Abstract
Objectives
Concerns have been expressed that some drugs may increase susceptibility to SARS-CoV-2 infection. In contrast, other drugs have generated interest as potential therapeutic agents.
Methods
All adults aged ≥18 years who were tested for COVID-19 were included. Exposure was defined as a prescription of study drugs which would have been continued until 7 days prior to test for COVID-19 or later. The outcome measures were the diagnosis of COVID-19 and severe COVID-19. Disease risk score matching and multiple logistic regression was used.
Results
Matched claims and testing results were available for 219,961 subjects, of whom 7,341 (3.34%) were diagnosed with COVID-19. Patients were matched to 36,705 controls, and the subset of 878 patients of severe COVID-19 also matched with 1,927 mild-to-moderate patients. Angiotensin receptor blockers were not associated with either the diagnosis of COVID-19 (adjusted OR [aOR], 1.02; 95% confidence interval [CI], 0.90–1.15) or severe disease (aOR, 1.11; 95% CI, 0.87–1.42). The use of hydroxychloroquine was not associated with a lower risk for COVID-19 (aOR, 0.94; 95% CI, 0.53–1.66) or severe disease (aOR, 3.51; 95% CI, 0.76–16.22).
Conclusions
In this national claims data-based case-control study, no commonly prescribed medications were associated with risk of COVID-19 infection or COVID-19 severity.
Keywords: COVID-19, Disease risk score, Prophylaxis, South Korea, Treatment
Introduction
Coronavirus disease (COVID-19) is a novel infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that has spread worldwide since the first reported case in late 2019. Host cell entry of SARS-CoV-2 requires angiotensin-converting enzyme 2 (ACE2) (Hoffmann et al., 2020). The action of ACE2 is not directly affected by ACE inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), although these drugs reportedly lead to increased expression of ACE2 in various tissues (Ferrario et al., 2005, Ferrario and Varagic, 2010). Thus, there is controversy regarding whether treatment using ACEIs or ARBs might increase the host’s susceptibility to COVID-19.
Most patients with COVID-19 have a mild course of the disease; however, respiratory failure and death are more common among older people and those with underlying conditions (Onder et al., 2020, Richardson et al., 2020, Wu and McGoogan, 2020). Thus, there is significant interest in repurposing medications that are used for other indications (Sanders et al., 2020). Hydroxychloroquine (HCQ) and lopinavir/ritonavir were drugs with promising results from preclinical studies; however, randomized controlled trials showed no benefit of these agents (Horby et al., 2020, Pan et al., 2020). Other agents have reportedly been effective in vitro against other coronaviruses, specifically those that cause severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS-CoV) (Chan et al., 2013, Hoffmann et al., 2020). However, there is no high-quality clinical evidence to support the use of these agents for treating COVID-19.
The possible association of commonly used drugs with the risk of COVID-19 and with its severity level is a particularly important issue. However, it is virtually impossible to design a prospective clinical trial on medications taken before a diagnosis of COVID-19. Conversely, results from randomized clinical trials using drugs with potential therapeutic effects are being published (Beigel et al., 2020, Pan et al., 2020). Still, most of these trials were designed to study the effect of the drugs after a diagnosis, and their effectiveness as prophylaxis or early treatment is yet to be tested thoroughly. Therefore, controversy persists regarding the possible benefits or harm that may be associated with the use of various drugs for patients with COVID-19.
In the absence of prospective clinical data, carefully curated and reliable data from a large cohort may be a useful alternative. Thus, we used nationwide medical insurance claims data and records of confirmed COVID-19 patients to evaluate the relationships between common medications prescribed prior to COVID-19 testing and the risk of and severity of COVID-19 in South Korea.
Materials and methods
Study design and data sources
We conducted a retrospective case-control study using information extracted from two national Korean databases. The Korean Health Insurance Review & Assessment Service (HIRA) is a quasi-governmental agency that reviews all claims made to the National Health Insurance Service, which is the universal single payer for healthcare in Korea. All reimbursement claims for COVID-19 tests in suspected cases are sent for review by the HIRA (Supplementary Figure S1). Claims for COVID-19 tests are made using a special “public crisis” code (MT043) and can thus identify all individuals tested for COVID-19 in Korea. Eligible subjects were all individuals ≥18 years old with an MT043 code. Patients were identified by matching this list with data from the Korea Centers for Disease Control and Prevention (KCDC) registry, which records all confirmed patients of COVID-19 in Korea. The study protocol was approved by the institutional review board of the Gil Medical Center, Gachon University College of Medicine and Science (GFIRB2020-118), with a waiver of consent. This study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline (von Elm et al., 2007).
Definitions and outcome measures
Exposure was defined as the prescription of medications that was continued up to 7 days or less before testing for COVID-19. The maximum interval of 7 days between the prescription ending and COVID-19 testing was selected to account for the incubation period and delays in diagnosis. Drugs of interest were selected from a list of pharmaceutical agents with reported inhibitory effects against SARS-CoV-2 infection in preclinical studies, as well as agents with theoretical concerns regarding an increased risk of COVID-19 (Supplementary Table S1). Two authors (KH and WJ) reviewed the literature and selected the drugs of interest; any disagreement was arbitrated by a third author (JJ).
Outcome measures were (1) the diagnosis of COVID-19 among all tested individuals and (2) severe disease among the patients diagnosed with COVID-19. All patients with COVID-19 were diagnosed through detection of SARS-CoV-2 from nasopharyngeal swabs or sputa using reverse transcription polymerase chain reaction, as outlined in national guidelines (Korea Centers for Disease Control and Prevention, 2020). Severe disease was defined as the requirement of any of the following during hospitalization or prior to death: supplementary oxygen, high-flow nasal cannula, non-invasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation.
Comorbidities were categorized into disease groups (Supplementary Table S2) and identified using ICD-10 codes that were entered in the subject’s record at least twice within 1 year before the COVID-19 tests. The list of comorbidities and their corresponding ICD-10 codes were determined before data extraction. The Charlson comorbidity index (CCI) was calculated according to standard methods (Charlson et al., 1987). Healthcare utilization was evaluated based on the numbers of claims for hospitalizations, outpatient visits, and emergency room visits within 1 year before the tests for COVID-19.
Statistical analysis
As the likelihoods of being tested for COVID-19, of testing positive, and of undergoing a severe clinical course are all affected by demographic characteristics and comorbidities, we used disease risk score (DRS) matching to control for discrepancies of baseline characteristics. DRS models were constructed to estimate the propensity for outcome measures with age, sex, coverage for low household income, CCI, and comorbidities (diabetes, hypertension, asthma/allergic rhinitis, chronic heart diseases, chronic lung diseases, malignancy, chronic kidney diseases, and chronic neurologic diseases) as covariates. Each case of COVID-19 was matched with up to five controls by greedy matching algorithms; each case of severe COVID-19 was matched with up to three controls with mild-to-moderate COVID-19 (Stuart, 2010).
A sensitivity analysis was conducted with the prescription of medications that was continued up to 14 days or less, instead of 7 days or less, before COVID-19 testing as exposure.
The baseline demographic characteristics and comorbidities of patients and controls were compared using the χ2 test, Fisher’s exact test, or Student’s t test, as appropriate. Exposures to the drugs of interest were compared between DRS-matched groups using multivariable logistic regression models with sex, age, region of residence, comorbidities, healthcare utilization, and other drugs of interest as covariates. All tests were two-tailed, and results were considered statistically significant at P values <0.05. All analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA).
Results
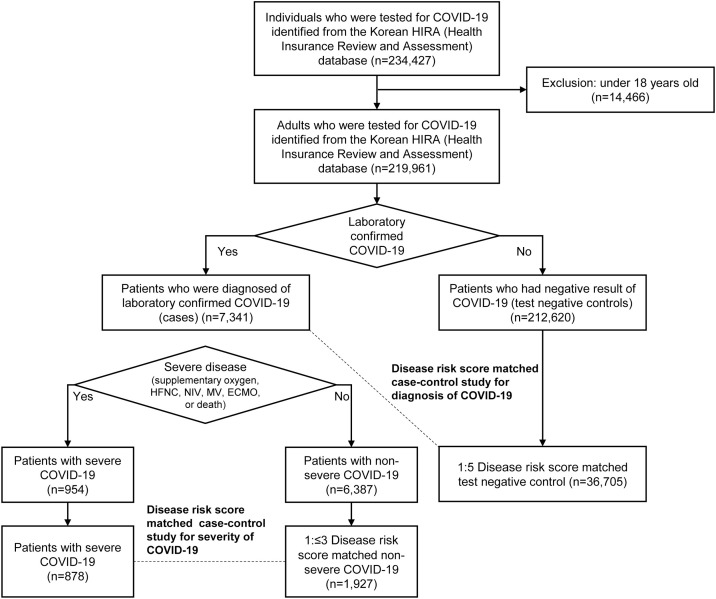
We identified 219,961 patients who had been tested for COVID-19, including 7,341 patients (3.34%) who were subsequently diagnosed with COVID-19 (Figure 1 ). The mean age of patients was 49.4 years (range, 18–116 years); 47.4% were male (Table 1 ). The most common comorbidities were hypertension (29.8%), chronic lung diseases (28.6%), chronic liver diseases (25.9%), and diabetes (20.9%).
Figure 1.
Flowchart of study design and subjects.
Table 1.
Baseline characteristics of subjects by diagnosis of COVID-19 and by severity of disease course. Severe disease was defined as the requirement of any one of the following: supplementary oxygen, high-flow nasal cannula, non-invasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation.
| Characteristic | By diagnosis of COVID-19 among tested individuals |
By severity among patients with COVID-19 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall |
Disease risk score matched |
Overall |
Disease risk score matched |
|||||||||
| Case (N = 7341) |
Control (N = 212620) |
P | Case (N = 7341) |
Control (N = 36705) |
P | Severe (N = 954) |
Mild-moderate (N = 6387) |
P | Severe (N = 878) |
Mild-moderate (N = 1927) |
P | |
| Demographic information | ||||||||||||
| Age, mean (SD), y* | 47.1 (19.0) | 49.5 (19.9) | <0.001 | 47.1 (19.0) | 47.3 (19.7) | 0.3786 | 67.0 (15.1) | 44.1 (17.7) | <0.001 | 65.5 (14.7) | 61.0 (14.1) | <0.001 |
| Sex* | ||||||||||||
| Male | 2970 (40.46) | 101361 (47.67) | <0.001 | 2970 (40.46) | 14882 (40.54) | 0.8895 | 458 (48.01) | 2512 (39.33) | <0.001 | 403 (45.90) | 879 (45.61) | 0.89 |
| Female | 2202 (59.54) | 111259 (52.33) | 4371 (59.54) | 21823 (59.46) | 496 (51.99) | 3875 (60.67) | 475 (54.10) | 1048 (54.39) | ||||
| Insurance coverage for low-income household* | 619 (8.43) | 12031 (5.66) | <0.001 | 619 (8.43) | 3034 (8.27) | 0.6374 | 120 (12.58) | 499 (7.81) | <0.001 | 114 (12.98) | 211 (10.95) | 0.12 |
| Comorbidities | ||||||||||||
| Charlson comorbidity index, mean (SD)* | 1.23 (1.65) | 1.94 (2.36) | <0.001 | 1.23 (1.65) | 1.21 (1.65) | 0.3431 | 2.68 (2.19) | 1.01 (1.43) | <0.001 | 2.72 (2.22) | 1.93 (1.78) | <0.001 |
| Diabetes* | 1179 (16.06) | 44684 (21.02) | <0.001 | 1179 (16.06) | 5673 (15.46) | 0.1918 | 370 (38.78) | 809 (12.67) | <0.001 | 354 (40.32) | 571 (29.63) | <0.001 |
| Hypertension* | 1576 (21.47) | 63868 (30.04) | <0.001 | 1576 (21.47) | 7973 (21.72) | 0.6306 | 506 (53.04) | 1070 (16.75) | <0.001 | 466 (53.08) | 755 (39.18) | <0.001 |
| Chronic heart disease* | 575 (7.83) | 31882 (14.99) | <0.001 | 575 (7.83) | 2661 (7.25) | 0.0805 | 220 (23.06) | 355 (5.56) | <0.001 | 198 (22.55) | 265 (13.75) | <0.001 |
| Chronic lung disease* | 1464 (19.94) | 61506 (28.93) | <0.001 | 1464 (19.94) | 7729 (21.06) | 0.032 | 314 (32.91) | 1150 (18.01) | <0.001 | 292 (33.26) | 477 (24.75) | <0.001 |
| Asthma and allergic rhinitis* | 4342 (59.15) | 133618 (62.84) | <0.001 | 4342 (59.15) | 21945 (59.79) | 0.3073 | 570 (59.75) | 3772 (59.06) | 0.69 | 537 (61.16) | 1146 (59.47) | 0.40 |
| Chronic liver disease | 1519 (20.69) | 55485 (26.10) | <0.001 | 1519 (20.69) | 7215 (19.66) | 0.0423 | 334 (35.01) | 1185 (18.55) | <0.001 | 319 (36.33) | 629 (32.64) | 0.06 |
| Chronic kidney disease* | 202 (2.75) | 15597 (7.34) | <0.001 | 202 (2.75) | 915 (2.49) | 0.1979 | 72 (7.55) | 130 (2.04) | <0.001 | 70 (7.97) | 82 (4.26) | <0.001 |
| Malignancy* | 320 (4.36) | 23540 (11.07) | <0.001 | 320 (4.36) | 1654 (4.51) | 0.5781 | 87 (9.12) | 233 (3.65) | <0.001 | 82 (9.34) | 132 (6.85) | 0.02 |
| RA, SLE, GCA, and JIA | 214 (2.92) | 7729 (3.614) | 0.0012 | 214 (2.92) | 993 (2.71) | 0.3149 | 41 (4.30) | 173 (2.71) | 0.007 | 41 (4.67) | 94 (4.88) | 0.81 |
| Other connective tissue disease | 22 (0.30) | 1242 (0.58) | 0.0015 | 22 (0.30) | 171 (0.47) | 0.0491 | 5 (0.52) | 17 (0.27) | <0.001 | 5 (0.57) | 6 (0.31) | 0.34 |
| Chronic neurologic disease* | 1001 (13.64) | 39495 (18.58) | <0.001 | 1001 (13.64) | 4834 (13.17) | 0.2824 | 361 (37.84) | 640 (10.02) | <0.001 | 315 (35.88) | 461 (23.92) | <0.001 |
| Pancreatitis | 120 (1.63) | 9518 (4.48) | <0.001 | 120 (1.63) | 1068 (2.91) | <0.001 | 30 (3.14) | 90 (1.41) | <0.001 | 28 (3.19) | 45 (2.34) | 0.19 |
| Healthcare utilization | ||||||||||||
| Number of hospitalizations, mean (SD) | 0.25 (0.90) | 0.83 (2.14) | <0.001 | 0.25 (0.90) | 0.48 (1.45) | <0.001 | 0.67 (1.48) | 0.19 (0.76) | <0.001 | 0.67 (1.51) | 0.37 (1.18) | <0.001 |
| Number of outpatient visit, mean (SD) | 17.19 (21.38) | 25.46 (31.94) | <0.001 | 17.19 (21.38) | 20.59 (25.37) | <0.001 | 29.44 (35.33) | 15.36 (17.70) | <0.001 | 29.89 (36.24) | 23.70 (22.54) | <0.001 |
| Number of ED visit, mean (SD) | 0.12 (0.47) | 0.44 (1.56) | <0.001 | 0.12 (0.47) | 0.30 (1.52) | <0.001 | 0.25 (0.70) | 0.10 (0.42) | <0.001 | 0.25 (0.67) | 0.13 (0.46) | <0.001 |
Abbreviations: COVID-19, coronavirus disease 2019; SD, standard deviation; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; GCA, giant cell arteritis; JIA, juvenile idiopathic arthritis; ED, emergency department.
Variables used for the calculation of disease risk score.
Relative to the controls, patients had a significantly lower mean age (47.1 years vs. 49.5 years, p < 0.001) and were significantly less likely to be male (40.46% male vs. 47.67% female, p < 0.001). The controls had more comorbidities and a higher mean CCI (1.94 vs. 1.23, p < 0.001), as well as more frequent hospitalizations, outpatient visits, and emergency department visits. Among patients diagnosed with COVID-19, 954 (13.0%) patients were categorized as having severe disease. Patients with severe COVID-19 were older and had more comorbidities and higher healthcare utilization. Through DRS matching, patients were matched with 36,705 controls; the 878 severe patients were matched to 1,927 mild-moderate patients. Differences in characteristics among each group were substantially reduced after DRS matching.
Drugs commonly used for comorbidities
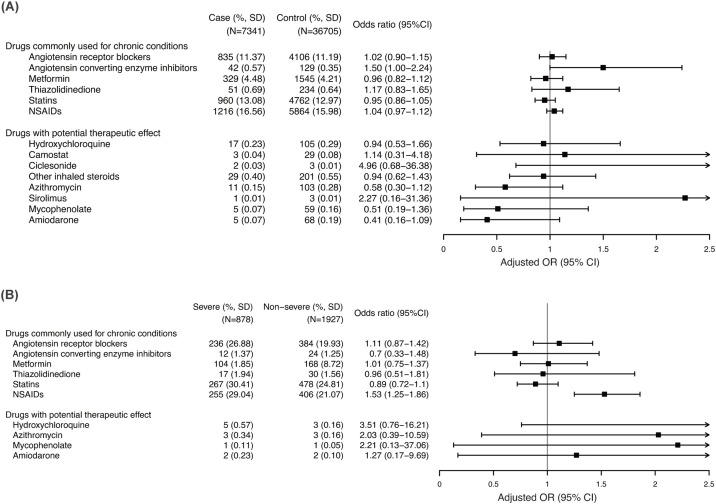
Angiotensin receptor blockers (ARBs) were not associated with either the diagnosis of COVID-19 (adjusted OR [aOR], 1.02; 95% confidence interval [CI], 0.90–1.15; P = 0.75) or severe disease (aOR, 1.11; 95% CI, 0.87–1.42; P = 0.38; Figure 2 A and Table 2 ). Angiotensin-converting enzyme inhibitors (ACEIs) showed a marginal association with COVID-19 (aOR, 1.50; 95% CI, 1.00–2.24; P = 0.05) but were not associated with severe disease (aOR, 0.70; 95% CI, 0.33–1.48; P = 0.35). Use of statins was not associated with the risk of COVID-19 (aOR, 0.95; 95% CI, 0.86–1.05; P = 0.28) or severe disease (aOR, 0.89; 95% CI, 0.72–1.10; P = 0.30). Similarly, no association was observed with the use of metformin and thiazolidinedione. We examined the effect of the combination of aforementioned drugs on the diagnosis of COVID-19 (Supplementary Table S3). No significant associations were observed with any of the combinations.
Figure 2.
Association of previously administered medications with the risk of COVID-19 and severity.
(A) Risk of COVID-19 among tested individuals. (B) Risk of severe disease among patients with COVID-19. Severe disease was defined as the requirement of any one of the following or death: supplementary oxygen, high-flow nasal cannula, non-invasive ventilation, mechanical ventilation, and extracorporeal membrane oxygenation.
Table 2.
Association of previously administered medications with the risk of COVID-19 among tested individuals.
| Drug | Case (N = 7341) |
Control (N = 36705) |
Crude |
Adjusted |
||
|---|---|---|---|---|---|---|
| OR (95% CI) |
P | OR (95% CI) |
P | |||
| Drugs commonly used for chronic conditions | ||||||
| Angiotensin receptor blockers | 835 (11.37) | 4106 (11.19) | 1.02 (0.94–1.10) | 0.64 | 1.02 (0.90–1.15) | 0.75 |
| Angiotensin-converting enzyme inhibitors | 42 (0.57) | 129 (0.35) | 1.63 (1.15–2.32) | 0.006 | 1.50 (1.00–2.24) | 0.05 |
| Metformin | 329 (4.48) | 1545 (4.21) | 1.07 (0.95–1.21) | 0.29 | 0.96 (0.82–1.12) | 0.58 |
| Thiazolidinedione | 51 (0.69) | 234 (0.64) | 1.09 (0.81–1.48) | 0.58 | 1.17 (0.83–1.65) | 0.36 |
| Statins | 960 (13.08) | 4762 (12.97) | 1.01 (0.94–1.09) | 0.81 | 0.95 (0.86–1.05) | 0.28 |
| NSAIDs | 1216 (16.56) | 5864 (15.98) | 1.05 (0.98–1.12) | 0.26 | 1.04 (0.97–1.12) | 0.30 |
| Drugs with potential therapeutic effect | ||||||
| Hydroxychloroquine | 17 (0.23) | 105 (0.29) | 0.81 (0.48–1.35) | 0.42 | 0.94 (0.53–1.66) | 0.82 |
| Camostat | 3 (0.04) | 29 (0.08) | 0.52 (0.16–1.70) | 0.28 | 1.14 (0.31–4.18) | 0.84 |
| Ciclesonide | 2 (0.03) | 3 (0.01) | 3.35 (0.56–20.04) | 0.19 | 4.96 (0.68–36.39) | 0.12 |
| Other inhaled steroids | 29 (0.40) | 201 (0.55) | 0.72 (0.49–1.06) | 0.09 | 0.94 (0.62–1.43) | 0.76 |
| Azithromycin | 11 (0.15) | 103 (0.28) | 0.53 (0.29–0.99) | 0.03 | 0.58 (0.30–1.12) | 0.10 |
| Sirolimus | 1 (0.01) | 3 (0.01) | 1.67 (0.17–16.04) | 0.66 | 2.27 (0.16–31.36) | 0.54 |
| Mycophenolate | 5 (0.07) | 59 (0.16) | 0.42 (0.17–1.06) | 0.07 | 0.51 (0.19–1.36) | 0.18 |
| Amiodarone | 5 (0.07) | 68 (0.19) | 0.37 (0.15–0.91) | 0.03 | 0.41 (0.16–1.09) | 0.07 |
| Demographic | ||||||
| Male sex | 2970 (40.46) | 14882 (40.54) | 1.00 (0.95–1.05) | 0.89 | 0.97 (0.91–1.02) | 0.22 |
| Age, mean (SD), y | 47.05 (18.99) | 47.27 (19.65) | 0.38 | 1.00 (0.99–1.00) | <0.001 | |
| Daegu/Gyeongsangbuk–do | 4027 (54.86) | 5601 (15.26) | 6.75 (6.39–7.12) | <0.001 | 6.77 (6.41–7.16) | <0.001 |
| Coverage for low–income households | 619 (8.43) | 3034 (8.27) | 1.02 (0.93–1.12) | 0.64 | 1.09 (0.98–1.21) | 0.11 |
| Comorbidities | ||||||
| Charlson comorbidity index, mean (SD) | 1.23 (1.65) | 1.21 (1.65) | 0.34 | |||
| Diabetes | 1179 (16.06) | 5673 (15.46) | 1.05 (0.98–1.12) | 0.19 | 1.16 (1.04–1.28) | 0.005 |
| Hypertension | 1576 (21.47) | 7973 (21.72) | 0.99 (0.93–1.05) | 0.63 | 1.05 (0.95–1.17) | 0.35 |
| Chronic heart disease | 575 (7.83) | 2661 (7.25) | 1.09 (0.99–1.19) | 0.08 | 1.36 (1.21–1.53) | <0.001 |
| Chronic lung disease | 1464 (19.94) | 7729 (21.06) | 0.93 (0.88–0.99) | 0.03 | 1.07 (1.00–1.16) | 0.05 |
| Asthma and allergic rhinitis | 4342 (59.15) | 21945 (59.79) | 0.97 (0.93–1.03) | 0.31 | 1.06 (1.00–1.13) | 0.04 |
| Chronic liver disease | 1519 (20.69) | 7215 (19.66) | 1.07 (1.00–1.14) | 0.04 | 1.13 (1.04–1.21) | 0.003 |
| Chronic kidney disease | 202 (2.75) | 915 (2.49) | 1.11 (0.95–1.29) | 0.20 | 1.40 (1.17–1.67) | <0.001 |
| Malignancy | 320 (4.36) | 1654 (4.51) | 0.97 (0.86–1.09) | 0.58 | 1.46 (1.27–1.69) | <0.001 |
| RA, SLE, GCA, and JIA | 214 (2.92) | 993 (2.71) | 1.08 (0.93–1.25) | 0.32 | 1.19 (1.00–1.42) | 0.05 |
| Other connective tissue disease | 22 (0.30) | 171 (0.47) | 0.64 (0.41–1.00) | 0.05 | 0.78 (0.48–1.26) | 0.31 |
| Chronic neurologic disease | 1001 (13.64) | 4834 (13.17) | 1.04 (0.97–1.12) | 0.28 | 1.14 (1.04–1.25) | 0.007 |
| Pancreatitis | 120 (1.63) | 1068 (2.91) | 0.56 (0.46–0.67) | <0.001 | 0.74 (0.60–0.91) | 0.005 |
| Healthcare utilization | ||||||
| Number of hospitalizations, mean (SD) | 0.25 (0.90) | 0.48 (1.45) | <0.001 | 0.892 (0.86–0.93) | <0.001 | |
| Number of outpatient visit, mean (SD) | 17.19 (21.38) | 20.59 (25.37) | <0.001 | 0.99 (0.99–0.99) | <0.001 | |
| Number of ED visit, mean (SD) | 0.12 (0.47) | 0.30 (1.52) | <0.001 | 0.67 (0.63–0.72) | <0.001 | |
Abbreviations: OR, odds ratio; CI, confidence interval; NSAID, nonsteroidal anti-inflammatory drug; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; GCA, giant cell arteritis; JIA, juvenile idiopathic arthritis; ED, emergency department.
In contrast, the prescription of nonsteroidal anti-inflammatory drugs (NSAIDs) was identified as an independent risk factor for severe disease among persons infected with SARS-CoV-2 (aOR, 1.53; 95% CI, 1.25–1.86; P < 0.001), although it was not associated with an overall increased risk of COVID-19 (aOR, 1.04; 95% CI, 0.97–1.12; P = 0.30). However, this higher risk only occurred when NSAIDs were newly prescribed within 7 days prior to the COVID-19 test (aOR, 1.36; 95% CI, 1.09–1.71; P = 0.006) and not when used for more than 30 days within 90 days prior to the test (aOR, 0.83; 95% CI, 0.62–1.10; P = 0.20).
Drugs with potential effect against SARS-CoV-2
Following a literature review, the following drugs were selected as having potential therapeutic effects: HCQ, camostat, ciclesonide, azithromycin, sirolimus, mycophenolate, and amiodarone (Figure 2B and Table 3 ). Lopinavir/ritonavir and other protease inhibitors, direct antiviral agents for hepatitis C, and tocilizumab were excluded from the analysis as the numbers of prescriptions for those agents were too small. The use of HCQ was not associated with the risk of COVID-19 infection (aOR, 0.94; 95% CI, 0.53–1.66; P = 0.82) or with severe disease (aOR, 3.51; 95% CI, 0.76–16.22; P = 0.11). Azithromycin also did not show an association with infection (aOR, 0.58; 95% CI, 0.30–1.12; P = 0.10) or severe disease (aOR, 2.03; 95% CI, 0.39–10.60; P = 0.40). A lack of significant associations was also observed for camostat, ciclesonide, sirolimus, mycophenolate, and amiodarone.
Table 3.
Association of previously administered medications with the risk of severe disease among patients with COVID-19. Severe disease was defined as the requirement of any one of the following or death: supplementary oxygen, high-flow nasal cannula, non-invasive ventilation, mechanical ventilation, and extracorporeal membrane oxygenation.
| Drug | Severe (N = 878) |
Mild–moderate (N = 1927) |
Crude |
Adjusted |
||
|---|---|---|---|---|---|---|
| OR (95% CI) |
P | OR (95% CI) |
P | |||
| Drugs commonly used for chronic conditions | ||||||
| Angiotensin receptor blockers | 236 (26.88) | 384 (19.93) | 1.48 (1.23–1.78) | <0.001 | 1.11 (0.87–1.42) | 0.38 |
| Angiotensin-converting enzyme inhibitors | 12 (1.37) | 24 (1.25) | 1.10 (0.55–2.21) | 0.79 | 0.70 (0.33–1.48) | 0.35 |
| Metformin | 104 (11.85) | 168 (8.72) | 1.41 (1.09–1.82) | 0.01 | 1.01 (0.75–1.37 | 0.94 |
| Thiazolidinedione | 17 (1.94) | 30 (1.56) | 1.25 (0.69–2.28) | 0.47 | 0.96 (0.51–1.81) | 0.90 |
| Statins | 267 (30.41) | 478 (24.81) | 1.33 (1.11–1.58) | 0.002 | 0.89 (0.72–1.10) | 0.30 |
| NSAIDs | 255 (29.04) | 406 (21.07) | 1.53 (1.28–1.84) | <0.001 | 1.53 (1.25–1.86) | <0.001 |
| Drugs with potential therapeutic effect | ||||||
| Hydroxychloroquine | 5 (0.57) | 3 (0.16) | 3.67 (0.88–15.40) | 0.08 | 3.51 (0.76–16.22) | 0.11 |
| Azithromycin | 3 (0.34) | 3 (0.16) | 2.20 (0.44–10.92) | 0.34 | 2.03 (0.39–10.60) | 0.40 |
| Mycophenolate | 1 (0.11) | 1 (0.05) | 2.20 (0.14–35.15) | 0.58 | 2.21 (0.13–37.06) | 0.58 |
| Amiodarone | 2 (0.23) | 2 (0.10) | 2.20 (0.31–15.62) | 0.43 | 1.27 (0.17–9.69) | 0.82 |
| Demographic | ||||||
| Male sex | 403 (45.90) | 879 (45.61) | 1.01 (0.86–1.19) | 0.89 | 1.09 (092–1.29) | 0.35 |
| Age, mean (SD), y | 65.47 (14.65) | 61.04 (14.14) | <0.001 | 1.01 (1.00–1.02) | 0.02 | |
| Daegu/Gyeongsangbuk–do | 647 (73.69) | 1225 (63.57) | 1.61 (1.35–1.92) | <0.001 | 1.30 (1.07–1.56) | 0.007 |
| Coverage for low–income households | 114 (12.98) | 211 (10.95) | 1.21 (0.95–1.55) | 0.12 | 0.98 (0.75–1.27) | 0.85 |
| Comorbidities | ||||||
| Charlson comorbidity index, mean (SD) | 2.72 (2.22) | 1.93 (1.78) | <0.001 | |||
| Diabetes | 354 (40.32) | 571 (29.63) | 1.60 (1.36–1.90) | <0.001 | 1.24 (1.00–1.54) | 0.05 |
| Hypertension | 466 (53.08) | 755 (39.18) | 1.76 (1.50–2.06) | <0.001 | 1.18 (0.94–1.49) | 0.16 |
| Chronic heart disease | 198 (22.55) | 265 (13.75) | 1.83 (1.49–2.24) | <0.001 | 1.31 (1.04–1.65) | 0.02 |
| Chronic lung disease | 292 (33.26) | 477 (24.75) | 1.52 (1.27–1.80) | <0.001 | 1.21 (0.99–1.47) | 0.06 |
| Asthma and allergic rhinitis | 537 (61.16) | 1146 (59.47) | 1.07 (0.91–1.26) | 0.40 | 0.92 (0.76–1.11) | 0.40 |
| Chronic liver disease | 319 (36.33) | 629 (32.64) | 1.18 (1.00–1.39) | 0.06 | 0.88 (0.73–1.070) | 0.20 |
| Chronic kidney disease | 70 (7.97) | 82 (4.26) | 1.95 (1.40–2.71) | <0.001 | 1.51 (1.05–2.17) | 0.02 |
| Malignancy | 82 (9.34) | 132 (6.85) | 1.40 (1.05–1.87) | 0.02 | 1.12 (0.82–1.54) | 0.47 |
| RA, SLE, GCA, and JIA | 41 (4.67) | 94 (4.88) | 0.96 (0.66–1.39) | 0.81 | 0.72 (0.47–1.10) | 0.13 |
| Other connective tissue disease | 5 (0.57) | 6 (0.31) | 1.83 (0.56–6.02) | 0.31 | 1.76 (0.50–6.21) | 0.38 |
| Chronic neurologic disease | 315 (35.88) | 461 (23.92) | 1.80 (1.50–2.12) | <0.001 | 1.303 (1.06–1.60) | 0.01 |
| Pancreatitis | 28 (3.19) | 45 (2.34) | 1.38 (0.85–2.22) | 0.19 | 0.88 (0.51–1.51) | 0.63 |
| Healthcare utilization | ||||||
| Number of hospitalizations, mean (SD) | 0.67 (1.51) | 0.37 (1.18) | <0.001 | 1.07 (0.99–1.15) | 0.08 | |
| Number of outpatient visit, mean (SD) | 29.89 (36.24) | 23.70 (22.54) | <0.001 | 1.00 (1.00–1.01) | 0.37 | |
| Number of ED visit, mean (SD) | 0.25 (0.67) | 0.13 (0.46) | <0.001 | 1.16 (0.97–1.38) | 0.11 | |
Abbreviations: OR, odds ratio; CI, confidence interval; NSAID, nonsteroid anti-inflammatory drug; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; GCA, giant cell arteritis; JIA, juvenile idiopathic arthritis; ED, emergency department.
Sensitivity analysis
Sensitivity analysis was conducted using an alternative definition of exposure: the prescription of drugs that was continued up to 14 days or less prior to testing for COVID-19 (Supplementary Table S4). The results were generally consistent with the main analysis with some exceptions. NSAIDs were associated with an increased risk for COVID-19 diagnosis (aOR, 1.10; 95% CI, 1.03–1.18; P = 0.006); however, chronic use (≥30 days within the last 90 days) was associated with a lower risk (aOR, 0.76; 95% CI, 0.68–0.86; P < 0.001), which was similar to their association with severity. Ciclesonide (aOR, 6.01; 95% CI, 1.01–35.59; P = 0.05) and mycophenolate (aOR, 0.37; 95% CI, 0.14–0.95, P = 0.04) showed statistically significant associations.
Discussion
Using Korean health insurance claims and SARS-CoV-2 testing results, this large case-control study found that none of the drugs commonly used to treat chronic conditions were associated with the risk of COVID-19 or its severity. ACEIs and ARBs inhibit the activity of ACE1, which is a homolog to ACE2 but is not used by SARS-CoV for host-cell entry (American College of Cardiology, 2020). However, several studies have shown that ACEIs/ARBs upregulate the expression of ACE2 which has been correlated with susceptibility to SARS-CoV infection in vitro (Ferrario et al., 2005, Ferrario and Varagic, 2010, Hattermann et al., 2005, Mossel et al., 2005). Thus, concerns have been raised about a theoretical risk for COVID-19 in patients taking ACEIs/ARBs. In contrast, other reports have indicated that high serum levels of angiotensin II and downregulation of ACE2 expression may be related to lung injury caused by respiratory viral infections (Gu et al., 2016, Kuba et al., 2005). Therefore, ACEIs/ARBs have a theoretical possibility of decreasing the risk for severe SARS-CoV-2 infections. However, there is no firm evidence that ACEIs/ARBs are associated with clinical outcome in either harmful or protective ways.
A retrospective study of 1,128 patients with COVID-19 and hypertension revealed that inpatient use of ACEIs/ARBs was associated with a lower risk of mortality (Zhang et al., 2020). Furthermore, two large-scale studies from the United States and Italy demonstrated that the use of ACEIs/ARBs was not associated with the likelihood of COVID-19 or of severe disease (Mancia et al., 2020, Reynolds et al., 2020). In general, our results are in line with those previous studies and affirm the safety of ACEIs/ARBs during the COVID-19 pandemic. We also observed a lack of association with other medications commonly used for diabetes and dyslipidemia—namely metformin, thiazolidinediones, and statins. Our results suggest that those drugs could be safely continued without increasing the risk of COVID-19 or of severe disease.
There was a brief controversy over whether the use of NSAIDs might increase the risk of severe COVID-19 (Little, 2020). We also observed that the prescription of NSAIDs was associated with a higher risk of severe COVID-19, albeit the risk of an overall COVID-19 diagnosis was not higher in patients taking NSAIDs. In our post-hoc analysis, the chronic use of NSAIDs (≥30 days within 90 days prior to testing) was not shown to increase the risk for severe COVID-19, while a new prescription within 7 days prior to testing did increase risk. Our finding suggests that NSAID-use might indicate the presence of symptoms severe enough to require pharmaceutical intervention; it seems unlikely that NSAIDs would directly increase the risk for severe disease among patients with COVID-19. A recent multicenter retrospective cohort study of hospitalized patients with COVID-19 also reported a lack of association between the pre-hospital use of NSAIDs and mortality (Imam et al., 2020).
Chloroquine (CQ) has been used to treat malaria since the 1940s and has also been used for the treatment of connective tissue diseases, including rheumatoid arthritis and systemic lupus erythematosus (SLE). CQ reportedly inhibits the SARS-CoV-2 in vitro by inhibiting glycosylation of host receptors and endosomal acidification (Devaux et al., 2020, Wang et al., 2020). In China, CQ and HCQ have been recommended for treating COVID-19, and a small trial indicated that HCQ treatment led to a faster negative conversion of viral shedding (Gao et al., 2020, Gautret et al., 2020). However, other studies have suggested a lack of clinical benefit and even an increased possibility of poor outcomes (Magagnoli et al., 2020, Molina et al., 2020). A randomized trial of HCQ as a post-exposure prophylaxis for household or occupational exposure found that HCQ did not prevent COVID-19-like illness (Boulware et al., 2020). The present study found that HCQ use was not associated with a lower risk of COVID-19 and did not prevent severe disease among infected patients. Together, our results and those of the recent clinical studies suggest that HCQ does not have a significant clinical effect against SARS-CoV-2 infection.
Azithromycin is a macrolide antibiotic with an immunomodulatory effect (Zimmermann et al., 2018), and in vitro studies have reported its effectiveness against Zika and Ebola viruses (Madrid et al., 2015, Retallack et al., 2016). The combination of azithromycin with HCQ was reported to result in a faster viral clearance in a small trial from France, which led to a high level of public interest (Gautret et al., 2020). However, a retrospective study of hospitalized patients with COVID-19 showed no reduction of in-hospital mortality with azithromycin treatment, either alone or in combination with HCQ (Rosenberg et al., 2020). We also found no association between the use of azithromycin and the risk for COVID-19 or severe disease.
SARS-CoV and SARS-CoV-2 engage ACE2 as the receptor for host-cell entry, with the spike (S) protein of the coronavirus binding to ACE2 (Hoffmann et al., 2020, Li et al., 2003). In this process, the S protein must be primed by host-cell proteinases which include a serine protease known as TMPRSS2 (Glowacka et al., 2011, Matsuyama et al., 2010). Camostat mesylate is a serine protease inhibitor that is approved for treating chronic pancreatitis in Korea and Japan. A recent in vitro study demonstrated that camostat, a known TMPRSS2 inhibitor, blocks the entry of SARS-CoV-2 into lung cells (Hoffmann et al., 2020). However, we failed to observe a protective effect of camostat in our study, although the number of individuals taking camostat was too small to draw a firm conclusion (Figure 2A). Results from ongoing clinical trials should provide more evidence regarding its potential effect against SARS-CoV-2 infections.
Sirolimus, mycophenolate, and amiodarone reportedly had inhibitory effects against SARS-CoV and/or MERS-CoV in preclinical studies (Aimo et al., 2020, Cheng et al., 2015, Kindrachuk et al., 2015). However, the present study failed to detect significant protective effects for these drugs. From a different point of view, our results are reassuring that these agents do not increase the likelihood of mild or severe COVID-19 and can be continued safely.
Ciclesonde and mycophenolate were shown to be associated with the diagnosis of COVID-19 in the sensitivity analysis only. As the sensitivity analysis used a less stringent definition of exposure, we suggest that its result be interpreted carefully. Further studies are needed to reach a firm conclusion regarding the two drugs.
Our study has several strengths. First, South Korea rapidly established a large testing capacity and has successfully contained the SARS-CoV-2 outbreak, suggesting that it is unlikely that a large number of COVID-19 patients were missed. Further, we were able to use “test-negative” controls owing to the vast testing capacity. Second, the single-payer universal healthcare system in Korea ensures that we had reliable data on medication use and comorbidities. Given that there is near-complete coverage of common illnesses in the HIRA database, only an extremely small proportion of patients would not have data on the drugs of interest. The comprehensiveness of our datasets enabled the use of DRS-matching to mitigate differences in baseline characteristics. Finally, our methods are transparent, and the results are reproducible. The HIRA dataset we used are available for public analysis, and our full coding protocol is included in the Supplements.
The present study does have several limitations. First, many of the drugs proposed for treatment of COVID-19 are not prescribed frequently enough to provide sufficient statistical power for analysis despite the large size of overall study population. Second, the use of claims data precludes an analysis of prescription compliance or the accuracy of comorbidity diagnoses. Further, over-the-counter use of drugs could not be captured. While all other study drugs require prescription in Korea, NSAID-use might be underestimated in our study. Third, we could not obtain data regarding the severity of the underlying conditions, performance status, and socioeconomic characteristics as this information was not available in the HIRA or KCDC databases. Finally, the differences in baseline characteristics between groups remained after DRS-matching, albeit to a substantially smaller degree. While we tried to further adjust for those variables using logistic regression, the possibility of residual confounding cannot be excluded.
In conclusion, we found no association between drugs of concern, including ARB and HCQ, and the risk of COVID-19 and its severity. Our results are reassuring in that drugs commonly used for chronic conditions do not increase the risk for COVID-19. However, our findings also suggest that repurposing pharmaceutical agents may not provide significant clinical benefits for patients with COVID-19. Clinical trials are needed to generate high-quality evidence regarding the efficacy of these agents.
Statement of ethics
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki and was approved by the appropriate institutional review board of the Gachon University College of Medicine, Incheon, Republic of Korea (GFIRB2020-118).
Conflict of interest
The authors have no conflict of interest to declare.
Funding/support
This work was supported by grants from the Gachon University Gil Medical Center (grant nos. 2018-17 and 2019-11). The sponsor of the study was not involved in the study design, analysis, and interpretation of data; writing of the report; or the decision to submit the study results for publication.
Author contributions
Drs. Huh and Jung had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Huh, Ji, Jung. Acquisition, analysis or interpretation of data: Huh, Ji, Kang, Hong, Bae, Lee, Na, Jung. Drafting of the manuscript: Huh, Ji, Jung. Statistical analysis: Huh, Kang, Na, Jung.
Acknowledgement
The authors thank the healthcare professionals dedicated to treating COVID-19 patients in Korea and the Ministry of Health and Welfare and the Health Insurance Review & Assessment Service of Korea for sharing invaluable national health insurance claims data in a prompt manner. We appreciate Dr. Cynthia Sung for the discussion of our results. Further, we express our sincere gratitude to Dr. Taegyu Park for his assistance in the graphic works.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.12.041.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aimo A., Baritussio A., Emdin M., Tascini C. Amiodarone as a possible therapy for coronavirus infection. Eur J Prev Cardiol. 2020 doi: 10.1177/2047487320919233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Cardiology . 2020. HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. Available from: https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19. [Accessed 25 April 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C. A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Chan K.H., Kao R.Y., To K.K., Zheng B.J., Li C.P. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67(6):606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Cheng K.W., Cheng S.C., Chen W.Y., Lin M.H., Chuang S.J., Cheng I.H. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antiviral Res. 2015;115:9–16. doi: 10.1016/j.antiviral.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID- 19? Int J Antimicrob Agents. 2020;55(5):105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Ferrario C.M., Varagic J. The ANG-(1-7)/ACE2/mas axis in the regulation of nephron function. Am J Physiol Renal Physiol. 2010;298(6):F1297–1305. doi: 10.1152/ajprenal.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Glowacka I., Bertram S., Muller M.A., Allen P., Soilleux E., Pfefferle S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Xie Z., Li T., Zhang S., Lai C., Zhu P. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci Rep. 2016;6(1):19840. doi: 10.1038/srep19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattermann K., Müller M.A., Nitsche A., Wendt S., Donoso Mantke O., Niedrig M. Susceptibility of different eukaryotic cell lines to SARS-coronavirus. Arch Virol. 2005;150(5):1023–1031. doi: 10.1007/s00705-004-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R. Effect of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020;383(21):2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam Z., Odish F., Gill I., O’Connor D., Armstrong J., Vanood A. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288(4):469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother. 2015;59(2):1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korea Centers for Disease Control and Prevention . 2020. COVID-19 Response Guidelines (Edition 8-1) Available from: http://ncov.mohw.go.kr/shBoardView.do?brdId=2&brdGubun=28&ncvContSeq=2447. [Accessed 7 June 2020] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020;368:m1185. doi: 10.1136/bmj.m1185. [DOI] [PubMed] [Google Scholar]
- Madrid P.B., Panchal R.G., Warren T.K., Shurtleff A.C., Endsley A.N., Green C.E. Evaluation of ebola virus inhibitors for drug repurposing. ACS Infect Dis. 2015;1(7):317–326. doi: 10.1021/acsinfecdis.5b00030. [DOI] [PubMed] [Google Scholar]
- Magagnoli J., Narendran S., Pereira F., Cummings T., Hardin J.W., Sutton S.S. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. Med (N Y) 2020;1(1):114–127.e3. doi: 10.1016/j.medj.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol. 2010;84(24):12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50(4):384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossel E.C., Huang C., Narayanan K., Makino S., Tesh R.B., Peters C.J. Exogenous ACE2 expression allows refractory cell lines to support severe acute respiratory syndrome coronavirus replication. J Virol. 2005;79(6):3846–3850. doi: 10.1128/JVI.79.6.3846-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., Abdool Karim Q. Repurposed antiviral drugs for covid-19 - interim WHO solidarity trial results. N Engl J Med. 2020 doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallack H., Di Lullo E., Arias C., Knopp K.A., Laurie M.T., Sandoval-Espinosa C. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A. 2016;113(50):14408–14413. doi: 10.1073/pnas.1618029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Stuart E.A. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Ziesenitz V.C., Curtis N., Ritz N. The immunomodulatory effects of macrolides-a systematic review of the underlying mechanisms. Front Immunol. 2018;9:302. doi: 10.3389/fimmu.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.