Abstract
Three randomized phase III trials have now conclusively proven that exposure to a PD-1 inhibitor prolongs survival in recurrent/metastatic (R/M) HNSCC, and it is clear that such agents should be used in the management of all patients who do not have contraindications to their use. Two of these phase III randomized trials showed that the anti-PD1 antibodies nivolumab and pembrolizumab were superior to investigators′ choice chemotherapy in second-line platinum-refractory R/M HNSCC. Recently, a third phase III randomized trial, KEYNOTE-048, showed that pembrolizumab with chemotherapy was superior to the EXTREME regimen (cis- or carboplatin, 5-fluorouracil (5-FU) and cetuximab) in all patients, and pembrolizumab monotherapy was superior in patients whose tumors express PD-L1 in first-line R/M HNSCC. Pembrolizumab is now approved as monotherapy in PD-L1 expressing disease (combined positive score ≥1) or in combination with chemotherapy for all patients with R/M HNSCC. Thus, PD-L1 biomarker testing will be routinely used in R/M HNSCC, and this employs a scoring system that incorporates immune cell staining, referred to as the combined positive score (CPS). Additionally, for the 85% of patients with PD-L1 CPS ≥1, clinical judgment will guide the choice of pembrolizumab monotherapy or pembrolizumab plus chemotherapy, until more detailed clinical data are forthcoming to better inform this decision. In this article we discuss the clinical trials leading to these therapeutic advances and we will review initial results from clinical trials in previously untreated, locally advanced disease, and those using novel combinations of checkpoint inhibitors, co-stimulatory agonists, and therapeutic vaccines.
Keywords: Head and neck squamous cell carcinoma, Head and neck cancer, Immunotherapy, Immune checkpoint inhibitor, Pembrolizumab, Nivolumab
Introduction
Worldwide, approximately 830,000 patients develop head and neck cancer each year.1 Approximately 430,000 will die from this disease [1]. Despite aggressive multimodal strategies to treat head and neck squamous cell carcinoma (HNSCC) using combinations of surgery, radiotherapy (RT) and chemotherapy, the 5-year overall survival of carcinogen-related HNSCC is only 40–50% [2]. In addition, the rapid emergence of the human papillomavirus (HPV)-associated subset of HNSCC has motivated novel, immune-based therapies. For recurrent/metastatic (R/M) disease, median survival is only 10.1 months with the historic standard first-line EXTREME regimen using the triplet: cis- or carboplatin, 5-fluorouracil (5-FU) and cetuximab [3]. The toxicity of the EXTREME regimen is considerable, with an 82% rate of grade 3–4 adverse events (AE) [3]. In HNSCC there is a considerable need to improve survival without further exacerbating toxicity.
Antitumor immunotherapy is based upon the principle that adaptations in immune surveillance and the tumor microenvironment allow immune escape. The biological rationale for antitumor immunotherapy specifically in HNSCC is built upon several observations. First, HNSCC has a relatively high tumor mutation burden (TMB) [4]. This is relevant because high TMB has been shown to be predictive of efficacy of immune checkpoint inhibitors (ICIs), presumed due to the production from mutated DNA of altered proteins which are antigenic, and which serve as tumoral immune targets [5]. Mutagenesis in HPV-mediated cancers is related to activity of the gene-editing apolipoprotein B mRNA editing catalytic polypeptide-like (APOBEC) proteins. These are known viral response genes, and expression of APOBEC3B, APOBEC3C, APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H are all increased in HPV-related HNSCC, [6,7] relative to HPV− HNSCC. APOBEC enzymatic activity results in a clustered (kaetegis) pattern of C → T and C → G mutations, classed as signatures 2 and 13 in the COSMIC database. Neopeptides translated from APOBEC mutated sequences exhibit higher degrees of hydrophobicity, predicted to enhance immunogenicity, and correlate with response to ICI [8]. Conversely, the tobacco mutagenesis and methylation signatures are also associated with improved ICI responsiveness. Second, while inflammation can contribute to development of HNSCC, [9] HNSCC can be immunosuppressive: many patients with HNSCC exhibit impaired tumor-infiltrating T lymphocytes via overexpression of PD-1 and other ICR, [10]impaired natural killer cells, [11] and poor antigen-presenting function [12]. Third, HNSCC is frequently infiltrated with immune cells that could be targeted towards anti-tumor effects. Fourth, an increasing proportion of HNSCC is caused by human papillomavirus (HPV), which signifies failed immunologic control of this chronic viral infection, as well as providing a convenient therapeutic and antigenic target.
The PD-1/PD-L1 pathway is a key mechanism of immune escape by cancers and a pathway that can be targeted. Anti-PD1/PD-L1 agents block tumors′ immunosuppressive signaling and boost the anti-tumor immune response [13]. The biological rationale for targeting the anti-PD1/PD-L1 pathway in HNSCC has been reinforced by recent large clinical trials, demonstrating improved outcomes from ICIs compared with standard of care therapy. In this review, we will discuss the latest advances in immunotherapy for HNSCC. This focus of this review will be on cancers of the oral cavity, oropharynx, larynx, and hypopharynx.
Discussion
Platinum-refractory, recurrent/metastatic HNSCC
Prior to the advent of ICIs, second-line treatment options for R/M HNSCC refractory to platinum-based chemotherapy included cetuximab, taxanes, or methotrexate [14,15]. The response proportions achieved by these agents varied from 4 to 14%, with a median progression free survival (PFS) of only 2–3 months [14,15].
KEYNOTE-012 was a phase Ib trial that was the first to demonstrate durable responses to pembrolizumab in patients with platinum-refractory R/M HNSCC with ≥1% PD-L1 expression with an overall response rate of 16% [16,17].
Soon afterwards data from the randomized phase III CheckMate 141 trial of 361 patients, showed improved overall survival (OS) and quality of life (QOL) relative to investigator′s choice (IC) of standard of care systemic therapy for platinum-refractory disease (Table 1) [18]. In this trial Nivolumab doubled OS at 1 year (36% with nivolumab vs. 17% with chemotherapy, hazard ratio 0.70, 95% confidence interval 0.51–0.96), a finding which has persisted at minimum 2 years of follow up [18]. Based on this data both pembrolizumab and nivolumab were approved by the US FDA in August and November of 2016 respectively.
Table 1.
Completed phase III trials investigating immune checkpoint inhibitors in HNSCC.
| Study | Number of patients | Treatment arms | Median overall survival | HR (CI) for overall survival | Objective response rate | Treatment related grade 3–5 adverse events |
|---|---|---|---|---|---|---|
| First-line R/M | ||||||
| KEYNOTE-048# [32] comparison 1: pembrolizumab plus chemo vs. EXTREME | 882 | Pembrolizumab, platinum, 5-FU combination (P + C) vs. cetuximab, platinum, 5-FU combination (E) | CPS ≥20: 14.7 mo (P + C) vs. 11.0 mo (E) CPS ≥1: 13.6 mo (P + C) vs. 10.4 mo (E) Total population: 13.0 mo (P + C) vs. 10.7 mo (E) |
CPS ≥20: 0.60 (0.45–0.82) CPS ≥1: 0.65 (0.53–0.80) Total population: 0.77 (0.63–0.93) |
CPS ≥20: 43% (P + C) vs. 38% (E) CPS ≥1: 36% (P + C) vs. 36% (E) Total population: 36% (P + C) vs. 36% (E) |
71% (P + C) vs. 69% (E) |
| KEYNOTE-048 comparison 2: pembrolizumab monotherapy vs. EXTREME | Pembrolizumab (P_ vs. cetuximab, platinum, 5-FU combination (E) | CPS ≥20: 14.8 mo (P) vs. 10.7 mo (E) CPS ≥1: 12.3 mo (P) (P + C) vs. 10.3 mo (E) Total population: 11.5 mo (P) vs. 10.7 mo (E) |
CPS ≥20: 0.58 (0.44–0.78) CPS ≥1: 0.74 (0.61–0.90) Total population: 0.83 (0.70–0.99); p = 0.0199* |
CPS ≥20: 23% (P) vs. 36% (E) CPS ≥1: 19% (P) vs. 35% (E) Total population: 17% (P) vs. 36% (E) |
17% (P) vs. 69 (E) | |
| Second-line R/M | ||||||
| Ferris et al CheckMate 141 [18] | 361 | Nivolumab vs. investigator′s choice chemotherapy | 7.5 mo (nivolumab) vs. 5.1 mo (chemotherapy) | 0.70 (0.50–0.96) for nivolumab | 13% | 13% (nivolumab) vs. 35% (chemotherapy) |
| Cohen et al. [20] KEYNOTE-040 | 497 | Pembrolizumab vs. investigator′s choice chemotherapy | 8.7 mo (pembrolizumab) vs. 7.1 mo (chemotherapy) | 0.80 (0.65–0.98) for pembrolizumab | 15% | 13% (pembrolizumab) vs. 36% (chemotherapy) |
| EAGLE# [56] | 736 | Durvalumab vs. durvalumab plus tremelimumab vs. investigator′s choice chemotherapy | 7.6 mo (durvalumab) vs. 6.5 mo (durvalumab plus tremelimumab) vs 8.3 mo (chemotherapy) | 0.88 (0.72–1.08)* for durvalumab vs. 1.04 (0.85–1.26)* for durvalumab plus tremelimumab | 18% (durvalumab) vs. 18% (durvalumab plus tremelimumab) vs. 17% (chemotherapy) | 10% (durvalumab) vs. 16% (durvalumab plus tremelimumab) vs. 24% (chemotherapy) |
Trial that has been presented but whose results have not been published in a peer reviewed journal.
Statistical superiority not demonstrated. Abbreviations: HR = hazard ratio, CI = 95% confidence interval, mo = months, CPS = combined proportion score.
The activity of pembrolizumab in the platinum-refractory R/M HNSCC setting was subsequently confirmed in the phase II KEYNOTE-055 [19] and the phase III KEYNOTE-040 trials [20]. Importantly, the KEYNOTE-040 registrational trial randomized 495 patients to pembrolizumab vs. standard of care chemotherapy [20]. On final analysis, this study demonstrated an improvement in OS to 8.4 months with pembrolizumab vs. 6.9 months with chemotherapy (HR 0.80, CI 0.65–0.98, p = 0.0161). The pre-specified efficacy boundary of p < 0.0175 was narrowly missed on initial presentation (p = 0.0204) [21]. However, with full acquisition of survival status from 11 outstanding patients the pre-specified efficacy boundary was met. In KEYNOTE-040 there was a confounding effect of subsequent ICIs in the standard of care arm (13% vs. 5% with pembrolizumab) that may have decreased the magnitude of benefit in OS observed in the pembrolizumab arm. In addition, the control arm of this trial had a higher median survival than previously described for platinum-resistant disease. Possible explanations are the exclusion of patients who had progressed within the first 3 months of platinum exposure and the higher dose every 3-week docetaxel regimen employed. Furthermore, perhaps informed by the results of Checkmate 141, investigators were more likely to choose docetaxel as standard of care therapy in this study, thus yielding a control arm with much higher survival than seen in an IC arm in Checkmate-141, although the agents permitted for IC were identical.
The available long-term data for both nivolumab and pembrolizumab appear to demonstrate a tail to the survival curve with a durable survival benefit in a minority of patients [16,18]. Analysis of patients in CheckMate 141 with a minimum of 2 years of follow-up demonstrates that nivolumab continued to improve OS compared with chemotherapy, with a near tripling of OS at 2-years (17% vs. 6%) [22]. Interestingly, long-term results for nivolumab demonstrate that OS benefit is maintained regardless of PD-L1 expression, supporting a dynamic effect of this type of therapy over time, further differentiating its mechanism of action from chemotherapy, where benefits are often static and short-lived [22].
Assessing response and treatment duration
One challenge in interpreting clinical trials of ICI vs. chemotherapy is assessing response to therapy. Response proportion has been conventionally measured using the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 guidelines developed for tumors treated with chemotherapy [23]. RECIST guidelines presume that early tumor growth on treatment indicates progressive disease. The kinetics of RECIST measurements may initially favor chemotherapy. Using standard RECIST criteria to assess response with ICIs could result in an incorrect finding of disease progression and premature discontinuation of treatment. While ICIs may exhibit a distinct response pattern, with early pseudoprogression as a result of immune cell infiltration of tumors followed by objective response, radiographically documented pseudoprogression in HSNCC is rare [24].
In CheckMate 141, patients who experienced progression as defined using RECIST v1.1 with stable performance status, were permitted to receive treatment beyond progression. These patients received nivolumab until further progression as defined by an additional > 10% increase in tumor volume [25]. One hundred forty-six patients experienced RECIST-defined progression on nivolumab, and 62 of them received treatment beyond progression [25]. Of 60 evaluable patients, 25% had no change in size, 25% had a 10–30% reduction in size and 5% had > 30% reduction. These results indicate that a substantial proportion of patients treated beyond progression with ICI experience benefit. The high proportion of patients who did not continue nivolumab beyond progression suggests an important element of patient selection, as nivolumab may have been less likely to be continued in the face of toxicity or rapidly progressive disease. Clinicians should be aware of the altered kinetics of response to ICI, and that delayed response may be seen even in initially PD-L1− patients.
A subset of patients with HNSCC treated with PD-1 or PD-L1 inhibitors may exhibit accelerated tumor growth, in a pattern referred to as hyperprogression [26]. Several definitions for hyperprogression have been proposed, including a doubling of growth rate over that documented on the last therapy prior to ICI, or a 50% increase in target lesions over 4 weeks. Typically, hyperprogression is the enhanced growth of existing lesions, rather than development of many new lesions, although such a pattern is also described. In HNSCC, the pattern is predominantly observed in patients with locoregional recurrence, rather than those being treated for distant metastases only, [27] and the rate of hyperprogression for ICI therapy alone may be as high as 29%. The effects of hyperprogression can be devastating when anatomic location of the progressing lesion leads to compromise of adjacent structures, as in a recent case report of blindness resulting from hyperprogression of a sinus cancer following a single dose of nivolumab [28]. Preclinical modeling of this phenomenon in non-small cell lung cancer indicates a role for tumor-associated macrophage reprogramming after PD-1 receptor engagement by the immune checkpoint inhibitor, an area for further study in HNSCC. Most critically, combinations of ICI with chemotherapy, antiangiogenic or other therapies which abrogate the risk for hyperprogression merit rapid development in this disease.
Toxicity
Anti-PD1 agents demonstrate different patterns of toxicity and overall improved QOL compared to standard cytotoxic chemotherapy [29]. Toxicity has been similar in both nivolumab and pembrolizumab trials. In both CheckMate 141 and KEYNOTE-040, 13% of patients treated with anti-PD-1 agents experienced grade 3–4 AE, compared to 35–36% of patients treated with chemotherapy [18,30]. ICIs introduce the potential for immune related AE that can affect any organ system [16,18,30]. In HNSCC, consistently reported immune related AE include rash, hepatitis, pneumonitis, colitis, and endocrinopathies like hypothyroidism.
On detailed QOL analysis, nivolumab improved QOL across domains, including social function, swallowing, talking, eating, and xerostomia. In contrast, standard cytotoxic chemotherapy led to deterioration in QOL [29].Similarly, KEYNOTE-040 found that pembrolizumab stabilized QOL domains, and that QOL declined with use of chemotherapy [31].
First-line R/M HNSCC
Recently, results of the randomized three-arm phase III KEYNOTE-048 trial in first-line R/M HNSCC were presented (NCT02358031) [32,33] The trial examined 882 patients who received either a) pembrolizumab monotherapy or b) a novel combination of pembrolizumab, 5-FU and cisplatin or carboplatin or c) the EXTREME regimen of cisplatin, 5-FU and cetuximab as a control arm [32]. Chemotherapy plus an anti-PD1 therapy is a rational combination strategy. Chemotherapy disrupts the architecture in the tumor microenvironment, which may help to overcome immune exclusion and produce antigen shedding [34]. Chemotherapy also produces rapid responses, and may do so in patients who would be unresponsive or progressive on ICI. Given the observation that pembrolizumab monotherapy response is lower in bulky tumors, chemotherapy-induced reduction in tumor volume might improve sensitivity simply on this basis [35]. Chemotherapy was given for up to 6 cycles, pembrolizumab for up to 24 months and cetuximab indefinitely, until treatment was discontinued for progressive disease or toxicity. Approximately 25% of patients had HPV-associated (p16+) cancers. This trial is the first trial in HNSCC to prospectively use a biomarker of PD-L1 expression level in the primary endpoint analysis. In this trial, combined proportion score (CPS) was used as a biomarker. CPS is defined as the sum of PD-L1 stained tumor cell and surrounding lymphocytes and macrophages divided by the total number of viable tumor cells multiplied by 100 [36]. In contrast, tumor proportion score (TPS) which is the proportion of viable tumor cells.
The primary outcomes were OS and progression-free survival (PFS) tested sequentially for CPS ≥20, CPS ≥1, and total population. The second interim analysis and final analysis have been publically presented [32,33]. Objective response rate (ORR) was 36% with EXTREME, 36% with pembrolizumab plus chemotherapy, or 16% with pembrolizumab monotherapy in the overall population. ORR was improved for pembrolizumab plus chemotherapy (43%) vs. the EXTREME regimen (38%) in the CPS ≥20 population. PFS for pembrolizumab plus chemotherapy in the CPS ≥20 group was HR 0.76 (CI 0.58–1.01) and in the CPS ≥1 was HR 0.84 (0.69–1.02) and in the total population was HR 0.92 (CI 0.77–1.10). PFS for pembrolizumab monotherapy in the total population was 2.3 months vs. 5.2 months (HR 1.34; CI 1.13–1.59). Although pembrolizumab plus chemotherapy had longer PFS than pembrolizumab monotherapy, at 2 years an identical percentage were progression-free, indicating that the chemotherapy effect is early but not durable, and further that chemotherapy does not blunt the durability of pembrolizumab. Final analysis of this study identified that pembrolizumab plus chemotherapy significantly improved OS for the CPS ≥20 (14.7 vs. 11.0 months, HR 0.60, CI 0.45–0.82, p = 0.0004), CPS ≥1 (13.6 vs. 10.4 months, HR 0.65, CI 0.53–0.80, p < 0.0001) and overall populations (13.0 vs. 10.7 months, HR 0.72, CI 0.60–0.87). Pembrolizumab monotherapy also significantly improved OS for the CPS ≥20 (14.8 vs. 10.7 months) and CPS ≥1 (12.3 vs. 10.3 months, HR 0.74) populations, and was noninferior to EXTREME in the overall population (11.5 vs. 10.7 months, HR 0.83, CI 0.70–0.99, P = 0.0199). Despite the inferior ORR, the OS benefit was driven by longer duration of response (DOR) in the pembrolizumab cohort (20.9 months vs. 4.5 months). Furthermore, the OS curve for the pembrolizumab cohort continues to run considerably above the PFS curve at 3 years suggesting that some patients who do not meet objective PFS criteria experience OS benefit from pembrolizumab. Potential mechanisms include an alteration in the natural history of disease or an increased sensitivity to subsequent therapies.
Grade 3–5 treatment related AE was observed in 71% of patients receiving pembrolizumab plus chemotherapy, 69% with the EXTREME regimen and 17% with pembrolizumab monotherapy. These findings indicate that pembrolizumab can be safely added to platinum-based combination chemotherapy with a similar safety profile to the EXTREME regimen. Exposure to subsequent ICIs after progression has not yet been reported, however, it would be expected that any exposure to subsequent ICIs in patients treated on study with the EXTREME regimen would blunt the OS benefit observed in this study. This trial led to US FDA approval of pembrolizumab as first-line for R/M HNSCC in combination with platinum and 5-FU in all patients, or as monotherapy in tumors with a PD-L1 combined positive score (CPS) ≥1.
In summary, three randomized phase III trials have now conclusively proven that exposure to a PD-1 inhibitor prolongs survival in R/M HNSCC, and it is clear that such agents should be used in the management of all patients who do not have contraindications to their use. In the 85% of R/M HNSCC whose tumors are PD-L1 positive (CPS ≥1), [32] these results also pose new questions about whether to choose pembrolizumab monotherapy or pembrolizumab plus chemotherapy as first-line therapy. Important factors to consider in treatment selection in patients with R/M HNSCC include prior exposure to systemic therapy, tumor burden and location, symptom burden, PD-L1 expression, and toxicity. Pembrolizumab plus chemotherapy should be considered in cases where response rate is critical, such as for symptomatic disease, or patients with bulky locoregional disease at risk of airway or bleeding complications. It may be preferred for patients with lower PD-L1 expression, given the somewhat lower pembrolizumab monotherapy response proportion in this subset. However, in patients with less symptomatic disease, those with lung metastases only, and high PD-L1 expression, pembrolizumab monotherapy will be highly attractive, as for these patients, the median OS with pembrolizumab monotherapy vs. pembrolizumab plus chemotherapy was similar (14.8 vs. 14.7 months) and was achieved with considerably less toxicity.
Nonetheless, many uncertainties remain regarding the relative benefit of these agents in the first line or platinum-refractory setting, the role of chemotherapy in increasing the likelihood of objective response, and the worth of novel combination immunotherapies in clinical testing. The treatment algorithm presented in Fig. 1 reflects the US FDA approved indications for ICI in R/M HNSCC. These treatment recommendations need to be tailored to account for patient preferences and individual circumstances. Additionally, consensus guidelines developed by the Society for the Immunotherapy of Cancer Head and Neck Guidelines Subcommittee provide some guidance for treatment decision-making [37] (see Fig. 2).
Fig. 1.
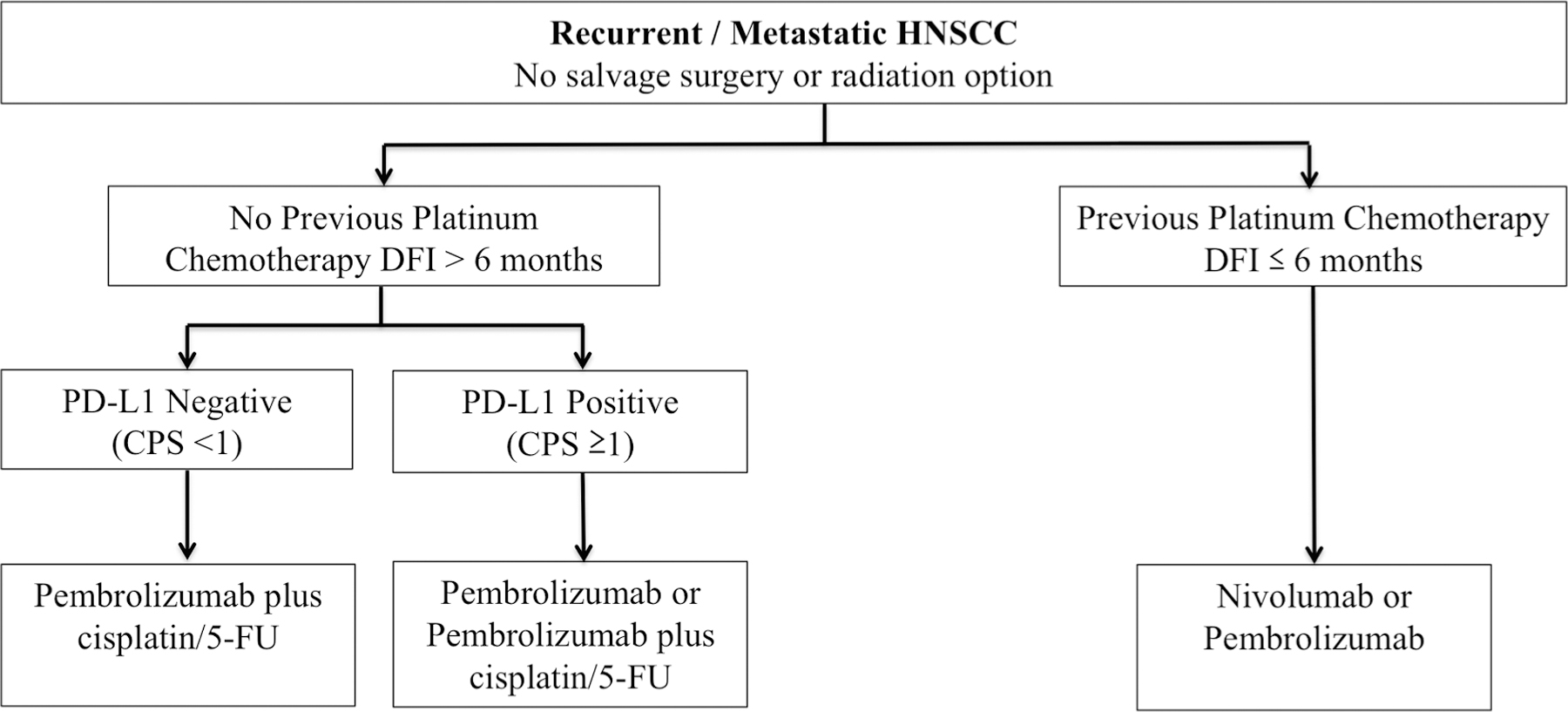
Algorithm for management of recurrent or metastatic HNSCC. Abbreviations: DFI = disease free interval, CPS = combined proportion score.
Fig. 2.
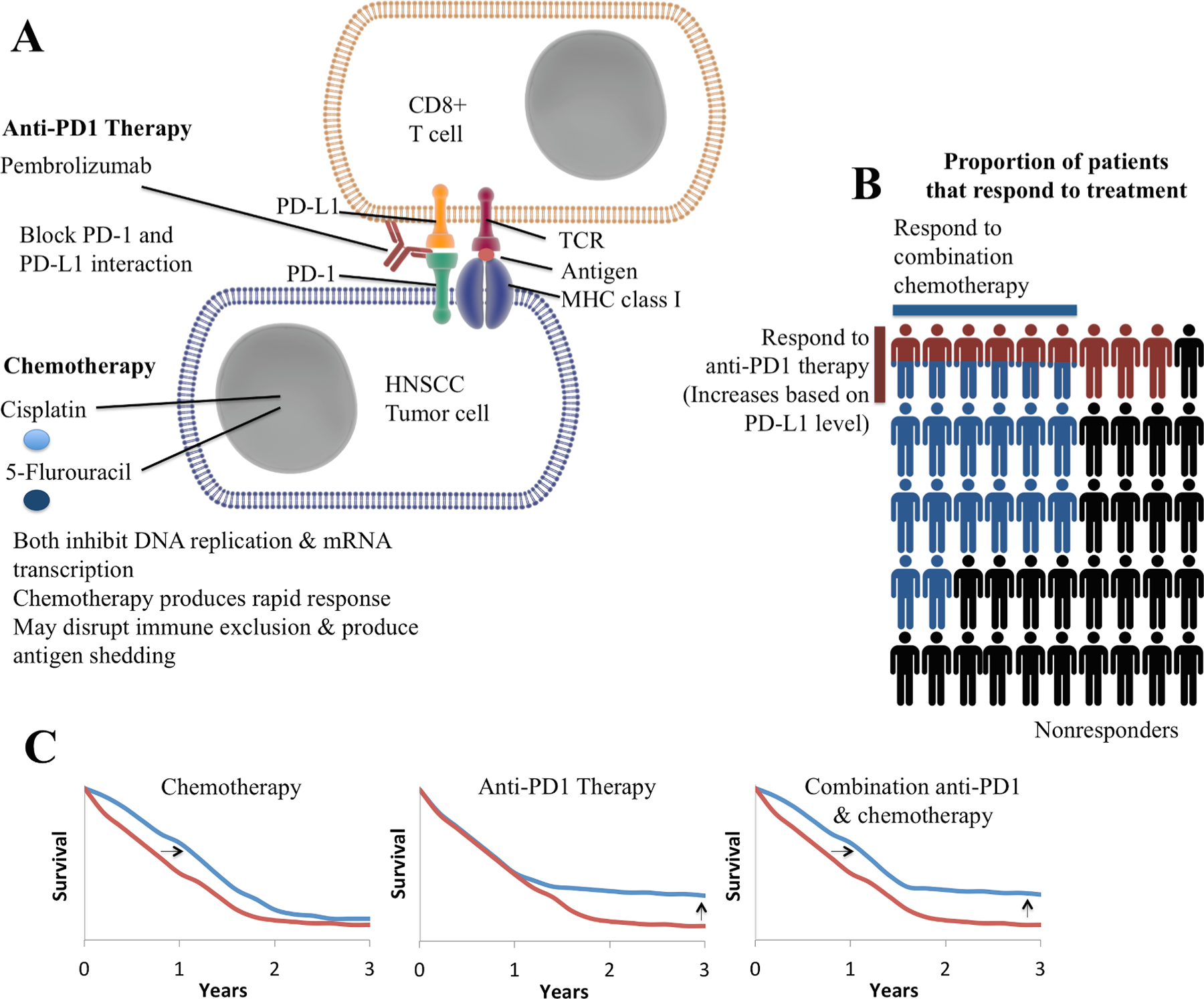
Mechanisms of combination immunotherapy and chemotherapy in HNSCC. A. Molecular mechanism of action of anti-PD1 therapy and chemotherapy. B. Depiction of response rate to anti-PD1 therapy and chemotherapy. Response rates will vary based on the specific chemotherapy regimen used. Each person represents approximately 2%. Red colored persons represent those that respond to anti-PD1 therapy and blue colored persons represent those that respond to combination chemotherapy. C. Depiction of overall survival curves observed in HNSCC with anti-PD1 immunotherapy and chemotherapy. Anti-PD1 therapy produces a dramatically improved duration of response (i.e. tail of the curve) that has powered the overall survival benefit with this therapy despite a modest response rate. The images in part A are a derivative of cell_membrane, antibody, receptor, nucleus and dimeric receptor by Idoya Lahortiga & Luk Cox (https://www.somersault1824.com/) used under Creative Commons BY-NC-SA 4.0 https://creativecommons.org/licenses/by-nc-sa/4.0/.
Biomarkers of response to ICIs
PD-L1 expression
As highlighted in KEYNOTE-048, higher levels of PD-L1 expression are associated with an increased likelihood of response to anti-PD1 therapy [16,18]. Around 50–60% of HNSCC tumor cells express PD-L1 (measured using TPS), [38] but this percentage increases to 85% when considering tumor cells and surrounding immune cells (measured with CPS) [32]. The predictive value of PD-L1 expression is increased when considering the combined expression on tumor cells and infiltrating immune cells. In the KEYNOTE-012 study the response rate was 21% PD-L1+ vs. 6% in PD-L1− by CPS compared with 18% in PD-L1+ vs. 19% in PD-L1− by TPS assessment [16]. In CheckMate 141, the presence of PD-L1 expressing immune cells in the tumor microenvironment was more predictive of response than were PD-L1 expression in tumor cells [39].
The ability to predict response to anti-PD1 therapy may be further improved by also considering other biomarkers. Expression of PD-L2, the other ligand of PD-1, is another potential biomarker of response to anti-PD1 therapy. Data from KEYNOTE-012 also showed that PD-L2 protein expression was a biomarker for response to anti-PD1 therapy, independent of PD-L1 expression [40].
Interpreting PD-L1 expression (or PD-L2) as a biomarker across trials is challenging beyond the companion diagnostic test approved in first-line R/M HNSCC. Different studies use a variety of different assays and cut points making cross study comparisons difficult. Importantly, the challenge of interpreting PD-L1 expression across studies has led to harmonization projects for PD-L1 assays in non-small cell lung cancer by regulatory agencies and the scientific community [41,42]. These studies have identified which anti-PDL1 antibodies are largely interchangeable. As future trials are reported using a variety of new agents, a similar understanding of PD-L1 assays will be necessary in HNSCC.
Relevantly, the FDA approval of pembrolizumab monotherapy only in patients with a CPS score ≥1 represents the first mandated biomarker testing for selection of immunotherapy in HNSCC in the United States [32]. In conjunction with the first-line approval for pembrolizumab in the US, the FDA also extended the use of the PD-L1 IHC 22C3 pharmDx kit as a companion diagnostic device to measure CPS and help in selecting patients for treatment with pembrolizumab monotherapy. Currently, most laboratories do not routinely employ CPS in reporting of PD-L1 expression. In response to the FDA approval of pembrolizumab monotherapy only in biomarker-selected populations, it will be critical to expand availability of CPS testing into routine practice.
While PD-L1 expression level may help to select patients in the first-line R/M setting for pembrolizumab in combination with chemotherapy vs. monotherapy, some patients with PD-L1 negative HNSCC also derive benefit from anti-PD1 agents. In KEYNOTE-012 patients with < 1% PD-L1 expression treated with pembrolizumab had a response rate of 4% compared with 22% for patients with ≥1% PD-L1 expression [43]. In Checkmate 141 patients with a TPS < 1% experienced a trend towards improved survival (HR 0.89, CI 0.54–1.45) compared with TPS ≥1% (HR 0.55, CI 0.36–0.83) [18]. The proportion of PD-L1 negative patients who respond is smaller, but even in the absence of PD-L1 expression, ICI naïve patients should be offered second-line ICI treatment with an approved PD-1 inhibitor or on a clinical trial of a novel immunotherapy strategy.
HPV status
HPV+ HNSCC is more likely to exhibit an immunologically active tumor microenvironment [44] highlighting the potential for improved activity in this population. Some data suggest that HPV status is predictive of response to anti-PD1 agents. KEYNOTE-012 showed an improved objective response rate in HPV+ (24%) compared with HPV− (16%) [16]. In CheckMate 141, patients with HPV+ cancers treated with nivolumab were more likely to experience benefit (HR 0.56, CI 0.32–0.99) compared with HPV− patients (HR 0.73, CI 0.42–1.25) [18]. In contrast, in KEYNOTE-040, HPV− cancers appeared to experience greater benefit from pembrolizumab (HR 0.77, CI 0.61–0.97) compared with HPV+ cancers (HR 0.97, CI 0.63–1.49). Overall, patients with both HPV+ and HPV− cancers appear to derive benefit from ICIs. In KEYNOTE-048, the HR of 0.54 for pembrolizumab plus chemotherapy was very significant for HPV+ making them potentially better candidates for combination therapy [45]. HPV specific regimens using DNA immunotherapy targeting HPV16/18 E6/E7 with IL12 plasmids or HPV-16 vaccines have shown promise in small phase Ib/II trials [46,47]. Outside of research studies, HPV status is not recommended to select patients for treatment with ICIs.
Tumor mutation burden
High tumor mutational burden has been strongly associated with response to ICIs in other solid tumors [5]. HNSCC exhibits moderate to high mutational load, as described above. Immune gene expression profile scores analyzed via RNA expression to identify a genetic signature of T cell activation has been used to predict anti-PD1 efficacy (GEP) in HNSCC [16,48]. At present, these biomarkers are investigational, and should not be used to select patients for ICI therapy.
Important patient subgroups
Cetuximab-treated patients
As the data supporting ICIs in HNSCC have matured, several important subgroups have emerged that may impact response to therapy. Recently, pre-planned subset analysis of the CheckMate 141 randomized trial revealed significant differences in response based on prior cetuximab use, which was a pre-specified stratification factor in this trial [49]. Nivolumab reduced the risk of death by 16% in patients with prior cetuximab use and by 48% in patients without prior cetuximab use [49]. Cetuximab down-regulates interferon γ-induced PD-L1 expression in HNSCC cell lines, [38] which may help to explain these differences. The reduced efficacy of nivolumab in patients with prior cetuximab exposure could also be attributed to patient or disease characteristics. However, Eastern Cooperative Oncology Group (ECOG) performance status was similar among patients with and without prior cetuximab use.
In contrast, in the larger KEYNOTE-040 trial prior cetuximab therapy was not a significant predictor of response to ICI. In this study, in patients without prior cetuximab median OS was 8.2 months with pembrolizumab vs. 6.9 months with standard of care (HR 0.78; CI 0.56–1.07) [50]. In contrast, for patients who had prior cetuximab survival was 8.4 months with pembrolizumab vs. 7.1 months with standard of care (HR 0.89; CI 0.68–1.16) [35]. However, KEYNOTE-040 did not pre-specify prior cetuximab therapy as a stratification factor and it is not yet clear whether differences in trial design or patient population explain this difference between the trials, or if the finding from Checkmate 141 was a random effect [50]. Trials are currently investigating strategies to combine anti-PD1 inhibitors with cetuximab and RT in a prospective fashion (NCT03258554).
Subgroup analysis based on age
As a high proportion of patients with HNSCC are older the 65 years, [51] an important question is whether the effectiveness of ICIs varies across age groups. Post hoc analysis of CheckMate 141 identified that nivolumab improved OS compared with chemotherapy in both young (< 65 years old HR 0.63, CI0.47–0.84) and old patients (≥65 years old HR 0.75, CI0.51–1.12) [52]. This study also showed that the incidence of treatment related AE was similar across age groups.
Understudied populations
Prospective clinical trials of ICIs in HNSCC have largely excluded patients with advanced performance status (ECOG ≥2) or a history of autoimmune disorders, HIV positivity or organ transplantation. Strategies to extend the benefit to ICIs to these populations are being cautiously examined. In patients with HIV, in a study of multiple different tumor types treatment with ICIs was safe and associated with a 22% partial response rate and 22% cancer stabilization rate [53]. In contrast the experience with ICIs in solid organ transplant recipients in another study of multiple different tumor types was associated with an allograft rejection rate was 41% with high mortality [54].
Combination immunotherapy strategies under investigation
While ICIs provide a major advance for patients with HNSCC, only a minority derives long-term clinical benefit from anti-PD1 monotherapy. In an effort to decrease the proportion that develops recurrent disease, ICIs are being investigated in the locally advanced setting. Multiple clinical trials are investigating ICIs in combination with immunostimulatory agents. In this review we will focus on phase II and III trials of combination strategies.
Combination anti-PDL1 and anti-CTLA4 therapy
Durvalumab, an antibody to PD-L1, has been extensively investigated, but it is not FDA approved in HNSCC [55,56]. The phase II CONDOR [57] and phase III EAGLE [56] studies both investigated durvalumab monotherapy vs. durvalumab combination therapy vs. IC. The EAGLE trial included 736 randomized patients with platinum-refractory R/M HNSCC [56]. Durvalumab monotherapy produced a similar response rate (18%) to other ICIs [56]. However, durvalumab monotherapy (HR 0.88, CI 0.72–1.08) did not improve OS, compared with chemotherapy [56].
One of the most anticipated agents combined with anti-PD1/PD-L1 therapy in HNSCC was anti-CTLA4 therapy. Anti-CTLA4 agents produce distinct and potentially complementary effects on T cells [58] and it was hoped that combination anti-CTLA4 and anti-PD1/PD-L1 agents would produce synergy in HNSCC, as has been seen in melanoma [59]. Combination studies adding anti-CTLA4 agents to anti-PD1/PD-L1 therapy in HNSCC proceeded rapidly despite limited evidence of single agent activity with anti-CTLA4 in HNSCC. In both the phase II CONDOR [57] and phase III EAGLE [56] trials, durvalumab was investigated in combination with the anti-CTLA4 antibody, tremelimumab. Disappointingly, durvalumab in combination with tremelimumab did not improve OS (HR 1.04, CI 0.85–1.26) compared with chemotherapy, in the phase III EAGLE trial [56]. Overall response rate with durvalumab plus tremelimumab was 18%, similar to durvalumab monotherapy. Combination therapy increased toxicity. Surprisingly, the IC arm out-performed historical results in this setting. Subsequent ICI was more common in the IC arm (15% vs. 2–5% in the durvalumab or durvalumab plus tremelimumab arms) and may have confounded OS results. Additionally, there was an imbalance in the ECOG performance status in favor of the IC arm, providing further potential for confounding. However, these potential confounding variables may not explain the lack of enhanced efficacy with adding anti-CTLA4 antibodies. The CheckMate 714 phase II randomized using an alternative combination anti-CTLA4 and anti-PD1 trial of nivolumab vs. nivolumab/ipilimumab trial did not meet its primary endpoint in the platinum refractory population, according to a April 2019 press release [60]. Ongoing phase III studies are investigating alternative combinations of anti-CTLA-4 and anti-PD1 therapy in HNSCC (NCT02741570, NCT02551159).
Use of ICIs prior to surgery has also been investigated in several small studies with promising efficacy. Uppaluri et al reported initial results of a single-arm phase II trial of 21 patients treated with neoadjuvant pembrolizumab prior to surgery [61]. They found neoadjuvant pembrolizumab to be safe and feasible and further found that 43% of patients showed pathologic treatment response to a single dose of neoadjuvant pembrolizumab. The Checkpoint Inhibitors Assessment in Oropharynx cancer (CIAO) investigated durvalumab vs. durvalumab plus tremelimumab, each given for two cycles prior to surgery in 28 patients with oropharyngeal cancer [62]. Interim results presented at the American Society of Clinical Oncology meeting on May 31, 2019 showed an overall response rate of 43% with equivalent efficacy with or without tremelimumab [62]. 79% of patients displayed treatment effects and 57% of patients did not require adjuvant RT after surgery [62]. Another small phase II study showed a similar 44% response rate using neoadjuvant nivolumab followed by surgical resection [63].
Toll-like receptor agonists
Toll-like receptors (TLR) are a family of innate and adaptive immunity receptors that help fight viral and other pathogens. The Active8 study was a randomized phase II study of the TLR8 agonist motolimod, with or without the EXREME regimen, in 195 patients with R/M HNSCC [65]. Adding motolimod to the EXTREME regimen did not improve OS in the overall population. However, prespecified subgroup analysis found that motolimod improved OS (15.2 vs. 12.6 months) in HPV-positive patients and in those with injection site reactions indicating potential activity in certain populations [65].
Alternative immunotherapy combination strategies
A variety of novel combination strategies of ICIs and other immunostimulatory agents are underway. Small phase I/II trials have shown encouraging responses to ICIs combined with: TLR9 agonists, [66] multiple therapeutic vaccines, [67–69] the anti-EGFR antibody cetuximab [70] or the anti-VEGF multiple kinase inhibitor lenvatinib [71]. Other co-stimulatory agonists being tested include OX40, CD40L, CD137, STING, IDO1 and STAT3 inhibitors [64,72].
Immunotherapy combined with radiation
The interaction of RT on the tumor microenvironment is complex, and may both enhance and suppress the immune system. In some circumstances RT may strengthen the anti-tumor immune response by releasing cytokines and tumor-associated antigens. ICIs are being investigated in various combinations with radiation in both the locally advanced and R/M setting. Unfortunately, data on the efficacy of anti-PD1/anti-PDL1 agents in combination with radiation is sparse at the present time.
In the R/M setting, radiation combined with ICIs may produce a response in both irradiated sites of disease and in distant un-irradiated sites [73]. This so-called abscopal effect of radiation occurs when radiation produces immune stimulatory properties that improve systemic anti-tumor effects. In R/M HNSCC, the abscopal effect was examined in a randomized phase II study of 53 patients examining nivolumab with or without stereotactic body radiotherapy (SBRT) [74]. The addition of SBRT did not improve efficacy. The objective response rate with nivolumab alone was 27%, compared to 22% with nivolumab plus SBRT [74].
ICIs are being tested in a variety of combinations with surgery, radiation and chemotherapy in the locally advanced setting. Phase I results from NRG-HN003 found that adding pembrolizumab to adjuvant cisplatin/radiation was feasible with manageable toxicity [75]. Phase I/II safety data from the GORTEC 2015–01 PembroRad and RTOG-3504 trials both found that adding anti-PD1 antibodies to definitive cetuximab/RT was feasible with manageable toxicity [76,77]. Multiple ongoing phase III trials are investigating combining anti-PD1 antibodies with definitive CRT in locally advanced HNSCC (NCT02952586, NCT3040999, NCT02999087, NCT03349710, NCT03258554, Table 2). Among these trials, the Javelin Head and Neck 100 trial examining adding avelumab to definitive CRT in high-risk HNSCC recently completed accrual and results are expected in the next 2 years (NCT02952586). KEYNOTE-412/MK-3475–412 another phase III randomized trial of adding neoadjuvant and adjuvant pembrolizumab versus placebo to definitive CRT that also recently completed accrual (NCT03040999). Another relevant study to several types of HNSCC in the locally advanced setting is CheckMate 358 is also recently completed accrual (NCT02488759). This trial is investigating neoadjuvant nivolumab or nivolumab plus combination therapy with anti-CTLA4 therapy vs. anti-LAG3 therapy vs. antiCD-38 therapy in virus-associated cancers including HPV-associated and EBV-associated cancers.
Table 2.
Ongoing Phase III Trials In Locally Advanced HNSCC.
| Study | Population | Treatment arms | Recruitment |
|---|---|---|---|
| Neoadjuvant | |||
| KEYNOTE-689 (NCT03765918) | Stage III-IVA oral cavity/larynx/hypopharynx and HPV− OPSCC or Stage III (AJCC 8th ed) HPV+ OPSCC | Neoadjuvant pembrolizumab and adjuvant pembrolizumab added to surgery and standard risk-based adjuvant therapy vs. surgery and standard risk-based adjuvant therapy | Recruitment ongoing, planned participant count 704 |
| Adjuvant immunotherapy after definitive CRT | |||
| WO420242 (NCT03452137) | HNSCC requiring multimodality therapy | Adjuvant atezolizumab vs. placebo after definitive local therapy (surgery or RT) | Recruitment ongoing, Planned participant count 400 |
| ECOG ACRIN EA3161 (NCT03811015) | Phase II/III trial, intermediate-risk HPV+ OPSCC (≥10 pack-year smoking and stage I AJCC 8th ed or < 10 pack-year smoking and stage II-III AJCC 8th ed) | Nivolumab plus cisplatin/RT vs. cisplatin/RT | Recruitment ongoing, planned participant count 744 |
| Definitive | |||
| JAVELIN (NCT02952586) | Stage III-IVB HPV− HNSCC or T4 or N2c/N3 (AJCC 7th ed) HPV+ OPSCC | Avelumab plus cisplatin/RT vs. cisplatin/RT | Accrual completed, Participant count 697 |
| GORTEC 2017–01 (REACH) (NCT02999087) | Stage III-IVB HNSCC | Cisplatin eligible patients: Avelumab plus cetuximab/RT vs. Cisplatin/RT Cisplatin ineligible patients: Avelumab plus cetuximab/RT vs. cetuximab/RT |
Recruitment ongoing, planned participant count 688 |
| NRG HN-004 (NCT03258554) | Phase II/III, Cisplatin ineligible, Stage III-IVB oral cavity/larynx/hypopharynx or HPV− OPSCC, Stage I-II HPV+ OPSCC with > 10 pack years or Stage III OPSCC | Durvalumab/RT vs. cetuximab/RT | Recruitment ongoing, Planned participant count 523 |
| NRG HN-005 (NCT03952585) | Phase II/III, HPV+ non-smoking associated OPSCC | Nivolumab/reduced dose RT (60 Gy) vs. Cisplatin/reduced dose RT (60 Gy) vs. cisplatin/standard dose RT (70 Gy) | Recruitment ongoing, Planned participant count 711 |
| KEYNOTE-412 (NCT03040999) | HNSCC, oral cavity cancers need to be unresectable | Pembrolizumab plus cisplatin/RT vs. cisplatin/RT | Accrual completed Participant count 780 |
| (NCT03349710) | LA HNSCC | Cisplatin eligible patients: Nivolumab plus cisplatin/RT vs. cisplatin/RT Cisplatin ineligible patients: Nivolumab/RT vs. cetuximab/RT |
Active, not recruiting Planned participant count 1046 |
Conclusion
Immunotherapy is rapidly altering the therapeutic landscape in HNSCC improving survival and reducing toxicity compared with established treatments. ICIs have now advanced from the second-line treatment for R/M HNSCC to the first-line treatment. ICIs are being investigated in earlier stages of HNSCC, to determine whether more patients may benefit from them. Data on the use of ICIs in the locally advanced setting, as intensification for high risk disease, or as de-intensification to permit reduced/eliminated chemotherapy or radiotherapy, are emerging and potentially practice changing phase III trials are maturing.
Acknowledgments
Financial Support
This work was supported by National Institute of Health grants P50 CA097190 and University of Pittsburgh Cancer Center Support Grant P30CA047904.
Role of funding source
Robert L. Ferris is supported by the above grants. The above funding agencies played no part in the design or conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011;11:9–22. [DOI] [PubMed] [Google Scholar]
- [3].Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–27. [DOI] [PubMed] [Google Scholar]
- [4].Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cannataro VL, Gaffney SG, Sasaki T, et al. APOBEC-induced mutations and their cancer effect size in head and neck squamous cell carcinoma. Oncogene 2019;38:3475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pan C, Issaeva N, Yarbrough WG. HPV-driven oropharyngeal cancer: current knowledge of molecular biology and mechanisms of carcinogenesis. Cancers Head Neck 2018;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Boichard A, Pham TV, Yeerna H, et al. APOBEC-related mutagenesis and neo-peptide hydrophobicity: implications for response to immunotherapy. Oncoimmunology 2019;8:1550341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cottin SC, Turcotte S, Douville P, et al. Predictors of circulating INTERLEUKIN-6 levels in head and neck cancer patients. Cancers Head Neck 2018:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Whiteside TL. Immune cells in the tumor microenvironment. Mechanisms responsible for functional and signaling defects. Adv Exp Med Biol 1998;451:167–71. [PubMed] [Google Scholar]
- [11].Bauernhofer T, Kuss I, Henderson B, et al. Preferential apoptosis of CD56dim natural killer cell subset in patients with cancer. Eur J Immunol 2003;33:119–24. [DOI] [PubMed] [Google Scholar]
- [12].Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res 2006;12:3890–5. [DOI] [PubMed] [Google Scholar]
- [13].Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol 2015;33:3293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Leon X, Hitt R, Constenla M, et al. A retrospective analysis of the outcome of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck refractory to a platinum-based chemotherapy. Clin Oncol (R Coll Radiol) 2005;17:418–24. [DOI] [PubMed] [Google Scholar]
- [15].Lala M, Chirovsky D, Cheng JD, et al. Clinical outcomes with therapies for previously treated recurrent/metastatic head-and-neck squamous cell carcinoma (R/M HNSCC): a systematic literature review. Oral Oncol 2018;84:108–20. [DOI] [PubMed] [Google Scholar]
- [16].Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956–65. [DOI] [PubMed] [Google Scholar]
- [17].Mehra R, Seiwert TY, Gupta S, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer 2018;119:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ferris RL, Blumenschein G Jr., Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm. Phase II Study. J Clin Oncol 2017;35:1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cohen EEW, Soulieres D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156–67. [DOI] [PubMed] [Google Scholar]
- [21].Cohen E, Harrington K, Tourneau C, et al. Pembrolizumab (pembro) vs standard of care (SOC) for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC): phase 3 KEYNOTE-040 trial. Madrid, Spain: European Society of Medical Oncology; 2017. [Google Scholar]
- [22].Ferris RL, Blumenschein G Jr., Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–47. 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- [24].Baxi SS, Dunn LA, Burtness BA. Amidst the excitement: a cautionary tale of immunotherapy, pseudoprogression and head and neck squamous cell carcinoma. Oral Oncol 2016;62:147–8. [DOI] [PubMed] [Google Scholar]
- [25].Haddad R, Concha-Benavente F, Blumenschein G Jr, et al. Nivolumab treatment beyond RECIST-defined progression in recurrent or metastatic squamous cell carcinoma of the head and neck in CheckMate 141: a subgroup analysis of a randomized phase 3 clinical trial. Cancer 2019. [DOI] [PMC free article] [PubMed]
- [26].Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920–8. [DOI] [PubMed] [Google Scholar]
- [27].Saada-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605–11. [DOI] [PubMed] [Google Scholar]
- [28].Xiang JJ, Uy NF, Minja FJ, et al. Hyperprogression after one dose of nivolumab in sinonasal cancer: a case report. Laryngoscope 2019. [DOI] [PubMed]
- [29].Harrington KJ, Ferris RL, Blumenschein G Jr, et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol 2017;18:1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cohen EEW, Soulieres D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2018. [DOI] [PubMed]
- [31].Cohen EEW, Soulieres D, Tourneau CL, et al. Health-related quality of life (HRQoL) of pembrolizumab (pembro) vs standard of care (SOC) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) in KEYNOTE-040. J Clin Oncol 2018;36 6013–6013. [Google Scholar]
- [32].Rischin D, Harrington KJ, Greil R, et al. Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol 2019;37 6000–6000. [Google Scholar]
- [33].Burtness B, Harrington K, Greil R, et al. KEYNOTE-048: Phase III study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). Germany: European Society of Medical Oncology. Munich; 2018. [Google Scholar]
- [34].Economopoulou P, Agelaki S, Perisanidis C, et al. The promise of immunotherapy in head and neck squamous cell carcinoma. Ann Oncol 2016;27:1675–85. [DOI] [PubMed] [Google Scholar]
- [35].Chow LQ, Burtness B, Weiss J, et al. LBA31A phase IB study of pembrolizumab (Pembro; MK-3475) in patients (PTS) with human papiilloma virus (HPV)-positive and negative head and neck cancer (HNC). Ann Oncol 2014;25. [Google Scholar]
- [36].Roach C, Zhang N, Corigliano E, et al. Development of a Companion Diagnostic PD-L1 Immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol 2016;24:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cohen EEW, Bell RB, Bifulco CB, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J ImmunoTher Cancer 2019;7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Concha-Benavente F, Srivastava RM, Trivedi S, et al. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNgamma that induce PD-L1 expression in head and neck cancer. Cancer Res 2016;76:1031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ferris RL, Blumenschein G, Harrington K, et al. Abstract CT021: tumor-associated immune cell PD-L1 expression and peripheral immune profiling: analyses from CheckMate 141. Cancer Res 2017;77 CT021–CT021. [Google Scholar]
- [40].Yearley JH, Gibson C, Yu N, et al. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res 2017;23:3158–67. [DOI] [PubMed] [Google Scholar]
- [41].Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between Programmed Cell Death Ligand-1 diagnostic assays across multiple protein expression cutoffs in non-small cell lung cancer. Clin Cancer Res 2017;23:3585–91. [DOI] [PubMed] [Google Scholar]
- [42].Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol 2018;13:1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chow LQM, Haddad R, Gupta S, et al. antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol 2016;34:3838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gameiro SF, Ghasemi F, Barrett JW, et al. Treatment-naive HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV-counterparts that has implications for immunotherapy. Oncoimmunology 2018;7:e1498439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Burtness B, Goldwasser MA, Flood W, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an eastern cooperative oncology group study. J Clin Oncol 2005;23:8646–54. [DOI] [PubMed] [Google Scholar]
- [46].Massarelli E, William W, Johnson F, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol 2019;5:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Aggarwal C, Cohen RB, Morrow MP, et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin Cancer Res 2019;25:110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Prat A, Navarro A, Pare L, et al. Immune-Related Gene Expression Profiling After PD-1 Blockade in Non-Small Cell Lung Carcinoma, Head and Neck Squamous Cell Carcinoma, and Melanoma. Cancer Res 2017;77:3540–50. [DOI] [PubMed] [Google Scholar]
- [49].Ferris RL, Licitra L, Fayette J, et al. Nivolumab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: efficacy and safety in CheckMate 141 by Prior cetuximab use. Clin Cancer Res 2019. [DOI] [PMC free article] [PubMed]
- [50].Le Tourneau C, Cohen EE, Harrington KJ, et al. Pembrolizumab for recurrent head and neck squamous cell carcinoma (HNSCC): post-hoc analyses of treatment options from the phase 3 KEYNOTE-040 trial. Germany: European Society for Medical Oncology; Munich; 2018. [Google Scholar]
- [51].Argiris A, Li Y, Murphy BA, et al. Outcome of elderly patients with recurrent or metastatic head and neck cancer treated with cisplatin-based chemotherapy. J Clin Oncol 2004;22:262–8. [DOI] [PubMed] [Google Scholar]
- [52].Saba NF, Blumenschein GR, Guigay J, et al. Nivolumab (nivo) vs investigator’s choice (IC) in patients (pts) with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): analysis of CheckMate 141 by age. J Clin Oncol 2018;36 6028–6028. [Google Scholar]
- [53].Spano JP, Veyri M, Gobert A, et al. Immunotherapy for cancer in people living with HIV: safety with an efficacy signal from the series in real life experience. AIDS 2019;33:F13–9. [DOI] [PubMed] [Google Scholar]
- [54].Abdel-Wahab N, Safa H, Abudayyeh A, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J ImmunoTher Cancer 2019;7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Segal NS, Ou SH, Balmanoukian AS, et al. Safety and efficacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. J Clin Oncol 2015;33 3011–3011. [Google Scholar]
- [56].Licitra LF, Haddad RI, Even C, et al. EAGLE: a phase 3, randomized, open-label study of durvalumab (D) with or without tremelimumab (T) in patients (pts) with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol 2019;37 6012–6012. [Google Scholar]
- [57].Siu LL, Even C, Mesia R, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol 2018. [DOI] [PMC free article] [PubMed]
- [58].Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sqibb B-M. Bristol-myers squibb reports first quarter financial results; 2019.
- [61].Uppaluri R, Zolkind P, Lin T, et al. Neoadjuvant pembrolizumab in surgically resectable, locally advanced HPV negative head and neck squamous cell carcinoma (HNSCC). J Clin Oncol 2017;35:6012. [Google Scholar]
- [62].Ferrarotto R, Bell D, Rubin ML, et al. Checkpoint inhibitors assessment in oropharynx cancer (CIAO): safety and interim results. J Clin Oncol 2019;37 6008–6008. [Google Scholar]
- [63].Horton JD, Knochelmann H, Armeson K, et al. Neoadjuvant presurgical PD-1 inhibition in oral cavity squamous cell carcinoma. J Clin Oncol 2019;37 2574–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hamid O, Bauer TM, Spira AI, et al. Epacadostat plus pembrolizumab in patients with SCCHN: preliminary phase I/II results from ECHO-202/KEYNOTE-037. J Clin Oncol 2017;35 6010–6010. [Google Scholar]
- [65].Ferris RL, Saba NF, Gitlitz BJ, et al. Effect of adding motolimod to standard combination chemotherapy and cetuximab treatment of patients with squamous cell carcinoma of the head and neck: the active8 randomized clinical trial. JAMA Oncol 2018;4:1583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cohen E, Bishnoi S, Laux DE, et al. Abstract CT098: phase Ib/II, open label, multicenter study of intratumoral SD-101 in combination with pembrolizumab in anti-PD-1 treatment naïve patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC). Cancer Res 2018;78 CT098–CT098. [Google Scholar]
- [67].Massarelli E, William W, Johnson F, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol 2018. [DOI] [PMC free article] [PubMed]
- [68].Schuler PJ, Harasymczuk M, Visus C, et al. Phase I dendritic cell p53 peptide vaccine for head and neck cancer. Clin Cancer Res 2014;20:2433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res 2010;16:4005–15. [DOI] [PubMed] [Google Scholar]
- [70].Sacco AG, Chen R, Ghosh D, et al. An open label, nonrandomized, multi-arm, phase II trial evaluating pembrolizumab combined with cetuximab in patients with recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): results of cohort 1 interim analysis. J Clin Oncol 2019;37 6033–6033. [Google Scholar]
- [71].Taylor MH, Rasco DW, Brose MS, et al. A phase 1b/2 trial of lenvatinib plus pembrolizumab in patients with squamous cell carcinoma of the head and neck. J Clin Oncol 2018;36 6016–6016. [Google Scholar]
- [72].Davis RJ, Ferris RL, Schmitt NC. Costimulatory and coinhibitory immune checkpoint receptors in head and neck cancer: unleashing immune responses through therapeutic combinations. Cancers of the Head Neck 2016;1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005;11:728–34. [PubMed] [Google Scholar]
- [74].McBride SM, Sherman EJ, Tsai CJ, et al. A phase II randomized trial of nivolumab with stereotactic body radiotherapy (SBRT) versus nivolumab alone in metastatic (M1) head and neck squamous cell carcinoma (HNSCC). J Clin Oncol 2018;36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bauman JE, Harris J, Uppaluri R, et al. NRG-HN003: phase I and expansion cohort study of adjuvant cisplatin, intensity-modulated radiation therapy (IMRT), and MK-3475 (Pembrolizumab) in high-risk head and neck squamous cell carcinoma (HNSCC). J Clin Oncol 2019;37 6023–6023. [Google Scholar]
- [76].Sun XS, Sire C, Tao Y, et al. A phase II randomized trial of pembrolizumab versus cetuximab, concomitant with radiotherapy (RT) in locally advanced (LA) squamous cell carcinoma of the head and neck (SCCHN): first results of the GORTEC 2015–01 “PembroRad” trial. J Clin Oncol 2018;36 6018–6018. [Google Scholar]
- [77].Ferris RL, Gillison ML, Harris J, et al. Safety evaluation of nivolumab (Nivo) concomitant with cetuximab-radiotherapy for intermediate (IR) and high-risk (HR) local-regionally advanced head and neck squamous cell carcinoma (HNSCC): RTOG 3504. J Clin Oncol 2018;36 6010–6010. [Google Scholar]


