Abstract
Background
Breast cancer (BC) is the most common cancer among women worldwide. At present, there is a need to search for new, accurate, reliable, minimally invasive and cheap biomarkers in addition to existing methods for the diagnosis and prognosis of BC. The main goal of this study was to test the diagnostic value of six circulating miRNAs in Kazakh women.
Materials and methods
TaqMan-based miRNA profiling was conducted using plasma specimens from 35 BC women patients and 33 healthy women samples (control group).
Results
The level of all seven miRNAs (including endogenous control) normalized by synthetic cel-miR-39 were significantly elevated in the group of BC patients. Normalization using miR-222-3p as endogenous control reduced differences in level of miRNAs between groups; as a result, only three miRNAs were significantly upregulated in the group of BC patients—miR-145-5p (P = 6.5e−12), miR-191-5p (P = 3.7e−10) and miR-21-5p (P = 0.0034). Moreover, ROC analysis showed that the use of miR-145-5p and miR-191-5p, both individually (AUC = 0.931 and 0.904, respectively) or in combination (AUC = 0.984), allows to accurately differentiate BC patients from healthy individuals.
Conclusions
Two plasma miRNAs—miR-145-5p and miR-191-5p—are potential biomarkers for diagnosis of BC in the Kazakh population. The findings need to be further substantiated using a more representative sample.
Keywords: Breast cancer, Circulating miRNA, Biomarker, Plasma, miR-145, miR-191, miR-21, Diagnosis, Kazakh population
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer type in women around the world. Just like most cancers, early BC is asymptomatic. This has resulted in late detection of the disease, at which point no therapy is very effective (Höfelmann, Anjos & Ayala, 2014). Mammographic screening of women, in the age range the most at risk to breast cancer, did make the tumor detection at early stages more common and therefore, caused significant reduction in mortality (Onega et al., 2016; Wang, 2017). However, mammography shows a significant number of false positives in women with dense breasts, especially at a younger age. In this regard, mammography screening is confidently recommended for women over 50 years old, although women aged 40–50 years are also at risk of BC (McDonald et al., 2016; Nelson et al., 2016; Phi et al., 2018). Various molecular subtypes of BC that require different therapy (EBCTCG, 2015; Guerrero-Zotano & Arteaga, 2017; Lee & Seo, 2018), individual patient susceptibility to drugs and side effects from drugs (Potosky et al., 2015; Greenlee et al., 2017; Moo et al., 2018) and the development of drug resistance (Li et al., 2020; Zhong et al., 2020) make treatment of this disease more difficult and complicated. The listed difficulties indicate the need for study of new biomarkers that can help in the early detection, diagnosis and prognosis of BC.
Nowadays miRNAs are promising markers for early diagnosis and prognosis of tumors. miRNAs are a large class of small non-coding RNAs that function as negative regulators of most genes in the genome and are involved in important biological processes, such as development, differentiation, apoptosis, proliferation, etc. (Jansson & Lund, 2012). Many studies have highlighted differential expression of certain miRNAs in several cancer types, including BC (Acunzo et al., 2015; Aggarwal, Priyanka & Tuli, 2020).
The property of miRNAs that they can be detected in both tumor cells and biological fluids (in a cell-free form) serves as a major advantage for using these molecules over other oncogenic biomarkers. miRNAs directly enter the bloodstream from primary or metastatic tumors by active secretion, apoptosis or necrosis, and thus changes in the amount of circulating miRNAs can reflect the pathological process (Schwarzenbach, 2017; Sun et al., 2018). In this regard, the level of miRNA-marker can be determined in a minimally invasive way. High stability of miRNA in biological fluids also makes them a very suitable choice as cancer biomarkers (Grasedieck et al., 2012; Glinge et al., 2017). Several miRNAs have been revealed to contribute to the pathological mechanisms of BC progression and many of them have been recommended by previous research studies as diagnostic or prognostic markers (McGuire, Brown & Kerin, 2015; Stückrath et al., 2015; Zhang et al., 2015; Schwarzenbach, 2017; Hamam et al., 2017; Nassar, Nasr & Talhouk, 2017; Shao et al., 2019). The main limitation of currently existing serum biomarkers, including the best of them CA15-3 and CEA, as a marker of BC is the lack of sensitivity for patients with early disease (Duffy, Evoy & McDermott, 2010); miRNA-markers seem to have no such limitations (Schwarzenbach, 2017). It is known that there are some ethnic differences in the pathogenesis of breast cancer (Nakshatri, Anjanappa & Bhat-Nakshatri, 2015; Özdemir & Dotto, 2017; Wu et al., 2020), which is also true for the applicability of miRNAs as markers of BC (Zhao et al., 2010; Nassar et al., 2017; Wu et al., 2020). For this reason, miRNA-markers need to be validated for specific ethnic groups.
The aim of our study was to test the diagnostic value of six circulating miRNAs recommended previously as plasma/serum markers of BC: miR-145-5p (Ng et al., 2013), miR-21-5p (Adhami et al., 2018), miR-210-3p (Jung et al., 2012), miR-29c-3p (Zhang et al., 2015), miR-16-5p (Usmani et al., 2017) and miR-191-5p (Mar-Aguilar et al., 2013) among Kazakh women. To do so, we compared plasma levels of the miRNAs between age-matched BC patients (n = 35) and healthy women (n = 33) from Almaty and Almaty region in Kazakhstan.
Materials & Methods
Subjects
Venous blood of 35 Kazakh women with primary BC was collected at the Kazakh Research Institute of Oncology and Radiology, Almaty, Kazakhstan before therapy in 2019. All patients analyzed had histologic proven BC. The average age of patients was 52.6 ± 11.66. Venous blood of 33 healthy Kazakh women was collected in the Karasai central district hospital in the Almaty region, Kazakhstan in 2019. All controls underwent mammography and were over 40 years old. The average age of the control group was 53.0 ± 7.61. Clinicopathological characteristics of BC patients and control group are presented in Table 1. The study was carried out in compliance with the principles of the Helsinki Declaration, and approved by the local ethics committee of the M. Aitkhozhin Institute of Molecular Biology and Biochemistry, Almaty, Kazakhstan (approval number 185/01-02). All participants provided written informed consent for the use of biomaterials in this study.
Table 1. Clinicopathological characteristics of BC patients group and control group.
| Characteristics | BC patients group | Control group |
|---|---|---|
| ER-/ER+ | 8/27 | – |
| PR-/PR+ | 10/25 | – |
| HER2-/HER2+ | 27/7 | – |
| Tumor size: T1/T2/T3/T4 | 4/28/2/1 | – |
| Lymph node: Nx/N0/N1-3 | 5/23/7 | – |
| Metastases: no/yes | 34/1 | – |
| Ki-67: <20%/≥20% | 17/18 | – |
| Tumor grade: G1/G2/G3 | 1/28/6 | – |
| Menarche age: early (≤14)/late (>14) | 27/8 | 16/17 |
| Menopausal status: pre-/post | 14/21 | 14/19 |
| Age of first birth: ≤22/≥23 | 17/16 | 18/15 |
| Number of children: 0/≤2/≥3 | 2/13/20 | 0/13/20 |
| Number of unsuccessful pregnancies: 0/1/≥2 | 11/10/14 | 8/9/16 |
| Family history of cancer: no/yes | 27/8 | 24/9 |
| Alcohol consumption: no/yes | 31/4 | 26/7 |
Plasma preparation
Blood was collected in vacuum tubes with sodium citrate, which showed considerable miRNA yield in preliminary tests. Blood was stored at 4 °C and plasma was obtained within 8 h after blood sampling. To obtain plasma, the blood was centrifuged at 1,000 g for 15 min at 4 °C; the upper aqueous phase was transferred to a fresh tube and centrifuged at 2,500 g for 15 min at 4 °C. The resulting plasma was divided into aliquots and stored at −70 °C until the isolation of miRNA step. Before being examined, the plasma was subjected to one freeze-thaw cycle.
Isolation of RNA
Isolation of total RNA from 200 µl of plasma was performed utilizing technique previously developed by Zununi Vahed et al., (2016) with minor modifications. Briefly, deproteinization was carried out according to the standard Trizol method. Then, to precipitate RNA, an equal volume of 2.5M lithium chloride and two volumes of cold ethanol were added and incubated overnight at −70 °C, then centrifuged for 16,000 g for 20 min at 4 °C. The pellets was dried and dissolved in 50 µL of DEPC water, incubating for 5 min at 65 °C. At the stage of Trizol treatment, 20 fmol of synthetic cel-miR-39 was added to the sample. The resulting total RNA sample was stored at −70 °C until use.
Obtaining cDNA and quantitative PCR
Reverse transcription and quantitative PCR was performed using primers and probes from TaqMan MicroRNA Assay (Applied Biosystems, USA). cDNA was obtained using TaqMan MicroRNA Reverse Transcription Kit reagents (Applied Biosystems) according to the manufacturer’s protocol. Quantitative PCR was performed in triplicates using TaqMan Universal Master Mix II with UNG reagents (Applied Biosystems) under the conditions recommended by the manufacturer on the StepOnePlus Real-Time PCR System (Applied Biosystems). Quantitative data was normalized to the level of exogenous spike-in control cel-miR-39 and endogenous control miR-222-3p.
Statistical analysis
Primary processing of the results was carried out in StepOne Software and ExpressionSuite Software. The suitability of endogenous control was evaluated using the NormFinder (Andersen, Jensen & Orntoft, 2004) and GeNorm (Vandesompele et al., 2002) programs. Relative quantification is carried out using the comparative Ct (ΔΔCt) method with modifications as described in the paper (Königshoff et al., 2009). Relative transcript abundance is expressed in ΔCt values (ΔCt = Ctreference − Cttarget). ΔΔCt value (ΔΔCt = ΔCtBC − ΔCtcontrol) was considered as log2 fold change.
Statistics were performed in the Jamovi program (https://www.jamovi.org). Statistical significance of the differences in ΔCt between the groups was calculated using the two-tailed Mann–Whitney U test. P <0.05 was considered statistically significant. Due to the explorative nature of the study no adjustment for multiple testing was performed. The characteristics of the markers were evaluated by ROC analysis using the web-tool easyROC (Goksuluk et al., 2016), and Jamovi. Youden’s index method was used to calculate optimal cut-off points.
Results
Endogenous control selection
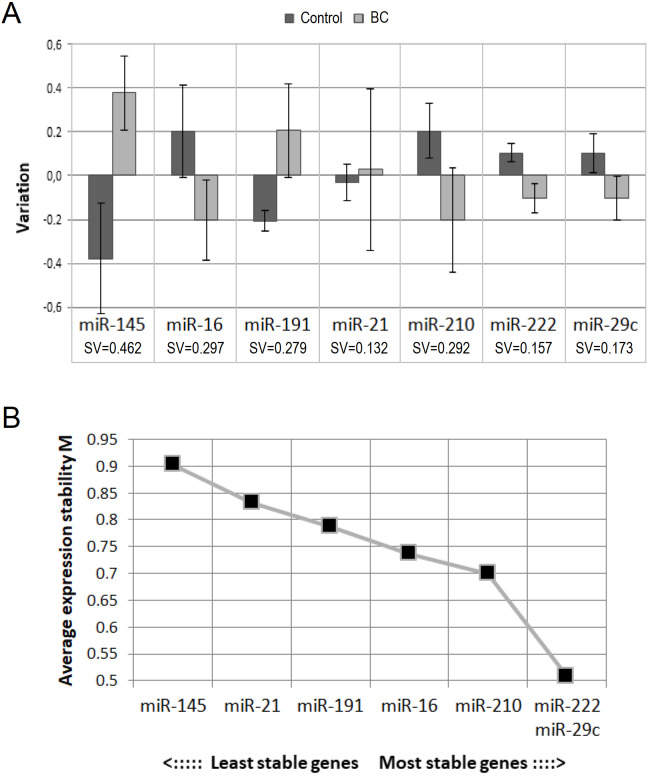
To select the best endogenous control, we evaluated the concentration stability of analyzed miRNAs in our sample with the help of NormFinder and GeNorm programs. According to NormFinder, the three best (the lowest) stability values were shown for miR-21-5p, miR-222-3p and miR-29c-3p (Fig. 1A). According to GeNorm, miR-222-3p and miR-29c-3p are the best internal controls for our sample (Fig. 1B). Thus, there are two miRNAs on the overlap of the results of two programs: miR-222-3p and miR-29c-3p.
Figure 1. Selection of endogenous control.
(A) Results from NormFinder: intergroup (bars) and intragroup (whiskers) variation plot and stability value (SV), calculated on their basis; (B) average expression stability values of remaining control candidates during stepwise exclusion of the least stable control candidate, obtained from GeNorm.
Unlike NormFinder, GeNorm does not recommend using miR-21-5p. Also, although NormFinder showed the best stability value for miR-21-5p, intragroup variation in the BC patient group was the largest. This may indicate the heterogeneity of the group and does not exclude the existence of an association between circulating miR-21-5p concentration and some clinicopathological parameter. These considerations, as well as the fact that circulating miR-21-5p has most often been found to be dysregulated in BC (Schwarzenbach, 2017; Adhami et al., 2018), prompted us to abandon it as an endogenous control.
One of the important criteria when choosing endogenous control is their relative abundance. It seems to us that miR-29c-3p is not abundant enough for this role (Ct mean 34.6). Taking into account all the mentioned above, we decided to use miR-222-3p as single endogenous control for our study.
The level of miRNA in the plasma of BC patients in comparison with the control group
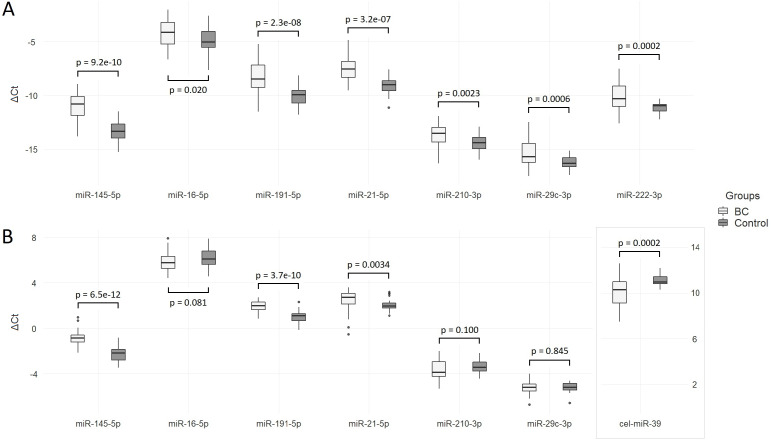
The Ct values of the analyzed miRNAs in two groups relative to the spike-in control cel-miR-39 level are shown in Fig. 2A. The concentration of all miRNAs, including miR-222-3p (used later as endogenous control), was significantly elevated in the plasma of BC patients compared to healthy controls. Log2 fold changes higher than one are obtained for miR-145-5p (2.36), miR-191-5p (1.87) and miR-21-5p (1.35) (Table 2).
Figure 2. Differences in ΔCt between BC patients and control group.
(A) Data are normalized to the spike-in control cel-miR-39; (B) data are normalized to the endogenous control miR-222-3p.
Table 2. Cycle threshold values (Ct) and comparative statistics of studied miRNAs between the BC patients group and control group.
| miRNA | BCCt mean ± SD | Control Ct mean ± SD | Cel-miR-39 normalization | miR-222-3p normalization | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BC ΔCt mean ± SE | Control ΔCt mean ± SE | ΔΔCt (95% CI), log2 fold change | P value | BC ΔCt mean ± SE | Control ΔCt mean ± SE | ΔΔCt (95% CI), log2 fold change | P value | |||
| miR-145-5p | 29.52 ± 1.52 | 32.41 ± 1.19 | −10.94 ± 0.21 | −13.30 ± 0.16 | 2.36 (1.84; 2.88) | 9.2e−10 | −0.84 ± 0.11 | −2.22 ± 0.12 | 1.38 (1.06; 1.72) | 6.5e−12 |
| miR-16-5p | 22.72 ± 1.70 | 23.94 ± 1.46 | −4.14 ± 0.20 | −4.83 ± 0.18 | 0.69 (0.15; 1.23) | 0.020 | 5.96 ± 0.15 | 6.25 ± 0.14 | −0.29(−0.69; 0.12) | 0.081 |
| miR-191-5p | 26.76 ± 1.76 | 29.17 ± 1.17 | −8.18 ± 0.26 | −10.05 ± 0.14 | 1.87 (1.28; 2.46) | 2.3e−08 | 1.92 ± 0.08 | 1.02 ± 0.09 | 0.89 (0.66; 1.13) | 3.7e−10 |
| miR-21-5p | 26.24 ± 1.84 | 28.13 ± 1.10 | −7.66 ± 0.18 | −9.02 ± 0.14 | 1.35 (0.89; 1.82) | 3.2e−07 | 2.44 ± 1.17 | 2.06 ± 0.09 | 0.38 (−0.04; 0.76) | 0.0034 |
| miR-210-3p | 32.31 ± 1.38 | 33.53 ± 1.09 | −13.73 ± 0.17 | −14.42 ± 0.15 | 0.69 (0.24; 1.14) | 0.0023 | −3.63 ± 0.15 | −3.35 ± 0.11 | −0.29(−0.65; 0.08) | 0.100 |
| miR-29c-3p | 33.87 ± 1.81 | 35.38 ± 0.96 | −15.29 ± 0.22 | −16.27 ± 0.10 | 0.98 (0.49; 1.47) | 0.0002 | −5.19 ± 0.10 | −5.19 ± 0.07 | 0.01 (−0.24; 0.25) | 0.845 |
| miR-222-3p | 28.68 ± 1.52 | 30.19 ± 0.84 | −10.10 ± 0.20 | −11.08 ± 0.08 | 0.98 (0.53; 1.42) | 0.0006 | – | – | – | – |
| cel-miR-39 | 18.58 ± 1.10 | 19.11 ± 0.67 | – | – | – | – | −10.10 ± 0.20 | −11.08 ± 0.08 | −0.98 (−1.42; −0.53) | 0.0002 |
When quantitative data were normalized to miR-222-5p, the levels of miR-145-5p, miR-191-5p and miR-21-5p in the BC group were significantly increased compared to healthy controls (Fig. 2B). Differences between groups in miR-16-5p, miR-210-3p, and miR-29c-3p concentrations were not significant. Compared to cel-miR-39 normalization, log2 fold change significantly decreased: only one miRNA exceeded one—miR-145-5p (1.38). Relative to the endogenous control, the level of cel-miR-39 was significantly lower in the group of BC patients (ΔΔCt = − 0.98, P = 0.0004) with a wider range of ΔCt values compared to the control group.
Associations with clinicopathological parameters
The results of comparisons between groups with different clinicopathological characteristics are presented in Table 3. When normalized to endogenous control miR-222-3p, the level of miR-145-5p was significantly higher (P = 0.043) and the level of miR-191-5p was significantly lower (P = 0.006) in patients with HER2 positive tumor compared to patients with HER2 negative tumor. The level of miR-21-5p in patients with high Ki-67 (≥20%) was significantly higher compared to patients with low Ki-67 (P = 0.003). The level of miR-210-3p and miR-145-5p in patients with poorly differentiated tumor (grade G3) were significantly higher compared to patients with moderately differentiated tumor (grade G2) (P = 0.007 and 0.033, respectively). In the group of BC patients, levels of miR-145-5p and miR-21-5p were significantly higher in women with early menarche compared to women with late menarche (P = 0.009 and 0.022, respectively). In the control group, the level of miR-21-5p in women with two or less children was significantly higher compared to women with more than two children (P = 0.011). In the control group, the level of miR-29c-3p in women over 50 years old was significantly lower compared to women younger than or 50 years old (P = 0.008). In the control group, the level of miR-191-5p in women with a positive family history of cancer was significantly lower compared to women without it (P = 0.029). Differences in the level of the analyzed miRNAs between the groups, categorized by other clinicopathological parameters were not significant.
Table 3. P values for ΔCt comparisons between groups with different clinicopathological characteristics, after normalization to miR-222-3p.
| Clinicopathological characteristics | miR-145-5p | miR-16-5p | miR-191-5p | miR-21-5p | miR-210-3p | miR-29c-3p |
|---|---|---|---|---|---|---|
| BC patients group | ||||||
| ER- vs ER+ | 0.630 | 0.714 | 0.862 | 0.269 | 0.832 | 0.428 |
| PR- vs PR+ | 0.287 | 0.627 | 0.553 | 0.339 | 0.577 | 0.122 |
| HER2- vs HER2+ | 0.043 | 0.559 | 0.006 | 0.379 | 0.191 | 0.771 |
| N0 vs N1-3 | 0.086 | 0.441 | 0.190 | 0.246 | 0.810 | 0.360 |
| Ki-67 <20% vs ≥20% | 0.096 | 0.134 | 0.089 | 0.003 | 0.708 | 0.405 |
| Tumor grade: G2 vs G3 | 0.033 | 0.066 | 0.644 | 0.297 | 0.007 | 0.676 |
| Age: <50 vs. ≥50 | 0.257 | 0.987 | 0.906 | 0.371 | 0.191 | 0.749 |
| Menarche age: ≤14 vs >14 | 0.009 | 0.743 | 0.166 | 0.022 | 0.802 | 0.862 |
| Menopausal status: pre- vs post | 0.096 | 0.908 | 0.517 | 0.249 | 0.118 | 0.881 |
| Age of first birth: ≤22 vs >22 | 0.063 | 0.683 | 0.345 | 0.873 | 0.102 | 0.276 |
| Number of children: ≤2 vs >2 | 0.987 | 0.347 | 0.139 | 0.107 | 0.521 | 0.099 |
| Unsuccessful pregnancies: 0 vs >0 | 0.316 | 0.061 | 0.713 | 0.163 | 0.300 | 0.099 |
| Family history of cancer: no vs yes | 0.576 | 0.499 | 0.550 | 0.143 | 0.286 | 0.143 |
| Alcohol consumption: no vs yes | 0.093 | 0.378 | 0.233 | 0.745 | 0.379 | 0.565 |
| Control group | ||||||
| Age: <50 vs. ≥50 | 0.501 | 0.986 | 0.102 | 0.842 | 0.137 | 0.008 |
| Menarche age: ≤14 vs >14 | 0.402 | 0.118 | 0.087 | 0.581 | 0.276 | 0.402 |
| Menopausal status: pre- vs post- | 0.240 | 0.186 | 0.872 | 0.553 | 0.815 | 0.114 |
| Age of first birth: ≤22 vs >22 | 0.486 | 0.929 | 0.166 | 0.442 | 0.401 | 0.901 |
| Number of children: ≤2 vs >2 | 0.478 | 0.316 | 0.392 | 0.011 | 0.235 | 0.730 |
| Unsuccessful pregnancies: 0 vs >0 | 0.696 | 0.272 | 0.886 | 0.067 | 0.127 | 0.726 |
| Family history of cancer: no vs yes | 0.796 | 0.438 | 0.029 | 0.592 | 0.179 | 0.564 |
| Alcohol consumption: no vs yes | 0.352 | 0.215 | 0.780 | 0.682 | 0.352 | 0.249 |
Note.
p values <0.05 are in bold.
We also found statistically significant differences in the distribution of women with early and late menarche between BC and control groups (P = 0.023, OR = 3.59, 95% CI [1.26–10.18]), and an inverse correlation between the level of Ki-67 and the age of BC patients (Spearman’s rho = −0.507, P = 0.0019).
We did not consider differences between groups divided by clinicopathological parameters based on data normalized to spike-in cel-miR-39, due to doubtful results (see Discussion for details).
ROC analysis
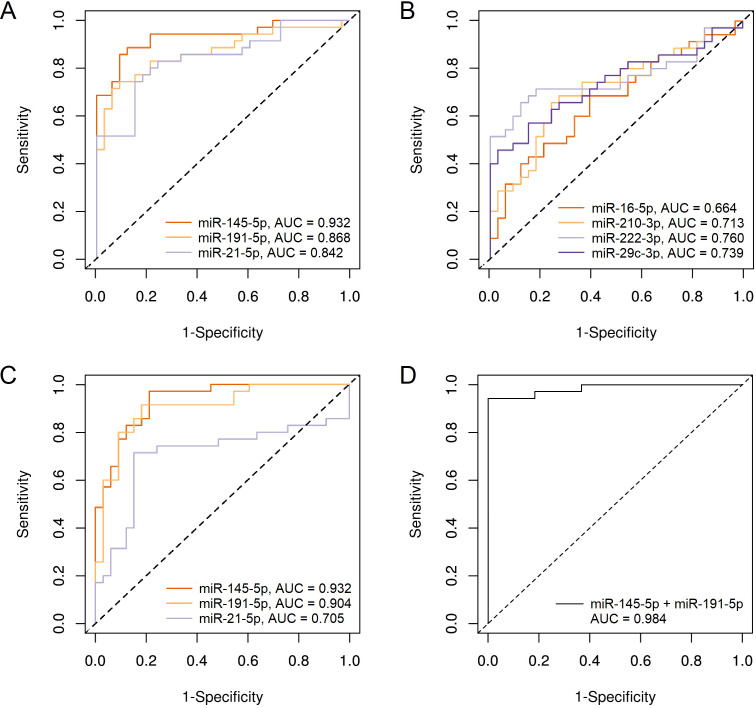
To test the ability of our miRNAs to distinguish BC patients from healthy individuals, we performed a ROC analysis, the results are presented in Table 4. When normalized to cel-miR-39, the largest area under the ROC curve (AUC) was obtained for miR-145-5p (0.932); miR-191-5p and miR-21-5p were far behind with values close to each other (0.868 and 0.842, respectively) (Fig. 3A). AUC for the remaining 4 miRNAs was lower than 0.8 (Fig. 3B). Using combination models of the three best markers did not increase at least a hundredth of the best individual AUC.
Table 4. ROC analysis results for potential markers and their combinations.
| Classes | Potential markers/ combinations | Cel-miR-39 normalization | miR-222-3p normalization | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Optimal cut-of value (point) | Specificity | Sensitivity | Accuracy | AUC | Optimal cut-of value (point) | Specificity | Sensitivity | Accuracy | ||
| Controls vs BC patients | miR-145-5p | 0.932 | 0.535 (−12.17) | 0.857 | 0.909 | 0.882 | 0.932 | 0.32 (−1.77) | 0.788 | 0.971 | 0.882 |
| miR-16-5p | 0.664 | 0.51 (−4.45) | 0.606 | 0.686 | 0.647 | – | – | – | – | – | |
| miR-191-5p | 0.868 | 0.60 (−9.00) | 0.939 | 0.714 | 0.824 | 0.904 | 0.421 (1.37) | 0.818 | 0.914 | 0.868 | |
| miR-21-5p | 0.842 | 0.58 (−8.21) | 0.848 | 0.743 | 0.794 | 0.705 | 0.54 (2.42) | 0.848 | 0.714 | 0.779 | |
| miR-210-3p | 0.713 | 0.55 (−13.86) | 0.758 | 0.657 | 0.706 | – | – | – | – | – | |
| miR-222-3p | 0.760 | 0.549 (−10.57) | 0.879 | 0.657 | 0.765 | – | – | – | – | – | |
| miR-29c-3p | 0.739 | 0.68 (−15.23) | 0.970 | 0.457 | 0.706 | – | – | – | – | – | |
| miR-145-5p + miR-191-5p | 0.930 | 0.52 | 0.879 | 0.886 | 0.882 | 0.984 | 0.72 | 1.000 | 0.943 | 0.971 | |
| miR-145-5p + miR-21-5p | 0.936 | 0.44 | 0.818 | 0.943 | 0.882 | 0.932 | 0.44 | 0.818 | 0.914 | 0.868 | |
| miR-191-5p + miR-21-5p | 0.875 | 0.36 | 0.697 | 0.914 | 0.809 | 0.919 | 0.53 | 0.879 | 0.857 | 0.868 | |
| miR-145-5p + miR-191-5p + miR-21-5p | 0.933 | 0.605 | 0.939 | 0.829 | 0.882 | 0.984 | 0.72 | 1.000 | 0.943 | 0.971 | |
| HER2- vs HER2+ | miR-145-5p | – | – | – | – | – | 0.751 | 0.15 (−0.98) | 0.481 | 1.000 | 0.588 |
| miR-191-5p | – | – | – | – | – | 0.831 | 0.147 (1.98) | 0.667 | 1.000 | 0.735 | |
| Ki-67: <20% vs ≥20% | miR-21-5p | – | – | – | – | – | 0.791 | 0.506 (2.55) | 0.706 | 0.944 | 0.829 |
| Tumor grade: G2 vs G3 | miR-145-5p | – | – | – | – | – | 0.780 | 0.32 (−0.25) | 0.667 | 0.964 | 0.912 |
| miR-210-3p | – | – | – | – | – | 0.845 | 0.10 (−3.73) | 1.000 | 0.679 | 0.735 | |
Figure 3. ROC plots for miRNAs, showing significant differences in plasma levels between BC patients group and control group.
(A) miR-145-5p, miR-191-5p and miR-21-5p, normalized to cel-miR-39; (B) miR-16-5p, miR-210-3p, miR-222-3p and miR-29c-3p, normalized to cel-miR-39; (C) miR-145-5p, miR-191-5p and miR-21-5p, normalized to miR-222-3p; (D) Combination of miR-145-5p and miR-191-5p, normalized to miR-222-3p.
When normalized to miR-222-3p, only three miRNAs, that showed significant differences in concentration between BC patients and controls, were tested for suitability as diagnostic markers. Although log2 fold change was significantly reduced relative to cel-miR-39 normalization, the AUC for miR-145-5p was the same 0.932, and for miR-191-5p even increased and amounted to 0.904 (Fig. 3C). The diagnostic effectiveness of miR-21-5p significantly decreased to AUC = 0.705. The combination of miR-145-5p and miR-191-5p in one model made it possible to increase AUC to 0.984 (Fig. 3D) with the highest specificity, good sensitivity (0.943) and accuracy of separation (97%). The addition of miR-21-5p to this combination did not lead to changes in indicators.
We also tested the ability of miRNAs to separate BC patients according to clinicopathological parameters. ROC analysis showed that using miR-145-5p and miR-191-5p it was possible to distinguish patients with HER2 negative tumors from patients with HER2 positive tumors with 58% and 74% accuracy, respectively; using miR-21-5p it was possible to divide patients into low and high Ki-67 groups (<20% vs ≥20%) with 83% accuracy; using miR-145-5p and miR-210-3p it was possible to distinguish patients with moderately differentiated and poorly differentiated tumors with 92% and 74% accuracy, respectively.
Discussion
When planning the study, in accordance with literature, we selected 5 miRNAs as candidate markers (miR-21-5p, miR-145-5p, miR-210-3p, miR-222-3p and miR-29c-3p), one miRNA as candidate marker or endogenous control (miR-16-5p), one miRNA as endogenous control (miR-191-5p), and one miRNA as exogenous spike-in control (cel-miR-39). However, for the reasons stated below, we decided not to use the spike-in control and chose miR-222-3p as the endogenous control.
When working with bio-fluids, the amount of input biomaterial is easily standardized by the specified volume of the sample, thereby it is possible to take into account the differences that arise during RNA isolation. This is achieved by adding to the sample a certain dose of synthetic miRNA at the step of lysis (Kroh et al., 2010). The lack of reliable and universally accepted endogenous control for miRNA data normalization (Schwarzenbach et al., 2015) determines the relevance of using a spike-in control. Therefore, we first tested spike-in control normalization method.
When we used cel-miR-39 as reference, the average ΔCt values for all 7 miRNAs in BC patients were significantly higher than in controls. These results seem suspicious, although it is possible that they reflect the actual difference between compared groups. Second explanation: blood specimens of the compared groups differed in the degree of hemolysis, although plasma with visually distinct hemolysis was excluded from the analysis in advance. However, Appierto et al. showed that the initial stages of hemolysis are visually indistinguishable (Appierto et al., 2014). In our case, the level of miR-16-5p, which is considered as a marker of hemolysis (Pizzamiglio et al., 2017), varied less in comparison with other miRNAs. The third explanation: two groups differed in the content of plasma proteins and lipids associated with miRNA, which may affect the efficiency of miRNA isolation, as suggested by Sourvinou, Markou & Lianidou (2013). They found that the Trizol method yielded a reduced amount of spike-in cel-miR-39 compared to endogenous miR-21. In our case, the average Ct value for cel-miR-39 in the group of BC patients was significantly lower than that in the control group (P = 0.003), but for targeted miRNAs the difference was even more considerable. The obtained data indicate better efficiency of RNA isolation in the group of BC patients, but it is unclear whether the yield of the added synthetic cel-miR-39 and endogenous miRNA in each of the two groups is equal. Due to the ambiguity in this matter, we could not confidently use the spike-in control to normalize our data. Perhaps using column-based RNA isolation methods would solve this problem, as shown by Sourvinou, Markou & Lianidou (2013).
Since the spike-in control was inappropriate, we evaluated the concentration stability of endogenous miRNAs to determine its suitability as an internal control. Surprisingly, both initial candidates for reference, miR-191-5p and miR-16-5p, were inferior in stability to other miRNAs. Based on an analysis of concentration stability of our miRNA, and also taking into account the relative abundance of transcripts, we chose miR-222-3p as reference, although initially we selected it as target miRNA for the study in accordance with literature screening (Hu et al., 2012; Song et al., 2017; Kim et al., 2019). Previously, this miRNA was already used as a reference in such studies (Tay et al., 2017). After replacing spike-in cel-miR-39 by endogenous miR-222-3p the difference in the target miRNAs level between the two groups considerably decreased, and as a result, the number of dysregulated miRNAs was reduced to three. Despite this, according to the ROC analysis, the ability of miR-145-5p to distinguish BC patients from controls remained the same; for miR-191-5p it even increased; and the combination of the two made it possible to further improve the separation efficiency. In addition, based on these data, we found associations with clinicopathological parameters for some miRNAs. These arguments suggest that we selected the endogenous control correctly, and our results reflect the real state of things.
miR-191-5p is probably the most commonly used as endogenous control in quantitative studies of circulating miRNAs. To date, there is evidence of important role of miR-191 in tumorigenesis and its dysregulation in a wide range of cancers, including BC (Gao et al., 2017; Zhang et al., 2018). Two studies showed the association of circulating miR-191 with BC (Ng et al., 2013; Mar-Aguilar et al., 2013). In agreement with these data, we also found a significant upregulation of circulating miR-191-5p in BC patients compared to healthy women. In addition, the concentration of miR-191-5p differed in plasma of BC patients depending on HER-2 status of the tumor.
miR-16-5p has also been frequently used previously as an endogenous control (McDermott, Kerin & Miller, 2013; Donati, Ciuffi & Brandi, 2019). At the same time, several studies report about increased miR-16-5p concentrations in plasma of BC patients compared to healthy controls (Hu et al., 2012; Ng et al., 2013; Stückrath et al., 2015; Usmani et al., 2017). A meta-analysis of the diagnostic and prognostic value of miR-16 showed that its use as a biomarker is more applicable in Asian populations (Cui, 2015). Our data are not consistent with the aforementioned studies: we found no significant differences in plasma levels of miR-16-5p between breast cancer patients and the controls in the Kazakh population.
miR-145-5p showed the most significant association with BC in our study. This miRNA inhibits the expression of certain oncogenes and thus acts as a tumor suppressor (Sachdeva et al., 2009). In accordance with this concept, most previous studies reported about reduced level of circulating miR-145 in BC patients compared to controls (Ng et al., 2013; Kodahl et al., 2014; Hu et al., 2015). In contrast, in the aforementioned study, Mar-Aguilar et al. (2013) found elevated mir-145-5p level in the serum of BC patients, which is consistent with our data. Thus, according to the identified associations of miR-145-5p and miR-191-5p, our Kazakh population is similar to the Mexican one, and differs from other studied populations. Our results in comparison with published data confirm the thesis that the applicability of the miRNA-marker needs to be verified for certain ethnic group. The revealed differences in plasma miR-145-5p concentration between BC patients with early and late menarche may help to further understand the role of this miRNA in the pathogenesis of BC.
The most frequently mentioned circulating miRNA in association with BC is miR-21-5p (Schwarzenbach, 2017; Adhami et al., 2018). We also confirm this association in the Kazakh population. The NormFinder showed a wide range of miR-21-5p variation in the BC patient group, which indicates the heterogeneity of this group. Indeed, we found significant differences in miR-21-5p level between groups separated by some clinicopathological parameters. We found its significantly increased concentration in the plasma of BC patients with high Ki-67, which is consistent with the data that miR-21 promotes BC proliferation (Qiu et al., 2018; Wang et al., 2019). Early menarche and reduced breastfeeding are considered as risk factors for BC (Jeong et al., 2017; Khalis et al., 2018). We found associations of both factors with elevation of miR-21-5p in plasma of Kazakh women. According to our data, miR-21-5p can play an important role in the development of BC in women with these risk factors.
miR-210 is known as a marker of hypoxia during tumor development; and in BC, hypoxia is associated with resistance to therapy and poor prognosis (Camps et al., 2008; Pasculli et al., 2019). Previous studies have shown that dysregulation of circulating miR-210 in BC is associated with tumor presence and lymph node metastasis in patients with HER-2 positive BC (Jung et al., 2012), metastases (Markou et al., 2016; Madhavan et al., 2016) and resistance to chemotherapy (Jung et al., 2012; Shao et al., 2019). In our study, unfortunately, patients with lymph node metastasis were insignificantly represented (N = 7); and there was only one patient with distant metastases. We found no difference in the plasma levels of miR-210-3p in these patients compared to other patients. Instead, we found increased levels of miR-210-3p in patients with poorly differentiated tumor (grade 3) compared with patients with moderate differentiated tumor (grade 2). The findings are consistent with the result of a previous study, which showed an increased expression of miR-210 in poorly differentiated tumors compared to well-differentiated tumors (Wu, 2020). Thus, we have shown that circulating miR-210-3p can be a marker of aggressive, poorly differentiated tumors.
miR-29 has been shown to have an important role in cancer development (Kwon et al., 2018). In most cancer, miR-29 acts as a tumor suppressor by promoting tumor cell apoptosis, by suppressing DNA methylation of tumor-suppressor genes and by reducing proliferation of tumors and by increasing chemosensitivity (Jiang et al., 2014). In contrast, in BC, miR-29 acts as an oncogene by inhibiting fibrosis and thereby promoting epithelial-mesenchymal transition (Jiang et al., 2014; Wang et al., 2017). In line with this, it has been shown that miR-29 is up-regulated both in breast tumors and in the serum of BC patients (Wu et al., 2012; Zhang et al., 2015). But, we found no significant differences in plasma miR-29c-3p concentration between BC patients and controls in the Kazakh population. Instead, we found that level of circulating miR-29c-3p decrease in women (healthy controls) after age 50 compared to younger women. Taking into account the anti-fibrotic activity of miR-29, our data are consistent with the fact that fibrotic processes increase with advancing age (Nho, 2015).
To evaluate the diagnostic effectiveness of potential markers, we performed a ROC analysis. We identified two miRNAs—miR-145-5p and miR-191-5p, which are able to accurately distinguish patients with BC from healthy women, both individually and in combination. The most effective is their combination model, which showed 97% accuracy in the separation of two groups—66 out of 68 women were classified correctly. The applicability of the revealed diagnostic capabilities of miRNAs according to clinicopathological parameters is debatable.
Although we found a promising combination of miRNA-markers to differentiate BC patients from healthy people, there are a few suggestions for further research. As the sample size is small, further validations in large cohort are recommended. The majority of BC patients in our study had T2 tumors; so, it is necessary to check whether the data obtained are valid for other stages of tumor progression. Also, it is desirable to investigate whether our miRNAs are reversed in plasma of BC patients undergoing treatment. In addition, it would be interesting to study the expression of this miRNAs in tumor tissue to test the secretory hypothesis.
Conclusions
When using spike-in cel-miR-39 as a reference, we obtained doubtful results. Some possible reasons are unequal isolation efficiency of endogenous and spike-in miRNA in each of the two groups, visually undetectable hemolysis, or other unknown factors. Endogenous controls selected according to the literature should be verified in the current study. Based on the results of the analysis of concentration stability as well as taking into account the relative abundance of transcripts, we selected miR-222-3p as the endogenous control for our samples.
We revealed three plasma miRNAs (miR-145-5p, miR-191-5p and miR-21-5p) significantly elevated in BC patients compared to control group. ROC analysis showed, that using miR-145-5p and miR-191-5p (both individually and in combination), it is possible to separate BC patients from healthy individuals quite accurately, therefore, these miRNAs should be considered as potential biomarkers for BC detection in Kazakh population. The inconsistency of some of our results with published data suggests that it is necessary to verify biomarkers for certain ethnic group. The findings need to be confirmed on a more representative cohort of samples.
Supplemental Information
Funding Statement
This research was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan (Grant No. AP05132207). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yeldar Ashirbekov conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Arman Abaildayev conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Nazgul Omarbayeva conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, collection of biomaterial, and approved the final draft.
Dauren Botbayev and Anel Askandirova performed the experiments, prepared figures and/or tables, collection of biomaterial, and approved the final draft.
Ayaz Belkozhayev performed the experiments, prepared figures and/or tables, and approved the final draft.
Alena Neupokoyeva, Kamalidin Sharipov and Nagima Aitkhozhina conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Gulzhakhan Utegenova analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Local ethics committee of the M. Aitkhozhin Institute of Molecular Biology and Biochemistry, Almaty, Kazakhstan (185/01-02).
Data Availability
The following information was supplied regarding data availability:
Experimental and clinico-pathological data is available as Supplemental File.
References
- Acunzo et al. (2015).Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer—a brief overview. Advances in Biological Regulation. 2015;57:1–9. doi: 10.1016/j.jbior.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Adhami et al. (2018).Adhami M, Haghdoost AA, Sadeghi B, Malekpour Afshar R. Candidate miRNAs in human breast cancer biomarkers: a systematic review. Breast Cancer. 2018;25(2):198–205. doi: 10.1007/s12282-017-0814-8. [DOI] [PubMed] [Google Scholar]
- Aggarwal, Priyanka & Tuli (2020).Aggarwal V, Priyanka K, Tuli HS. Emergence of circulating microRNAs in breast cancer as diagnostic and therapeutic efficacy biomarkers. Molecular Diagnosis & Therapy. 2020;24(2):153–173. doi: 10.1007/s40291-020-00447-w. [DOI] [PubMed] [Google Scholar]
- Andersen, Jensen & Orntoft (2004).Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research. 2004;64(15):5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- Appierto et al. (2014).Appierto V, Callari M, Cavadini E, Morelli D, Daidone MG, Tiberio P. A lipemia-independent NanoDrop-based score to identify hemolysis in plasma and serum samples. Bioanalysis. 2014;6(9):1215–1226. doi: 10.4155/bio.13.344. [DOI] [PubMed] [Google Scholar]
- Camps et al. (2008).Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clinical Cancer Research. 2008;14(5):1340–1348. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- Cui (2015).Cui J. MiR-16 family as potential diagnostic biomarkers for cancer: a systematic review and meta-analysis. International Journal of Clinical and Experimental Medicine. 2015;8(2):1703–1714. [PMC free article] [PubMed] [Google Scholar]
- Donati, Ciuffi & Brandi (2019).Donati S, Ciuffi S, Brandi ML. Human circulating miRNAs real-time qRT-PCR-based analysis: an overview of endogenous reference genes used for data normalization. International Journal of Molecular Sciences. 2019;20(18):4353. doi: 10.3390/ijms20184353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, Evoy, McDermott (2010).Duffy MJ, Evoy D, McDermott EW. CA 15-3: uses and limitation as a biomarker for breast cancer. Clinica Chimica Acta. 2010;411(23–24):1869–1874. doi: 10.1016/j.cca.2010.08.039. [DOI] [PubMed] [Google Scholar]
- EBCTCG (2015).Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2017).Gao X, Xie Z, Wang Z, Cheng K, Liang K, Song Z. Overexpression of miR-191 predicts poor prognosis and promotes proliferation and invasion in esophageal squamous cell carcinoma. Yonsei Medical Journal. 2017;58(6):1101–1110. doi: 10.3349/ymj.2017.58.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinge et al. (2017).Glinge C, Clauss S, Boddum K, Jabbari R, Jabbari J, Risgaard B, Tomsits P, Hildebrand B, Kaab S, Wakili R, Jespersen T, Tfelt-Hansen J. Stability of circulating blood-based MicroRNAs—pre-analytic methodological considerations. PLOS ONE. 2017;12(2):e0167969. doi: 10.1371/journal.pone.0167969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goksuluk et al. (2016).Goksuluk D, Korkmaz S, Zararsiz G, Karaağaoğlu AE. EasyROC: an interactive web-tool for ROC curve analysis using R language environment. The R Journal. 2016;8(2):213–230. doi: 10.32614/RJ-2016-042. [DOI] [Google Scholar]
- Grasedieck et al. (2012).Grasedieck S, Scholer N, Bommer M, Niess JH, Tumani H, Rouhi A, Bloehdorn J, Liebisch P, Mertens D, Dohner H, Buske C, Langer C, Kuchenbauer F. Impact of serum storage conditions on microRNA stability. Leukemia. 2012;26:2414–2444. doi: 10.1038/leu.2012.106. [DOI] [PubMed] [Google Scholar]
- Greenlee et al. (2017).Greenlee H, DuPont-Reyes MJ, Balneaves LG, Carlson LE, Cohen MR, Deng G, Johnsom JA, Mumber M, Seely D, Zick SM, Boyce LM, Tripathy D. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA: A Cancer Journal for Clinicians. 2017;67(3):194–232. doi: 10.3322/caac.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Zotano & Arteaga (2017).Guerrero-Zotano AL, Arteaga CL. Neoadjuvant trials in ER+ breast cancer: a tool for acceleration of drug development and discovery. Cancer Discovery. 2017;7(6):561–574. doi: 10.1158/2159-8290.CD-17-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamam et al. (2017).Hamam R, Hamam D, Alsaleh KA, Kassem M, Zaher W, Alfayez M, Aldahmash A, Alajez NM. Circulating microRNAs in breast cancer: novel diagnostic and prognostic biomarkers. Cell Death & Disease. 2017;8(9):e3045. doi: 10.1038/cddis.2017.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfelmann, Anjos & Ayala (2014).Höfelmann DA, Anjos JC, Ayala AL. Survival for ten years and prognostic factors for women with breast cancer in Joinville in the State of Santa Catarina, Brazil. Ciencia e Saude Coletiva. 2014;19(6):1813–1824. doi: 10.1590/1413-81232014196.03062013. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2012).Hu Z, Dong J, Wang LE, Ma H, Liu J, Zhao Y, Tang J, Chen X, Dai J, Wei Q, Zhang C, Shen H. Serum microRNA profiling and breast cancer risk: the use of miR-484/191 as endogenous controls. Carcinogenesis. 2012;33(4):828–834. doi: 10.1093/carcin/bgs030. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2015).Hu J, Xu J, Wu Y, Chen Q, Zheng W, Lu X, Zhou C, Jiao D. Identification of microRNA-93 as a functional dysregulated miRNA in triple-negative breast cancer. Tumor Biology. 2015;36(1):251–258. doi: 10.1007/s13277-014-2611-8. [DOI] [PubMed] [Google Scholar]
- Jansson & Lund (2012).Jansson MD, Lund AH. MicroRNA and cancer. Molecular Oncology. 2012;6(6):590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong et al. (2017).Jeong SH, An YS, Choi JY, Park B, Kang D, Lee MH, Han W, Noh DY, Yoo K-Y, Park SK. Risk reduction of breast cancer by childbirth, breastfeeding, and their interaction in Korean women: heterogeneous effects across menopausal status, hormone receptor status, and pathological subtypes. Journal of Preventive Medicine and Public Health. 2017;50(6):401–410. doi: 10.3961/jpmph.17.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2014).Jiang H, Zhang G, Wu JH, Jiang CP. Diverse roles of miR-29 in cancer (review) Oncology Reports. 2014;31(4):1509–1516. doi: 10.3892/or.2014.3036. [DOI] [PubMed] [Google Scholar]
- Jung et al. (2012).Jung EJ, Santarpia L, Kim J, Esteva FJ, Moretti E, Buzdar AU, Leo AD, Le XF, Jr RCBast, Park ST, Pusztai L, Calin GA. Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer. 2012;118(10):2603–2614. doi: 10.1002/cncr.26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalis et al. (2018).Khalis M, Charbotel B, Chajès V, Rihaldi S, Moskal A, Biessy C, Dossus L, Huybrechts I, Fort E, Mellas N, Elfakir S, Charaka H, Nejjari C, Romieu I, Rhazi RE. Menstrual and reproductive factors and risk of breast cancer: a case-control study in the Fez region, Morocco. PLOS ONE. 2018;3(1):e0191333. doi: 10.1371/journal.pone.0191333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2019).Kim J, Oh S, Park S, Ahn S, Choi Y, Kim G, Kim SI, Lee H. Circulating miR-221 and miR-222 as potential biomarkers for screening of breast cancer. Biomedical Science Letters. 2019;25:185–189. doi: 10.15616/BSL.2019.25.2.185. [DOI] [Google Scholar]
- Kodahl et al. (2014).Kodahl AR, Lyng MB, Binder H, Cold S, Gravgaard K, Knoop AS, Ditzel HJ. Novel circulating microRNA signature as a potential non-invasive multi-marker test in ER-positive early-stage breast cancer: a case control study. Molecular Oncology. 2014;8(5):874–883. doi: 10.1016/j.molonc.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Königshoff et al. (2009).Königshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Günther A, Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. Journal of Clinical Investigation. 2009;119(4):772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroh et al. (2010).Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR) Methods. 2010;50(4):298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon et al. (2018).Kwon JJ, Factora TD, Dey S, Kota J. A systematic review of miR-29 in cancer. Molecular Therapy—Oncolytics. 2018;12:173–194. doi: 10.1016/j.omto.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee & Seo (2018).Lee SY, Seo JH. Current strategies of endocrine therapy in elderly patients with breast cancer. BioMed Research International. 2018;2018:6074808. doi: 10.1155/2018/6074808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2020).Li Q, Qin T, Bi Z, Hong H, Ding L, Chen J, Wu W, Lin X, Fu W, Zheng F, Yao Y, Luo M, Saw PEr, Wulf GM, Xu X, Song E, Yao H, Hu H. Rac1 activates non-oxidative pentose phosphate pathway to induce chemoresistance of breast cancer. Nature Communications. 2020;11(1):1456. doi: 10.1038/s41467-020-15308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan et al. (2016).Madhavan D, Peng C, Wallwiener M, Zucknick M, Nees J, Schott S, Rudolph A, Riethdorf S, Trumpp A, Pantel K, Sohn C, Chang-Claude J, Schneeweiss A, Burwinkel B. Circulating miRNAs with prognostic value in metastatic breast cancer and for early detection of metastasis. Carcinogenesis. 2016;37(5):461–470. doi: 10.1093/carcin/bgw008. [DOI] [PubMed] [Google Scholar]
- Mar-Aguilar et al. (2013).Mar-Aguilar F, Mendoza-Ramírez JA, Malagón-Santiago I, Espino-Silva PK, Santuario-Facio SK, Ruiz-Flores P, Rodriguez-Padilla C, Resendez-Perez D. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Disease Markers. 2013;34(3):163–169. doi: 10.3233/DMA-120957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou et al. (2016).Markou A, Zavridou M, Sourvinou I, Yousef G, Kounelis S, Malamos N, Georgoulias V, Lianidou E. Direct comparison of metastasis-related miRNAs expression levels in circulating tumor cells, corresponding plasma, and primary tumors of breast cancer patients. Clinical Chemistry. 2016;62(7):1002–1011. doi: 10.1373/clinchem.2015.253716. [DOI] [PubMed] [Google Scholar]
- McDermott, Kerin & Miller (2013).McDermott AM, Kerin MJ, Miller N. Identification and validation of miRNAs as endogenous controls for RQ-PCR in blood specimens for breast cancer studies. PLOS ONE. 2013;8(12):e83718. doi: 10.1371/journal.pone.0083718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald et al. (2016).McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM. Clinical diagnosis and management of breast cancer. The Journal of Nuclear Medicine. 2016;57(Suppl 1):9S–16S. doi: 10.2967/jnumed.115.157834. [DOI] [PubMed] [Google Scholar]
- McGuire, Brown & Kerin (2015).McGuire A, Brown JA, Kerin MJ. Metastatic breast cancer: the potential of miRNA for diagnosis and treatment monitoring. Cancer and Metastasis Reviews. 2015;34(1):145–155. doi: 10.1007/s10555-015-9551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moo et al. (2018).Moo TA, Sanford R, Dang C, Morrow M. Overview of breast cancer therapy. PET Clinics. 2018;13(3):339–354. doi: 10.1016/j.cpet.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakshatri, Anjanappa & Bhat-Nakshatri (2015).Nakshatri H, Anjanappa M, Bhat-Nakshatri P. Ethnicity-dependent and -independent heterogeneity in healthy normal breast hierarchy impacts tumor characterization. Scientific Reports. 2015;5:13526. doi: 10.1038/srep13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar, Nasr & Talhouk (2017).Nassar FJ, Nasr R, Talhouk R. MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharmacology & Therapeutics. 2017;172:34–49. doi: 10.1016/j.pharmthera.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Nassar et al. (2017).Nassar FJ, Talhouk R, Zgheib NK, Tfayli A, Sabban MEl, El Saghir NS, Boulos F, Jabbour MN, Chalala C, Boustany M, Kadara H, Zhang Z, Zheng Y, Joyce B, Hou L, Bazarbachi A, Calin G, Nasr R. MicroRNA expression in ethnic specific early stage breast cancer: an integration and comparative analysis. Scientific Reports. 2017;7(1):16829. doi: 10.1038/s41598-017-16978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson et al. (2016).Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. preventive services task force recommendation. Annals of Internal Medicine. 2016;164(4):244–255. doi: 10.7326/M15-0969. [DOI] [PubMed] [Google Scholar]
- Ng et al. (2013).Ng EK, Li R, Shin VY, Jin HC, Leung CP, Ma ES, Pang R, Chua D, Chu KM, Law WL, Law SYK, Poon RTP, Kwong A. Circulating microRNAs as specific biomarkers for breast cancer detection. PLOS ONE. 2013;8(1):e53141. doi: 10.1371/journal.pone.0053141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nho (2015).Nho RS. Alteration of aging-dependent MicroRNAs in idiopathic pulmonary fibrosis. Drug Development Research. 2015;76(7):343–353. doi: 10.1002/ddr.21272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onega et al. (2016).Onega T, Goldman LE, Walker RL, Miglioretti DL, Buist DS, Taplin S, Geller BM, Hill DA, Smith-Bindman R. Facility mammography volume in relation to breast cancer screening outcomes. Journal of Medical Screening. 2016;23(1):31–37. doi: 10.1177/0969141315595254. [DOI] [PubMed] [Google Scholar]
- Özdemir & Dotto (2017).Özdemir BC, Dotto GP. Racial differences in cancer susceptibility and survival: more than the color of the skin? Trends in Cancer. 2017;3(3):181–197. doi: 10.1016/j.trecan.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasculli et al. (2019).Pasculli B, Barbano R, Rendina M, Fontana A, Copetti M, Mazza T, Valori VM, Morritti M, Maiello E, Graziano P, Murgo R, Fazio VM, Esteller M, Parrella P. Hsa-miR-210-3p expression in breast cancer and its putative association with worse outcome in patients treated with Docetaxel. Scientific Reports. 2019;9:14913. doi: 10.1038/s41598-019-51581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phi et al. (2018).Phi XA, Tagliafico A, Houssami N, Greuter MJW, De Bock GH. Digital breast tomosynthesis for breast cancer screening and diagnosis in women with dense breasts—a systematic review and meta-analysis. BMC Cancer. 2018;18(1):380. doi: 10.1186/s12885-018-4263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzamiglio et al. (2017).Pizzamiglio S, Zanutto S, Ciniselli CM, Belfiore A, Bottelli S, Gariboldi M, Verderio P. A methodological procedure for evaluating the impact of hemolysis on circulating microRNAs. Oncology Letters. 2017;13(1):315–320. doi: 10.3892/ol.2016.5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potosky et al. (2015).Potosky AL, O’Neill SC, Isaacs C, Tsai HT, Chao C, Liu C, Ekezue BF, Selvam N, Kessler LG, Zhou Y, Schwartz MD. Population-based study of the effect of gene expression profiling on adjuvant chemotherapy use in breast cancer patients under the age of 65 years. Cancer. 2015;121(22):4062–4070. doi: 10.1002/cncr.29621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu et al. (2018).Qiu Y-F, Wang M-X, Meng L-N, Zhang R, Wang W. MiR-21 regulates proliferation and apoptosis of oral cancer cells through TNF- α. European Review for Medical and Pharmacological Science. 2018;22(22):7735–7741. doi: 10.26355/eurrev_201811_16395. [DOI] [PubMed] [Google Scholar]
- Sachdeva et al. (2009).Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo Y-Y. P53 represses c-Myc through induction of the tumor suppressor miR-145. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenbach (2017).Schwarzenbach H. Clinical relevance of circulating, cell-free and exosomal microRNAs in plasma and serum of breast cancer patients. Oncology Research and Treatment. 2017;40(7–8):423–429. doi: 10.1159/000478019. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach et al. (2015).Schwarzenbach H, Da Silva AM, Calin G, Pantel K. Data normalization strategies for MicroRNA quantification. Clinical Chemistry. 2015;61(11):1333–1342. doi: 10.1373/clinchem.2015.239459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao et al. (2019).Shao B, Wang X, Zhang L, Li D, Liu X, Song G, Cao H, Zhu J, Li H. Plasma microRNAs predict chemoresistance in patients with metastatic breast cancer. Technology in Cancer Research and Treatment. 2019;18:1533033819828709. doi: 10.1177/1533033819828709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al. (2017).Song J, Ouyang Y, Che J, Li X, Zhao Y, Yang K, Zhao X, Chen Y, Fan C, Yuan W. Potential value of miR-221/222 as diagnostic, prognostic, and therapeutic biomarkers for diseases. Frontiers in Immunology. 2017;8:56. doi: 10.3389/fimmu.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourvinou, Markou & Lianidou (2013).Sourvinou IS, Markou A, Lianidou ES. Quantification of circulating miRNAs in plasma: effect of preanalytical and analytical parameters on their isolation and stability. The Journal of Molecular Diagnostics. 2013;15(6):827–834. doi: 10.1016/j.jmoldx.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Stückrath et al. (2015).Stückrath I, Rack B, Janni W, Jäger B, Pantel K, Schwarzenbach H. Aberrant plasma levels of circulating miR-16, miR-107, miR-130a and miR-146a are associated with lymph node metastasis and receptor status of breast cancer patients. Oncotarget. 2015;6(15):13387–13401. doi: 10.18632/oncotarget.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al. (2018).Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Yuan W. Effect of exosomal miRNA on cancer biology and clinical applications. Molecular Cancer. 2018;17(1):147. doi: 10.1186/s12943-018-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay et al. (2017).Tay JW, James I, Hughes QW, Tiao JY, Baker RI. Identification of reference miRNAs in plasma useful for the study of oestrogen-responsive miRNAs associated with acquired Protein S deficiency in pregnancy. BMC Research Notes. 2017;10(1):312. doi: 10.1186/s13104-017-2636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usmani et al. (2017).Usmani A, Shoro AA, Shirazi B, Memon Z, Hussain M. MiR-16: a novel hereditary marker in breast cancer and their offspring. Journal of Pakistan Medical Association. 2017;67(3):446–450. [PubMed] [Google Scholar]
- Vandesompele et al. (2002).Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3(7):34.1–34.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang (2017).Wang L. Early diagnosis of breast cancer. Sensors. 2017;17(7):1572. doi: 10.3390/s17071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang H, An X, Yu H, Zhang S, Tang B, Zhang X, Li Z. MiR-29b/TET1/ZEB2 signaling axis regulates metastatic properties and epithelial-mesenchymal transition in breast cancer cells. Oncotarget. 2017;8(60):102119–102133. doi: 10.18632/oncotarget.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2019).Wang H, Tan Z, Hu H, Liu H, Wu T, Zheng C, Wang X, Luo Z, Wang J, Liu S, Lu Z, Tu J. MicroRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer. 2019;19(1):738. doi: 10.1186/s12885-019-5951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu (2020).Wu X. Expressions of miR-21 and miR-210 in breast cancer and their predictive values for prognosis. Iranian Journal of Public Health. 2020;49(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2020).Wu SM, Tsai WS, Chiang SF, Lai YH, Ma CP, Wang JH, Lin J, Lu P-S, Yang C-Y, Tan BCM, Liu H. Comprehensive transcriptome profiling of Taiwanese colorectal cancer implicates an ethnic basis for pathogenesis. Scientific Reports. 2020;10(1):4526. doi: 10.1038/s41598-020-61273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2012).Wu Q, Wang C, Lu Z, Guo L, Ge Q. Analysis of serum genome-wide microRNAs for breast cancer detection. Clinica Chimica Acta. 2012;413(13–14):1058–1065. doi: 10.1016/j.cca.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2018).Zhang X, Wu M, Chong QY, Zhang W, Qian P, Yan H, Qian W, Zhang M, Lobie PE, Zhu T. Amplification of hsa-miR-191/425 locus promotes breast cancer proliferation and metastasis by targeting DICER1. Carcinogenesis. 2018;39(12):1506–1516. doi: 10.1093/carcin/bgy102. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2015).Zhang L, Xu Y, Jin X, Wang Z, Wu Y, Zhao D, Chen G, Li D, Wang X, Cao H, Xie Y, Liang Z. A circulating miRNA signature as a diagnostic biomarker for non-invasive early detection of breast cancer. Breast Cancer Research and Treatment. 2015;154(2):423–434. doi: 10.1007/s10549-015-3591-0. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2010).Zhao H, Shen J, Medico L, Wang D, Ambrosone CB, Liu S. A pilot study of circulating miRNAs as potential biomarkers of early stage breast cancer. PLOS ONE. 2010;5(10):e13735. doi: 10.1371/journal.pone.0013735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong et al. (2020).Zhong P, Chen X, Guo R, Chen X, Chen Z, Wei C, Li Y, Wang W, Zhou Y, Qin L. Folic acid-modified nanoerythrocyte for codelivery of paclitaxel and tariquidar to overcome breast cancer multidrug resistance. Molecular Pharmaceutics. 2020;17(4):1114–1126. doi: 10.1021/acs.molpharmaceut.9b01148. [DOI] [PubMed] [Google Scholar]
- Zununi Vahed et al. (2016).Zununi Vahed S, Barzegari A, Rahbar Saadat Y, Mohammadi S, Samadi N. A microRNA isolation method from clinical samples. Bioimpacts. 2016;6(1):25–31. doi: 10.15171/bi.2016.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Experimental and clinico-pathological data is available as Supplemental File.