total hip areal bone mineral density.
P values were obtained using the Hosmer–Lemeshow tests. P > 0.05 indicates that the null hypothesis (observed event rates match expected event rates in subgroups of the model population) is not rejected.
Figure 4.

Receiver operating characteristic curves for muscle and bone parameters in propensity score‐matched population: (A) femoral neck fracture, (B) intertrochanteric fracture, and (C) any hip fracture. CI, confidence interval; FN aBMD, femoral neck areal bone mineral density; G.MaxM, gluteus maximus muscle; G.Med/MinM, gluteus medius and minimus muscle.
Association with fracture was also determined for four selected models (Models 1–4) combining bone and muscle parameters. Numerically, the highest AUC values were obtained for Model 1 (FNF: AUC 0.912; ITF: AUC 0.958), but AUC values for selected Model 2 (FNF: AUC 0.908; ITF: AUC 0.957) and Model 3 (FNF: AUC 0.902; ITF: AUC 0.956) were not dominantly lower (Model 2 vs. Model 1: FNF: P = 0.417; ITF: P = 0.149; Model 3 vs. Model 1: FNF: P = 0.115; ITF: P = 0.551). AUC results of Model 4 (FNF: AUC 0.755; ITF: AUC 0.858) showed that association with fracture significantly dropped when not including muscle density (Model 4 vs. Model 1: FNF: P < 0.001; ITF: P < 0.001). AUC values for Model 1 were higher than for the other three models in any HF (Model 2: P = 0.311; Model 3: P = 0.132; and Model 4: P < 0.001).
For all multivariate and selected models, AUC values for any type of HF were in between those of FNF and trochanteric fracture. Separate results for the two age groups younger and older than 70 years were only performed for the any fracture due to the limited number of participants' age differentiation (Table 5). AUC values of multivariate models were numerically higher in the younger age group with the exception of Model 4, but none of the differences were statistically significant.
Table 5.
ORs of any fracture risk per SD increase in PS‐matched population by age group
| Model/variables | <70 years old (N = 215) | ≥70 years old (N = 191) | ||
|---|---|---|---|---|
| ORs (95% CIs) | P value | ORs (95% CIs) | P value | |
| Univariate muscle parameters | ||||
| G.MaxM area (cm2) | 0.66 (0.50, 0.87) | <0.01 | 0.49 (0.35, 0.69) | <0.01 |
| G.MaxM density (HU) | 0.36 (0.25, 0.53) | <0.01 | 0.25 (0.17, 0.39) | <0.01 |
| G.Med/MinM density (HU) | 0.07 (0.03, 0.14) | <0.01 | 0.06 (0.02, 0.13) | <0.01 |
| Multivariate muscle model | ||||
| G.MaxM area (cm2) | 1.05 (0.68, 1.60) | 0.84 | 0.91 (0.56, 1.5) | 0.72 |
| G.MaxM density (HU) | 2.45 (1.23, 4.88) | 0.01 | 1.07 (0.55, 2.08) | 0.85 |
| G.Med/MinM density (HU) | 0.03 (0.01, 0.09) | <0.01 | 0.05 (0.02, 0.16) | <0.01 |
| AUC (95% CIs), P value a | 0.917 (0.879, 0.954), 0.12 | 0.904 (0.861, 0.947), 0.64 | ||
| Univariate BMD variables | ||||
| TH aBMD (g/cm2) | 0.22 (0.14, 0.36) | <0.01 | 0.32 (0.21, 0.48) | <0.01 |
| FN aBMD (g/cm2) | 0.22 (0.14, 0.34) | <0.01 | 0.27 (0.17, 0.41) | <0.01 |
| Multivariate BMD model | ||||
| TH aBMD (g/cm2) | 0.63 (0.29, 1.40) | 0.25 | 0.77 (0.40, 1.51) | 0.45 |
| FN aBMD (g/cm2) | 0.31 (0.15, 0.66) | <0.01 | 0.33 (0.16, 0.66) | <0.01 |
| AUC (95% CIs), P value a | 0.806 (0.748, 0.864), 0.31 | 0.788 (0.723, 0.853), 0.52 | ||
| Bone structure model | ||||
| FN CortThick (mm) | 0.59 (0.43, 0.81) | <0.01 | 0.53 (0.36, 0.78) | <0.01 |
| AUC (95% CIs), P value a | 0.589 (0.510, 0.668), 0.01 | 0.658 (0.577, 0.74), 0.09 | ||
| Selected Model 1 | ||||
| G.MaxM area (cm2) | 1.69 (1.01, 2.82) | 0.04 | 1.22 (0.68, 2.17) | 0.51 |
| G.MaxM density (HU) | 2.59 (1.20, 5.58) | 0.02 | 1.02 (0.50, 2.08) | 0.95 |
| G.Med/MinM density (HU) | 0.03 (0.01, 0.09) | <0.01 | 0.07 (0.02, 0.20) | <0.01 |
| FN aBMD (g/cm2) | 0.17 (0.08, 0.36) | <0.01 | 0.40 (0.22, 0.75) | <0.01 |
| AUC (95% CIs), P value a | 0.946 (0.918, 0.974), 0.18 | 0.923 (0.884, 0.961), 0.29 | ||
| Selected Model 2 | ||||
| G.MaxM density (HU) | 2.76 (1.29, 5.91) | 0.01 | 1.07 (0.53, 2.16) | 0.85 |
| G.Med/MinM density (HU) | 0.03 (0.01, 0.09) | <0.01 | 0.07 (0.02, 0.21) | <0.01 |
| FN aBMD (g/cm2) | 0.22 (0.11, 0.43) | <0.01 | 0.44 (0.25, 0.77) | <0.01 |
| AUC (95% CIs), P value a | 0.943 (0.915, 0.971), 0.88 | 0.923 (0.884, 0.961), 0.53 | ||
| Selected Model 3 | ||||
| G.Med/MinM density (HU) | 0.07 (0.03, 0.15) | <0.01 | 0.07 (0.03, 0.18) | <0.01 |
| FN aBMD (g/cm2) | 0.23 (0.12, 0.44) | <0.01 | 0.44 (0.25, 0.77) | <0.01 |
| AUC (95% CIs), P value a | 0.934 (0.904, 0.964), 0.27 | 0.923 (0.884, 0.961), 0.58 | ||
| Selected Model 4 | ||||
| G.MaxM area (cm2) | 1.13 (0.80, 1.58) | 0.50 | 0.72 (0.48, 1.09) | 0.12 |
| FN aBMD (g/cm2) | 0.20 (0.12, 0.33) | <0.01 | 0.30 (0.19, 0.47) | <0.01 |
| AUC (95% CIs), P value a | 0.801 (0.742, 0.861), 0.55 | 0.802 (0.738, 0.865), 0.65 | ||
AUC, area under curve; BMD, bone mineral density; CIs, confidence intervals; FN aBMD, femoral neck areal bone mineral density; FN CortThick, cortical thickness of femoral neck; G.MaxM, gluteus maximus muscle; G.Med/MinM, gluteus medius and minimus muscle; HU, Hounsfield units; ORs, odds ratios; PS, propensity score; SD, standard deviation; TH aBMD, total hip areal bone mineral density.
P values were obtained using the Hosmer–Lemeshow tests. P > 0.05 indicates that the null hypothesis (observed event rates match expected event rates in subgroups of the model population) is not rejected.
Results for handgrip strength and a corresponding muscle strength model are not shown in Tables 4 and 5, because in the HF group, handgrip measurements were available only from 66 subjects. Using these limited numbers, AUC results for a muscle strength model to discriminate fracture were as follows: HF: AUC 0.751, P for Hosmer–Lemeshow test = 0.53; ITF: AUC 0.722, P = 0.58; and FNF: AUC 0.706, P = 0.02. For age stratified, AUC results for any HF were as follows: age <70 years: AUC 0.771, P = 0.49, and age >70 years: AUC 0.714, P = 0.16.
Discussions
Based on the analysis of 739 participant of the large China Action on Spine and Hip Status case–control cohort, our study demonstrates that CT muscle density is higher associated with acute HF than muscle size or aBMD measured by CTXA of the hip. To our knowledge, this is the largest CT study so far that demonstrated an important contribution of muscle characteristic to HF discrimination. In addition, the relevance of muscle size vs. muscle density for HF discrimination has not been addressed so far.
Muscle density as determined by CT is measured as X‐ray attenuation of muscle reported as CT value in HU. Elevated levels of intramuscular fat accumulation 24 reduce CT muscle density but do not affect muscle area. Thus, muscle area is insensitive to intramuscular fat accumulation, which is an important cause of age‐related muscle degradation. 25 , 26 , 27 However, in the vast majority of imaging studies targeting muscle, muscle area, not density, was obtained as a primary outcome. 14 , 16 An abundance of previous research has shown that fatty infiltration of muscle gradually increases with age. 26 A study of the spine showed that muscle density profoundly declined with age (−17% per decade in women and −11% in men). 28 In another longitudinal study of trunk muscle with 6 year follow‐up, a 20–30% decline of lumber muscles density was observed. 29 Age‐related decreases in muscle density were also reported from several studies. In the AGES Reykjavik Study, quadriceps muscle density decreased by about 12.5% between 65 and 85 years. 30 Several cross‐sectional or longitudinal studies of the Health ABC project also confirmed decreases of thigh muscle density or increases of thigh muscle fatty infiltration with age. 12 , 24 , 31 Another study 32 found that fat content of the hip muscles remained relatively stable between 20 and 50 years of age and then increased with age. Further, the increase in fat content with age in individuals over 50 years of age was greater for the gluteal muscles than for the other hip muscles. Therefore, we believe that changes in muscle density precede HF and may be a long‐term predictor of frailty leading to HF. Of course, under certain circumstances, for example, treatment with corticosteroids, there may be an additional, rapid increase in fatty infiltration leading to progressive frailty shortening the time to HF.
In agreement with the three basic studies cited earlier, 25 , 26 , 27 our results confirm that with respect to HF discrimination, muscle density is the more important parameter than muscle area. This is in particular obvious when comparing Models 3 and 4. The combination of aBMD with muscle area resulted in the same AUC value as the BMD model alone, while the combination of aBMD with G.med/min muscle density significantly improved HF discrimination. The G.med/min muscle, referred to as the ‘rotator cuff of the hip’, plays an important role in gait stability. 18 It inserts on the greater trochanter of the femur and serves as major abductors and rotators of the hip during normal gait for maintenance of balance. 33 , 34 A recent study also found a significant correlation of smaller gluteus muscle with higher loads during daily activities like stair climbing and sit‐down/stand‐up from a chair or walking for patients after TH arthroplasty. 35 Therefore, ageing of the gluteus muscle may lead to walking impairments and fall‐related fractures.
Our emphasis of the importance of muscle density was further supported by the separate analysis of the two age groups. Muscle density was significantly lower at older age, in both the control participants and the HF patients, while in the control participants, muscle area did not change between the two age groups. In the control group, muscle degradation by fatty infiltration did not result in muscle atrophy; this combined effect was only observed in patients with acute HF.
In data from the Health ABC Study, Lang et al. showed that mid‐thigh muscle CSA and density predicted fracture risk after adjustment for age, height, BMI, and some additional covariates, but only muscle density remained after an additional adjustment for DXA aBMD. 36 In a case–control study based on a Chinese female population, Lang et al. 17 showed that subjects with HFs had lower hip muscle density and lower muscle CSA values compared with controls, similar to our results. However, Lang et al. did not observe gluteus maximus muscle (extensor muscle) density differences between HF patients and controls. The discordance of Lang study to our study results might be due to a small sample size (45 HFs) and a slightly different muscle level of the measurement. In the EEFECT study, Mühlberg et al. found that the amount of adipose tissue and the distribution of the adipocytes in the muscles of the upper thigh were significantly associated with acute HF (AUC, 0.85; 95% CI: 0.78, 0.93). Muscle area did not discriminate HF, and muscle density became insignificant after adjustment for age height and weight. 19 But again, the sample size was small (40 HFs) and the location of the analysed muscles differed from the current study.
A strength of our study was the rather large number of included subjects. Controls and fractured cases could be matched with respect to age, gender, and BMI independently for FNF, trochanteric fracture, and all HFs as well as for the two age groups. In contrast to other studies, it was not necessary to use age, gender, and BMI as covariates or adjustments in the multivariate analyses. Another strength was that patients with incident HFs were scanned within 48 h, which would mostly minimize fracture‐related changes of bone and muscle tissues, apart from oedema around the fractured hip.
A major limitation of the study was the cross‐sectional design, limiting the analysis to evaluation associations with HF instead of HF prediction. Another limitation was the muscle assessments, which were based on the single slice instead of a full 3D analysis of the complete muscles. Further, a more advanced muscle analysis as performed in the EFFECT was beyond the scope of this study because it requires a 3D analysis and a complicated segmentation of the fascia. 14 , 37 Finally, we did not answer the question whether the HF risk varies with age. This requires more than two age groups and a separate analysis by gender, which requires a higher number of subjects than were available in this study the missing gender specific analysis.
In conclusion, our study results show that muscle density performs better than aBMD from hip CTXA and muscle size in discrimination of HF. Combination of aBMD and muscle density provided the best discrimination. The integration of muscle assessments may trigger a paradigm shift in HF prediction. Gluteus muscle density should also be evaluated as treatment outcome, and its use as a treatment endpoint for therapies that reduce HF risk should be further investigated.
Conflict of interest
K.E. is a part‐time employee of BioClinica, Inc. Other authors declare no conflict of interest.
Funding
This work is supported in part by the National Natural Science Foundation of China (Grant Nos. 81901718, 81771831, and 81971617), the Beijing Natural Science Foundation–Haidian Primitive Innovation Joint Fund (Grant No. L172019), and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (Code: XMLX201843).
Supporting information
Table S1. Comparisons between area and density of gluteus medius and minimus muscle at 3rd sacral (S3) and non‐3rd sacral (S4&S5) vertebrae levels
Acknowledgement
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. 38
Wang L., Yin L., Zhao Y., Su Y., Sun W., Liu Y., Yang M., Yu A., Blake G. M., Cheng X., Wu X., Veldhuis A., and Engelke K. (2020) Muscle density discriminates hip fracture better than computed tomography X‐ray absorptiometry hip areal bone mineral density, Journal of Cachexia, Sarcopenia and Muscle, 11, 1799–1812, doi: 10.1002/jcsm.12616.
Contributor Information
Xiaoguang Cheng, Email: xiao65@263.net.
Xinbao Wu, Email: wuxinbao_jst@126.com.
References
- 1. Cooper C, Cole ZA, Holroyd CR, Earl SC, Harvey NC, Dennison EM, et al. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporosis internl: j establi result coope betw the European Found for Osteops and the Nat Osteop Found the USA 2011;22:1277–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johansson H, Kanis JA, Oden A, Johnell O, McCloskey E. BMD, clinical risk factors and their combination for hip fracture prevention. Osteoporosis internl: j establi result coope betw the European Found for Osteops and the Nat Osteop Found the USA 2009;20:1675–1682. [DOI] [PubMed] [Google Scholar]
- 3. Rivadeneira F, Zillikens MC, De Laet CE, Hofman A, Uitterlinden AG, Beck TJ, et al. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam Study. J Bone Miner Res 2007;22:1781–1790. [DOI] [PubMed] [Google Scholar]
- 4. Bousson VD, Adams J, Engelke K, Aout M, Cohen‐Solal M, Bergot C, et al. In vivo discrimination of hip fracture with quantitative computed tomography: results from the prospective European Femur Fracture Study (EFFECT). J Bone Miner Res 2011;26:881–893. [DOI] [PubMed] [Google Scholar]
- 5. Engelke K. Quantitative computed tomography—current status and new developments. J Clin Densitom 2017;20:309–321. [DOI] [PubMed] [Google Scholar]
- 6. Engelke K, Lang T, Khosla S, Qin L, Zysset P, Leslie WD, et al. Clinical use of quantitative computed tomography (QCT) of the hip in the management of osteoporosis in adults: the 2015 ISCD official positions—part I. J Clin Densitom 2015;18:338–358. [DOI] [PubMed] [Google Scholar]
- 7. Anderson DE, Quinn E, Parker E, Allaire BT, Muir JW, Rubin CT, et al. Associations of computed tomography‐based trunk muscle size and density with balance and falls in older adults. J Gerontol A Biol Sci Med Sci 2016;71:811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Addison O, Young P, Inacio M, Bair WN, Prettyman MG, Beamer BA, et al. Hip but not thigh intramuscular adipose tissue is associated with poor balance and increased temporal gait variability in older adults. Curr Aging Sci 2014;7:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, et al. Trunk muscle composition as a predictor of reduced functional capacity in the Health, Aging and Body Composition Study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci 2005;60:1420–1424. [DOI] [PubMed] [Google Scholar]
- 10. Frank‐Wilson AW, Farthing JP, Chilibeck PD, Arnold CM, Davison KS, Olszynski WP, et al. Lower leg muscle density is independently associated with fall status in community‐dwelling older adults. Osteoporosis internl: j establi result coope betw the European Found for Osteops and the Nat Osteop Found the USA 2016;27:2231–2240. [DOI] [PubMed] [Google Scholar]
- 11. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 13. Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 2001;56:B209–B217. [DOI] [PubMed] [Google Scholar]
- 14. Engelke K, Museyko O, Wang L, Laredo JD. Quantitative analysis of skeletal muscle by computed tomography imaging—state of the art. J Orthop Translat 2018;15:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee K, Shin Y, Huh J, Sung YS, Lee IS, Yoon KH, et al. Recent issues on body composition imaging for sarcopenia evaluation. Korean J Radiol 2019;20:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Linder N, Schaudinn A, Langenhan K, Krenzien F, Hau HM, Benzing C, et al. Power of computed‐tomography‐defined sarcopenia for prediction of morbidity after pancreaticoduodenectomy. BMC Med Imaging 2019;19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lang T, Koyama A, Li C, Li J, Lu Y, Saeed I, et al. Pelvic body composition measurements by quantitative computed tomography: association with recent hip fracture. Bone 2008;42:798–805. [DOI] [PubMed] [Google Scholar]
- 18. Chi AS, Long SS, Zoga AC, Parker L, Morrison WB. Association of gluteus medius and minimus muscle atrophy and fall‐related hip fracture in older individuals using computed tomography. J Comput Assist Tomogr 2016;40:238–242. [DOI] [PubMed] [Google Scholar]
- 19. Mühlberg A, Museyko O, Bousson V, Pottecher P, Laredo JD, Engelke K. Three‐dimensional distribution of muscle and adipose tissue of the thigh at CT: association with acute hip fracture. Radiology 2019;290:426–434. [DOI] [PubMed] [Google Scholar]
- 20. Wang L, Museyko O, Su Y, Brown K, Yang R, Zhang Y, et al. QCT of the femur: comparison between QCTPro CTXA and MIAF Femur. Bone 2019;120:262–270. [DOI] [PubMed] [Google Scholar]
- 21. Su YB, Wang L, Wu XB, Yi C, Yang MH, Yan D, et al. The spatial differences in bone mineral density and hip structure between low‐energy femoral neck and trochanteric fractures in elderly Chinese using quantitative computed tomography. Bone 2019;124:62–68. [DOI] [PubMed] [Google Scholar]
- 22. Lu X, Chu H, Wang L, Yang R, Li Y, Sun W, et al. Age‐ and sex‐related differences in muscle strength and physical performance in older Chinese. Aging Clin Exp Res 2019. [DOI] [PubMed] [Google Scholar]
- 23. Wang L, Khoo BCC, Cheng XG, Brown K, Lewis JR, Su YB, et al. Differences in femoral neck structure between elderly Caucasian and Chinese populations: a cross‐sectional study of Perth–Beijing cohorts. Archives of osteop 2017;12:72. [DOI] [PubMed] [Google Scholar]
- 24. Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol 2001;90:2157–2165. [DOI] [PubMed] [Google Scholar]
- 25. Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, et al. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging 2014;18:532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol 2014;2014:309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loenneke JP, Buckner SL, Dankel SJ, Abe T. Exercise‐induced changes in muscle size do not contribute to exercise‐induced changes in muscle strength. Sports Med 2019;49:987–991. [DOI] [PubMed] [Google Scholar]
- 28. Johannesdottir F, Allaire B, Anderson DE, Samelson EJ, Kiel DP, Bouxsein ML. Population‐based study of age‐ and sex‐related differences in muscle density and size in thoracic and lumbar spine: the Framingham study. Osteoporosis intern j established as result coope betw the Europ Found for Osteop Natil Osteop Found the USA 2018;29:1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lorbergs AL, Allaire BT, Yang L, Kiel DP, Cupples LA, Jarraya M, et al. A longitudinal study of trunk muscle properties and severity of thoracic kyphosis in women and men: the Framingham Study. J Gerontol A Biol Sci Med Sci 2019;74:420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frank‐Wilson AW, Chalhoub D, Figueiredo P, Jonsson PV, Siggeirsdottir K, Sigurdsson S, et al. Associations of quadriceps torque properties with muscle size, attenuation, and intramuscular adipose tissue in older adults. J Gerontol A Biol Sci Med Sci 2018;73:931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez‐Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 2009;90:1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Daguet E, Jolivet E, Bousson V, Boutron C, Dahmen N, Bergot C, et al. Fat content of hip muscles: an anteroposterior gradient. J Bone Joint Surg Am 2011;93:1897–1905. [DOI] [PubMed] [Google Scholar]
- 33. Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking. Gait Posture 2003;17:159–169. [DOI] [PubMed] [Google Scholar]
- 34. Addison O, Inacio M, Bair WN, Beamer BA, Ryan AS, Rogers MW. Role of hip abductor muscle composition and torque in protective stepping for lateral balance recovery in older adults. Arch Phys Med Rehabil 2017;98:1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Damm P, Brackertz S, Streitparth F, Perka C, Bergmann G, Duda GN, et al. ESB Clinical Biomechanics Award 2018: muscle atrophy‐related increased joint loading after total hip arthroplasty and their postoperative change from 3 to 50 months. Clini biomec 2019;65:105–109. [DOI] [PubMed] [Google Scholar]
- 36. Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB, et al. Computed tomographic measurements of thigh muscle cross‐sectional area and attenuation coefficient predict hip fracture: the Health, Aging, and Body Composition Study. J Bone Miner Res 2010;25:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mühlberg A, Museyko O, Laredo JD, Engelke K. A reproducible semi‐automatic method to quantify the muscle‐lipid distribution in clinical 3D CT images of the thigh. PLoS ONE 2017;12:e0175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparisons between area and density of gluteus medius and minimus muscle at 3rd sacral (S3) and non‐3rd sacral (S4&S5) vertebrae levels


