Abstract
Objective
To estimate the budget impact from the perspective of the Irish health-care system attributable to a reconfiguration in the diagnostic care pathway for patients with suspected RA by adopting an early identification and referral model (EIM).
Methods
The budget impact model evaluated the total health-care use and costs attributable to an EIM to diagnose patients with suspected RA relative to the reference scenario of current practice. The modelling also assessed a primary outcome of effect, which examined how many patients can be diagnosed by a rheumatologist within 3 months of symptom onset. The budget impact analysis model was estimated over a 5-year time frame.
Results
The EIM generated a cost saving for the Irish health-care system of €237 547 over the time frame relative to current practice. The cost savings were realized owing to a reduction in the number of general practitioner (GP) visits of 18 790 and a reduction in diagnostic tests carried out by GPs. The results showed that 1027 (510%) more patients were diagnosed within 3 months of symptom onset in the EIM compared with current practice.
Conclusion
This paper has presented an alternative rheumatologist-led service design that can be used in diagnosing patients with suspected RA. The rheumatologist-led service provision detailed in this study has the potential simultaneously to reduce demand for primary care services and to improve the health outcomes of patients. The use of an EIM sees rheumatologist activity incorporate patient demand.
Keywords: Primary care, rheumatoid arthritis, budget, care pathways, referrals
Key messages
The use of an early identification and referral model for RA will generate budget savings.
Significantly more RA patients can be diagnosed within 3 months of symptom onset.
A rheumatologist-led service design for diagnosis of RA patients can reduce demand for primary care.
Introduction
RA is a chronic inflammatory disease characterized by inflammation of synovial joints and other tissues, including the eyes, heart, lungs and vasculature. This inevitably results in tissue damage and loss of function associated with significant morbidity, reduction in quality of life, increased health-care expenditure, reduced labour productivity and early mortality without effective treatment [1–6]. Recent advances in our understanding of this disease and developments in pharmacological treatments have resulted in improved management of RA, with significantly better health outcomes. The early introduction of DMARDs and the availability of new therapies, such as biological medications, has transformed this disease and the outlook for patients [7]. Today, achieving and sustaining disease remission is the desired therapeutic goal [8]. The increased availability and early initiation of these treatments has resulted in improved health outcomes for patients by controlling the symptoms and halting the progression of the disease, which reduces the development of joint deformities, disability and illness burden [9, 10]. Inflammation can be controlled and damage prevented, but damage is not reversible. Therefore, in order for treatments to be most effective and of greatest benefit to patients, a correct and timely diagnosis must ensue.
The early identification and diagnosis of RA allows effective treatments to begin earlier in the care pathway, with the early suppression of disease activity (inflammation) being essential for limiting joint damage [11–13]. RA is not a common disease, affecting <1% of European adults [6, 7]. As an approximate estimate, an Irish general practitioner (GP) is confronted with 0.6 incidence cases of RA per year, which is comparable to the 0.4 incidence cases for Austrian GPs [14]. In Ireland, many GPs lack training and confidence in diagnosing and managing musculoskeletal diseases, including RA [15]. Rheumatologists are best placed to diagnose and manage this disease in practice [16], but Ireland continues to have one of the lowest per capita numbers of rheumatologists in Europe. It is estimated that a ratio of 1:58 000 rheumatologists per capita should be employed in the Irish health-care system to comply with the European Working Time Directive [17]. As such, the current ratio is 1:100 000 rheumatologists per capita, demonstrating that rheumatologists are under-staffed in Ireland [18]. It is paramount that the most appropriate health professional diagnoses the individual with suspected RA at an early stage, because any delay in the diagnosis process can result in the delay of the initiation of treatment and worsen the health outcomes for the patient [7, 19]. The importance of early diagnosis and treatment is echoed by the Irish Health Service Executive in the National Clinical Programme for Rheumatology Model of Care published in 2018, ‘Adopting a chronic disease model of care so as to facilitate a right person, right place, first-time approach to patients’ [20]. In order for a patient with suspected RA to receive a timely diagnosis by the appropriate health professional, the reconfiguration of the diagnostic care pathway into a more streamlined, rheumatologist-led approach might be warranted. Such pathways could result in considerable cost savings for national health systems while simultaneously improving the health outcomes of patients [14, 21, 22]. An intervention that is deemed to be cost saving while also being more effective than the standard of care is worthy of further application in multiple jurisdictions.
This purpose of this study is to undertake, for the first time, a budget impact analysis, from the perspective of the Irish health-care system, attributable to the reconfiguration of the diagnostic care pathway for patients with suspected RA. More specifically, we provide an estimation of the use of health-care services and costs associated with an early identification and referral model (EIM) for patients with suspected RA in comparison to current practice (CPr) in Ireland. This model would entail earlier referral of patients with suspected RA by GPs to a rheumatologist to be assessed through the use of acceptable referral criteria as outlined by Puchner et al. [14] and the national early arthritis referral form (designed by the Irish Rheumatology Community in conjunction with the primary care community as part of a national initiative to enhance early referrals in 2011), as seen in Supplementary Data S1, available at Rheumatology Advances in Practice online. The criteria include the presence of tender and swollen joints, family history of RA, morning stiffness, extra-articular features and laboratory investigations for inflammation and antibodies. Given the lack of research in an Irish context, such an analysis will be of considerable interest to decision-makers charged with the health-care service design of rheumatic disease in Ireland and elsewhere.
Methods
The budget impact analysis presented in this paper estimates the impact of the reconfiguration in the diagnostic care pathway for patients with suspected RA in the Irish health-care system over a 5-year time frame. The reference scenario for the analysis is CPr. The budget impact analysis was compiled in accordance with both sets of guidelines for such works by the International Society for Pharmacoeconomics and Outcomes Research [23] and by the Irish regulatory body, the Health Information and Quality Authority [24]. The perspective for the analysis is the Irish health-care system [25]. No ethics requirement was needed for this research.
Modelling framework
An incidence-based, BIA model is estimated for Ireland. The model evaluates the impact of the reconfiguration in the diagnostic care pathway for patients with suspected RA by comparing the total healthcare use and costs to the reference scenario of current practice. The reconfiguration entails of an early identification and referral model (EIM) to substitute wholly the existing current practice (CPr). Due to the nature of this analysis, we explored the following budget impact scenario: Reconfiguration scenario (EIM): An early identification and referral model to substitute the existing current practice of diagnosing a patient with suspected RA.
The model tracks all health-care usage and costs for patients with suspected RA from symptom onset to diagnosis by a rheumatologist. The model incorporates all incident cases over a 5-year time frame. It is important to note that no follow-up, monitoring or treatment costs are included in this model. The model also examines the number of patients who are diagnosed by a rheumatologist within 3 months of symptom onset relative to those diagnosed outside of 3 months but within a year. As such, it is a primary endpoint of the present study and is calculated using the same method proposed by Xu & Groom [21]. Following Brodszky et al. [26], the total costs of the scenarios are estimated as aggregations of the product of the patients with suspected RA in the different model states and the costs associated with these states (see Supplementary Fig. S1, available at Rheumatology Advances in Practice online). The net budget impact was calculated as the difference between EIM and CPr. Estimates are reported in Euros (€) at 2018 prices, with no future discounting of values. We focused on a primary outcome of effect, which is the number of patients diagnosed by a rheumatologist within 3 months of symptom onset, as noted above. All costs were adjusted for inflation to their 2018 equivalent and converted to Irish Euros using the Organisation for Economic Co-operation and Development’s Purchasing Power Parity conversion rates [27].
Incident patient population
All individuals enter the model with suspected undifferentiated arthritis (UA) and will potentially receive an ultimate diagnosis of RA later in model. To calculate the total incident cases of UA in the Irish population, we multiplied the total population of Ireland [28] by three times the incidence rate of RA, following Abhishek et al. [29] and Xu & Groom [21]. The model also accounts for the increasing size of the population, with UA based on population increase estimates for the 5-year time frame of the model [28].
Modelling assumptions
The model is based on several assumptions guided by the published literature [14, 21] and by expert clinical opinion [personal communication, Professor David Kane, Department of Medicine (Rheumatology), Trinity College Dublin, Ireland]. For this analysis and in the absence of any primary data, we construct a snapshot of the diagnostic care pathway for a patient with suspected RA in Ireland and their associated health-care usage and costs. All modelling assumption probabilities and their references are presented in Table 1.
Table 1.
Model parameters
| Variables | Base case parameter value | Reference |
|---|---|---|
| Population of Ireland | 4 857 000 | [28] |
| Overall incidence of RA in the population | 0.00032 | [29] |
| Probability of individual having inflammatory arthritis or non-inflammatory arthritis | 0.5 | [21] |
| Probability an individual with UA presents to the A&E | 0.2 | [14] |
| Probability an individual with UA who presents to the A&E obtains a referral to a rheumatologist | 1 | [21] |
| Probability of presenting to a GP within 3 months of symptom onset | 0.4 | [21] |
| Probability of presenting to a GP outside of 3 months of symptom onset | 0.6 | 1–# from above |
| Probability of being referred to the rheumatologist first time upon first GP visit under the CPr | 0.2 | [30] |
| Probability of being referred to the rheumatologist after a further three GP visits under the CPr | 0.8 | 1–# from above |
| Probability of being referred to the rheumatologist first time upon first GP visit under the EIM | 0.8 | [21] |
| Probability of being referred to the rheumatologist after a further three GP visits under the EIM | 0.2 | 1–# from above |
| Probability of being diagnosed by a rheumatologist within 3 months of symptom onset once referred under the CPr | 0.4 | [21] with a range of 0.33–0.51 |
| Probability of being diagnosed by a rheumatologist within 3 months of symptom onset once referred under the EIM | 0.51 | [21] with a range of 0.4–0.57 |
| Probability of a GP carrying out a RF testa | 0.49 | [21] |
| Probability of a GP carrying out a CRP testa | 0.56 | [21] |
| Probability of a GP carrying out an ESR testa | 0.60 | [21] |
| Probability of a GP carrying out an anti-CCP testa | 0.12 | [21] |
| Probability of a GP carrying out a full blood count testa | 0.75 | [21] |
| Probability of a GP carrying out an X-raya | 0.09 | [21] |
| Probability of a rheumatologist carrying out a RF test | 0.6 | [21] |
| Probability of a rheumatologist carrying out an anti-CCP test | 0.68 | [21] |
| Probability of a rheumatologist carrying out an X-ray | 0.69 | [21] |
| Probability of a rheumatologist/GP carrying out the above tests on non-inflammatory arthritis patients | 0.5 | [21] |
Only individuals who were not referred upon their first GP visit have tests carried out by the GP before referral to a rheumatologist. A&E: Accident and Emergency; CPr: current practice; EIM: early identification and referral model; GP: general practitioner; UA: undifferentiated arthritis.
Unit costs associated with model states
Only direct health-care costs are included in the analysis, with all unit costs and references presented in Table 2. The health-care utilization for each scenario includes the total number of Accident and Emergency (A&E) department visits, GP visits, rheumatologist consultations and the total volume of diagnostic tests carried out per state. The states reflect each stage of the diagnostic care pathway for each scenario in the model. Only individuals who are not referred upon their first GP visit have tests carried out by the GP before referral to a rheumatologist. Once at the rheumatologist consultation, a series of diagnostics tests are potentially carried out regardless of whether an individual is referred to the rheumatologist after only one GP visit or after multiple visits.
Table 2.
Unit costs
| Variable | Unit cost (€) | Reference |
|---|---|---|
| Accident and Emergency visit | 288.03 | [31] |
| General practitioner visit | 53.85 | [32] |
| Rheumatologist visit | 215 | [21] |
| Full blood count test | 9.53 | [21] |
| RF test | 15.06 | [21] |
| ESR test | 7.44 | [21] |
| CRP test | 12.9 | [33] |
| Anti-CCP test | 24.77 | [21] |
| X-ray | 53.74 | [33] |
All costs are updated to 2018 Euro prices.
In Ireland, GPs act as gatekeepers, and a patient’s first point of contact with the health-care system comes through a GP visit or a visit to the A&E department of a hospital [34]. Therefore, in both models, patients enter into the model through contact with their GP or, occasionally, through a visit to the A&E after symptom onset [14, 21]. The analysis presented in this study incorporates all patients with UA, of whom 50% have inflammatory arthritis and 50% non-inflammatory arthritis. The model estimates that 20% of all UA patients will attend the A&E upon symptom onset, as noted by Puchner et al. [14], irrespective of whether an EIM exists. Of the remaining 80% of patients with UA, 40% will visit their GP within and 60% outside of the 3 months of symptom onset. These probabilities are uniform across both the CPr and EIM.
Referral behaviour
Both models differ with respect to the referral behaviour of the GPs. Of the 50% of patients with inflammatory arthritis who present to the GP either within or outside of 3 months of symptom onset, 20% and 80% will be referred to a rheumatologist after one GP visit in the CPr and EIM, respectively. With the remainder in both models being referred to a rheumatologist after a further three GP visits (four GP visits in total). Of the 20% of patients who attend the A&E, all will obtain a referral to a rheumatologist. For patients with non-inflammatory arthritis, the probabilities of GP referral to a rheumatologist, a GP carrying out diagnostic tests and a rheumatologist carrying out diagnostic tests are 50% less than those with inflammatory arthritis. The number of GP visits for those with non-inflammatory arthritis is reduced by 50% also for those not referred to a rheumatologist on their first GP visit in both the CPr and the EIM, for a total of two GP visits before being referred to a rheumatologist. For the patients with non-inflammatory arthritis, 50% and 100% of patients will receive a rheumatologist consultation in the CPr and EIM, respectively [21]. A final key model assumption is that there will be the necessary rheumatologist capacity to incorporate the extra volume of consultations in the EIM.
Sensitivity analysis
We performed one-way sensitivity analysis following methods outlined by Brodszky et al. [26]. Based on this, we vary key model parameters by ±10%. This includes the referral behaviour of GPs in the EIM arm only, the number of GP visits for those not referred after their first GP visit in the EIM arm only, the incidence rate of UA in both arms, and the number of those who present to the A&E in both arms. These are presented as shown in Fig. 1A. We also vary the costs by ±20% as recommended by the Health Information and Quality Authority [24], which are shown in Fig. 1B. We also investigated a scenario where the diagnosis rate once referred to a rheumatologist is increased from 0.51 to 0.75 when compared with our estimate for CPr of 0.4.
Fig. 1.
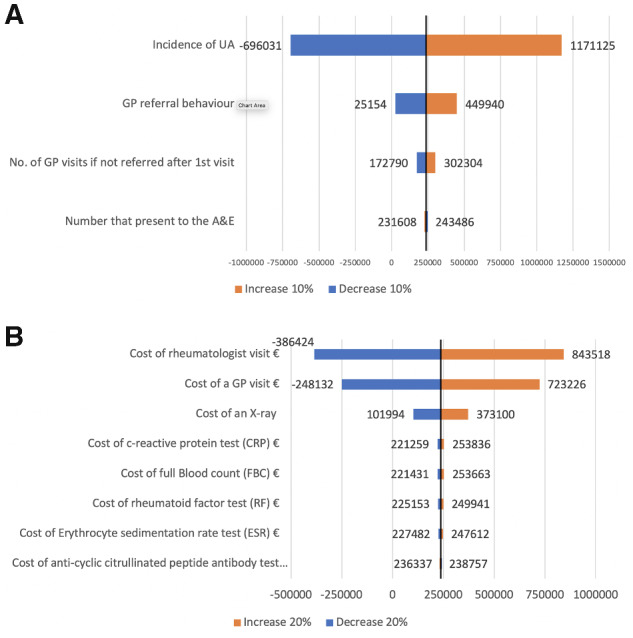
One-way sensitivity analysis on model parameters and model costs included in the analysis
(A) Sensitivity analysis on model parameters (increase or decrease by 10%). (B) Sensitivity analysis on model costs (increase or decrease by 20%). Note that estimates are presented as deviations from the baseline result of a potential cost saving of €237 547. A&E: Accident & Emergency; GP: general practitioner; UA: undifferentiated arthritis.
Results
The results of the budget impact analysis are presented in Table 3. In the base year (2019), there were 4604 incident cases of UA amongst the population of Ireland. The results show that the reconfiguration of the diagnostic care pathway for patients with suspected RA through the use of an EIM generates a potential cost saving for the Irish health-care system of €237 547 over the 5-year time frame. The cost savings were principally attributable to a reduction in the number of GP visits of 18 790 over this time frame and the reduction in diagnostic tests carried out by GPs. The results of the incremental differences between both arms are presented in Table 4. The primary end point of the analysis was the number of patients diagnosed by a rheumatologist within 3 months of symptom onset. The EIM was able to diagnose 1027 (510%) or 1628 (750%) more patients in this period than the CPr. This assumed that the rate at which a rheumatologist maked a diagnosis within 3 months once a patient was referred was 51% or 75%, respectively, where 51% was the mean rate, with a range of 0.4–0.75, as noted by Xu & Groom [21]. These results are displayed in Table 5. The results from the sensitivity analysis for the model parameters, presented in Fig. 1A, demonstrated that the main cost drivers were the incidence rate of UA and GP referral behaviour. The results from the sensitivity analysis for the cost parameters, presented in Fig. 1B, demonstrated that the main cost drivers were the cost of the rheumatologist visit and the cost of a GP visit.
Table 3.
Budget impact analysis results
| Scenario | Year 1 (2019) | Year 2 (2020) | Year 3 (2021) | Year 4 (2022) | Year 5 (2023) |
|---|---|---|---|---|---|
| Scenario 1: (EIM) | (€46 576) | (€47 042) | (€47 512) | (€47 987) | (€48 430) |
The use of brackets represents a minus figure denoting the savings that can be made after subtracting current practice from the early identification and referral model (EIM).
Table 4.
Incremental differences over the 5-year time frame
| Variable | Unit difference | Mean annual difference | Cost difference (€) | Mean annual cost difference (€) |
|---|---|---|---|---|
| A&E visitsa | – | – | – | – |
| GP visits | (18 790) | (3758) | (1 011 831) | (202 366) |
| Rheumatologist visits | 4697 | 939 | 1 009 952 | 201 990 |
| Full blood count test | (8455) | (1691) | (80 580) | (16 116) |
| RF test | (4904) | (981) | (60 219) | (12 044) |
| ESR test | (6764) | (1353) | (50 327) | (10 065) |
| CRP test | (6313) | (1263) | (81 443) | (16 289) |
| Anti-CCP test | 244 | 49 | 6051 | 1210 |
| X-ray | 552 | 110 | 32 602 | 6520 |
| Total cost difference | (237 547) | (47 509) |
The use of brackets represents a minus figure denoting the savings that can be made after subtracting CPr from the EIM. aA&E visits were held constant across the two model arms, the EIM and CPr, and as such there is no difference between them. In each arm there was a total of 4697 A&E visits at a total cost of €1 353 006 over the 5-year time frame. A&E: Accident and Emergency; CPr: current practice; EIM: early identification and referral model; GP: general practitioner.
Table 5.
Total number of patients diagnosed by a rheumatologist within 3 months once referred
| Current practice | Scenario 1 (EIM, with mean 51%) | Scenario 1 (EIM, with maximum 75%) | |
|---|---|---|---|
| Total incidence of people with RA over time frame | 7829 | 7829 | 7829 |
| Percentage of people with RA presenting to the GP within 3 months | 0.4 | 0.4 | 0.4 |
| Percentage of people with RA referred to a rheumatologist within 3 months | 0.2 | 0.8 | 0.8 |
| Percentage of people being diagnosed by a rheumatologist within 3 months once referred | 0.4 | 0.51 | 0.75 |
| Total number of people diagnosed with RA within 3 months | 251 | 1278 | 1878 |
| Percentage of total annual incidence (7829) | 3% | 16% | 24% |
EIM: early identification and referral model; GP: general practitioner.
Discussion
In this study, we have, for the first time, estimated the potential budget impact of the reconfiguration in the diagnostic care pathway for patients with suspected RA in the Irish health-care system as proposed by the National Clinical Programme for Rheumatology Model of Care (2018) [20]. The reconfigured care pathway scenario examined in this analysis was presented as an EIM. More specifically, the EIM would see patients with suspected RA identified earlier by the use of the Irish national referral criteria and the criteria noted by Puchner et al. [14]. This approach leads to earlier rheumatologist diagnosis and earlier initiation of treatment. We find a positive budget impact, with potential savings of €237 547 to the Irish health-care system over the 5-year time frame, owing to the reconfiguration of the diagnostic care pathway. As noted previously, the cost savings are the result of significantly lower use of GPs services and fewer diagnostic tests being carried out by GPs, which are later replicated by rheumatologists. A primary endpoint of the current analysis was the estimation of the number of patients who would be diagnosed by a rheumatologist within 3 months of symptom onset. Under the EIM, 1027 (510%) more patients are diagnosed by a rheumatologist within 3 months of symptom onset compared with CPr, using the approach by Xu & Groom [21].
Although less common than osteoporosis or cardiovascular disease, RA is a costly disease, and a multitude of studies have estimated the economic cost, health-care burden and economic impact of treatments relating to RA once a patient has been diagnosed [35–39]. However, there is scant health economic evidence on health-care use and cost on the lead-up to diagnosis, especially in an Irish context. The overall budget impact results demonstrate that a reconfiguration in the diagnostic care pathway for patients with suspected RA will lead to a cost saving for the Irish health-care system. Where the reconfiguration in the care pathway will generate significant value for the Irish health-care system, economy and wider society will be in the number of patients diagnosed by a rheumatologist within 3 months of symptom onset, allowing these patients to commence treatment promptly. The EIM presented in this study will be able to diagnose within 3 months of symptom onset between 1027 (510%) and 1628 (750%) more RA patients compared with CPr, depending on the rate at which a rheumatologist can make a diagnosis. By taking the mean estimate of the rheumatologist diagnosis rate of 51% compared with an estimate of 40% in CPr, 510% more patients will be diagnosed within 3 months of symptom onset. The merits of an EIM approach to diagnosis of patients with RA is seen in the early arthritis clinic, as noted by van der Horst-Bruinsma et al. [40], who found that use of the early arthritis clinic provides reliable diagnosis of RA when compared with CPr. Previous work in this area by Puchner et al. [14] noted that the reconfiguration in the diagnostic care pathway for patients with suspected RA in the Austrian health-care system is a cost-effective service offering, noting that the Austrian health-care system could potentially save more than €100 000 per annum from an EIM.
In this paper, we found that under the current system there is a potential over-reliance on the use of primary health-care services, which are ill-equipped to manage RA patients, as noted previously [15]. Under an EIM there will potentially be 18 790 fewer GP visits over the 5-year time frame. Patients with a confirmed diagnosis of RA are frequent users of primary care services and place considerable demand on these services in Ireland. For example, Bevan et al. [41] found that patients with rheumatic disease account for 30% of all GP visits in Ireland, and in a later study, Doherty & O'Neill [42] found that patients with RA have significantly greater use of GP services in Ireland compared with non-rheumatic individuals. In addition, well-managed RA patients place considerably less burden on health-care services too, requiring fewer visits, investigations and treatments over time, and have less disability and illness burden [7, 43]. At a time in Irish health care when primary health-care services are under considerable pressure [44], any service provision that can alleviate this pressure and can reduce the demand for such a vital service offering should be considered by policymakers. If policy objectives, such as reducing the demand on GP services and providing more cost-effective care for patients, are to be adhered to in accordance with the Slàintecare report (policy paper on reforming the Irish health-care system) [45], it is important to understand the significant determinants of GP service use better. Nonetheless, this alone is not enough, because viable alternative services are required to alleviate the excessive demand constraints placed upon GP services. This paper has presented an alternative rheumatologist-led service design that can be used in the diagnosis of patients with suspected RA that will reduce the demand of GP services through the use of an EIM that sees rheumatologist activity incorporate patient demand.
There is a lack of Irish data examining the diagnostic care pathway for patients with suspected RA in Ireland, and given that such data from published sources were used, we acknowledge this to be a limitation to the present study. A modelling assumption was that the EIM will necessarily be staffed and resourced in order to be able to incorporate the influx of new patients with suspected RA. It is assumed that if a rheumatologist were to be dedicated to RA, they could see 500 new inflammatory arthritis patients per year (personal communication, Professor John Carey, School of Medicine, National University of Ireland Galway). As such, under the EIM where every patient with suspected RA receives a rheumatologist appointment, more than nine rheumatologists would be required to meet the capacity demands of the 4604 patients with suspected UA in Ireland. Considering how a rheumatologist’s time is split across the varying rheumatic diseases and how diagnosis is only one facet of their duties, we did not include personnel investments required to deliver the proposed service. Given that RA patients are receiving an earlier diagnosis and, therefore, earlier treatment too, this should also present cost savings. We believe the results presented here provide a conservative estimate of the resources that would be available with which to consider such investments. Beyond the cost associated with each visit, we have not considered the extra benefit that would be incurred from the reduction in the demand for primary care, given the 18 790 fewer GP visits that would occur in the EIM scenario. Further research in this area would be to examine the costs associated with treatments, monitoring and follow-up for patients who have commenced treatment within 3 months vs >3 months after symptom onset.
In conclusion, the timely initiation of treatment of patients with RA is imperative to slow the progression of this chronic condition and reduce the illness burden. The present study demonstrates the potential value of the early diagnosis of patients within 3 months of symptom onset, in order that they can begin treatment earlier in the care pathway, in the Irish health-care system. Through this reconfiguration in the diagnostic care pathway, potential cost savings to the Irish health-care system would probably be realized, with a greater number of patients diagnosed within 3 months of symptom onset, further increasing their ability to remain economically active. The rheumatologist-led service provision detailed in the paper has the potential simultaneously to reduce demand for primary care services and to improve health outcomes. The results from this study will be of interest to policymakers in Ireland and elsewhere.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the funding and support from the Patient Improvement Fund for Ireland (RPIF) from the Irish Society of Rheumatology.
Each author fully, fairly and equally contributed to the drafting of this manuscript.
Dan Kelleher: literature review, data analysis, writing original draft of manuscript and redrafting of manuscript. Luke Barry: data analysis and redrafting of manuscript. Bernie McGowen: Literature review and redrafting of manuscript. Edel Doherty: redrafting of manuscript. John Carey: redrafting of manuscript. David Kane: redrafting of manuscript.
Funding: This work was supported by the Patient Improvement Fund for Ireland (RPIF) from the Irish Society of Rheumatology.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
Supplementary data
Supplementary data are available at Rheumatology Advances in Practice online.
Contributor Information
Dan Kelleher, Health Economics and Policy Analysis Centre, National University of Ireland, Galway, Galway, Ireland.
Luke Barry, Centre for Public Health, Queen’s University Belfast, Belfast, Northern Ireland.
Bernie McGowan, School of Medicine, National University of Ireland, Galway.
Edel Doherty, Health Economics and Policy Analysis Centre, National University of Ireland, Galway, Galway, Ireland.
John J Carey, School of Medicine, National University of Ireland, Galway.
David Kane, Department of Medicine (Rheumatology), Trinity College Dublin, Dublin, Ireland; National Clinical Programme for Rheumatology, HSE, Ireland.
References
- 1. Scott DL, Symmons DP, Coulton BL, Popert AJ.. Long-term outcome of treating rheumatoid arthritis: results after 20 years. Lancet 1987;329:1108–11. [DOI] [PubMed] [Google Scholar]
- 2. Mitchell DM, Spitz PW, Young DY. et al. Survival, prognosis, and causes of death in rheumatoid arthritis. Arthritis Rheum 1986;29:706–14. [DOI] [PubMed] [Google Scholar]
- 3. Pincus T, Callahan LF, Sale WG. et al. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum 1984;27:864–72. [DOI] [PubMed] [Google Scholar]
- 4. Isomäki H. Long-term outcome of rheumatoid arthritis. Scand J Rheumatol 1992;21:3–8. [DOI] [PubMed] [Google Scholar]
- 5. Wolfe F. The natural history of rheumatoid arthritis. J Rheumatol Suppl 1996;44:13–22. [PubMed] [Google Scholar]
- 6. Kobelt G, Kasteng F.. Access to innovative treatments in rheumatoid arthritis in Europe. A report prepared for the European Federation of Pharmaceutical Industry Associations (EFPIA). Brussels, Belgium: European Federation of Pharmaceutical Industry Associations (EFPIA), 2009;1–92.
- 7. Smolen JS, Aletaha D, McInnes IB.. Rheumatoid arthritis. Lancet 2016;388:2023–38. [DOI] [PubMed] [Google Scholar]
- 8. Emery P, Breedveld FC, Hall S. et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375–82. [DOI] [PubMed] [Google Scholar]
- 9. van der Heijde DM, van 't Hof M, van Riel PL, van de Putte LB.. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 1993;20:579–81. [PubMed] [Google Scholar]
- 10. Prevoo ML, van 't Hof MA, Kuper HH. et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 11. Widdifield J, Bernatsky S, Paterson JM. et al. Quality care in seniors with new-onset rheumatoid arthritis: a Canadian perspective. Arthritis Care Res 2011;63:53–7. [DOI] [PubMed] [Google Scholar]
- 12. Aletaha D, Nell VPK, Stamm T. et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Le Loet X, Strotz V, Lequerre T. et al. Combining anti-cyclic citrullinated peptide with the American College of Rheumatology 1987 criteria failed to improve early rheumatoid arthritis diagnosis in the community-based very early arthritis cohort. Rheumatology 2011;50:1901–7. [DOI] [PubMed] [Google Scholar]
- 14. Puchner R, Hochreiter R, Pieringer H, Vavrovsky A.. Improving patient flow of people with rheumatoid arthritis has the potential to simultaneously improve health outcomes and reduce direct costs. BMC Musculoskelet Disord 2017;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conway R, Kavanagh R, Coughlan RJ, Carey JJ.. Expanding access to rheumatology care: the rheumatology general practice toolbox. Irish Med J 2015;108:48–50. [PubMed] [Google Scholar]
- 16. Smolen JS, Landewe RBM, Bijlsma JWJ. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79. doi:10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 17. Department of Health. Report of the national task force on medical staffing (Hanly report). Ireland: Government Publications Office Dublin, 2003. [Google Scholar]
- 18. Health Service Executive. Speciality-Specific reviews (rheumatology). Dublin, Ireland: Health Service Executive, 2014. [Google Scholar]
- 19. Möttönen T, Hannonen P, Korpela M. et al. Delay to institution of therapy and induction of remission using single‐drug or combination–disease‐modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum 2002;46:894–8. [DOI] [PubMed] [Google Scholar]
- 20. Health Service Executive. Model of care for rheumatology in Ireland In: National clinical programme for rheumatology. Dublin, Ireland, 2018. https://www.hse.ie/eng/about/who/cspd/ncps/rheumatology/achievements/model-of-care-for-rheumatology-in-ireland.pdf [Google Scholar]
- 21. Xu D, Groom C.. Economic models of identification and treatment of early rheumatoid arthritis. National Audit Office 2009;1:1–41. [Google Scholar]
- 22. Sokka T, Haugeberg G, Asikainen J. et al. Similar clinical outcomes in rheumatoid arthritis with more versus less expensive treatment strategies. Observational data from two rheumatology clinics. Clin Exp Rheumatol 2013;31:409–14. [PubMed] [Google Scholar]
- 23. Sullivan SD, Mauskopf JA, Augustovski F. et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014;17:5–14. [DOI] [PubMed] [Google Scholar]
- 24. Health Information and Quality Authority. Guidelines for the budget impact analysis of health technologies in Ireland. Dublin, Ireland, 2018. https://www.hiqa.ie/reports-and-publications/health-technology-assessment/guidelines-budget-impact-analysis-health
- 25. Gillespie P, Carter L, McIntosh C, Gethin G.. Estimating the health-care costs of wound care in Ireland. J Wound Care 2019;28:324–30. [DOI] [PubMed] [Google Scholar]
- 26. Brodszky V, Baji P, Balogh O, Péntek M.. Budget impact analysis of biosimilar infliximab (CT-P13) for the treatment of rheumatoid arthritis in six Central and Eastern European countries. Eur J Health Econ 2014;15: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The Campbell and Cochrane Economics Methods Group (CCEMG) and the Evidence for Policy and Practice Information and Coordinating Centre (EPPI-Centre). Cost Converter. 2019. https://eppi.ioe.ac.uk/costconversion/default.aspx
- 28. Central Statistics Office. Population and migration estimates. Dublin, Ireland, 2019. https://www.cso.ie/en/statistics/population/populationandmigrationestimates [Google Scholar]
- 29. Abhishek A, Doherty M, Kuo CF. et al. Rheumatoid arthritis is getting less frequent—results of a nationwide population-based cohort study. Rheumatology 2017;56:736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steward K, Land M. Perceptions of patients and professionals on rheumatoid arthritis care: Kings Fund; 2009.
- 31.National Casemix Programme of the Health Services Executive (NCPHS). Ready Reckoner of Acute Hospital inpatient and daycase activity and costs (summarised by DRG) relating to 2011 costs and activities. Health Service Executive. Dublin, Ireland: Health Service Executive, 2013.
- 32. Danyliv A, Gillespie P, O'Neill C. et al. Short- and long-term effects of gestational diabetes mellitus on healthcare cost: a cross-sectional comparative study in the ATLANTIC DIP cohort. Diabet Med 2015;32:467–76. [DOI] [PubMed] [Google Scholar]
- 33. O'Brien C, Fogarty E, Walsh C. et al. The cost of the inpatient management of febrile neutropenia in cancer patients – a micro-costing study in the Irish healthcare setting. Eur J Cancer Care 2015;24:125–32. [DOI] [PubMed] [Google Scholar]
- 34. Heavey P. The Irish Healthcare System: a morality tale. Camb Q Healthc Ethics 2019;28:276–302. [DOI] [PubMed] [Google Scholar]
- 35. Horváth CZ, Sebestyén A, Österle A. et al. Economic burden of long-term care of rheumatoid arthritis patients in Hungary. Eur J Health Econ 2014;15: 131– 5. [DOI] [PubMed] [Google Scholar]
- 36. Chen CI, Wang L, Wei W, Yuce H, Phillips K.. Burden of rheumatoid arthritis among US Medicare population: co-morbidities, health-care resource utilization and costs. Rheumatol Adv Pract 2018;2:rky005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nieuwenhuis WP, van Steenbergen HW, Mangnus L. et al. Evaluation of the diagnostic accuracy of hand and foot MRI for early rheumatoid arthritis. Rheumatology 2017;56:1367–77. [DOI] [PubMed] [Google Scholar]
- 38. Li X, Anis A.. Cost sharing of prescription drugs and demand for health-care utilization among seniors with rheumatoid arthritis. Appl Econ Lett 2013;20:23–7. [Google Scholar]
- 39. Jha A, Upton A, Dunlop WCN, Akehurst R.. The budget impact of biosimilar infliximab (Remsima®) for the treatment of autoimmune diseases in five European countries. Adv Ther 2015;32:742–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Horst-Bruinsma I, Speyer I, Visser H, Breedveld F, Hazes J.. Diagnosis and course of early-onset arthritis: results of a special early arthritis clinic compared to routine patient care. Br J Rheumatol 1998;37:1084–8. [DOI] [PubMed] [Google Scholar]
- 41. Bevan S, Quadrello T, McGee R. et al. Fit for work In: Musculoskeletal disorders in the European workforce. London, UK: The Work Foundation Part of Lancaster University, 2009: 2009. [Google Scholar]
- 42. Doherty E, O'Neill C.. Estimating the health-care usage associated with osteoarthritis and rheumatoid arthritis in an older adult population in Ireland. J Public Health 2014;36:504–10. [DOI] [PubMed] [Google Scholar]
- 43. Rantalaiho V, Korpela M, Laasonen L. et al. Early combination disease-modifying antirheumatic drug therapy and tight disease control improve long-term radiologic outcome in patients with early rheumatoid arthritis: the 11-year results of the Finnish Rheumatoid Arthritis Combination Therapy trial. Arthritis Res Ther 2010;12:R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCombe G, Conneally N, Harrold A. et al. How does the introduction of free GP care for children impact on GP service provision? A qualitative study of GPs. Ir J Med Sci 2019;188:1245–9. [DOI] [PubMed] [Google Scholar]
- 45. Burke S, Barry S, Siersbaek R. et al. Sláintecare – A ten-year plan to achieve universal healthcare in Ireland. Health Policy 2018;122:1278–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.


