Abstract
A healthy nutritional state is required for all aspects of reproduction and is signaled by the adipokine leptin. Leptin acts in a relatively narrow concentration range: too much or too little will compromise fertility. The leptin signal timing is important to prepubertal development in both sexes. In the brain, leptin acts on ventral premammillary neurons which signal kisspeptin (Kiss1) neurons to stimulate gonadotropin releasing hormone (GnRH) neurons. Suppression of Kiss1 neurons occurs when agouti-related peptide neurons are activated by reduced leptin, because leptin normally suppresses these orexigenic neurons. In the pituitary, leptin stimulates production of GnRH receptors (GnRHRs) and follicle-stimulating hormone at midcycle, by activating pathways that derepress actions of the messenger ribonucleic acid translational regulatory protein Musashi. In females, rising estrogen stimulates a rise in serum leptin, which peaks at midcycle, synchronizing with nocturnal luteinizing hormone pulses. The normal range of serum leptin levels (10-20 ng/mL) along with gonadotropins and growth factors promote ovarian granulosa and theca cell functions and oocyte maturation. In males, the prepubertal rise in leptin promotes testicular development. However, a decline in leptin levels in prepubertal boys reflects inhibition of leptin secretion by rising androgens. In adult males, leptin levels are 10% to 50% of those in females, and high leptin inhibits testicular function. The obesity epidemic has elucidated leptin resistance pathways, with too much leptin in either sex leading to infertility. Under conditions of balanced nutrition, however, the secretion of leptin is timed and regulated within a narrow level range that optimizes its trophic effects.
Keywords: leptin, leptin receptors, pubertal development, ventral premammillary nucleus (PMV), gonadotrope, granulosa and theca cells, oocytes, Leydig cells, Sertoli cells, spermatocytes
The Impact of Nutrition on Reproduction
Food availability is considered the single most important environmental factor that influences mammalian reproduction (1). Healthy reproduction requires adequate nutrition for appropriately timed maturation events and the development of secondary sex characteristics (2, 3). Maternal nutrition is required for the timing of normal reproductive cyclicity to promote the development of healthy female gametes and prepare the female to support a pregnancy and provide milk for the young (4, 5). Adequate nutrition is also important for female receptivity to mating, the timing of which ensures that the short-lived sperm can reach a viable oocyte (6).
Nutritional deficiency results in reduced pituitary luteinizing hormone (LH) release in response to decreased hypothalamic gonadotropin-releasing hormone (GnRH) stimulation (7-10). When reproduction occurs in spite of nutritional deprivation, the initial outcomes are a reduction in the number and/or the size of the young (2, 3). Severe nutritional deficiency inhibits reproduction altogether as the immediate survival of the animal takes priority over its reproduction (2, 3).
In order to respond to nutritional challenges, metabolic signals relay information on energy status to the hypothalamic–pituitary–gonadal (HPG) axis. One of the most powerful signals is leptin, the 167 amino acid product of the Lep (formerly ob) gene. Leptin has the distinction of being the only known biomarker of adiposity, as its circulating levels are in linear proportion to fat mass (11, 12).
The first recognized role for leptin was to send a negative feedback signal to the brain when energy stores were adequate, resulting in decreased food intake and increased energy expenditure (11, 13-16). However, studies of animals that are leptin deficient (10, 17-19) revealed that leptin also plays a dynamic role in reproduction (17-27).
History of the Discovery of Leptin
Researchers sought answers to questions related to nutritional state and reproduction long before leptin was discovered (28), as it has been recognized that an animal must grow to maturity and attain a threshold of body fat before it can breed (2). Clues to a factor that signaled the amount of adiposity were uncovered 70 years ago in a mouse line bearing spontaneous gene mutations that rendered it obese (ob/ob) (29) or diabetic (db/db) (30). Studies of these obese mouse lines also reported that females and most males were infertile because their HPG axis was immature (31).
Classical parabiosis experiments by D.L. Coleman showed that the db/db mice carried a blood-borne factor that caused significant weight loss and starvation in its parabiotic partner in pioneering work which led to the Lasker prize (28, 32). Coleman hypothesized that the ob/ob mouse lacked the secreted factor, and the db/db mouse overproduced it, but could not respond to it (28, 32, 33). Coleman’s group reported that the factor was a component of adipose tissue but were never able to isolate it. In 1994, Zhang et al. (in Dr. Friedman’s laboratory) successfully isolated the factor by positional cloning, naming it leptin after the Greek “leptos” meaning thin (13). The ob/ob mice carried a mutation in the leptin gene (Ob or Lep) and db/db mice had a mutated leptin receptor gene (Db or Lepr) (34-37).
Regulation of Leptin Secretion from Adipocytes
As reviewed by Cammisotto et al. (38), studies of humans and rodents since its discovery have shown that leptin transcription and secretion in adipocytes may be stimulated by dexamethasone and other glucocorticoids and peroxisome proliferator-activated receptor-gamma agonists. Leptin is inhibited by catecholamines, free fatty acids, and thyroid hormones. Studies of rats reported that both glucose and insulin are stimulatory (39).
Cammisotto et al. (38) also reported that leptin secretion from male rat adipocytes was stimulated by nutrient signals, including glycolytic substrates (glucose, fructose, or pyruvate). In addition, amino acid L-glutamate stimulated leptin alone; L-aspartate, L-valine, L-methionine, and L-phenylalanine potentiated glucose action and L-leucine was stimulatory only in the presence of glucose.
In adult human male or female adipose tissue (40), pituitary thyroid-stimulating hormone (TSH) stimulated leptin by directly regulating adipocytes. However, this same group reported no stimulation by prolactin, adrenocorticotropin (ACTH), follicle-stimulating hormone (FSH), or luteinizing hormone (LH). As part of its lipolytic function, however, growth hormone (GH) reduced leptin gene expression and leptin secretion in bovine or rat adipocytes (41, 42).
With regard to regulation by reproductive hormones, rising estradiol levels are correlated with rising serum leptin in human and rodent females and estrogen treatment stimulates serum leptin (43, 44). In contrast, androgens inhibit leptin expression (45) and testosterone treatment reduced leptin levels in adult men (46). Similarly, studies of 3T3-L1 adipocyte tumor cells treated with dihydrotestosterone showed an 80% reduction in Lep messenger ribonucleic acid (mRNA) while estradiol increased Lep mRNA by 140% (46).
This mini-review will describe the current state of our knowledge regarding the importance of leptin to reproduction. Its importance was recognized historically in classical studies of ob/ob mice, in which exogenous leptin restored reproduction before it cured the obesity (10, 17-19). However, the mechanism by which leptin influences reproduction is only now becoming clear. Questions remain, including whether leptin regulates the timing of events in the reproductive system under normal energy conditions and whether it is involved in the timing of the reproductive cycle. We will address the target tissues and cells involved and discuss how leptin influences each.
Control of Leptin Signaling
Pleiotropic leptin receptors
The leptin receptor (LEPR) (47, 48), a product of the Lepr gene and a member of the class I cytokine receptor superfamily, has 6 isoforms, which may be expressed in different proportions depending on the cell type and the species (47-53). LEPRa,b,c,d,f have identical extracellular and transmembrane domains but differ in the length of their intracellular tail. Short isoforms (LEPRa,c,d,f) have 30 to 40 residue intracellular tails and unique C-termini, suggesting unique roles (47). LEPRa is broadly expressed and may facilitate leptin transfer across the blood–brain barrier (47). The sixth isoform, LEPRe, is soluble, secreted into the bloodstream, and may transport leptin, modulating its bioavailability (47).
LEPR forms dimers or oligomers and can heterodimerize in the presence and absence of leptin (47). LEPRs are distributed intracellularly in target cells in the receptor-mediated endocytic pathway, with only 10% to 20% of receptors found on the extracellular surface and remaining molecules in the endoplasmic reticulum, trans-Golgi, and endosomes, which are available for recycling to the plasma membrane as needed (48).
LEPR signaling pathways
The best characterized signaling pathway activated by the long isoform LEPRb is the Janus kinase (JAK) and signal transducer and activator of transcription proteins (STAT3 and STAT5 (48)). Paradoxically, STAT5 has been implicated in reproductive competence (54, 55). However, ablation of STAT5 and/or STAT3 in cells expressing LEPR (56) results in normal puberty onset, cyclicity, and fertility over a 4-month period, suggesting that HPG target cells process leptin signals through multiple signaling pathways. These leptin-signaling pathways involve mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase (ERK) 1/2 (57, 58); phosphoinositol 3 kinase (PI3K) (59, 60), mammalian target of rapamycin (mTOR) (21, 61, 62), and/or nitric oxide (63). Investigators studying the individual target cells in the HPG axis have included tests of each of these pathways to determine which are involved in leptin actions. Some of these studies will be discussed in later sections focused on the leptin target cells.
Leptin resistance
As leptin operates in a narrow concentration range (64), elevated leptin levels, such as those seen in obesity, are often accompanied by resistance to leptin signaling, (65). This multifactorial event largely centers on the trafficking and signaling of LEPR (47, 48, 66) and involves feedback inhibition via suppressor of cytokine signaling 3 (SOCS3), protein tyrosine phosphatase 1B, and T-cell PTP (47, 48, 66). The expression of these negative regulators is elevated in the hypothalamus of obese animals. Deletion of SOCS3 leads to enhanced leptin actions and attenuation of leptin resistance in models of diet-induced obesity (47, 48).
Circulating Leptin: Which Is More Important, Levels or Timing?
Proof that adipocytes are the source of circulating leptin
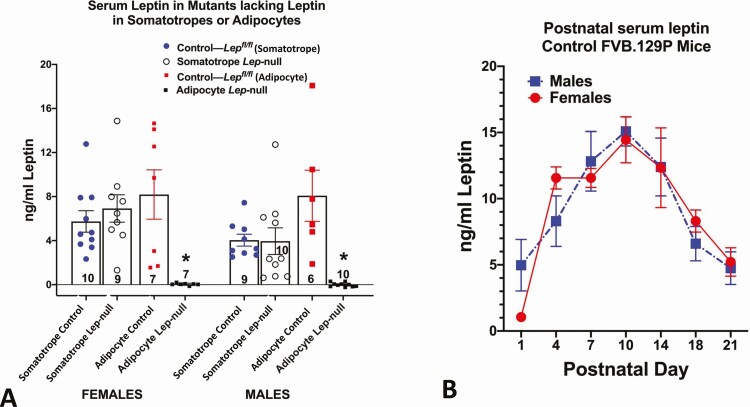
Many reviews have identified changes in serum leptin levels critical to metabolic health, puberty, and the reproductive cycle (17-27). However, in addition to adipocytes, leptin production has been detected in numerous organs, leading researchers to speculate that each of the organs may contribute to differential circulating leptin (reviewed in (67)). Variation in leptin levels may reflect differential secretion from these organs, or differential regulation of adipocytes. At this point, no systemic functions for extra-adipocyte leptin have been reported. Our laboratory addressed this with tissue-specific Lep-null mouse models that selectively ablated leptin in adipocytes or pituitary somatotropes (67). The ablation of adipocyte leptin resulted in undetectable serum leptin levels in adult or neonatal mice (Fig. 1A), because it caused obesity similar to that of ob/ob mice. In contrast, mice lacking leptin only in somatotropes did not show a decrease in serum leptin (Fig. 1A). These findings point to adipocytes as the main, if not the only, source of circulating leptin (67) and are consistent with the tight association between circulating leptin and adiposity.
Figure 1.
Serum leptin levels in different physiological states. (A) Serum leptin in mice in which leptin was ablated in somatotropes (Cre-Gh × Lepfl/fl or adipocytes (Cre-Adipoq × Lepfl/fl). Controls for each group are littermates from each line, which bore only floxed leptin (Lepfl/fl). In both females and males, only mice lacking Leptin in adipocytes showed a significant loss in serum leptin (*significantly lower than all other values, ANOVA followed by Tukey’s post hoc test). Method: Serum leptin was assayed by enzyme immunoassay (R&D Systems, Quantikine, MOB00). (B) Sera were collected from male and female FVB.129P mice during neonatal development and assayed for leptin as in Fig. 1A. In both males and females, the peak levels were seen on day 10. Analysis by 2-way ANOVA showed no significant sex differences and a significant postnatal age variance: F = 14.44. DFn = 6, DFd = 64, P < .0001. Tukey’s post hoc test identified significant differences. *Data points that are different from day 1 values. Day 10 values are higher than all others P < .0001) n/age group ranges from 3 to 15 mice.
The neonatal leptin surge, puberty, and metabolic health
The association between the prepubertal increase in fat stores and the onset of puberty originally supported a hypothesis that the leptin signal is vital for the timing of puberty. Reports that leptin accelerates puberty (16, 68, 69) suggested that leptin might be the primary metabolic trigger. However, there was no correlation between prepubertal serum leptin levels and the timing of puberty in normal rodents (70-72) or primates (73-76) arguing against this hypothesis. There is evidence, however, for a rise in leptin during the third trimester in the human fetus (77, 78) or postnatally in rodents (71, 72, 79, 80), shown in Fig. 1B. The data in Fig. 1B are the first to compare postnatal male and female mice, showing a postnatal age variance but no sex differences (2-way analysis of variance [ANOVA] followed by Tukey’s post hoc test; see Fig. 1 legend). These studies are important because previous studies reported data only from female mice (79), male rats (81), or data from rats (82) or mice (83) which combined the sexes. Studies show that this neonatal leptin rise does not coincide with an increase in fat mass and is unrelated to appetite regulation. Indeed, during this period of development, leptin does not inhibit food intake (84, 85). However, the period of the rise is critical, because, in leptin-deficient mice, leptin restored maturation and development only if given during the neonatal period (86, 87). The importance of the neonatal leptin rise to reproduction and metabolism was highlighted recently in a study by Ramos-Lobo et al. (88), who studied an inducible mouse model in which mice were LEPR-null at birth and then were rescued globally during the fourth or tenth week of age (88). Some systems recovered, but the reproductive system remained immature or dysfunctional in most animals. Males retained low testicular weights and exhibited low GnRH fiber density and kisspeptin (Kiss1) mRNA. Only 10% of the wild-type females mated with rescued males became pregnant. However, 50% of rescued females became pregnant in spite of dysfunctional cycling as defined by vaginal smears. Collectively, the study pointed to the importance of leptin signals early in development for metabolic and reproductive function in the adult.
Studies that manipulated the neonatal leptin surge have reported that it can be blunted or altered by maternal undernutrition (81, 83) or administration of a leptin antagonist (89), with diverse consequences, including the improper formation of hypothalamic networks involved in feeding and dysfunctional metabolic responses (81, 90, 91). Maternal undernutrition blunted or shifted the neonatal leptin surge, with either result having lifelong detrimental effects on the metabolic health of the offspring (81, 83, 90, 91). Progeny exhibit sensitivity to a high-fat diet (HFD) (82). Another study (83) replicated the conditions of an early leptin surge by injecting leptin on postnatal day 5, resulting in high sensitivity to a HFD in the treated adults. This study thus introduced the concept that the timing of the leptin rise may be as important as serum levels for early developmental processes.
Researchers investigating effects of altering the leptin surge on the reproductive system found that blockade or alteration of the postnatal leptin surge decreased testicular growth and FSH in male rat pups and decreased ovarian growth and FSH in females (92). Puberty onset was delayed in both sexes (92) and ovarian primordial follicles were reduced in females (89). There were sex-dependent effects on the development of the hypothalamic circuits that regulate reproduction (especially the kisspeptin network) (93-95).
Our recent studies of mice lacking LEPR in gonadotropes have demonstrated a leptin dependency for expression of GnRH receptor proteins, Fshb mRNA, and the mRNAs for the FSH regulator activin (96-98), which may have begun during neonatal development in females. Female mice lacking LEPR in gonadotropes have significantly reduced levels of GnRHR, and Fshb, Actβa, and Actβb mRNAs (96-98). We propose that this dependency may be manifested as early as neonatal development, because studies of developing purified gonadotropes by Wen et al. reported that full coexpression of GnRHR and FSH coincides with the rise in serum leptin (by postnatal day 7) (99). Additional studies of female rat pituitaries reported a rise in Fshb mRNA during the time of the postnatal rise in leptin (100-102), along with a parallel rise in pituitary activin βa and βb subunits (103).We have recently performed a maternal undernutrition study on mice with a 20% food-restricted diet. The underfed progeny had an early leptin surge, and female mice showed delayed puberty, in spite of the fact that they had sufficient leptin at the time of the normal surge. On day 29, 90.1% of 11 controls and only 12.5% of the 8 underfed progeny showed vaginal opening. The remaining underfed progeny did not show vaginal opening until day 32 (Miles et al., ms in preparation). These ongoing studies continue to indicate that the timing of the neonatal leptin surge is as important to reproductive and metabolic health as the level.
The importance of leptin pulses to the timing of puberty
The timing of the leptin signal was initially implicated as a positive regulator of puberty, because injections of leptin in weanling female mice caused early onset of puberty (16). In male monkeys, when the time of puberty onset is defined by the nocturnal LH pulses, studies showed that nocturnal leptin levels increased significantly before puberty onset along with a gradual increase in growth hormone (GH) and insulin-like growth factor-1 (IGF-1) levels (104). The investigators suggested that leptin stimulates the GH–IGF-1 axis, which in turn stimulates GnRH and LH. Nocturnal leptin secretion was also increased by 28 days of pubertal development in female rats (105). Thus, studies of leptin secretion during postnatal and peripubertal development point to the importance of both nocturnal timing and amplitude as mechanisms leptin uses to permit development of adult reproductive competence.
Sex differences in leptin secretion in the adult
Figure 1B shows identical leptin secretory patterns in postnatal male and female mice. However, in adult humans there are striking sex differences in serum leptin concentrations beginning in the peripubertal period (106). In humans, this begins as early as 10 years of age in boys, who show a decline in serum leptin during puberty. Normal weight (body mass index <30) human women have leptin levels averaging 23.5 ± 1.5 ng/mL, with a range between 4.7 and 46 ng/mL (107). In contrast, healthy weight human men average 9 ± 0.83 ng/mL serum leptin, with a range of 2.65 to 20.7 ng/mL. Indeed, the average leptin levels in women are close to obesity levels for men (29 ± 1.55 ng/mL). This difference is likely due to sex steroids, as estrogens stimulate leptin release by adipocytes whereas androgens inhibit leptin release (reviewed in (108)). This sets up a negative association between leptin and testosterone levels in males, which reflects androgen’s inhibitory influence upon adipocyte leptin (46, 109) and explains the peripubertal reduction in serum leptin in males (108).
Cyclicity of leptin secretion in the female
Not only is leptin secretion higher in the human female, it exhibits cyclicity, which was first reported as an increase from 14.9 ng/mL in the early follicular phase to 20.4 ng/mL at the midluteal phase (64). Further studies also reported synchrony between nocturnal leptin and LH pulses in normal cycling women (110, 111). This synchrony with leptin, LH, and estradiol pulses was best seen at night, supporting a hypothesis that leptin regulates LH and estradiol oscillations. Many additional studies of human females showed cyclic changes in serum leptin as cited in (112), which reports a comprehensive study of 259 healthy premenopausal women (509 cycles) documenting hormonal changes during both ovulatory and anovulatory cycles (112). They assayed serum leptin, estradiol, progesterone, LH, FSH, and testosterone 8× per cycle and factored exercise and body mass index into their analysis. They reported an increase in serum leptin from 16.7 ng/mL to 20.4 ng/mL in the luteal phase, with leptin levels averaging 21.7 ng/mL at the time of the LH surge (112). The highest levels of leptin were associated with higher estradiol, progesterone, ovulatory LH, and testosterone, but lower FSH. It is unclear why high levels of leptin were not correlated with high FSH levels. The explanation may be that the best correlation between leptin levels and gonadotropins is evident following nocturnal assays of leptin and LH pulses (110, 112). It is significant however that overall leptin levels were lower in anovulatory cycles, which pointed to the need for normal development of ovarian follicles to produce the estrogen. This study suggested that the source of the rising leptin might be from ovarian follicles (112). However, our recent studies showing undetectable serum leptin levels in mice lacking leptin only in adipocytes argue against an ovarian contribution to serum leptin (67) (Fig. 1A).
Leptin Interactions with Hypothalamic–Pituitary–Gonadal Target Cells
Leptin target cells in the hypothalamus
The search for leptin-regulatory pathways in the brain was begun in pioneering studies (113) which partially ablated exon 17 of LEPR (thus eliminating the signaling domain of LEPRb) in hypothalamic neurons. They reported fertility with a partial ablation, but infertility only after LEPRb was ablated in all target neurons. The search then began for the subset of LEPR neurons specifically involved in fertility. This search was challenging because GnRH neurons lacked LEPR (114, 115). Moreover, as reviewed by Elias and Purohit, LEPR expression in kisspeptin neurons was low or varied with the species or physiological state (22). Furthermore, ablation of LEPR in Kiss1 neurons had no effect on reproduction (116). This field has been reviewed comprehensively, and the reader is referred to a series of excellent papers for further details on early work in this area (1, 19-23, 25, 117-119). This mini-review will concentrate mainly on recent findings focusing on 2 major sets of leptin-responsive neurons that mediate either stimulation or suppression of the reproductive system.
Donato et al. (116, 120) discovered that neurons in the ventral premammillary nucleus (PMV) formed a pathway mediating leptin stimulation of kisspeptin neurons, which then stimulated GnRH neurons (Fig. 2). This was initially based on studies in rats showing that PMV lesions blunted kisspeptin and GnRH neuronal activity during the afternoon of proestrus, decreasing LH and estrogen secretion, and disrupting estrous cyclicity. As stated above, this group had also determined that deletion of LEPR in Kiss1 neurons did not affect the timing of puberty or fertility (116), ruling out Kiss1 neurons themselves as direct leptin mediators of reproduction. They then developed a mouse model, which re-expressed LEPR in the PMV of a LEPR knockout mouse model and reported that restoration of LEPR in the PMV induced puberty, improved estrous cycles, and allowed pregnancy (116, 120). However, only a fraction of the females (20%) carried pups to term. A follow-up study (121) correlated restoration of a leaner weight with the delivery of healthy pups in 1/5 females. In a different model in which Cre recombinase was delivered to the PMV by an adenovirus (121), puberty was restored in all 11 mice which had correctly targeted the PMV (out of 18 tested). Five of these mice became pregnant, and 1 of these had a healthy litter. Thus, collectively, these studies demonstrated the importance of the PMV neurons as leptin targets for stimulation of kisspeptin neurons in the anteroventral periventricular and caudal arcuate nuclei, and regulation of pubertal development, cyclicity, and pregnancy (116, 120, 121). The low pregnancy success rate following selective restoration of LEPR may have reflected the mouse obesity and the lack of LEPR on extrahypothalamic target cells.
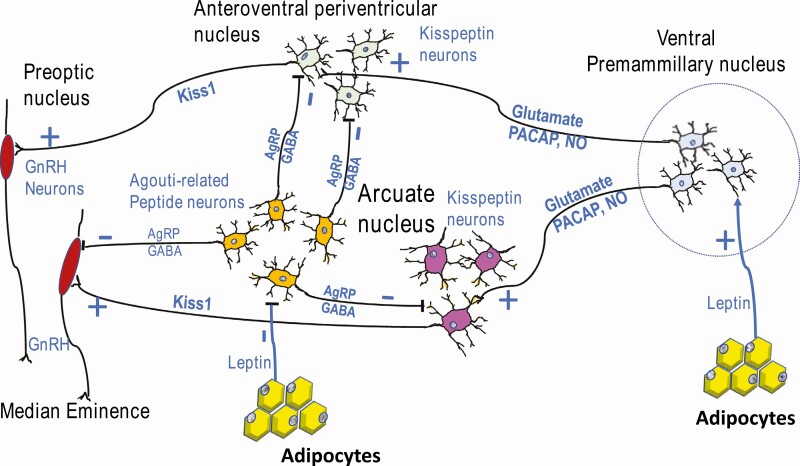
Figure 2.
Pathways for leptin’s permissive actions in the hypothalamus. Stimulation by leptin is mediated by neurons in the ventral premammillary nucleus (PMV), which send connections to kisspeptin neurons in the anteroventral periventricular and caudal arcuate nuclei and stimulate Kiss1 expression by glutamate, PACAP or nitric oxide. Also, leptin normally inhibits the orexigenic AgRP–NPY–GABAergic neurons in the arcuate. When leptin signals are reduced (by food deprivation, for example), these neurons signal kisspeptin neurons in the AVPV and caudal arcuate, inhibiting the expression of Kiss1 by AgRP or GABA. These actions ultimately stimulate or inhibit GnRH neurons and modulate pulsatile activity to effect gonadotropin secretion and the LH surge.
The investigations of stimulatory pathways then continued with studies to determine the identity of the neurotransmitters in PMV neurons responsible for leptin-mediated regulation of either GnRH neurons or kisspeptin neurons. As shown in Fig. 2, 3 candidate regulators are glutamate (22, 119, 122), pituitary adenylate cyclase activating polypeptide (PACAP) (122), and nitric oxide (63, 119, 123). PACAP regulates both food intake and gonadotropin release (122). Ross et al. (122) reported that PACAP was expressed in LEPR-bearing PMV neurons that produce glutamate and had the highest levels of pSTAT3 activity, an indicator of LEPR function. They selectively ablated PACAP in all LEPR-expressing neurons and reported that females showed delayed onset of puberty, a blunted LH surge, dysregulated estrous cycling, and low numbers of pups/litter (122). Tests of responses to exogenous leptin showed a blunted LH response. However, exogenous kisspeptin given to these PMV neuron PACAP-null mice resulted in stimulated LH secretion, suggesting that GnRH neurons were intact and that the dysfunction in the connection was at the level of the kisspeptin neurons. When PACAP was deleted selectively in adult animals (with adenovirus carrying Cre-recombinase) females had longer cycle lengths, fewer cycles in a 25-day period, reduced corpora lutea, delays in the time to pregnancy and a failure to nurture their pups. Tests of LH responses to exogenous kisspeptin showed intact signaling between kisspeptin and GnRH neurons. However, exogenous leptin stimulated higher levels of LH. This aspect of the study suggested that PACAP from the PMV may be needed for normal Kiss1 to GnRH neuronal relays but perhaps not for the relay of the leptin signal. Nevertheless, their studies mapped distinct connections from the leptin receptive PMV PACAP neurons to both sets of kisspeptin neurons and they showed PACAP stimulation of a subset of these neurons (122).
As investigators were identifying pathways mediating the stimulation of Kiss1 and GnRH, parallel studies investigated pathways that may mediate the suppression of GnRH secretion during times when leptin signals are low or absent, such as during food deprivation. Candidate pathways involved GABAergic leptin receptor-bearing neurons (117) based on findings showing that deletion of LEPR specifically from GABAergic, but not glutaminergic neurons caused infertility in females (124, 125). Zuure et al. (124) reported that deletion of LEPR in GABA neurons in mice did not interfere with the estrogen negative feedback to GnRH neurons. However, the ablation of leptin signals clearly blunted a preovulatory-like LH surge. In their comprehensive discussion of possible sites for these GABAergic neurons (124), they pointed to studies of agouti-related peptide (AgRP) neurons in the arcuate nucleus, which also produce neuropeptide Y (NPY). These are illustrated in Fig. 2. Zuure et al. hypothesized that these factors may serve as intermediates that suppress HPG neuronal secretion when leptin signaling is reduced (in fasting, for example). This hypothesis was based on the fact that normally leptin inhibits this group of orexigenic neurons, so the lack of leptin signals would result in increased AgRP and NPY. Zurre et al. cited evidence for AgRP and NPY inhibitory influences on GnRH and Kiss1 neurons (124). In addition, NPY and AgRP are elevated in mice lacking leptin (ob/ob mice) (126, 127).
Early evidence for the involvement of AgRP neurons in reproduction was strengthened when Wu et al. (127) reported that ablation of AgRP neurons in adult ob/ob mice restored fertility. Five years later, Egan et al. (128) selectively deleted leptin receptors in AgRP neurons and reported delays in puberty in female but not male mice. However, reproduction was not prevented. When LEPR was restored in AgRP neurons in infertile Lepr-null mice, they reported restoration of all reproductive attributes, although puberty was delayed in females. The restored animals remained subfertile, however, and females were prone to dystocia complications because of their obesity. They did show a normal LH response to removal of estrogen feedback indicating that the Kiss1-GnRH circuit was intact in the rescued animals.
Most recently, the AgRP pathways were further delineated in mice by Padilla et al. (126) as they stimulated AgRP fibers and detected inhibitory synaptic connections with kisspeptin neurons in both arcuate and anteroventral periventricular (AVPV) neurons, which were reduced when AgRP neurons were ablated (Fig. 2). In addition, when the AgRP neurons were chemogenetically activated, the females showed delayed estrous cycle length and decreased fertility. Their tests of GABA responses by GnRH neurons indicated that the AgRP neurons mediate their suppression of fertility by directly inhibiting Kiss1 neurons.
To summarize, the studies thus far indicate that leptin-dependent PMV neurons synapse on Kiss1 neurons and their signaling with PACAP, glutamate, or nitric oxide as neurotransmitters is required for reproduction and the onset of puberty. In addition, reduced leptin signals in states of low energy stores or food deprivation permit arcuate orexigenic neurons to produce AgRP, NPY, and GABA to suppress reproduction and thus conserve energy for food gathering. The next sections of this mini-review will focus on the downstream target cells in the HPG axis to present evidence for leptin integration with HPG regulatory hormones to amplify or limit its metabolic signal to the system.
Pituitary gonadotropes as leptin target cells
Many early studies have shown that pituitary gonadotropes bear leptin receptors and their expression of this receptor varied with the stage of the cycle. Lepr mRNA was first detected in rat pituitary cells by Zamerano et al. (53), and LEPRa and LEPRb expression by gonadotropes was first reported by Jin et al. (129, 130). Evidence supporting gonadotropes as leptin target cells also came from studies showing that LEPRs were functional (96, 129-136). In addition, their leptin dependency was evident by the reduced numbers of gonadotropes in leptin-deficient mice (134, 137-139).
Our cytochemical studies of mice and rats showed that pituitary LEPRb immunolabeling increased during the luteal phase (96, 140), which correlated with the midcycle rise in serum leptin (43, 44, 141-143). This increase in LEPRb cells reflected additional cells that were dual labeled for LH-beta and LEPR (96).
Multiple in vivo studies have reported leptin modulation of the expression and/or secretion of gonadotropins in vivo (31, 96, 114, 142, 144-148). For example, leptin restored LH secretion in fasted mice, rats, and monkeys (143, 149-156) and normalized reproductive hormone levels in a leptin-deficient prepubertal child (157), as well as in adult men (158) and women with amenorrhea (159, 160).
In vitro studies have also demonstrated that leptin stimulates LH secretion (63, 142, 144, 148, 161). Our laboratory reported that in vitro exposure to 10 to 100 pg/mL leptin restored the numbers of gonadotropes reduced by 24 hours of fasting (162). Leptin also stimulated FSH secretion from monolayer pituitary cultures derived from 2 groups of female monkeys (163). The leptin-mediated pathway to pituitary gonadotropes is illustrated in Fig. 3. Our studies of mice lacking LEPR in gonadotropes indicate that leptin may be involved directly or indirectly in the translation of GnRHR and FSH proteins, in partnership with estradiol positive feedback and pulses of GnRH (96).
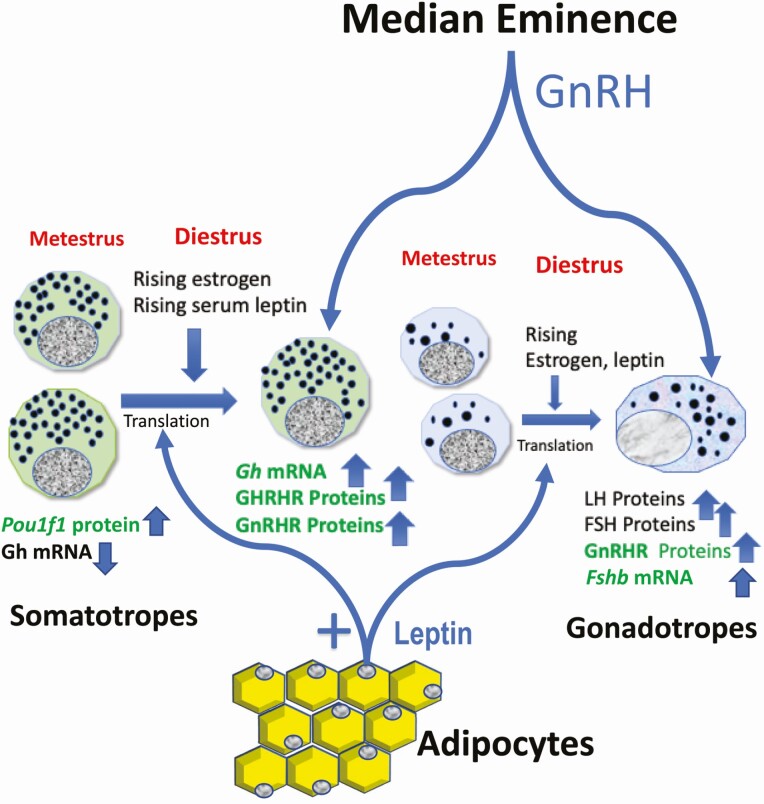
Figure 3.
Leptin’s role in the anterior pituitary. Leptin receptors on gonadotropes and somatotropes receive rising serum leptin which peaks at midcycle. Leptin stimulates gonadotropes to produce GnRHR proteins and Fshb and activin mRNAs in partnership with pulsatile GnRH and estradiol positive feedback. Leptin also stimulates the translation of GH and GHRHR proteins to support the cogonadotropic function of somatotropes. The products in green are the known leptin targets.
Whereas there is agreement about gonadotrope responses to leptin, the importance of gonadotropes as direct metabolic sensors has been a subject of controversy. Are gonadotropes passive mediators of hypothalamic or gonadal responses to metabolic changes? Does leptin play an active trophic role in the regulation and differentiation of the gonadotrope population? We addressed these questions using a deletion mutant mouse model in which exon 17 of Leprb (which encodes the STAT3 binding site) was ablated selectively in gonadotropes (96). This loss of LEPR signaling in gonadotropes did not affect the timing of puberty nor did it affect ovarian, uterine, or testicular weights (96). However, mutant females showed delayed time to first pregnancy and lower numbers of pups. The remaining pups survived, indicating normal lactation. With respect to pituitary hormone expression, mutant diestrous females had reduced serum LH and FSH and reduced mRNA levels of Fshb and activin. Mutant males had reduced levels of Fshb and Cga mRNAs (96).
More recently, we ablated exon 1 in LEPR in gonadotropes (97), which removes the signal peptide common to all LEPR isoforms, thus preventing the LEPR molecules getting translocated to the endoplasmic reticulum (ER) or targeted to the cell membrane. Comparing the phenotype of our 2 lines will be useful as Lepr exon 17-null gonadotropes may still express short forms of LEPRa and signal through the MAPK or nitric oxide pathways (63, 148). Our ongoing studies indicate that gonadotrope Lepr exon 1-null mice are more severely affected by loss of LEPR in gonadotropes, including more severe subfertility or infertility and abnormal estrous cycling (97).
The most striking change in LEPR-null gonadotropes has been the reduced expression of GnRH receptors (GnRHRs) (96) as detected by biotinylated GnRH or immunolabeling for GnRHR, especially in females. Furthermore, leptin stimulated GnRHR proteins but did not change mRNA levels (98). These data indicated that GnRHRs were an important post-transcriptional leptin target.The gonadotrope Lepr exon 17-null mice also exhibited some additional surprising findings, suggesting that leptin’s actions on gonadotropes have a broader impact, specifically on cells belonging to the Pou1f1/Pit1 transcription factor expressing lineage. Both males and females showed reduced serum GH and males showed reduced serum TSHb along with reduced Gh and GH releasing hormone receptor (Ghrhr) mRNA levels. Figure 3 shows the leptin influence on somatotropes as well.
There are several possible explanations for these off-target effects reviewed recently by us (164). First, we have shown that Pou1f1/Pit1 is another post-transcriptional leptin target (165, 166). It is possible that the targeting construct used to delete gonadotrope LEPR may also function in a multipotential Pou1f1/Pit1 pituitary precursor cell population that perhaps become somatogonadotropes (164, 167-171), as they are stimulated by estradiol (172) and following their expression of GnRHR (169) and GHRHR (170). Alternatively, deletion of LEPR may have affected the production of paracrine factors secreted by gonadotropes, which stimulate somatotropes and thyrotropes (173). Further studies are needed to explore roles for leptin in transdifferentiation of pituitary cells, as well as modulating paracrine interactions in the pituitary. Figure 3 shows that leptin stimulates gene expression in somatotropes, and more details are reported elsewhere (165, 166, 174).
Other investigators have also recently addressed the relative importance of gonadotrope LEPR by restoring it in mice genetically engineered to be ubiquitously deficient in LEPR (175). They reported that although FSH levels were elevated, fertility was not restored (175). This finding is expected, because the hypothalamic neuronal target cells remained LEPR null and the mice remained morbidly obese. Without GnRH from the hypothalamus, gonadotropes will remain in a prepubertal state. Whereas GnRHR was not assayed (175), their findings showed elevated FSH levels confirming FSH as a downstream leptin target (96).
Leptin’s actions on gonadotropes: a role for Musashi
After we discovered that leptin regulated GnRHR through post-transcriptional pathways (96, 97), our in silico analysis showed that the 3′ untranslated region (3′ UTR) of murine Gnrhr mRNA contains 3 consensus binding sequences for the translational regulatory protein Musashi (MSI, MSI binding elements [MBEs]). The Musashi1 isoform (MSI1) and Musashi2 isoform (MSI2) are sequence-specific RNA binding proteins that repress target mRNA translation. These proteins promote stem and progenitor cell self-renewal, as they oppose translation of mRNAs that encode prodifferentiation factors and inhibitors of cell cycle progression (176). Although MSI was reportedly expressed in the mouse pituitary (177), no functional role had been determined.
We reported that MSI binds to the Gnrhr mRNA 3′ UTR and represses translation of a reporter mRNA driven by this UTR (98). Notably, MSI is a target of regulation by leptin signaling where leptin stimulation results in decreased Msi mRNA and MSI1 protein levels in GnRHR cells detected by immunolabeling and enzyme immunoassay (98). Our findings suggest that Musashi functions to regulate differentiated gonadotropes by mediating their expression of GnRHR. Collectively, these data support the hypothesis that leptin functions to stimulate hormone expression in the gonadotrope, through its up regulation of GnRHR, activin, and Fshb mRNA levels. We propose that when nutrition is optimal, the midcycle rise in serum leptin would be permissive for the translation of Gnrhr mRNA resulting in an activated gonadotrope population (97).
Biphasic actions of leptin on target cells in the ovary
Early studies showed that ovaries were among the organs containing the highest levels of Lepr mRNA (178), which stimulated investigations to determine leptin’s direct effects on the ovary. However, following an early report suggesting that both lean and obese women with polycystic ovarian syndrome had 30% higher leptin levels (179), researchers hypothesized that leptin was inhibitory (180). Subsequent studies did not confirm this early study and in fact found that leptin levels were high only in obese PCOS women, correlating with adiposity (181-184). Unfortunately, however, the earliest in vitro studies of human (185, 186), rodent (187, 188), or bovine (180, 189) granulosa or theca cells used obesity levels of leptin (>30 ng/mL). These high leptin levels inhibited steroid secretion stimulated by gonadotropins added with a growth factor (either IGF-1, transforming growth factor-alpha) or insulin. High leptin levels also inhibited mouse follicular growth stimulated by FSH, with and without GH, IGF-1, 8 Br-cAMP, or forskolin (190) and lowered numbers of ovulated oocytes in rats (191). In humans, 50 to 200 ng/mL leptin also decreased follicles and reduced oocyte maturation (192). By 2000, the early consensus was that leptin inhibited the synergistic responses to gonadotropins and a growth factor (186-189, 193). This conclusion was based on exposure to obesity levels of leptin. Whereas this supported the hypothesis that obesity impaired ovarian functions (186-189, 191), none of the early studies tested the relatively low serum levels of leptin (10-20 ng/mL) found in normal weight women, in spite of the fact that these data were contemporaneously available (64).More recent studies utilized in vivo and in vitro models with a full dose range of leptin levels and discovered leptin’s biphasic effects on ovarian target cells. Figure 4 illustrates leptin stimulated pathways which influenced follicular development (from primary to antral follicles) by stimulating theca and granulosa cells in partnership with FSH, IGF-1, and GH. Leptin also stimulated ovulation and oocyte maturation in partnership with LH. Fig. 4 summarizes results from studies in a number of species, which are reviewed in the following sections. One study showed that induction of early puberty in rats resulted in granulosa cell hypertrophy, a 2-fold increase in ovulated oocytes and increases in serum steroid and gonadotropins and ovarian steroidogenic acute regulatory protein and adrenotoxin levels (194). In porcine granulosa cells, 10 ng/mL leptin stimulated but 1000 ng/mL inhibited progesterone accumulation and phosphorylation of STAT3 (195). Similarly, in mice, lower concentrations of leptin increased LH or FSH-stimulated ovarian steroids and high concentrations decreased follicular growth (196). Leptin stimulated 36% of rat oocytes to resume meiosis compared with 83% in the presence of LH and FSH (197). Leptin also stimulated meiotic progression of bovine oocytes at 12.5 ng/mL but inhibited progression at 100 ng/mL (198).Studies of mechanisms underlying leptin actions in rat ovaries showed similar biphasic effects in the regulation of Lepr (199), the production of progesterone, and stimulation of ovarian STAT3 and MAPK (at 3-10 ng/mL) (200). The latter study indicated that ovarian cells use short forms of the leptin receptor. Their in vivo rat ovulation model showed that leptin enhanced ovulation, increasing phosphorylated STAT3, MAPK and decreasing inhibitory SOCS3 protein, effects that were inhibited by inhibitors of MAPK or JAK/STAT signaling pathways (200). In contrast, an acute high-dose treatment model resulted in reduced ovulation and activated MAPK and STAT3 along with increased inhibitory SOCS3 protein, pointing to mechanisms underlying the development of leptin resistance as a result of exposure to high concentrations.More examples of leptin biphasic actions were reported in multiple species. In humans, low leptin (1 and 10 ng/mL) stimulated estradiol and progesterone secretion from granulosa cells, while higher concentrations were suppressive (201). In mice, 10 ng/mL but not 100 ng/mL leptin stimulated germinal vesicle breakdown and appearance of the first polar body (202). In luteinized mouse ovaries, LH stimulated mRNA levels of the short form LEPRa (Lepra) but not the long form LEPRb (Leprb), which supported the hypothesis that these cells may use LEPRa (202). In rabbit cumulus–oocyte complexes, physiological levels stimulated an increase in metaphase II oocytes, actions that were impaired with inhibitors of either the JAK/STAT or MAPK pathways (201).Considering the dynamic changes in the reproductive system, both inhibitory and stimulatory roles may be physiologically relevant when viewed in the context of the changing serum levels of leptin during the cycle and with obesity. As described in an earlier section, the nadir point for leptin secretion in the early follicular phase is 14 to 16 ng/mL, which rises to reach 21.7 ng/mL at midcycle (112). Leptin’s stimulatory actions described during the last 20 years are evident in those studies that used that concentration range. It is clear that the HPG system is not geared to respond positively to obesity levels of leptin in the microgram range let alone 50 to 200 ng/mL, which were used in the studies before 2000. Such high levels shut down leptin signaling pathways and explain the obesity-induced infertility or subfertility. Indeed, a recent study by Wołodko et al. details changes in ovarian gene expression associated with leptin resistance caused by obesity (65). Collectively, these studies attest to the importance of maintaining optimal levels of serum leptin for female reproductive competence.
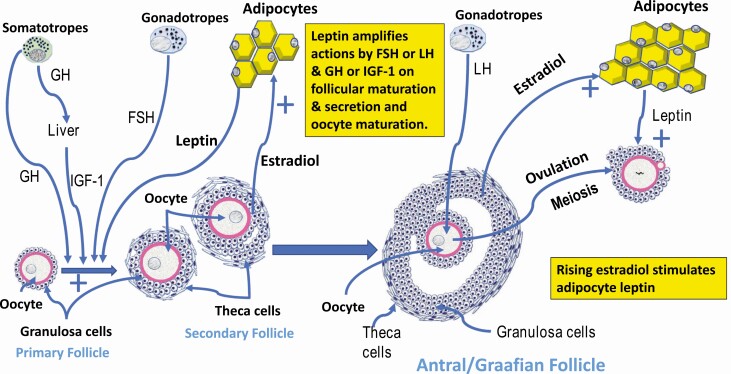
Figure 4.
Leptin’s role in ovarian follicular and oocyte maturation. All ovarian follicular cells have leptin receptors and physiological levels of leptin stimulate granulosa and theca cells and oocyte maturation. This drawing is based on many studies showing leptin stimulation of these target cells. Physiological levels of leptin stimulates, in partnership with growth factors (IGF-1), GH, and FSH, to promote the development of follicles to the antral stage. Leptin also promotes ovulation and oocyte maturation in partnership with LH. Leptin levels rise in association with rising estradiol from the follicles (granulosa cells). Leptin also acts with LH and growth factors to promote oocyte meiosis and formation of the polar body.
Testicular Leptin target cells: a little leptin goes a long way in the male
Zamorano et al. (53) first reported that Lepr mRNAs were expressed in the rat testes with subsequent studies identifying all isoforms (203-205). In 1996, leptin treatment of ob/ob mice elevated serum FSH levels, increasing testicular and seminal vesicle weights and sperm counts (14).
In the male, leptin is stimulatory before puberty. Suter et al. (206) correlated the rise in serum leptin levels with the pubertal nocturnal LH pulses in male monkeys showing that leptin is clearly important in development. In isolated mouse seminiferous tubules, or 5-day-old mouse Leydig cells, leptin induced an increase in phosphorylated STAT3 but not MAPK (207). In essence, in a number of species, many studies of pubertal development in the male indicate that leptin is permissive and stimulatory at the level of the hypothalamus and pituitary (23, 63, 105, 148, 152, 153, 206, 208, 209).
However, in the earlier section on sex differences in serum leptin levels, we reported that adult males have relatively low serum leptin, when compared with females (46, 108, 109). Leptin levels in normal adult males are presumably limited by androgens, which inhibit secretion of adipocyte leptin (46, 108, 109). This is fortuitous because, as will be shown in the following paragraphs, leptin inhibits testicular function if serum levels rise above a certain threshold.With regard to Leydig cells, in vitro studies in cultured rat Leydig cells and a Leydig tumor line demonstrated that obesity levels of leptin inhibited gonadotropin-mediated testosterone secretion (23, 209-212). The leptin suppression of testosterone was accompanied by parallel reductions in androstenedione and a rise in progesterone and pregnenolone metabolites (23, 211). In rat testicular slices, leptin downregulated testicular LEPRb (203, 204, 209, 210). Researchers have pointed to the positive correlation between high leptin in serum of obese men and reduced androgen levels (23).After a report showed that circulating leptin could pass through the mouse blood–testes barrier and thus enter the seminiferous tubules (213), researchers investigated leptin actions on spermatogonia, spermatocytes and Sertoli cells. One report showed that leptin impaired mouse sperm development and elements of the blood–testis barrier (214). Mice receiving 3 mg/kg leptin for 2 weeks showed lower sperm counts (by 50.9%) and motility along with abnormal morphology. Leptin also induced leakiness in the blood–testes barrier by reducing tight junction proteins (occludin, claudin 5, and Zonula Occudins-1), actions that were prevented by inhibitors of leptin signaling pathways: JAK2, PI3K, and MAPK. Collectively these studies explain the adverse impact of high leptin on male mouse fertility and sperm counts. The actions on the tight junction proteins also pointed to leptin interactions with Sertoli cells, which form the tight junctions and the blood–testes barrier.Sertoli cells are vital for the physical and nutritional support of spermatogenesis, producing acetate from glucose. In isolated human Sertoli cells in culture (215), 3 concentrations of leptin were tested, including adult male physiological levels (5 ng/mL) and levels found in obese (25 ng/mL) or morbidly obese (50 ng/mL) patients. Physiological concentration of leptin increased glucose transporter 2 proteins (215), indicating a route by which normal leptin levels may maintain spermatogenesis. However, with obesity levels of leptin, there was a dose-dependent decrease in acetate production, suggesting that rising leptin levels impair Sertoli cell nutritional support for spermatocytes. These findings are important to our understanding of how too much leptin can interfere with Leydig cell production of testosterone and Sertoli cell metabolic support of spermatogenesis.
Integrating Leptin Actions along the HPG Axis
We have proposed an integrative hypothesis for female reproduction that supports a “leptin permissive” partnership with the products of the HPG target cells to effect the optimal regulation of reproduction (97) (Figs. 2–4). We have further proposed that in the adult postpubertal state, leptin’s support for reproduction is optimal only in a narrow concentration range, which has been shown to be stimulatory to all target cells tested. Figures 2–4 summarize the major leptin target cells in the HPG axis.In females, the rise in leptin early in the cycle (from 14 to 20 ng/mL) is stimulated by estrogens from the growing follicles (108) (Fig. 4). Peak leptin levels synchronize with nocturnal LH pulses at midcycle (110, 111) because rising leptin has stimulated PMV neurons, which signal Kiss1 neurons (116, 120) (Figs. 2 and 3) and, subsequently, Kiss1 stimulates GnRH neurons (21, 22). During the follicular phase, rising estrogen from the developing follicles continues to stimulate the highest leptin levels at the time of the LH surge (112), as it provides positive feedback to promote higher amplitude and frequency GnRH pulses. Estrogen is also known to stimulate hormone and receptor expression in somatotropes, which promotes its role as a “somatogonadotrope,” or secretion of GH as a cogonadotropin (164, 168-170, 172) (Fig. 3). Leptin’s actions at the level of the ovary may enhance or permit the synergy between GH, IGF-1, and gonadotropins (186-189, 193) (Fig. 4).In the pituitary, rising leptin levels stimulate LEPR expression (96) peaking at midcycle and remaining high during the luteal phase. The combination of rising leptin and rapid GnRH pulses stimulate GnRHR (97, 98), thus facilitating the partnership between GnRH neurons and gonadotropes by preparing them to receive the more rapid midcycle GnRH pulses. This molecular partnership results in synchronized leptin–LH pulses and ovulation. We have determined that leptin uses a post-transcriptional signaling pathway to increase pituitary GnRHR levels and increase the sensitivity of gonadotropes to the more rapid GnRH pulses at midcycle (97, 98). It is notable that Fshb mRNA also has MBEs in its 3′ UTR, suggesting that cyclic derepression of Fshb mRNA translation in response to leptin signaling could enhance translation during the late luteal phase and may facilitate the early rise in FSH and the beginning of follicular development for the next cycle.In the ovary, we propose that rising serum leptin during the follicular phase can promote the production of estrogen directly (201), or in the context of the FSH growth factor synergy to produce testosterone and estrogen. Hyperleptinemia caused by obesity may block estrogen production due to leptin resistance (186-189, 193). We also propose that the slightly higher luteal phase leptin levels (20 ng/mL) maintain corpus luteum function, possibly promoting the synergy between LH and growth factor mediated estrogen and progesterone secretion. However, a higher leptin level in obesity will be inhibitory (186-189, 193). During the periovulatory period, we propose that normal leptin levels promote meiotic progression of the oocytes after the LH surge. Thus, appropriate levels of leptin are required at all levels of the HPG as a permissive signal leading to successful reproduction.
Acknowledgments
Financial Support: Grants supporting this work: NIH R01 HD059056 (G.V.C.); NIH R01HD087057 (G.V.C. and A.M.M.); NIH R01HD093461 (A.MM., G.V.C., and M.C.M.), NIH R01DK113776-01 (G.V.C., A.M.M., and M.C.M.); NIGMS P20 GM103425 and P30GM11070 (Dr. Edgar Garcia-Rill).
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- AgRP
agouti-related peptide
- ANOVA
analysis of variance
- FSH
follicle-stimulating hormone
- ERK
Extracellular signal regulated kinase
- GH
growth hormone
- GnRH
gonadotropin releasing hormone
- GnRHR
gonadotropin releasing hormone receptor
- HFD
high-fat diet
- HPG
hypothalamic–pituitary–gonadal
- IGF
insulin-like growth facto
- JAK
Janus kinase
- Kiss1
kisspeptin
- LEPR
leptin receptor
- LH
luteinizing hormone
- MAPK
mitogen-activated protein kinase
- mRNA
messenger ribonucleic acid
- MSI
Musashi
- mTOR
mammalian target of rapamycin
- NPY
neuropeptide Y
- PACAP
pituitary adenylate cyclase activating polypeptide
- PI3K
phosphoinositol 3 kinase
- PMV
ventral premammillary nucleus
- SOCS
suppressor of cytokine signaling
- STAT
signal transducer and activator of transcription
- TSH
thyroid-stimulating hormone
- UTR
untranslated region
Additional Information
Disclosure Summary: The authors have nothing to disclose
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in published articles that were reviewed by the authors. Also, because this is a review, data sharing of findings by others may not be applicable for many sections as no datasets were generated or analyzed during the writing of this review.
References
- 1. Hileman SM, Pierroz DD, Flier JS. Leptin, nutrition, and reproduction: timing is everything. J Clin Endocrinol Metab. 2000;85(2):804-807. [DOI] [PubMed] [Google Scholar]
- 2. Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol. 1963;166:408-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foster DL, Olster DH. Effect of restricted nutrition on puberty in the lamb: patterns of tonic luteinizing hormone (LH) secretion and competency of the LH surge system. Endocrinology. 1985;116(1):375-381. [DOI] [PubMed] [Google Scholar]
- 4. Schillo KK. Effects of dietary energy on control of luteinizing hormone secretion in cattle and sheep. J Anim Sci. 1992;70(4):1271-1282. [DOI] [PubMed] [Google Scholar]
- 5. Wade GN, Schneider JE, Li HY. Control of fertility by metabolic cues. Am J Physiol. 1996;270(1 Pt 1):E1-19. [DOI] [PubMed] [Google Scholar]
- 6. Gill CJ, Rissman EF. Female sexual behavior is inhibited by short- and long-term food restriction. Physiol Behav. 1997;61(3):387-394. [DOI] [PubMed] [Google Scholar]
- 7. Hamilton GD, Bronson FH. Food restriction and reproductive development: male and female mice and male rats. Am J Physiol. 1986;250(3 Pt 2):R370-R376. [DOI] [PubMed] [Google Scholar]
- 8. Cameron JL, Nosbisch C. Suppression of pulsatile luteinizing hormone and testosterone secretion during short term food restriction in the adult male rhesus monkey (Macaca mulatta). Endocrinology. 1991;128(3):1532-1540. [DOI] [PubMed] [Google Scholar]
- 9. Cameron JL, Weltzin TE, McConaha C, Helmreich DL, Kaye WH. Slowing of pulsatile luteinizing hormone secretion in men after forty-eight hours of fasting. J Clin Endocrinol Metab. 1991;73(1):35-41. [DOI] [PubMed] [Google Scholar]
- 10. Foster DL, Nagatani S. Physiological perspectives on leptin as a regulator of reproduction: role in timing puberty. Biol Reprod. 1999;60(2):205-215. [DOI] [PubMed] [Google Scholar]
- 11. Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292-295. [DOI] [PubMed] [Google Scholar]
- 12. Ruhl CE, Harris TB, Ding J, et al. Body mass index and serum leptin concentration independently estimate percentage body fat in older adults. Am J Clin Nutr. 2007;85(4):1121-1126. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425-432. [DOI] [PubMed] [Google Scholar]
- 14. Barash IA, Cheung CC, Weigle DS, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137(7):3144-3147. [DOI] [PubMed] [Google Scholar]
- 15. Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12(3):318-320. [DOI] [PubMed] [Google Scholar]
- 16. Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest. 1997;99(3):391-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kiess W, Blum WF, Aubert ML. Leptin, puberty and reproductive function: lessons from animal studies and observations in humans. Eur J Endocrinol. 1998;138(1):26-29. [DOI] [PubMed] [Google Scholar]
- 18. Casanueva FF, Dieguez C. Neuroendocrine regulation and actions of leptin. Front Neuroendocrinol. 1999;20(4):317-363. [DOI] [PubMed] [Google Scholar]
- 19. Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413-437. [DOI] [PubMed] [Google Scholar]
- 20. Chou SH, Mantzoros C. 20 years of leptin: role of leptin in human reproductive disorders. J Endocrinol. 2014;223(1):T49-T62. [DOI] [PubMed] [Google Scholar]
- 21. Elias CF. Leptin action in pubertal development: recent advances and unanswered questions. Trends Endocrinol Metab. 2012;23(1):9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elias CF, Purohit D. Leptin signaling and circuits in puberty and fertility. Cell Mol Life Sci. 2013;70(5):841-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caprio M, Fabbrini E, Isidori AM, Aversa A, Fabbri A. Leptin in reproduction. Trends Endocrinol Metab. 2001;12(2):65-72. [DOI] [PubMed] [Google Scholar]
- 24. Pralong FP, Gaillard RC. Neuroendocrine effects of leptin. Pituitary. 2001;4(1-2):25-32. [DOI] [PubMed] [Google Scholar]
- 25. Blüher S, Mantzoros CS. Leptin in reproduction. Curr Opin Endocrinol Diabetes Obes. 2007;14(6):458-464. [DOI] [PubMed] [Google Scholar]
- 26. Mantzoros CS, Magkos F, Brinkoetter M, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301(4):E567-E584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paz-Filho G, Wong ML, Licinio J. Ten years of leptin replacement therapy. Obes Rev. 2011;12(5):e315-e323. [DOI] [PubMed] [Google Scholar]
- 28. Coleman DL. A historical perspective on leptin. Nat Med. 2010;16(10):1097-1099. [DOI] [PubMed] [Google Scholar]
- 29. Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317-318. [DOI] [PubMed] [Google Scholar]
- 30. Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127-1128. [DOI] [PubMed] [Google Scholar]
- 31. Swerdloff RS, Batt RA, Bray GA. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology. 1976;98(6):1359-1364. [DOI] [PubMed] [Google Scholar]
- 32. Coleman DL, Hummel KP. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol. 1969;217(5):1298-1304. [DOI] [PubMed] [Google Scholar]
- 33. Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9(4):294-298. [DOI] [PubMed] [Google Scholar]
- 34. Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632-635. [DOI] [PubMed] [Google Scholar]
- 35. Chen H, Charlat O, Tartaglia LA, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84(3):491-495. [DOI] [PubMed] [Google Scholar]
- 36. Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A. 1996;93(13):6231-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263-1271. [DOI] [PubMed] [Google Scholar]
- 38. Cammisotto PG, Gélinas Y, Deshaies Y, Bukowiecki LJ. Regulation of leptin secretion from white adipocytes by insulin, glycolytic substrates, and amino acids. Am J Physiol Endocrinol Metab. 2005;289(1):E166-E171. [DOI] [PubMed] [Google Scholar]
- 39. Saladin R, De Vos P, Guerre-Millo M, et al. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377(6549):527-529. [DOI] [PubMed] [Google Scholar]
- 40. Menendez C, Baldelli R, Camiña JP, et al. TSH stimulates leptin secretion by a direct effect on adipocytes. J Endocrinol. 2003;176(1):7-12. [DOI] [PubMed] [Google Scholar]
- 41. Isozaki O, Tsushima T, Miyakawa M, Demura H, Seki H. Interaction between leptin and growth hormone (GH)/IGF-I axis. Endocr J. 1999;46(Suppl):S17-S24. [DOI] [PubMed] [Google Scholar]
- 42. Isozaki O, Tsushima T, Miyakawa M, Nozoe Y, Demura H, Seki H. Growth hormone directly inhibits leptin gene expression in visceral fat tissue in fatty Zucker rats. J Endocrinol. 1999;161(3):511-516. [DOI] [PubMed] [Google Scholar]
- 43. Fungfuang W, Nakada T, Nakao N, et al. Serum leptin concentrations, leptin mRNA expression, and food intake during the estrous cycle in rats. Lab Anim Res. 2013;29(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fungfuang W, Terada M, Komatsu N, Moon C, Saito TR. Effects of estrogen on food intake, serum leptin levels and leptin mRNA expression in adipose tissue of female rats. Lab Anim Res. 2013;29(3):168-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jenks MZ, Fairfield HE, Johnson EC, Morrison RF, Muday GK. Sex steroid hormones regulate leptin transcript accumulation and protein secretion in 3T3-L1 cells. Sci Rep. 2017;7(1):8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luukkaa V, Pesonen U, Huhtaniemi I, et al. Inverse correlation between serum testosterone and leptin in men. J Clin Endocrinol Metab. 1998;83(9):3243-3246. [DOI] [PubMed] [Google Scholar]
- 47. Peelman F, Zabeau L, Moharana K, Savvides SN, Tavernier J. 20 years of leptin: insights into signaling assemblies of the leptin receptor. J Endocrinol. 2014;223(1):T9-23. [DOI] [PubMed] [Google Scholar]
- 48. Wauman J, Zabeau L, Tavernier J. The leptin receptor complex: heavier than expected? Front Endocrinol (Lausanne). 2017;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baumann H, Morella KK, White DW, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93(16):8374-8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chua SC Jr, Koutras IK, Han L, et al. Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts. Genomics. 1997;45(2):264-270. [DOI] [PubMed] [Google Scholar]
- 51. White DW, Kuropatwinski KK, Devos R, Baumann H, Tartaglia LA. Leptin receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. J Biol Chem. 1997;272(7):4065-4071. [DOI] [PubMed] [Google Scholar]
- 52. Yamashita T, Murakami T, Otani S, Kuwajima M, Shima K. Leptin receptor signal transduction: OBRa and OBRb of fa type. Biochem Biophys Res Commun. 1998;246(3):752-759. [DOI] [PubMed] [Google Scholar]
- 53. Zamorano PL, Mahesh VB, De Sevilla LM, Chorich LP, Bhat GK, Brann DW. Expression and localization of the leptin receptor in endocrine and neuroendocrine tissues of the rat. Neuroendocrinology. 1997;65(3):223-228. [DOI] [PubMed] [Google Scholar]
- 54. Patterson CM, Villanueva EC, Greenwald-Yarnell M, et al. Leptin action via LepR-b Tyr1077 contributes to the control of energy balance and female reproduction. Mol Metab. 2012;1(1-2):61-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gao Q, Wolfgang MJ, Neschen S, et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101(13):4661-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Singireddy AV, Inglis MA, Zuure WA, Kim JS, Anderson GM. Neither signal transducer and activator of transcription 3 (STAT3) or STAT5 signaling pathways are required for leptin’s effects on fertility in mice. Endocrinology. 2013;154(7):2434-2445. [DOI] [PubMed] [Google Scholar]
- 57. Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393(Pt 1):7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bjørbaek C, Buchholz RM, Davis SM, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276(7):4747-4755. [DOI] [PubMed] [Google Scholar]
- 59. Myers MG Jr. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287-304. [DOI] [PubMed] [Google Scholar]
- 60. Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413(6858):794-795. [DOI] [PubMed] [Google Scholar]
- 61. Roa J, García-Galiano D, Castellano JM, Gaytan F, Pinilla L, Tena-Sempere M. Metabolic control of puberty onset: new players, new mechanisms. Mol Cell Endocrinol. 2010;324(1-2):87-94. [DOI] [PubMed] [Google Scholar]
- 62. Roa J, Garcia-Galiano D, Varela L, et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology. 2009;150(11):5016-5026. [DOI] [PubMed] [Google Scholar]
- 63. Yu WH, Walczewska A, Karanth S, McCann SM. Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology. 1997;138(11):5055-5058. [DOI] [PubMed] [Google Scholar]
- 64. Riad-Gabriel MG, Jinagouda SD, Sharma A, Boyadjian R, Saad MF. Changes in plasma leptin during the menstrual cycle. Eur J Endocrinol. 1998;139(5):528-531. [DOI] [PubMed] [Google Scholar]
- 65. Wołodko K, Walewska E, Adamowski M, Castillo-Fernandez J, Kelsey G, Galvão A. Leptin resistance in the ovary of obese mice is associated with profound changes in the transcriptome of cumulus cells. Cell Physiol Biochem. 2020;54(3):417-437. [DOI] [PubMed] [Google Scholar]
- 66. Bjørbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272(51):32686-32695. [DOI] [PubMed] [Google Scholar]
- 67. Odle AK, Haney A, Allensworth-James M, Akhter N, Childs GV. Adipocyte versus pituitary leptin in the regulation of pituitary hormones: somatotropes develop normally in the absence of circulating leptin. Endocrinology. 2014;155(11):4316-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chehab FF, Mounzih K, Lu R, Lim ME. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997;275(5296):88-90. [DOI] [PubMed] [Google Scholar]
- 69. Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138(2):855-858. [DOI] [PubMed] [Google Scholar]
- 70. Bronson FH. Food-restricted, prepubertal, female rats: rapid recovery of luteinizing hormone pulsing with excess food, and full recovery of pubertal development with gonadotropin-releasing hormone. Endocrinology. 1986;118(6):2483-2487. [DOI] [PubMed] [Google Scholar]
- 71. Bronson FH. Puberty in female mice is not associated with increases in either body fat or leptin. Endocrinology. 2001;142(11):4758-4761. [DOI] [PubMed] [Google Scholar]
- 72. Cheung CC, Thornton JE, Nurani SD, Clifton DK, Steiner RA. A reassessment of leptin’s role in triggering the onset of puberty in the rat and mouse. Neuroendocrinology. 2001;74(1):12-21. [DOI] [PubMed] [Google Scholar]
- 73. Mann DR, Akinbami MA, Gould KG, Castracane VD. Leptin and thyroxine during sexual development in male monkeys: effect of neonatal gonadotropin-releasing hormone antagonist treatment and delayed puberty on the developmental pattern of leptin and thyroxine secretion. Eur J Endocrinol. 2002;146(6):891-898. [DOI] [PubMed] [Google Scholar]
- 74. Mann DR, Bhat GK, Ramaswamy S, Stah CD, Plant TM. Regulation of circulating leptin and its soluble receptor during pubertal development in the male rhesus monkey (Macaca mulatta). Endocrine. 2007;31(2):125-129. [DOI] [PubMed] [Google Scholar]
- 75. Plant TM, Durrant AR. Circulating leptin does not appear to provide a signal for triggering the initiation of puberty in the male rhesus monkey (Macaca mulatta). Endocrinology. 1997;138(10):4505-4508. [DOI] [PubMed] [Google Scholar]
- 76. Urbanski HF, Pau KY. A biphasic developmental pattern of circulating leptin in the male rhesus macaque (Macaca mulatta). Endocrinology. 1998;139(5):2284-2286. [DOI] [PubMed] [Google Scholar]
- 77. Jaquet D, Leger J, Levy-Marchal C, Oury JF, Czernichow P. Ontogeny of leptin in human fetuses and newborns: effect of intrauterine growth retardation on serum leptin concentrations. J Clin Endocrinol Metab. 1998;83(4):1243-1246. [DOI] [PubMed] [Google Scholar]
- 78. Cetin I, Morpurgo PS, Radaelli T, et al. Fetal plasma leptin concentrations: relationship with different intrauterine growth patterns from 19 weeks to term. Pediatr Res. 2000;48(5):646-651. [DOI] [PubMed] [Google Scholar]
- 79. Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101(5):1020-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Devaskar SU, Ollesch C, Rajakumar RA, Rajakumar PA. Developmental changes in ob gene expression and circulating leptin peptide concentrations. Biochem Biophys Res Commun. 1997;238(1):44-47. [DOI] [PubMed] [Google Scholar]
- 81. Delahaye F, Breton C, Risold PY, et al. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149(2):470-475. [DOI] [PubMed] [Google Scholar]
- 82. Vickers MH, Gluckman PD, Coveny AH, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146(10):4211-4216. [DOI] [PubMed] [Google Scholar]
- 83. Yura S, Itoh H, Sagawa N, et al. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005;1(6):371-378. [DOI] [PubMed] [Google Scholar]
- 84. Mistry AM, Swick A, Romsos DR. Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am J Physiol. 1999;277(3):R742-R747. [DOI] [PubMed] [Google Scholar]
- 85. Proulx K, Richard D, Walker CD. Leptin regulates appetite-related neuropeptides in the hypothalamus of developing rats without affecting food intake. Endocrinology. 2002;143(12):4683-4692. [DOI] [PubMed] [Google Scholar]
- 86. Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108-110. [DOI] [PubMed] [Google Scholar]
- 87. Bouret SG, Simerly RB. Minireview: leptin and development of hypothalamic feeding circuits. Endocrinology. 2004;145(6):2621-2626. [DOI] [PubMed] [Google Scholar]
- 88. Ramos-Lobo AM, Teixeira PD, Furigo IC, et al. Long-term consequences of the absence of leptin signaling in early life. Elife. 2019;8:e40970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Attig L, Larcher T, Gertler A, Abdennebi-Najar L, Djiane J. Postnatal leptin is necessary for maturation of numerous organs in newborn rats. Organogenesis. 2011;7(2):88-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Coupé B, Amarger V, Grit I, Benani A, Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology. 2010;151(2):702-713. [DOI] [PubMed] [Google Scholar]
- 91. López-Gallardo M, Antón-Fernández A, Llorente R, et al. Neonatal treatment with a pegylated leptin antagonist induces sexually dimorphic effects on neurones and glial cells, and on markers of synaptic plasticity in the developing rat hippocampal formation. J Neuroendocrinol. 2015;27(8):658-669. [DOI] [PubMed] [Google Scholar]
- 92. Léonhardt M, Lesage J, Croix D, Dutriez-Casteloot I, Beauvillain JC, Dupouy JP. Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol Reprod. 2003;68(2):390-400. [DOI] [PubMed] [Google Scholar]
- 93. Mela V, Díaz F, Lopez-Rodriguez AB, et al. Blockage of the neonatal leptin surge affects the gene expression of growth factors, glial proteins, and neuropeptides involved in the control of metabolism and reproduction in peripubertal male and female rats. Endocrinology. 2015;156(7):2571-2581. [DOI] [PubMed] [Google Scholar]
- 94. Castellano JM, Bentsen AH, Sánchez-Garrido MA, et al. Early metabolic programming of puberty onset: impact of changes in postnatal feeding and rearing conditions on the timing of puberty and development of the hypothalamic kisspeptin system. Endocrinology. 2011;152(9):3396-3408. [DOI] [PubMed] [Google Scholar]
- 95. Iwasa T, Matsuzaki T, Murakami M, et al. Sensitivities of mRNA expression levels of Kiss1 and its receptor, Kiss1r, to nutritional status are changed during the developmental period in female rats. J Endocrinol. 2010;207(2):195-202. [DOI] [PubMed] [Google Scholar]
- 96. Akhter N, CarlLee T, Syed MM, et al. Selective deletion of leptin receptors in gonadotropes reveals activin and GnRH-binding sites as leptin targets in support of fertility. Endocrinology. 2014;155(10):4027-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Odle AK, Akhter N, Syed MM, et al. Leptin regulation of gonadotrope gonadotropin-releasing hormone receptors as a metabolic checkpoint and gateway to reproductive competence. Front Endocrinol (Lausanne). 2017;8:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Odle AK, Beneš H, Melgar Castillo A, et al. Association of Gnrhr mRNA with the stem cell determinant Musashi: a mechanism for leptin-mediated modulation of GnRHR expression. Endocrinology. 2018;159(2):883-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci U S A. 2010;107(37):16372-16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Döhler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97(4):898-907. [DOI] [PubMed] [Google Scholar]
- 101. Bjelobaba I, Janjic MM, Kucka M, Stojilkovic SS. Cell type-specific sexual dimorphism in rat pituitary gene expression during maturation. Biol Reprod. 2015;93(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wilson ME, Handa RJ. Ontogeny of gene expression in the gonadotroph of the developing female rat. Biol Reprod. 1997;56(2):563-568. [DOI] [PubMed] [Google Scholar]
- 103. Wilson ME, Handa RJ. Activin subunit, follistatin, and activin receptor gene expression in the prepubertal female rat pituitary. Biol Reprod. 1998;59(2):278-283. [DOI] [PubMed] [Google Scholar]
- 104. Suter KJ, Pohl CR, Plant TM. The pattern and tempo of the pubertal reaugmentation of open-loop pulsatile gonadotropin-releasing hormone release assessed indirectly in the male rhesus monkey (Macaca mulatta). Endocrinology. 1998;139(6):2774-2783. [DOI] [PubMed] [Google Scholar]
- 105. Nagatani S, Guthikonda P, Foster DL. Appearance of a nocturnal peak of leptin secretion in the pubertal rat. Horm Behav. 2000;37(4):345-352. [DOI] [PubMed] [Google Scholar]
- 106. Garcia-Mayor RV, Andrade MA, Rios M, Lage M, Dieguez C, Casanueva FF. Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. J Clin Endocrinol Metab. 1997;82(9):2849-2855. [DOI] [PubMed] [Google Scholar]
- 107. Isidori AM, Strollo F, Morè M, et al. Leptin and aging: correlation with endocrine changes in male and female healthy adult populations of different body weights. J Clin Endocrinol Metab. 2000;85(5):1954-1962. [DOI] [PubMed] [Google Scholar]
- 108. Wauters M, Considine RV, Van Gaal LF. Human leptin: from an adipocyte hormone to an endocrine mediator. Eur J Endocrinol. 2000;143(3):293-311. [DOI] [PubMed] [Google Scholar]
- 109. Wabitsch M, Blum WF, Muche R, et al. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J Clin Invest. 1997;100(4):808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Licinio J, Negrão AB, Mantzoros C, et al. Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proc Natl Acad Sci U S A. 1998;95(5):2541-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sir-Petermann T, Piwonka V, Pérez F, Maliqueo M, Recabarren SE, Wildt L. Are circulating leptin and luteinizing hormone synchronized in patients with polycystic ovary syndrome? Hum Reprod. 1999;14(6):1435-1439. [DOI] [PubMed] [Google Scholar]
- 112. Ahrens K, Mumford SL, Schliep KC, et al. Serum leptin levels and reproductive function during the menstrual cycle. Am J Obstet Gynecol. 2014;210(3):248.e1-248.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. McMinn JE, Liu SM, Liu H, et al. Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. Am J Physiol Endocrinol Metab. 2005;289(3):E403-E411. [DOI] [PubMed] [Google Scholar]
- 114. Chan JL, Mantzoros CS. Leptin and the hypothalamic-pituitary regulation of the gonadotropin-gonadal axis. Pituitary. 2001;4(1-2):87-92. [DOI] [PubMed] [Google Scholar]
- 115. Quennell JH, Mulligan AC, Tups A, et al. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150(6):2805-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Donato J Jr, Cravo RM, Frazão R, et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121(1):355-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Navarro VM. Metabolic regulation of kisspeptin - the link between energy balance and reproduction. Nat Rev Endocrinol. 2020;16(8):407-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Evans MC, Anderson GM. Integration of circadian and metabolic control of reproductive function. Endocrinology. 2018;159(11):3661-3673. [DOI] [PubMed] [Google Scholar]
- 119. Donato J Jr, Elias CF. The ventral premammillary nucleus links metabolic cues and reproduction. Front Endocrinol (Lausanne). 2011;2:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Donato J Jr, Silva RJ, Sita LV, et al. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J Neurosci. 2009;29(16):5240-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mahany EB, Han X, Borges BC, et al. Obesity and high-fat diet induce distinct changes in placental gene expression and pregnancy outcome. Endocrinology. 2018;159(4):1718-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ross RA, Leon S, Madara JC, et al. PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Petrine JCP, Franci CR, Del Bianco-Borges B. Leptin actions through the nitrergic system to modulate the hypothalamic expression of the kiss1 mRNA in the female rat. Brain Res. 2020;1728:146574. [DOI] [PubMed] [Google Scholar]
- 124. Zuure WA, Roberts AL, Quennell JH, Anderson GM. Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function. J Neurosci. 2013;33(45):17874-17883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Martin C, Navarro VM, Simavli S, et al. Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. J Neurosci. 2014;34(17):6047-6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Padilla SL, Qiu J, Nestor CC, et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci U S A. 2017;114(9):2413-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci U S A. 2012;109(8):3155-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Egan OK, Inglis MA, Anderson GM. Leptin signaling in AgRP neurons modulates puberty onset and adult fertility in mice. J Neurosci. 2017;37(14):3875-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Jin L, Burguera BG, Couce ME, et al. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999;84(8):2903-2911. [DOI] [PubMed] [Google Scholar]
- 130. Jin L, Zhang S, Burguera BG, et al. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology. 2000;141(1):333-339. [DOI] [PubMed] [Google Scholar]
- 131. Iqbal J, Kurose Y, Canny B, Clarke IJ. Effects of central infusion of ghrelin on food intake and plasma levels of growth hormone, luteinizing hormone, prolactin, and cortisol secretion in sheep. Endocrinology. 2006;147(1):510-519. [DOI] [PubMed] [Google Scholar]
- 132. Iqbal J, Pompolo S, Considine RV, Clarke IJ. Localization of leptin receptor-like immunoreactivity in the corticotropes, somatotropes, and gonadotropes in the ovine anterior pituitary. Endocrinology. 2000;141(4):1515-1520. [DOI] [PubMed] [Google Scholar]
- 133. Lloyd RV, Jin L, Qian X, Zhang S, Scheithauer BW. Nitric oxide synthase in the human pituitary gland. Am J Pathol. 1995;146(1):86-94. [PMC free article] [PubMed] [Google Scholar]
- 134. Lloyd RV, Jin L, Tsumanuma I, et al. Leptin and leptin receptor in anterior pituitary function. Pituitary. 2001;4(1-2):33-47. [DOI] [PubMed] [Google Scholar]
- 135. Giusti M, Bocca L, Florio T, et al. In vitro effect of human recombinant leptin and expression of leptin receptors on growth hormone-secreting human pituitary adenomas. Clin Endocrinol (Oxf). 2002;57(4):449-455. [DOI] [PubMed] [Google Scholar]
- 136. Sone M, Osamura RY. Leptin and the pituitary. Pituitary. 2001;4(1-2):15-23. [DOI] [PubMed] [Google Scholar]
- 137. Mann DR, Plant TM. Leptin and pubertal development. Semin Reprod Med. 2002;20(2):93-102. [DOI] [PubMed] [Google Scholar]
- 138. Urbanski HF. Leptin and puberty. Trends Endocrinol Metab. 2001;12(10):428-429. [DOI] [PubMed] [Google Scholar]
- 139. Yura S, Ogawa Y, Sagawa N, et al. Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J Clin Invest. 2000;105(6):749-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kaminski T, Smolinska N, Gajewska A, et al. Leptin and long form of leptin receptor genes expression in the hypothalamus and pituitary during the luteal phase and early pregnancy in pigs. J Physiol Pharmacol. 2006;57(1):95-108. [PubMed] [Google Scholar]
- 141. Carro E, Pinilla L, Seoane LM, et al. Influence of endogenous leptin tone on the estrous cycle and luteinizing hormone pulsatility in female rats. Neuroendocrinology. 1997;66(6):375-377. [DOI] [PubMed] [Google Scholar]
- 142. De Biasi SN, Apfelbaum LI, Apfelbaum ME. In vitro effect of leptin on LH release by anterior pituitary glands from female rats at the time of spontaneous and steroid-induced LH surge. Eur J Endocrinol. 2001;145(5):659-665. [DOI] [PubMed] [Google Scholar]
- 143. Schneider JE, Goldman MD, Tang S, Bean B, Ji H, Friedman MI. Leptin indirectly affects estrous cycles by increasing metabolic fuel oxidation. Horm Behav. 1998;33(3):217-228. [DOI] [PubMed] [Google Scholar]
- 144. Ogura K, Irahara M, Kiyokawa M, et al. Effects of leptin on secretion of LH and FSH from primary cultured female rat pituitary cells. Eur J Endocrinol. 2001;144(6):653-658. [DOI] [PubMed] [Google Scholar]
- 145. Spicer LJ. Leptin: a possible metabolic signal affecting reproduction. Domest Anim Endocrinol. 2001;21(4):251-270. [DOI] [PubMed] [Google Scholar]
- 146. Tezuka M, Irahara M, Ogura K, et al. Effects of leptin on gonadotropin secretion in juvenile female rat pituitary cells. Eur J Endocrinol. 2002;146(2):261-266. [DOI] [PubMed] [Google Scholar]
- 147. Swerdloff RS, Peterson M, Vera A, Batt RA, Heber D, Bray GA. The hypothalamic-pituitary axis in genetically obese (ob/ob) mice: response to luteinizing hormone-releasing hormone. Endocrinology. 1978;103(2):542-547. [DOI] [PubMed] [Google Scholar]
- 148. Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci U S A. 1997;94(3):1023-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250-252. [DOI] [PubMed] [Google Scholar]
- 150. Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139(11):4652-4662. [DOI] [PubMed] [Google Scholar]
- 151. Gonzalez LC, Pinilla L, Tena-Sempere M, Aguilar E. Leptin(116-130) stimulates prolactin and luteinizing hormone secretion in fasted adult male rats. Neuroendocrinology. 1999;70(3):213-220. [DOI] [PubMed] [Google Scholar]
- 152. Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology. 1998;67(6):370-376. [DOI] [PubMed] [Google Scholar]
- 153. Nagatani S, Zeng Y, Keisler DH, Foster DL, Jaffe CA. Leptin regulates pulsatile luteinizing hormone and growth hormone secretion in the sheep. Endocrinology. 2000;141(11):3965-3975. [DOI] [PubMed] [Google Scholar]
- 154. Schneider JE, Blum RM, Wade GN. Metabolic control of food intake and estrous cycles in Syrian hamsters. I. Plasma insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2000;278(2):R476-R485. [DOI] [PubMed] [Google Scholar]
- 155. Schneider JE, Buckley CA, Blum RM, et al. Metabolic signals, hormones and neuropeptides involved in control of energy balance and reproductive success in hamsters. Eur J Neurosci. 2002;16(3):377-379. [DOI] [PubMed] [Google Scholar]
- 156. Schneider JE, Zhou D. Interactive effects of central leptin and peripheral fuel oxidation on estrous cyclicity. Am J Physiol. 1999;277(4):R1020-R1024. [DOI] [PubMed] [Google Scholar]
- 157. Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879-884. [DOI] [PubMed] [Google Scholar]
- 158. Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111(9):1409-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Welt CK. Will leptin become the treatment of choice for functional hypothalamic amenorrhea? Nat Clin Pract Endocrinol Metab. 2007;3(8):556-557. [DOI] [PubMed] [Google Scholar]
- 160. Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351(10):987-997. [DOI] [PubMed] [Google Scholar]
- 161. Walczewska A, Yu WH, Karanth S, McCann SM. Estrogen and leptin have differential effects on FSH and LH release in female rats. Proc Soc Exp Biol Med. 1999;222(2):170-177. [DOI] [PubMed] [Google Scholar]
- 162. Crane C, Akhter N, Johnson BW, et al. Fasting and glucose effects on pituitary leptin expression: is leptin a local signal for nutrient status? J Histochem Cytochem. 2007;55(10):1059-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Sarmento-Cabral A, Peinado JR, Halliday LC, et al. Adipokines (leptin, adiponectin, resistin) differentially regulate all hormonal cell types in primary anterior pituitary cell cultures from two primate species. Sci Rep. 2017;7:43537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Childs GV, MacNicol AM, MacNicol MC. Molecular mechanisms of pituitary cell plasticity. Front Endocrinol (Lausanne). 2020;11:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Allensworth-James ML, Odle AK, Lim J, et al. Metabolic signalling to somatotrophs: transcriptional and post-transcriptional mediators. J Neuroendocrinol. 2020;32(11):e12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Odle AK, Allensworth-James ML, Akhter N, et al. A sex-dependent, tropic role for leptin in the somatotrope as a regulator of POU1F1 and POU1F1-dependent hormones. Endocrinology. 2016;157(10):3958-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Childs GV. Growth hormone cells as co-gonadotropes: partners in the regulation of the reproductive system. Trends Endocrinol Metab. 2000;11(5):168-175. [DOI] [PubMed] [Google Scholar]
- 168. Childs GV. Development of gonadotropes may involve cyclic transdifferentiation of growth hormone cells. Arch Physiol Biochem. 2002;110(1-2):42-49. [DOI] [PubMed] [Google Scholar]
- 169. Childs GV, Unabia G, Miller BT. Cytochemical detection of gonadotropin-releasing hormone-binding sites on rat pituitary cells with luteinizing hormone, follicle-stimulating hormone, and growth hormone antigens during diestrous up-regulation. Endocrinology. 1994;134(4):1943-1951. [DOI] [PubMed] [Google Scholar]
- 170. Childs GV, Unabia G, Miller BT, Collins TJ. Differential expression of gonadotropin and prolactin antigens by GHRH target cells from male and female rats. J Endocrinol. 1999;162(2):177-187. [DOI] [PubMed] [Google Scholar]
- 171. Childs GV, Unabia G, Rougeau D. Cells that express luteinizing hormone (LH) and follicle-stimulating hormone (FSH) beta-subunit messenger ribonucleic acids during the estrous cycle: the major contributors contain LH beta, FSH beta, and/or growth hormone. Endocrinology. 1994;134(2):990-997. [DOI] [PubMed] [Google Scholar]
- 172. Childs GV, Iruthayanathan M, Akhter N, Unabia G, Whitehead-Johnson B. Bipotential effects of estrogen on growth hormone synthesis and storage in vitro. Endocrinology. 2005;146(4):1780-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Denef C. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol. 2008;20(1):1-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Odle AK, Allensworth-James M, Haney A, Akhter N, Syed M, Childs GV. Adipocyte versus somatotrope leptin: regulation of metabolic functions in the mouse. Endocrinology. 2016;157(4):1443-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Allen SJ, Garcia-Galiano D, Borges BC, Burger LL, Boehm U, Elias CF. Leptin receptor null mice with reexpression of LepR in GnRHR expressing cells display elevated FSH levels but remain in a prepubertal state. Am J Physiol Regul Integr Comp Physiol. 2016;310(11):R1258-R1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Fox RG, Park FD, Koechlein CS, Kritzik M, Reya T. Musashi signaling in stem cells and cancer. Annu Rev Cell Dev Biol. 2015;31:249-267. [DOI] [PubMed] [Google Scholar]
- 177. Szabat M, Kalynyak TB, Lim GE, et al. Musashi expression in β-cells coordinates insulin expression, apoptosis and proliferation in response to endoplasmic reticulum stress in diabetes. Cell Death Dis. 2011;2:e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Cioffi JA, Shafer AW, Zupancic TJ, et al. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat Med. 1996;2(5):585-589. [DOI] [PubMed] [Google Scholar]
- 179. Brzechffa PR, Jakimiuk AJ, Agarwal SK, Weitsman SR, Buyalos RP, Magoffin DA. Serum immunoreactive leptin concentrations in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81(11):4166-4169. [DOI] [PubMed] [Google Scholar]
- 180. Spicer LJ, Francisco CC. The adipose obese gene product, leptin: evidence of a direct inhibitory role in ovarian function. Endocrinology. 1997;138(8):3374-3379. [DOI] [PubMed] [Google Scholar]
- 181. Chapman IM, Wittert GA, Norman RJ. Circulating leptin concentrations in polycystic ovary syndrome: relation to anthropometric and metabolic parameters. Clin Endocrinol (Oxf). 1997;46(2):175-181. [DOI] [PubMed] [Google Scholar]
- 182. Mantzoros CS, Dunaif A, Flier JS. Leptin concentrations in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(6):1687-1691. [DOI] [PubMed] [Google Scholar]
- 183. Laughlin GA, Morales AJ, Yen SS. Serum leptin levels in women with polycystic ovary syndrome: the role of insulin resistance/hyperinsulinemia. J Clin Endocrinol Metab. 1997;82(6):1692-1696. [DOI] [PubMed] [Google Scholar]
- 184. Rouru J, Anttila L, Koskinen P, et al. Serum leptin concentrations in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(6):1697-1700. [DOI] [PubMed] [Google Scholar]
- 185. Karlsson C, Lindell K, Svensson E, et al. Expression of functional leptin receptors in the human ovary. J Clin Endocrinol Metab. 1997;82(12):4144-4148. [DOI] [PubMed] [Google Scholar]
- 186. Agarwal SK, Vogel K, Weitsman SR, Magoffin DA. Leptin antagonizes the insulin-like growth factor-I augmentation of steroidogenesis in granulosa and theca cells of the human ovary. J Clin Endocrinol Metab. 1999;84(3):1072-1076. [DOI] [PubMed] [Google Scholar]
- 187. Zachow RJ, Magoffin DA. Direct intraovarian effects of leptin: impairment of the synergistic action of insulin-like growth factor-I on follicle-stimulating hormone-dependent estradiol-17 beta production by rat ovarian granulosa cells. Endocrinology. 1997;138(2):847-850. [DOI] [PubMed] [Google Scholar]
- 188. Zachow RJ, Weitsman SR, Magoffin DA. Leptin impairs the synergistic stimulation by transforming growth factor-beta of follicle-stimulating hormone-dependent aromatase activity and messenger ribonucleic acid expression in rat ovarian granulosa cells. Biol Reprod. 1999;61(4):1104-1109. [DOI] [PubMed] [Google Scholar]
- 189. Spicer LJ, Francisco CC. Adipose obese gene product, leptin, inhibits bovine ovarian thecal cell steroidogenesis. Biol Reprod. 1998;58(1):207-212. [DOI] [PubMed] [Google Scholar]
- 190. Kikuchi N, Andoh K, Abe Y, Yamada K, Mizunuma H, Ibuki Y. Inhibitory action of leptin on early follicular growth differs in immature and adult female mice. Biol Reprod. 2001;65(1):66-71. [DOI] [PubMed] [Google Scholar]
- 191. Duggal PS, Van Der Hoek KH, Milner CR, et al. The in vivo and in vitro effects of exogenous leptin on ovulation in the rat. Endocrinology. 2000;141(6):1971-1976. [DOI] [PubMed] [Google Scholar]
- 192. Brannian JD, Hansen KA. Leptin and ovarian folliculogenesis: implications for ovulation induction and ART outcomes. Semin Reprod Med. 2002;20(2):103-112. [DOI] [PubMed] [Google Scholar]
- 193. Ricci AG, Di Yorio MP, Faletti AG. Inhibitory effect of leptin on the rat ovary during the ovulatory process. Reproduction. 2006;132(5):771-780. [DOI] [PubMed] [Google Scholar]
- 194. Almog B, Gold R, Tajima K, et al. Leptin attenuates follicular apoptosis and accelerates the onset of puberty in immature rats. Mol Cell Endocrinol. 2001;183(1-2):179-191. [DOI] [PubMed] [Google Scholar]
- 195. Ruiz-Cortés ZT, Martel-Kennes Y, Gévry NY, Downey BR, Palin MF, Murphy BD. Biphasic effects of leptin in porcine granulosa cells. Biol Reprod. 2003;68(3):789-796. [DOI] [PubMed] [Google Scholar]
- 196. Swain JE, Dunn RL, McConnell D, Gonzalez-Martinez J, Smith GD. Direct effects of leptin on mouse reproductive function: regulation of follicular, oocyte, and embryo development. Biol Reprod. 2004;71(5):1446-1452. [DOI] [PubMed] [Google Scholar]
- 197. Ryan NK, Woodhouse CM, Van der Hoek KH, Gilchrist RB, Armstrong DT, Norman RJ. Expression of leptin and its receptor in the murine ovary: possible role in the regulation of oocyte maturation. Biol Reprod. 2002;66(5):1548-1554. [DOI] [PubMed] [Google Scholar]
- 198. Paula-Lopes FF, Boelhauve M, Habermann FA, Sinowatz F, Wolf E. Leptin promotes meiotic progression and developmental capacity of bovine oocytes via cumulus cell-independent and -dependent mechanisms. Biol Reprod. 2007;76(3):532-541. [DOI] [PubMed] [Google Scholar]
- 199. Di Yorio MP, Bilbao MG, Pustovrh MC, Prestifilippo JP, Faletti AG. Leptin modulates the expression of its receptors in the hypothalamic-pituitary-ovarian axis in a differential way. J Endocrinol. 2008;198(2):355-366. [DOI] [PubMed] [Google Scholar]
- 200. Di Yorio MP, Bilbao MG, Biagini-Majorel AM, Faletti AG. Ovarian signalling pathways regulated by leptin during the ovulatory process. Reproduction. 2013;146(6):647-658. [DOI] [PubMed] [Google Scholar]
- 201. Karamouti M, Kollia P, Kallitsaris A, Vamvakopoulos N, Kollios G, Messinis IE. Modulating effect of leptin on basal and follicle stimulating hormone stimulated steroidogenesis in cultured human lutein granulosa cells. J Endocrinol Invest. 2009;32(5):415-419. [DOI] [PubMed] [Google Scholar]
- 202. Ye Y, Kawamura K, Sasaki M, et al. Leptin and ObRa/MEK signalling in mouse oocyte maturation and preimplantation embryo development. Reprod Biomed Online. 2009;19(2):181-190. [DOI] [PubMed] [Google Scholar]
- 203. Tena-Sempere M, Manna PR, Zhang FP, et al. Molecular mechanisms of leptin action in adult rat testis: potential targets for leptin-induced inhibition of steroidogenesis and pattern of leptin receptor messenger ribonucleic acid expression. J Endocrinol. 2001;170(2):413-423. [DOI] [PubMed] [Google Scholar]
- 204. Tena-Sempere M, Pinilla L, Zhang FP, et al. Developmental and hormonal regulation of leptin receptor (Ob-R) messenger ribonucleic acid expression in rat testis. Biol Reprod. 2001;64(2):634-643. [DOI] [PubMed] [Google Scholar]
- 205. Hoggard N, Mercer JG, Rayner DV, Moar K, Trayhurn P, Williams LM. Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochem Biophys Res Commun. 1997;232(2):383-387. [DOI] [PubMed] [Google Scholar]
- 206. Suter KJ, Pohl CR, Wilson ME. Circulating concentrations of nocturnal leptin, growth hormone, and insulin-like growth factor-I increase before the onset of puberty in agonadal male monkeys: potential signals for the initiation of puberty. J Clin Endocrinol Metab. 2000;85(2):808-814. [DOI] [PubMed] [Google Scholar]
- 207. El-Hefnawy T, Ioffe S, Dym M. Expression of the leptin receptor during germ cell development in the mouse testis. Endocrinology. 2000;141(7):2624-2630. [DOI] [PubMed] [Google Scholar]
- 208. Zhang J, Gong M. Review of the role of leptin in the regulation of male reproductive function. Andrologia. 2018. [DOI] [PubMed] [Google Scholar]
- 209. Tena-Sempere M, Barreiro ML. Leptin in male reproduction: the testis paradigm. Mol Cell Endocrinol. 2002;188(1-2):9-13. [DOI] [PubMed] [Google Scholar]
- 210. Tena-Sempere M, Pinilla L, González LC, Diéguez C, Casanueva FF, Aguilar E. Leptin inhibits testosterone secretion from adult rat testis in vitro. J Endocrinol. 1999;161(2):211-218. [DOI] [PubMed] [Google Scholar]
- 211. Caprio M, Isidori AM, Carta AR, Moretti C, Dufau ML, Fabbri A. Expression of functional leptin receptors in rodent Leydig cells. Endocrinology. 1999;140(11):4939-4947. [DOI] [PubMed] [Google Scholar]
- 212. Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab. 1999;84(10):3673-3680. [DOI] [PubMed] [Google Scholar]
- 213. Banks WA, McLay RN, Kastin AJ, Sarmiento U, Scully S. Passage of leptin across the blood-testis barrier. Am J Physiol. 1999;276(6):E1099-E1104. [DOI] [PubMed] [Google Scholar]
- 214. Wang X, Zhang X, Hu L, Li H. Exogenous leptin affects sperm parameters and impairs blood testis barrier integrity in adult male mice. Reprod Biol Endocrinol. 2018;16(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Martins AD, Moreira AC, Sá R, et al. Leptin modulates human Sertoli cells acetate production and glycolytic profile: a novel mechanism of obesity-induced male infertility? Biochim Biophys Acta. 2015;1852(9):1824-1832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in published articles that were reviewed by the authors. Also, because this is a review, data sharing of findings by others may not be applicable for many sections as no datasets were generated or analyzed during the writing of this review.