Abstract
Mitochondrial dysfunction can be a major cause of a wide range of age‐related diseases. Maintaining the normal homeostasis of mitochondria population plays an important role in ensuring people's health, which is done through the mitophagy process. Among the various stimuli for the onset of mitophagy, caloric restriction (CR) is one of the strongest non‐genetic triggers for initiating the mitophagy process. The primary objective of this paper is to review the literature assessing the effect of CR on mitophagy. Medline, Web of Science, Scopus, and Google Scholar databases was searched from inception to 1 August 2019. Reference lists from all selected articles were also examined for additional relevant studies. The evidence regarding the effect of fasting or CR on mitophagy is still limited. In addition, the methodological approaches of the studies are too heterogeneous in terms of types of food restriction, study duration, and targeted tissues. Most of the studies showed that fasting or CR induced mitophagy and mitophagy‐related markers such as Binp3 and Parkin. However, some studies demonstrated that mitophagy occurred both in fasting and fed state with no significant differences or may be induced in fed state. Study on the muscle tissue of subjects after exercise showed that mitophagy was upregulated in the fed state. It has been demonstrated that mitophagy in the muscle was lowered in the absence of AMP‐dependent kinase and fibroblast growth factor 21 genes, both in fasted and fed conditions. Current evidence overwhelmingly suggests that CR and fasting induce mitophagy and mitophagy‐related markers. Based on the current evidence that we reviewed here, it could be concluded that fasting or CR has a promising role as a novel and practical approach in the prevention of age‐related diseases without any side effects by inducing mitophagy in different organs of the body. More studies will be required in future to clarify the relationship between food deprivation and mitophagy. Further studies using a variety of different types of CR and fasting states are also warranted to determine the best approach for inducing mitophagy and improving health.
Keywords: Mitophagy, PINK1, BNip3, Fasting, Calorie restriction
Introduction
Mitochondria are double‐membrane‐bound organelles responsible for many process in eukaryotic cells, such as the production of adenosine triphosphate and reactive oxygen species (ROS), cell death, regulation of calcium haemostasis, and innate immunity. 1 , 2 Thus, regulation of mitochondria function is important for cell health. Adenosine triphosphate is produced by oxidative phosphorylation within the respiratory chain inside the mitochondria. This process can result in the generation of ROS, such as peroxides, superoxide, hydroxyl radical, and singlet oxygen. 3 If the overproduction of ROS is higher than the antioxidative capacity of the cell, this causes oxidative stress and cell death, which can occur in mitochondria due to their low antioxidant capacity. Impaired mitochondrial function leads to enhanced ROS production, stimulating apoptotic cell death. 3 Mitochondrial quality and quantity is mediated by the mitophagy process in which damaged or dysfunctional mitochondria are degraded. 4 The half‐life of mitochondria in the human body is 10–25 days, and the mitophagy process is important in maintaining the balance between degradation and biosynthesizing of mitochondria. 5 Furthermore, mitophagy plays an important role in removing mutated mitochondrial DNA. 6 Two types of mitophagy exist, one of them is micromitophagy in which vacuolar/lysosomal membrane invades the mitochondria directly, 7 while macromitophagy refers to the removal of targeted mitochondria by autophagosomes and fusion of autophagosomes to vacuolar/lysosomal leading to degradation dysfunction mitochondria by hydrolases. 8 Only macromitophagy exists in mammalian cells, which plays a major role in several biologic process in the body, including the development of diseases. For example, dysfunctional mitochondria are degraded by PINK1/Parkin‐dependent process in dopaminergic neurons in the substantia nigra, which contributes to Parkinson's disease pathology. 9 Mitophagy was initially termed in 2005 when Uth1p, specific outer membrane protein in yeast mitochondria, was introduced and demonstrated to be responsible for mitochondrial autophagy. 10 Two years later, Tal et al. 11 revealed that the localization of Aup1p in the intermembrane space of yeast mitochondria is important for efficient mitophagy in stationary phase cells. Further research demonstrated that evacuation of a mitochondrial inner membrane protein, Mdm38, leads to the loss of mitochondrial K+/H+ exchange activity and mitochondrial swelling occurred, resulting in fragmentation of the mitochondrial reticulum. 12 Atg32 is located in the intramitochondrial domain and acts as a mitochondrial receptor for mitophagy in the yeast. 13 Nitrogen starvation phosphorylates Atg32, which is required for mitophagy to occur. 14 Atg11 is phosphorylated by the mitogen‐activated protein kinase, Hog1. Hog1 is also required to phosphorylated the downstream mitogen‐activated protein kinase, Pbs2. 15 In addition to removing dysfunctional mitochondria, mitophagy also plays an important role in adaptive responses to conditions such as starvation, hypoxia, or developmental signals. 16 Mammalian mitophagy is induced by two main pathways, specifically damage‐induced mitophagy or developmental‐induced mitophagy. 17 Damage‐induced mitophagy is stimulated by two main proteins: (i) Parkin is a E3 ubiquitin protein ligase and (ii) serine/threonine‐protein kinase PINK1 that encodes the PTEN‐induced putative kinase. 18 In normal polarized conditions, PINK1 is preserved at basal levels and is located in the inner mitochondrial membrane. When depolarization occurs due to damaged mitochondria, the mitochondrial membrane potential is not sufficient to pass PINK1 from the outer mitochondrial membrane to the inner mitochondrial membrane. 19 Then, PINK1 is located on the outer membrane of mitochondria leading to phosphorylation of Mitofusin‐1, Mitofusin‐2, ubiquitin, and ubiquitin‐like domain of Parkin to activate the E3 ligase of parkin1. 20 Parkin promotes ubiquitination of voltage dependent anion channels. These are required to preserve the LC3‐interacting region containing p62 that acts as an adaptor molecule to recruit autophagosomal membranes in mitochondria. 21 Developmental process‐induced mitophagy occurs by the proapoptotic protein, Nip3‐like protein X (Nix) or Bnip3L, and adenovirus E1B 19 kDa‐interacting protein (Bnip) 3 that they are part of the B‐cell lymphoma 2 (Bcl‐2) family. 22 Mitophagy can be induced by the Nix protein that interacts with LC3. Then, LC3 interacts with gamma‐aminobutyric acid receptor‐associated protein to produce the LC3/gamma‐aminobutyric acid receptor‐associated protein complex that transfers the autophagosome to the mitochondria for removal. 20 Also, the beclin1/Bcl‐2 interaction is broken by the binding of Nix and Bnip3 to Bcl‐2, so beclin1 can initiate mitochondrial autophagy. 17 FUNDC1 is a mitochondrial outer‐membrane receptor of mitophagy, which is induced by hypoxia. When FUNDC1 is dephosphorylated due to hypoxia, it interacts with LC3‐II to initiate mitophagy process. 23
Fasting or calorie restriction
Accumulated evidence suggest that dietary restriction (DR), including modifications in calorie intake, dietary composition (e.g. protein content), or timing of food intake (e.g. intermittent fasting [IF]), has a substantial role in preventing or treating chronic disorders. 24 DR also has beneficial effects on longevity, likely through a delay in onset and reduction in severity of chronic aging‐related diseases, including cancer. 24 The major role of DR on longevity and health span is regulated by overlapping pathways: in the presence of sufficient nutrient supply, nutrient‐sensing signalling cascades will be activated and cellular growth will be promoted, which lead to organismal growth and proliferation. These signalling pathways are downregulated in periods of low food availability or the lack of specific macromolecules, resulting in activation of protective metabolic pathways to ameliorate the accumulation of cellular damage, block cellular proliferation, and activate stress resistance transcription factors that negatively regulate pro‐aging pathways. 24 , 25 , 26 , 27 , 28 , 29 One of the dietary interventions is calorie restriction (CR) that reduces calorie intake without creating malnutrition. 30 CR alters physiological processes, such as reduction of cell proliferation rates, decreased generation of ROS, and a decline in body temperature. 31 , 32 , 33 , 34 Insulin‐like growth factor‐1 receptor‐dependent pathways and target of rapamycin (TOR)‐dependent activities are inhibited by CR, these processes are involved in cell proliferation and glycolysis. 35 CR also has anti‐inflammatory effects; it reduces nuclear factor‐kB activity and decreases pro‐inflammatory profile of aging. 35 Research has also revealed that CR induces mitochondrial activity through activation of AMP‐dependent kinase (AMPK) and sirtuins. Simultaneously, a higher activity of Forkhead box proteins occurs which activates both auto/mitophagy and antioxidant expression. 36 Therefore, both TOR inhibition and AMPK activation can active Forkhead box protein. CR also activates nuclear factor erythroid 2‐related factor 2, resulting in the expression of antioxidant proteins. 36 CR leads to decreased accumulation of damaged proteins through two mechanisms. Firstly, CR produces short‐term starvation that leads to increase proteolysis and reduce accumulation of damaged protein. 37 Secondly, CR leads to decrease protein turn over, reducing the need for protein replacement. CR improves glucose metabolism, reduces insulin levels, and has protective effects on neurodegeneration in animal models of Huntington's disease, Alzheimer's disease, Parkinson's disease, and stroke. 38 Additionally, CR can reduce the incidence of inflammatory disorders, such as diabetes, cancer, and cardiovascular disorders (Figure 1). 38 , 39
Figure 1.
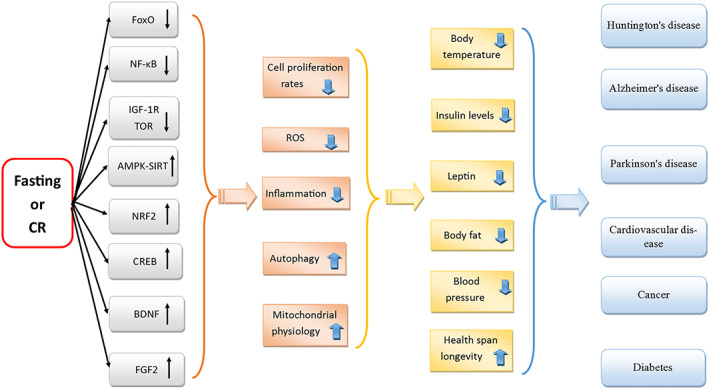
Schematic summary of pathways depicting the possible effects of fasting or calorie restriction (CR) on various cellular pathways, which induces responses of the whole organism, leading results in several health benefits for the body. Fasting or CR inhibits IGF‐1 receptor‐dependent pathways and TOR‐dependent activities. Fasting or CR inhibit nuclear actor‐kB (NF‐kB) activity. Fasting or CR also activate AMP‐dependent kinase (AMPK) and sirtuins. At the same time, fasting or CR activate FoxOs. Fasting or CR also induces the nuclear factor (erythroid‐derived 2)‐like 2 (Nrf2) activation. Moreover, fasting or CR upregulate cyclic AMP response element‐binding protein (CREB) and neurotrophic factors, including brain‐derived neurotrophic factor (BDNF) and fibroblast growth factor 2 (FGF2). All of these processes have several beneficial effects on organisms including upregulation of autophagy, reduction of inflammation and stress oxidative as well as cell proliferation rates, and increase mitochondrial health. These processes have several health benefits such as reduction in body temperature, insulin level, leptin, body fat, blood pressure, and increase longevity. Finally, several positive health outcomes will achieve such as age related disorders such as Huntington's disease, Alzheimer's disease, and Parkinson disease, as well as metabolic disorders such as cardiovascular diseases, and diabetes mellitus, and cancer. ROS, reactive oxygen species.
Due to the wide range of beneficial effects, fasting has recently attracted significant attention as a novel strategy to promote protection against multiple diseases. 40 , 41 A common type of fasting is IF (including alternate day fasting [ADF]), which refers to eating patterns where individual intake little or no energy for extended time periods (e.g. 16–48 h), with intervening periods of normal food intake on a recurring basis. Periodic fasting is another type of fasting, which refer to IF with periods of fasting or ‘fasting mimicking diets’ lasting from 2 to as many as 21 or more days. Time‐restricted feeding indicates an eating pattern in which food access is limited to a time window of 8–9 h or less every day. 24 , 41 , 42 Research demonstrates that IF/ADF has beneficial impacts on metabolic conditions because it reduces insulin and leptin level, reduces body fat, resting heart rate, blood pressure, inflammation, increases insulin and leptin sensitivity, and improve the resistance of the brain and heart to stress (e.g. reduced tissue damage and improved functional outcome in models of stroke and myocardial infarction). 41 Similarly, animal studies have demonstrated the beneficial effects of IF/ADF, periodic fasting, and time‐restricted feeding on preventing and/or treating, delaying onset, and slowing the progression of a wide range of age‐related disorders including cardiovascular disease, stroke, diabetes, and neurological disorders such as Alzheimer's disease and Parkinson's disease. 41 Research suggests that IF improves memory performance, 43 which could be beneficial in diseases such as dementia and Alzheimer's disease. Additionally, evidence demonstrates that IF should be considered as a novel approach to prevent malignancies and increase the efficacy of cancer therapies. 40 Activation of adaptive cellular stress response signalling pathways results in increased mitochondrial health, DNA repair, and autophagy. These effects are considered the main cellular and molecular mechanisms by which IF improves health and counteracts disease progression. 41 Several signalling pathways could be affected by IF, such as a reduction in mammalian TOR signalling, improvement of mitochondrial function, stimulation of mitochondrial biogenesis, and upregulation of autophagy, upregulation of cyclic AMP response element‐binding protein and neurotrophic factors, including brain‐derived neurotrophic factor and fibroblast growth factor 2 (FGF2). 41 Research has revealed that fasting stimulates anti‐aging processes and prolongs lifespan. 44 , 45 , 46 The molecular mechanism of the process by which fasting causes lifespan‐extension in many spices is its salient role in autophagy induction. 47 , 48 , 49 Similar to CR, fasting is the most common and popular approach of autophagy induction which causes increased in cellular clean‐up, prevention of the accumulation of toxic components and promotes longevity. 49
However, according to our knowledge, the effect of CR or fasting on induction of mitophagy or mitochondrial autophagy has not been assessed until now. In this review, we examine the impact of CR on autophagy stimulation 50 and assessed whether fasting or CR can stimulate mitophagy.
Materials and methods
This narrative review was designed and reported in accordance with the guidelines of the preferred reporting items for systematic reviews and meta‐analyses (PRISMA). Medline, Web of Science, Scopus, and Google Scholar databases were search using the following terms in titles abstracts: mitophagy OR mitochondrial autophagy AND fating OR fast OR fasted OR CR OR CR OR food restriction OR food deprivation or low calorie OR low calorie diet. We included studies that assessed the effect of fasting or CR on mitophagy. The literature search was conducted from inception to 1 August 2019. Two researchers (SM and MB) independently and systematically screened the major bibliographic databases. Reference lists from all selected articles were also examined for additional relevant studies.
Results
Fasting or calorie restriction and mitophagy
The effect of CR or fasting on inducing mitochondrial autophagy has been assessed in preclinical experiments and a few clinical studies. A summary of results is shown in Table 1. In addition, a schematic summary pathway of the effect of fasting or CR on signalling pathways of mitophagy stimulation is presented in Figure 2.
Table 1.
The effect of fasting or calorie restriction on mitophagy
| Author (year) | Intervention/main component | Duration | Target tissue | Human/animal | Main outcomes |
|---|---|---|---|---|---|
| Price et al. (2012) 30 | Ad libitum NIH‐31/NIA fortified diet or only 3 g/day NIH‐31/NIA fortified food | 32 days | Liver | Mice | Hepatic mitochondrial biogenesis and mitophagy ↓ |
| Cui et al. (2013) 51 |
Control diet: (3.42 kcal/g) High‐caloric diet: (4.52 kcal/g) CR: (consumed 30% less calorie than control) |
20 months | Kidney | Rats |
↓ PINK1 expression ↑ Bnip3 expression |
| Zhao et al. (2018) 52 |
Control diet CR (40% less than control) Exercise training CR + exercise training |
10 weeks | Heart | Mice |
Drp1 levels ↑ Nix ↓ Mitophagy in cardiac tissue ↑ |
| No | CR condition on a 0.2% concentration of glucose | no‐state | — | Yeast | atg32Δ mutant↓ CR, This indicating that macromitophagy is a key factor to assure longevity process in chronologically aging yeast |
| Tarpey et al. (2017) 55 | 12 h fasting then intake of high fat meal | 12 h | Muscle | Human | A greater amount of phospho‐P1NK1 and phospho‐Parkin in skeletal muscle of runners compared with sedentary subjects in both fasted and fed state. |
| Schwalm et al. (2017) 56 |
Fasting state: consumption of 150 mL water every 15 min during exercise Fed state: consumed 150 mL drink containing 6% carbohydrate every 15 min during the exercise period |
2 h | Muscle | Human |
Bnip3 expression ↑in fed state immediately after exercise compared with fasted state. mRNA levels of Bnip3 and Bnip3L was higher in fed state after exercise compared with fasted state LC3bII/LC3bI ratio was significantly decreased in the fasted state |
| Linden et al. (2015) 57 | CR diet that was approximately 70% of ad libitum diet | 12 week | Liver | Rat | BINP3 was higher in combination of caloric restriction and metformin therapy |
| Glick et al. (2013) 58 | 6 and 24 h fasting | 6 and 24 h | Liver | Mice | BNpi3 protein expression was higher in fasted state than basal level |
| Jamart et al. (2013) 59 | Fasting | — | Muscle | Mice | Mitophagy and its variation (BNIP3 a↑ during exercise in the fasted state |
| Bujak et al. (2015) 60 | Fasting | 12, 24, or 48 h | Muscle | Mice | Level of parkin protein was lower in AMPK‐MKO rather than wild mice both in fed or fasted state |
| Shirakabe et al. (2016) 62 | Fasting | 48 h | Heart | Mice | Mitochondrial autophagy ↑ |
| Oost et al. (2019) 61 | Fasting | 24 h | Muscle | Mice | Mitophagy flux reduced in FGF‐21 knockout mice whether in fasting or fed condition |
| Gubbiotti et al. (2015) 65 | Fasting | 25 h | Heart | Mice | Decorin that plays a role in mitophagy of breast carcinoma cell increased in fasting condition |
| Gutierrez‐Casado et al. (2015) 64 | CR 40% | — | Muscle | Mice | The highest values of PINK1 and Parkin were seen after 18 months of CR in lard group |
| Dongil et al. (2018) 63 | Fasting | 48 h | Liver | Mice |
Protein and mRNA expression of Fis1 ↑ in PASK deficient mice |
| Kim and Lemasters (2011) 67 | Nutrient deprivation | 90 min | Hepatocyte | Mice |
Mitophagy ↑ |
| McWilliams et al. (2018) 68 | Fasted condition | — | Pancreas | Mouse | No differences between fasted and fed state according to PINK1 level |
| Luevano‐Martinez et al. (2017) 69 | CR 40% | 8 week | Liver | Mice | Biosynthesize and remodelling of cardiolipin↑ |
| Rambold et al. (2011) 70 | Nutrient deprivation | 2 and 6 h | — | Mouse embryonic fibroblasts | Mitochondrial tubulation ↑ |
| Sacks et al. (2018) 66 | RYGB surgery | — | Liver | Rat | Gene expression for mitochondrial fusion genes, m (Mfn1 and Mfn2) and optic atrophy 1 increased following RYGB surgery |
CR, calorie restriction; PASK, PAS Domain Kinase; RYGB, ROX‐en‐Y gastric bypass surgery.
Figure 2.
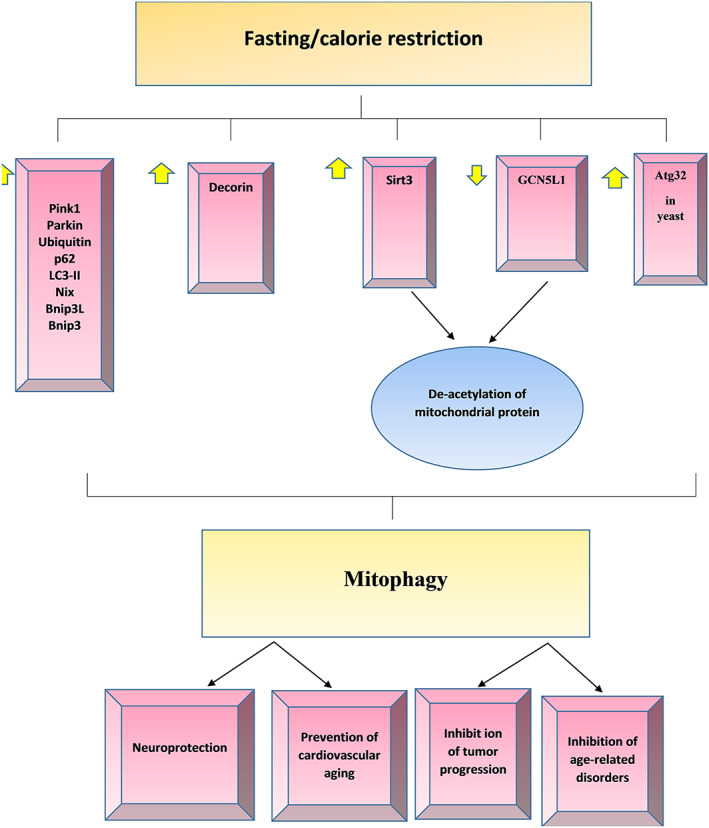
The effect of fasting or calorie restriction (CR) on signalling pathways of mitophagy stimulation. CR result in upregulating of protein markers of mitophagy, such as PINK1, Parkin, ubiquitin, p62/LC3‐II, Nix, Bnip3L, and Bnip3 in mammalian. Also, CR can induce atg32 in yeast, which leads to increased mitophagy processes. Activation of Sirt3 and downregulation of GCN5L1 induced by fasting or CR lead to deacetylation of mitochondrial proteins, and mitophagy may be initiated by unknown process through retrograde signalling or direct ubiquitinylation. In tumour cells, decorin stabilized mitostatin mRNAs, leading to the accumulation of mitostatin and mitophagy initiated by modulation of the PINK1/Parkin signalling axis.
Price et al. 30 assessed the effect of long‐term CR on in vivo hepatic mitophagy in mice. Twenty CR male C57/BL6 mice and 20 age‐matched ad libitum (AL) group controls were used in this study. Mice were allocated into two groups to receive either a CR diet where the mice were provided with 3.0 g of NIH‐31/NIA fortified diet once a day (this is an autoclavable diet formulated used in biological and biomedical research as a standard reference diet) or an AL diet in which animals freely accessed the NIH‐31/NIA fortified feed. Animals were sacrificed at various time points (0.5, 1, 4, 8, 15, or 32 days) following their allocated diet. Food intake and body weight of mice in each group measured weekly and while anaesthetising them. Following prolonged CR, global protein synthesis and breakdown rates were reduced in the majority of cellular proteins in the liver. The half‐life of mitochondrial protein increased in CR‐conditioned mice compared with AL mice. Additionally, a considerable reduction was observed in hepatic mitochondrial biogenesis and global rate of proteolysis including mitophagy, as well as general protein metabolic rates in response to chronic CR. Similarly, Cui et al. 51 assessed the effect of long‐term CR or high‐calorie diet on the kidney, specifically mitochondrial function and protein expression of autophagy and mitophagy markers. Three‐month‐old rats were purchased and placed on adopting feeding for 1 month. Then, rats were randomly divided into three groups to receive either a normal rat chow providing 3.42 kcal/g (control group n = 20), high calorie diet group consumed modified chow that supplied 4.51 kcal/g (n = 25), or a CR diet in which animals were fed 30% less calorie compared with control rats (n = 16). After 20 months of intervention, six rats from each group were sacrificed and blood samples and renal tissue waste collected. This study showed that a high‐caloric diet resulted in increased kidney weight, creatinine concentration, cholesterol, and triglyceride levels compared with the control group. Also, a significant increase was observed in the expression of PINK1 in the high‐calorie kidneys, indicating increased mitochondrial damage. Nevertheless, in the CR group, the expression of PINK1 in the kidneys was dramatically decreased, demonstrating that mitochondrial damage could be mitigated by CR. While Parkin expression did not significantly differ between the three groups. Bnip3 expression was significantly upregulated in the CR rat kidneys, suggesting mitophagy can be increased by CR while the expression of Binp3 decreased in high‐caloric condition that indicated possibility of preventing mitophagy in this condition. In another study, Zhao et al. 52 examined the effects of exercise training and DR on cardiac mitophagy. Eight‐week‐old male mice were given AL water and standard mouse food before being randomly allocated in one of three groups; control (n−8), exercise trained (n = 8, mice underwent swim training 5 days/week for 10 weeks), CR (n = 8 mice were fed 40% less calories than normal for 10 weeks), and exercise training + CR (T + CR) groups. The average food intake of the control group was measured every week; then, 60% of calculated energy were given to caloric restriction group. Food composition used in this study (specific‐pathogen‐free food) included 23% protein, 9% water, 4% crude fibre, and 6% fat. The average intake of food in the control group was 2.5 to 4.5 g during the study. Based on the findings of this study, autophagosomes enclosing suspectable mitochondria were found in CR and CR + T groups, which indicated that CR and exercise training can increase mitophagy. Following the 10 week intervention, PINK1 protein and mRNA expression was significantly increased in the T and CR + T group compared with control group. However, Parkin mRNA expression was significantly reduced in the T + CR group relative to control group, demonstrating a higher sensitivity of PINK1 than Parkin in PINK1/Parkin pathway. Additionally, Binp3 was significantly reduced in T and T + CR group compared with control group. The expression of NIX protein was decreased in all intervention groups compared with the control group. Drp1 that controls the final part of mitochondrial fission was significantly increased in CR and T + CR group. These results demonstrate that exercise or CR stimulates mitophagy in the heart due to PINK1 or Drp1 upregulation, respectively. Conversely, Bnip3 and Nix were reported to have no effect on mitophagy activation in mouse cardiac tissue.
Kanki et al. 53 aimed to determine what genes are essential for mitophagy by screening for mitophagy‐deficient mutants. In this study, yeasts were grown under CR conditions on a nutrient‐rich medium containing a low (0.2%) concentration of glucose. Results showed that both the mean and maximum chronological lifespan of yeast was substantially reduced in the atg32Δ mutant. Atg32Δ damage only occurs within the selective macroautophagy pathway, and it is not related to other pathways of non‐selective macroautophagy. 13 , 53 Under limited calorie supply, macromitophagy is a key factor to assure longevity processes in chronologically aging yeast. 54 Tarpey et al. 55 compared healthy male subjects who lead a sedentary lifestyle or endurance runners, in order to assess whether skeletal muscle from runners would be associated with elevated autophagy and mitophagy. Nine healthy sedentary males and 10 males with endurance‐training were recruited in the referred study. Participants withheld from exercise for 36 h before being exposed to either fasting conditions or a high‐fat meal. Muscle biopsies were taken after 12 h of fasting and 4 h after a high‐fat meal (contained 52 g carbohydrate, 24 g protein, and 58 g fat) to assess mitophagy biomarkers. Four days food diaries were used to assess the compliance of subjects. Muscle biopsies were collected from the vastus lateralis of subjects to determine autophagy and mitophagy protein markers. The results of this study demonstrated that total P1NK1 levels were not different between sedentary and endurance‐trained subjects either before or after a high‐fat meal. However, skeletal muscle from runner subjects contains a greater amount of phospho‐P1NK1 compared with sedentary subjects in both fasted and fed states. Additionally, total Parkin protein was higher in sedentary participants, especially following the intake of a high fat meal, while skeletal muscle of endurance‐trained subjects contained higher amount of phospho‐Parkin following both fasted and high‐fat meal intervention. Mitochondrial fusion protein (Mfn2) significantly increased in endurance‐trained subjects in both fasted and high‐fat diet state. Mfn2 protein content tended to be higher in the fasting state but significantly increased following the high fat meal in the endurance‐trained subjects. Elevated levels of phospho‐PINK1 and phospho‐Parkin in endurance runners confirmed that endurance‐training enhances mitophagy and mitochondrial dynamics in skeletal muscle.
Similarly, Schwalm et al. 56 aimed to determine the effect of exercise and diet on mitophagy in skeletal muscle. Seven healthy cycle athletes (minimum VO2 max of 50 mL/min/kg) were included in this study. Subjects were fed a high‐carbohydrate diet containing 63% carbohydrate the day before they were randomly allocated into a fed group or fasted group. In a cross‐over design, participants were allocated into a fed or fasted group. The fed group consumed 150 mL drink containing 6% carbohydrate every 15 min while subjects in the fasted group consumed 150 mL water every 15 min during the 2 h exercise session. Following a two weeks wash‐out period, subjects in each group receive the another intervention. Muscle biopsies were collected from the mid‐portion of the vastus lateralis muscle under local anaesthesia at baseline, before initiation of exercise, immediately after and 1 h after exercise to assess mRNA level and protein expression of related mitophagy biomarkers. Based on the results of this study, Mfn2 mRNA level were lowered in the fed state compared with baseline values. Conversely, mitochondrial DRP1 and Fis1 protein expression were increased post‐exercise in fed condition. This study revealed that mitochondrial Parkin was similar among the two nutritional states, as well as before and after exercise. While protein expression and mRNA levels of Bnip3 were significantly higher in the fed group immediately after exercise compared with fasted state. Phospho‐DRP1 involved in mitochondrial fission, increased post‐exercise and 1 h post‐exercise in the fed state. The ratio of LC3bII/LC3bI was significantly decreased in the fasted state post‐exercise compared with fed state, demonstrating decreased mitophagy in this condition. In another study, Linden et al. 57 used diabetic Otsuka Long‐Evans Tokushima fatty rats to assess the effect of CR, metformin therapy, and combination therapy on managing of non‐alcoholic fatty liver and diabetes. Twenty‐week‐old rats were allocated to one these four groups: AL diet, metformin (300 mg/kg/day) with AL diet, CR (~30% less than AL diet; 21 g/day), or combination of metformin + CR for 12 weeks. Liver and blood samples were collected at 32 weeks to assess biochemical markers attributed to liver function and insulin resistance. This study demonstrated that BNip3 concentrations were elevated in CR + metformin group compared with AL diet. Also, fasting glucose and insulin were improved in the CR group. Combination of CR + metformin therapy led to improved glucose tolerance. Reduction in serum alanine aminotransferases was shown in the CR + metformin group. Markers of de novo lipogenesis in liver such as fatty acid synthase, acetyl‐CoA carboxylase, and stearoylCoA desaturase‐1 were also decreased in the CR group while hepatic mitochondrial activity such as palmitate oxidation and β‐hydroxyacyl CoA dehydrogenase activity was increased in this group. In another study conducted by Glic et al., 58 3–5 month BNip3 heterozygous and BNip3 null mice were used to assess the effect of Binp3 protein in regulation of mitochondrial function. Mice were fasted for 16 h prior to conducting a glucose tolerance test, glucagon stimulation test, pyruvate challenge, or alanine challenge, or 4 h fasted for assessment of the insulin tolerance tests, 6 and 24 h fasted for mitophagy protein marker assessment. Results from this study revealed high levels of Binp3 expression in the liver and heart of BNip3 heterozygous mice. This study also revealed that BNip3 protein expression was higher during the fasted state than basal levels. BNip3 protein levels were further induced following 24 h of fasting than 6 h of fasting. However, no change in nix protein levels were observed in the fasted state compared with their initial condition. Similar results were obtained by Jamart et al. 59 , who randomly allocated thirty‐six 12‐week‐old mice into four intervention groups: fed state and rested, fed state and exercised (90 min running with speed of 10 m/min), fasted state (9.5 h of food withdrawal), and rested (deprived of food during the dark cycle) or fasted state and exercised (running began 8 h after starvation). A biopsy of the gastrocnemius muscle of mice was used to assess biomarkers of mitophagy. According to result of this study, Drp1 mRNA decreased in fasted state, but it remains unchanged in exercise state. Binp3 protein level increased in fasted plus rest condition but decreased after exercise in the fasted mice group. BNip3 mRNA increased during the fasted state whether the mice underwent a rest or run session. Parkin protein levels were significantly increased in the fasted + rest group, but parkin mRNA remained unchanged in all intervention condition. Additionally, mitochondrial fusion was not affected by any intervention, as Mfn1 and Mfn2 levels remained unchanged across the groups.
Bujak et al. 60 examined the link between AMPK, autophagy, and fasting. AMPK is known to activate autophagy; however, the role of AMPK in muscle function during fasting periods has not been established. This study examined mice lacking skeletal muscle AMPK (AMPK‐MKO) which were either 2 (young) or 18 (aged) month old wild‐type mice. The mice were placed under either fasting conditions for 12, 24, or 48 h or fed AL before sacrificing, and muscle was removed for autophagy and mitophagy biomarker assessment. This study revealed that protein level and mRNA expression of autophagy adaptor protein, p62, was increased in fasted condition. Also Parkin protein level increased by more than two‐fold in AMPK‐MKO mice compared with wild‐type, regardless of the diet condition, demonstrating that AMPK in skeletal muscle is needed for induction of mitophagy and autophagy. Similar to Bujak's group, Oost et al. 61 also studied muscle function and mitophagy, but instead examined FGF21. This was done using a knockout muscle derived FGF21 knockout model, where mice were either fasted for 24 h or remained on a fed diet. Mitochondrially targeted mKeima plasmid (mt‐mKeima) was injected to assay mitophagy. In fasted wild‐type mice, the levels of FGF21 mRNA were significantly increased suggesting physiological stress as FGF21 typically has low expression in healthy muscle. Fasting FGF21 knockout mice presented with normal muscle histology and no muscle loss, similar to that of wild‐type fed mice demonstrating that the deletion protects against fasting‐induced muscle damage. The mt‐mKeima injection also revealed a decrease in mitophagy flux in FGF21 knockout mice in the fasting group. Bnip3 levels were reduced in the absence of FGF‐21 in both diet conditions, indicating that this protein is essential for FGF21‐mediated muscle wasting. This study was able to demonstrate a link between fasting and autophagy using FGF21.
Shirakabe et al. 62 also utilized mt‐mKeima transduction (adeno‐associated virus 9 vector) to assess mitochondrial autophagy in the heart. Keima targeted to mitochondria is a fluorescent protein that can emit several colour signals in different situation of pH; therefore, it can be used to assess mitophagy in cardiomyocytes when administered intravenously to mice. Mice were divided into two groups; a control group that was fed chow normally and a 48 h fasted group. Cardiac tissue was removed, and total DNA extract was used to assess mitochondrial DNA. The results demonstrated an increase in mitochondrial autophagy, as indicated by the presence of autophagosomes containing mitochondria in the 48 h fasted group compared with the fed group. The mitochondrial DNA content and COX1/GAPDH levels can be used as an indicator of mitochondrial mass were significantly lower in the fasted group compared with the control group. This paper further provides evidence that fasting can induce mitophagy and could provide be cardioprotective.
Dongil et al. 63 examined fasting and mitophagy using a PAS Domain Kinase (PASK) deficient model. This study recruited both wild type or PASK deficient male mice that were 12–20 weeks old. Mice were kept on an AL diet or fasted for 24–48 h, and liver samples were collected to assess the expression of mitophagy biomarkers. This study demonstrated that the mitochondrial DNA content of PASK‐deficient mice was higher than in wild type under fasted and fed conditions. Expression of Mnf1 and Opa1 genes that are related to mitochondrial fusion were higher in PASK‐deficient mice under non‐fasting conditions relative to wild‐type mice. PASK deficient mice also had greater protein levels and mRNA expression of genes involved in mitophagy (Fis1 and PINK1) compared with the wild‐type group in the 48 h fasted condition. Also, Binp3 mRNA increased in fasted state in PASK deficient mice and wild‐type mice. Genes involved in the expression of antioxidant enzymes (GPx, MnSOD, HO1, Cu/ZnSOD, and GCLm) were also overexpressed in the PASK deficient fasting group. Collectively, these findings demonstrate improved cell survival and mitophagy during fasting periods.
Muscle mass loss is considered normal with age; however, calorie restricted diets can slow aging and protect muscle. Therefore, Gutierrez‐Casado et al. 64 assessed CR and age on markers of mitophagy. Ten‐week‐old mice were divided into four groups: a control group and three CR groups with different sources of dietary fat (lard, fish oil, or soybean oil) for 6 or 18 months. After the interventions, a period of fasting was induced for 18 h followed by dissection of the hind limb muscle. Mitophagy markers were assessed using monoclonal and polyclonal sera raised against PINK1 and Parkin. Primary antibodies of Mfn1, Mfn2, OPA1 and Drp1 were used to detection the concentration of mitochondrial fusion and fission proteins. This study revealed that the CR with lard intervention group for 18 months produced the highest levels of PINK1 compared with 6 months intervention. Additionally, mice that were in the CR + lard group had a highest level of PINK1 compared with sunflower or fish oil CR. The soybean intervention group had a lower Parkin level compared with control group. The levels of Parkin were affected by age and dietary fat within the CR groups, among the three CR groups 18 months CR + lard group had the highest Parkin level. In the case of fusion/fission markers, Mfn1 was not affected by type of fat intervention and age. Mfn2 increased in 18 months sunflower CR group compared with control group. There was also an increasing linear trend for OPA1 levels seen for lard, sunflower, and fish CR group, respectively, after 18 month. According to result of this study, it can be considered that the lard intervention CR diet had the greatest protective effect on preventing muscle loss during aging.
Gubbiotti et al. 65 examined decorin, a secreted proteoglycan known to play a role in the mitophagy of breast carcinoma cells. Four‐month‐old to six‐month‐old male and female mice were placed on a fed diet or under fasting conditions for 25 h. Mice in the fasted condition had food withheld for 25 h, but they could consume water AL. Fasting resulted in a significant increase in cardiac decorin transcript levels. Additionally, immunoblotting of whole tissue extracts confirm that the fast state lead to increased decorin proteoglycan transcript and protein levels of cardiac decorin increased after fasting, indicating again that fasting can induce autophagy.
The previously mentioned studies all examined fasting on normal, healthy rodents; however, Sacks et al. 66 assess gastric bypass surgery in obese animals and the effect this has on mitochondrial dynamics. Rats (12 weeks of age) were placed on a high‐fat diet to establish obesity and then divided into sham surgery or ROX‐en‐Y gastric bypass surgery (RYGB). Gastric bypass procedure was performed after overnight fasting. Seven days post‐surgery rats were placed on an unrestricted liquid diet prior to returning to an AL high‐fat diet. Ninety days after surgery, all rat were euthanized, and livers were collected to evaluate biomarkers of mitophagy. This study showed that the mRNA and protein expression of Bnip3 was significantly higher in the RYGB group than sham group. Other gene markers for mitochondrial fusion (Mfn1, Mfn2, and OPA1) and biogenesis regulators (PGC1 α and Nrf1) were also increased in the RYGB rats compared to sham group. Nutrient deprivation also increases organelle recycling through autophagy as demonstrated by Kim and Lemasters. 67 Green fluorescent protein‐light chain 3 protein transgenic mice were used to determine hepatocyte mitochondrial turnover by mitophagy during nutrient deprivation. Green fluorescent protein‐light chain 3 protein fluorescence was incorporated in small patches in the vicinity of mitochondria representing pre‐autophagic structures. This study revealed that after 90 min of nutrient deprivation, pre‐autophagic structures grew into phagophores and then into autophagosomes that surrounded the mitochondria. Following this, degradation of the mitochondrial content occurred due to mitochondrial depolarization. These results indicate that nutrient deprivation was able to accelerate mitophagy. McWilliams et al. 68 assessed basal mitophagy in brain, heart, eye, spleen, liver, and kidney tissues with high rates of metabolic demand and assessed the contribution of PINK1 to mitophagy in above tissues. They also assessed the effect of fasted and non‐fasted condition on mitophagy in pancreas mouse model. Based on their results, high degree of mitophagy occurred in neural cells such as dopaminergic neurons and microglia. Also in all tissues except pancreatic islets, loss of PINK1 despite disrupting depolarization did not affect basal mitophagy, and there was no significant difference observed between fasted and non‐fasted conditions on the mitophagy process according to PINK1 level. Luevano‐Martinez et al. 69 examined the effect of CR on mitochondria membrane. For this reason, phospholipids from rat liver mitochondria isolated in CR and fed condition. In this study 8‐week‐old mice were divided in two groups: AL group, fed AIN‐93‐M diet and CR group, fed 40% less calorie than AL group. The amount of food given to CR group was adjusted weekly according to their weight. Intervention lasted for 4 months, and then, rats were sacrificed after 12 h of fasting, and the livers were extracted for assessment of study objectives. The result demonstrated that the expression of enzymes involved in biosynthesize and remodelling of cardiolipin (is a mitochondrial anionic phospholipid that acts as a signalling protein in mitophagy biosynthesize pathway) was increased in the CR group compared with the fed AL group. Also in CR group redistribution of cardiolipin phospholipid occurred when mitochondrial membranes were fractionated. Also, expression of Drp1 and Mfn2 that they were involved in fission and fusion of mitochondria increased in CR group compared with AL group. So CR lead to prompts mitochondrial membrane redistribution. Rambold et al. 70 used mouse embryonic fibroblasts to determine the tubular network formation during starvation to protect mitochondria degradation by autophagosomal. Mouse embryonic fibroblasts were transfected with mitochondrial matrix‐targeted YFP and starved for 2 and 6 h with different nutrient deprivation (Dulbecco's phosphate buffered saline contained 1 g/L glucose without serum, amino acid and glutamine; D‐GSG contained 1 g/L glucose and amino acid without serum and glutamine; and no Ser contained 4.5 g/L glucose + amino acid + Gln without serum) or 6 h (no glucose contained amino acid + serum + Gln without glucose, low glucose contained 1 g/L glucose + amino acid + Gln + serum, no amino acid contained 4.5 g/L glucose + serum + Gln without amino acid, no glutamine contained 4.5 g/L glucose + serum + amino acid without Gln and D‐GG contained 1 g/L glucose + serum + amino acid without Gln. Result of this study showed that nutrient deprivation protected the mitochondria from degradation by post‐translational modification of Drp1. Assessment of mitochondrial morphology with live imaging showed that additive nutrient deprivation led to induced mitochondrial tubulation. Mitochondrial tubulation increased significantly in Dulbecco's phosphate buffered saline, low glucose and no amino acid condition. Additionally, additive nutrient starvation (6 h compared with 2 h) produced more efficient mitochondrial tubulation. Mitochondrial tubulation ameliorates degradation of mitochondria by autophagosoma during nutrient deprivation.
Conclusions
Evidence regarding fasting or CR are limited and varied, such as the type of intervention, duration of intervention, target tissue, and target population (Table 1). Current evidence overwhelmingly suggests that CR and fasting induce mitophagy and mitophagy‐related markers. Based on the current evidence that we reviewed here, it could be concluded that fasting or CR has a promising role as a novel, practical approach without any side effects in the regulation of health by inducing mitochondria autophagy in different organs of body. More studies are required to clarify the relationship between food deprivation and mitophagy. Additionally, assessing different types of CR, such as restricting carbohydrate, fat, or protein, or different types of fasting such as intermittent, time‐restricted feeding, and alternative day fasting, should be used to determine the best approach in terms of mitophagy induction and health status. Additionally, it is important that further research is required to link the effect of CR and mitophagy on the progression of diseases, such as diabetes, neurodegenerative disease, and cancer.
Conflict of interest
The authors have no competing interests to declare.
Acknowledgements
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle. 71
Mehrabani S., Bagherniya M., Askari G., Read M. I., and Sahebkar A. (2020) The effect of fasting or calorie restriction on mitophagy induction: a literature review, Journal of Cachexia, Sarcopenia and Muscle, 11, 1447–1458, 10.1002/jcsm.12611
References
- 1. Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 2007;8:870–879. [DOI] [PubMed] [Google Scholar]
- 2. Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev 2008;22:1577–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS‐induced ROS release. Physiol Rev 2014;94:909–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas RL, Gustafsson AB. Mitochondrial autophagy—an essential quality control mechanism for myocardial homeostasis. Circ J 2013;77:2449–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pfeifer U. Inhibition by insulin of the formation of autophagic vacuoles in rat liver. A morphometric approach to the kinetics of intracellular degradation by autophagy. J Cell Biol 1978;78:152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suen DF, Narendra DP, Tanaka A, Manfredi G, Youle RJ. Parkin overexpression selects against a deleterious mtDNA mutation in heteroplasmic cybrid cells. Proc Natl Acad Sci U S A 2010;107:11835–11840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhatia‐Kissova I, Camougrand N. Mitophagy in yeast: actors and physiological roles. FEMS Yeast Res 2010;10:1023–1034. [DOI] [PubMed] [Google Scholar]
- 8. Kanki T, Klionsky DJ. The molecular mechanism of mitochondria autophagy in yeast. Mol Microbiol 2010;75:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richard VR, Leonov A, Beach A, Burstein MT, Koupaki O, Gomez‐Perez A, et al. Macromitophagy is a longevity assurance process that in chronologically aging yeast limited in calorie supply sustains functional mitochondria and maintains cellular lipid homeostasis. Aging 2013;5:234–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res 2005;8:3–5. [DOI] [PubMed] [Google Scholar]
- 11. Tal R, Winter G, Ecker N, Klionsky DJ, Abeliovich H. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. J Biol Chem 2007;282:5617–5624. [DOI] [PubMed] [Google Scholar]
- 12. Nowikovsky K, Reipert S, Devenish RJ, Schweyen RJ. Mdm38 protein depletion causes loss of mitochondrial K+/H+ exchange activity, osmotic swelling and mitophagy. Cell Death Differ 2007;14:1647–1656. [DOI] [PubMed] [Google Scholar]
- 13. Okamoto K, Kondo‐Okamoto N, Ohsumi Y. Mitochondria‐anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev Cell 2009;17:87–97. [DOI] [PubMed] [Google Scholar]
- 14. Aoki Y, Kanki T, Hirota Y, Kurihara Y, Saigusa T, Uchiumi T, et al. Phosphorylation of Serine 114 on Atg32 mediates mitophagy. Mol Biol Cell 2011;22:3206–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mao K, Wang K, Zhao M, Xu T, Klionsky DJ. Two MAPK‐signaling pathways are required for mitophagy in Saccharomyces cerevisiae . J Cell Biol 2011;193:755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Springer MZ, Macleod KF. In brief: mitophagy: mechanisms and role in human disease. J Pathol 2016;240:253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Novak I. Mitophagy: a complex mechanism of mitochondrial removal. Antioxid Redox Signal 2012;17:794–802. [DOI] [PubMed] [Google Scholar]
- 18. Gouspillou G, Sgarioto N, Norris B, Barbat‐Artigas S, Aubertin‐Leheudre M, Morais JA, et al. The relationship between muscle fiber type‐specific PGC‐1alpha content and mitochondrial content varies between rodent models and humans. PLoS One. 2014;9:e103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 2012;393:547–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamacher‐Brady A, Brady NR. Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci 2016;73:775–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palikaras K, Tavernarakis N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol 2014;56:182–188. [DOI] [PubMed] [Google Scholar]
- 22. Ney PA. Mitochondrial autophagy: origins, significance, and role of BNIP3 and NIX. Biochim Biophys Acta 2015;1853:2775–2783. [DOI] [PubMed] [Google Scholar]
- 23. Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, et al. Mitochondrial outer‐membrane protein FUNDC1 mediates hypoxia‐induced mitophagy in mammalian cells. Nat Cell Biol 2012;14:177–185. [DOI] [PubMed] [Google Scholar]
- 24. Brandhorst S, Harputlugil E, Mitchell JR, Longo VD. Protective effects of short‐term dietary restriction in surgical stress and chemotherapy. Ageing Res Rev 2017;39:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madia F, Wei M, Yuan V, Hu J, Gattazzo C, Pham P, et al. Oncogene homologue Sch9 promotes age‐dependent mutations by a superoxide and Rev1/Polζ‐dependent mechanism. J Cell Biol 2009;186:509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science 2010;328:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science 2003;299:1342–1346. [DOI] [PubMed] [Google Scholar]
- 28. Kenyon C. A conserved regulatory system for aging. Cell 2001;105:165–168. [DOI] [PubMed] [Google Scholar]
- 29. Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature 2000;408:255–262. [DOI] [PubMed] [Google Scholar]
- 30. Price JC, Khambatta CF, Li KW, Bruss MD, Shankaran M, Dalidd M, et al. The effect of long term calorie restriction on in vivo hepatic proteostatis: a novel combination of dynamic and quantitative proteomics. Mol Cell Proteomics 2012;11:1801–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caro P, Gomez J, Lopez‐Torres M, Sanchez I, Naudi A, Jove M, et al. Forty percent and eighty percent methionine restriction decrease mitochondrial ROS generation and oxidative stress in rat liver. Biogerontology 2008;9:183–196. [DOI] [PubMed] [Google Scholar]
- 32. Soare A, Cangemi R, Omodei D, Holloszy JO, Fontana L. Long‐term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging (Albany NY) 2011;3:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bruss MD, Thompson AC, Aggarwal I, Khambatta CF, Hellerstein MK. The effects of physiological adaptations to calorie restriction on global cell proliferation rates. Am J Physiol Endocrinol Metab 2011;300:E735–E745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shaddock JG, Chou MW, Casciano DA. Effects of age and caloric restriction on cell proliferation in hepatocyte cultures from control and hepatectomized Fischer 344 rats. Mutagenesis 1996;11:281–284. [DOI] [PubMed] [Google Scholar]
- 35. Picca A, Pesce V, Lezza AMS. Does eating less make you live longer and better? An update on calorie restriction Clinical interventions in aging 2017;12:1887–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. López‐Lluch G, Navas P. Calorie restriction as an intervention in ageing. J Physiol 2016;594:2043–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boya P, Gonzalez‐Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 2005;25:1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 2005;6:298–305. [DOI] [PubMed] [Google Scholar]
- 39. Fontana L, Meyer TE, Klein S, Holloszy JO. Long‐term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci 2004;101:6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brandhorst S, Longo VD. Fasting and caloric restriction in cancer prevention and treatment. Metabolism in Cancer: Springer 2016;241–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev 2017;39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chaix A, Zarrinpar A, Miu P, Panda S. Time‐restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 2014;20:991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cherif A, Roelands B, Meeusen R, Chamari K. Effects of intermittent fasting, caloric restriction, and Ramadan intermittent fasting on cognitive performance at rest and during exercise in adults. Sports Med 2016;46:35–47. [DOI] [PubMed] [Google Scholar]
- 44. Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab 2014;19:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Castrogiovanni P, Volti GL, Sanfilippo C, Tibullo D, Galvano F, Vecchio M, et al. Fasting and fast food diet play an opposite role in mice brain aging. Mol Neurobiol 2018;1–13. [DOI] [PubMed] [Google Scholar]
- 46. Longo VD, Antebi A, Bartke A, Barzilai N, Brown‐Borg HM, Caruso C, et al. Interventions to slow aging in humans: are we ready? Aging Cell 2015;14:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Madeo F, Zimmermann A, Maiuri MC, Kroemer G. Essential role for autophagy in life span extension. J Clin Invest 2015;125:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol 2010;12:842–846. [DOI] [PubMed] [Google Scholar]
- 49. Petrovski G, Das DK. Does autophagy take a front seat in lifespan extension? J Cell Mol Med 2010;14:2543–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bagherniya M, Butler AE, Barreto GE, Sahebkar A. The effect of fasting or calorie restriction on autophagy induction: a review of the literature. Ageing Res Rev 2018;47:183–197. [DOI] [PubMed] [Google Scholar]
- 51. Cui J, Shi S, Sun X, Cai G, Cui S, Hong Q, et al. Mitochondrial autophagy involving renal injury and aging is modulated by caloric intake in aged rat kidneys. PLoS One 2013;8:e69720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao Y, Zhu Q, Song W, Gao B. Exercise training and dietary restriction affect PINK1/Parkin and Bnip3/Nix‐mediated cardiac mitophagy in mice. Gen Physiol Biophys 2018;37:657–666. [DOI] [PubMed] [Google Scholar]
- 53. Kanki T, Wang K, Cao Y, Baba M, Klionsky DJ. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev Cell 2009;17:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Richard VR, Leonov A, Beach A, Burstein MT, Koupaki O, Gomez‐Perez A, et al. Macromitophagy is a longevity assurance process that in chronologically aging yeast limited in calorie supply sustains functional mitochondria and maintains cellular lipid homeostasis. Aging (Albany NY) 2013;5:234–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tarpey MD, Davy KP, McMillan RP, Bowser SM, Halliday TM, Boutagy NE, et al. Skeletal muscle autophagy and mitophagy in endurance‐trained runners before and after a high‐fat meal. Molecular metabolism 2017;6:1597–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schwalm C, Deldicque L, Francaux M. Lack of activation of mitophagy during endurance exercise in human. Med Sci Sports Exerc 2017;49:1552–1561. [DOI] [PubMed] [Google Scholar]
- 57. Linden MA, Lopez KT, Fletcher JA, Morris EM, Meers GM, Siddique S, et al. Combining metformin therapy with caloric restriction for the management of type 2 diabetes and nonalcoholic fatty liver disease in obese rats. Appl Physiol Nutr Metab 2015;40:1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Glick D, Zhang W, Beaton M, Marsboom G, Gruber M, Simon MC, et al. BNip3 regulates mitochondrial function and lipid metabolism in the liver. Mol Cell Biol 2012;32:2570–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jamart C, Naslain D, Gilson H, Francaux M. Higher activation of autophagy in skeletal muscle of mice during endurance exercise in the fasted state. Am J Physiol Endocrinol Metab 2013;305:E964–E974. [DOI] [PubMed] [Google Scholar]
- 60. Bujak AL, Crane JD, Lally JS, Ford RJ, Kang SJ, Rebalka IA, et al. AMPK activation of muscle autophagy prevents fasting‐induced hypoglycemia and myopathy during aging. Cell Metab 2015;21:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Oost LJ, Kustermann M, Armani A, Blaauw B, Romanello V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J Cachexia Sarcopenia Muscle 2019;10:630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shirakabe A, Fritzky L, Saito T, Zhai P, Miyamoto S, Gustafsson AB, et al. Evaluating mitochondrial autophagy in the mouse heart. J Mol Cell Cardiol 2016;92:134–139. [DOI] [PubMed] [Google Scholar]
- 63. Dongil P, Perez‐Garcia A, Hurtado‐Carneiro V, Herrero‐de‐Dios C, Blazquez E, Alvarez E, et al. Pas kinase deficiency triggers antioxidant mechanisms in the liver. Sci Rep 2018;8:13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gutierrez‐Casado E, Khraiwesh H, Lopez‐Dominguez JA, Montero‐Guisado J, Lopez‐Lluch G, Navas P, et al. The impact of aging, calorie restriction and dietary fat on autophagy markers and mitochondrial ultrastructure and dynamics in mouse skeletal muscle. J Gerontol A Biol Sci Med Sci 2019;74:760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gubbiotti MA, Neill T, Frey H, Schaefer L, Iozzo RV. Decorin is an autophagy‐inducible proteoglycan and is required for proper in vivo autophagy. Matrix Biol 2015;48:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sacks J, Mulya A, Fealy CE, Huang H, Mosinski JD, Pagadala MR, et al. Effect of Roux‐en‐Y gastric bypass on liver mitochondrial dynamics in a rat model of obesity. Physiol Rep 2018;6:e13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim I, Lemasters JJ. Mitochondrial degradation by autophagy (mitophagy) in GFP‐LC3 transgenic hepatocytes during nutrient deprivation. Am J Physiol Cell Physiol 2011;300:C308–C317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McWilliams TG, Prescott AR, Montava‐Garriga L, Ball G, Singh F, Barini E, et al. Basal mitophagy occurs independently of PINK1 in mouse tissues of high metabolic demand. Cell Metabolism 2018;27:439–49.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Luevano‐Martinez LA, Forni MF, Peloggia J, Watanabe IS, Kowaltowski AJ. Calorie restriction promotes cardiolipin biosynthesis and distribution between mitochondrial membranes. Mech Ageing Dev 2017;162:9–17. [DOI] [PubMed] [Google Scholar]
- 70. Rambold AS, Kostelecky B, Elia N, Lippincott‐Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A 2011;108:10190–10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


