Abstract
The formation of axons and dendrites during development, and their regeneration following injury, are energy intensive processes. The underlying assembly and dynamics of the cytoskeleton, axonal transport mechanisms and extensive signaling networks all rely on ATP and GTP consumption. Cellular ATP is generated through oxidative phosphorylation (OxP) in mitochondria, glycolysis and “regenerative” kinase systems. Recent investigations have focused on the role of the mitochondrion in axonal development and regeneration emphasizing the importance of this organelle and OxP in axon development and regeneration. In contrast, the understanding of alternative sources of ATP in neuronal morphogenesis and regeneration remains largely unexplored. This review focuses on the current state of the field of neuronal bioenergetics underlying morphogenesis and regeneration and considers the literature on the bioenergetics of non-neuronal cell motility to emphasize the potential contributions of non-mitochondrial energy sources.
Keywords: mitochondrion, growth cone, glycolysis, creatine kinase, cytoskeleton
INTRODUCTION
Neurons are characterized by complex morphologies. Following their terminal mitosis neuronal cell bodies migrate to their destination by generating leading edge processes that guide their migration (Jossin, 2020). As with other forms of cell migration, the nucleus follows in the direction of leading edge advance while the rear of the cell undergoes contraction allowing displacement of the cell as a whole. Upon arriving at their proper position neuronal cell bodies then generate multiple processes that develop into the axon and dendrites. The morphogenesis of neuronal processes is fundamental to the establishment of neuronal circuits and thus nervous system function. Depending on the neuron type, the axon can then extend up to meters in length in large animals. Dendrites tend to attain lengths from a few tens of microns to a few hundred depending on neuron type. Individual axons and dendrites form intricate patterns of branching. The branching of neuronal processes is crucial to the establishment of complex patterns of connectivity and in the case of axons allows the single axon to establish contacts with disparate targets within the nervous system. The final stage of morphogenesis involves the establishment of synaptic contacts and the refinement of neuronal projections (Riccomagno and Kolodkin, 2015). As a general rule, neurons develop more axon and dendrite branches than are present in the final configuration of the active circuit. The refinement process involves activity-dependent dynamic branch additions and retractions, even if in the absence of any further axon or dendrite elongation.
The formation of axons and dendrites involves the assembly and dynamics of the cytoskeleton (Dent and Gertler, 2003). The structure of axons and dendrites is supported by the underlying microtubule cytoskeleton. Depolymerization of microtubules results in the thinning and eventual fragmentation of axons and dendrites. The actin filament cytoskeleton is responsible for the surface features of axons and dendrites. All protrusive structures generated by axons and dendrites (e.g., filopodia, lamellipodia, synaptic structures) are dependent on actin filaments for their formation and maintenance. The tips of developing axons and dendrites are the sites of active elongation. In both cases, the tips develop structures termed growth cones.
Growth cones are highly dynamic subcellular domains that undergo complex changes in morphology and consist of a central (C) and peripheral (P) domain (Figure 1). Lamellipodia and filopodia are structures fully dependent of actin filaments for their formation and restructuring that characterize the P-domain of growth cones (Figure 1A,B) during the process of elongation and guidance to their targets. While actin filaments and thus lamellipodia and filopodia are not strictly required for axon elongation, in a context dependent manner (e.g., Abosch and Lagenaur, 1993; Selzer et al., 2006), they are required for the guidance of growth cones and are integral components of both the signaling and mechanical aspects of guidance (McCormick LE, Gupton, 2020). Myosin II is an ATPase molecular motor protein that generates contractile forces on actin filaments in an ATP hydrolysis dependent manner. Myosin II based contractility of filaments antagonizes the forward advance of axonal and dendritic growth cones and the regulation of actomyosin contractility is required for proper axon growth cone guidance (Turney and Bridgman, 2005; Loudon et al., 2006; Ketschek et al., 2007; Kollins et al., 2009; Turney et al., 2016). In growth cones the activity of myosin II at the interface of the P and C domains serves to pull the actin filaments in the P-domain toward to C-domain in a process termed actin retrograde flow (Figure1C; Lin and Forscher, 1995; Brown and Bridgman, 2003; Kerstein et al., 2015). Microtubule plus tips are highly dynamic within the C-domain of growth cones (Figure 1D) and through cycles of polymerization and depolymerization, termed dynamic instability, probe the intracellular environment and allow the subcellular targeting and delivery of cargoes rides along them. Microtubule plus tip polymerization is required for the elongation of axons, although in some cases the transport of short microtubules within the axon to its tip may suffice. Microtubule dynamic instability is required for growth cone guidance. It is generally considered that interactions between actin filaments and the plus tips of microtubules are responsible for the directed turning of growth cones in response to extracellular signals. Myosin II-driven actin retrograde flow counters the ability of microtubules plus tips to advance into the P-domain (Ketschek et al., 2007; Schaefer et al., 2008; Turney et al., 2016). Myosin II contractility at the region where the growth cone splays out from the main axon, often termed the “neck” of the growth cone, serves to maintain the growth cone polarized at the end of the axon and bundle microtubules into the axon as the growth cone advances (Burnette et al., 2008).
FIGURE 1. The growth cone cytoskeleton.
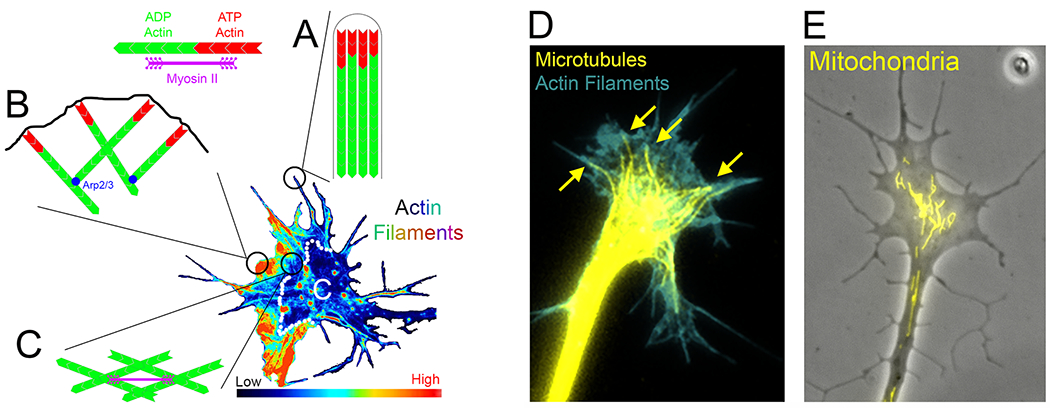
(A-C) The actin filament cytoskeleton. In the center of the (A-C) is shown a growth cone stained with fluorescent phalloidin that specifically stains actin filaments. The signal is false colored to show the relative levels of actin filaments. The C-domain is denoted by a C and the border between the C and P domains is approximated by the line of white dots. (A) Shows the organization of actin filaments at the tip of a filopodium. Within filopodia actin filaments are bundled and form an aligned array. The polymerizing barbed ends, containing ATP-bound actin, are found beneath the distal end of the filopodium just beneath the membrane. (B) In lamellipodia actin filaments are organized as mesh works of varying geometries. The Arp2/3 filament nucleating system is responsible for the array of filament in lamellipodia that have a branched organization wherein filaments extend off the sides of other filaments. Many of the polymerizing barbed ends are found in close proximity to the leading edge of the lamellipodium. (C) Within the P-domain lamellipodial filaments and the filaments that form the base of filopodia intertwine into an interconnected network. At the border between the P and C domains, where the P-domain actin filament levels decrease sharply and referred to as the transition zone, the contractile motor protein myosin II pulls the P-domain network of filaments toward the C-domain resulting in actin retrograde flow. (D) Example of a growth cone stained with phalloidin and anti-α-tubulin using a preparation protocol that removes soluble proteins (e.g., soluble tubulin) and allows clear visualization of polymerized microtubules (Gallo and Letourneau, 1999). The microtubules are organized into a bundle within the axon shaft. Upon entering the growth cone the dynamic plus ends of microtubule splay out (yellow arrowheads). (E) Example of a sensory axon and its growth cone, visualized using phase contrast optics, with mitochondria fluorescently labeled using MitoTracker Green (as in Spillane et al., 2013).
The formation of axon branches along the axon shaft, independent of the activity of the growth cone, also requires the dynamic reorganization of the axonal cytoskeleton (Armijo-Weingart L, Gallo, 2017; Menon and Gupton, 2018). Branches are initiated as filopodia that in turn emerge from transient localized “patches” of actin filaments. Axonal filopodia are transient and only a few mature into branches. The maturation requires the targeting and retention of a microtubule plus tip into the filopodium that subsequently loses its filopodial actin organization and develops a polarized accumulation of actin filaments at the tip of the nascent branch. As the branch matures further microtubules become stabilized to support continued elongation and the tip develops a growth cone, albeit usually smaller than that present at the tip of the main axon.
BIOENERGETIC DEMANDS OF AXON AND DENDRITE DEVELOPMENT
Actin is an ATPase. The loading of an actin monomer with ATP greatly increases the polymerization competency of the monomer into a filament relative to its ADP bound state (Pollard, 2016). The importance of this aspect of actin biology is emphasized by the existence of actin regulatory proteins that regulate the loading of ATP onto actin (Goldschmidt-Clermont et al., 1992). Following polymerization into a filament the ATPase activity of actin hydrolyzes the ATP to ADP and following depolymerization from the filament that actin submit must be reloaded with ATP prior to reutilization (Figure 1A,B). Similarly, tubulin is a GTPase and has similar requirements for its polymerization into a microtubule as actin into a filament (Bowne-Anderson et al., 2015). Following polymerization into a microtubule the GTPase activity of tubulin hydrolyzes the bound GTP. The dynamic plus ends of microtubules thus contain a high content of GTP-bound tubulin relative to the rest of the microtubule lattice.
A difference between microtubules and actin filaments is that the latter are overall much more dynamic and undergo much faster turnover. While there are differences in the relative durations of filaments in different actin filament based structures (e.g., lamellipodia relative to stress fibers), overall actin filaments have life spans ranging from a few seconds to minutes. These high rates of filament turnover are particularly representative of filaments underlying protrusive structures, such as the P-domain of the growth cone. In contrast, in axons and dendrites microtubules have stable lattices that undergo relatively little tubulin exchange while their plus tips are the dynamic ends of the microtubules that exhibit dynamic instability and thus depend on GTP-loaded tubulin for their dynamics. The growth cone C-domain contains mostly the dynamic plus tips of axonal microtubules. Thus, the growth cone is a subcellular domain that is a sink for both ATP and GTP utilization by cytoskeletal turnover and assembly. Having said that, the axon shaft also contains dynamic microtubule plus tips and populations of dynamic actin filaments.
The bioenergetic drain (e.g., the relative utilization of ATP/GTP levels) of cellular processes in axons and dendrites is not well understood. In developing neurons undergoing process extension, the maintenance of membrane potential through the activity of ATP utilizing Na+/K+ pump systems is estimated to account for approximately 50% of the bioenergetic drain for ATP (Bernstein and Bamburg, 2003; Engl and Attwell, 2015). Importantly, in the same developing neurons the drain of actin filament turnover is estimated to account for the remaining 50% of ATP utilization (Bernstein and Bamburg, 2003). A similar estimate for the bioenergetic drain of actin filament turnover was obtained for platelets (Daniel et al. 1986). Analysis of the relative contributions of actin turnover, through preventing filament polymerization, in slices of postnatal rat hippocampus arrived at estimates of oxygen utilization of approximately 25% (Engl et al., 2017), but this reflects the averaged bioenergetic drain of the various cell types present in slices and it should be noted that this experimental system is at post-developmental stages reflecting established circuitry and not developing axons and dendrites. The same study estimated a 22% utilization of oxygen by microtubule dependent processes as determined by treatment of the slices with high doses of the microtubule depolymerizing agent nocodazole, but this result cannot be attributed directly to the drain of microtubule dynamics as depolymerization of microtubules is expected to have occurred that would result in a variety of changes in cellular physiology including indirect alterations in the actin cytoskeleton. A specific analysis of the bioenergetic drain of microtubule dynamics under more controlled conditions is lacking, as is an analysis of the drain of axonal transport systems. Analysis of the bioenergetic drain of cytoskeletal dynamics in developing, and later stage, neurons under well controlled conditions is thus warranted. However, the current experimental evidence indicates that actin filament dynamics constitute a major bioenergetic drain in cells that exhibit high filament turnover rates such as developing neurons, and by inference specifically at the growth cone.
MITOCHONDRIA AND OXIDATIVE PHOSPHORYLATION
Although a role for mitochondria in regulating cell migration in non-neuronal cells has been thoroughly investigated (Denisenko et al., 2019; Majumdar et al., 2019), the role of the mitochondrion in neuronal migration remains minimally considered. Intriguingly, the role of mitochondria in the migration of cortical neurons to their target positions is neuron type dependent (Lin-Hendel et al., 2016). Glutamatergic projection neurons (PN) are guided to their position by radial glial fibers that the neurons attach to and follow along a relatively linear trajectory. In contrast, interneuron GABAergic neurons (IN) take a more circuitous tangential route to reach their target positions. Mitochondria in IN neurons are dynamic and undergo changes in subcellular position and target to the leading edge, while in PN neurons they tend to reside perinuclearly. Pharmacological and genetic inhibition of OxP impaired the migration of IN neurons but not PN neurons. These observations pave the way for continued analysis of the role of mitochondria and OxP in the migration of the many other neuron types during both central and peripheral nervous system development.
In fully differentiated neurons, mitochondria undergo bidirectional transport along axons and dendrites. The molecular motor systems and aspects of the regulation of mitochondrial transport are well understood (Melkov and Abdu, 2018). Mitochondria undergo long distance transport on microtubules using kinesin and dynein motors and more local movements through association with actin filament interacting myosin motors (Smith and Gallo, 2018). Mitochondria have multiple physiological roles; the regulation of cytoplasmic calcium levels, the generation of ATP and the reactive oxygen species. These roles are in turn interlinked as mitochondrial calcium levels and ROS positively and negatively regulate OxP, respectively (Griffiths and Rutter , 2009; Wang et al., 2017). Mitochondria are found interspersed throughout axons and at any given time only a subpopulation are undergoing active transport. Many mitochondria along both extending and stabilized axons integrated into circuits remain stalled in place for extended time periods in vitro and in vivo (Lewis et al., 2016; Cheng and Sheng, 2020) likely reflecting their requirement at specific subcellular locations. Similarly, mitochondria remain stalled at specific locations along dendrites as dendrites develop and incorporate into circuitry (Faits et al., 2016 ). Mitochondria undergo transport into nascent axons from the cell body soon after axon specification (Ruthel and Hollenbeck, 2003) and similarly into nascent dendrites (Fukumitsu et al., 2015). The biogenesis of mitochondria, presumably at the cell body, is required for normal axon development in cortical neurons (Vaarmann et al., 2916). The subsequent transport of mitochondria into axons and dendrites, which is under the control of TRAK1 and TRAK2 respectively, is required for normal axon and dendrite development (van Spronsen et al., 2013). In actively extending axons mitochondria exhibit higher densities at growth cones than more proximal segments of the axon shaft (Figure 1E; Morris and Hollenbeck, 1993; Ketschek and Gallo, 2010), and the membrane potential of mitochondria at the growth cone is more hyperpolarized than that along the main axon (Verburg and Hollenbeck, 2008), indicating that the intracellular environment of the growth cone positively regulates their bioenergetic output.
Inhibition of OxP in embryonic sensory neurons results in growth cone collapse (Sainath et al., 2017a), characterized by the loss of filopodia and lamellipodia and a decrease in actin filament levels in the distal axon. Using microfluidic chambers, treatment of distal axon with inhibitors of OxP independent of the cell body blocks the extension of embryonic sensory and cortical neuron axons (Zhou et al., 2016; Sainath et al., 2017a). Time lapse analysis of the distribution of mitochondria at growth cones and the extension of axons showed that mitochondria positioning to the growth cone correlates positively with bouts of axon extension (Ruthel and Hollenbeck, 2000, 2003; Sainath et al., 2017a). In axons with bifurcations, wherein the growth cone split into two giving rise to two branches of the main axon, mitochondria are preferentially sorted at the branch point into the branch that is actively undergoing extension (Ruthel and Hollenbeck, 2003). Conversely, when axon extension is halted by a physical barrier, or by pharmacologically induced growth cone collapse, mitochondria redistribute from the growth cone into the more proximal axon (Morris and Hollenbeck, 1993). The transport of mitochondria is also required for the elongation of developing dendrites in Purkinje cells and hippocampal neurons (van Spronsen et al., 2013; Fukumitsu et al., 2015).
Mitochondria have emerged as important organelles in axon regeneration and are regulated by extracellular signals that inhibit or promote regeneration. The positioning and respiration of mitochondria in distal axons is of functional significance in regenerating axons following injury (Smith and Gallo, 2018; Cheng and Sheng, 2020). Axon regeneration correlates with the positioning of mitochondria at the tip of the severed axon (Han et al., 2016) and promotion of mitochondria transport to the tips of regenerating axons increases the rate of regeneration in vitro and in vivo both in the peripheral and central nervous system (Zhou et al., 2016; Cartoni et al., 2016, 2017; Han et al., 2020). Mitochondrial calcium uptake after axon injury also promotes regenerative competency (Lee et al, 2019; Tang et al., 2020), possibly through the regulation of OxP. Chondroitin sulfate proteoglycans (CSGPs) are components of the extracellular matrix that inhibit axon extension during regenerative attempts (Tran et al., 2018) and stabilize axonal circuitry in the mature nervous system (Fawcett et al., 2019). On CSPGs mitochondria can target to growth cones but then exhibit increased rates of retrograde evacuation resulting in growth cones with lower mitochondria content than those on control growth permissive substrata (Sainath et al., 2017a). Myelin associated glycoprotein, an inhibitor of axon regeneration, and CSPGs impact the transport of mitochondria along axons in adult sensory neurons (Kalinski et al., 2019). CSPGs also induce depolarization of the mitochondrial membrane potential (Sainath et al., 2017a, 2017b; Kalinski et al., 2019) that is expected to result in decreased ATP production. In contrast, axon extension/regeneration promoting factors (e.g., neurotrophins) hyperpolarize the mitochondrial membrane potential (Huang et al., 2005; Verburg and Hollenbeck, 2008).
Axon branching is dependent on the positioning of stalled mitochondria along axons. The sites of the formation of axonal filopodia and in turn branching correlate with sites populated by stalled axonal mitochondria along both sensory and central nervous system axons (Courchet et al., 2013; Spillane et al., 2013; Tao et al., 2014). Experimentally decreasing the proportion of stalled mitochondria through suppression of syntaphilin, a microtubule based mitochondria anchoring molecule, suppresses branching of cortical axons in vitro and in vivo (Courchet et al., 2013). Inhibition of OxP prevents branching along sensory axons in response to nerve growth factor (NGF; Spillane et al., 2013). Although stalled mitochondria determine the sites of axons where branching may occur, following the initial maturation of a branchlet mitochondria undergoing transport then populate the branch as it develops further (Armijo-Weingart et al., 2019). Developing branches are preferentially populated by mitochondria representing the shorter subpopulation of axonal mitochondria (Armijo-Weingart et al., 2019). In the case of NGF induced sensory axon branching NGF drives a rapid bout of mitochondria fission and then maintains a new steady state of shorter mitochondria in axons that provide a greater number of ideally sized mitochondria for branch development (Armijo-Weingart et al., 2019). In contrast to axons, mitochondria aggregate in the proximal dendrites of cortical pyramidal neurons and locally negatively regulate branching (Kimura et al., 2014). The mechanism through which mitochondria suppress branching along proximal dendrites is not known but may involve the mitochondrion’s role in calcium buffering (Konur and Ghosh, 2005).
The contribution of mitochondrial OxP to axonal actin dynamics is local. Axonal and dendritic filopodia arise from precursors axonal actin patches (Andresen et al., 2005; Gallo, 2013). Treatment of sensory axons NGF increases the proportion of patches that form in axon segments populated by stalled mitochondria (Ketschek and Gallo, 2010). Patches that form in axon segments populated by stalled mitochondria exhibit longer durations relative to those that form in segments lacking mitochondria (Sainath et al., 2017b; Armijo-Weingart et al., 2019). CSPGs depolarize axonal mitochondria (Sainath et al., 2017a, 2017b; Kalinski et al., 2019) and decrease the duration of patches formed in association with mitochondria (Sainath et al., 2017b) but do not affect actin patches not associated with mitochondria. The effect of CSPGs on the duration of patches associated with mitochondria is reversed by pharmacologically promoting OxP (Sainath et al., 2017b). Furthermore, patches that form associated with mitochondria account for approximately 80% of sites of axonal filopodia formation from actin patches (Armijo-Weingart et al., 2019) and CSPGs suppress the formation of filopodia from patches (Sainath et al., 2017b), an effect partially reversed by pharmacologically promoting OxP. The induction of branches by NGF is dependent of the intra-axonal translation of mRNAs coding for actin regulatory proteins (Spillane et al., 2012). Stalled axonal mitochondria define hotspots of high intra-axonal translation of these mRNAs that is dependent on OxP (Spillane et al., 2013). A similar local role for mitochondria in defining sites of localized translation has also been described for dendrites (Rangaraju et al., 2019). CSPGs suppress axon branching and the intra-axonal translation of cortactin mRNA (Sainath et al., 2017b), one of the mRNAs whose mitochondria-dependent axonal translation contributes to branching. Pharmacological promotion of OxP on CSPGs restores cortactin translation along axons and the localized hotspots of translation along axons (Sainath et al., 2017b). Motile mitochondria can also contribute to axonal physiology. Mitochondria undergoing transport near a presynaptic site regulate synaptic vesicle release through local alterations in ATP levels (Sun et al., 2013).
GLYCOLYSIS
Although glycolysis yield much less ATP than OxP per molecule of glucose, the rate of ATP production is greater through glycolysis than OxP and the two processes can generate equivalent amounts of ATP over equivalent time periods (Pfeiffer et al., 2001; Epstein et al., 2014; Shestov et al., 2014; Slavov et al., 2014) and glycolytic activity can be promoted to rapidly generate ATP (Epstein et al., 2014). Glycolysis is active in developing neurons. Glycolysis contributes proportionally more to ATP levels than OxP in embryonic neurons relative to later developmental stage neurons in both rat retinal ganglion cells (E20 relative to P5; Steketee et al., 2012) and rat hippocampal neurons (E17/18 relative to P2/3; Surin et al., 2013). Glycolysis accounts for approximately 30% of cellular ATP during the early stages of cortical neuron differentiation in vitro (Agostini et al, 2016). In embryonic E12 chicken sympathetic neurons glycolysis accounts for the majority of cellular ATP (Wakade and Wakade, 1985; Wakade et al., 1985). In P1–2 rat superior cervical ganglion neurons glycolysis and OxP each contribute approximately one third and 2 thirds to ATP levels in the neurites of these neurons (Tolkovsky and Suidan, 1987). In unpublished work, using the ATP/ADP ratiometric sensor PercevalHR (Tarasov and Rutter, 2014), we find that OxP and glycolysis each account for approximately 50% of ATP in the distal axons of E7 chicken sensory neurons, respectively. These observations indicate that glycolysis may be a significant contributor to axonal bioenergetics, particularly during development. The full spectrum of the contribution of glycolysis in adult neurons remains to be determined. However, glycolysis has been involved in the regulation of synaptic physiology (reviewed in Ashrafi G, Ryan, 2017). Glycolytic enzymes associate with vesicles undergoing fast axonal transport and “on-board” glycolysis on the vesicle’s surface contributes the ATP to power its transport (Zala et al., 2013; Hinckelmann et al.k 2016). In contrast, the axonal transport of mitochondria is powered by OxP independent of glycolysis (Zala et al., 2013; Spillane et al., 2013). Membrane addition is an important aspect of axon development (Winkle and Gupton, 2016). Glycolysis may thus contribute to neuronal morphogenesis through its role in fast axonal transport, but this remains to be determined.
Recent evidence has challenged the dogma that neurons rely on astrocytic lactate during activity (Díaz-García et al., 2017; Yellen, 2018; Díaz-García CM, Yellen G, 2019), further emphasizing the emerging significance of glycolysis in neuronal function. In vitro, cortical neurons initially form multiple “minor” processes (Stage II) and subsequently one of these processes differentiates into the axon (Stage III) and the remainder become dendrites. Inhibition of glycolysis using 2-deoxyglucose treatment starting at the time of culturing prevented cortical neurons from developing beyond stage II (Agostini et al., 2016), indicating a requirement for glycolysis to commence the differentiation of axons and dendrites from the undifferentiated precursor minor processes. However, the role of glycolysis in axon and dendrite development has not been specifically addressed. The observations that during in vivo development of cortical pyramidal cell dendrites there is a low density of mitochondria is distal dendritic segments, that mitochondria inhibit the branching of the more proximal apical dendrite and that suppression of mitochondria targeting into dendrites does not adversely impact the overall growth of the dendrites (Kimura et al., 2014) raise the question of whether glycolysis or other bioenergetic sources may contribute to the development of the more apical dendrite in this neuron type. GAPDH associates with GluA2- containing AMPA receptors (Lee at al, 2016). Treatment of cultured neurons with a peptide that blocks the association of GAPDH with AMPA receptors decreases dendritic growth in vitro, results in aggregation of the axonal marker TAU-1 and diminishes growth cone morphology (Lee et al., 2016). The effects of the inhibitory peptide on glycolytic activity have not been assessed and the effects have been ascribed to changes in the acetylation of p53 downstream of the GAPDH-AMPA receptor interaction (Lee et al., 2016). Thus, it is not clear whether these data are indicative of a role for GAPDH mediated glycolysis, and perhaps unlikely.
Although the migration of whole cells is not the same process as axon extension, growth cones share similarities to the leading edges of migratory cells. Some glycolytic enzymes (e.g., phosphofructokinase, aldolase, pyruvate kinase, lactate dehydrogenase, glyceraldehyde 3-phosphate dehydrogenase) have actin binding sequences and target to subsets of actin filaments in cells (Masters et al., 1987), ideally placing them for the regulation of actin based processes related to cell motility. Cancer cells have to contend with low oxygen environments and under these conditions rely on glycolysis for their bioenergetic needs (Dias et al., 2019; Yang et al., 2020) and cell types such as renal podocytes and astrocytes can survive without OxP and rely on glycolysis (Supplie et al., 2017; Brinkkoetter et al., 2019; Yang et al., 2020). A growing literature demonstrates that the migration of cancer cells, crucial for their metastatic potential, is dependent on glycolysis (Beckner et al., 1990; Sottnik et al., 2011; Shiraishi et al., 2015; Verdone et al., 2015; Cantelmoet al., 2016; Kathagen-Buhmann et al., 2016; Zhou et al., 2016; Yan et al., 2017). A role for glycolysis in mediating cell migration is not limited to cancer cells and has been shown in the regulation of lamellipodia, filopodia and cell migration has been shown in a variety of cell types (Nguyen et al., 2000; De Bock et al., 2013; Semba et al., 2016; Kishore et al., 2017; Guak et al., 2018; Liu et al., 2019; Qiao et al., 2019). Two salient examples are renal glomerular podocytes and endothelial cells. In renal glomerular podocytes mitochondria do not target to the peripheral domains of the cell and also do not contribute to the morphology and migration of these cells which are instead under the control of glycolysis occurring the peripheral domains (Ozawa et al., 2015). Endothelial cells give rise to vasculature through their migration and tubulogenesis and derive approximately 80-90% of their ATP from glycolysis (De Bock et al., 2013). During migration endothelial cells form filopodia and lamellipodia. PFKFB3, an activator or glycolysis, and glycolytic enzymes target to the leading edge of migrating endothelial cells (De Bock et al., 2013). Suppression of glycolysis due to conditional knock out of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) in endothelial cells in vivo decreased their migration and formation of filopodia (De Bock et al., 2013). Similarly, in vitro the formation of filopodia and lamellae was also impaired.
Although it is known that glycolytic enzymes undergo axonal transport (Yuan et al., 1999; Hinckelmann et al., 2016) and most have axonal mRNAs that may undergo local translation (Gumy et al., 2011), our understanding of the distribution and subcellular targeting of glycolytic enzymes in developing or regenerating adult axons and dendrites is minimal. Hexokinase is the first enzyme in the glycolytic pathway. The activity and the targeting of hexokinase to mitochondria is required for axon extension in small and medium sized adult dorsal root ganglion neurons in vitro (Wang et al., 2008). It has been argued that reliance on glycolysis downstream of mitochondrial dysfunction contributes to the impairment of collateral sprouting and axon regeneration in diabetes (Fernyhough, 2015). Glycolytic enzymes are subcellularly recruited to synapses in response to energy stresses (Jang et al., 2016), presumably representative of a metabolic compensatory response. Investigation of the role of glycolysis in neuronal morphogenesis and regeneration is expected to be a fruitful area of future investigation.
KINASE BASED ATP REGENERATION
ATP regeneration refers to the phosphorylation of ADP to ATP by kinases. Of specific interest to the nervous system are creatine kinase and the arthropod expression specific arginine kinase. ATP regeneration system are used by cells to rapidly replenish ATP in subcellular domains that have high bioenergetic drains. Creatine kinases is localized to ion pumps in the plasma membrane, actomyosin complexes and calcium pumps in muscle cell sarcoplasmic reticulum (Andres et al., 2008). Creatine kinase has two major forms, a mitochondrial and a cytoplasmic (CK-B) that is strongly expressed in brain tissue and neurons (Wallimann and Hemmer, 1994). CK- B localizes to the membrane of electrically active cells to provide a steady supply of ATP to the pump systems that control membrane potential (Wallimann and Hemmer, 1994) and also serves to provide ATP to the actomyosin system during muscular contraction (Wallimann et al., 1983, 1984; Hornemann et al., 2000, 2003). Creatine kinase is found throughout dendrites and axons of embryonic hippocampal neurons at all stages of in vitro morphogenesis (Kupier et al, 2008). Arginine kinase was shown to target to growth cones of embryonic grasshopper neurons, align with actin filaments and target to filopodia (Wang et al., 1998). The localization of CK-B to the plasma membrane places it in an ideal location to also provide ATP for actin filament polymerization as most polymerizing filament barbed ends are located just beneath the membrane (Figure 1A,B).
The functions of ATP regenerating systems in neuronal morphogenesis have been minimally investigated. Double CK knock out mice have behavioral deficits but the underlying circuitry has not been thoroughly investigated (Streijger et al., 2005). However, pharmacological inhibition of creatine kinase or shRNA-mediated depletion of either mitochondrial or CK-B impairs the growth of the dendrites of Purkinje cells (Fukumitsu et al., 2015). Whether CK contributes to axon development has not been addressed, but loss of CK-B increases the proportion of motile mitochondria without affecting APP vesicle movements (Kuiper et al., 2008a). In contrast, roles for CK-B in promoting actin filament protrusive structures and cell motility (Kuiper et al., 2009; Venter et al., 2015) and the regulation of actin filaments during phagocytosis (Kuiper et al., 2008b) have been established in non-neuronal cells. CK-B colocalizes with actin protrusive structures and targeting it to the membrane promotes protrusive activity.
Investigation of the roles of CK and arginine kinase in neuronal morphogenesis may yield interesting new observations. Considering that these ATP regeneration systems may be acting to provide energy under conditions of energy depletion or high levels of utilization, the roles of these systems may be in the context of specific signaling cues, environments or insults (e.g., hypoglycemia). The guidance of axons involves both attractant and repellent cues. Thrombin, a signal that causes cellular responses after vascular injury and extravasation into nervous tissue, elicits contractile responses resulting in growth cone collapse and axon retraction (Jalink et al., 1994; Fritsche et al., 1999). Similarly, developmentally relevant repellent cues cause growth cone collapse and subsequent contractile actomyosin dependent retraction of the axon tip (Gallo et al., 2002; Gallo, 2006; Brown and Bridgman, 2009; Brown et al.,2009). CK-B was found to associate with PAR-1, the receptor mediating the effects of thrombin, and was required for thrombin induced contractile responses (Mahajan et al., 2000). A role for CK-B in a contractile responses is consistent with its role in locally providing ATP regeneration for actomyosin contractility in muscle cell sarcomeres (Wallimann et al., 1983, 1984; Hornemann et al., 2000, 2003). It will be of interest to consider whether other extracellular signal receptor systems of relevance to neuronal morphogenesis may associate with ATP regenerating systems and the contributions thereof to the neuronal responses to these signals.
FUTURE DIRECTIONS
This review emphasizes recent developments in the understanding of the role of mitochondria in neuronal morphogenesis and regeneration, but also highlights the relative void in the understanding of other bioenergetic sources. Figure 2 summarizes the aspects of neuronal morphogenesis to which specific ATP sources have been determined to contribute, and also notes the areas of investigation that remain unexplored/unreported (ND in Figure 2). While there is an extensive literature on the bioenergetics of the nervous system at the tissue level (for a recent and thorough review see Dienel , 2019), this knowledge base is devoid of information about the bioenergetics of specific cell types in the nervous system. As the nervous system has a high degree of cellular heterogeneity it will be important to address the issue of the bioenergetics of specific cell types during development, the response to injury and regeneration and normal activity. The availability of genetically encoded sensors for ATP, GTP and many signaling pathways and cellular processes dependent on these energy sources in conjunction with modern imaging modalities will allow for revision and updating of our understanding of the bioenergetics of the nervous system. These new developments have already led to challenges to, and revision of, prior dogma regarding neuronal bioenergetics (Díaz-García et al., 2017; Yellen, 2018; Díaz-García CM, Yellen G, 2019).
FIGURE 2.
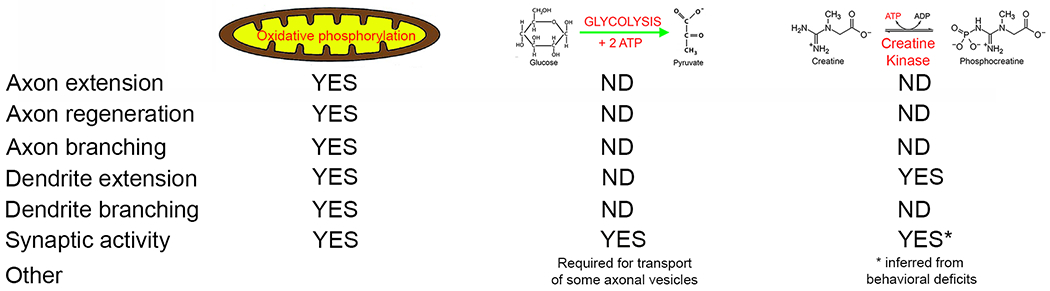
At a glance summary of investigations into the bioenergetics underlying aspects of neuronal development and regeneration. ND = not determined. YES = has been investigated and found to contribute, see main text for details.
The subcellular localization of bioenergetic sources is an important issue that promises to yield advances. Mitochondria distribution in cells is a controlled process and these organelles are recruited to subcellular sites where their functions are needed. This is also the case in developing and regenerating axons as mitochondria accumulate at growth cones, are recruited to actively growing branches of the same axon, and target to the ends of severed axons to promote their regeneration. Similarly, the localization of stalled mitochondria along axons allows these axon segments to give rise to branches. Mitochondria locally promote actin filament dynamics in axonal segments populated by mitochondria. Glycolysis is also spatio-temporally regulated. In neurons, populations of vesicles undergoing fast axonal transport associate with glycolytic enzymes and glycolysis occurs “on-board” and powers the transport of these vesicles. A number of glycolytic enzymes have actin binding domains and target to actin filaments in cells, indicating that glycolysis can be targeted to subcellular domains where actin filament dynamics are highest. Glycolytic enzymes can also be subcellularly recruited to specific sites such as synapses in response to energy stresses (Jang et al., 2016). The ATP regenerating systems are similarly targeted to subcellular domains where they are most needed. Creatine kinases associates with membranes where it is needed to power pump activity and the actomyosin system where it is required to power myosin II activity. How each one of the bioenergetic power sources available to the neuron is utilized and subcellularly regulated remains to be fully investigated and understood.
Whether bioenergetics are regulated during axon guidance has not been investigated. Although both attractant and repellent signals that can guide growth cones have been shown to alter mitochondrial membrane potential (e.g., neurotrophins, CSPGs, semaphorin 3A) whether this regulation occurs in the context of guidance remains to be directly addressed. It is possible that guidance signals may differentially impact bioenergetics across the growth cone. Single filopodial contacts with guidance cues elicit localized reorganization of the cytoskeleton and directed growth cone extension toward or away from the contact depending on the nature of the cue (Dent and Gertler, 2003). Similarly, when guidance cues are presented as soluble gradients across the growth cone they elicit graded intracellular responses. For example, some guidance signals use calcium fluxes and when presented as gradients across the growth cone establish intracellular spatio-temporal heterogeneities in calcium levels within the growth cone (Gasperini et al., 2017). Cytosolic calcium may then be taken up by mitochondria and in turn differentially control OxP in mitochondria across the growth cone. Mitochondria may also undergo regulated targeting within the growth cone during guidance. When growth cones encounter borders between a permissive substratum and CSPGs they turn and grow preferentially on the permissive substratum. Since when growth cones are growing on homogeneous CSPGs the mitochondria tend to be evacuated from growth cones, the same phenomenon may occur on a local scale within the portion of the growth cone making contact with the repellent signals (e.g., CSPGs). Alternatively, other bioenergetic sources such as glycolytic enzymes or creatine kinase may be differentially targeted or activated across the growth cone during guidance. As at least some glycolytic enzymes associate with actin filaments, and actin filaments levels are increased and decreased respectively by attractant and repellent guidance cues, the subcellular localization of the glycolytic system may regulated during guidance by changes in actin filament levels across the growth cone.
In addition to ATP, GTP is also an energy source used by the cell to regulate a variety of physiological processes of direct relevance to neuronal morphogenesis; microtubule plus tip dynamics, GTPases signaling mechanisms and protein synthesis. The understanding of the regulation of the level of GTP in developing neurons and its subcellular dynamics are an open frontier. The availability of GTP sensos for live imaging in cells should allow for rapid advance on this issue (Bianchi-Smiraglia et al., 2017). Furthermore, GTP biosynthetic enzymes are found at the leading edge of renal carcinoma cells (Wolfe et al., 2019), but their distribution in neurons is not known.
In conclusion, the understanding of the mitochondrion in neuronal morphogenesis and regeneration has advanced significantly in recent years. However, investigations into the contributions of other bioenergetic sources are lagging. The information reviewed in this manuscript indicates that glycolysis and ATP regenerating systems are likely to be of significance to neuronal morphogenesis and may also be of relevance to regeneration. As with other cellular systems, it is possible that in neurons different bioenergetic resources may be utilized in different contexts, such as different local environments, in response to different extracellular signals and at different developmental stages or following an injury. Continued analysis of neuronal bioenergetics can be expected to reveal a complex spatio-temporal and cell type specific landscape.
Acknowledgements:
work was supported by NIH awards to GG (NS095471, NS118000). The author does not have any conflicts of interests to disclose.
REFERENCES
- Abosch A, Lagenaur C. 1993. Sensitivity of neurite outgrowth to microfilament disruption varies with adhesion molecule substrate. J Neurobiol. 1993 March;24(3):344–55. [DOI] [PubMed] [Google Scholar]
- Agostini M, Romeo F, Inoue S, Niklison-Chirou MV, Elia AJ, Dinsdale D, Morone N, Knight RA, Mak TW, Melino G. 2016. Metabolic reprogramming during neuronal differentiation. Cell Death Differ. 23:1502–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen R, Li Y, Resseguie M, Brenman JE. 2005. Calcium/calmodulin-dependent protein kinase II alters structural plasticity and cytoskeletal dynamics in Drosophila. J Neurosci. 25:8878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. 2008. Functions and effects of creatine in the central nervous system. Brain Res Bull. 76:329–43. [DOI] [PubMed] [Google Scholar]
- Armijo-Weingart L, Gallo G. 2017. It takes a village to raise a branch: Cellular mechanisms of the initiation of axon collateral branches. Mol Cell Neurosci. 84:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armijo-Weingart L, Ketschek A, Sainath R, Pacheco A, Smith GM, Gallo G. 2019. Neurotrophins induce fission of mitochondria along embryonic sensory axons. Elife. 8:e49494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi G, Ryan TA. 2017. Glucose metabolism in nerve terminals. Curr Opin Neurobiol. 45:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckner ME, Stracke ML, Liotta LA, Schiffmann E. 1990. Glycolysis as primary energy source in tumor cell chemotaxis. 82:1836–40. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Actin-ATP hydrolysis is a major energy drain for neurons. J Neurosci. 23:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi-Smiraglia A, Rana MS, Foley CE, Paul LM, Lipchick BC, Moparthy S, Moparthy K, Fink EE, Bagati A, Hurley E, Affronti HC, Bakin AV, Kandel ES, Smiraglia DJ, Feltri ML, Sousa R, Nikiforov MA. 2017. Internally ratiometric fluorescent sensors for evaluation of intracellular GTP levels and distribution. Nat Methods. 14:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowne-Anderson H, Hibbel A, Howard J. 2015. Regulation of Microtubule Growth and Catastrophe: Unifying Theory and Experiment. Trends Cell Biol. 25:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkkoetter PT, Bork T, Salou S, Liang W, Mizi A, Özel C, Koehler S, Hagmann HH, Ising C, Kuczkowski A, Schnyder S, Abed A, Schermer B, Benzing T, Kretz O, Puelles VG, Lagies S, Schlimpert M, Kammerer B, Handschin C, Schell C, Huber TB. 2019. Anaerobic Glycolysis Maintains the Glomerular Filtration Barrier Independent of Mitochondrial Metabolism and Dynamics. Cell Rep. 27:1551–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Bridgman PC. 2003. Role of myosin II in axon outgrowth. J Histochem Cytochem. 51:421–8. [DOI] [PubMed] [Google Scholar]
- Brown JA, Bridgman PC. 2009. Disruption of the cytoskeleton during Semaphorin 3A induced growth cone collapse correlates with differences in actin organization and associated binding proteins. Dev Neurobiol. 69:633–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Wysolmerski RB, Bridgman PC. 2009. Dorsal root ganglion neurons react to semaphorin 3A application through a biphasic response that requires multiple myosin II isoforms. Mol Biol Cell. 20:1167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette DT, Ji L, Schaefer AW, Medeiros NA, Danuser G, Forscher P. 2008. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev Cell. 15:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, Pircher A, Chaturvedi P, Hol J, Thienpont B, Teuwen LA, Schoors S, Boeckx B, Vriens J, Kuchnio A, Veys K, Cruys B, Finotto L, Treps L, Stav-Noraas TE, Bifari F, Stapor P, Decimo I, Kampen K, De Bock K, Haraldsen G, Schoonjans L, Rabelink T, Eelen G, Ghesquière B, Rehman J, Lambrechts D, Malik AB, Dewerchin M, Carmeliet P. 2016. Inhibition of the Glycolytic Activator PFKFB3 in Endothelium Induces Tumor Vessel Normalization, Impairs Metastasis, and Improves Chemotherapy. Cancer Cell. 30:968–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni R, Norsworthy MW, Bei F, Wang C, Li S, Zhang Y, Gabel CV, Schwarz TL, He Z. 2016. The Mammalian-Specific Protein Armcx1 Regulates Mitochondrial Transport during Axon Regeneration. Neuron. 92:1294–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni R, Pekkurnaz G, Wang C, Schwarz TL, He Z. 2017. A high mitochondrial transport rate characterizes CNS neurons with high axonal regeneration capacity. PLoS One. 12:e0184672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng XT, Sheng ZH. 2020. Developmental regulation of microtubule-based trafficking and anchoring of axonal mitochondria in health and diseases. Dev Neurobiol. doi: 10.1002/dneu.22748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchet J, Lewis TL Jr, Lee S, Courchet V, Liou DY, Aizawa S, Polleux F. 2013. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell. 2013 153:1510–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JL, Molish IR, Robkin L, Holmsen H. 1986. Nucleotide exchange between cytosolic ATP and F-actin-bound ADP may be a major energy-utilizing process in unstimulated platelets. Eur J Biochem. 156:677–84. [DOI] [PubMed] [Google Scholar]
- De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquière B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P. 2013. Role of PFKFB3-driven glycolysis in vessel sprouting. 154:651–63. [DOI] [PubMed] [Google Scholar]
- Denisenko TV, Gorbunova AS, Zhivotovsky B. 2019. Mitochondrial Involvement in Migration, Invasion and Metastasis. Front Cell Dev Biol. 7:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Gertler FB. 2003. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 40:209–27. [DOI] [PubMed] [Google Scholar]
- Dias AS, Almeida CR, Helguero LA, Duarte IF. 2019. Metabolic crosstalk in the breast cancer microenvironment. Eur J Cancer. 121:154–171. [DOI] [PubMed] [Google Scholar]
- Díaz-García CM, Mongeon R, Lahmann C, Koveal D, Zucker H, Yellen G. 2017. Neuronal Stimulation Triggers Neuronal Glycolysis and Not Lactate Uptake. Cell Metab. 26:361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-García CM, Yellen G. 2019. Neurons rely on glucose rather than astrocytic lactate during stimulation. J Neurosci Res. 97:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. 2019. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol Rev. 99:949–1045. [DOI] [PubMed] [Google Scholar]
- Engl E, Attwell D. 2015. Non-signalling energy use in the brain. J Physiol. 593:3417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engl E, Jolivet R, Hall CN, Attwell D. 2017. Non-signalling energy use in the developing rat brain. J Cereb Blood Flow Metab. 37:951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein T, Xu L, Gillies RJ, Gatenby RA. 2014. Separation of metabolic supply and demand: aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane. Cancer Metab. 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faits MC, Zhang C, Soto F, Kerschensteiner D. 2016. Dendritic mitochondria reach stable positions during circuit development. Elife. 5:e11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Oohashi T, Pizzorusso T. 2019. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci. 20:451–465. [DOI] [PubMed] [Google Scholar]
- Fernyhough P 2015. Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Curr Diab Rep. 15:89. [DOI] [PubMed] [Google Scholar]
- Fritsche J, Reber BF, Schindelholz B, Bandtlow CE. 1999. Differential cytoskeletal changes during growth cone collapse in response to hSema III and thrombin. Mol Cell Neurosci. 14:398–418. [DOI] [PubMed] [Google Scholar]
- Fukumitsu K, Fujishima K, Yoshimura A, Wu YK, Heuser J, Kengaku M. 2015. Synergistic action of dendritic mitochondria and creatine kinase maintains ATP homeostasis and actin dynamics in growing neuronal dendrites. J Neurosci. 35:5707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. 1999. Different contributions of microtubule dynamics and transport to the growth of axons and collateral sprouts. J Neurosci. 19:3860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G 2006. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J Cell Sci. 119:3413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G 2013. Mechanisms underlying the initiation and dynamics of neuronal filopodia: from neurite formation to synaptogenesis. Int Rev Cell Mol Biol. 301:95–156. [DOI] [PubMed] [Google Scholar]
- Gallo G, Yee HF Jr, Letourneau PC. 2002. Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J Cell Biol. 158:1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperini RJ, Pavez M, Thompson AC, Mitchell CB, Hardy H, Young KM, Chilton JK, Foa L. 2017. How does calcium interact with the cytoskeleton to regulate growth cone motility during axon pathfinding? Mol Cell Neurosci. 84:29–35. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont PJ, Furman MI, Wachsstock D, Safer D, Nachmias VT, Pollard TD. 1992. The control of actin nucleotide exchange by thymosin beta 4 and profilin. A potential regulatory mechanism for actin polymerization in cells. Mol Biol Cell. 3:1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths EJ, Rutter GA. 2009. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim Biophys Acta. 1787(11):1324–33. [DOI] [PubMed] [Google Scholar]
- Guak H, Al Habyan S, Ma EH, Aldossary H, Al-Masri M, Won SY, Ying T, Fixman ED, Jones RG, McCaffrey LM, Krawczyk CM. 2018. Glycolytic metabolism is essential for CCR7 oligomerization and dendritic cell migration. Nat Commun. 9:2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumy LF, Yeo GS, Tung YC, Zivraj KH, Willis D, Coppola G, Lam BY, Twiss JL, Holt CE, Fawcett JW. 2011. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA. 17:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SM, Baig HS, Hammarlund M. 2016. Mitochondria Localize to Injured Axons to Support Regeneration. Neuron. 92:1308–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Xie Y, Ordaz JD, Huh AJ, Huang N, Wu W, Liu N, Chamberlain KA, Sheng ZH, Xu XM. 2020. Restoring Cellular Energetics Promotes Axonal Regeneration and Functional Recovery after Spinal Cord Injury. Cell Metab. 31:623–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckelmann MV, Virlogeux A, Niehage C, Poujol C, Choquet D, Hoflack B, Zala D, Saudou F. 2016. Self-propelling vesicles define glycolysis as the minimal energy machinery for neuronal transport. Nat Commun. 7:13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornemann T, Stolz M, Wallimann T. 2000. Isoenzyme-specific interaction of muscle-type creatine kinase with the sarcomeric M-line is mediated by NH2-terminal lysine charge-clamps. J. Cell Biol. 149:1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornemann T, Kempa S, Himmel M, Hayess K, Furst DO, Wallimann T. 2003. Muscle-type creatine kinase interacts with central domains of the M-band proteins myomesin and M-protein. J. Mol. Biol 332:877–887 [DOI] [PubMed] [Google Scholar]
- Huang TJ, Verkhratsky A, Fernyhough P. 2005. Insulin enhances mitochondrial inner membrane potential and increases ATP levels through phosphoinositide 3-kinase in adult sensory neurons. Mol Cell Neurosci. 28:42–54. [DOI] [PubMed] [Google Scholar]
- Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. 1994. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 126:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Nelson JC, Bend EG, Rodríguez-Laureano L, Tueros FG, Cartagenova L, Underwood K, Jorgensen EM, Colón-Ramos DA. 2016. Glycolytic Enzymes Localize to Synapses under Energy Stress to Support Synaptic Function. Neuron. 90:278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Selzer ME, Gallo G. 2006. Developmental regulation of sensory axon regeneration in the absence of growth cones. J Neurobiol. 66:1630–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y 2020. Molecular mechanisms of cell polarity in a range of model systems and in migrating neurons. Mol Cell Neurosci. 106:103503. [DOI] [PubMed] [Google Scholar]
- Kalinski AL, Kar AN, Craver J, Tosolini AP, Sleigh JN, Lee SJ, Hawthorne A, Brito-Vargas P, Miller-Randolph S, Passino R, Shi L, Wong VSC, Picci C, Smith DS, Willis DE, Havton LA, Schiavo G, Giger RJ, Langley B, Twiss JL. 2019. Deacetylation of Miro1 by HDAC6 blocks mitochondrial transport and mediates axon growth inhibition. J Cell Biol. 218:1871–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathagen-Buhmann A, Schulte A, Weller J, Holz M, Herold-Mende C, Glass R, Lamszus K. 2016. Glycolysis and the pentose phosphate pathway are differentially associated with the dichotomous regulation of glioblastoma cell migration versus proliferation. Neuro Oncol. 18:1219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstein PC, Nichol RH IV, Gomez TM. 2015. Mechanochemical regulation of growth cone motility. Front Cell Neurosci. 9:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek A, Gallo G. 2010. Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia. J Neurosci. 30:12185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek AR, Jones SL, Gallo G. 2007. Axon extension in the fast and slow lanes: substratum-dependent engagement of myosin II functions. Dev Neurobiol. 67:1305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Murakami F. 2014. Evidence that dendritic mitochondria negatively regulate dendritic branching in pyramidal neurons in the neocortex. J Neurosci. 34:6938–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore M, Cheung KCP, Fu H, Bonacina F, Wang G, Coe D, Ward EJ, Colamatteo A, Jangani M, Baragetti A, Matarese G, Smith DM, Haas R, Mauro C, Wraith DC, Okkenhaug K, Catapano AL, De Rosa V, Norata GD, Marelli-Berg FM. 2017. Regulatory T Cell Migration Is Dependent on Glucokinase-Mediated Glycolysis. Immunity. 47:875–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins KM, Hu J, Bridgman PC, Huang YQ, Gallo G. 2009. Myosin-II negatively regulates minor process extension and the temporal development of neuronal polarity. Dev Neurobiol. 69:279–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konur S, Ghosh A. 2005. Calcium signaling and the control of dendritic development. Neuron. 46:401–5. [DOI] [PubMed] [Google Scholar]
- Kuiper JW, Oerlemans FT, Fransen JA, Wieringa B. 2008a. Creatine kinase B deficient neurons exhibit an increased fraction of motile mitochondria. BMC Neurosci. 9:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JW, Pluk H, Oerlemans F, van Leeuwen FN, de Lange F, Fransen J, Wieringa B. 2008b. Creatine kinase-mediated ATP supply fuels actin-based events in phagocytosis. PLoS Biol. 6:e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JW, van Horssen R, Oerlemans F, Peters W, van Dommelen MM, te Lindert MM, ten Hagen TL, Janssen E, Fransen JA, Wieringa B. 2009. Local ATP generation by brain-type creatine kinase (CK-B) facilitates cell motility. PLoS One. 4:e5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FH, Su P, Xie YF, Wang KE, Wan Q, Liu F. 2016. Disrupting GluA2-GAPDH Interaction Affects Axon and Dendrite Development. Sci Rep. 6:30458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Wang W, Hwang J, Namgung U, Min KT. 2019. Increased ER-mitochondria tethering promotes axon regeneration. Proc Natl Acad Sci U S A. 116:16074–16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Forscher P. 1995. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron. 14:763–71. [DOI] [PubMed] [Google Scholar]
- Lin-Hendel EG, McManus MJ, Wallace DC, Anderson SA, Golden JA. 2016. Differential Mitochondrial Requirements for Radially and Non-radially Migrating Cortical Neurons: Implications for Mitochondrial Disorders. Cell Rep. 15:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang X, Chen K, Cheng Y, Liu S, Xia M, Chen Y, Zhu H, Li Z, Cao X. 2019. CCR7 Chemokine Receptor-Inducible lnc-Dpf3 Restrains Dendritic Cell Migration by Inhibiting HIF-1α-Mediated Glycolysis. Immunity. 50:600–615 [DOI] [PubMed] [Google Scholar]
- Masters CJ, Reid S, Don M. 1987. Glycolysis--new concepts in an old pathway. Mol Cell Biochem. 76:3–14. [DOI] [PubMed] [Google Scholar]
- Loudon RP, Silver LD, Yee HF Jr, Gallo G. 2006. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol. 66:847–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan VB, Pai KS, Lau A, Cunningham DD. 2000. Creatine kinase, an ATP-generating enzyme, is required for thrombin receptor signaling to the cytoskeleton. Proc Natl Acad Sci U S A. 97:12062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick LE, Gupton SL. 2020. Mechanistic advances in axon pathfinding. Curr Opin Cell Biol. 63:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkov A, Abdu U. 2018. Regulation of long-distance transport of mitochondria along microtubules. Cell Mol Life Sci. 75:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S, Gupton S. 2018. Recent advances in branching mechanisms underlying neuronal morphogenesis. F1000Res. 7:F1000 Faculty Rev-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. 1993. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci. 104:917–27. [DOI] [PubMed] [Google Scholar]
- Majumdar R, Steen K, Coulombe PA, Parent CA. 2019. Non-canonical processes that shape the cell migration landscape. Curr Opin Cell Biol. 57:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TN, Wang HJ, Zalzal S, Nanci A, Nabi IR. 2000. Purification and characterization of beta-actin-rich tumor cell pseudopodia: role of glycolysis. Exp Cell Res. 258:171–83. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Ueda S, Imamura H, Mori K, Asanuma K, Yanagita M, Nakagawa T. 2015. Glycolysis, but not Mitochondria, responsible for intracellular ATP distribution in cortical area of podocytes. Sci Rep. 5:18575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer T, Schuster S, Bonhoeffer S. 2001. Cooperation and competition in the evolution of ATP-producing pathways. Science 292:504–507. [DOI] [PubMed] [Google Scholar]
- Pollard TD. 2016. Actin and Actin-Binding Proteins. Cold Spring Harb Perspect Biol. 8:a018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, He X, Zhang Q, Yuan H, Wang D, Li L, Hui Y, Wu Z, Li W, Zhang N. 2019. Alpha-synuclein induces microglial migration via PKM2-dependent glycolysis. Int J Biol Macromol. 129:601–607. [DOI] [PubMed] [Google Scholar]
- Rangaraju V, Lauterbach M, Schuman EM. 2019. Spatially Stable Mitochondrial Compartments Fuel Local Translation during Plasticity. Cell. 176:73–84. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Kolodkin AL. 2015. Sculpting neural circuits by axon and dendrite pruning. Annu Rev Cell Dev Biol. 31:779–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. 2000. Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons. J Neurosci. 20:2266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. 2003. Response of mitochondrial traffic to axon determination and differential branch growth. J Neurosci. 23:8618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainath R, Armijo-Weingart L, Ketscheck A, Xu Z, Li S, Gallo G. 2017a. Chondroitin sulfate proteoglycans negatively regulate the positioning of mitochondria and endoplasmic reticulum to distal axons. Dev Neurobiol. 77:1351–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainath R, Ketschek A, Grandi L, Gallo G. 2017b. CSPGs inhibit axon branching by impairing mitochondria-dependent regulation of actin dynamics and axonal translation. Dev Neurobiol. 77:454–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AW, Schoonderwoert VT, Ji L, Mederios N, Danuser G, Forscher P. 2008. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev Cell. 15:146–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba H, Takeda N, Isagawa T, Sugiura Y, Honda K, Wake M, Miyazawa H, Yamaguchi Y, Miura M, Jenkins DM, Choi H, Kim JW, Asagiri M, Cowburn AS, Abe H, Soma K, Koyama K, Katoh M, Sayama K, Goda N, Johnson RS, Manabe I, Nagai R, Komuro I. 2016. HIF-1α-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat Commun. 7:11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestov AA, Liu X, Ser Z, Cluntun AA, Hung YP, Huang L, Kim D, Le A, Yellen G, Albeck JG, Locasale JW. 2014. Quantitative determinants of aerobic glycolysis identify flux through the enzyme GAPDH as a limiting step. Elife. 3:e03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi T, Verdone JE, Huang J, Kahlert UD, Hernandez JR, Torga G, Zarif JC, Epstein T, Gatenby R, McCartney A, Elisseeff JH, Mooney SM, An SS, Pienta KJ. 2015. Glycolysis is the primary bioenergetic pathway for cell motility and cytoskeletal remodeling in human prostate and breast cancer cells. Oncotarget. 6:130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavov N, Budnik BA, Schwab D, Airoldi EM, van Oudenaarden A. 2014. Constant growth rate can be supported by decreasing energy flux and increasing aerobic glycolysis. Cell Rep. 7:705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Gallo G. 2018. The role of mitochondria in axon development and regeneration. Dev Neurobiol. 78:221–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottnik JL, Lori JC, Rose BJ, Thamm DH. 2011. Glycolysis inhibition by 2-deoxy-D-glucose reverts the metastatic phenotype in vitro and in vivo. Clin Exp Metastasis. 28:865–75. [DOI] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Donnelly CJ, Pacheco A, Twiss JL, Gallo G. 2012. Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. J Neurosci. 32:17671–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G. 2013. Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep. 5:1564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streijger F, Oerlemans F, Ellenbroek BA, Jost CR, Wieringa B, Van der Zee CE. 2005. Structural and behavioural consequences of double deficiency for creatine kinases BCK and UbCKmit. Behav Brain Res. 157:219–34. [DOI] [PubMed] [Google Scholar]
- Steketee MB, Moysidis SN, Weinstein JE, Kreymerman A, Silva JP, Iqbal S, Goldberg JL. 2012. Mitochondrial dynamics regulate growth cone motility, guidance, and neurite growth rate in perinatal retinal ganglion cells in vitro. Invest Ophthalmol Vis Sci. 53:7402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH. 2013. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 4:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supplie LM, Düking T, Campbell G, Diaz F, Moraes CT, Götz M, Hamprecht B, Boretius S, Mahad D, Nave KA. 2017. Respiration-Deficient Astrocytes Survive As Glycolytic Cells In Vivo. J Neurosci. 37:4231–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surin AM, Khiroug S, Gorbacheva LR, Khodorov BI, Pinelis VG, Khiroug L. 2013. Comparative analysis of cytosolic and mitochondrial ATP synthesis in embryonic and postnatal hippocampal neuronal cultures. Front Mol Neurosci. 5:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang NH, Kim KW, Xu S, Blazie SM, Yee BA, Yeo GW, Jin Y, Chisholm AD. 2020. The mRNA Decay Factor CAR-1/LSM14 Regulates Axon Regeneration via Mitochondrial Calcium Dynamics. Curr Biol. 30(5):865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao K, Matsuki N, Koyama R. 2014. AMP-activated protein kinase mediates activity-dependent axon branching by recruiting mitochondria to axon. Dev Neurobiol. 74:557–73. [DOI] [PubMed] [Google Scholar]
- Tarasov AI, Rutter GA. 2014. Use of genetically encoded sensors to monitor cytosolic ATP/ADP ratio in living cells. Methods Enzymol. 542:289–311. [DOI] [PubMed] [Google Scholar]
- Tolkovsky AM, Suidan HS. 1987. Adenosine 5′-triphosphate synthesis and metabolism localized in neurites of cultured sympathetic neurons. Neuroscience 23:1133–42. [DOI] [PubMed] [Google Scholar]
- Tran AP, Warren PM, Silver J. 2018. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol Rev. 98:881–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turney SG, Bridgman PC. 2005. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat Neurosci. 8:717–9. [DOI] [PubMed] [Google Scholar]
- Turney SG, Ahmed M, Chandrasekar I, Wysolmerski RB, Goeckeler ZM, Rioux RM, Whitesides GM, Bridgman PC. 2016. Nerve growth factor stimulates axon outgrowth through negative regulation of growth cone actomyosin restraint of microtubule advance. Mol Biol Cell. 27:500–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaarmann A, Mandel M, Zeb A, Wareski P, Liiv J, Kuum M, Antsov E, Liiv M, Cagalinec M, Choubey V, Kaasik A. 2016. Mitochondrial biogenesis is required for axonal growth. Development. 143:1981–92. [DOI] [PubMed] [Google Scholar]
- van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, Kuijpers M, Wulf PS, Keijzer N, Demmers J, Kapitein LC, Jaarsma D, Gerritsen HC, Akhmanova A, Hoogenraad CC. 2013. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron. 77:485–502. [DOI] [PubMed] [Google Scholar]
- Venter G, Polling S, Pluk H, Venselaar H, Wijers M, Willemse M, Fransen JA, Wieringa B. 2015. Submembranous recruitment of creatine kinase B supports formation of dynamic actin-based protrusions of macrophages and relies on its C-terminal flexible loop. Eur J Cell Biol. 94:114–27. [DOI] [PubMed] [Google Scholar]
- Verburg J, Hollenbeck PJ. 2008. Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J Neurosci. 28:8306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdone JE, Zarif JC, Pienta KJ. 2015. Aerobic glycolysis, motility, and cytoskeletal remodeling Cell Cycle. 14:169–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade AR, Prat JC, Wakade TD. 1985. Sympathetic neurons extend neurites in a culture medium containing cyanide and dinitrophenol but not iodoacetate. FEBS Lett. 190:95–8. [DOI] [PubMed] [Google Scholar]
- Wakade AR, Wakade TD. 1985. Sympathetic neurons grown in culture generate ATP by glycolysis: correlation between ATP content and [3H]norepinephrine uptake and storage. Brain Res. 359:397–401. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Moser H, Eppenberger HM. 1983. Isozyme-specific localization of M-line bound creatine kinase in myogenic cells. J. Muscle Res. Cell Motil 4:429–441 [DOI] [PubMed] [Google Scholar]
- Wallimann T, Schlosser T, Eppenberger HM. 1984. Function of M-line-bound creatine kinase as intramyofibrillar ATP regenerator at the receiving end of the phosphorylcreatine shuttle in muscle. J. Biol. Chem 259:5238–5246. [PubMed] [Google Scholar]
- Wallimann T, Hemmer W. 1994. Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem. 133–134:193–220. [DOI] [PubMed] [Google Scholar]
- Wang YE, Esbensen P, Bentley D. 1998. Arginine kinase expression and localization in growth cone migration. J Neurosci. 18:987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gardiner NJ, Fernyhough P. 2008. Blockade of hexokinase activity and binding to mitochondria inhibits neurite outgrowth in cultured adult rat sensory neurons. Neurosci Lett. 434:6–11. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang X, Wu D, Huang Z, Hou T, Jian C, Yu P, Lu F, Zhang R, Sun T, Li J, Qi W, Wang Y, Gao F, Cheng H. 2017. Mitochondrial flashes regulate ATP homeostasis in the heart. Elife. 6:e23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle CC, Gupton SL. 2016. Membrane Trafficking in Neuronal Development: Ins and Outs of Neural Connectivity. Int Rev Cell Mol Biol. 322:247–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe K, Kofuji S, Yoshino H, Sasaki M, Okumura K, Sasaki AT. 2019. Dynamic compartmentalization of purine nucleotide metabolic enzymes at leading edge in highly motile renal cell carcinoma. Biochem Biophys Res Commun. 516(1):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XL, Zhang XB, Ao R, Guan L. 2017. Effects of shRNA-Mediated Silencing of PKM2 Gene on Aerobic Glycolysis, Cell Migration, Cell Invasion, and Apoptosis in Colorectal Cancer Cells. J Cell Biochem. 118:4792–4803. [DOI] [PubMed] [Google Scholar]
- Yang J, Ren B, Yang G, Wang H, Chen G, You L, Zhang T, Zhao Y. 2020. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol Life Sci. 77:305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G 2018. Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J Cell Biol. 217:2235–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Mills RG, Bamburg JR, Bray JJ. 1999. Cotransport of glyceraldehyde-3-phosphate dehydrogenase and actin in axons of chicken motoneurons. Cell Mol Neurobiol. 19:733–44. [DOI] [PubMed] [Google Scholar]
- Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelières FP, Marco S, Saudou F. 2013. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 152:479–91. [DOI] [PubMed] [Google Scholar]
- Zhou B, Yu P, Lin MY, Sun T, Chen Y, Sheng ZH. 2016. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J Cell Biol. 214:103–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Jiang S, Fu Q, Smith K, Tu K, Li H, Zhao Y. 2016b. FASN, ErbB2-mediated glycolysis is required for breast cancer cell migration. Oncol Rep. 35:2715–22. [DOI] [PubMed] [Google Scholar]


