Abstract
Background and purpose
An increasing number of reports have observed thrombosis in severe cases of COVID-19. The aim of this study was to evaluate the incidence of thromboembolism in mild/moderate cases of COVID-19. All of the patients had normal coagulation tests and none had any overt thrombotic complications. Our findings indicate that it is important to screen the thrombotic status of cases with mild/moderate COVID-19.
Methods
Between 11 June and 8 July 2020, 23 patients with mild/moderate COVID-19 pneumonia consented to having computed tomography pulmonary angiography (CPTA) and computed tomography venography (CTV) scans of the lungs and extremity veins. Doppler ultrasound (DUS) was also performed in all patients for screening. The incidence, clinical manifestations, laboratory examinations, imaging features, and prognosis, of patients with venous thromboembolism (VTE) were analyzed and compared with those of patients with COVID-19 pneumonia without VTE.
Results
Nineteen patients (82.6%) had VTE, mainly distal limb thrombosis. Only one of the VTE patients was positive when screened by DUS; the other VTE patients were negative by DUS. All of the mild/moderate patients with VTE were screened by CTPA + CTV. Blood tests for inflammatory, coagulation, and biochemical, parameters were all within the normal range, except for WBC and LDH.
Conclusions
When using CTV screening for DVT, we found that the incidence of thrombosis in patients with mild/moderate COVID-19 markedly increased to 82.6% (19/23). Screening for thrombosis is therefore important in patients with COVID-19. CTV is more sensitive than DUS for the detection of thrombosis. More research is now needed to evaluate the significance of thrombosis in COVID-19 pneumonia.
Keywords: COVID-19, Venous thromboembolism, Thrombotic complications, Mild/moderate COVID-19
Introduction
As the number of confirmed cases of severe COVID-19 has increased, an increasing number of reports have suggested that thrombotic complications in severe to critical COVID-19 cases may represent an important risk (Helms et al., 2020). An increasing body of evidence, acquired via autopsy or imaging, also supports the existence of thrombo-embolism in severe cases of COVID-19 (James et al., 2020, Maximilian et al., 2020). However, very little is known with regards to the specific characteristics of thromboembolic complications in cases of mild/moderate COVID-19.
Thrombotic complications include pulmonary thromboembolism (PE) and venous thromboembolism (VTE). Most previous reports have focused on PE but have rarely considered venous clots, especially sacral thrombosis (Jean-Marie et al., 2020). Coagulopathy is known to contribute to the severity of COVID-19 infection; this is associated with an excess of venous, arterial, or microvascular-associated events.
The activated coagulation pathway and endothelial dysfunction are the leading factors associated with the development of thrombosis (Ning et al., 2020). The combination of thrombocytopenia, prolonged prothrombin time, and increased levels of d-dimer, are all known markers of thrombosis (Marcel et al., 2020). The measurement of d-dimer levels in the plasma is emerging as a direct prognostic marker for patientsd with COVID-19. However, most previous reports concerned patients with severe COVID-19. In the present study, we showed that thrombotic complications also occurred in mild to moderately ill patients with COVID-19; most of these patients had normal d-dimer levels and prothrombin time.
Doppler ultrasound (DUS) is the method that is normally used to screen for thrombosis in patients with COVID-19 (Anna Maria, 2020). A previous study, involving patients in the Intensive Care Unit (ICU), reported that the incidence of VTE ranged from 9% to 65% when screened by routine ultrasound (Fatimah et al., 2020). However, another study failed to identify any patients with DVT after undergoing routine lower extremity ultrasound screening (Marco et al., 2020). CT venography (CTV) is another method that can be used to screen for thrombosis. In this study, we compared different screening methods for thrombosis in patients with COVID-19.
Methods
Patients
Consecutive patients with confirmed COVID-19 were enrolled in Beijing Ditan Hospital, the designated hospital for COVID-19 in Beijing. COVID-19 was formally diagnosed according to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) (Zhou et al., 2020). This study was approved by the Ethics Committee of Beijing Ditan Hospital (Beijing, China).
We recruited patients with mild and moderate COVID-19 who were diagnosed during their hospital stay; 23 patients were enrolled between 11 June and 8 July 2020. All patients had consented to undergo computed tomography pulmonary angiography (CPTA) and computed tomography venography (CTV) scans of the lungs and extremity veins. Doppler ultrasound (DUS) was also used to screen for thrombosis in the same patients. For each patient, we collated a range of clinical data, including demographic information, comorbidities, symptoms on admission, blood test results, lower limb venous ultrasound Doppler, time between admission and CT scan diagnosis, and patient outcome.
Procedures
CT scans were performed on a 256-slice spiral CT system (Philips, iCT, Netherlands). CT images were independently reviewed by two experienced thoracic radiologists (CBD and XRM). Disagreements were resolved by consensus. The radiologists assessed both lung and lower veins and were blinded to all clinical data. All images were examined via dedicated windows. We also used by artificial intelligence (AI) technology to evaluate inflammation caused by pneumonia.
Statistical analysis
Data are given as mean ± standard deviation, median (interquartile range), or numbers (percentages), where appropriate. P < 0.05 was considered to be statistically significant. Data were analyzed using SPSS 21.0 for Windows (SPSS Inc.).
Results
Patient population and clinical characteristics
We recruited 23 patients (14 males and 9 females); each patient had a complete set of clinical information and laboratory data. Table 1 shows the baseline patient characteristics for the VTE and non-VTE groups. The mean age was 42.7 ± 12.0 years. We performed CTPA + CTV all patients 10–14 days after admission.
Table 1.
Demographic and clinical characteristics of 23 patients with COVID-19.
| Variable | Total (N = 23) | VTE (n = 19) | Non-VTE (n = 4) | P value |
|---|---|---|---|---|
| Male, n (%) | 14 (60.1) | 12 (63.2) | 2 (50.0) | 1.000 |
| Age (years) | 42.7 ± 12.0 | 43.2 ± 12.1 | 40.8 ± 13.5 | 0.725 |
| BMI | 23.6 ± 2.8 | 23.3 ± 2.4 | 25.1 ± 4.4 | 0.260 |
| Onset of symptoms | ||||
| Fever, n (%) | 15 (65.2) | 12 (63.2) | 3 (75.0) | 1.000 |
| Cough, n (%) | 12 (52.2) | 10 (52.6) | 2 (50.0) | 1.000 |
| Sputum, n(%) | 3 (13.0) | 3 (15.8) | 0 (0.0) | 1.000 |
| Fatigue, n (%) | 6 (26.1) | 5 (26.3) | 1 (25.0) | 1.000 |
| Dyspnea, n (%) | 1 (4.3) | 0 (0.0) | 1 (25.0) | 0.174 |
| Diarrhea, n (%) | 5 (21.7) | 4 (21.1) | 1 (25.0) | 1.000 |
| Sore throat, n (%) | 4 (17.4) | 3 (15.8) | 1 (25.0) | 1.000 |
| Anosmia, n(%) | 4 (17.4) | 3 (15.8) | 1 (25.0) | 1.000 |
| Vital signs | ||||
| Temperature (℃) | 37.7 ± 0.7 | 37.7 ± 0.8 | 37.8 ± 0.8 | 0.798 |
| Respiratory rate (breaths per min) | 19.4 ± 1.0 | 19.4 ± 1.0 | 19.0 ± 1.2 | 0.466 |
| Heart rate (beats per min) | 86.4 ± 10.6 | 84.8 ± 9.6 | 93.8 ± 13.4 | 0.128 |
| SBP (mmHg) | 125.7 ± 16.7 | 125.3 ± 17.0 | 127.5 ± 17.5 | 0.826 |
| DBP (mmHg) | 80.3 ± 10.8 | 79.7 ± 10.9 | 83.3 ± 11.2 | 0.560 |
| Padua prediction score | 1 (1, 4) | 1 (1, 4) | 1 | 0.794 |
| Hematological and infection-related indices | ||||
| White blood cells (×109/L) | 6.7 ± 1.7 | 7.1 ± 1.6 | 5.1 ± 1.3 | 0.042 |
| Lymphocytes (×109/L) | 2.3 ± 0.7 | 2.4 ± 0.7 | 1.8 ± 0.4 | 0.115 |
| Neutrophil (×109/L) | 4.1 ± 1.5 | 4.4 ± 1.4 | 2.8 ± 1.1 | 0.056 |
| Neutrophils/lymphocytes | 2.5 ± 1.1 | 2.5 ± 1.1 | 2.1 | 0.458 |
| Platelets (×109/L) | 187.3 ± 61.8 | 182.2 ± 61.0 | 211.4 ± 68.9 | 0.404 |
| Hemoglobin (g/L) | 134.8 ± 20.0 | 136.6 ± 18.2 | 126.5 ± 29.1 | 0.374 |
| Coagulation function index | ||||
| D-dimer (μg/mL) | 3.3 ± 13.2 | 0.5 ± 0.5 | 0.4 ± 0.3 | 0.441 |
| PT (seconds) | 12.0 ± 0.8 | 12.0 ± 0.9 | 12.0 ± 0.6 | 0.982 |
| APTT (seconds) | 35.0 ± 10.7 | 35.6 ± 11.6 | 31.8 ± 2.7 | 0.239 |
| Liver function index | ||||
| Aspartate aminotransferase (U/L) | 32.5 ± 16.0 | 32.4 ± 15.7 | 32.7 ± 20.2 | 0.981 |
| Alanine aminotransferase (U/L) | 43.7 ± 35.8 | 44.3 ± 37.8 | 40.9 ± 28.0 | 0.866 |
| Total bilirubin (μmol/L) | 11.0 ± 3.4 | 10.9 ± 3.3 | 11.6 ± 4.3 | 0.703 |
| Direct bilirubin (μmol/L) | 3.7 ± 1.3 | 3.6 ± 1.2 | 3.9 ± 1.8 | 0.691 |
| Lactic dehydrogenase (U/L) | 241.1 ± 72.0 | 254.6 ± 71.6 | 177.1 ± 25.0 | 0.048 |
| Kidney function index | ||||
| Blood urea nitrogen (mmol/L) | 5.1 ± 1.0 | 5.1 ± 1.0 | 5.0 ± 1.6 | 0.885 |
| Serum creatinine(μmol/L) | 70.6 ± 12.8 | 70.9 ± 12.9 | 69.2 ± 14.0 | 0.821 |
| K+ (mmol/L) | 3.2 ± 0.3 | 3.2 ± 0.3 | 3.2 ± 0.2 | 0.953 |
| Na+ (mmol/L) | 141.9 ± 1.3 | 141.9 ± 1.2 | 141.8 ± 1.8 | 0.828 |
| CRP (mg/L) | 16.2 ± 25.0 | 14.7 ± 21.2 | 23.3 ± 42.5 | 0.465 |
| SAA(mg/L) | 63.2 ± 93.1 | 67.4 ± 98.4 | 42.8 ± 69.6 | 0.641 |
| Pneumonia index | 136.7 ± 166.6 | 145.8 ± 174.9 | 93.5 ± 130.5 | 0.581 |
BMI: body mass index; COVID-19: coronavirus disease 2019; DBP: diastolic blood pressure; SBP: systolic blood pressure; SD: standard deviation; VTE: venous thromboembolism; PT:Prothrombin time; APTT:Activated partial thromboplastin time; CRP: C-reactive protein; SAA: serum amyloid A.
The initial symptoms of our patient group included fever, cough, fatigue, and other non-specific symptoms. Only three patients had a significant past medical history; one had hypertension, one had pulmonay fibrosis (case 10), and the third had breast cancer (case 12). All of these conditions were stable at the time of admission.
All 23 patients had pneumonia when they were enrolled, as determined by according to CT scan. Table 1 shows AI scores for pulmonary pathology, as determined from CT characterization data. The mean inflammation value, as determined by AI, was 136.7 ± 166.6.
Thrombotic events and the incidence of such events
In total, 82.6% (19/23) of the patients had deep venous thrombosis (DVT), as determined by radiographic scans; none of these patients had any related symtoms. Padua prediction scores for embolism were normal in all patients. Blood tests for inflammation, coagulation, and biochemistry, were not statistically significant when compared between the DVT and the non-DVT group, other than plasma LDH and total WBC. Analysis showed that LDH and WBC were significantly higher in VTE group than in the non-VTE group. We also performed univariable and multivariable OR; however, only univariable analysis showed that LDH and WBC were significant risk factors for VTE.
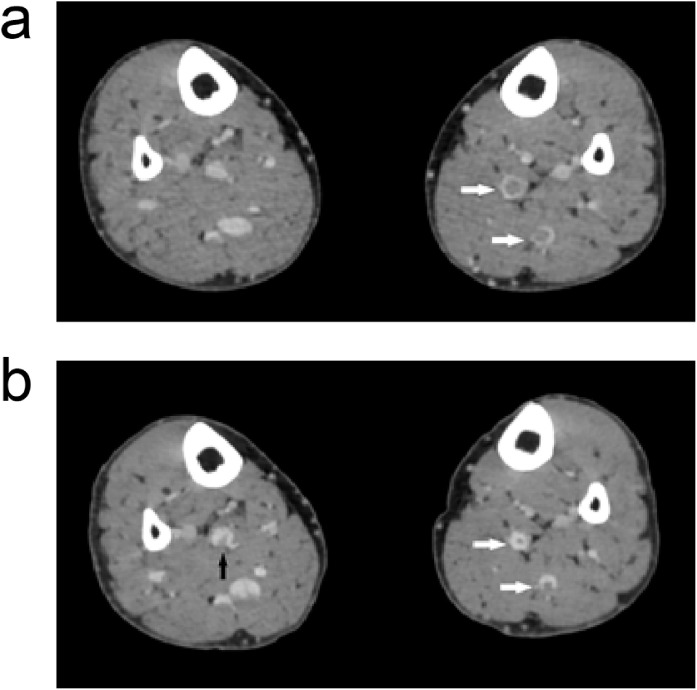
In the radiographically-confirmed VTE patients, only one patient (patient 8) showed VTE when screened by ultrasound; this patient had the most severe VTE of all patients. Patient 8 was a 64 year old female who had a history of pulmonary fibrosis. Her pulmonary fibrosis was mild and she did not require need oxygen support prior to hospitalisation. Following CTPA + CTV, we identified thrombosis in the anterior tibial, posterior tibial, and fibular veins (Figure 1a). Subsequently, she received low molecular weight heparin (LMWH) at therapeutic doses. After two weeks of treatment, we repeated the CTV; her thrombosis had resolved or improved (Figure 1b).
Figure 1.
An image of patient 8 before and after LMWH treatment. (a) Before LMWH treatment showing thrombosis in the left plexus venosus leg muscle (arrow). (b) After LMWH treatment. The thrombosis in the left plexus venosus leg muscle (white arrow) had reduced and a thrombosis in the right plexus venosus leg muscle (black arrow) had emerged.
Thrombosis characteristics
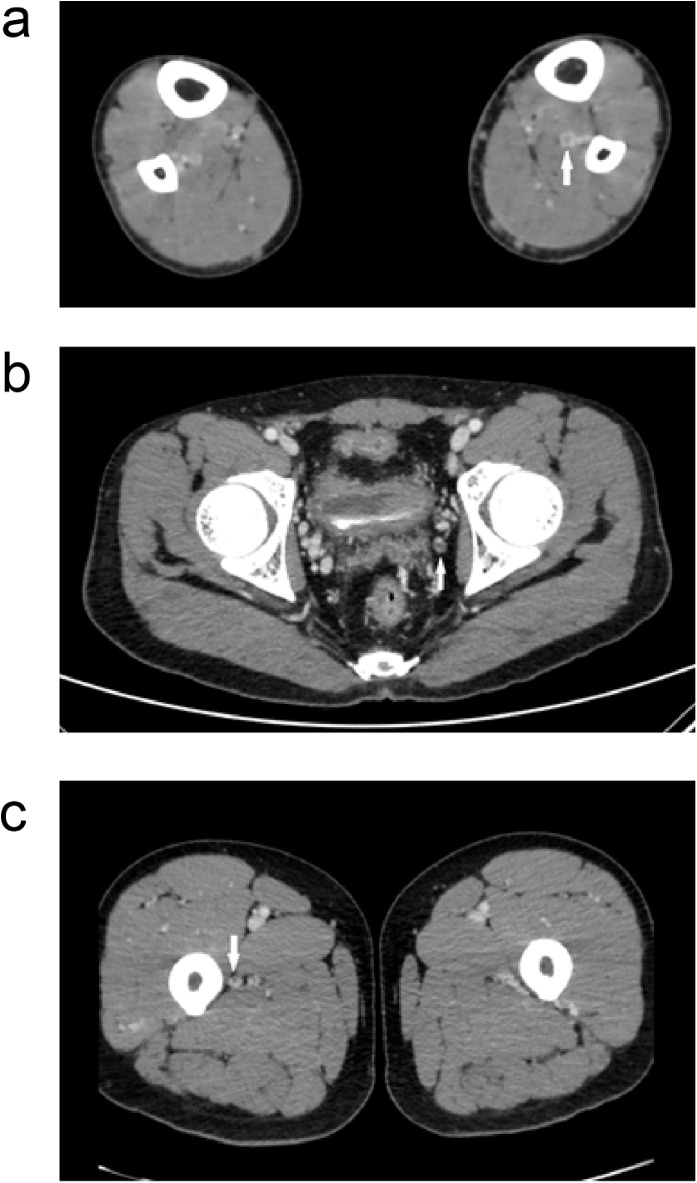
We found that the 19 VTEs were distributed in different veins (Table 2 ). Most cases had a distal DVT (18/19). Onne patient had thrombosis in the prostatic venous plexus and one other patient had a proximal DVT (Figure 2 ). Other than patient 8, none of the other patients received anti-coagulation treatment.
Table 2.
The characteristics of thromboembolism complications.
| No. | Sex | Age (year) | Plt | PT | APTT | D-D | Padua | CRP | Pneumonia index | Site of DVT |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 37 | 283 | 11.2 | 33.8 | 0.51 | 1 | 0.7 | 0.7 | PTV (L,R); FV (L,R) |
| 2 | M | 49 | 273 | 12.3 | 83 | 0.18 | 1 | 3.1 | 139.9 | FV (L,R); DFV |
| 3 | M | 29 | 298 | 11.2 | 31.9 | 0.11 | 1 | 4.1 | 210.2 | ATV (R) |
| 4 | M | 36 | 227 | 11.5 | 33.8 | 0.24 | 1 | 0.3 | 3.5 | PTV (R,L) |
| 5 | M | 45 | 224 | 10.2 | 30.1 | 0.27 | 1 | 3.5 | 3.6 | ATV (L,R), PTV (L,R) |
| 6 | M | 52 | 208 | 11.2 | 32.5 | 0.19 | 1 | 2.9 | 456.9 | ATV (L,R), PTV (L,R), FV (R) |
| 7 | F | 55 | 257 | 12.6 | 35 | 0.43 | 1 | 3.8 | 84.3 | ATV (L) |
| 8 | F | 64 | 166 | 10.9 | 30.9 | 0.12 | 2 | 5.2 | 439.1 | ATV (L), PTV (L), FV (L,R) |
| 9 | F | 34 | 441 | 10.4 | 29.1 | 0.18 | 4 | 1.3 | 0.4 | PTV (L), FV (L,R), DFV |
| 10 | M | 30 | 339 | 11 | 31.9 | 0.08 | 1 | 6.7 | 97.49 | PTV (L), FV (L,R) |
| 11 | F | 43 | 286 | 10.9 | 29.7 | 0.1 | 1 | 0.6 | 6.9 | ATV (R) |
| 12 | F | 65 | 389 | 11.7 | 33.6 | 0.17 | 2 | 53 | 336.7 | ATV (L) |
| 13 | F | 29 | 124 | 13.9 | 26.8 | 0.13 | 1 | 0.8 | 90.9 | PTV (R) |
| 14 | M | 58 | 143 | 11.5 | 34.1 | 0.3 | 1 | 9.6 | 102.3 | PV |
| 15 | M | 36 | 298 | 12.3 | 30 | 0.35 | 1 | 0.8 | 3.9 | Prostatic venous |
| 16 | M | 39 | 301 | 10 | 30.5 | 0.14 | 1 | 2.8 | 159.6 | ATV (L), FV (R) |
| 17 | F | 50 | 314 | 11.3 | 34.1 | 0.31 | 1 | 0.5 | 60.3 | ATV (R), PTV (R), FV (R) |
| 18 | M | 24 | 252 | 12.6 | 32.1 | 0.36 | 1 | 7.7 | 15.5 | PTV (R) |
| 19 | M | 45 | 249 | 12.4 | 32.4 | 0.24 | 1 | 8.9 | 557.3 | ATV (R), PTV (R) |
right: R; left: L; posterior tibial vein: PTV; fibular veins: FV; deep femoral vein: DFV; anterior tibial veins: ATV; popliteal vein: P.
Figure 2.
Embolism in different veins. (a) Posterior tibial vein thrombosis (arrow), (b) prostatic venous thrombosis (arrow), and (c) fibular vein thrombosis (arrow).
All of these patients were discharged according to our clinical guidelines. None of the patients became severe or critical. None of the patients developed sequelae related to thrombosis.
Discussion
The most important and simple way to diagnose COVID-19 is by chest CT scan without the administration of contrast agent (Fang et al., 2020). Research has shown that the incidence of PE in patients with COVID-19 ranges from 20.4% to 28% (Julien et al., 2020). However, this data is potentially biased because the number of CTPAs performed is lower in patients with COVID-19.
In the present study, we performed a prospective cross-sectional study and investigated 19 patients with mild to moderate COVID-19 who had experienced thrombotic events. The proportion of patients with VTE, as detected by CT, was as high as 82.6%. We found that radiographic examination resulted in far more thromboembolic diagnoses (19/23) than duplex ultrasound (1/23). None of these patients had any evidence of VTE on routine blood counts or by clinical symptoms or signs. Higher WBC and LDH levels might be associated with thrombosis; these were significantly higher in the VTE group than in the non-VTE group, but only in our univariate analysis. In contrast to severe cases (Fontana et al., 2020), patients with mild/moderate disease in both the VTE and non-VTE groups, showed no specific blood test results that were indicative of infection, immunology, viral load, or coagulation.
Thrombosis is an important part of the clinical picture of in COVID-19 that needs to be taken into consideration. Ultrasound is one of the most common methods used by clinicians to detect thrombosis. Some cases of thrombosis in COVID-19 were detected by CTPA or at autopsy (Barnes et al., 2020, Kosior et al., 2020, Moores et al., 2020, Spyropoulos et al., 2020). Almost all of the existing reports in this field have focused on severe or critically ill patients; very little attention has been paid to those with mild to moderate COVID-19 (Chen et al., 2020).
In the present study, we found that thrombosis was much more common in case of mild disease (82.6%) than in patients with severe disease (49%) (Linnemann et al., 2020). In the current study, we used CTPA + CVT; this increased the sensitivity of clot detection, particularly in the distal veins. None of our patients had any risk factors for thrombosis in their medical history and their Padua scores were all within the normal range. Although we did not treat most of these patients with anticoagulant therapy, this study highlights the need for us to pay more attention to the risk of thromboembolism in patients with COVID-19. The mechanism involved may be a direct effect of SARS-CoV-2 or some indirect effects related to vascular endothelial cells (Varga et al., 2020). We might speculate that some of this occult thrombosis could be associated with some of the post-COVID-19 syndromes that have been described (Danying et al., 2020). In our report, it is evident that the use of routine tests and clinical evaluations, such as the Padua score (Chen et al., 2020; Demelo-Rodriguez et al., 2020), are associated with certain limitations when sued toevaluate the risk of thrombosis. The accuracy of the diagnosis of thrombosis in CTV is more sensitive than by DUS. Surveillance for thrombosis may therefore be important when evaluating patients with COVID-19 (Hanny et al., 2020; Zadow et al., 2020).
Our study has certain limitations that need to be considered. For example, this is a relatively small, single-center study. We do not have long term follow-up of these cases to determine whether these thromboses were associated with sequelae. Furthermore, this was a cross sectional study. We need to obtain more serial data to evaluate the value of early surveillance for thrombosis in the vessels of patients with COVID-19.
Ethical approval
This study was approved by the Ethics Committee of Beijing Ditan Hospital. Informed consent was obtained from each patient prior to the study.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported by Grant DTZLX201709. We thank all of the medical staff involved in the treatment of the patients described herein.
References
- Anna Maria I., Andrea C., Stefano F., Elvira S., Stefano A., Maria Carmela A. Early detection of deep vein thrombosis in patients with coronavirus disease 2019: who to screen and who not to with Doppler ultrasound. J Ultrasound. 2020 doi: 10.1007/s40477-020-00515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes G.D., Burnett A., Allen A., Blumenstein M., Clark N.P., Cuker A. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhang D., Zheng T., Yu Y., Jiang J. DVT incidence and risk factors in critically ill patients with COVID-19. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02181-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danying L., Fen Z., Lili L., Min X., Hongbo W., Jiahong X. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7(9) doi: 10.1016/S2352-3026(20)30217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demelo-Rodriguez P., Cervilla-Munoz E., Ordieres-Ortega L., Parra-Virto A., Toledano-Macias M., Toledo-Samaniego N. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P., Ji W. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020:200432. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatimah A.-A., Samer C., Alejandro L.-L. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192 doi: 10.1016/j.thromres.2020.05.039-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana P., Casini A., Robert-Ebadi H., Glauser F., Righini M., Blondon M. Venous thromboembolism in COVID-19: systematic review of reported risks and current guidelines. Swiss Med Wkly. 2020;150:w20301. doi: 10.4414/smw.2020.20301. [DOI] [PubMed] [Google Scholar]
- Hanny A.-S., Fei S., Elizabeth Van C., David J.K., Rachel R. Evaluation of the prothrombin fragment 1.2 in patients with coronavirus disease 2019 (COVID-19) Am J Hematol. 2020 doi: 10.1002/ajh.25962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D.M., Hannah S., Karlheinz P., Hannah S., Karlheinz P. The emerging threat of (Micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res. 2020;127(4) doi: 10.1161/CIRCRESAHA.120.317447-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Marie B., Ludovic D., Frédéric L. Kawasaki-like diseases and thrombotic coagulopathy in COVID-19: delayed over-activation of the STING pathway. Emerg Microb Infect. 2020 doi: 10.1080/22221751.2020.1785336-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien P., Julien G., Morgan C., Erika P., Thibault D., Fanny L. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [Google Scholar]
- Kosior D.A., Undas A., Kopec G., Hryniewiecki T., Torbicki A., Mularek-Kubzdela T., Windyga J. Guidance for anticoagulation management in venous thromboembolism during the coronavirus disease 2019 pandemic in Poland: an expert opinion of the Section on Pulmonary Circulation of the Polish Cardiac Society. Kardiol Pol. 2020;78(6):642–646. doi: 10.33963/KP.15425. [DOI] [PubMed] [Google Scholar]
- Linnemann B., Bauersachs R., Grebe M., Klamroth R., Muller O., Schellong S. Venous thromboembolism in patients with COVID-19 (SARS-CoV-2 infection)—a position paper of the German Society of Angiology (DGA) Vasa. 2020;49(4):259–263. doi: 10.1024/0301-1526/a000885. [DOI] [PubMed] [Google Scholar]
- Marcel L., Jecko T., Toshiaki I., Jerrold H.L. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6) doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco C., Elena M.B., Simone B., Carolina B., Daniele M., Marco M. Pulmonary embolism or pulmonary thrombosis in COVID-19? is the recommendation to use high-dose heparin for thromboprophylaxis justified. Thromb Haemost. 2020 doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximilian A., Stijn E.V., Mark K., Axel H., Tobias W., Florian L. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2) doi: 10.1056/NEJMoa2015432-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K. Prevention, diagnosis, and treatment of VTE in patients with COVID-19: CHEST guideline and expert panel report. Chest. 2020 doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning T., Dengju L., Xiong W., Ziyong S. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost: JTH. 2020;18(4) doi: 10.1111/jth.14768-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadow E.K. Coronavirus (COVID-19), coagulation, and exercise: interactions that may influence health outcomes. Semin Thromb Hemost. 2020 doi: 10.1055/s-0040-1715094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Liu Q., Yang Z., Liao J., Yang K., Bai W. Preliminary prediction of the basic reproduction number of the Wuhan novel coronavirus 2019-nCoV. J Evid Based Med. 2020 doi: 10.1111/jebm.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]