Abstract
Objective:
To investigate the role of baseline gonadotropins in predicting the biochemical response to clomiphene citrate (CC) treatment.
Methods:
We conducted a retrospective review of data from hypogonadal men treated with CC in two high-volume fertility centers between 2013 and 2018. Patient age, body mass index (BMI), and baseline hormones (follicle stimulating hormone [FSH], luteinizing hormone [LH], and total testosterone [TT]) were obtained. Response to treatment was measured as changes in TT levels within six months of initiating CC treatment. Linear regression models adjusted for age, BMI, and time on CC therapy were fitted to assess the associations between baseline LH and FSH levels with treatment response.
Results:
A total of 332 men with mean ± standard deviation age of 36.2±8.2 years were included. Median time to initial follow-up was 6 weeks (25th–75th interquartile range [IQR]: 4–9 weeks). TT levels increased significantly on CC treatment (mean change: 329.2 ng/dL, 95% CI: 307.4 to 351.0) with 73% of men having at least 200 ng/dL increase over baseline TT levels. In univariable linear regression models, only age was significantly associated with TT response. Neither the baseline LH nor FSH significantly predicted TT response in linear regression models.
Conclusion:
CC treatment results in significant increases in testosterone levels in most men. Baseline gonadotropins are not strong predictors for treatment response to CC. Adequate biochemical response with CC trial can be expected in most patients with normal or slightly elevated baseline gonadotropin levels.
Keywords: testosterone deficiency, clomiphene citrate, gonadotropin, hypogonadism, testosterone, Testosterone replacement therapy
Introduction
The American Urological Association (AUA) guidelines define hypogonadism as a total testosterone level of <300 ng/dL in the presence of hypogonadal signs or symptoms.1 Primary hypogonadism is a result of abnormalities at the testicular level and is usually associated with reduced testosterone concentration with high gonadotropin levels. Conversely, secondary hypogonadism can be due to hypothalamic-pituitary dysfunction and be associated with low or inappropriately normal levels of gonadotropins.2 Patients can also present with combined primary and secondary hypogonadism with variable levels of baseline gonadotropins.2 Testosterone levels decline with aging, and lower levels can lead to infertility, decreased libido, and decreased muscle mass, among other effects.3 Therefore, testosterone therapy and its alternatives are commonly used in clinical practice to improve symptoms and quality of life in men with hypogonadism.4,5
Exogenous testosterone therapy is the mainstay of male hypogonadism treatment and results in an effective increase in testosterone levels.6 However, alternatives to testosterone therapy, with minimal detrimental effects on the hypothalamic-pituitary-gonadal axis, are appealing therapeutic options, especially for younger men and those interested in fertility preservation. Different classes of medications such as human chorionic gonadotropin (hCG), aromatase inhibitors, and selective estrogen receptor modulators (SERMs) have been successfully used to treat hypogonadism.5 Clomiphene citrate (CC) is a SERM that increases gonadotropin levels by inhibiting the negative feedback from estrogen on the hypothalamic-pituitary-gonadal axis. CC indirectly and effectively increases testosterone with minimal side effects and without negatively impacting spermatogenesis.7
Not all hypogonadal patients respond equally to CC treatment. Intuitively, patients with primary hypogonadism will not have an optimal response to medications working at the hypothalamic-pituitary level. Thus, anecdotally, patients with higher levels of gonadotropins are not considered good candidates for CC treatment and some have used arbitrary cut-offs to exclude them from CC treatment.8–11 However, attempts to find predictors of a successful response to CC have produced inconsistent results.12–16 Although some suggest that baseline gonadotropin levels can be used to predict testosterone response to CC, the effect size was small and not clinically significant.12,15,16 Additionally, most these studies had small sample size from single institutions and might have been underpowered to answer this question. Thus, gonadotropin levels below which response to CC is optimized are not established1 and it remains unclear if a baseline hormonal panel can be used to predict the response to CC treatment. We aimed to use a larger cohort of hypogonadal men to test the hypothesis that pre-treatment gonadotropin levels can predict the biochemical testosterone response after CC treatment.
Methods
Study population:
A retrospective review of data from all hypogonadal men treated with CC in two high-volume centers was performed from 2013–2018. Patient age, body mass index (BMI), and baseline hormones (follicle-stimulating hormone [FSH], luteinizing hormone [LH], estradiol [E], and total testosterone [TT]) were obtained. All hormone levels were based on early morning blood draws. Patients were excluded if they: 1) were diagnosed with known genetic causes of primary hypogonadism (e.g., Klinefelter syndrome); 2) were on exogenous testosterone or gonadotropin therapy within 6 months of CC treatment; or 3) did not have any follow-up with hormonal assessment within 6 months of CC initiation.
Definitions:
Hypogonadism was defined as the presence of a TT <300 ng/dL combined with signs and/or symptoms of low testosterone.1 Any of the following was considered a hypogonadal symptom: (i) positive Androgen Deficiency in Aging Males (ADAM)17 questionnaire (a ‘yes’ answer to questions on decreased libido [Q1] or erectile dysfunction [Q7], or any other three questions); or (ii) a chief complaint of reduced energy or endurance, unexplained fatigue, decreased libido, erectile dysfunction, or infertility.1 Biochemical response was assessed as the amount of increase in TT at the first follow-up within six months of CC therapy compared to pre-treatment levels. Furthermore, a categorical (yes vs. no) variable for response to treatment was defined as attaining a total TT increase of 200 ng/dL as previously suggested in the literature.12,16
Treatment and follow-up protocol:
Starting CC dose varied from 25–50 mg; 50% and 27% of patients were started on a 50 mg and 25 mg every other day regimens; 18% and 5% were started on 50 mg and 25 mg daily regimens. We base our starting dose on patient preferences, symptom severity, BMI, and bioavailable testosterone when available and will consider a dose increase if bioavailable testosterone is <250 ng/dL at first follow-up. Our biochemical treatment goal is a TT between 600–900 ng/dL, and we try to keep TT levels <1000 ng/dL with dose adjustment as needed. Follow-up hormonal assessment was different between the centers, but all patients were followed up with a measurement of TT usually 3–4 weeks after initiation of CC therapy to assess initial response and then every 3–6 months. We do not regularly monitor hematocrit values given the negligible risk of hematologic adverse effects with CC.18 PSA was monitored only for high-risk patients or those who remained on CC therapy for >6 months and was not part of our assessment in this study.
Statistical Analysis:
Values are presented as median (25th-75th percentile interquartile range [IQR]), mean (standard deviation [SD]), or mean (standard error [SE]) as appropriate. Independent sample t-test or Wilcoxon rank-sum test were used to compare baseline characteristics and hormone values between independent groups. Changes in TT values from baseline to the first follow-up were calculated and are presented as mean changes (95% confidence interval [CI]). A paired-sample t-test was used to compare the TT changes from baseline.
Linear and curvilinear (quadratic fit) correlations were assessed between baseline gonadotropins and changes in TT levels. Scatter plots were used to graph the data. As the primary goal was to find potential cut-points that are associated with decreased TT response, we subjectively chose the visual transition points that were associated with an increasing downward slope on the quadratic fit regression lines. Using this method, we attempted to find the thresholds that were potentially associated with decreased TT response ignoring the lower values on the x-axis associated with upward or flat parts of the graph.
Univariable and multivariable linear regression models adjusted for age and time on CC therapy were fitted to assess the associations between continuous values of baseline LH and FSH levels with treatment response as measured as changes in TT levels. The same analyses were repeated using the subjective thresholds derived from the curvilinear regression lines for baseline LH and FSH as predictors. Statistical analyses were conducted using STATA 15 (Stata Corp, College Station, TX, USA) with a two-sided p-value <0.05 considered as statistically significant.
Results
A total of 332 men met the inclusion criteria with 66% also having a male infertility complaint. Mean±SD age was 36.2±8.2 years. Median FSH and LH levels at baseline were 4.3 mIU/mL (IQR: 2.7–7.3) and 4.2 mIU/mL (IQR: 2.8–6.2), respectively. Median baseline TT before treatment was 249.5 ng/dL (200.5–298.0). Baseline variables were not significantly different between the two centers except for patient age (38.2±9.9 vs. 35.4±7.3 years, p=0.005); ADAM scores were only available from one center. Baseline patient characteristics and hormonal values are summarized in Table-1.
Table-1.
Baseline characteristics and hormonal values of hypogonadal cohort on clomiphene citrate
| n | Median (IQR) | |
|---|---|---|
| Age, years | 332 | 35 (31–40) |
| BMI, kg/m2 | 317 | 30.3 (26.4–35.1) |
| ADAMa | 175 | 4 (2–6) |
| LH, mIU/mL | 256 | 4.2 (2.8–6.2) |
| FSH, mIU/mL | 306 | 4.3 (2.7–7.3) |
| Total Testorsterone, ng/dL | 332 | 249.5 (200.5–298.0) |
| Bioavailable Testosterone, ng/dL | 184 | 136 (108–160) |
| Estradiol, pg/mL | 265 | 18.9 (14.2–23.1) |
| Albumin g/dL | 234 | 4.5 (4.2–4.7) |
IQR, 25th-75th interquartile range; BMI, body mass index; LH, luteinizing hormone; FSH, follicle stimulating hormone
ADAM data only available from one center
The median time to initial follow-up was six weeks (IQR: 4–9). Median TT at follow-up was 553.5 ng/dL (IQR: 433.0–706.5). On average, TT levels increased 329.2 ng/dL (95% CI: 307.4 to 351.0) in follow-up when compared to pre-treatment levels (p<0.001) and 241 men (73%) had at least 200 ng/dL increase over their baseline TT. No significant linear correlation existed between TT response and any of the baseline hormones (Figure-1). In univariable linear regression, only age was significantly associated with TT response (beta coefficient: −5.7, 95% CI: −8.3 to −3.1). Neither the baseline LH nor FSH significantly predicted TT response using univariable linear regression or multivariable regression adjusted for age and time on CC therapy.
Figure-1:
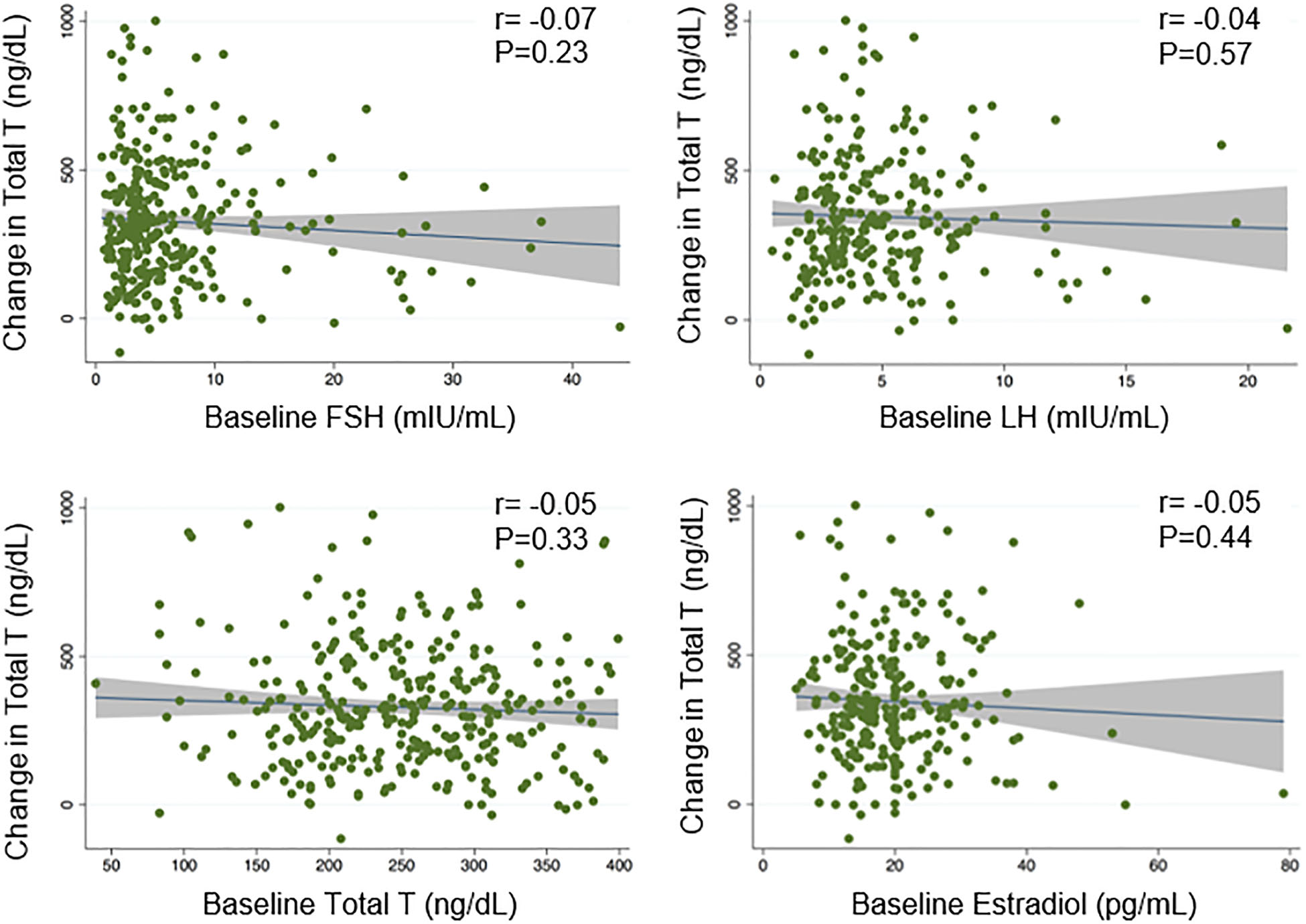
Scatter plots depicting the associations between testosterone response and hormone levels at baseline. None of the baseline hormones had statistically significant linear correlation with testosterone response in patients taking clomiphene citrate.
The subjective visual transition points were chosen at 10 mIU/mL for FSH and 8 mIU/mL for LH (Figure-2). Using the multivariable models, no correlation between FSH and TT response was observed when baseline FSH was ≤10 mIU/mL. For patients with FSH>10 (n=42, 14%), TT response decreased for each unit increase in FSH (slope: −10.7, 95% CI: −18.4 to −3.0) although they still demonstrated a robust average increase in TT, which was not different from those with FSH≤10 mIU/mL (Table-2). For patients with LH >8 (n=28; 11%), TT response decreased for each unit increase in LH (slope: −23.1 per unit, 95% CI: −44.6 to −1.6) although they still demonstrated a robust average increase in TT, which was not different from those with LH ≤8 mIU/mL (Table-2). The linear associations of baseline gonadotropins and TT response for the subgroups of patients with high LH or FSH are presented in Supplementary Figure 1.
Figure-2:
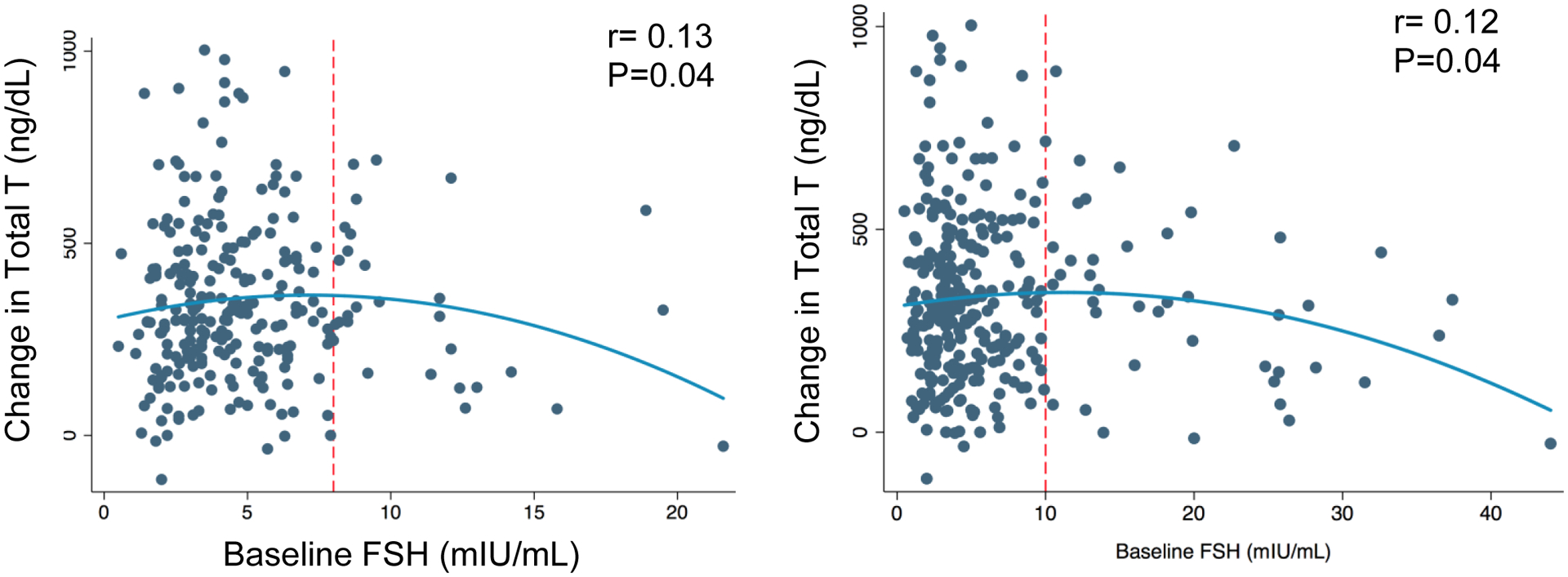
Scatter plots depicting the curvilinear associations between testosterone response and hormone levels at baseline. Red dashed lines indicate the subjective transition points for response to CC treatment.
Table-2.
Average increase in total testosterone levels in patients receiving clomiphene citrate
| TT response (ng/dL) | ||
|---|---|---|
| All patients | 329.2 (95% CI: 307.4–351.0) | |
| FSH categories | ||
| ≤10 mIU/mL | 327.1 (95% CI: 302.0–352.1) | |
| >10 mIU/mL | 323.6 (95% CI: 257.6–389.6) | |
| P-value | 0.92 | |
| LH categories | ||
| ≤8 mIU/mL | 345.6 (95% CI: 266.3–424.9) | |
| >8 mIU/mL | 346.5 (95% CI: 319.2–373.7) | |
| P-value | 0.98 |
Discussion
This study confirms that CC is an effective treatment for increasing testosterone concentration in hypogonadal men and provides an inexpensive orally available alternative to exogenous testosterone and other injectable regimens. Seventy-three percent of men achieved at least a 200 ng/dL increase in TT compared to their baseline levels. However, neither baseline LH nor FSH levels were strong predictors for biochemical response to CC treatment and we were not able to find an LH or FSH threshold that provides a meaningful distinction between those who will or will not have a biochemical response to CC.
Our results showed that patients had an average of 329 ng/dL increase in their TT levels throughout treatment with CC, which are in line with the observed biochemical responses reported in the literature ranging from 200 to 360 ng/dL.16,19–23 For example, in a randomized controlled trial comparing CC and Anastrozole, Helo et al. reported an average of 317 ng/dL increase in TT levels after 12 weeks of treatment on 25 mg daily dose of CC.22 Similarly, in another randomized trial comparing CC and hCG, the authors observed a 304 ng/dL increase in TT levels after 12 weeks of treatment on a 50 mg daily dose of CC.21 The differences in average TT response in these studies could be attributed to differences in CC dosage, follow-up time, study sample size, as well as differences in laboratory standards.
Objective monitoring of treatment response in hypogonadal patients receiving testosterone therapy is not straightforward. In the absence of validated questionnaires to reliably monitor outcomes24, hormonal testing and subjective symptomatic relief is commonly used to monitor patients’ response and adjust treatment dose.1,24 In previous studies, successful treatment of hypogonadism was defined as a TT increase >200 ng/dL within six months of receiving treatment.12,16 Our observed response rate of 73% aligns with those observed by Mazzola et al., which had a successful treatment rate of 64%.16 Our slightly higher response rate could be attributed to different dosing regimens used in our retrospective cohort.16 Although using this definition (TT increase >200 ng/dL) the majority of men with a positive response will potentially fall within what is considered a normal TT range (300–1000 ng/dL), the 200 ng/dL threshold seems to be arbitrary and does not account for the magnitude of TT response as captured in the approach using the continuous TT response. Thus, we mainly used the average change in TT on CC therapy to define the primary outcome in our analyses and believe that this approach provides a more objective way to define treatment response.
Male reproductive physiology is controlled via the hypothalamic-pituitary-testicular axis involving multiple feedback loops regulating gonadotropin secretion. CC works by inhibiting the negative feedback of estradiol on gonadotropins and thus is an effective alternative to increase LH and FSH, and subsequently testosterone levels, in men with reserved testicular function. Thus, in theory, patients with lower pre-treatment LH and FSH levels might have better responses to CC treatment. In fact, patients with elevated gonadotropins at baseline were anecdotally considered poor candidates for CC treatment8–11 as this could reflect primary hypogonadism with limited testicular reserve. However, the thresholds to define high gonadotropins for this purpose are poorly defined and in reality, not all men with higher FSH or LH will fail to respond to CC treatment. Interestingly, most attempts to use baseline gonadotropin levels to predict response to CC treatment (either defined as an increase in testosterone levels or improvement in semen parameters) have been either unsuccessful or inconsistent.12,13,16,23 In theory, this could be related to different factors such as the underlying causes of hypogonadism, the function of the hypothalamic-pituitary axis, testicular reserve for testosterone production, and also patients’ metabolism of CC and sensitivity to the medication.
Considering the predictors for biochemical response to CC, Mazzola et al. studied 32 men taking 25 mg of CC every other day and suggested that greater testicular size and lower LH levels at baseline are associated with a successful response.16 Specifically, the authors found those with LH<6 IU/mL to have 3.5-fold (95% CI:1.9–8.7) higher chances of response to CC in a multivariable analysis including several factors such as age, testicular volume, varicocele grade, baseline free T and TT. In a more recent report, Salter et al.12 performed a similar analysis to find predictors of biochemical response to CC and built a predictive nomogram incorporating age, baseline LH, and baseline TT. However, none of the included factors in their preliminary analysis, including the baseline LH levels, reached the threshold for statistical significance and the overall predictive performance of the model was limited (concordance index=0.54).12 In another study, prolactin levels rather than pre-treatment LH or FSH were associated with response to CC therapy measured as improvements in semen parameters14 although we do not routinely check prolactin levels in hypogonadal men. Our results are more in line with the findings of Salter et al. as none of the included hormonal values in our univariable analyses were significant predictors for biochemical response to CC. We believe that in the absence of univariate statistical significance and given the low overall effect size of baseline gonadotropins in predicting the CC response, a multivariable analysis would not be appropriate.
As our initial hypothesis was that men with higher baseline LH or FSH will have a weaker response to CC treatment, we further tried to find LH and FSH thresholds that will be associated with a decrease in testosterone response. In patients with baseline FSH>10 mIU/mL, the testosterone response slightly decreased for each unit increase in FSH. Of note, these patients still demonstrated a robust biochemical response to CC treatment. Similar results were observed for LH levels of >8 mIU/mL. Although the clinical significance of these small changes per unit is unknown, this decreased response could indicate some degree of testicular dysfunction in men with higher values of LH and FSH. However, we were not able to find an LH or FSH threshold that provides a meaningful distinction between those who will or will not have a biochemical response to CC. We hypothesize that high LH and FSH could be predictors of poor CC response only in patients with primary hypogonadism and greatly diminished testicular reserve, a patient population that is not well-represented in our study cohort. Thus, based on our findings, FSH and LH are not strong predictors for the success of CC treatment in men with normal or slightly elevated baseline gonadotropins.
Our study has some limitations worth discussing. We acknowledge that TT measurement is an imperfect method to assess accurate treatment response, and this should ideally be based on symptom alleviation. However, we do not routinely use ADAM scores as part of our follow-up evaluations and baseline scores were only available from one center. Furthermore, the sensitivity and specificity of the questionnaires such as ADAM are limited and inconsistent with biochemical responses, and they are not currently recommended to monitor symptom response.1,7,24 Some previous studies have suggested that prolactin levels14 and testicular volume16 might be useful predictors of response to CC treatment, however, we did not have consistent data on these variables to include in our analyses. About 15% of the records initially screened did not have any follow-up information and were excluded, as well as additional 3% who did not have an initial early follow-up (within 6 months of starting treatment); we did not gather baseline data on these patients and cannot comment on whether the baseline characteristics and hormonal profile in the excluded group were different from the included cohort. Our patients were started on different doses of CC with some dose adjustments on treatment, which can impact our results. However, there is no standard regimen for CC, and this reflects the real-world practice to select different doses based on initial symptoms and patient characteristics. Importantly, we also used the first hormonal follow-up to define initial response to CC. Thus, dose escalations did not affect our findings. However, using our design, we were not able to assess whether baseline gonadotropins can predict the need for dose adjustments or the final and long-term treatment response on a stable CC dose. Most importantly, the majority of the patients included in this study had lower or near-normal values of baseline LH and FSH; thus, the subgroup analyses for patients with higher values of LH and FSH might lack statistical power. It is possible that the clinical judgment process that led the cohort to be considered for CC treatment, has introduced selection bias to the study by excluding those with primary hypogonadism and very high baseline LH and FSH levels. Additionally, our cohort might represent patients having degrees of mixed primary and secondary hypogonadism who have the testicular reserve to respond to higher values of stimulated LH and FSH even if the pre-treatment values were within normal limits or slightly above normal limits.
Conclusion
Treatment with clomiphene citrate results in a significant increase in testosterone levels in most men. However, pre-treatment LH and FSH serum concentrations are not strong predictors of biochemical treatment response to CC. We were not able to find an LH or FSH threshold that provides a meaningful distinction between those who will or will not have a biochemical response to CC. Thus, adequate biochemical response with CC trial can be expected in most patients with normal or slightly elevated baseline gonadotropin levels.
Supplementary Material
Supplementary Figure-1: Scatter plots depicting the linear associations between testosterone response and gonadotropins for patients with higher pre-treatment levels.
Source of Funding:
None
Footnotes
Conflict of Interest: None of the authors declare any conflicts of interests related to this work.
Statement: The abstract of this work is presented at the 20th annual Fall meeting of the Sexual Medicine Society of North America (SMSNA), October 24–27, 2019, in Nashville, TN, USA.
References
- 1.Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, et al. : Evaluation and Management of Testosterone Deficiency: AUA Guideline. J Urol. 2018; 200(2): 423–432. [DOI] [PubMed] [Google Scholar]
- 2.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. : Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018; 103(5): 1715–1744. [DOI] [PubMed] [Google Scholar]
- 3.Sussman EM, Chudnovsky A and Niederberger CS: Hormonal evaluation of the infertile male: has it evolved? Urol Clin North Am. 2008; 35(2): 147–155, vii. [DOI] [PubMed] [Google Scholar]
- 4.Ko EY, Siddiqi K, Brannigan RE and Sabanegh ES Jr.: Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012; 187(3): 973–978. [DOI] [PubMed] [Google Scholar]
- 5.Lo EM, Rodriguez KM, Pastuszak AW and Khera M: Alternatives to Testosterone Therapy: A Review. Sex Med Rev. 2018; 6(1): 106–113. [DOI] [PubMed] [Google Scholar]
- 6.Traish AM: Benefits and Health Implications of Testosterone Therapy in Men With Testosterone Deficiency. Sex Med Rev. 2018; 6(1): 86–105. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler KM, Sharma D, Kavoussi PK, Smith RP and Costabile R: Clomiphene Citrate for the Treatment of Hypogonadism. Sex Med Rev. 2019; 7(2): 272–276. [DOI] [PubMed] [Google Scholar]
- 8.Chehab M, Madala A and Trussell JC: On-label and off-label drugs used in the treatment of male infertility. Fertil Steril. 2015; 103(3): 595–604. [DOI] [PubMed] [Google Scholar]
- 9.Pasqualotto FF and Pasqualotto EB: Is there a special subgroup of males with adult-onset idiopathic hypogonadotrophic hypogonadism who may respond with increases in sperm concentration after clomiphene citrate? Fertil Steril. 2007; 88(1): 249; author reply 249–250. [DOI] [PubMed] [Google Scholar]
- 10.Paulson DF: Clomiphene citrate in the management of male hypofertility: predictors for treatment selection. Fertil Steril. 1977; 28(11): 1226–1229. [PubMed] [Google Scholar]
- 11.Rambhatla A, Mills JN and Rajfer J: The Role of Estrogen Modulators in Male Hypogonadism and Infertility. Rev Urol. 2016; 18(2): 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salter CA, Zajichek A, Benfante N, Kattan M and Mulhall JP: MP58–05 A Nomogram Predicting Testosterone Response in Men on Clomiphene. Journal of Urology. 2019; 201(Supplement 4): e854–e854. [Google Scholar]
- 13.Sharma D, Zillioux J, Khourdaji I, Reines K, Wheeler K, Costabile R, et al. : Improvements in semen parameters in men treated with clomiphene citrate-A retrospective analysis. Andrologia. 2019; 51(5): e13257. [DOI] [PubMed] [Google Scholar]
- 14.Hammami MM: Hormonal evaluation in idiopathic oligozoospermia: correlation with response to clomiphene citrate therapy and sperm motility. Arch Androl. 1996; 36(3): 225–232. [DOI] [PubMed] [Google Scholar]
- 15.Masterson JM, Cohen J, Blachman-Braun R, Machen GL, Sandlow J and Ramasamy R: Pre-treatment estradiol does not predict testosterone response to clomiphene citrate. Translational Andrology and Urology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzola CR, Katz DJ, Loghmanieh N, Nelson CJ and Mulhall JP: Predicting biochemical response to clomiphene citrate in men with hypogonadism. J Sex Med. 2014; 11(9): 2302–2307. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed O, Freundlich RE, Dakik HK, Grober ED, Najari B, Lipshultz LI, et al. : The quantitative ADAM questionnaire: a new tool in quantifying the severity of hypogonadism. Int J Impot Res. 2010; 22(1): 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavoussi PK, Machen GL, Wenzel JL, Ellis AM, Kavoussi M, Kavoussi KM, et al. : Medical Treatments for Hypogonadism do not Significantly Increase the Risk of Deep Vein Thrombosis Over General Population Risk. Urology. 2019; 124: 127–130. [DOI] [PubMed] [Google Scholar]
- 19.Chandrapal JC, Nielson S, Patel DP, Zhang C, Presson AP, Brant WO, et al. : Characterising the safety of clomiphene citrate in male patients through prostate-specific antigen, haematocrit, and testosterone levels. BJU Int. 2016; 118(6): 994–1000. [DOI] [PubMed] [Google Scholar]
- 20.Da Ros CT and Averbeck MA: Twenty-five milligrams of clomiphene citrate presents positive effect on treatment of male testosterone deficiency - a prospective study. Int Braz J Urol. 2012; 38(4): 512–518. [DOI] [PubMed] [Google Scholar]
- 21.Habous M, Giona S, Tealab A, Aziz M, Williamson B, Nassar M, et al. : Clomiphene citrate and human chorionic gonadotropin are both effective in restoring testosterone in hypogonadism: a short-course randomized study. BJU Int. 2018; 122(5): 889–897. [DOI] [PubMed] [Google Scholar]
- 22.Helo S, Ellen J, Mechlin C, Feustel P, Grossman M, Ditkoff E, et al. : A Randomized Prospective Double-Blind Comparison Trial of Clomiphene Citrate and Anastrozole in Raising Testosterone in Hypogonadal Infertile Men. J Sex Med. 2015; 12(8): 1761–1769. [DOI] [PubMed] [Google Scholar]
- 23.Smith R, Fantus J, Doerge EJ, Lipshultz L, Kovac JR and Coward R: Baseline FSH Predicts Semen Parameter Response in Infertile Men on Clomiphene Citrate. Journal of Mens Health. 2015; 11(5): 22–29. [Google Scholar]
- 24.Trost LW and Mulhall JP: Challenges in Testosterone Measurement, Data Interpretation, and Methodological Appraisal of Interventional Trials. J Sex Med. 2016; 13(7): 1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure-1: Scatter plots depicting the linear associations between testosterone response and gonadotropins for patients with higher pre-treatment levels.


