Abstract
Reliable biomarkers of memory decline are critical for the early detection of Alzheimer’s disease. Previous work has found three EEG measures, namely the event-related brain potential P600, suppression of oscillatory activity in the alpha frequency range (∼10 Hz) and cross-frequency coupling between low theta/high delta and alpha/beta activity, each of which correlates strongly with verbal learning and memory abilities in healthy elderly and patients with mild cognitive impairment or prodromal Alzheimer’s disease. In the present study, we address the question of whether event-related or oscillatory measures, or a combination thereof, best predict the decline of verbal memory in mild cognitive impairment and Alzheimer’s disease. Single-trial correlation analyses show that despite a similarity in their time courses and sensitivities to word repetition, the P600 and the alpha suppression components are minimally correlated with each other on a trial-by-trial basis (generally |rs| < 0.10). This suggests that they are unlikely to stem from the same neural mechanism. Furthermore, event-related brain potentials constructed from bandpass filtered (delta, theta, alpha, beta or gamma bands) single-trial data indicate that only delta band activity (1–4 Hz) is strongly correlated (r = 0.94, P < 0.001) with the canonical P600 repetition effect; event-related potentials in higher frequency bands are not. Importantly, stepwise multiple regression analyses reveal that the three event-related brain potential/oscillatory measures are complementary in predicting California Verbal Learning Test scores (overall R2’s in 0.45–0.63 range). The present study highlights the importance of combining EEG event-related potential and oscillatory measures to better characterize the multiple mechanisms of memory failure in individuals with mild cognitive impairment or prodromal Alzheimer’s disease.
Keywords: EEG, event-related potential, mild cognitive impairment, memory, Alzheimer’s disease
Xia et al. found that combining cognitive event-related potential (e.g. P600 word repetition effect) and EEG oscillatory measures (e.g. alpha suppression) of memory processes were complementary and produced highly predictive models of verbal memory abilities in an elderly cohort which included 25 persons with mild cognitive impairment or prodromal Alzheimer’s disease.
Graphical Abstract
Graphical Abstract.
Introduction
Episodic memory decline, often assessed via verbal memory tests, is typically the earliest and most prominent cognitive change that occurs before the onset of dementia of the Alzheimer’s type (Salmon and Bondi, 2009). Neuropsychological studies have shown that deficits in episodic memory can distinguish mild cognitive impairment (MCI) from normal aging and predict imminent conversion to Alzheimer’s disease (e.g. Rabin et al., 2009). Amnestic MCI is considered a precursor to Alzheimer’s disease based on the high rate of conversion (10–15% per year) from this stage to Alzheimer’s disease dementia (Petersen et al., 2001; Petersen and Knopman, 2006), and the high prevalence of Alzheimer’s disease neuropathology (Galvin et al., 2005). Sensitive neural biomarkers of episodic memory decline thus may provide a tool for accurate diagnosis of prodromal Alzheimer’s disease, which in turn would enable earlier, and potentially more effective treatment.
Scalp-recorded EEG is well-suited to provide insight into the electrophysiological basis of episodic memory decline as it reflects instantaneous scalp voltage fluctuations, mostly resulting from synchronized activity (i.e. post-synaptic potentials) of large populations of neocortical neurons. Neuronal synchronization is thought to be the fundamental mechanism of information transfer across neural circuits and networks essential for complex cognitive functions (Buzsáki, 2006). Synaptic dysfunction, one of the earliest and most dominant features of Alzheimer’s disease (Selkoe, 2002), severely compromises the integrity of neural networks underlying episodic memory among other cognitive functions (Sperling et al., 2010). A large body of research has identified EEG oscillatory and event-related brain potential (ERP) features of Alzheimer’s disease and related cognitive impairments (Olichney et al., 2011; Yener and Başar, 2013; Horváth et al., 2018). Moreover, we have identified a number of EEG/ERP features specifically associated with verbal memory impairments in individuals with MCI and prodromal Alzheimer’s disease (Olichney et al., 2008; Mazaheri et al., 2018).
Using a word repetition paradigm with semantically congruous and incongruous words, we have observed a large decrement in P600 amplitude to repeated (old), relative to new, congruous words in healthy elderly individuals; this is known as the P600 word repetition effect (Olichney et al., 2008). Similarly, oscillatory power suppression in the alpha range (9–11 Hz) has been found to be attenuated for repeated relative to new words after about 0.5 s after word onset (Mazaheri et al., 2018). Both of these repetition effects were reportedly smaller in individuals with MCI. Additionally, the P600 effect was absent in individuals with MCI who converted to Alzheimer’s disease within 3 years (Olichney et al., 2008). Word repetition typically facilitates lexical processing if the memory traces (implicit and possibly declarative) for that word were laid down upon its initial presentation (Schacter et al., 1993). Word repetition effects such as P600 and alpha suppression are hypothesized to reflect this lexical facilitation for words that have been successfully encoded.
Mazaheri et al. (2018) identified a functional connectivity measure that also was associated with verbal memory encoding, namely the cross-frequency anti-coupling between low theta/high delta power increase at the midline parietal electrode site Pz (3–5 Hz, 0.4–0.5 s after word onset) and alpha/beta suppression at the mid-frontal electrode site Fz (10–20 Hz, 0.5–1.0 s). Assessing connectivity between brain regions using scalp EEG is difficult, however, due to volume conduction: any given electrodes at the scalp pick up activity from nearby as well as distant generators/sources. One approach that circumvents this problem is to examine the trial-by-trial negative (i.e. anti) correlations between different oscillatory activities over distinct brain regions. This method, called ‘cross-frequency power correlation’ (Mazaheri et al., 2009), circumvents the volume conduction problem, given that it is highly unlikely for a common source to generate an increase in power of one frequency across one region of the brain and a simultaneous decrease of power in another frequency at a distant region.
The cross-frequency low theta/high delta to alpha/beta anti-coupling (Mazaheri et al., 2018) was quantified on a trial-by-trial basis and was present only for congruous words, i.e., words that followed a statement about a category with which they were semantically congruent, in healthy elderly participants. The low theta/high delta power increase we observed, larger for congruent than incongruent words, was thought to reflect the retrieval of semantic information associated with the target word. The suppression of beta activity over the frontal cortices may be related to semantic unification (i.e. the integration of pieces of semantic information into an unfolding representation of the context) (Wang et al., 2012). Thus, the cross-frequency anti-coupling between theta/delta power increase and alpha/beta suppression we observe may reflect the interplay between semantic retrieval and the integration of the retrieved information with the context established by the preceding statement. Based on these findings, all three EEG/ERP features appear to be related to verbal memory in individuals with MCI. Indeed, we found that all three features were strongly correlated with scores on standard neuropsychological measures of verbal memory such as the California Verbal Learning Test (CVLT; Delis et al., 2000).
In the current study, we aimed to determine if these three memory-related EEG/ERP measures contribute synergistically to the prediction of verbal memory test performance in healthy elderly individuals and in patients with MCI or prodromal Alzheimer’s disease. Before assessing this hypothesis, we tested whether the measures are independent. The independence issue arises given the similarities in timing and functional interpretation of the P600 and alpha word repetition effects. Research has demonstrated that slow ERPs can be generated by a modulation of ongoing alpha oscillations, i.e., asymmetric alpha amplitude modulation (Nikulin et al., 2007; Mazaheri and Jensen, 2008; van Dijk et al., 2010; Iemi et al., 2019). It has been proposed that as neural oscillations do not vary symmetrically around zero, modulation of such asymmetrical fluctuation by stimuli cannot be eliminated by averaging across trials as in ERP analysis. On this account, the P600 and alpha power suppression in response to words may represent different manifestations of a single neural phenomenon. In the present study, we conducted trial-by-trial correlation and bandpass filtered ERP analyses to assess the relationship between the P600 and alpha suppression effects. Stepwise multiple regression analyses were then carried out to determine whether each of the three EEG/ERP measures independently contributes to the prediction of CVLT scores.
Methods
Participants and General Procedures
EEG and behavioural data were collected from 25 patients with amnestic MCI (15 converters: mean age 74.6 years, range 60–84; 10 non-converters: mean age 71.1 years, range 55–81) and 11 healthy elderly controls (mean age 74.1 years, range 57–79) who were recruited for a previously published longitudinal study (Olichney et al., 2008). The participants were recruited primarily from the Shiley-Marcos Alzheimer’s Disease Research Center at the University of California, San Diego and provided informed consent according to the guidelines of the University of California, San Diego University of California, San Diego Human Research Protection Program. Participants received annual clinical, neurological and neuropsychological assessments, as well as evaluation of daily function using the Clinical Dementia Rating (Hughes et al., 1982), a functional activities questionnaire, or an instrumental activities of daily living scale (Pfeffer et al., 1982). All participants were screened for treatable causes of cognitive impairments such as vitamin B12 deficiency and thyroid dysfunction and underwent a brain scan (generally clinical MRI). Exclusion criteria for the Alzheimer’s Disease Research Center included stroke, epilepsy and chronic psychiatric conditions (e.g. schizophrenia, bipolar disorder). Exclusion from the EEG study also included current use of several classes of CNS-active medications (e.g. benzodiazepines).
Participants were tested annually with an EEG word repetition paradigm. At the initial baseline recording session, all participants met Petersen criteria for amnestic MCI (Petersen et al., 1999; Petersen, 2004), but not for dementia (American Psychiatric Association, 2000) or Alzheimer’s disease dementia (McKhann et al., 1984). Of the 25 MCI participants, 15 converted to Alzheimer’s disease (MCI converters) within 3 years of their initial baseline session (mean number of years 1.62 ± 0.7). A diagnosis of Alzheimer’s disease dementia was made according to National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria for probable or possible Alzheimer’s disease (McKhann et al., 1984) that require decline in two or more cognitive domains and functional decline. The remaining 10 MCI participants had all remained in the MCI stage for ≥3 years (mean follow-up = 4.9 ± 1.5 years). The normal control group comprised age-controlled robust normal elderly who remained cognitively normal (based on annual neuropsychological testing) for a minimum of 3 years after the ERP session, and for the duration of all available follow-up.
In the present study, we focus on the initial baseline EEG and neuropsychological assessment data (specifically, the CVLT) to determine if three memory-related EEG/ERP measures contribute synergistically to the prediction of verbal memory test performance in healthy elderly individuals and patients with MCI or prodromal Alzheimer’s disease. The CVLT assesses an individual’s ability to learn a 16-item word list over five presentation trials with immediate recall, recall after short (1 min) and long (20 min) delay intervals (with or without semantic cues) and to recognize the words versus distractor words (i.e. discriminability) after a delay (20 min).
Word repetition paradigm
For each trial, participants were presented with an auditory phrase describing a category (e.g. ‘a type of wood’) followed by a written target word ∼1 s later (presentation duration = 0.3 s, visual angle ∼= 0.4°). The target words were either congruous (e.g. cedar) or incongruous (e.g. pancake) with the meaning of the preceding category phrase with a probability of 0.5. Congruous and incongruous words were matched on frequency of usage (Francis and Kučera, 1982) and word length. Participants were instructed to wait for 3 s after the onset of each target word, then read the word aloud, and give a ‘yes/no’ decision indicating whether the word fit the preceding category. No time limit was imposed on response execution. One-third of the category-word pairs (50% congruous, 50% incongruous) were repeated only once, and the lag between the first and the second presentation was short (0–3 intervening trials, spanning ∼10–40 s). One-third of the category-word pairs (50% in/congruous) were repeated twice, and the lags between presentations were longer (10–13 intervening trials, spanning ∼100–140 s). One-third of the category-word pairs appeared only once. A total of 432 trials were performed in 3 blocks of 144 trials each. Further details of the experimental paradigm can be found in Olichney et al. (2000, 2008).
EEG recordings
Across participants, EEG was recorded from 19 to 32 channels including midline (Fz, Cz, Pz) and lateral (F7/F8, T5/T6, O1/O2) sites in the International 10–20 System and additional sites which approximate Broca’s area (BL/BR), Wernicke’s area (WL/WR) and Brodmann area 41 (41L/41R). EEG signals were recorded with a 250 Hz sampling rate, the band passed between 0.016 and 100 Hz, and re-referenced offline to averaged mastoids. EEG epochs were extracted time-locked to the onset of target words, 2 s before and 2 s after the word onset. Non-physiological artefacts were removed via visual inspection before the application of independent component analysis to remove eye movements (Jung et al., 2001). EEG pre-processing and artefact rejection were performed using EEGLAB (Delorme and Makeig, 2004) and Fieldtrip (Oostenveld et al., 2011).
ERP analyses
For each participant, ERPs were averaged across artefact-free epochs of congruous and incongruous words for new and repeated presentations using a pre-stimulus baseline of 100 ms. The P600 repetition effect was quantified as the difference in amplitude between new (1st presentation) and repeated (collapsing 2nd and 3rd presentations) semantically congruous words at electrode Pz within the 500–800 ms time window after word onset, as in Olichney et al. (2008). For trial-by-trial analyses, an automated denoising method (Ahmadi and Quiroga, 2013) was used to extract the P600 component from single-trials (see Results section for more details).
Oscillatory analyses
Frequency bands of interest in the present study were a priori determined as low theta/high delta (3–5 Hz), alpha (9–11 Hz) and alpha/beta (10–20 Hz), in line with Mazaheri et al. (2018). For each trial, time-frequency representations (TFRs) of power were derived using sliding Hanning tapers with varying time windows of three cycles of each frequency. Target word induced oscillatory activity was computed as the proportional change in power relative to baseline (−500 to −100 ms before word onset). Two oscillatory features, alpha suppression and cross-frequency coupling, were extracted using methods as described in Mazaheri et al. (2018). The alpha repetition effect was quantified as the difference in the magnitude of word induced alpha suppression between new words and the third presentation of the same words, collapsing across congruous and incongruous conditions. The quantification of the alpha repetition effect as in Mazaheri et al. (2018) was based on the findings that (i) the magnitude of alpha suppression diminishes with each word repetition (i.e. new > 2nd presentation > 3rd presentation; see Fig. 2 in Mazaheri et al., 2018) and (ii) the alpha repetition effect was not affected by semantic congruency. For each participant, the amplitude envelope of the early low theta/high delta activity (400–500 ms) at the midline parietal electrode (Pz) and the late alpha/beta activity (500–1000 ms) at the midline frontal electrode (Fz) were correlated (Spearman) on a trial-by-trial basis. Cross-frequency coupling was computed as a normalized correlation coefficient using Fischer’s r-to-z transformation.
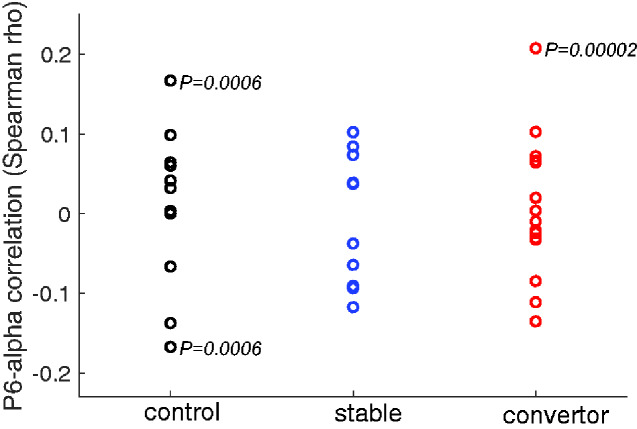
Figure 2.
Trial-by-trial correlations between the P600 and alpha suppression. Spearman correlations between the two measures at electrode Pz for each participant in the three groups. For 3 out of 36 participants, the two measures were significantly correlated across trials (uncorrected P values presented).
Frequency band-specific ERPs
Artefact-removed EEG epochs were mirror-padded to 8 s (adding 2 s to the beginning and 2 s to the end) and bandpass filtered into five conventional frequency bands (delta 1–4 Hz, theta 4–8 Hz, alpha 8–13 Hz, beta 13–30 Hz and gamma 30–45 Hz) using zero-phase Hamming-windowed sinc FIR filters as implemented in EEGLAB (pop_eegfiltnew). This function automatically selects the optimal filter order and transition bandwidth to minimize filter distortions and maximize time precision. ERPs were then computed and the P600 repetition effect quantified using the same method as described above in ERP analyses.
Statistical analyses
The analysis was focused on the three previously identified EEG/ERP measures: the P600 congruous word repetition effect, the alpha repetition effect and the cross-frequency coupling. We used a priori defined latencies and electrodes of interest for statistical testing, guided by our previous studies (Olichney et al., 2008; Mazaheri et al., 2018). Between-subjects one-way ANOVAs were used to compare the magnitude of each EEG/ERP word repetition effect between participant groups (control, MCI non-converters and MCI converters). Trial-by-trial correlations between P600 and alpha suppression were examined using Spearman’s rank correlation test, as single-trial alpha power changes were not normally distributed. Correlation coefficients for each participant were converted to z-values with Fischer’s r-to-z transform [Z = ½*ln[(1 + r)/(1 − r)]] for comparisons across groups and conditions (Mazaheri et al., 2018), using a repeated-measures ANOVA with factors of group, congruency and repetition. Bayes Factors were calculated for non-significant effects via Bayesian ANOVA using default prior probabilities in JASP version 0.10.2. Bayes factors provide relative evidence of both the null (H0) and alternative hypothesis (H1). An estimated Bayes Factor (BF10: H0/H1) value < 1 was considered as evidence in favour of H0 (Wagenmakers et al., 2018). For example, a BF10 of 0.25 indicates that the H0 is 4 times (1:0.25) more likely than the H1. Using the classification criteria of Wagenmakers et al. (2018), a BF10 between 0.33 and 1 provides anecdotal evidence for the H0 and a BF10 between 0.1 and 0.33 provides moderate evidence for H0. A BF10 < 0.10 provides strong evidence for H0.
Pearson’s correlation coefficients were used to examine the relationships between the EEG/ERP measures, as well as between these measures and neuropsychological test scores. Multiple linear regression models were constructed to examine the contribution of each EEG/ERP measure in predicting variance in the CVLT memory scores. With CVLT scores as dependent variables, the three EEG/ERP measures were each allowed to enter into or be removed from the stepwise regression models (α-to-enter: P < 0.05, α-to-remove: P ≥ 0.1) after controlling for age, education and gender. All participants across the three groups were combined in these analyses to capitalize on the heterogeneity in verbal memory abilities and electrophysiological measures.
Data availability
If required, the clinical data used in the current paper can be made available upon reasonable request. However, because of the sensitive nature of patients’ clinical information, any shared data will be de-identified and all potentially identifying personal health information removed. In addition, a shared data use agreement may be required.
Results
Participant demographics and behavioural data are shown in Table 1. Please see Olichney et al. (2008) for more information.
Table 1.
Mean (SD) values of demographics and CVLT scores in the three groups
| MCI stable | MCI converter | Control | |
|---|---|---|---|
| N | 10 | 15 | 11 |
| Age (years) | 71.1(7.5) | 74.6 (6.9) | 74.1 (6.8) |
| Sex | 4 F, 6 M | 5 F, 10 M | 7 F, 4 M |
| Education (years) | 14.3 (3.8) | 16.8 (2.8) | 15.8 (2.8) |
| CVLT list A, trials 1–5 | 38.9 (6.3) | 26.2 (8.0)* | 60.3 (10.0)** |
| CVLT short delay free recall | 5.3 (2.7) | 3.1 (2.7) | 13.2 (2.0)** |
| CVLT short delay cued recall | 7.3 (2.2) | 4.9 (3.2)* | 13.9 (1.6)** |
| CVLT long delay free recall | 5.7 (2.0) | 3.5 (2.8)* | 13.0 (2.0)** |
| CVLT long delay cued recall | 7.3 (2.4) | 3.9 (2.8)* | 13.3 (1.8)** |
| CVLT discriminability (%) | 86. (5.0) | 72.9 (15.7)* | 96.9 (4.1)** |
P < 0.05, MCI stable vs. MCI converter;
P < 0.05, MCI stable vs. control;
CVLT = California Verbal Learning Test.
Characteristics of the P600 and alpha word repetition effects
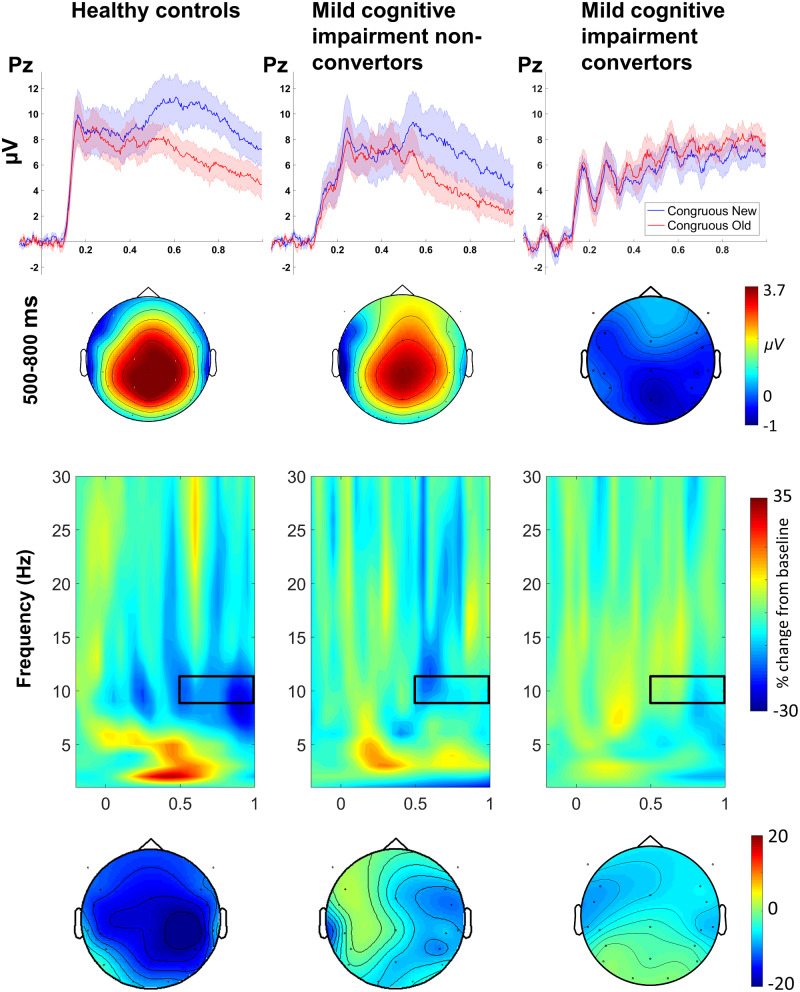
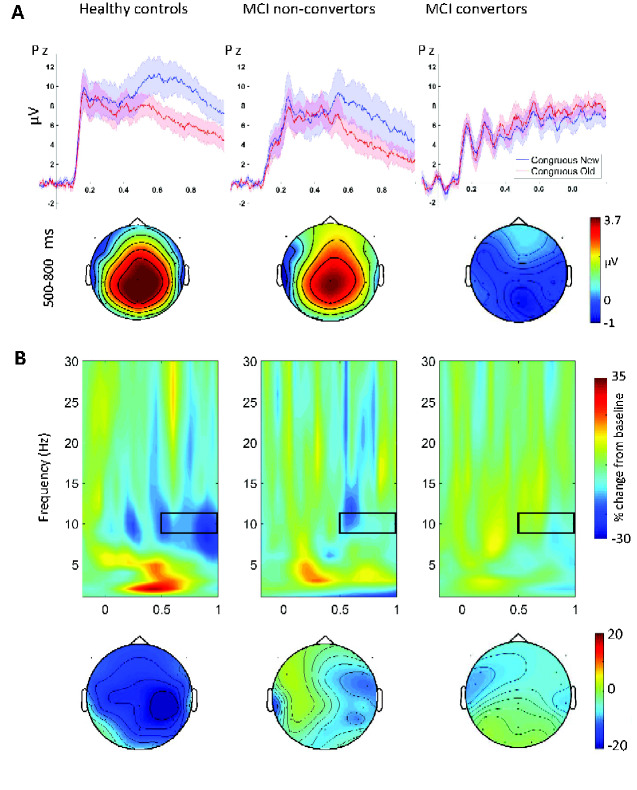
The grand averaged ERP waveforms representing the P600 repetition effect, the TFRs of the alpha suppression effect, and the corresponding scalp distribution plots are shown in Fig. 1. TFRs of power change separated for new and old words are shown in Supplementary Fig. 4. Visual inspection shows that both effects were smaller in the MCI non-converters than in healthy controls, and further diminished (alpha suppression) or even reversed (P600) in MCI converters. The onset latencies of these effects appeared to be delayed in the MCI group averages compared to those of the control group. Between-subject ANOVAs confirmed that the magnitudes of these word repetition effects differed significantly across the participant groups (P600: F(2, 33) = 9.90, P < 0.001; alpha suppression: F(2, 33) = 7.47, P < 0.005). The P600 repetition effect was diminished in MCI converters compared to healthy controls (t24 = 4.07, P < 0.001) and non-converters (t23 = 4.01, P < 0.001), and no statistical difference was found between healthy control and MCI non-converters (t19 = 0.50, P = 0.625). In contrast, the alpha suppression effect was significantly reduced in both MCI patient groups (non-converter vs. control: t19 = 2.77, P = 0.012; converter vs. control: t24 = 4.07, P < 0.001) and there was no significant difference between the two MCI patient groups (t23 = 0.83, P = 0.416).
Figure 1.
The P600 and alpha word repetition effects. (A) Upper: grand averaged ERP waveforms (standard errors presented as shaded areas) elicited by new and repeated semantically congruous words at the midline-parietal electrode Pz are shown for the three groups. Lower: scalp distributions of the P600 word repetition effect measured as the difference in amplitude between congruous new and congruous old words (new–old) from 0.5 to 0.8 s after word onset. (B) Upper: TFRs of the differences in power change between new words and the third presentations of these words (new–3rd presentation) at electrode Pz for the three groups. The alpha (9–11 Hz) suppression effects are measured between 0.5 and 1 s as highlighted by the black boxes. Lower: scalp distributions of the alpha suppression effects.
Trial-by-trial correlations between P600 and alpha suppression
For each trial, P600 was quantified as the mean amplitude at Pz in the 500–800 ms time window, and alpha suppression was quantified as the mean percentage change in alpha power at Pz during the 500–1000 ms epoch compared to the baseline (−500 to −100 ms). To overcome the low signal-to-noise ratio in single-trial ERPs, an automated denoising method was used to obtain single trial level P600 (Ahmadi and Quiroga, 2013). Following the denoising method, ERPs for new or old congruous words were subjected to multi-scale wavelet decomposition to extract frequency- and time-specific coefficients. An automatic thresholding procedure (equations as reported in Ahmadi and Quiroga, 2013) was then used to remove coefficients related to ongoing background EEG (noise). Finally, denoised wavelet coefficients were used to construct single-trial ERPs. This method has been shown to significantly improve estimates of single-trial amplitude and latency of ERP components (Ahmadi and Quiroga, 2013).
The trial-by-trial relationship between P600 and alpha suppression was assessed using Spearman’s rank-order correlation for each participant, with adjustment for multiple tests using Bonferroni correction (two-tailed α = 0.05/36 ≈ 0.0014). Trial-by-trial correlations were significant for three participants (two healthy controls and one MCI converter) out of all 36 participants (see Fig. 2). Note, however, that the directions of the three significant correlations were not consistent (i.e. two positive and one negative), and that most rho’s were small (between −0.10 and 0.10) and not statistically significant.
Since the magnitudes of the P600 and alpha suppressions effects vary with word repetition, congruency and participant group, trial-by-trial correlations between the two measures might also be affected by these experimental manipulations. To test this possibility, the correlation coefficients were normalized using Fischer’s r-to-z transformation (Supplementary Fig. 1) and subjected to repeated-measures ANOVA with factors of group, congruency and repetition. The results revealed no significant effect for any of these factors or interactions between them (F’s < 1.39, P’s > 0.26), suggesting that the trial-by-trial correlations between P600 and alpha suppression were unlikely to be influenced by experimental conditions. Bayesian repeated-measures ANOVA also showed moderate (congruency: BF10 = 0.285; repetition: BF10 = 0.239; group: BF10 = 0.163) to strong (all interactions: BF10 ≤ 0.069) evidence in favour of the null hypothesis (H0: no effect of any of the factors or interactions between factors).
Frequency band-specific ERP
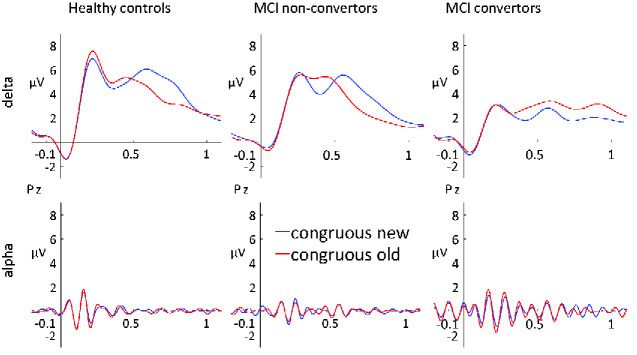
ERPs constructed from bandpass filtered single-trial data reveal that delta band activity (1–4 Hz) alone was strongly correlated (r = 0.94, P < 0.001) with the traditional P600 repetition effect. ERPs in the alpha band did not contribute to the P600 effect (r = 0.12, P = 0.50). Figure 3 shows bandpass filtered ERP repetition effects in delta and alpha bands, separated for the three participant groups (see Supplementary Fig. 2 for bandpass filtered ERP waveforms in all five frequency bands).
Figure 3.
Frequency band-specific ERPs. Delta (upper) and alpha (lower) bandpass filtered ERP waveforms elicited by new and repeated semantically congruous words at electrode Pz for the three groups.
Correlations between the EEG/ERP measures and verbal memory scores
Between the three EEG/ERP memory measures, the P600 congruous repetition effect correlated significantly with cross-frequency coupling (r = −0.34, P = 0.044) and marginally with the alpha repetition effect (r = −0.32, P = 0.06). There was no reliable relationship between the alpha repetition effect and cross-frequency coupling (r = 0.007, P = 0.97). Correlations between each of the three EEG/ERP measures and CVLT scores are displayed in Table 2 (see Supplementary Table 1 for correlations broken down by congruity and for correlations between EEG/ERP measures and subjects’ age and education). The overall correlation structure between all EEG/ERP and CVLT measures is shown in Supplementary Fig. 3. Note that cross-frequency coupling measures the anti-correlation between low theta/high delta and alpha/beta power modulation, i.e., an increase in theta/delta and decrease in alpha/beta power (relative to baseline) following word onset (see Fig. 3 in Mazaheri et al., 2018). All three EEG/ERP measures are correlated with verbal memory scores on the CVLT, raising the possibility that correlations among the electrophysiological measures could be spurious, and mediated by a shared relationship with memory ability.
Table 2.
Pearson correlations between each of the three EEG/ERP measures and CVLT scores across all subjects
| CVLT | P600 repetition effect (congruous) |
Alpha repetition effect (congruous + incongruous) |
Theta/delta (Pz) − alpha/beta (Fz) coupling (congruous) |
|||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| List A, trials 1–5 | 0.55*** | <0.001 | −0.40* | 0.016 | −0.53** | 0.001 |
| Short delay free recall | 0.43** | 0.009 | −0.45** | 0.006 | −0.50** | 0.002 |
| Short delay cued recall | 0.53** | 0.001 | −0.40* | 0.017 | −0.46** | 0.005 |
| Long delay free recall | 0.55** | 0.001 | −0.44** | 0.008 | −0.51** | 0.002 |
| Long delay cued recall | 0.59*** | <0.001 | −0.47** | 0.004 | −0.49** | 0.002 |
| Discriminability | 0.53** | 0.001 | −0.33* | 0.047 | −0.54** | 0.001 |
CVLT = California Verbal Learning Test.
P < 0.05;
P < 0.01;
P < 0.001.
Multiple regression models
Hierarchical linear regression models of verbal memory (i.e. CVLT) scores were constructed to examine the effectiveness of EEG/ERP word repetition measures in predicting memory after controlling for the demographic factors of age, education and gender (Table 3). The three demographic factors were entered first in block, and then followed by stepwise regression that allowed each of the three ERP/EEG measures to be independently entered (α-to-enter: P ≤ 0.05) or removed (α-to-remove: P ≥ 0.1). All three EEG/ERP word repetition measures contributed independently to predict CVLT learning (trials 1–5) and long-delay cued and free recall performance. The P600 congruous repetition effect and the cross-frequency coupling measure contributed independently to prediction of CVLT short-delay cued recall and recognition discriminability. The alpha suppression effect and cross-frequency coupling measure contributed independently to prediction of CVLT short-delay free recall. Note that the beta coefficients for the alpha repetition effect and cross-frequency coupling are consistently negative, as these effects are normally in the negative direction (i.e. more alpha suppression to new than repeated words and anti-coupling between early low theta/high delta synchronization and late alpha/beta desynchronization).
Table 3.
Hierarchical stepwise multiple regression models for California Verbal Learning Test (CVLT; Delis et al., 2000) scores
| Predictor variables | R 2 | Adjusted R2 | Standardized β | P-value |
|---|---|---|---|---|
| CVLT list A, trial 1–5 | ||||
| Block 1 | 0.051 | −0.038 | ||
| Age | 0.157 | 0.258 | ||
| Education | 0.242 | 0.097 | ||
| Gender (male) | −0.061 | 0.667 | ||
| P600 repetition—congruous | 0.361 | 0.278 | 0.386 | 0.012 |
| θ/δ-α/β coupling—congruous | 0.509 | 0.428 | −0.471 | 0.002 |
| Alpha repetition effect | 0.574 | 0.486 | −0.272 | 0.045 |
| CVLT short delay free recall | ||||
| Block 1 | 0.036 | −0.054 | ||
| Age | 0.189 | 0.194 | ||
| Education | 0.182 | 0.217 | ||
| Gender (male) | −0.039 | 0.797 | ||
| θ/δ-α/β coupling—congruous | 0.307 | 0.217 | −0.580 | <0.001 |
| Alpha repetition effect | 0.504 | 0.421 | −0.444 | 0.002 |
| CVLT short delay cued recall | ||||
| Block 1 | 0.031 | −0.060 | ||
| Age | 0.296 | 0.059 | ||
| Education | 0.168 | 0.292 | ||
| Gender (male) | −0.003 | 0.983 | ||
| P600 repetition—congruous | 0.318 | 0.230 | 0.477 | 0.003 |
| θ/δ-α/β coupling—congruous | 0.451 | 0.359 | −0.421 | 0.012 |
| CVLT long delay free recall | ||||
| Block 1 | 0.075 | −0.012 | ||
| Age | 0.216 | 0.106 | ||
| Education | 0.300 | 0.034 | ||
| Gender (male) | −0.134 | 0.323 | ||
| P600 repetition—congruous | 0.400 | 0.323 | 0.393 | 0.007 |
| θ/δ-α/β coupling—congruous | 0.534 | 0.456 | −0.452 | 0.002 |
| Alpha repetition effect | 0.614 | 0.534 | −0.303 | 0.021 |
| CVLT long delay cued recall | ||||
| Block 1 | 0.030 | −0.061 | ||
| Age | 0.265 | 0.044 | ||
| Education | 0.206 | 0.126 | ||
| Gender (male) | −0.016 | 0.900 | ||
| P600 repetition—congruous | 0.404 | 0.327 | 0.427 | 0.003 |
| θ/δ-α/β coupling—congruous | 0.540 | 0.464 | −0.457 | 0.002 |
| Alpha repetition effect | 0.633 | 0.558 | −0.327 | 0.011 |
| CVLT discriminability | ||||
| Block 1 | 0.095 | 0.010 | ||
| Age | 0.101 | 0.502 | ||
| Education | 0.157 | 0.318 | ||
| Gender (male) | −0.131 | 0.403 | ||
| P600 repetition—congruous | 0.334 | 0.248 | 0.428 | 0.007 |
| θ/δ-α/β coupling—congruous | 0.464 | 0.375 | −0.418 | 0.011 |
Discussion
The present study assessed relationships among three previously identified electrophysiological measures—the P600 ERP and two oscillatory effects (i.e. alpha suppression and θ/δ-α/β coupling) and their potential as biomarkers of verbal memory functioning in healthy aging, MCI and prodromal Alzheimer’s disease. All three ERP/EEG measures were significantly correlated with verbal memory abilities across individuals. Our results further suggest that the P600 and alpha word repetition effects are dissociable. Importantly, we show that combining all three measures provides the best model to account for variance in CVLT scores (overall R2’s in 0.45–0.63 range) and is superior to any single measure alone. Our results also suggest that the three EEG/ERP measures reflect different aspects of verbal memory processing, and each uniquely contributing to individual verbal memory performance.
Minimal correlation between the P600 and alpha suppression in trial-by-trial correlation analyses suggests that they are independent measures despite similarities in their time courses and scalp topographies, as well as in their correlation with memory. These word repetition effects, therefore, are not likely to be generated by the same neural mechanism as the asymmetric alpha amplitude modulation theory suggests (Mazaheri and Jensen, 2008). The dissociation between P600 and alpha suppression effects is supported by the results of our bandpass filtered ERPs analyses. The posterior P600 correlates most strongly with delta activity, and not with higher frequencies such as alpha. Research has revealed a link between event-related delta modulation and the P300 ERP component (see Güntekin and Başar, 2016 for a review). Furthermore, the present results demonstrate that the vast majority of the P600 word repetition effect is mediated by slow oscillations in the delta band. Slow oscillations are thought to underlie large-scale information integration across long time intervals and multiple neural networks (Buzsáki, 2006; Buzsáki and Watson, 2012). This aligns well with the role of delta band activity in high-level language processing such as chunking words into syntactic phrases to facilitate sentence comprehension (see Meyer, 2018 for a review). Alpha suppression, by comparison, has been related to successful sentence encoding (Vassileiou et al., 2018) and maintenance in working memory (Meltzer et al., 2017). In memory research, a decrease in alpha power often has been associated with successful encoding of individual words (Sederberg et al., 2006; Fell et al., 2008; Hanslmayr et al., 2009; Fellner et al., 2013) and has generally been taken to reflect word-level semantic processing (Klimesch, 1997; Hanslmayr et al., 2012; Hanslmayr and Staudigl, 2014; Vogelsang et al., 2018).
Functional dissociation between the P600 and alpha suppression is supported by their correlations with the CVLT measures. Consistent with our prior reports (MCI: Olichney et al., 2002, 2008; Alzheimer’s disease: Olichney et al., 2006), the magnitude of the P600 word repetition effect to congruous words are strongly correlated with all CVLT measures, but the P600 effect to incongruous words are not (Supplementary Table 1). By comparison, correlations between the alpha repetition effect and CVLT scores are strong for either congruous or incongruous words and strongest across all words. These results suggest that the P600 component is related to the successful integration of a target word with the preceding categorical statement (i.e. memory binding) which is fostered by semantically congruous words. On the other hand, alpha suppression appears sensitive to the processing of both congruous and incongruous target words. Correlations between alpha suppression and memory are stronger for incongruous than congruous words. Based on the role of alpha suppression in verbal working memory and semantic encoding as discussed above, the alpha repetition effect in the present study may reflect semantic elaboration of the novel target words during sustained attention, which in turn promotes effective memory encoding. Taken together, these results suggest that although both the P600 and alpha suppression effects may mediate verbal memory, they likely reflect different aspects of memory encoding.
Multiple regression models combining the three EEG/ERP measures accounted for 57% of the variance in CVLT total learning (the sum of the total recall across five learning trials) and 61–63% of the variance in delayed recall scores (free and cued). To demonstrate the generalizability of these models, we repeated the analyses without controlling for demographic factors. The pattern of results remains largely unchanged (overall R2s in 0.43–0.56 range across all CVLT scores) although the alpha suppression effect now also enters the short delay cued recall model as a significant predictor (results not shown). It has been shown that CVLT total learning is one of the best verbal memory measures to distinguish amnestic MCI from normal aging, whereas CVLT delayed recall is highly sensitive to imminent conversion from MCI to Alzheimer’s disease (Rabin et al., 2009). Consistent with these findings, research on 895 participants has shown that CVLT total learning was the first score to decline in preclinical Alzheimer’s disease, followed by short- and long-delayed free recall scores as a diagnosis of MCI approached (Bilgel et al., 2014). In another report, decline in immediate recall in the preclinical stage of Alzheimer’s disease was associated specifically with regional tau measures, whereas decline in delayed recall in a combined sample of prodromal Alzheimer’s disease and Alzheimer’s disease dementia was associated with β-amyloid and cortical thickness (Ossenkoppele et al., 2019). These findings suggest that various verbal memory measures and the processes they assess are selectively affected in the different stages of Alzheimer’s disease. Thus, aggregate EEG/ERP measures that index these processes may prove to be effective tools for both early diagnosis and staging of Alzheimer’s disease (Olichney et al., 2008; Mazaheri et al., 2018). Although there are a number of conventional Alzheimer’s disease biomarkers such as Amyloid-β (Aβ), tau and cortical atrophy (McDonald et al., 2012; Ossenkoppele et al., 2016, 2019; Schöll et al., 2016; Bejanin et al., 2017; Mattsson et al., 2019), the EEG/ERP measures investigated in the present study might have added value as cost-effective, sensitive biomarkers of verbal memory dysfunction in early-stage Alzheimer’s disease. Research is needed to better understand the relationship between EEG/ERP components, memory decline and underlying Alzheimer’s disease pathology.
Subtle differences in the relationships between the P600 or alpha repetition effects and individual CVLT scores were also revealed in the multiple regression analyses. The P600 was the strongest predictor of all CVLT measures except short-delay free recall, for which alpha suppression was a strong predictor. These differences could be explained by the functional dissociation between the two word repetition effects (as discussed above): the P600 effect is related to the integration of a target word with its context (e.g. the category statement), whereas alpha suppression is related to word-level processing during sustained attention. It may be that neural mechanisms supporting the target-context integration are closely linked to cued recall, which requires associative encoding between a cue and a target word, while those supporting word-level processing are critical for successful free recall after a short delay. As for the cross-frequency anti-coupling, it may be that the effect also reflects binding of the target word with the preceding context since it was found with congruous trials only, and was strongly correlated with CVLT verbal memory scores (Table 2 and Supplementary Table 1). One plausible interpretation is that the increase in posterior low theta/high delta power is related to semantic processing in parieto-temporal cortex and the late alpha/beta suppression allows encoding into working memory by the prefrontal cortex. Testing these hypotheses will advance our understanding of these EEG/ERP features and our knowledge of how to best use them in Alzheimer’s disease research.
Despite the close link to learning and memory, EEG/ERP measures are currently underutilized as biomarkers or outcome markers in Alzheimer’s disease clinical trials. One of the main limitations of using EEG/ERP as biomarkers for clinical conditions is that the inter-subject variability in EEG/ERP measures may not be tightly coupled with behaviour. This limitation is well illustrated by MacLeod and Donaldson (2017) who tested the left parietal old/new effect, widely considered the ERP signature of memory retrieval. Using two large samples of healthy young participants, these authors found that the magnitude of the left parietal old/new effect was consistently modulated by recollective processes across old/new recognition, item source accuracy and Remember/Know/Guess tasks within participants. Across participants, however, left parietal old/new effect amplitude did not correlate with recollection. These findings suggest that even though within-subject variance in the left parietal old/new effect reflect differences in recollection, across-subject variation at least in young healthy samples is likely to arise from cognitive processes beyond recollection. In contrast, significant across-subject correlation between the P600 word repetition effect and behavioural measures of verbal memory has been found in the current study, as well as in individuals with amnesia, MCI and mild Alzheimer’s disease (Olichney et al., 2000, 2002, 2006), suggesting that word repetition effects in this paradigm may provide a useful biomarker of memory decline in early Alzheimer’s disease, and perhaps other conditions. As discussed above, the P600 repetition effect is thought related to memory ‘binding’ between a word and a preceding category phrase. Such verbal associative encoding may be specifically impaired in early Alzheimer’s disease (Lowndes and Savage, 2007). In comparison, participants in a typical memory recognition task receive a retrieval cue and are asked to decide whether or not they have encountered the item before. It is possible that the left parietal old/new effect reflects cognitive processes that are not recollection per se, for example, those supporting the active maintenance of target information in working memory which facilitates retrieval. Furthermore, the behavioural measures used in the current study, i.e., CVLT scores which include free and cued recall, may be more sensitive to individual differences in verbal memory ability than those typically used in memory retrieval experiments. Future studies could test this by adding CVLT to retrieval paradigms, or including retrieval tests on words used in word repetition tasks.
A limitation of this study is the relatively small sample size of each participant group, which is a common problem in clinical patient studies. Future study will benefit from having larger samples to ensure non-biased and reproducible effects. Our published studies have shown consistently reduced or absent P600 repetition effects in MCI and Alzheimer’s disease patients (Olichney et al., 2002, 2006, 2013). The effect of alpha suppression in verbal memory encoding is also well supported in the literature (e.g. Sederberg et al., 2006; Fell et al., 2008; Hanslmayr et al., 2009; Fellner et al.,2013; Vassileiou et al., 2018). The combined utility of the three ERP/EEG measures (one fairly novel, i.e., the cross-frequency anti-coupling), however, needs to be replicated.
In conclusion, the present study reveals that the three EEG/ERP features identified during our word repetition paradigm are independent, complementary measures of verbal memory in a sample of non-demented elderly with MCI or normal cognition. Importantly, our results highlight the utility and value of combining phase-locked (i.e. ERP) and non-phase-locked EEG oscillatory measures of brain activity to characterize memory processes more comprehensively. Taken together these measures explain more than half of the variance in most of the key CVLT measures, including more than 60% of the variance in long delay free and cued recall. Our results further show that ERP and EEG oscillations capture different aspects of memory dysfunction in patients with MCI or prodromal Alzheimer’s disease. In conjunction with our previous research (Olichney et al., 2002, 2008, 2013; Mazaheri et al., 2018), we also have demonstrated the potential utility of EEG and ERP measures of memory as diagnostic tools for detecting very early-stage Alzheimer’s disease and possibly for tracking memory decline as well. EEG/ERP memory biomarkers have great potential utility, particularly for assessing treatments that aim to improve memory, synaptic plasticity, or to reduce the synaptic dysfunction thought to be characteristic of early Alzheimer’s disease.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
Special thanks to Ralph Nowacki, the Shiley-Marcos UCSD ADRC and the UC Davis Center for Mind & Brain. We would like to thank Dr. Tom Campbell for writing the program ‘Rawhide’ to convert EEG data in binary format to a format readable by EEGLab. We would also like to thank Chris Brick and Jeremy Smith for technical assistance.
Funding
This work was supported by National Institute of Health grants RO1-AG048252, RO1-AG18442, RO1-AG08313, P30 AG10129 and P30 AG062429.
Competing interests
David Salmon serves as a paid consultant for Aptinyx Inc. and Biogen Inc. All the authors report no competing interest.
Glossary
- CVLT =
California Verbal Learning Test
- ERP =
event-related potential
- MCI =
mild cognitive impairment
References
- Ahmadi M, Quiroga RQ.. Automatic denoising of single-trial evoked potentials. NeuroImage 2013; 66: 672–80. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn Washington, DC: American Psychiatric Publishing; 2000. [Google Scholar]
- Bejanin A, Schonhaut DR, La Joie R, Kramer JH, Baker SL, Sosa N, et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer's disease. Brain 2017; 140: 3286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgel M, An Y, Lang A, Prince J, Ferrucci L, Jedynak B, et al. Trajectories of Alzheimer disease-related cognitive measures in a longitudinal sample. Alzheimers Dement 2014; 10: 735–42.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Buzsáki G, Watson BO.. Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci 2012; 14: 345–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA, California Verbal Learning Test – 2nd edn (CVLT-II). San Antonio: Psychological Corporation, 2000. [Google Scholar]
- Delorme A, Makeig S.. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004; 134: 9–21. [DOI] [PubMed] [Google Scholar]
- Fell J, Ludowig E, Rosburg T, Axmacher N, Elger C.. Phase‐locking within human mediotemporal lobe predicts memory formation. Neuroimage 2008; 43: 410–9. [DOI] [PubMed] [Google Scholar]
- Fellner MC, Bauml KH, Hanslmayr S.. Brain oscillatory subsequent memory effects differ in power and long-range synchronization between semantic and survival processing. Neuroimage 2013; 79: 361–70. [DOI] [PubMed] [Google Scholar]
- Francis W, Kučera H.. Frequency analysis of English usage: Lexicon and grammar. Boston: Houghton Mifflin; 1982. [Google Scholar]
- Galvin JE Powlishta KK Wilkins K Mckeel DW Xiong C Grant E, et al. Predictors of preclinical Alzheimer disease and Dementia: A clinicopathologic study. Arch Neurol 2005; 62: 758–65. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Başar E.. Review of evoked and event-related delta responses in the human brain [Review]. Int J Psychophysiol 2016; 103: 43–52. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Spitzer B, Bauml KH.. Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb Cortex 2009; 19: 1631–40. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Fellner MC.. Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Front Hum Neurosci 2012; 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T.. How brain oscillations form memories – a processing based perspective on oscillatory subsequent memory effects. Neuroimage 2014; 85: 648–55. [DOI] [PubMed] [Google Scholar]
- Horváth A, Szucs A, Csukly G, Sákovics A, Stefanics G, Kamondi A.. EEG and ERP biomarkers of Alzheimer's disease: a critical review [Review]. Front Biosci 2018; 23: 183–220. [DOI] [PubMed] [Google Scholar]
- Hughes C, Berg L, Danziger W, Coben L, Martin R.. A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140: 566–72. [DOI] [PubMed] [Google Scholar]
- Iemi L, Busch NA, Laudini A, Haegens S, Samaha J, Villringer A, et al. Multiple mechanisms link prestimulus neural oscillations to sensory responses. eLife 2019; 8: e43620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TP, Makeig S, McKeown MJ, Bell AJ, Lee TW, Sejnowski TJ.. Imaging brain dynamics using independent component analysis. Proc IEEE 2001; 89: 1107–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG-alpha rhythms and memory processes. Int J Psychophysiol 1997; 26: 319–40. [DOI] [PubMed] [Google Scholar]
- Lowndes G, Savage G.. Early detection of memory impairment in Alzheimer's disease: a neurocognitive perspective on assessment. Neuropsychol Rev 2007; 17: 193–202. [DOI] [PubMed] [Google Scholar]
- MacLeod CA, Donaldson DI.. Investigating the functional utility of the left parietal ERP old/new effect: brain activity predicts within but not between participant variance in episodic recollection. Front Hum Neurosci 2017; 11: 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Insel PS, Donohue M, Jögi J, Ossenkoppele R, Olsson T, et al. Predicting diagnosis and cognition with 18F-AV-1451 tau PET and structural MRI in Alzheimer's disease. Alzheimer's Dement 2019; 15: 570–80. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Jensen O.. Asymmetric amplitude modulations of brain oscillations generate slow evoked responses. J Neurosci 2008; 28: 7781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Nieuwenhuis IL, van Dijk H, Jensen O.. Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Hum Brain Mapp 2009; 30: 1791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Segaert K, Olichney J, Yang J-C, Niu Y-Q, Shapiro K, et al. EEG oscillations during word processing predict MCI conversion to Alzheimer's disease. NeuroImage Clin 2018; 17: 188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E.. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34: 939–44. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Gharapetian L, McEvoy LK, Fennema-Notestine C, Hagler DJ Jr, Holland D, et al. Alzheimer's Disease Neuroimaging Initiative. Relationship between regional atrophy rates and cognitive decline in mild cognitive impairment. Neurobiol Aging 2012; 33: 242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JA, Kielar A, Panamsky L, Links KA, Deschamps T, Leigh RC.. Electrophysiological signatures of phonological and semantic maintenance in sentence repetition. Neuroimage 2017; 156: 302–14. [DOI] [PubMed] [Google Scholar]
- Meyer L. The neural oscillations of speech processing and language comprehension: state of the art and emerging mechanisms. Eur J Neurosci 2018; 48: 2609–21. [DOI] [PubMed] [Google Scholar]
- Nikulin VV, Linkenkaer-Hansen K, Nolte G, Lemm S, Müller KR, Ilmoniemi RJ, et al. A novel mechanism for evoked responses in the human brain. Eur J Neurosci 2007; 25: 3146–54. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Van Petten C, Paller KA, Salmon DP, Iragui VJ, Kutas M.. Word repetition in amnesia: electrophysiological measures of impaired and spared memory. Brain 2000; 123: 1948–63. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Morris SK, Ochoa C, Salmon DP, Thal LJ, Kutas M, et al. Abnormal verbal event related potentials in mild cognitive impairment and incipient Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2002; 73: 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Iragui VJ, Salmon DP, Riggins BR, Morris SK, Kutas M.. Absent event-related potential (ERP) word repetition effects in mild Alzheimer's disease. Clin Neurophysiol 2006; 117: 1319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Taylor JR, Gatherwright J, Salmon DP, Bressler AJ, Kutas M, et al. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology 2008; 70: 1763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Yang JC, Taylor J, Kutas M.. Cognitive event-related potentials: biomarkers of synaptic dysfunction across the stages of Alzheimer's disease. J Alzheimer's Dis 2011; 26: 215–28. [Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Pak J, Salmon DP, Yang J-C, Gahagan T, Nowacki R, et al. Abnormal P600 word repetition effect in elderly persons with preclinical Alzheimer's disease. Cogn Neurosci 2013; 4: 143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM.. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011; 2011: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 2016; 139: 1551–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Smith R, Ohlsson T, Strandberg O, Mattsson N, Insel PS, et al. Associations between tau, Aβ, and cortical thickness with cognition in Alzheimer disease. Neurology 2019; 92: e601–e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R, Smith G, Waring S, Ivnik R, Tangalos E, Kokmen E.. Mild cognitive impairment. Arch Neurol 1999; 56: 303–8. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001; 58: 1985–92. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256: 183–94. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Knopman DS.. MCI is a clinically useful concept. Int Psychogeriatr 2006; 18: 394–402. [DOI] [PubMed] [Google Scholar]
- Pfeffer R, Kurosaki T, Harrah C Jr, Chance J, Filos S.. Measurement of functional activities in older adults in the community. J Gerontol 1982; 37: 323–9. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Paré N, Saykin AJ, Brown MJ, Wishart HA, Flashman LA, et al. Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimer’s disease. Aging Neuropsychol Cogn 2009; 16: 357–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP, Bondi MW. Neuropsychological assessment of dementia. Annu Rev Psychol 2009; 60: 257–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Chiu CY, Ochsner KN.. Implicit memory: a selective review. Annu Rev Neurosci 1993; 16: 159–82. [DOI] [PubMed] [Google Scholar]
- Schöll M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, et al. PET imaging of tau deposition in the aging human brain. Neuron 2016; 89: 971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, et al. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex 2006; 17: 1190–6. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science 2002; 298: 789–91. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, et al. Functional alterations in memory networks in early Alzheimer’s disease. Neuromol Med 2010; 12: 27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Jensen O, van den Brink D, Weder N, Schoffelen J-M, Magyari L, et al. Beta oscillations relate to the N400m during language comprehension. Hum Brain Mapp 2012; 33: 2898–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, van der Werf J, Mazaheri A, Medendorp WP, Jensen O.. Modulations in oscillatory activity with amplitude asymmetry can produce cognitively relevant event-related responses. Proc Natl Acad Sci USA 2010; 107: 900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileiou B, Meyer L, Beese C, Friederici AD.. Alignment of alpha-band desynchronization with syntactic structure predicts successful sentence comprehension. NeuroImage 2018; 175: 286–96. [DOI] [PubMed] [Google Scholar]
- Vogelsang DA, Gruber M, Bergström ZM, Ranganath C, Simons JS.. Alpha oscillations during incidental encoding predict subsequent memory for new “foil” information. J Cogn Neurosci 2018; 30: 667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers EJ, Love J, Marsman M, Jamil T, Ly A, Verhagen J, et al. Bayesian inference for psychology. Part II: Example applications with JASP. Psychon Bull Rev 2018; 25: 58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yener GG, Başar E.. Biomarkers in Alzheimer's disease with a special emphasis on event-related oscillatory responses. [Review]. Suppl Clin Neurophysiol 2013; 62: 237–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
If required, the clinical data used in the current paper can be made available upon reasonable request. However, because of the sensitive nature of patients’ clinical information, any shared data will be de-identified and all potentially identifying personal health information removed. In addition, a shared data use agreement may be required.