Abstract
CaWRKY40 was previously found to be transcriptionally up‐regulated by Ralstonia solanacearum inoculation (RSI) or heat stress (HS), but the underlying mechanism remains unknown. Herein, we report that a double‐W box‐element (DWE) in the promoter of CaWRKY40 is critical for these responses. The upstream W box unit WI of this composite element is crucial for preferential binding by CaWRKY40 and responsiveness to RSI or HS. DWE‐driven CaWRKY40 can be transcriptionally and nonspecifically regulated by itself and by CaWRKY58 and CaWRKY27. The DWE was also found in the promoters of CaWRKY40 orthologs, including AtWRKY40, VvWRKY40, GmWRKY40, CplWRKY40, SaWRKY40, SpWRKY40, NtWRKY40, and NaWRKY40. DWEAtWRKY40 was analogous to DWECaWRKY40 by responding to RSI or HS and AtWRKY40 expression. These data suggest that a conserved response of plants to pathogen infection or HS is probably mediated by binding of the DWE by WRKY40.
Keywords: double‐W box‐element, pepper, promoter, Ralstonia solanacearum, transcription factor, WRKY
Our study reports that a conserved response of plants to pathogen infection or heat shock is probably mediated by the binding of the double‐W box to WRKY40.
1. INTRODUCTION
Growing in natural habitats, plants are inevitably exposed to a great variety of biotic and abiotic stresses, either as a single stress or multiple stresses. Plants have evolved sophisticated defence machineries to perceive and discriminate different types of stresses, transmit information via signalling networks, and transcriptionally modulate the expression of a large number of response genes by a multitude of transcription factors (TFs). Stress responses are finely modulated to maximize defence and reduce cost. Typically, stress‐responsive genes are functionally interconnected within transcriptional networks. Unravelling how such stress‐responsive transcriptional networks operate is a major goal in current plant research.
WRKY TFs are one of the largest TF families in plants (Rushton et al., 2010). A large number of WRKY TFs have been reported from Arabidopsis (Wu et al., 2005; Zhang and Wang, 2005), rice (Wu et al., 2005; Zhang and Wang, 2005), soybean (Yin et al., 2013), papaya (Pan and Jiang, 2014), poplar (Abbruscato et al., 2012), Physcomitrella patens (Tripathi et al., 2012), cucumber (Ling et al., 2011), and Brachypodium distachyon (Wen et al., 2014). The members of this family are characterized by the conserved WRKY domain of approximately 60 amino acids. These DNA‐binding domains feature an N‐terminal, nearly invariant, amino acid sequence WRKYGQK and a C‐terminal C2H2 (C‐X4‐5‐C‐X22‐23‐H‐X‐H) or C2HC (C‐X7‐C‐X23‐H‐X‐C) zinc finger motif. WRKY members are grouped into three families and additional subfamilies based on the number of WRKY domains, types of zinc finger motifs, and presence of additional domains (Eulgem et al., 2000). WRKY TFs specifically bind via their WRKY domains to W box‐type cis‐elements in the promoters of their target genes, and have been implicated in growth and developmental processes such as trichome development (Johnson et al., 2002), seed development (Luo et al., 2005), and leaf senescence (Kim et al., 2008; Miao et al., 2004). However, the major function of WRKY TFs appears to be mediating biotic and abiotic stress responses, such as those triggered by pathogens (Eulgem et al., 1999; 2000; Kim et al., 2008), herbivores (Skibbe et al., 2008), viruses (Park et al., 2006), drought (Jiang et al., 2012), cold (Yokotani et al., 2013), salinity (Hu et al., 2013), osmotic stress (Ding et al., 2014; Raghothama et al., 1993), and heat stress (HS) (Dang et al., 2013).
WRKY genes often contain functional W boxes in their promoters and can be regulated by their own gene product or other WRKYs (Chen et al., 2003; Cormack et al., 2002; Eulgem et al., 1999; Eulgem and Somssich, 2007; Lippok et al., 2007; Turck et al., 2004). Thus WRKY genes are functionally connected and form a transcriptional network (Eulgem, 2006; Eulgem and Somssich, 2007). Subsets of WRKY family members often cooperatively participate in plant response to a single environmental cue (Liu et al., 2012), while in other cases a single WRKY TF is involved in several apparently incongruent or unlinked processes (Ding et al., 2014; Knoth et al., 2007; Ulker et al., 2007; Yokotani et al., 2013). This hints at complex contributions of WRKY TFs and WRKY network interactions in plant response to single stresses as well as crosstalk between distinct response pathways.
Critical for the diversity and distinction of their roles is the specificity of their interactions with target promoters. While the majority of WRKY TFs seem to bind to derivatives of W box elements (general consensus: TTGACC/T), some exceptions have been reported (Sun et al., 2003). The DNA‐sequence specificity of WRKY TFs seems to be at least partially determined by the WRKYGQK motif, which resides within a larger β‐sheet secondary structure at the N‐terminal end of most WRKY domains and protrudes into the major grove of double‐stranded DNA (Yamasaki et al., 2012). This nearly invariant motif is engaged in specific contacts with the nucleobases of W box‐related motifs. However, variants of this conserved sequence, such as WRKYGKK, WRKYGMK, WSKYGQK, WQKYGQK, and WIKYGEN (Rushton et al., 2012; Zhang and Wang, 2005), have been identified, along with variants of the typical W box, including CGACTTTT (Machens et al., 2014), TGCGCTT, SURE element (TAAAGATTACTAATAGGAA), and SURE‐like element and the WK box (TTTTCCAC) as WRKY binding sites (Rushton et al., 2012). Novel WRKY‐associated zinc‐finger variants, such as Cx29HxH and Cx7Cx24HxC (Huang et al., 2012), have also been identified. Additional regulators, such as VQ‐motif‐containing proteins (Pecher et al., 2014) have been reported to be implicated in stimulus‐specific WRKY binding to target promoters (Cheng et al., 2012). A full understanding of how WRKY networks regulate stimulus‐specific responses remains to be elucidated.
Belonging to the Solanaceae family, Capsicum annuum (pepper) is an agriculturally important crop. Under the influence of a continuous cropping barrier, pepper is sensitive to soilborne diseases such as bacterial wilt and Phytophthora blight (Lamour et al., 2012; Lebeau et al., 2011). High temperature and high humidity aggravate the disease conditions. Some pepper germplasms might have developed specific defence strategies against this combination of stresses (Cai et al., 2015), but the underlying mechanism remains unknown. We previously found that CaWRKY40, a group IIb WRKY member, was up‐regulated by Ralstonia solanacearum inoculation (RSI), HS, and exogenous application of the defence‐inducing compounds salicylic acid (SA), jasmonic acid (JA), and ethephon (ETH) (Dang et al., 2013). It has been previously described that ectopic expression of CaWRKY40 conferred thermotolerance and enhanced resistance to R. solanacearum infection in transgenic tobacco. Furthermore, virus‐induced gene silencing of CaWRKY40 in pepper plants reduced thermotolerance and resistance to R. solanacearum inoculation (Dang et al., 2013). However, how CaWRKY40 is transcriptionally regulated and linked to defence and thermotolerance pathways in Solanaceae remains unknown.
In the present study, we isolated the CaWRKY40 promoter and performed 5ʹ deletion analysis to identify the promoter regions and cis‐elements responsive to R. solanacearum infection and HS treatment. We analysed the effects of the expression of several WRKY TFs associated with plant immunity, such as CaWRKY40 (Dang et al., 2013), CaWRKY58 (Wang et al., 2013), and CaWRKY27 (Dang et al., 2014), on the CaWRKY40 promoter. Our results suggest that CaWRKY40 responses to R. solanacearum infection and HS depend on an arrangement of two W boxes located in pCaWRKY40− 1,802 to −1,464, and that CaWRKY40 expression is regulated by its own gene product and other WRKY proteins.
2. RESULTS
2.1. Cloning the CaWRKY40 promoter and sequence analysis
To gain insight into the regulation mechanism of CaWRKY40 in response to R. solanacearum inoculation and heat shock, the promoter region of CaWRKY40 (accession number NM_001325081.1) was amplified using a genome‐walking strategy. The cloned CaWRKY40 promoter region was determined to be 1,802 bp long. A search for cis‐acting elements in the promoter region by PLANTCARE software (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) identified three groups of putative regulatory motifs, including core promoter motifs (TATA‐box), defence‐associated motifs (W box motif, ABRE motif etc.), and motifs that might be associated with growth and development (ARF binding site and the GA‐box motif) (Figure S1). The presence of these motifs is consistent with the proposed role of CaWRKY40 in responses to pathogen infection, HS, and exogenously applied hormones in pepper plants (Dang et al., 2013). The presence of these motifs also suggests that, besides pathogen defence, CaWRKY40 might participate in other biological processes, such as stress responses to drought and wounding, or regulation of growth and development.
2.2. Transiently overexpressed CaWRKY40, CaWRKY58, and CaWRKY27 up‐regulate the transcriptional expression of CaWRKY40
The presence of nine W box motifs in pCaWRKY40 suggested that CaWRKY40 may be regulated by itself and/or other WRKY TFs (Figures 1a and S1). To test this, expression vectors for haemagglutinin (HA)‐epitope‐tagged CaWRKY58 (a pepper WRKY TF that suppresses immunity, Wang et al., 2013), CaWRKY27 (a pepper WRKY TF promoting plant immunity, Dang et al., 2014), or CaWRKY40 were cotransformed with the reporter vector pCaWRKY40::GUS by agroinfiltration in pepper leaves and by particle bombardment in onion cells (Figure S2). Immunoblot analysis revealed that the CaWRKY40, CaWRKY58, and CaWRKY27 proteins were successfully expressed in pepper leaves and accumulated to similar levels (Figure 1b). At 48 hr after agroinfiltration, the overexpression of all three tested HA‐tagged CaWRKY TFs in infiltrated pepper leaves triggered enhanced GUS expression (Figure 1c). Onion cells cotransformed with the effector vector (CaWRKY40‐HA, CaWRKY58‐HA, or CaWRKY27‐HA) and reporter vector exhibited an obvious dark blue colour, while cells cotransformed with empty effector vector and reporter vector were not stained blue (Figure S2). In addtion, we found accumulation of CaWRKY40 transcript in pepper leaves transfomed with the effector vector (CaWRKY40‐HA, CaWRKY58‐HA, or CaWRKY27‐HA; Figure 1d). These results suggest that CaWRKY40 can be transcriptionally and nonspecifically regulated by itself and by CaWRKY58 and CaWRKY27.
FIGURE 1.
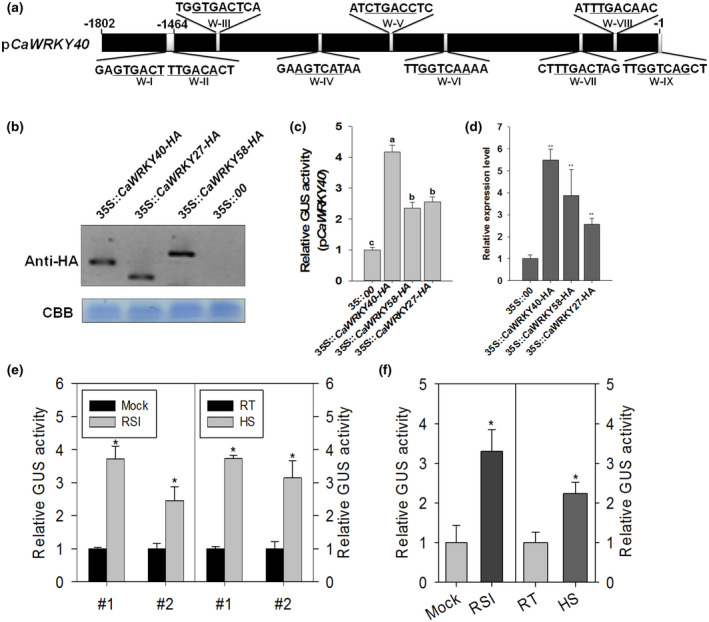
β‐Glucuronidase (GUS) activity analysis of CaWRKY40 promoter triggered by CaWRKY40, CaWRKY58, and CaWRKY27 overexpression, as well as Ralstonia solanacearum inoculation (RSI) and heat shock (HS). (a) Schematic representation of the locations of nine W box motifs (WI to WIX) in the promoter regions of the pepper CaWRKY40 gene isolated from the pepper genomic DNA using a genome‐walking strategy, with the primers from the CaWRKY40 cDNA sequence (accession number NM_001325081.1). The underlined nucleotides represent the sequences of W boxes. White boxes in the long black box represent the location of the nine W box motifs. Numbers above the black box represent the positions where the promoter was truncated. (b) Immunoblot assay was performed to detect the protein levels of all effector constructs transiently expressed in pepper leaves 48 hr after agroinfiltration. Antibody α‐HA was used to detect the haemagglutinin (HA)‐tagged fusion proteins. Coomassie blue staining of the gel to show equal loading. (c)–(d) Transiently overexpressed CaWRKY40‐HA, CaWRKY58‐HA, and CaWRKY27‐HA activate pCaWRKY40‐driven GUS expression (c) and CaWRKY40 transcript (d) in pepper plants. The leaves were harvested for GUS activity measurement at 48 hr after agroinfiltration. The GUS activity of pepper leaves cotransformed with pCaWRKY40::GUS and empty effector vector, and the CaWRKY40 transcript level in pepper leaves expressed empty vector were set to 1. (e)–(f) GUS activities driven by the full‐length CaWRKY40 promoter in response to RSI and HS were studied in transgenic tobacco plants of pCaWRKY40::GUS (d) and further confirmed by Agrobacterium‐mediated transient expression assay (e). RT, room temperature. The 6‐week‐old transgenic tobacco plants harbouring pCaWRKY40::GUS were treated with R. solanacearum or heat shock, and the leaves were harvested for GUS activity measurement at 24 hr posttreatment. The GUS activity of the leaves of pCaWRKY40::GUS tobacco plants without treatment was set to 1. For the transient expression assay, Agrobacterium GV3101 cells carrying the pCaWRKY40::GUS construct were infiltrated into pepper leaves and maintained in the greenhouse. At 24 hr postinfiltration, the infiltrated pepper leaves were treated with R. solanacearum or heat shock for 24 hr and harvested for GUS activity assay. The GUS activity of pepper leaves agroinfiltrated with pCaWRKY40::GUS without treatment was set to 1. (d)–(f) Data represent the mean ± SD of three independent biological experiments. Error bars represent ±SD (n = 3) from three independent experiments. Different letters (a or b) and asterisks indicate significant differences, as determined by Fisher's protected least significant difference test (p < .05)
2.3. CaWRKY40 promoter responses to pathogen infection and heat shock stress
We previously showed CaWRKY40 transcripts accumulate in response to RSI and HS. To confirm whether the CaWRKY40 promoter is responsive to the treatments above, transgenic tobacco lines of pCaWRKY40::GUS were generated. In two independent transgenic pCaWRKY40::GUS tobacco lines (transgenic lines with low β‐glucuronidase [GUS] expression background were chosen), GUS expression was consistently and significantly induced by RSI and HS (Figure 1e). To further confirm the results from transgenic tobacco plants, we used a transiently transformed pCaWRKY40::GUS by agroinfiltration into six pepper leaves (Yang et al., 2000). Consistently, GUS expression was significantly induced by RSI and HS (Figure 1f). These data support the positive role of CaWRKY40 in responses of pepper to pathogen infection and HS as we previously proposed (Dang et al., 2013), and show that the tested 1,802 bp promoter fragment is at least partially responsible for the transcriptional responses to stress‐related treatment.
2.4. Deletion analysis of pCaWRKY40
To identify the core functional region of pCaWRKY40 that is involved in the response to RSI and HS, five 5ʹ deletions of pCaWRKY40 based on the distribution of motifs were generated. Deletions, beginning with the locations −1,802 bp, −1,464 bp, −1,060 bp, −746 bp, and −360 bp to the transcriptional start site, were fused to the GUS gene (Figure 2a). Two independent transgenic tobacco lines harbouring the pCaWRKY40 −1464 construct consistently exhibited no GUS induction upon treatment with RSI or HS, while the 1,802 bp promoter region conferred significant levels of GUS induction upon these stress‐related treatments (Figure 2b,c). The other shorter pCaWRKY40 5ʹ deletions we tested did not show any responsiveness to the tested stimuli (data not shown). These results indicate that the motifs responsible for responses to pathogen inoculation and HS are located in the region of pCaWRKY40 −1,802 to −1,464. To confirm this result, a further experiment was performed by transient expression analysis in leaves of pepper plants following the protocol of Yang et al. (2000). Using Agrobacterium tumefaciens GV3101 strains containing the same set of deletion constructs we got a similar result, confirming that pCaWRKY40 −1,802 to −1,464 harbours functional regions critically important for the stress‐responsiveness of CaWRKY40 (Figure S3).
FIGURE 2.
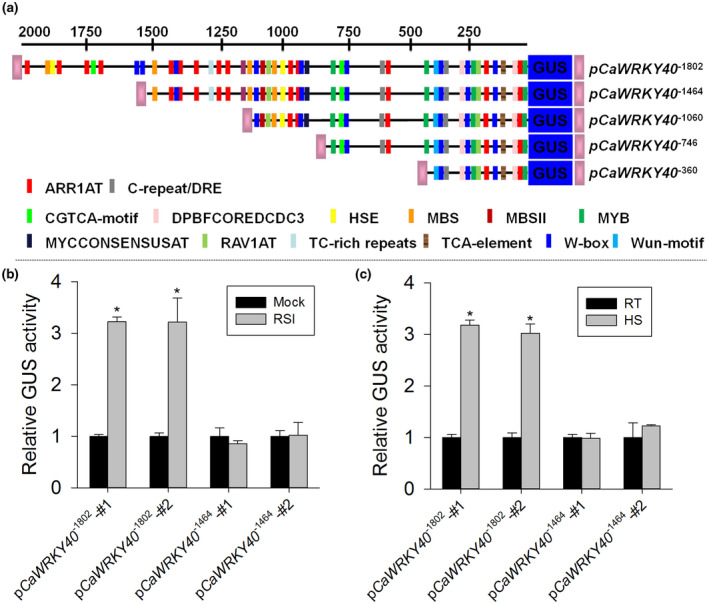
Activation analyses of pCaWRKY40 promoter and its deletion in response to Ralstonia solanacearum inoculation (RSI) and heat shock (HS). (a) Schematic diagram of the reporter vector constructs with five 5' deletions of pCaWRKY40 fused to the reporter gene β‐glucuronidase (GUS). The five 5′ deletions of pCaWRKY40 located at −1,802, −1,464, −1,060, −746, and 360 bp relative to the CaWRKY40 transcription start site were fused to the GUS reporter gene in the vector pMDC163. Boxes with different colours represent putative cis‐elements. (b)–(c) Five terminal deletion analysis of pCaWRKY40 in transgenic tobacco leaves at 24 hr after RSI (2 × 108 cfu/ml) (b) and HS treatment (c). RT, room temperature. The samples were harvested at 24 hr after pathogen and heat shock treatments for GUS activity measurement. (b)–(c) Two different transgenic lines of each promoter deletion were used for GUS measurement. The GUS activity of transgenic tobacco plants without treatment was set to 1. All the data represent the mean ± SD of three independent biological experiments. Error bars indicate SD (n = 3) from three independent experiments. Asterisks indicate significant differences determined by Student's t test (*p < .05)
2.5. A double‐W box element in pCaWRKY40 is responsible for the inducible expression of CaWRKY40
Besides several other known promoter motifs, the pCaWRKY40 −1,802 to −1,464 promoter region contains an arrangement of two directly repeated W box‐related motifs (GTGACTTTGACA) we termed the double W box element (DWE) (Figure 3a). To examine if the DWE is responsible for the responsiveness of this promoter region to overexpression of WRKY TFs and stresses, we tested wild‐type and mutated versions of its upstream (WI) and downstream (WII) W box units fused to the CaMV 35S core promoter (−46 to +8 bp; Figure 3a). The resulting reporter constructs CaDWE‐WT, CaDWE‐M1, CaDWE‐M2, and CaDWE‐DM contained the wild‐type DWE, or a DWE with a mutated version of WI, WII, or both W box units, respectively. Agroinfiltration‐based cotransformation of pepper leaves with the respective reporter constructs and the CaWRKY40‐overexpression vector indicated that CaWRKY40 can trigger GUS expression of CaDWE‐WT, but not any of the reporter constructs with double‐mutated DWE versions, while the WII mutation reduced CaDWE‐WT‐driven GUS expression in response to CaWRKY40‐overexpression to a lesser extent than the WI mutation (Figure 3b), suggesting that CaWRKY40 can bind to the CaDWE‐WT and trigger GUS expression.
FIGURE 3.
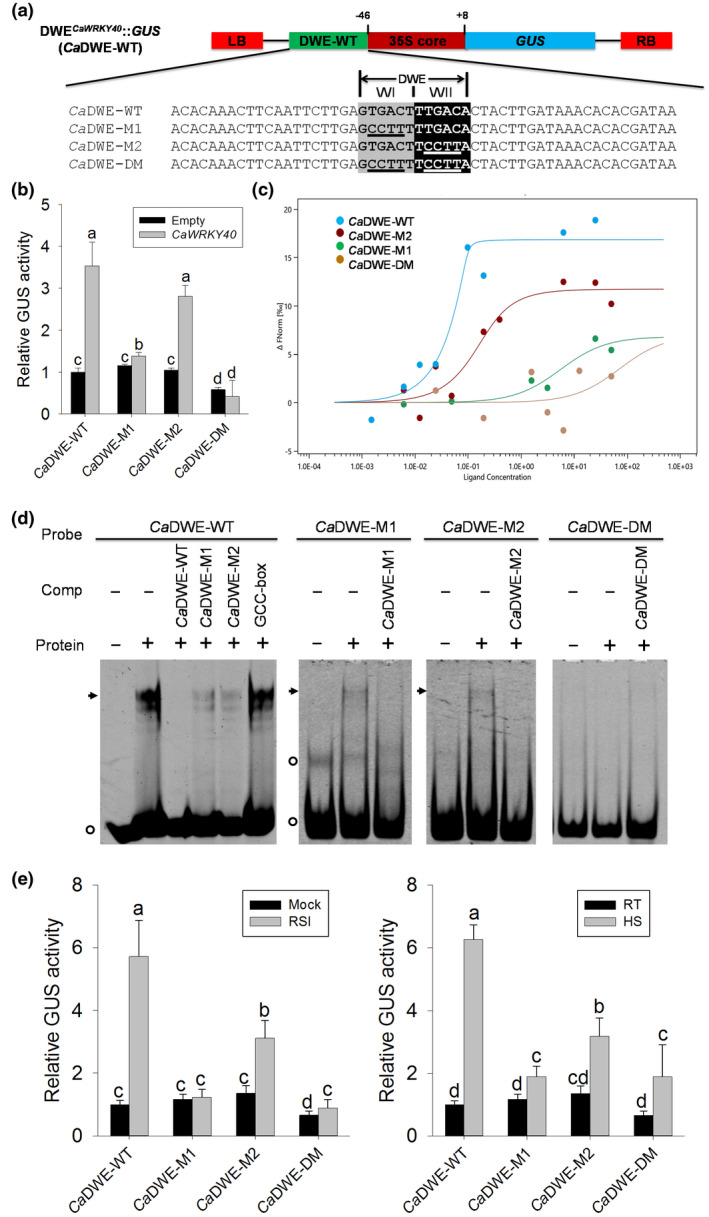
Trans‐activation analysis of the CaDWE in CaWRKY40−1,802 to −1,464 in response to CaWRKY40 overexpression, Ralstonia solanacearum inoculation (RSI) and heat shock (HS). (a) Linear structure of the DWECaWRKY40::GUS vector (CaDWE‐WT), sequences of DWECaWRKY40 motif and its three mutated versions (CaDWE‐M1, CaDWE‐M2, and CaDWE‐DM). DWE, double W boxes element; LB, left border; RB, right border; M, mutant; DM, double mutant. Nucleotides highlighted with grey and black represent the W box motif. Nucleotides underlined indicate the mutated sequences of W box motif. (b) β‐Glucuronidase (GUS) activity analysis of CaDWE‐WT and its three mutated versions transiently expressed in pepper plants in response to CaWRKY40 overexpression. (c) A microscale thermophoresis (MST)‐based binding experiment was performed to confirm the binding of purified CaWRKY40 to CaDWE‐WT and its mutated versions. The bound states of the molecule display different thermophoretic depletion profiles. The difference in thermophoresis can be plotted as a change in normalized fluorescence versus ligand concentration to obtain a binding curve. (d) The electrophoretic mobility shift assays (EMSAs) were performed with purified CaWRKY40‐6×His protein and Cy5‐labelled probes. One hundred‐fold excess of unlabelled probes were used as competitors. The GCC‐box (2 × AGCCGCC) was used as a negative competitor. Black arrows indicate specific shifts. Circles indicate nonspecific shifts or a free probe. Experiments were repeated at least three times. Protein, purified CaWRKY40‐6×His protein. (e) GUS activity analysis of CaDWE‐WT and its three mutated versions in pepper plants in response to RSI and HS. (b) and (e) The CaDWE‐WT and its mutated versions were transformed by agroinfiltration in pepper leaves. At 24 hr postinfiltration, CaWRKY40 was coexpressed in the infiltrated pepper leaves (b), treated with RSI and HS (e), respectively, followed by harvesting for GUS measurement at 24 hr posttreatment. The GUS activities of pepper leaves transformed with CaDWE‐WT without treatment or CaWRKY40 overexpression were both set to 1. Data represent the mean ± SD of three independent biological experiments. Error bars represent ± SD (n = 3) from three independent experiments and different letters (a to d) indicate significant differences, as determined by Fisher's protected least significant difference test
To investigate the ability of DWECaWRKY40 and its mutants to interact with CaWRKY40 protein, microscale thermophoresis (MST) experiments and electrophoretic mobility shift assays (EMSAs) were carried out using Escherichia coli‐expressed recombinant CaWRKY40‐6×His fusion protein purified by HisPur Ni‐NTA Superflow agarose (Figure S4a). For MST experiments, CaWRKY40‐6×His labelled with NT‐647‐NHS fluorescent dye (at a constant concentration of 10 μM; NanoTemper Technologies) and the synthetic DWECaWRKY40 motif (increasing concentration ranging from 10−3 to 103 μM) were together subjected to thermophoresis experiments. The thermophoresis signal was plotted against the ligand concentration to obtain a dose–response curve, from which the binding affinity could be evaluated (Figure 3c). The resulting binding curves showed that both wild‐type DWECaWRKY40 and DWECaWRKY40‐M2 showed a clear association with purified CaWRKY40 protein at a low concentration (10−2 μM). However, the CaWRKY40 binding affinity of DWECaWRKY40 was stronger than that of DWECaWRKY40‐M2, and the binding intensities both increased as the concentration of the targeted DNA increased. As the concentration of the DNA reached 1 and 100 μM, DWECaWRKY40‐M1 and DWECaWRKY40‐DM, respectively, showed weak associations with CaWRKY40. The thermophoretic traces and capillary shapes were scanned and recorded (Figure S4b,c). The results clearly show that under in vitro conditions DWECaWRKY40 can be bound by CaWRKY40 at low concentrations (10−3 μM) and that both of its W box units need to be intact for high affinity binding to occur. In addition, EMSAs were performed with purified recombinant CaWRKY40‐6×His protein and several Cy5‐labelled probes derived from the 54‐bp segments (Figure 3d). A probe containing the CaDWE‐WT led to intense shifts with purified CaWRKY40 protein. To determine which W box contained in the CaDWE‐WT was responsible for the observed shift, several mutated versions of CaDWE were tested including CaDWE‐M1, CaDWE‐M2, and CaDWE‐DM. CaDWE‐M1 and CaDWE‐M2 represent mutated versions of upstream (WI) and downstream (WII) W box units of CaDWE, respectively; CaDWE‐DM contains double mutations in the W box of CaDWE. The upper shift was efficiently competed by 100‐fold excess of unlabelled wild‐type probe CaDWE‐WT. Competition of CaDWE‐M1 or CaDWE‐M2 was unable to fully produce the shift; the negative competitor GCC‐box exhibited no competitiveness. As expected, EMSAs with labelled CaDWE‐M1 and CaDWE‐M2 both produced a weaker shift compared to the wild‐type CaDWE, and EMSA with labelled CaDWE‐DM failed to produce any shift (Figure 3d). These data show that CaWRKY40 can directly bind to both the W boxes of CaDWE.
To investigate the significance of DWECaWRKY40 in response to RSI and HS, and whether the DWE can function independently of its flanking regions, CaDWE‐WT, CaDWE‐M1, CaDWE‐M2, or CaDWE‐DM were agroinfiltrated into leaves of 4–5‐week‐old pepper plants prior to challenge with RSI and HS. Both treatments induced high levels of DWECaWRKY40‐mediated GUS expression (Figure 3e), while compared to this, GUS expression driven by mutated WI or WII was significantly reduced. The WII mutation reduced GUS expression in response to RSI and HS to a lesser extent than the WI mutation (Figure 3e). Thus, WI contributes more than WII in pepper's responses to RSI and HS mediated by the DWE element.
We further tested the effect of DWE mutation on the activities of the 1,802 bp pCaWRKY40 promoter in response to RSI and HS by transient expression in pepper leaves. The results revealed that GUS activities driven by the wild‐type pCaWRKY40 promoter (pCaWRKY40::GUS) were significantly induced in response to RSI and HS. However, GUS activities driven by the pCaWRKY40 promoter with mutations in both W box units of DWE (pCaWRKY40‐DWE‐DM::GUS) did not exhibit any inducibility by the stimuli above (Figure S5).
2.6. DWE‐like elements are conserved in CaWRKY40 orthologs from other eudicots
We searched the promoters of CaWRKY40 orthologs in other plant species and identified DWE‐like sequences in the promoters of AtWRKY40 (from Arabidopsis thaliana; Xu et al., 2006), GmWRKY40 (from Glycine max; Cui et al., 2019), VvWRKY40 (from Vitis vinifera; Wang et al., 2014), and CplWRKY40 (from Carica papaya; Pan and Jiang, 2014) (Figure 4a), as well as AtWRKY18 and AtWRKY60 (Chen et al., 2010), the two Arabidopsis WRKY genes structurally most closely related to AtWRKY40 (Chen et al., 2010; Liu et al., 2012; Figure S6), and WRKY40 candidate orthologs in the Solanaceae family (Figure S6), including Sl (U566777), Sl (U566776), Sl (Scfld179), three of five SlWRKY40 candidate orthologs from Solanum lycopersicum, SaWRKY40 from Solanum arcanum (KU674832.1), SpWRKY40 from Solanum pennellii (XM_015224511.2), NtWRKY40 from Nicotiana tabacum (NM_001325650.1), and NaWRKY40 from Nicotiana attenuata (XM_016651389.1). These DWE‐like sequences consist of a conserved arrangement of two directly tandemly repeated W box hexamers, including an invariant TGAC core motif. Besides CaWRKY40, similar W box arrangements are also present in the promoter region of other pepper WRKY genes (Table S1; Diao et al., 2016). The orthologs vary in the length of their spacings between the W box hexamers, while they all have a spacing of 0–2 bp. Promoter fragments of CaWRKY40 paralogs of approximately 50‐bp length harbouring the conserved DWE in their centre were fused to a cassette containing the CaMV35S core promoter linked to GUS and tested by Agrobacterium‐mediated transient expression in pepper leaves. In tissue cotransformed with the respective reporter constructs and either CaWRKY40 (Figure 4b) or AtWRKY40 (Figure 4c) effector constructs (35S::CaWRKY40‐HA or 35S::AtWRKY40‐HA), all wild‐type DWE versions mediated elevated GUS expression compared to cotransformation with the empty expression vector (Mock). DWE versions with combined block mutations in both W box units did not mediate enhanced GUS activity in the presence of either CaWRKY40 or AtWRKY40 expression.
FIGURE 4.
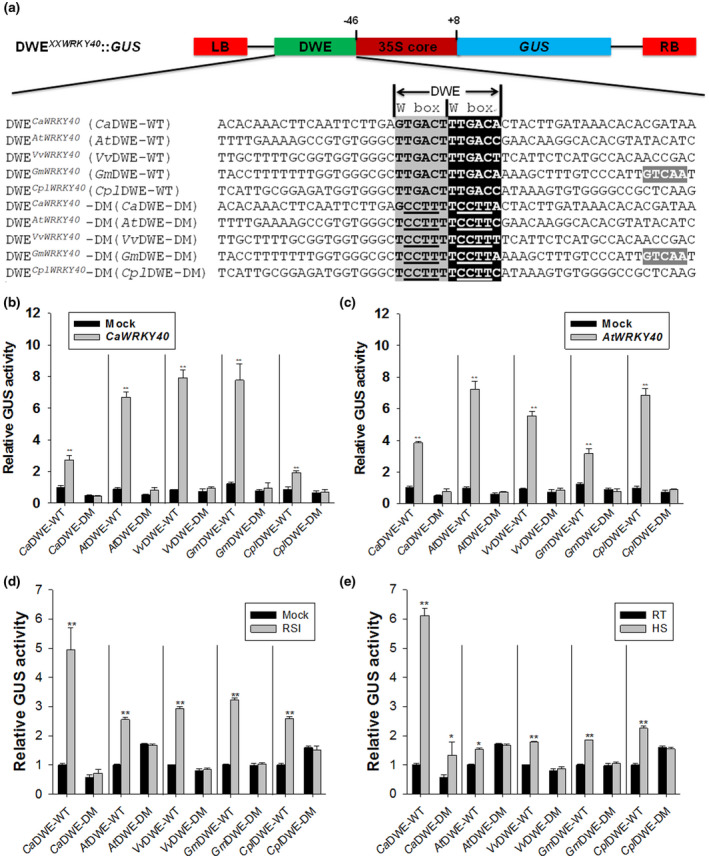
Analysis of CaWRKY40 ortholog promoter in response to Ralstonia solanacearum inoculation (RSI) and heat shock (HS) in other plant species. (a) Sequences analysis of DWECaWRKY40, DWEAtWRKY40, DWEVvWRKY40, DWEGmWRKY40, DWECplWRKY40, and their mutated versions. Underlined nucleotides indicate the mutated sequences of W box motifs. The accession numbers of CaWRKY40 orthologs were as follow: Arabidopsis AtWRKY40 (At1g80840; Xu et al., 2006), grape VvWRKY40 (Wang et al., 2014), soybean GmWRKY40 (ABC26914; Cui et al., 2019), and papaya CplWRKY40 (Pan and Jiang, 2014). (b)–(e) β‐Glucuronidase (GUS) activity driven by DWECaWRKY40 (CaDWE), DWEAtWRKY40 (AtDWE), DWEVvWRKY40 (VvDWE), DWEGmWRKY40 (GmDWE), DWECplWRKY40 (CplDWE), and their mutated versions in response to CaWRKY40 expression (b), AtWRKY40 expression (c), RSI (d), and HS (e). Agrobacterium tumefaciens GV3101 cells harbouring the CaDWE‐WT, AtDWE‐WT, VvDWE‐WT, GmDWE‐WT, CplDWE‐WT, and their double‐W box‐element (DWE) mutated version were vacuum‐infiltrated into the leaves of pepper plants. The infiltrated pepper leaves were subjected to CaWRKY40 overexpression, AtWRKY40 overexpression, RSI, and HS treatments, respectively, and then kept in the greenhouse. At 48 hr (CaWRKY40 and AtWRKY40 overexpression) or 24 hr (RSI and HS treatments) posttreatments, the treated and agroinfiltrated pepper leaves were harvested for GUS activity measurement. The GUS activity driven by CaDWE‐WT without treatment was set to 1. Data in (b)–(e) represent the mean ± SD of three independent biological experiments. Error bars indicate SD (n = 3) from three independent experiments. Asterisks indicate significant differences determined by Student's t test (*p < .05, **p < .01)
Similarly, all wild‐type DWE versions, but not the mutated DWE versions, mediated enhanced GUS expression after RSI treatment (Figure 4d). We observed a similar trend with HS treatment (Figure 4e).
These results suggest that DWE‐like promoter elements are probably conserved within the clade of eudicots and that they share common functional features, such as the ability to interact with WRKY TFs and responsiveness to defence‐ and HS‐related stimuli.
2.7. DWE is necessary for the inducible expression of CaWRKY40 and its role as a positive regulator in pepper's response to R. solanacearum attack and HS
The result that DWE confers the inducible expression of CaWRKY40 against RSI or HS by binding to CaWRKY40 itself suggests that this element is probably necessary for the function of CaWRKY40 during pepper's response to RSI and HS. To test this, the DWE was fused upstream of the CaMV35S core promoter to generate a chimeric promoter, which was further inserted upstream of CaWRKY40‐GUS to generate the fusion construct (CaDWE::CaWRKY40‐GUS), and its mutated version was also constructed (CaDWE‐DM::CaWRKY40‐GUS; Figure 5a). CaDWE::CaWRKY40‐GUS or its mutated version was transformed by agroinfiltration into pepper leaves. At 48 hr after agroinfiltration, the infiltrated pepper leaves were inoculated with R. solanacearum or treated with HS. The results of quantitative reverse transcription PCR (RT‐qPCR) showed that both RSI and HS enhanced the transcript expression of CaWRKY40‐GUS driven by DWECaWRKY40, but not driven by CaDWE‐DM CaWRKY40 (Figure 5b). The result of the GUS activity assay showed that the biosynthesis of the CaWRKY40‐GUS fusion protein was significantly induced by RSI or HS in a DWECaWRKY40‐dependent manner, while DWE mutation did not induce the biosynthesis of the CaWRKY40‐GUS fusion protein (Figure 5c). We next examined H2O2 accumulation by 3,3'‐diaminobenzidine (DAB) staining and hypersensitive response (HR)‐like cell death by trypan blue staining in CaDWE::CaWRKY40‐GUS infiltrated pepper leaves inoculated with R. solanacearum (Figure 5d,e). In contrast to the CaDWE‐DM mutated version, CaDWE drove the transcriptional expression of CaWRKY40 and thereby enhanced its protein production in pepper leaves challenged with R. solanacearum. Compared with the control plants, CaDWE::CaWRKY40‐GUS‐transformed pepper leaves infected by R. solanacearum exhibited clearly increased intensities of both DAB and trypan blue staining at 24 hr postinoculation (hpi). To further confirm the role of CaDWE in disease resistance and elucidate its possible mode of action, transcriptional responses of immunity‐ and thermotolerance‐related genes in CaDWE::CaWRKY40‐GUS transformed pepper leaves were investigated by RT‐qPCR (Figure S7a). The result showed that transcript levels of the SA‐signalling dependent NPR1 and PR1, JA‐responsive DEF1, and heat shock‐responsive HSP24 genes were significantly enhanced by CaDWE::CaWRKY40‐GUS transformation but not by that of CaDWE‐DM::CaWRKY40‐GUS. Interestingly, HS can also trigger the transcript levels of the tested defence‐related marker genes in pepper leaves transformed with DWECaWRKY40::CaWRKY40‐GUS (Figure S7b). These results indicate that DWE is probably important for the inducible expression of CaWRKY40 and a crucial cis‐element in pepper's responses to R. solanacearum infection and heat shock stress.
FIGURE 5.
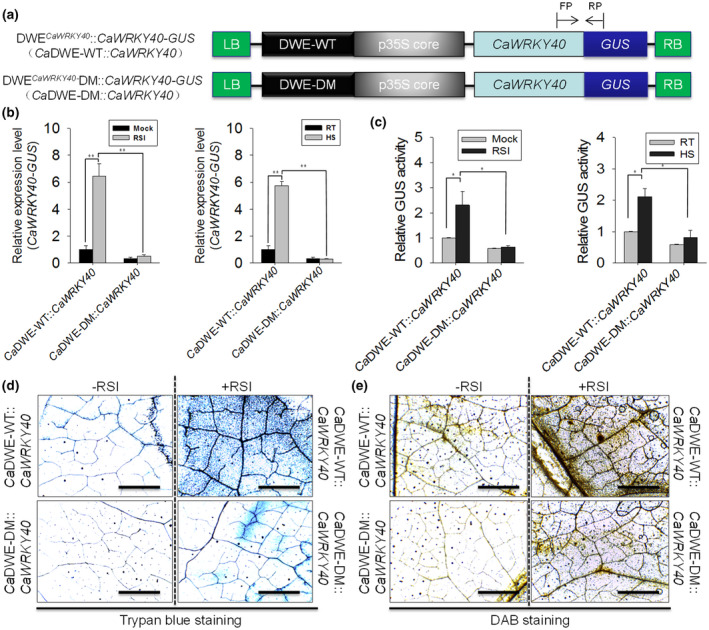
DWE is essential for the pathogen‐induced expression and functional role of CaWRKY40 in response to Ralstonia solanacearum inoculation. (a) Schematic representation of the construct of synthetic promoter of DWECaWRKY40 fused to the open reading frame of CaWRKY40 and β‐glucuronidase (GUS) (CaDWE::CaWRKY40) and its mutated construct (CaDWE‐DM:: CaWRKY40). FP and RP indicate the primer pair used to amplify the specific fragment of CaWRKY40‐GUS fusion. (b) Transcript levels of CaWRKY40‐GUS fusion detected in pepper leaves transiently expressing CaDWE::CaWRKY40 (or CaDWE‐DM::CaWRKY40) in response to Ralstonia solanacearum inoculation (RSI) and heat shock (HS), respectively. The transcript level of CaWRKY40‐GUS fusion in pepper leaves without RSI or HS treatment was set to 1. (c) GUS activity measurement of pepper leaves transiently overexpressing CaDWE::CaWRKY40 (or CaDWE‐DM::CaWRKY40) in response to RSI and HS, respectively. The GUS activity of pepper leaves transiently overexpressing CaDWE::CaWRKY40 without RSI or HS treatment was set to 1. (d)–(e) Trypan blue (d) and 3,3'‐diaminobenzidine (DAB) (e) staining were performed to detect the hypersensitive response‐like cell death and H2O2 accumulation, respectively, in CaDWE::CaWRKY40 transformed pepper leaves challenged with RSI. Bars = 500 μm. CaDWE::CaWRKY40 (or CaDWE‐DM::CaWRKY40) were transformed in pepper leaves and were immediately treated with RSI and HS, respectively. The treated pepper leaves were harvested at 24 hr posttreatment for RNA extraction, GUS activity measurement, trypan blue staining, or DAB staining. Data in (b) and (c) represent the mean ± SD of three independent biological experiments. Error bars represent ± SD (n = 3) from three independent experiments and asterisks indicate significant differences, as determined by Fisher's protected least significant difference test (*p < .05, **p < .01)
3. DISCUSSION
Pepper, an important solanaceous vegetable worldwide, is frequently affected by pathogen infection, which causes heavy losses of its production, especially under conditions of high temperature. CaWRKY40 is a crucial regulator of pepper thermotolerance and resistance to R. solanacearum infection. Investigation of the transcriptional regulation of CaWRKY40 might provide new insights into the underlying mechanism of disease resistance under high temperature. Herein, our data provide evidence that pCaWRKY40 −1,802 to −1,464, a core stretch in the CaWRKY40 promoter, is primarily responsible for CaWRKY40 responses to RSI or HS. In addition, we report that a conserved DWE‐like element in CaWRKY40 orthologs from different plant species responds to RSI or HS.
A set of potential motifs in pCaWRKY40 was identified, including the W box previously confirmed to be involved in thermotolerance and plant immunity by binding to WRKY TFs (Eulgem, 2006; Eulgem et al., 2000; Eulgem and Somssich, 2007; Pandey and Somssich, 2009; Rushton et al., 2010; Shang et al., 2010) or heat shock factors (HSFs) (Bechtold et al., 2013; Pajerowska‐Mukhtar et al., 2012; Rana et al., 2012). We found GUS expression driven by pCaWRKY40 to be enhanced by RSI or HS. These results are consistent with our previous study, which showed that CaWRKY40 acts as a crucial regulator of pepper's coordinated response to R. solanacearum inoculation and HS (Dang et al., 2013). The present study indicates that pCaWRKY40 −1,802 to −1,464, a 339‐bp CaWRKY40 promoter region, was primarily responsible for the CaWRKY40’s response to RSI and HS independent of its flanking sequence. Similar minimal or core regions have been identified in promoters of LURP1 (Knoth and Eulgem, 2008), RFP1 (Yu et al., 2013), and OsPIANK1 (Mou et al., 2013).
Due to the indispensable roles of WRKY networks in plant immunity and abiotic stress, our study focused on the two directly repeated W box‐related motifs (DWE) in the pCaWRKY40 −1,802 to −1,464. Mutation of both W box motifs significantly blocked pCaWRKY40 responses to RSI and HS, suggesting that DWE is involved in pepper's responses to RSI and HS. A spectrum of target genes is transcriptionally regulated by various WRKY TFs via indiscriminate binding to the typical W box within their promoters (Hussain et al., 2018). Similarly, transient overexpression of two positive regulators, CaWRKY27 and CaWRKY40, involved in plant immunity and/or thermotolerance (Dang et al., 2014; Wang et al., 2013), enhanced pCaWRKY40‐driven GUS expression in pepper. It is worth pointing out that transient overexpression of CaWRKY58, a negative regulator of plant immunity, also significantly triggered CaWRKY40 expression. This finding is similar to the observation that the transcripts of HSR203, a negative regulator of hypersensitive death (Tronchet et al., 2001), was highly activated in tobacco in the line overexpressing AtMYB30, a positive regulator of hypersensitive cell death, during the incompatible interaction with pathogens (Vailleau et al., 2002). We speculate that CaWRKY58 is never overexpressed in vivo to avoid inappropriate activation of defence responses against RSI.
Numerous studies have demonstrated sequence‐specific binding of WRKY TFs to W box, which is also influenced by the flanking sequence (Agarwal et al., 2011). Similarly, the two directly repeated W box‐related motifs of DWE, WI and WII, also exhibit differential binding affinity to CaWRKY40 in response to RSI and HS, confirmed by transient coexpression assay, MST, and EMSA. It was also found that the mutation of WI significantly attenuated the response of DWECaWRKY40 to transient overexpression of CaWRKY40 and HS, while dramatically blocking the response of DWECaWRKY40 to RSI. In contrast, mutation of WII only weakly impaired the response of DWE CaWRKY40 to RSI and HS as well as transient overexpression of CaWRKY40. The different binding affinities of WI and WII motifs by CaWRKY40 and the corresponding trans‐activation activity of CaWRKY40 in response to RSI and HS might be due to their specific flanking sequences. It is worth pointing out that the DWE in CaWRKY40 is also present in the promoter of CaWRKY40 orthologs in other plant species, including AtWRKY40 in Arabidopsis, VvWRKY40 in V. vinifera, GmWRKY40 in G. max, CplWRKY40 in C. papaya, SpWRKY40 in S. pennellii, NtWRKY40 in N. tabacum, NaWRKY40 in N. attenuata, as well as AtWRKY18 and AtWRKY60. Conserved expression and function have been found among these orthologs; for example, AtWRKY40, AtWRKY18, and AtWRKY60 respond synergistically to abscisic acid, HS (Chen et al., 2010; Liu et al., 2012), and pathogen attack (Xu et al., 2006). The data in our study indicate that the role of DWE might be conserved in the tested WRKY40s, as all DWEs containing synthetic promoters mediated elevated GUS expression in response to CaWRKY40 or AtWRKY40 transient overexpression, as well as RSI and HS treatments. These results suggest a conserved mechanism across different plant species that mediates responses to pathogen infection and HS due to the W box element arrangement, the enhanced affinity of WRKY40 to DWE, and possibly the DWE flanking sequences. Interestingly, AtWRKY40 is a negative regulator of immunity against Pseudomonas syringae DC3000 and several fungal pathogens such as Golovinomyces orontii. Nevertheless, CaWRKY40 plays a positive role in immunity against R. solanacearum. The dual role of WRKY40 as an activator and repressor in the defence response of different plant species upon challenge by different pathogens might be determined by the different pathogen lifestyles and invasive strategies. For example, P. syringae and G. orontii are foliar pathogens, while R. solanacearum is a root pathogen. The infection of these pathogens might activate different signalling components that differentially modulate the function of WRKY40, because the targeting and transcriptional regulation of a given WRKY can be significantly modulated by other proteins in a protein–protein interaction (Chi et al., 2013).
Our data indicate that DWE in pCaWRKY40 −1,802 to −1,464 might be primarily responsible for CaWRKY40’s responses to both RSI and HS by binding to CaWRKY40 itself and result in positive autoregulation, because mutation of this element significantly blocked the responses of CaWRKY40 to RSI or HS, as well as to transient overexpression of CaWRKY40. This result was further supported by the data that CaWRKY40 transcription driven by DWECaWRKY4 was up‐regulated significantly by RSI or HS and thus enhanced the resistance or tolerance of pepper plants to both RSI and HS. Consistently, DWE‐DMCaWRKY40 significantly blocked the response of CaWRKY40 and thereby resistance/tolerance of pepper plants to RSI or HS.
Collectively, the presented study provides support for the conclusion that responses of pepper to RSI or HS mediated by CaWRKY40 are determined primarily by the DWE within its promoter. The DWE‐mediated response of WRKY40 to RSI or HS is probably a conserved mechanism across plant species.
4. EXPERIMENTAL PROCEDURES
4.1. Plant material
Seeds of pepper inbred line L 68‐2 (C. annuum) and tobacco cultivar K326 (N. tabacum) were provided by the pepper breeding group in Fujian Agriculture and Forestry University (http://www.fafu.edu.cn). Pepper seeds were germinated in stream‐sterilized soil mix (peat moss: perlite [2:1, vol/vol]) in plastic pots, and plants were transferred to larger pots 2 weeks after germination. Seeds of wild‐type and transgenic tobacco were surface sterilized with 75% ethanol for 30 s, 10% H2O2 for 10 min, washed five times with sterile double‐distilled water, sown on Murashige and Skoog (MS) medium with (for transgenic seeds) or without (for wild‐type seeds) 60 mg/L kanamycin, and grown for 2–3 weeks. The surviving individual plants were transferred to soil mix (peat moss: perlite [2:1, vol/vol]) in plastic pots and kept in the greenhouse for an additional 2–3 weeks. Pepper and tobacco plants were grown in a greenhouse with 16hr light/8hr dark, 60% humidity, and day/night temperatures of 25 °C/22 °C.
4.2. Isolation of CaWRKY40 promoter
Purified genomic DNA was isolated from young pepper leaves by the hexadecyl trimethylammonium bromide (CTAB) method. The CaWRKY40 promoter was amplified using the APAgene Genome Walking Kit (Bio S&T; http://www.biost.com/page/default.aspx). Three nested CaWRKY40 antisense gene‐specific primers (GSPs) with 5′ walking direction, named GSPa (5′‐CACTGCTCACTCTATTTAGTTCCTCT‐3′), GSPb (5′‐CTCAAATCCAATGAAGTATC AACCA‐3′), and GSPc (5′‐GCTCTTCTAGTCTTTCTTCAGGTGT‐3′), were designed for promoter isolation. Single‐stranded DNA (ssDNA) fragments were produced by a single primer extension reaction using GSPa. The degenerate random tag (DRT) primer provided by the kit was added to the amplified ssDNA fragments to generate double‐stranded DNA (dsDNA) in the second PCR. Eventually, GSPb and GSPc were used for two rounds of nested PCR. The final amplified product was cloned into the pMD‐18T vector (TaKaRa) and sequenced. The cis‐acting elements in the promoter were analysed online using the PLANTCARE (http://bioinformatics.psb.ugent.be/webtools/ plantcare/html) and PLACE (http://www.dna.affrc.go.jp/PLACE/signalscan.html) databases.
4.3. Construction of reporter vectors containing 5′ deletions or site‐directed mutagenesis of the CaWRKY40 promoter
To elucidate the mechanism regulating CaWRKY40 expression, CaWRKY40 promoter and its 5′ deletion derivatives (−1,802, −1,464, −1,060, −746, −360 to −1; the first nucleotide A in the transcriptional start site was set as +1) were generated by high‐fidelity PCR (TaKaRa) with primers that incorporated the Gateway technique by flanking the attB sequence (Table S1). Site‐directed mutagenesis was performed according to Li and Rice (1989). Primers generated for site‐directed mutagenesis were pCaWRKY40‐DWE‐DM‐F (5′‐AGCCTTTTCCTTACTACTTGAT‐3′) and pCaWRKY40‐DWE‐DM‐R (5′‐ATCAAGTA GTAAGGAAAAGGCT‐3′), where mutagenized nucleotides are identified by bold underlined letters.
4.4. NCBI BLASTP of CaWRKY40 orthologs containing DWE‐like elements
To confirm the orthologs of CaWRKY40 in other plant species containing the DWE‐like motifs, NCBI BLASTP searches against the online database using the protein sequences of CaWRKY40 were performed, and only the proteins with more than 50% sequence identity were collected. The corresponding full‐length cDNA sequences of the selected potential CaWRKY40 orthologs were subjected to the NCBI BLASTN using the whole‐genome shotgun contigs (wgs) database to obtain the upstream promoter sequences. The cis‐acting motifs of the CaWRKY40 orthologs promoters were analysed via the PLANTCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) and only promoters containing the DWE‐like motif (TGACTTTGAC) were selected for further analysis.
4.5. Histochemical and fluorometric assays for GUS activity
Histochemical assays for GUS activity were performed according to the method of Jefferson et al. (1987). The GUS staining solution contained 2 mM X‐Gluc (200 mM phosphate buffer, pH 7.0, containing 100 mM K3[Fe(CN)6], 100 mM K4[Fe(CN)6], 1M EDTA‐Na2, and 0.1% 5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐glucuronide) in 10 mM phosphate buffer salt at pH 7.4. The X‐Gluc solution was preheated to 37 °C before use.
To quantitatively measure GUS activity, protein extraction buffer (10% glycerol, 25 mM Tris‐HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X‐100, 10 mM DTT, 1 × complete protease inhibitor cocktail [Sigma‐Aldrich], and 2% [wt/vol] polyvinylpolypyrrolidone [Choi et al., 2012]) was used to extract total proteins of pepper or tobacco leaves transiently expressing the reporter vectors. To measure GUS activity, the rate of p‐nitrophenol (λ = 415 nm) release was quantified using a microplate reader (Biotek).
4.6. Pathogen inoculation and HS
R. solanacearum strain FJC100301 was isolated previously in our laboratory from wilted pepper plants grown in Fujian province (Dang et al., 2013). FJC100301 cells were cultivated in potato sucrose agar (PSA; 200 g potato starch, 20 g sucrose, 3 g beef extract, 5 g tryptone, and adjusted to 1 L with water) overnight, and cultures were adjusted to 108 cfu/ml with 10 mM MgCl2 before inoculation. For inoculation, 5 μl of FJC100301 suspension was injected into tobacco or pepper leaves expressing the reporter vectors and control leaves expressing empty vectors using a syringe with a needle. Inoculated leaves were harvested for GUS staining or GUS activity assays at different time points after inoculation.
For HS treatment, tobacco and pepper plants expressing reporter vectors were placed in the growth chamber at 42 °C and 100% humidity, and control plants were kept at 25 °C and 100% humidity. Plants were harvested for GUS staining or GUS activity assay at appropriate time points after the treatment.
4.7. Agrobacterium‐mediated transient expression assay
Expression analysis of CaWRKY40 promoter was performed in pepper plants using Agrobacterium‐mediated transient expression (Hong and Hwang, 2009). A. tumefaciens GV3101 cells harbouring reporter vectors were cultured in Luria‐Bertani (LB) broth at 28 °C overnight, harvested by centrifugation for 10 min at 6,000 × g, suspended in infiltration media (10 mM MgCl2, 10 mM MES, 200 μM acetosyringone, pH 5.4), and adjusted to OD600 = 0.8. Bacterial suspensions harbouring different reporter vectors were infiltrated into pepper leaves with a syringe without a needle. After infiltration, plants were maintained in an illuminated incubator at 25 °C for 48 hr, followed by various treatments for GUS activity analysis.
4.8. Transient coexpression experiments using onion epidermal peels
The CaWRKY27‐HA, CaWRKY40‐HA, and CaWRKY58‐HA constructs generated previously in our laboratory (Dang et al., 2013, 2014; Wang et al., 2013) were used as effector vectors in this study. The vector pCaWRKY40::GUS constructed in this study was used as reporter vector. Effector vectors and reporter vector were cotransformed by particle bombardment into the inner epidermal peels of onion bulbs. After bombardment, the inner epidermal peels were incubated on MS medium for 12 hr at 25 °C in the dark, followed by histochemical GUS assays. The peels were placed on glass slides and examined with a Nikon microscope.
4.9. Recombinant expression of CaWRKY40 in E. coli
When the OD600 of E. coli BL21 containing the recombinant plasmid CaWRKY40‐17 reached 0.6, 1 mM isopropyl‐β‐d‐thiogalactopyranoside (IPTG) was added to the culture and growth continued at 37 °C for 3 hr. Induced cultures were centrifuged and the pelleted cells were suspended in 0.01 M phosphate‐buffered saline (PBS, pH 7.0). DNase I was added to the suspension and incubated on ice for 30 min to digest the DNA. The DNase I‐treated suspensions were centrifuged and the pelleted cells were suspended in the same volume of PBS. The supernatant and suspended pellets were both subjected to sodium dodecyl sulphate‐polyacrylamide gel elecrophoresis (SDS‐PAGE) to confirm the presence of CaWRKY40‐6×His fusion protein. HisPur Ni‐NTA super‐flow agarose (General Electric Company) was used to purify the suspension cells and the purified protein was eluted with 10 mM reduced glutathione in 50 mM Tris‐HCl (pH 8.0). Then 50 mM Tris‐HCl (pH 8.0) was used to dialyse the eluted protein to remove the glutathione. SDS‐PAGE was used for analysing the purified fusion protein and empty vector (pDEST17) was used as a control.
4.10. MST analysis
The Monolith NT.115 system (Nano Temper Technologies) was used to performed MST experiments using 100% LED and 20% infra‐red‐laser power. The purified CaWRKY40‐6×His fusion protein was labelled using the monolith NT protein labelling kit RED‐NHS (Nano Temper Technologies), which reacts with the primary amines of targeted protein to form stable dye–protein conjugates efficiently. The labelling procedure was performed according to the manual. The oligonucleotides of DWECaWRKY40 and its mutants were synthesized by the company and dissolved in water (100 μM). The samples were filled into Premium capillaries. Measurements were carried out in 20 mM HEPES pH 7.5, 200 mM KCl, 10 mM TCEP, 0.05% Tween20. Data were analysed during acquisition (on‐the‐fly data analysis) with the provided NT‐Analysis software.
4.11. Electrophoretic mobility shift assays
The purified CaWRKY40‐6×His fusion protein and the oligonucleotides described in Figure S4 and 3a were used for EMSAs. Double‐stranded synthetic oligonucleotides were labelled with Cy5. The Cy5‐labelled probe (500 nM) was incubated with 0.5 μg recombinant protein for 30 min at room temperature. For competition assays, 100‐fold excess of unlabelled dsDNA was added to the binding reaction. The EMSA reactions were subjected to electrophoresis on 6% polyacrylamide gels in 0.5×Tris borate EDTA (TBE) buffer at room temperature. Electrophoresis was performed on a cooled support (4 °C) at 120 V for 60 min. The fluorescence measurement of the polyacrylamide gel was detected on a LICOR Odyssey CLx system at 635 nm for excitation and 700 nm for emission. The images of the shifts were inverted to black‐and‐white.
4.12. Western blotting and chromatin immunoprecipitation assay
To test the expression level of fused protein, A. tumefaciens GV3101 cells harbouring CaWRKY40‐HA, CaWRKY27‐HA, or CaWRKY58‐HA were coinfiltrated with pCaWRKY40::GUS into pepper leaves. Infiltrated plants were grown in the greenhouse for another 2 days. The total protein of the infiltrated leaves was extracted using protein extraction buffer (10% glycerol, 25 mM Tris‐HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 2% Triton X‐100, 10 mM DTT, 1 × complete protease inhibitor cocktail [Sigma‐Aldrich], and 2% [wt/vol] polyvinylpolypyrrolidone [Choi et al., 2012]). The proteins were analysed by immunoblotting using an anti‐HA‐peroxidase antibody (Abcam).
4.13. Total RNA isolation and real‐time PCR analysis
First, 500 ng of the extracted RNA from each sample was used for the generation of the first cDNA strand using a One Step PrimeScript cDNA Synthesis Kit (TaKaRa). The products were diluted 10‐fold before analysis. The diluted cDNAs were used for further quantitative PCR, performed with SYBR‐Green PCR Mastermix (TaKaRa). The Cq (threshold cycle), defined as the PCR cycle at which a statistically significant increase of reporter fluorescence was first detected, was used as a measure for the starting copy numbers of the target gene. CaActin (GQ339766) and 18S ribosomal RNA (EF564281), which have been confirmed previously (Choi et al., 2012; Kim et al., 2014), were used to normalize the transcript levels in pepper. All gene‐specific primers are listed in Table S2.
Supporting information
FIGURE S1 Nucleotide sequences of 5′‐flanking regions of the CaWRKY40 promoter and distributions of potential cis‐acting elements. Nucleotide sequences highlighted with yellow indicate DWE motif. Nucleotide sequences in red represent the position of the transcriptional start site. ARFAT and ARF (auxin response factor), binding sites identified in the promoters of primary/early auxin response genes of Arabidopsis thaliana; WBOXNTCHN48, a W box identified in the region between –125 and –69 bp of the tobacco Class I basic chitinase gene CHN48; DPBFCOREDCDC3, a bZIP transcription factor DPBF‐1 and 2 (Dc3 promoter‐binding factor‐1 and 2) binding core sequence; ABRELATERD1, an ABRE‐like sequence (from –199 to –195) required for etiolation‐induced expression of erd1 (early responsive to dehydration) in Arabidopsis; ARE, a cis‐acting regulatory element essential for anaerobic induction; ARR1AT (ARR1), a response regulator; ATGCAAAT motif, a cis‐acting regulatory element associated with the TGAGTCA motif; C‐repeat/DRE, a regulatory element involved in cold and dehydration responses; TCA‐element, a cis‐acting element involved in salicylic acid responses; WUN‐motif, a wound‐responsive element; CGTCA‐motif, a cis‐acting regulatory element involved in MeJA responses; CuRE, the GTAC core of a CuRE (copper‐response element) found in Cyc6 and Cpx1 genes in Chlamydomonas; W box, a WRKY transcription factor binding site; HSE, a cis‐acting element involved in HS responses; MBS, a MYB binding site involved in drought inducibility; MBSII, a MYB binding site involved in flavonoid biosynthetic gene regulation; MYB, a MYB binding site; MYCCONSENSUSAT, a MYC recognition site found in the promoters of the dehydration‐responsive gene rd22 and many other genes in Arabidopsis; GA‐box, part of an auxin‐responsive element; TC‐rich repeats, cis‐acting elements involved in defense and stress responses; RAV1AT, a binding consensus sequence of the Arabidopsis transcription factor RAV1; WBOXHVISO1 and SUSIBA2 bind to the W box element in the barley iso1 (isoamylase1) promoter; WRKY710S, a core of the TGAC‐containing W box
FIGURE S2 Transactivation of CaWRKY40 promoter by overexprssion of CaWRKY40, CaWRKY58, and CaWRKY27 in onion cell by patical bombardment. Effector vector of CaWRKY40, CaWRKY58, or CaWRKY27, respectively, were co‐transformed with the reporter construct of pCaWRKY40::GUS into onion epidermal cells by particle bombardment followed by histochemical GUS staining for GUS expression detection. Onion cells co‐transformed with the effector vector (CaWRKY40‐HA, CaWRKY58‐HA, or CaWRKY27‐HA) and reporter vector exhibited obvious dark blue, while cells co‐transformed with empty effector vector and reporter vector were never stained blue
FIGURE S3 Effects of R. solanacearum infection and heat shock on the transient expression of the CaWRKY40 promoter deletion in pepper leaves. Activity of the 5ʹ deletion of the CaWRKY40 promoter was examined in pepper plants 24 hr after treatment with RSI and HS. The samples were harvested 24 hr postpathogen and heat shock treatments for GUS activity measurement. The GUS activity of transient expressed pepper leaves without treatment was set to 1. All the data represent the mean ± SD of three independent biological experiments. Error bars indicate SD (n = 3) from three independent experiments. Asterisks indicate significant differences determined by Student’s t test (*p < .05)
FIGURE S4 Confirmation of CaWRKY40 expression in E. coli strain BL21 and MST traces (on the left) and the capillary shape (on the right) of the MST experiment. (A) SDS‐PAGE analysis to confirm CaWRKY40 expression in E. coli. The gel was stained with Coomassie Brilliant Blue and scanned. M, protein ladder marker; 1, sample with IPTG induction; 2, sample without IPTG induction; 3, empty E. coli strain BL21; 4, purified CaWRKY40 protein. (B) and (C) MST traces (on the left) and capillary shape (on the right) of the MST experiment
FIGURE S5 Mutation analyses of DWE in pCaWRKY40 ‐1802 to ‐1464 and the responses of pCaWRKY40 to HS and RSI. (A) Diagrammatic representation of DWE in pCaWRKY40 ‐1802 to ‐1464 (pCaWRKY40) and its mutated version (pCaWRKY40‐DWE‐DM); GUS, GUS report gene. The number above the green box indicates the location of DWE in the promoter of CaWRKY40. (B) Mutation analyses of CaWRKY40‐1802 to ‐1464 DWE and the response of pCaWRKY40 to HS at 6 and 12 hr. GV3101 cells containing the pCaWRKY40 or pCaWRKY40‐DWE‐DM were infiltrated into the leaves of pepper plants. The agro‐infiltrated leaves were subjected to heat shock. At 24 and 48 hours post treatment (hpt) posttreatment, the Agro‐infiltrated leaves were harvested for GUS activity measurement. The GUS activity of pCaWRKY40‐infiltrated pepper without HS treatment was set to 1. (C) Mutation analyses of pCaWRKY40 ‐1802 to ‐1464 DWE and the response of pCaWRKY40 to RSI at 24 and 48 hr after infection. Agrobacterium cells harbouring the pCaWRKY40 or pCaWRKY40‐DWE‐DM were vacuum‐infiltrated into the pepper leaves using a needleless syringe, followed by inoculation withR. solanacearum. The pathogen‐inoculated pepper leaves were collected for further GUS activity measurement. The GUS activity of pCaWRKY40‐infiltrated pepper without RSI was set to 1. Data in (B) and (C) represent the mean ± SD of four independent experiments. Error bars indicate SD. Asterisks indicate significant differences determined by Fisher’s protected least significant difference test (*p < .05)
FIGURE S6 Sequences analysis of DWEs in the promoters of CaWRKY40 orthologs in other plant species. Highlighted are nucleotides matching the W box consensus (T)(T)TGACC/T. AtWRKY40, Arabidopsis thaliana WRKY transcription factor WRKY40 ((AT1G80840)); CplWRKY40, WRKY40 from Carica papaya L. (http://www.plantgdb.org/CpGDB/); GmWRKY40, WRKY40 from Glycine max (ABC26914); VvWRKY40, WRKY40 from Vitis vinifera (GI:225443178 in database http://www.genoscope.cns.fr/); Sl(U566777, U566776, Scfld179), three of five SlWRKY40 candidate orthologs from Solanum lycopersicum; SaWRKY40, Solanum arcanum WRKY40; SpWRKY40, Solanum pennellii probable WRKY transcription factor 40; NtWRKY40, Nicotiana tabacum probable WRKY transcription factor 40 (LOC107798622); NaWRKY40, Nicotiana attenuata probable WRKY transcription factor 40 (LOC109244974); SiWRKY40, Sesamum indicum probable WRKY transcription factor 40 (LOC105165167); CsWRKY40, Camellia sinensis WRKY transcription factor 40 (JQ820201)
FIGURE S7 Real‐time RT‐PCR analysis of the defense‐associated marker genes in pepper leaves transiently expressing CaDWE::CaWRKY40 in response to R. solanacearum inoculation and heat shock. Real‐time RT‐PCR was performed to detect the transcript levels of immunity and heat shock‐associated marker genes in pepper leaves transiently expressing CaDWE::CaWRKY40 (or CaDWE‐DM::CaWRKY40) in response to RSI and HS. Pepper leaves were transformed with CaDWE::CaWRKY40 (or CaDWE‐DM:: CaWRKY40) and subjected to immediate RSI and HS treatments, respectively. The transcript levels of marker genes in the mock pepper leaves (without RSI or HS treatment) were set to 1. Data represent the mean ± SD of three independent biological experiments. Error bars represent ±SD (n = 3) from three independent experiments and asterisks indicate significant differences, as determined by Fisher’s protected least significant difference test (*p < .05)
TABLE S1 Double W box motifs in the promoter region of pepper WRKYs
TABLE S2 Primers used in this study
ACKNOWLEDGEMENTS
We thank Eulgem Thomas (University of California at Riverside, CA 92521, USA) for experimental suggestions and language improvement. This work was supported by the Excellent Youth Foundation of Fujian Agriculture and Forestry University (xjq201913), the National Natural Science Foundation of China (31501767, 30971718, and 31372061), and the State Key Laboratory of Ecological Pest Control for Fujian and Taiwan Crops (SKB2017003). The funders had no role in study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
Liu Z‐Q, Shi L‐P, Yang S, et al. A conserved double‐W box in the promoter of CaWRKY40 mediates autoregulation during response to pathogen attack and heat stress in pepper. Mol. Plant Pathol. 2021;22:3–18. 10.1111/mpp.13004
Zhi‐Qin Liu and Lan‐Ping Shi contributed equally to this work.
Contributor Information
Zong‐Hua Wang, Email: Zonghuaw@163.com.
Shui‐Lin He, Email: shlhe201304@aliyun.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abbruscato, P. , Nepusz, T. , Mizzi, L. , Del Corvo, M. , Morandini, P. , Fumasoni, I. et al. (2012) OsWRKY22, a monocot WRKY gene, plays a role in the resistance response to blast. Molecular Plant Pathology, 13, 828–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal, P. , Reddy, M.P. and Chikara, J. (2011) WRKY: its structure, evolutionary relationship, DNA‐binding selectivity, role in stress tolerance and development of plants. Molecular Biology Reports, 38, 3883–3896. [DOI] [PubMed] [Google Scholar]
- Bechtold, U. , Albihlal, W.S. , Lawson, T. , Fryer, M.J. , Sparrow, P.A. , Richard, F. et al. (2013) Arabidopsis HEAT SHOCK TRANSCRIPTION FACTORA1b overexpression enhances water productivity, resistance to drought, and infection. Journal of Experimental Botany, 64, 3467–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, H. , Yang, S. , Yan, Y. , Xiao, Z. , Cheng, J. , Wu, J. et al. (2015) CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high‐temperature and high‐humidity tolerance in pepper. Journal of Experimental Botany, 66, 3163–3174. [DOI] [PubMed] [Google Scholar]
- Chen, H. , Lai, Z. , Shi, J. , Xiao, Y. , Chen, Z. and Xu, X. (2010) Roles of Arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biology, 10, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K. , Du, L. and Chen, Z. (2003) Sensitization of defense responses and activation of programmed cell death by a pathogen‐induced receptor‐like protein kinase in Arabidopsis. Plant Molecular Biology, 53, 61–74. [DOI] [PubMed] [Google Scholar]
- Cheng, Y. , Zhou, Y. , Yang, Y. , Chi, Y.J. , Zhou, J. , Chen, J.Y. et al. (2012) Structural and functional analysis of VQ motif‐containing proteins in Arabidopsis as interacting proteins of WRKY transcription factors. Plant Physiology, 159, 810–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, D.S. , Hwang, I.S. and Hwang, B.K. (2012) Requirement of the cytosolic interaction between PATHOGENESIS‐RELATED PROTEIN10 and LEUCINE‐RICH REPEAT PROTEIN1 for cell death and defense signaling in pepper. The Plant Cell, 24, 1675–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack, R.S. , Eulgem, T. , Rushton, P.J. , Kochner, P. , Hahlbrock, K. and Somssich, I.E. (2002) Leucine zipper‐containing WRKY proteins widen the spectrum of immediate early elicitor‐induced WRKY transcription factors in parsley. Biochimica et Biophysica Acta, 1576, 92–100. [DOI] [PubMed] [Google Scholar]
- Cui, X. , Yan, Q. , Gan, S. , Xue, D. , Wang, H. , Xing, H. et al. (2019) GmWRKY40, a member of the WRKY transcription factor genes identified from Glycine max L., enhanced the resistance to Phytophthora sojae . BMC Plant Biology, 19, 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, Y. , Yang, Y. , Zhou, Y. , Zhou, J. , Fan, B. , Yu, J.Q. et al. (2013) Protein–protein interactions in the regulation of WRKY transcription factors. Molecular Plant, 6, 287–300. [DOI] [PubMed] [Google Scholar]
- Dang, F. , Wang, Y. , She, J. , Lei, Y. , Liu, Z. , Eulgem, T. et al. (2014) Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection. Physiologia Plantarum, 150, 397–411. [DOI] [PubMed] [Google Scholar]
- Dang, F.F. , Wang, Y.N. , Yu, L. , Eulgem, T. , Lai, Y. , Liu, Z.Q. et al. (2013) CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant, Cell and Environment, 36, 757–774. [DOI] [PubMed] [Google Scholar]
- Diao, W.P. , Snyder, J.C. , Wang, S.B. , Liu, J.B. , Pan, B.G. , Guo, G.J. et al. (2016) Genome‐wide identification and expression analysis of WRKY gene family in Capsicum annuum L. Frontiers in Plant Science, 7, 211. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ding, Z.J. , Yan, J.Y. , Xu, X.Y. , Yu, D.Q. , Li, G.X. , Zhang, S.Q. et al. (2014) Transcription factor WRKY46 regulates osmotic stress responses and stomatal movement independently in Arabidopsis. The Plant Journal, 79, 13–27. [DOI] [PubMed] [Google Scholar]
- Eulgem, T. (2006) Dissecting the WRKY web of plant defense regulators. PLoS Pathogens, 2, e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem, T. , Rushton, P.J. , Robatzek, S. and Somssich, I.E. (2000) The WRKY superfamily of plant transcription factors. Trends in Plant Science, 5, 199–206. [DOI] [PubMed] [Google Scholar]
- Eulgem, T. , Rushton, P.J. , Schmelzer, E. , Hahlbrock, K. and Somssich, I.E. (1999) Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO Journal, 18, 4689–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem, T. and Somssich, I.E. (2007) Networks of WRKY transcription factors in defense signaling. Current Opinion in Plant Biology, 10, 366–371. [DOI] [PubMed] [Google Scholar]
- Hong, J.K. and Hwang, B.K. (2009) The promoter of the pepper pathogen‐induced membrane protein gene CaPIMP1 mediates environmental stress responses in plants. Planta, 229, 249–259. [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Chen, L. , Wang, H. , Zhang, L. , Wang, F. and Yu, D. (2013) Arabidopsis transcription factor WRKY8 functions antagonistically with its interacting partner VQ9 to modulate salinity stress tolerance. The Plant Journal, 74, 730–745. [DOI] [PubMed] [Google Scholar]
- Hussain, A. , Li, X. , Weng, Y. , Liu, Z. , Ashraf, M.F. , Noman, A. et al. (2018) CaWRKY22 acts as a positive regulator in pepper response to Ralstonia solanacearum by constituting networks with CaWRKY6, CaWRKY27, CaWRKY40, and CaWRKY58. International Journal of Molecular Sciences, 19, 1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. , Gao, Y. , Liu, J. , Peng, X. , Niu, X. , Fei, Z. et al. (2012) Genome‐wide analysis of WRKY transcription factors in Solanum lycopersicum . Molecular Genetics and Genomics, 287, 495–513. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A. , Kavanagh, T.A. and Bevan, M.W. (1987) GUS fusions: β‐glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal, 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y. , Liang, G. and Yu, D. (2012) Activated expression of WRKY57 confers drought tolerance in Arabidopsis. Molecular Plant, 5, 1375–1388. [DOI] [PubMed] [Google Scholar]
- Johnson, C.S. , Kolevski, B. and Smyth, D.R. (2002) TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. The Plant Cell, 14, 1359–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K.C. , Lai, Z. , Fan, B. and Chen, Z. (2008) Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. The Plant Cell, 20, 2357–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, N.H. , Kim, D.S. , Chung, E.H. and Hwang, B.K. (2014) Pepper suppressor of the G2 allele of skp1 interacts with the receptor‐like cytoplasmic kinase1 and type III effector AvrBsT and promotes the hypersensitive cell death response in a phosphorylation‐dependent manner. Plant Physiology, 165, 76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth, C. and Eulgem, T. (2008) The oomycete response gene LURP1 is required for defense against Hyaloperonospora parasitica in Arabidopsis thaliana . The Plant Journal, 55, 53–64. [DOI] [PubMed] [Google Scholar]
- Knoth, C. , Ringler, J. , Dangl, J.L. and Eulgem, T. (2007) Arabidopsis WRKY70 is required for full RPP4‐mediated disease resistance and basal defense against Hyaloperonospora parasitica . Molecular Plant‐Microbe Interactions, 20, 120–128. [DOI] [PubMed] [Google Scholar]
- Lamour, K.H. , Stam, R. , Jupe, J. and Huitema, E. (2012) The oomycete broad‐host‐range pathogen Phytophthora capsici . Molecular Plant Pathology, 13, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeau, A. , Daunay, M.C. , Frary, A. , Palloix, A. , Wang, J.F. , Dintinger, J. et al. (2011) Bacterial wilt resistance in tomato, pepper, and eggplant: genetic resources respond to diverse strains in the Ralstonia solanacearum species complex. Phytopathology, 101, 154–165. [DOI] [PubMed] [Google Scholar]
- Li, G.P. and Rice, C.M. (1989) Mutagenesis of the in‐frame opal termination codon preceding nsP4 of Sindbis virus: studies of translational readthrough and its effect on virus replication. Journal of Virology, 63, 1326–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, J. , Jiang, W. , Zhang, Y. , Yu, H. , Mao, Z. , Gu, X. et al. (2011) Genome‐wide analysis of WRKY gene family in Cucumis sativus . BMC Genomics, 12, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippok, B. , Birkenbihl, R.P. , Rivory, G. , Brummer, J. , Schmelzer, E. , Logemann, E. et al. (2007) Expression of AtWRKY33 encoding a pathogen‐ or PAMP‐responsive WRKY transcription factor is regulated by a composite DNA motif containing W box elements. Molecular Plant‐Microbe Interactions, 20, 420–429. [DOI] [PubMed] [Google Scholar]
- Liu, Z.Q. , Yan, L. , Wu, Z. , Mei, C. , Lu, K. , Yu, Y.T. et al. (2012) Cooperation of three WRKY‐domain transcription factors WRKY18, WRKY40, and WRKY60 in repressing two ABA‐responsive genes ABI4 and ABI5 in Arabidopsis. Journal of Experimental Botany, 63, 6371–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, M. , Dennis, E.S. , Berger, F. , Peacock, W.J. and Chaudhury, A. (2005) MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine‐rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America, 102, 17531–17536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machens, F. , Becker, M. , Umrath, F. and Hehl, R. (2014) Identification of a novel type of WRKY transcription factor binding site in elicitor‐responsive cis‐sequences from Arabidopsis thaliana . Plant Molecular Biology, 84, 371–385. [DOI] [PubMed] [Google Scholar]
- Miao, Y. , Laun, T. , Zimmermann, P. and Zentgraf, U. (2004) Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Molecular Biology, 55, 853–867. [DOI] [PubMed] [Google Scholar]
- Mou, S. , Liu, Z. , Guan, D. , Qiu, A. , Lai, Y. and He, S. (2013) Functional analysis and expressional characterization of rice ankyrin repeat‐containing protein, OsPIANK1, in basal defense against Magnaporthe oryzae attack. PLoS One, 8, e59699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajerowska‐Mukhtar, K.M. , Wang, W. , Tada, Y. , Oka, N. , Tucker, C.L. , Fonseca, J.P. et al. (2012) The HSF‐like transcription factor TBF1 is a major molecular switch for plant growth‐to‐defense transition. Current Biology, 22, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, L.J. and Jiang, L. (2014) Identification and expression of the WRKY transcription factors of Carica papaya in response to abiotic and biotic stresses. Molecular Biology Reports, 41, 1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, S.P. and Somssich, I.E. (2009) The role of WRKY transcription factors in plant immunity. Plant Physiology, 150, 1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, C.J. , Shin, Y.C. , Lee, B.J. , Kim, K.J. , Kim, J.K. and Paek, K.H. (2006) A hot pepper gene encoding WRKY transcription factor is induced during hypersensitive response to tobacco mosaic virus and Xanthomonas campestris . Planta, 223, 168–179. [DOI] [PubMed] [Google Scholar]
- Pecher, P. , Eschen‐Lippold, L. , Herklotz, S. , Kuhle, K. , Naumann, K. , Bethke, G. et al. (2014) The Arabidopsis thaliana mitogen‐activated protein kinases MPK3 and MPK6 target a subclass of 'VQ‐motif'‐containing proteins to regulate immune responses. New Phytologist, 203, 592–606. [DOI] [PubMed] [Google Scholar]
- Raghothama, K.G. , Liu, D. , Nelson, D.E. , Hasegawa, P.M. and Bressan, R.A. (1993) Analysis of an osmotically regulated pathogenesis‐related osmotin gene promoter. Plant Molecular Biology, 23, 1117–1128. [DOI] [PubMed] [Google Scholar]
- Rana, R.M. , Dong, S. , Tang, H. , Ahmad, F. and Zhang, H. (2012) Functional analysis of OsHSBP1 and OsHSBP2 revealed their involvement in the heat shock response in rice (Oryza sativa L.). Journal of Experimental Botany, 63, 6003–6016. [DOI] [PubMed] [Google Scholar]
- Rushton, D.L. , Tripathi, P. , Rabara, R.C. , Lin, J. , Ringler, P. , Boken, A.K. et al. (2012) WRKY transcription factors: key components in abscisic acid signalling. Plant Biotechnology Journal, 10, 2–11. [DOI] [PubMed] [Google Scholar]
- Rushton, P.J. , Somssich, I.E. , Ringler, P. and Shen, Q.J. (2010) WRKY transcription factors. Trends in Plant Science, 15, 247–258. [DOI] [PubMed] [Google Scholar]
- Shang, Y. , Yan, L. , Liu, Z.Q. , Cao, Z. , Mei, C. , Xin, Q. et al. (2010) The Mg‐chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA‐responsive genes of inhibition. The Plant Cell, 22, 1909–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibbe, M. , Qu, N. , Galis, I. and Baldwin, I.T. (2008) Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. The Plant Cell, 20, 1984–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, C. , Palmqvist, S. , Olsson, H. , Boren, M. , Ahlandsberg, S. and Jansson, C. (2003) A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar‐responsive elements of the iso1 promoter. The Plant Cell, 15, 2076–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi, P. , Rabara, R.C. , Langum, T.J. , Boken, A.K. , Rushton, D.L. , Boomsma, D.D. et al. (2012) The WRKY transcription factor family in Brachypodium distachyon . BMC Genomics, 13, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchet, M. , Ranty, B. , Marco, Y. and Roby, D. (2001) HSR203 antisense suppression in tobacco accelerates development of hypersensitive cell death. The Plant Journal, 27, 115–127. [DOI] [PubMed] [Google Scholar]
- Turck, F. , Zhou, A. and Somssich, I.E. (2004) Stimulus‐dependent, promoter‐specific binding of transcription factor WRKY1 to Its native promoter and the defense‐related gene PcPR1‐1 in parsley. The Plant Cell, 16, 2573–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulker, B. , Shahid Mukhtar, M. and Somssich, I.E. (2007) The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta, 226, 125–137. [DOI] [PubMed] [Google Scholar]
- Vailleau, F. , Daniel, X. , Tronchet, M. , Montillet, J.L. , Triantaphylides, C. and Roby, D. (2002) A R2R3‐MYB gene, AtMYB30, acts as a positive regulator of the hypersensitive cell death program in plants in response to pathogen attack. Proceedings of the National Academy of Sciences of the United States of America, 99, 10179–10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Vannozzi, A. , Wang, G. , Liang, Y.H. , Tornielli, G.B. , Zenoni, S. et al. (2014) Genome and transcriptome analysis of the grapevine (Vitis vinifera L.) WRKY gene family. Horticulture Research, 1, 14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Dang, F. , Liu, Z. , Wang, X. , Eulgem, T. , Lai, Y. et al. (2013) CaWRKY58, encoding a group I WRKY transcription factor of Capsicum annuum, negatively regulates resistance to Ralstonia solanacearum infection. Molecular Plant Pathology, 14, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, F. , Zhu, H. , Li, P. , Jiang, M. , Mao, W. , Ong, C. et al. (2014) Genome‐wide evolutionary characterization and expression analyses of WRKY family genes in Brachypodium distachyon . DNA Research, 21, 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, K.L. , Guo, Z.J. , Wang, H.H. and Li, J. (2005) The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Research, 12, 9–26. [DOI] [PubMed] [Google Scholar]
- Xu, X. , Chen, C. , Fan, B. and Chen, Z. (2006) Physical and functional interactions between pathogen‐induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. The Plant Cell, 18, 1310–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki, K. , Kigawa, T. , Watanabe, S. , Inoue, M. , Yamasaki, T. , Seki, M. et al. (2012) Structural basis for sequence‐specific DNA recognition by an Arabidopsis WRKY transcription factor. Journal of Biological Chemistry, 287, 7683–7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Li, R. and Qi, M. (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. The Plant Journal, 22, 543–551. [DOI] [PubMed] [Google Scholar]
- Yin, G. , Xu, H. , Xiao, S. , Qin, Y. , Li, Y. , Yan, Y. et al. (2013) The large soybean (Glycine max) WRKY TF family expanded by segmental duplication events and subsequent divergent selection among subgroups. BMC Plant Biology, 13, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokotani, N. , Sato, Y. , Tanabe, S. , Chujo, T. , Shimizu, T. , Okada, K. et al. (2013) WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. Journal of Experimental Botany, 64, 5085–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. , Xu, W. , Wang, J. , Wang, L. , Yao, W. , Xu, Y. et al. (2013) A core functional region of the RFP1 promoter from Chinese wild grapevine is activated by powdery mildew pathogen and heat stress. Planta, 237, 293–303. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. and Wang, L. (2005) The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evolutionary Biology, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Nucleotide sequences of 5′‐flanking regions of the CaWRKY40 promoter and distributions of potential cis‐acting elements. Nucleotide sequences highlighted with yellow indicate DWE motif. Nucleotide sequences in red represent the position of the transcriptional start site. ARFAT and ARF (auxin response factor), binding sites identified in the promoters of primary/early auxin response genes of Arabidopsis thaliana; WBOXNTCHN48, a W box identified in the region between –125 and –69 bp of the tobacco Class I basic chitinase gene CHN48; DPBFCOREDCDC3, a bZIP transcription factor DPBF‐1 and 2 (Dc3 promoter‐binding factor‐1 and 2) binding core sequence; ABRELATERD1, an ABRE‐like sequence (from –199 to –195) required for etiolation‐induced expression of erd1 (early responsive to dehydration) in Arabidopsis; ARE, a cis‐acting regulatory element essential for anaerobic induction; ARR1AT (ARR1), a response regulator; ATGCAAAT motif, a cis‐acting regulatory element associated with the TGAGTCA motif; C‐repeat/DRE, a regulatory element involved in cold and dehydration responses; TCA‐element, a cis‐acting element involved in salicylic acid responses; WUN‐motif, a wound‐responsive element; CGTCA‐motif, a cis‐acting regulatory element involved in MeJA responses; CuRE, the GTAC core of a CuRE (copper‐response element) found in Cyc6 and Cpx1 genes in Chlamydomonas; W box, a WRKY transcription factor binding site; HSE, a cis‐acting element involved in HS responses; MBS, a MYB binding site involved in drought inducibility; MBSII, a MYB binding site involved in flavonoid biosynthetic gene regulation; MYB, a MYB binding site; MYCCONSENSUSAT, a MYC recognition site found in the promoters of the dehydration‐responsive gene rd22 and many other genes in Arabidopsis; GA‐box, part of an auxin‐responsive element; TC‐rich repeats, cis‐acting elements involved in defense and stress responses; RAV1AT, a binding consensus sequence of the Arabidopsis transcription factor RAV1; WBOXHVISO1 and SUSIBA2 bind to the W box element in the barley iso1 (isoamylase1) promoter; WRKY710S, a core of the TGAC‐containing W box
FIGURE S2 Transactivation of CaWRKY40 promoter by overexprssion of CaWRKY40, CaWRKY58, and CaWRKY27 in onion cell by patical bombardment. Effector vector of CaWRKY40, CaWRKY58, or CaWRKY27, respectively, were co‐transformed with the reporter construct of pCaWRKY40::GUS into onion epidermal cells by particle bombardment followed by histochemical GUS staining for GUS expression detection. Onion cells co‐transformed with the effector vector (CaWRKY40‐HA, CaWRKY58‐HA, or CaWRKY27‐HA) and reporter vector exhibited obvious dark blue, while cells co‐transformed with empty effector vector and reporter vector were never stained blue
FIGURE S3 Effects of R. solanacearum infection and heat shock on the transient expression of the CaWRKY40 promoter deletion in pepper leaves. Activity of the 5ʹ deletion of the CaWRKY40 promoter was examined in pepper plants 24 hr after treatment with RSI and HS. The samples were harvested 24 hr postpathogen and heat shock treatments for GUS activity measurement. The GUS activity of transient expressed pepper leaves without treatment was set to 1. All the data represent the mean ± SD of three independent biological experiments. Error bars indicate SD (n = 3) from three independent experiments. Asterisks indicate significant differences determined by Student’s t test (*p < .05)
FIGURE S4 Confirmation of CaWRKY40 expression in E. coli strain BL21 and MST traces (on the left) and the capillary shape (on the right) of the MST experiment. (A) SDS‐PAGE analysis to confirm CaWRKY40 expression in E. coli. The gel was stained with Coomassie Brilliant Blue and scanned. M, protein ladder marker; 1, sample with IPTG induction; 2, sample without IPTG induction; 3, empty E. coli strain BL21; 4, purified CaWRKY40 protein. (B) and (C) MST traces (on the left) and capillary shape (on the right) of the MST experiment
FIGURE S5 Mutation analyses of DWE in pCaWRKY40 ‐1802 to ‐1464 and the responses of pCaWRKY40 to HS and RSI. (A) Diagrammatic representation of DWE in pCaWRKY40 ‐1802 to ‐1464 (pCaWRKY40) and its mutated version (pCaWRKY40‐DWE‐DM); GUS, GUS report gene. The number above the green box indicates the location of DWE in the promoter of CaWRKY40. (B) Mutation analyses of CaWRKY40‐1802 to ‐1464 DWE and the response of pCaWRKY40 to HS at 6 and 12 hr. GV3101 cells containing the pCaWRKY40 or pCaWRKY40‐DWE‐DM were infiltrated into the leaves of pepper plants. The agro‐infiltrated leaves were subjected to heat shock. At 24 and 48 hours post treatment (hpt) posttreatment, the Agro‐infiltrated leaves were harvested for GUS activity measurement. The GUS activity of pCaWRKY40‐infiltrated pepper without HS treatment was set to 1. (C) Mutation analyses of pCaWRKY40 ‐1802 to ‐1464 DWE and the response of pCaWRKY40 to RSI at 24 and 48 hr after infection. Agrobacterium cells harbouring the pCaWRKY40 or pCaWRKY40‐DWE‐DM were vacuum‐infiltrated into the pepper leaves using a needleless syringe, followed by inoculation withR. solanacearum. The pathogen‐inoculated pepper leaves were collected for further GUS activity measurement. The GUS activity of pCaWRKY40‐infiltrated pepper without RSI was set to 1. Data in (B) and (C) represent the mean ± SD of four independent experiments. Error bars indicate SD. Asterisks indicate significant differences determined by Fisher’s protected least significant difference test (*p < .05)
FIGURE S6 Sequences analysis of DWEs in the promoters of CaWRKY40 orthologs in other plant species. Highlighted are nucleotides matching the W box consensus (T)(T)TGACC/T. AtWRKY40, Arabidopsis thaliana WRKY transcription factor WRKY40 ((AT1G80840)); CplWRKY40, WRKY40 from Carica papaya L. (http://www.plantgdb.org/CpGDB/); GmWRKY40, WRKY40 from Glycine max (ABC26914); VvWRKY40, WRKY40 from Vitis vinifera (GI:225443178 in database http://www.genoscope.cns.fr/); Sl(U566777, U566776, Scfld179), three of five SlWRKY40 candidate orthologs from Solanum lycopersicum; SaWRKY40, Solanum arcanum WRKY40; SpWRKY40, Solanum pennellii probable WRKY transcription factor 40; NtWRKY40, Nicotiana tabacum probable WRKY transcription factor 40 (LOC107798622); NaWRKY40, Nicotiana attenuata probable WRKY transcription factor 40 (LOC109244974); SiWRKY40, Sesamum indicum probable WRKY transcription factor 40 (LOC105165167); CsWRKY40, Camellia sinensis WRKY transcription factor 40 (JQ820201)
FIGURE S7 Real‐time RT‐PCR analysis of the defense‐associated marker genes in pepper leaves transiently expressing CaDWE::CaWRKY40 in response to R. solanacearum inoculation and heat shock. Real‐time RT‐PCR was performed to detect the transcript levels of immunity and heat shock‐associated marker genes in pepper leaves transiently expressing CaDWE::CaWRKY40 (or CaDWE‐DM::CaWRKY40) in response to RSI and HS. Pepper leaves were transformed with CaDWE::CaWRKY40 (or CaDWE‐DM:: CaWRKY40) and subjected to immediate RSI and HS treatments, respectively. The transcript levels of marker genes in the mock pepper leaves (without RSI or HS treatment) were set to 1. Data represent the mean ± SD of three independent biological experiments. Error bars represent ±SD (n = 3) from three independent experiments and asterisks indicate significant differences, as determined by Fisher’s protected least significant difference test (*p < .05)
TABLE S1 Double W box motifs in the promoter region of pepper WRKYs
TABLE S2 Primers used in this study
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


