Abstract
Background
Handgrip strength (HGS) is associated with poor clinical outcomes, including all‐cause, non‐cardiovascular, and cardiovascular mortalities. The published cut‐off points for HGS are mostly based on community populations from Western countries, lacking information on cancer patients from China. The objective of this study was to establish sex‐specific cut‐off points for Chinese cancer patients and investigate the effect of low HGS on cancer mortality.
Methods
We did a retrospective cohort study of patients who were diagnosed with malignant cancer from June 2012 to December 2018. HGS was measured using a hand dynamometer in 8257 cancer patients. Optimal stratification was used to solve threshold points. The hazard ratio (HR) of all cancer mortality and cancer‐specific mortality was calculated using Cox proportional hazard regression models.
Results
Among all participants, there were 3902 (47.3%) women and 4355 (52.7%) men. The median age was 58 years old. The cut‐off points of HGS to best classify patients with respect to time to mortality were <16.1 kg for women and <22 kg for men. Low HGS was associated with overall cancer mortality in both women and men [HR = 1.339, 95% confidence interval (CI) = 1.170–1.531, P < 0.001; HR = 1.346, 95% CI = 1.176–1.540, P < 0.001, respectively]. For specific cancer types, low HGS was associated with breast cancer (HR = 1.593, 95% CI = 1.230–2.063, P < 0.001) in women, and lung cancer (HR = 1.369, 95% CI = 1.005–1.866, P = 0.047) and colorectal cancer (HR = 1.399, 95% CI = 1.007–1.944, P = 0.045) in men.
Conclusions
On the basis of our sex‐specific cut‐off points, low HGS was strongly associated with cancer mortalities. These results indicate the usefulness of HGS measurement in routine clinical practice for improving patient assessments, cancer prognosis, and intervention.
Keywords: Handgrip strength, Cut‐offs, Cancer, Mortality, Nutrition status, Sex difference
Introduction
Handgrip strength (HGS) is the force involving the movement of fingers and wrist and the use of the forearm muscles. Generally, HGS declines with increasing age at a rate of approximately 1% annually after midlife. 1 However, a sex‐specific difference in HGS is apparent, where men have higher HGS than women on average levels and have faster HGS decline. 2 , 3 The HGS of cancer patients was different from that of the healthy populations. Cancer accelerates the decline process owing to its chronic consumptive characteristics for resulting syndromes or diseases such as fatigue, cancer cachexia, and sarcopenia. 4 , 5 , 6 Recently, HGS was recommended to be a criterion in the definition of cancer cachexia. 7 More strikingly, HGS replaced the muscle mass as the primary criterion to define sarcopenia. 8 However, most HGS cut‐off points were from Western research studies based on healthy populations. 9 , 10 , 11 Thus, whether the cut‐off points of low HGS for normal populations can be applied to cancer patients remains unclear. Therefore, establishing sex‐specific cut‐off points for Chinese cancer patients is warranted.
HGS is positively associated with overall body strength, 12 and negatively associated with all‐cause, 13 non‐cardiovascular, 14 and cardiovascular mortalities. 14 , 15 , 16 Addition of HGS enhanced the predictive capability of an established office‐based risk scoring system for all‐cause and cardiovascular mortalities. 17 Its prognostic value, simplicity, accessibility, and low cost makes HGS an ideal tool to detect physical status in clinical practice. However, the impacts of HGS on cancer patients remain controversial. 17 , 18 , 19 A study with 420 727 cases from the UK biobank showed that low HGS was inversely related with the survival outcomes of colorectal cancer in men and breast cancer in women, but no significant association was found with lung cancer in both men and women. 17 Conversely, an existing evidence suggested that HGS was associated with cancer mortality only in men but not in women with a 24 years follow‐up. 19 By contrast, another study indicated that no significant difference between HGS and cancer‐related death in men before 55 years old was found. 20 However, these data were based on community residents, and data on patients with different cancer types are insufficient to prove the predictive ability of HGS for stratifying mortality risks.
The purpose of this study was to establish sex‐specific cut‐off points of low HGS based on Chinese cancer populations according to time to cancer mortality. In addition, we assessed hazard risks of low HGS for overall cancer mortality and cancer‐specific mortality stratified by sex, aiming to investigate whether the impacts of low HGS differs among various cancer types.
Patients and methods
We did a retrospective cohort study of patients who were pathologically diagnosed with malignant cancer and were admitted specifically for cancer treatments (including surgery, chemotherapy, radiotherapy and other anti‐cancer therapy) were included in this study from June 2012 to December 2018 in multicenter. Patients with multiple hospitalizations were regarded as one case and the data on the first survey were analysed. No special selection criteria were imposed for cancer types or demographic characteristics except for excluding patients whose HGS could not be measured or those who refused to participate in the study. All patients were regularly followed up by telephone interviews or outpatient visits. This study was approved by the Ethics Committee and the Institutional Review Boards of all participating institutions.
All data were collected by trained personnel once patients were hospitalized. For each patient enrolled in this study, the following data were collected: age, sex, height, weight, smoking history, alcohol drinking, tea drinking, body mass index (BMI), haemoglobin concentration, serum albumin concentration, nutritional risk screening 2002 (NRS 2002) scores, Karnofsky performance scores (KPS), patient‐generated subjective global assessment (PG‐SGA) score, physical activity, intake status, weight loss, mid‐arm circumference (MAC), triceps skinfold thickness (TSF), maximum calf girth, HGS, cancer types, tumour‐node‐metastasis stage, previous treatments (surgery, chemotherapy, and radiotherapy), types of chemotherapy (curative, neoadjuvant, adjuvant, maintenance, and palliative chemotherapy), comorbidities (diabetes mellitus, hypertension, coronary heart disease, cirrhosis, chronic hepatitis, chronic obstructive pulmonary disease, and stroke), total length of hospital stay, hospitalization costs, and quality of life (QoL). Physical activity was divided into three degrees as follows: low was defined as bedridden, moderate wasdefined as limited activity, and normal was defined as normal and unlimited activity. Intake status was divided into four degrees as follows: fasting was defined as medical fasting, low was defined as cannot eat by mouth at all, moderate was defined as partial eating restrictions, and normal was defined as fully independent food intake.
Handgrip strength was measured using a hand dynamometer (Jamar Hand Dynamometer, IL, USA). Patients were seated comfortably at an upright position with their shoulder adducted and neutrally rotated, posed their elbow flexed at 90° as well as the forearm and wrist in a neutral position. 21 Patients held the dynamometer with their dominant hand at maximum strength. Tests were performed three consecutive times with a 1 min rest after each set. 22 The maximal hand strength was recorded.
Quality of life was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ‐C30 Version 3.0), which including five functional scales (physical, role, social, emotional, and cognitive function), a global QoL scale, three symptom scales (fatigue, pain, and nausea & vomiting), and six single items (dyspnoea, insomnia, appetite loss, constipation, diarrhoea, and financial impact). 23 Summary score was calculated by = [physical functioning + role functioning + social functioning + emotional functioning + cognitive functioning + (100−fatigue) + (100−pain) + (100−nausea & vomiting) + (100−dyspnea) + (100−insomnia) + (100−appetite loss) + (100−constipation) + (100−diarrhoea)]/13. 24
Statistical analyses
In terms of baseline characteristics, the normality of continuous variables was checked using the Kolmogorov–Smirnov test. Continuous variables with normal distribution were analysed using the Student t‐test and were presented as mean and standard deviation (SD), while continuous variables with non‐normal distribution were analysed using the Mann–Whitney U test and were presented as median and interquartile range. Categorical data were analysed using the Pearson χ²‐squared test or Fisher exact test. Spearman correlation analysis was performed for correlation analysis, and linear associations were tested using a linear regression analysis.
Optimal stratification was used to solve the threshold points of the continuous covariates by using of the log‐rank statistics as reported in a previous study. 25 Briefly, we used the log‐rank statistics to find the best cut‐off points to best stratify patients with or without low HGS with respect to time to mortality. Cut‐off points for HGS associated with overall survival were <16.1 kg for women and <22 kg for men based on our calculation. Cut‐off points obtained with this method were then used to classify patients as low HGS and normal HGS group.
Cox proportional hazard models were used to investigate the association between potential predictors and mortality. The results are shown as hazard ratios (HRs) together with 95% confidence intervals. The proportional hazards assumption was verified for all variables by inspecting Kaplan–Meier curves or locally weighted scatterplot smoothing plot of the Schoenfeld residual for covariates. A total of four incremental models with increasing numbers of varieties were created. Model 0 was unadjusted. Model 1 was adjusted for age, gender, BMI, albumin, haemoglobin, weight loss, KPS, NRS 2002, PG‐SGA scores, physical activity, intake status, MAC, TSF, maximum calf girth, smoking, alcohol drinking, and tea drinking. Model 2 was adjusted for Model 1 plus previous treatments, types of chemotherapy, and cancer stages. Model 3 was adjusted for Model 2 plus cancer types, QoL, and comorbidities.
To test if these relationships varied in age and sex, we firstly investigated potential age and sex interactions by including interaction terms as low HGS × age and low HGS × sex in the final model. Significant interactions (P < 0.05) indicated that the association between low HGS and mortality was dependent on the age or sex distribution. Thus, analysis was performed at specific strata. Finally, we found a trend of statistical significance (P = 0.060) between sex and low HGS. Therefore, we stratified all subsequent analyses by sex.
Two sensitivity analyses were performed as follows: one analysis excluded patients who died within 3 months to reduce the potential impact of reverse causation. Besides, sex‐specific HGS/BMI cut‐off points were calculated in the other analysis to compare results with those at low HGS, in accordance with the suggestion of the Foundation for the National Institutes of Health to adjustment for BMI. 26 Cut‐off points for low HGS/BMI ratio were <0.997 for women and <1.102 for men based on our calculation. All data were analysed using spss statistical Version 23.0 (IBM, Armonk, NY, USA).
Results
Of the 8651 patients recruited in our study, 8257 (95.4%) patients had data on HGS. As a result, a total of 3902 women and 4355 men were included in the analyses. The sex‐specific cut‐off points for HGS associated with overall survival were <16.1 kg for women and <22 kg for men. In accordance with these cut‐off points, 2123 patients were diagnosed as low HGS. The comparison of the patients' demographic and clinicopathological characteristics between low and normal HGS groups is presented in Table 1. Low HGS was associated with old age, female, increased weight loss, higher NRS 2002 scores and tumour‐node‐metastasis stages, reduced physical activity and food intake, and lower height, weight, BMI, haemoglobin concentration, serum albumin concentration, KPS, MAC, TSF, and maximum calf girth. Previous alcohol drinking, tea drinking, and smoking history were associated with low HGS. More proportion of patents with low HGS underwent surgical treatment, and more proportion of patents with normal HGS received curative chemotherapy and radiotherapy. The presence of comorbid disorders including diabetes, hypertension, coronary heart disease, chronic obstructive pulmonary disease, and stroke was related with low HGS. HGS showed significant relationships with various nutritional indices including age, height, weight, BMI, albumin concentration, haemoglobin concentration, KPS, PG‐SGA, NRS 2002, MAC, TSF, and maximum calf girth (supporting information, Table S1), and all items of QoL (Table 2, supporting information, Table S2), although the strength of the correlation seems not strong enough. We further performed linear regression analysis to determine regression coefficients (slopes) of HGS on nutritional indices (supporting information, Table S3, Figure 1). The slopes for men were generally stronger than those for women. Patients with colorectal cancer, gastric cancer, breast cancer, cervical cancer, and ovarian cancer were more likely to have low HGS (Table 1). The HGS values between different cancer types were heterogeneous (Figure 2). For men, patients with oesophageal cancer (mean 27.5 kg) had the lowest mean HGS values, those with gastric cancer (29.1 kg) had the second lowest HGS, and those with nasopharyngeal cancer (34.4 kg) had the highest HGS. For women, patients with hepatic cancer (17.9 kg) have the lowest mean HGS values, those with gastric cancer (18.2 kg) had the second lowest HGS, and those with nasopharyngeal cancer (22.6 kg) had the highest HGS. Patients with low HGS had similar total lengths of hospital stay and hospitalization costs with those in the normal HGS group.
Table 1.
Patient characteristics stratified by sex‐specific cut‐off points of handgrip strength
| Characteristic | Total (n = 8257) | Low grip strength (n = 2123) | Normal grip strength (n = 6134) | P values |
|---|---|---|---|---|
| Socio‐demographics | ||||
| Age, median (IQR), year | 58 (16) | 61 (16) | 56 (16) | <0.001 |
| Gender | <0.001 | |||
| Female | 3902 (47.3) | 1237 (58.3) | 2665 (43.4) | |
| Male | 4355 (52.7) | 886 (41.7) | 3469 (56.6) | |
| Height, median (IQR), cm | 162 (11) | 160 (10) | 163 (12) | <0.001 |
| Weight, median (IQR), kg | 59.9 (15) | 55 (13) | 60 (14) | <0.001 |
| Smoking history | <0.001 | |||
| Yes | 3274 (39.7) | 691 (32.5) | 2583 (42.1) | |
| No | 4983 (60.3) | 1442 (67.5) | 3551 (57.9) | |
| Alcohol drinking | <0.001 | |||
| Yes | 1521 (18.4) | 282 (13.3) | 1239 (20.2) | |
| No | 6736 (81.6) | 1841 (86.7) | 4895 (79.8) | |
| Tea drinking | ||||
| Yes | 2120 (25.7) | 450 (21.2) | 1670 (27.2) | <0.001 |
| No | 6137 (74.3) | 1673 (78.8) | 4464 (72.8) | |
| Nutritional indices | ||||
| BMI, median (IQR), kg/m2 | 22.5 (4.6) | 21.5 (4.8) | 22.8 (4.3) | <0.001 |
| Haemoglobin | 125 (26) | 118 (28) | 127 (25) | <0.001 |
| Serum albumin | 39.2 (6.8) | 37.6 (7.9) | 39.7 (6.4) | <0.001 |
| NRS 2002 scores | <0.001 | |||
| <3 | 5732 (69.4) | 1211 (57.0) | 4521 (73.7) | |
| ≥3 | 2525(30.6) | 912 (43.0) | 1613 (26.3) | |
| PG‐SGA | <0.001 | |||
| 0–1 | 1946 (23.6) | 241 (11.4) | 1705 (27.8) | |
| 2–3 | 1281 (15.5) | 273 (12.9) | 1008 (16.4) | |
| 4–8 | 1393 (16.9) | 351 (16.5) | 1042 (17.0) | |
| ≥9 | 3637 (44.0) | 1258 (59.2) | 2379 (38.8) | |
| KPS, median (IQR), scores | 90 (10) | 90 (10) | 90 (0) | <0.001 |
| Physical activity | <0.001 | |||
| Low | 201 (2.4) | 127 (6.0) | 76 (1.2) | |
| Moderate | 1332 (16.2) | 581 (27.4) | 747 (12.2) | |
| Normal | 6724 (81.4) | 1415 (66.6) | 5311 (86.6) | |
| Intake status | <0.001 | |||
| Fasting | 190 (2.3) | 91 (4.3) | 99 (1.6) | |
| Low | 98 (1.2) | 54 (2.5) | 44 (0.7) | |
| Moderate | 1718 (20.8) | 645 (30.4) | 1073 (17.5) | |
| Normal | 6251 (75.7) | 1333 (62.8) | 4918 (80.2) | |
| Weight loss | <0.001 | |||
| 0–1.9% | 5390 (65.3) | 1181 (55.6) | 4209 (68.6) | |
| 2–2.9% | 569 (6.9) | 171 (8.1) | 398 (6.5) | |
| 3–4.9% | 821 (9.9) | 228 (10.7) | 593 (9.7) | |
| 5–9.9% | 1029 (12.5) | 358 (16.9) | 671 (10.9) | |
| ≥10% | 448 (5.4) | 185 (8.7) | 263 (4.3) | |
| MAC, median (IQR), cm | 26.5 (4.8) | 25.2 (4.5) | 27 (4) | <0.001 |
| TSF, median (IQR), mm | 16.5 (10.5) | 15 (12) | 18 (11) | <0.001 |
| Maximum calf girth, median (IQR), mm | 33 (4.5) | 31.8 (4.8) | 33.5 (4.5) | <0.001 |
| Tumour indices | ||||
| Nasopharyngeal cancer | 769 (9.3) | 69 (3.3) | 700 (11.4) | <0.001 |
| Lung cancer | 1706 (20.7) | 381 (17.9) | 1325 (21.6) | <0.001 |
| Breast cancer | 1218 (14.8) | 354 (16.7) | 864 (14.1) | 0.004 |
| Colorectal cancer | 1665 (20.2) | 459 (21.6) | 1206 (19.7) | 0.052 |
| Gastric cancer | 995 (12.1) | 315 (14.8) | 680 (11.1) | <0001 |
| Hepatic cancer | 331 (4.0) | 64 (3.0) | 267 (4.4) | 0.007 |
| Esophageal cancer | 517 (6.3) | 138 (6.5) | 379 (6.2) | 0.598 |
| Cervical cancer | 410 (5.0) | 132 (6.2) | 278 (4.5) | 0.002 |
| Ovarian cancer | 215 (2.6) | 73 (3.4) | 142 (2.3) | 0.005 |
| Others | 431 (5.2) | 138 (6.5) | 293 (4.8) | 0.001 |
| TNM stages | 0.001 | |||
| 0 | 89 (1.1) | 37 (1.7) | 52 (0.8) | |
| I | 1017 (12.3) | 257 (12.1) | 760 (12.4) | |
| II | 1923 (23.3) | 506 (23.8) | 1417 (23.1) | |
| III | 2860 (34.6) | 687 (32.4) | 2173 (35.4) | |
| IV | 2401 (29.0) | 661 (30.7) | 1740 (28.4) | |
| Previous treatments | ||||
| Surgery | 5262 (63.7) | 1427 (67.2) | 3835 (62.5) | <0.001 |
| Chemotherapy | 3736 (45.2) | 938 (44.2) | 2798 (45.6) | 0.253 |
| Curative chemotherapy | 1201 (14.5) | 277 (13.0) | 924 (15.1) | 0.023 |
| Neoadjuvant chemotherapy | 368 (4.5) | 96 (4.5) | 272 (4.4) | 0.866 |
| Adjuvant chemotherapy | 1838 (22.3) | 474 (22.3) | 1364 (22.2) | 0.931 |
| Maintenance chemotherapy | 76 (0.9) | 25 (1.2) | 51 (0.8) | 0.150 |
| Palliative chemotherapy | 521 (6.3) | 121 (5.7) | 400 (6.5) | 0.180 |
| Radiotherapy | 1737 (21.0) | 352 (16.6) | 1385 (22.6) | <0.001 |
| Comorbidity | ||||
| Diabetes mellitus | 624 (7.6) | 192 (9.0) | 432(7.0) | 0.003 |
| Hypertension | 1373 (16.6) | 414 (19.5) | 959 (15.6) | <0.001 |
| Coronary heart disease | 323 (3.9) | 114 (5.4) | 209 (3.4) | <0.001 |
| Cirrhosis | 120 (1.5) | 22 (1.0) | 98 (1.6) | 0.062 |
| Chronic hepatitis | 419 (5.1) | 96 (4.5) | 323 (5.3) | 0.178 |
| COPD | 67 (0.8) | 28 (1.3) | 39 (0.6) | 0.002 |
| Stroke | 57 (0.7) | 27 (1.3) | 30 (0.5) | <0.001 |
| Total hospital stay, median (IQR), days | 12 (12) | 12 (12) | 12 (12) | 0.962 |
| Hospitalization costs, median (IQR), yuan | 20 721 (32 659) | 20 819 (34 506) | 20 715 (31 743) | 0.446 |
Data are represented as median (interquartile range) or number (%).
BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; KPS, Karnofsky performance scores; MAC, mid‐arm circumference; NRS 2002, nutrition risk screening 2002; PG‐SGA, patient‐generated subjective nutrition assessment; TNM, tumour‐lymph node‐metastasis; TSF, triceps fold thickness.
Table 2.
Quality of life (QoL) stratified by sex‐specific cut‐off points of handgrip strength
| Characteristic | Total (n = 8257) | Low grip strength (n = 2123) | Normal grip strength (n = 6134) | P values |
|---|---|---|---|---|
| Physical function | 93.3 (20.0) | 86.7 (26.7) | 93.3 (13.3) | <0.001 |
| Role function | 100.0 (33.3) | 66.7 (33.3) | 100 (33.3) | <0.001 |
| Social function | 66.7 (33.3) | 66.7 (33.3) | 83.3 (33.3) | <0.001 |
| Emotional function | 91.7 (16.7) | 91.7 (25.0) | 100 (16.7) | <0.001 |
| Cognitive function | 100.0 (16.7) | 83.3 (33.3) | 100 (16.7) | <0.001 |
| Global QoL | 66.7 (33.3) | 58.3 (16.7) | 66.7 (33.3) | <0.001 |
| Fatigue | 11.1 (33.3) | 22.2 (33.3) | 11.1 (33.3) | <0.001 |
| Nausea & vomiting | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | <0.001 |
| Pain | 0.0 (16.7) | 0.0 (33.3) | 0.0 (16.7) | <0.001 |
| Dyspnoea | 0.0 (0.0) | 0.0 (33.3) | 0.0 (0.0) | <0.001 |
| Insomnia | 0.0 (33.3) | 33.3 (33.3) | 0.0 (33.3) | <0.001 |
| Appetite loss | 0.0 (33.3) | 0.0 (33.3) | 0.0 (0.0) | <0.001 |
| Constipation | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | <0.001 |
| Diarrhoea | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.005 |
| Financial impact | 33.3 (33.3) | 33.3 (66.7) | 33.3 (33.3) | <0.001 |
| Summary score | 90.5 (14.0) | 85.6 (18.5) | 91.8 (12.2) | <0.001 |
Data are represented as median (interquartile range).
Figure 1.
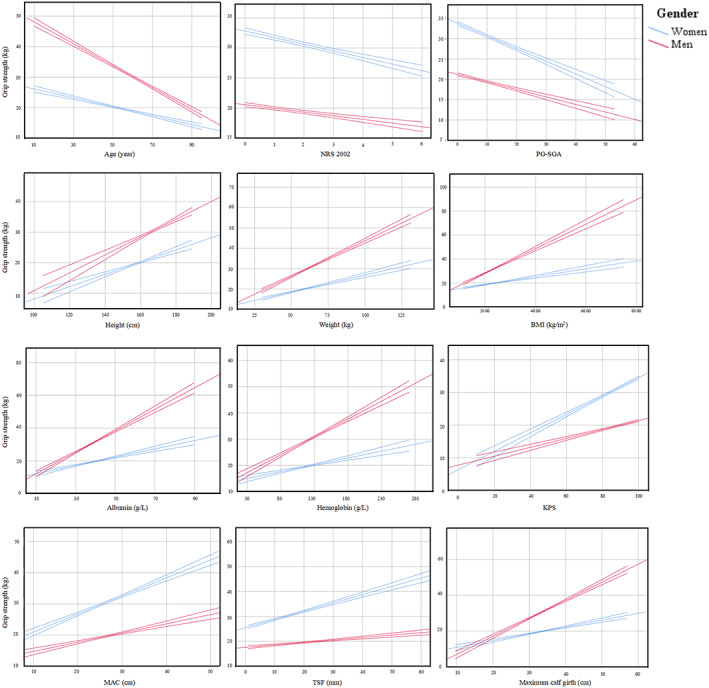
Linear associations between handgrip strength and nutritional indices with 95% confidence intervals. BMI, body mass index; KPS, Karnofsky performance scores; MAC, mid‐arm circumference; NRS 2002, nutrition risk screening 2002; PG‐SGA, patient‐generated subjective global assessment.
Figure 2.
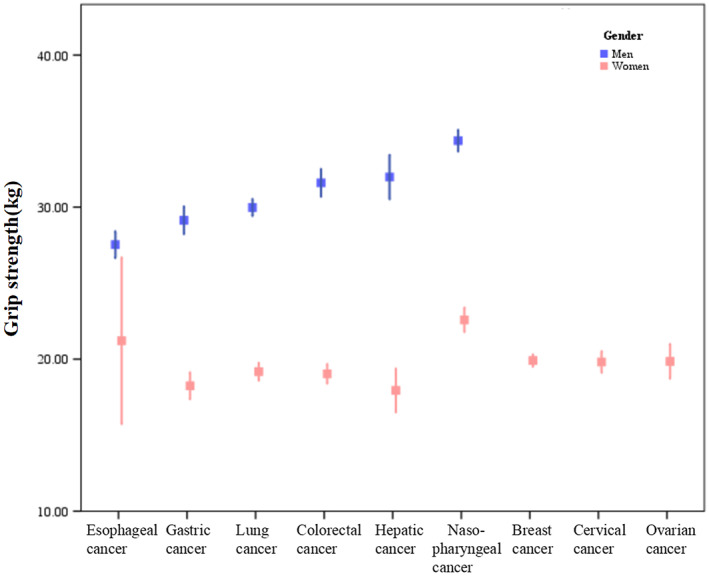
Handgrip strength in different cancer types stratified by sex.
Table 3 showed the HRs of low HGS for overall cancer mortality stratified by sex and age. The association between low HGS and cancer mortality was attenuated by increasing age. The HRs in the final model were, respectively, 1.337 (1.107–1.615), 1.324 (1.062–1.652), and 1.965 (0.887–4.352) in patients aged <60, between 60 and 75 years, and ≥75 years old for women and 1.487 (1.156–1.912), 1.298 (1.074–1.568), and 1.065 (0.716–1.585) for men. Further analysis of the relationship between low HGS and specific cancer types stratified by sex is summarized in Figure 3 and Table 4. When only low HGS was considered, cancer mortality in patients with low HGS was significant in overall cancer, lung cancer, colorectal cancer, gastric cancer, oesophageal cancer for both women and men and was significant in breast cancer for women and hepatic cancer for men (Figure 3). In the final model, low HGS was associated with breast cancer mortality for women and was associated with lung cancer and colorectal cancer mortalities for men (Table 4).
Table 3.
Hazard risk for all cancer mortality in patients with low handgrip strength stratified by sex and age
| Mortality | HR | 95% CI | P values | Mortality | HR | 95% CI | P values |
|---|---|---|---|---|---|---|---|
| Women | Men | ||||||
| <60 | <60 | ||||||
| Model 0 | 1.441 | 1.212 to 1.715 | <0.001 a | Model 0 | 2.381 | 1.920 to 2.952 | <0.001 a |
| Model 1 | 1.279 | 1.066 to 1.536 | 0.008 a | Model 1 | 1.596 | 1.256 to 2.028 | <0.001 a |
| Model 2 | 1.335 | 1.109 to 1.608 | 0.002 a | Model 2 | 1.506 | 1.180 to 1.922 | 0.001 a |
| Model 3 | 1.337 | 1.107 to 1.615 | 0.003 a | Model 3 | 1.487 | 1.156 to 1.912 | 0.002 a |
| 60–75 | 60–75 | ||||||
| Model 0 | 1.408 | 1.155 to 1.716 | <0.001 a | Model 0 | 1.681 | 1.429 to 1.978 | <0.001 a |
| Model 1 | 1.372 | 1.114 to 1.691 | 0.003 a | Model 1 | 1.319 | 1.104 to 1.576 | 0.002 a |
| Model 2 | 1.340 | 1.086 to 1.654 | 0.006 a | Model 2 | 1.339 | 1.117 to 1.607 | 0.002 a |
| Model 3 | 1.324 | 1.062 to 1.652 | 0.013 a | Model 3 | 1.298 | 1.074 to 1.568 | 0.007 a |
| ≥75 | ≥75 | ||||||
| Model 0 | 1.400 | 0.856 to 2.291 | 0.180 | Model 0 | 1.455 | 1.084 to 1.952 | 0.012 a |
| Model 1 | 1.270 | 0.725 to 2.225 | 0.403 | Model 1 | 1.039 | 0.739 to 1.463 | 0.824 |
| Model 2 | 1.606 | 0.889 to 2.901 | 0.116 | Model 2 | 1.113 | 0.783 to 1.582 | 0.551 |
| Model 3 | 1.965 | 0.887 to 4.352 | 0.096 | Model 3 | 1.065 | 0.716 to 1.585 | 0.755 |
CI, confidence interval; HR, hazard ratio.
Model 0 was unadjusted. Model 1 was adjusted for age, gender, body mass index, albumin, haemoglobin, weight loss, Karnofsky performance scores, nutritional risk screening 2002, patient‐generated subjective global assessment scores, physical activity, intake status, mid‐arm circumference, triceps skinfold thickness, maximum calf girth, smoking, alcohol drinking, and tea drinking. Model 2 was adjusted for Model 1 plus previous treatments, types of chemotherapy, and cancer stages. Model 3 was adjusted for Model 2 plus cancer types, quality of life, and comorbidities.
Data reach statistical significance.
Figure 3.
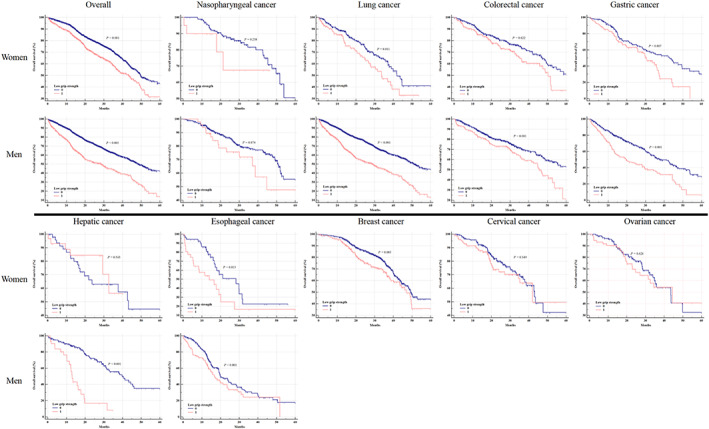
Kaplan–Meier curves of overall survival in different cancer types stratified by sex.
Table 4.
Hazard risk for all cancer mortality and cancer specific mortality in patients with low handgrip strength stratified by sex
| Mortality | HR b | 95% CI | P values |
|---|---|---|---|
| Overall | |||
| Women | 1.339 | 1.170 to 1.531 | <0.001 a |
| Men | 1.346 | 1.176 to 1.540 | <0.001 a |
| Specific tumour types | |||
| Nasopharyngeal cancer | |||
| Women | 1.000 | 0.180 to 5.553 | 1.000 |
| Men | 0.839 | 0.354 to 1.989 | 0.690 |
| Lung cancer | |||
| Women | 1.254 | 0.820 to 1.918 | 0.296 |
| Men | 1.369 | 1.005 to 1.866 | 0.047 a |
| Colorectal cancer | |||
| Women | 1.341 | 0.921 to 1.953 | 0.125 |
| Men | 1.399 | 1.007 to 1.944 | 0.045 a |
| Gastric cancer | |||
| Women | 1.000 | 0.642 to 1.558 | 1.000 |
| Men | 1.285 | 0.969 to 1.705 | 0.082 |
| Hepatic cancer | |||
| Women | 0.681 | 0.011 to 40.984 | 0.854 |
| Men | 1.428 | 0.532 to 3.836 | 0.479 |
| Esophageal cancer | |||
| Women | 0.992 | 0.110 to 7.731 | 0.941 |
| Men | 1.200 | 0.804 to 1.792 | 0.372 |
| Breast cancer | 1.593 | 1.230 to 2.063 | <0.001 a |
| Cervical cancer | 1.506 | 0.877 to 2.585 | 0.138 |
| Ovarian cancer | 1.074 | 0.434 to 2.658 | 0.878 |
CI, confidence interval; HR, hazard ratio.
Data reach statistical significance.
HRs are adjusted for Model 3.
Risk factors of cancer mortality were determined using univariate and multivariate Cox proportional hazards regression analyses. Factors with P value < 0.1 were shown in the supporting information, Table S4. In the univariate analysis, poor conditions of most baseline characteristics were associated with reduced survival time. In the multivariate analysis, age, PG‐SGA, NRS 2002, TSF, MAC, weight loss, low HGS, impaired intake status, metastasis, radiotherapy, and dyspnoea remained independent factors of cancer mortality. Using those risk factors, we performed ROC curve analyses with and without low HGS to predict 1 and 3 years cancer mortalities (supporting information, Tables S5 and S6). A difference of 0.025 from reference was considered a better discrimination. 27 We found that adding low HGS enhanced the predictive ability of 1 year mortality for gastric cancer and oesophageal cancer in women and colorectal cancer and hepatic cancer in men.
The sensitivity analysis yielded similar results as the main analysis (supporting information, Tables S7, S8, and S9).
Discussion
In the present study, we calculated our own sex‐specific HGS cut‐off points for Chinese cancer patients as <16.1 kg for women and <22 kg for men based on our large national sample. Using these cut‐off points, we found that low HGS was strongly associated with overall mortality in cancer patients regardless of sex. The associations observed in specific cancers were inconsistent across sexes, which were significant in breast cancer for women and were significant in lung cancer and colorectal cancer for men when nutritional indices were considered. Moreover, we found that low HGS enhanced the predictive ability for 1 year mortality in gastric cancer and oesophageal cancer for women and colorectal cancer and hepatic cancer for men.
Malnutrition is prevalent in cancer patients and is an independent determinant of HGS. 28 Cut‐off points of low HGS for cancer patients in our study (women < 16.1 kg and men < 22 kg) were much lower than those for community residents in other Western or Asian studies. 9 , 10 , 29 It was also lower than cut‐off points for defining sarcopenia in European Working Group on Sarcopenia in Older People guidelines. 8 , 30 Although cut‐off point of low HGS for women in the latest European Working Group on Sarcopenia in Older People guideline was similar to that of our study, the cut‐off point for men was higher than us. 8 The relatively high cut‐off points may diminish the impact of sarcopenia on clinical outcomes in cancer patients.
Poor nutritional status is a negative predictor of cancer mortality. HGS significantly correlated with nutritional indices such as BMI, PG‐SGA, MAC, and TSF in previous studies, 31 , 32 , 33 and it was a useful indicator to distinguish patients with chronic malnutrition from those who were underweight and had similar low BMI but were not undernourished. 34 In our study, patients with low HGS were shown correlated with poorer QoL, higher NRS 2002 scores and weight loss, lower BMI, haemoglobin concentration, serum albumin concentration, KPS, MAC, TSF, and maximum calf girth. After adjustment for nutritional indices in the Cox proportional hazard models, we found that low HGS remained a hazard factor for cancer mortality. Consistent with our study, a prospective cohort study involving 8677 men showed that HGS was inversely associated with cancer mortality, independent of BMI, percent body fat, waist circumference, and cardiorespiratory fitness. 35
Current studies seldom focused on the impacts of low HGS on cancer mortality. HGS was an excellent predictor of functional decline in patients with breast cancer. 36 In oesophageal cancer, low HGS had high predictive value for morbidity and surgical mortality. 37 Consistent with our study, Kilgour et al. 38 reported a shorter survival for patients whose HGS values were within the lowest 10th percentile of HGS values in advanced non‐small lung cancer and gastrointestinal cancer. However, they did not show concrete cut‐off points and enough information such as nutritional indices or sex‐stratified analysis. Celis‐Morales et al. 17 conducted a large‐scale prospective cohort that included half a million participants and showed that every 5 kg decrease in HGS was associated with a higher mortality hazard for colorectal cancer and lung cancer in both women and men, and for breast cancer in women. But strangely, when applied sex‐specific cut‐off points to define low HGS (<16 kg for women and <26 kg for men), the association diminished as low HGS was not associated with lung cancer mortality in both women and men and not associated with colorectal cancer mortality in women. 17 Unfortunately, they were unable to show data on more cancer types. In our study, we included more cancer types from multicentre and found that low HGS was associated with breast cancer mortality in women and was associated with lung cancer and colorectal cancer mortalities in men. Low HGS also showed a trend to statistically significant association in men patients with gastric cancer.
Low HGS seems to have a different impact on cancer mortality in men. Gynaecological cancer was reported have no association with HGS. 39 A study with 24 years follow‐up reported a 19% decrease in cancer mortality with per standard deviation increase in HGS in men but not in women 19 when nutritional indices were considered. However, the study did not list cancer types. Regrettably, we could not find the exact reasons for these differences in associations with sex in the present study. HGS is influenced by multiple factors, including health status, inactive lifestyles, and socio‐economic conditions. 40 A sex‐specific difference was found in the factors associated with HGS performance. 3 The HGS values of women are more likely to be influenced by stress, smoking, and dementia, while those of men are more likely to be influenced by chronic diseases, marital status, mean arterial pressure, and physical activity at work. This indicates that HGS values in two sexes are a reflection of the combination of different factors. Moreover, women have generally weaker HGS and slower decline in HGS than men, indicating that the HGS value of women may not change as significantly as that of men. Thus, low HGS in women may have fewer impacts.
Interestingly, we found no strong association between low HGS and overall cancer mortality in patients aged >75 years old. Consistent with our study, the Health ABC study found no association between the HGS tertiles and survival in older patients with malignancy. 41 Celis‐Morales et al. 17 had a similar finding that the HRs for all cancer mortality were higher in younger population in both women and men. We speculated that the age‐related decline in HGS concealed the cancer‐related HGS decline in older patients.
The clinical impacts of muscle strength have been well studied in non‐malignant diseases, such as diabetes 42 and cardiovascular diseases. 43 , 44 , 45 , 46 Patients with higher muscle strength have significant lower risk of type 2 diabetes. 42 In addition, the incidence of sudden cardiac death decreases by 69% for those with middle third of muscle strength compared with the lower third of muscle strength. 43 Lower incidence of heart failure is also observed in patients with higher handgrip strength. 44 However, there is a paucity of previous studies to systematically analyse the impacts of HGS on various cancer types. Our study extended previous evidence by reporting the findings that the HGS had varied impacts on different age, gender, and cancer type stratification. Chronic reduced oxygen delivery leads to metabolic alterations and muscle fibre changes while acute imbalance of oxygen supply and demand at the onset of activity lead to skeletal muscle fatigue and reduced cardiorespiratory fitness in patients with heart failure. 45 However, exact mechanism of cancer‐related muscle dysfunction is remained unclear, which may involve a wide range of behavioural‐related, tumour‐related, and therapy‐related factors. 5 Our results are supported by interventional research that resistance exercise is found to be strongly associated with lower risk of overall mortality. 46 Further studies are warrant to examine the impacts of resistance exercise on cancer patients.
Although this is a multicentre observational study involving more than eight thousand cancer patients, it has some limitations. First, although the posture and method of HGS measurement were referred from the American Society of Hand Therapists, 21 no official consensus has been reached on the protocol for HGS measurement. 47 Second, patients whose HGS could not be measured because of coma, paralysis, or limited mobility may have a higher risk of low HGS, and excluding them can induce bias in our study. Furthermore, some unmeasured or measured confounders could have an effect on the results in our analyses.
In conclusion, we firstly established sex‐specific cut‐off points for Chinese cancer patients. Low HGS adversely affects overall cancer mortality in both women and men. In specific cancer types, the effects of low HGS on cancer mortality varied between sexes. In women, low HGS was associated with breast cancer mortality. In men, it was associated with lung cancer and colorectal cancer mortalities. Our study is a new step to reveal the relationship between low HGS and mortality in different cancer types. These results indicate the usefulness of HGS measurement in routine clinical practice for improving patient assessments, cancer prognosis, and intervention. Further studies are imperative to investigate effects of interventions such as resistance exercise for patients with low HGS and distinguish patients who can acquire the greatest benefits.
Conflict of interest
The authors have declared no conflicts of interest.
Funding
This work was supported by the National Key Research and Development Program: The Key Technology of Palliative Care and Nursing for Cancer Patients (2017YFC1309200), the National Natural Science Foundation of China (81800795).
Supporting information
Table S1. Correlation between HGS and nutritional indices.
Table S2. Correlation between HGS and quality of life (QoL).
Table S3. Regression coefficients for each variables in the linear regression model for the relationship between HGS and nutritional indices stratified by sex.
Table S4. Univariate and multivariate Cox proportional hazards regression analysis of factors associated with overall survival.
Table S5. Effects of low HGS on the C‐index to predict cancer‐specific 1‐year mortality.
Table S6. Effect of low HGS on the C‐index to predict cancer‐specific 3‐year mortality
Table S7. Hazard risk for all cancer mortality in patients with low HGS by excluding patients dying within 3 months and stratifying by sex and age.
Table S8. Hazard risk for all tumour mortality in patients with the low HGS/BMI ratio stratified by sex and age.
Table S9. Hazard risk for all cancer mortality and cancer specific mortality in patients with low HGS/BMI ratio stratified by sex.
Acknowledgements
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. 48
Zhuang C.‐L., Zhang F.‐M., Li W., Wang K.‐H., Xu H.‐X., Song C.‐H., Guo Z.‐Q., and Shi H.‐P. (2020) Associations of low handgrip strength with cancer mortality: a multicentre observational study, Journal of Cachexia, Sarcopenia and Muscle, 11, 1476–1486, 10.1002/jcsm.12614
Cheng‐Le Zhuang and Feng‐Min Zhang contributed equally to this work.
References
- 1. Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik J. Grip strength changes over 27 yr in Japanese‐American men. J Appl Phys 1998;85:2047–2053. [DOI] [PubMed] [Google Scholar]
- 2. Frederiksen H, Hjelmborg J, Mortensen J, Mcgue M, Vaupel JW, Christensen K. Age trajectories of grip strength: cross‐sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol 2006;16:554–562. [DOI] [PubMed] [Google Scholar]
- 3. Sternäng O, Reynolds CA, Finkel D, Ernsth‐Bravell M, Pedersen NL, Dahl Aslan AK. Factors associated with grip strength decline in older adults. Age Ageing 2014;44:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kilgour RD, Vigano A, Trutschnigg B, Hornby L, Lucar E, Bacon SL, et al. Cancer‐related fatigue: the impact of skeletal muscle mass and strength in patients with advanced cancer. J Cachexia Sarcopenia Muscle 2010;1:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christensen JF, Jones L, Andersen J, Daugaard G, Rorth M, Hojman P. Muscle dysfunction in cancer patients. Ann Oncol 2014;25:947–958. [DOI] [PubMed] [Google Scholar]
- 6. Stene GB, Balstad TR, Leer ASM, Bye A, Kaasa S, Fallon M, et al. Deterioration in muscle mass and physical function differs according to weight loss history in cancer cachexia. Cancer 2019;11:1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vanhoutte G, van de Wiel M, Wouters K, Sels M, Bartolomeeussen L, De Keersmaecker S, et al. Cachexia in cancer: what is in the definition? BMJ Open Gastroenterol 2016;3:e000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2018;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, et al. Grip strength across the life course: normative data from twelve British studies. PLoS ONE 2014;9:e113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age‐associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 2003;95:1851–1860. [DOI] [PubMed] [Google Scholar]
- 11. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M157. [DOI] [PubMed] [Google Scholar]
- 12. Wind AE, Takken T, Helders PJ, Engelbert RH. Is grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? Eur J Pediatr 2010;169:281–287. [DOI] [PubMed] [Google Scholar]
- 13. Grøntved A, Hu FB. Walking pace and handgrip strength: simple measures of fitness and mortality risk? Eur Heart J 2017;38:3241–3243. [DOI] [PubMed] [Google Scholar]
- 14. Leong DP, Teo KK, Rangarajan S, Lopez‐Jaramillo P, Avezum A Jr, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386:266–273. [DOI] [PubMed] [Google Scholar]
- 15. Celis‐Morales CA, Lyall DM, Anderson J, Iliodromiti S, Fan Y, Ntuk UE, et al. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 UK‐Biobank participants. Eur Heart J 2016;38:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yates T, Zaccardi F, Dhalwani NN, Davies MJ, Bakrania K, Celis‐Morales CA, et al. Association of walking pace and handgrip strength with all‐cause, cardiovascular, and cancer mortality: a UK Biobank observational study. Eur Heart J 2017;38:3232–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Celis‐Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 2018;361:k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu MA, DuMontier C, Murillo A, Hshieh TT, Bean JF, Soiffer RJ, et al. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood 2019;134:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol 2006;36:228–235. [DOI] [PubMed] [Google Scholar]
- 20. Ortega FB, Silventoinen K, Tynelius P, Rasmussen F. Muscular strength in male adolescents and premature death: cohort study of one million participants. BMJ 2012;345:e7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fess E. Grip Strength In Casanova JS, ed. Clinical Assessment Recommendations. Journal of Hand Therapy; 1992. p 41–45. [Google Scholar]
- 22. Watanabe T, Owashi K, Kanauchi Y, Mura N, Takahara M, Ogino T. The short‐term reliability of grip strength measurement and the effects of posture and grip span. J Hand Surg Am 2005;30:603–609. [DOI] [PubMed] [Google Scholar]
- 23. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 24. Nordin K, Steel J, Hoffman K, Glimelius B. Alternative methods of interpreting quality of life data in advanced gastrointestinal cancer patients. Br J Cancer 2001;85:1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 26. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH Sarcopenia Project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Apfel CC, Kranke P, Greim C‐A, Roewer N. What can be expected from risk scores for predicting postoperative nausea and vomiting? Br J Anaesth 2001;86:822–827. [DOI] [PubMed] [Google Scholar]
- 28. Norman K, Stobäus N, Smoliner C, Zocher D, Scheufele R, Valentini L, et al. Determinants of hand grip strength, knee extension strength and functional status in cancer patients. Clin Nutr 2010;29:586–591. [DOI] [PubMed] [Google Scholar]
- 29. Woo J, Leung J, Sham A, Kwok T. Defining sarcopenia in terms of risk of physical limitations: a 5‐year follow‐up study of 3,153 Chinese men and women. J Am Geriatr Soc 2009;57:2224–2231. [DOI] [PubMed] [Google Scholar]
- 30. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flood A, Chung A, Parker H, Kearns V, O'Sullivan TA. The use of hand grip strength as a predictor of nutrition status in hospital patients. Clin Nutr 2014;33:106–114. [DOI] [PubMed] [Google Scholar]
- 32. Norman K, Stobäus N, Reiß J, Schulzke J, Valentini L, Pirlich M. Effect of sexual dimorphism on muscle strength in cachexia. J Cachexia Sarcopenia Muscle 2012;3:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pieterse S, Manandhar M, Ismail S. The association between nutritional status and handgrip strength in older Rwandan refugees. Eur J Clin Nutr 2002;56:933–939. [DOI] [PubMed] [Google Scholar]
- 34. Vaz M, Thangam S, Prabhu A, Shetty PS. Maximal voluntary contraction as a functional indicator of adult chronic undernutrition. Br J Nutr 1996;76:9–15. [DOI] [PubMed] [Google Scholar]
- 35. Ruiz JR, Sui X, Lobelo F, Lee D‐c, Morrow JR, Jackson AW, et al. Muscular strength and adiposity as predictors of adulthood cancer mortality in men. Cancer Epidemiol Biomarkers Prev 2009;18:1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Owusu C, Margevicius S, Schluchter M, Koroukian SM, Berger NA. Short physical performance battery, usual gait speed, grip strength and vulnerable elders survey each predict functional decline among older women with breast cancer. J Geriatr Oncol 2017;8:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sato S, Nagai E, Taki Y, Watanabe M, Watanabe Y, Nakano K, et al. Hand grip strength as a predictor of postoperative complications in esophageal cancer patients undergoing esophagectomy. Esophagus 2018;15:10–18. [DOI] [PubMed] [Google Scholar]
- 38. Kilgour RD, Vigano A, Trutschnigg B, Lucar E, Borod M, Morais JA. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer 2013;21:3261–3270. [DOI] [PubMed] [Google Scholar]
- 39. Cesari M, Cerullo F, Zamboni V, Di Palma R, Scambia G, Balducci L, et al. Functional status and mortality in older women with gynecological cancer. J Gerontol A Biol Sci Med Sci 2013;68:1129–1133. [DOI] [PubMed] [Google Scholar]
- 40. Kuh D, Bassey EJ, Butterworth S, Hardy R, Wadsworth MEJ. Grip strength, postural control, and functional leg power in a representative cohort of British men and women: associations with physical activity, health status, and socioeconomic conditions. J Gerontol A Biol Sci Med Sci 2005;60:224–231. [DOI] [PubMed] [Google Scholar]
- 41. Klepin HD, Geiger AM, Tooze JA, Newman AB, Colbert LH, Bauer DC, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc 2010;58:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y, Lee DC, Brellenthin AG, Sui X, Church TS, Lavie CJ, et al. Association of muscular strength and incidence of type 2 diabetes. Mayo Clin Proc 2019;94:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jiménez‐Pavón D, Brellenthin AG, Lee DC, Sui X, Blair SN, Lavie CJ. Role of muscular strength on the risk of sudden cardiac death in men. Mayo Clin Proc 2019;94:2589–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sillars A, Celis‐Morales CA, Ho FK, Petermann F, Welsh P, Iliodromiti S, et al. Association of fitness and grip strength with heart failure: findings from the UK Biobank population‐based study. Mayo Clin Proc 2019;94:2230–2240. [DOI] [PubMed] [Google Scholar]
- 45. Carbone S, Billingsley HE, Rodriguez‐Miguelez P, Kirkman DL, Garten R, Franco RL, et al. Lean mass abnormalities in heart failure: the role of sarcopenia, sarcopenic obesity, and cachexia. Curr Probl Cardiol 2019;100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lavie CJ, Kachur S, Sui X. Impact of fitness and changes in fitness on lipids and survival. Prog Cardiovasc Dis 2019;62:431–435. [DOI] [PubMed] [Google Scholar]
- 47. Norman K, Stobäus N, Gonzalez MC, Schulzke J‐D, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr 2011;30:135–142. [DOI] [PubMed] [Google Scholar]
- 48. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Correlation between HGS and nutritional indices.
Table S2. Correlation between HGS and quality of life (QoL).
Table S3. Regression coefficients for each variables in the linear regression model for the relationship between HGS and nutritional indices stratified by sex.
Table S4. Univariate and multivariate Cox proportional hazards regression analysis of factors associated with overall survival.
Table S5. Effects of low HGS on the C‐index to predict cancer‐specific 1‐year mortality.
Table S6. Effect of low HGS on the C‐index to predict cancer‐specific 3‐year mortality
Table S7. Hazard risk for all cancer mortality in patients with low HGS by excluding patients dying within 3 months and stratifying by sex and age.
Table S8. Hazard risk for all tumour mortality in patients with the low HGS/BMI ratio stratified by sex and age.
Table S9. Hazard risk for all cancer mortality and cancer specific mortality in patients with low HGS/BMI ratio stratified by sex.


