Abstract
Psychiatric and neurologic disorders are often characterized by both neuroinflammation and cognitive dysfunction. To date, however, the relationship between neuroinflammation and cognitive dysfunction remains understudied in humans. Preclinical research indicates that experimental induction of neuroinflammation reliably impairs memory processes. In this paradigm development study, we translated those robust preclinical findings to humans using positron emission tomography (PET) imaging with [11C]PBR28, a marker of microglia, and lipopolysaccharide (LPS), a potent neuroimmune stimulus. In a sample of 18 healthy adults, we confirmed our previous findings that LPS administration increased whole-brain [11C]PBR28 availability by 3150%, demonstrating a robust neuroimmune response (Cohen’s ds>1.6). We now show that LPS specifically impaired verbal learning and recall, hippocampal memory processes, by 11% and 22%, respectively (Cohen’s ds>0.9), but did not alter attention, motor, or executive processes. The LPS-induced increase in [11C]PBR28 binding was correlated with significantly greater decrements in verbal learning performance in the hippocampus (r=−0.52, p=.028), putamen (r=−0.50, p=.04), and thalamus (r=−0.55, p=.02). This experimental paradigm may be useful in investigating mechanistic relationships between neuroinflammatory signaling and cognitive dysfunction in psychiatric and neurologic disorders. It may also provide a direct approach to evaluate medications designed to rescue cognitive deficits associated with neuroinflammatory dysfunction.
Keywords: neuroinflammation, hippocampus, verbal memory, PBR28, microglia
Introduction
Aberrant neuroimmune signaling is implicated in cognitive dysfunction that is characteristic of both neurologic (e.g., Alzheimer’s disease) and psychiatric (e.g., major depression) disorders (McAfoose and Baune, 2009). However, these relationships are complex and clinical investigations conducted to date have been limited, perhaps due to the absence of human experimental models. Lipopolysaccharide (LPS) administration produces a robust proinflammatory response, which can be quantified using positron emission tomography (PET) imaging with [11C]PBR28, a radiotracer that binds the 18kDa translocator protein (TSPO), a marker found primarily in microglia, but also present in endothelial cells, neurons, and astrocytes (Hannestad et al., 2012; Hillmer et al., 2017; Sandiego et al., 2015; Tournier et al., 2019). LPS administration increases proinflammatory cytokine/chemokine levels (Sandiego et al., 2015), upregulates brain TSPO availability (Hannestad et al., 2012; Sandiego et al., 2015), and activates microglia (Hannestad et al., 2012; Tournier et al., 2019). Using fluorescence-activated cell sorting and TSPO imaging, Tournier and colleagues showed that the cellular source of LPS-induced TSPO increase was microglial proliferation and that TSPO binding did not change in neurons, astrocytes, or endothelial cells after LPS (Tournier et al., 2019). Whereas LPS-induced neuroimmune stimulation reliably impairs cognitive function in animals, especially hippocampal memory processes (Jo et al., 2001); the extent to which these brain-behavior relationships translate to humans is not known.
To address this gap, we developed an experimental paradigm to determine the effect of proinflammatory signaling, induced by systemic LPS administration, on cognitive function and TSPO availability in healthy adults. In this paradigm, the magnitude of LPS-induced neuroimmune stimulation was quantified via brain PET TPSO imaging with [11C]PBR28. This within-subject, repeated-measures design facilitated quantification of the main effects of LPS on cognitive function and regional TSPO availability, as well as examination of correlations between the change in cognitive performance and regional TSPO availability. For each cognitive domain affected by LPS, follow-up brain-behavior correlation analyses investigated the relationship between the magnitude change in cognitive performance and TSPO availability in the brain region/s thought to underly that cognitive domain.
Materials and Methods
Healthy adult volunteers (N=18; 11 ‘high’ and 7 ‘mixed’ affinity binders) were 28.0±8.3 years old [range=19–50], mostly male (15M; 83.3%) and Caucasian (38.9%), and on average, had normal body mass (BMI=26.7±2.64 [range=22.1–32.0]). Eligibility for this study has been described previously(Sandiego et al., 2015). Briefly, subjects were medically- and psychiatrically-healthy, and did not use anti-inflammatory medications within 72hr of this study. Subjects were genotyped for the rs6971 polymorphism and [11C]PBR28 low affinity binders were excluded. The Yale School of Medicine Human Investigation and Radioactive Drug Research Committees approved this study and all subjects provided written informed consent prior to participation. [11C]PBR28 data from 8 subjects have been published previously (Sandiego et al., 2015). Due to the sensitive nature of human participant information, data are available upon reasonable request by contacting the corresponding author.
Full PET acquisition details have been described previously (Sandiego et al., 2015). Briefly, participants completed two 120min [11C]PBR28 PET scans, with optical head motion tracking (Vicra, NDI Systems), in the High Resolution Research Tomograph (Siemens, Knoxville, TN) on the same day. During each scan, arterial blood samples were collected to measure the metabolite-corrected arterial input function. Regional TSPO VT values were calculated using multilinear analysis-1 (t*=30). E. coli LPS (1.0ng/kg; IV) was administered 3hr prior to the second PET scan. LPS timing was based on our prior research indicating peak TSPO availability between 1–4hr after LPS (Hannestad et al., 2012; Sandiego et al., 2015). Prior research indicates our test-retest variability with [11C]PBR28 is very good (≤9% (Park et al., 2015)). Before and ~75min after LPS, subjects completed a computerized cognitive battery (Cogstate) shown to reliably measure attention, motor, memory, and executive processes (Lim et al., 2013). Finally, throughout the experimental session, plasma samples were collected and assayed for cytokine/chemokine levels.
Repeated measures analyses of variance were conducted to evaluate LPS effects on [11C]PBR28 VT with ROIs as the within-subject factor and rs6971 genotype (high vs. mixed affinity binders) as the between-subject factor. We first analyzed the new cohort of subjects separately (n=10) and then the full sample (N=18), including data from 8 subjects published previously (Sandiego et al., 2015). [11C]PBR28 VT values shown in Table 1 and Figure 1C are corrected for rs6971 genotype. Post-hoc paired t-tests were conducted to evaluate changes in ROI VT and cognitive performance. For each cognitive domain that was impaired by LPS, Pearson correlations were conducted to evaluate relationships between the magnitude of cognitive decrement and neuroimmune response in the ROI/s implicated in that cognitive process. For completeness, follow-up exploratory correlations evaluated brain-behavior relationships in each ROI. Pearson correlation analyses confirmed that subject age was not related to LPS effects on TSPO availability or cognitive function (ps>.05). Prior to correlation analyses, the Shapiro-Wilk test was used to verify normal variable distributions. To control Type I error, Bonferroni-corrected significance thresholds were implemented, effect sizes were calculated, and small effects (Cohen’s d≤0.3), irrespective of statistical significance, were not interpreted. Power analyses indicated that 18 subjects were sufficient to detect moderate within-subject effects (Cohen’s d≥0.60) for auto-correlated outcome measures (r≥0.7) with >85% power at α<0.05.
Table 1.
Corrected [11C]PBR28 VT values and cognitive task scores pre- and post-LPS administration
| Region | Pre-LPS | Post-LPS | % Change | t value | Pvalue | Cohen’s d | |
|---|---|---|---|---|---|---|---|
| Corrected [11C]PBR28 VT Values | Composite of regions* | 5.38 (1.18) | 7.51 (1.50) | 41.0% | 10.15 | .000 | 2.39 |
| Caudate* | 4.16 (0.85) | 6.14 (1.57) | 47.1% | 7.62 | .000 | 1.80 | |
| Cerebellum* | 5.72 (1.22) | 7.89 (1.48) | 39.4% | 9.92 | .000 | 2.34 | |
| Frontal* | 5.54 (1.11) | 7.80 (1.64) | 41.5% | 9.40 | .000 | 2.21 | |
| Hippocampus* | 5.19 (1.35) | 7.05 (1.32) | 38.6% | 9.47 | .000 | 2.23 | |
| Insula* | 5.32 (1.17) | 7.20 (1.36) | 36.5% | 10.64 | .000 | 2.51 | |
| Occipital* | 6.07 (1.34) | 8.55 (2.23) | 41.3% | 6.91 | .000 | 1.63 | |
| Parietal* | 5.73 (1.17) | 8.60 (2.14) | 50.3% | 7.65 | .000 | 1.80 | |
| Putamen* | 4.85 (1.14) | 6.78 (1.34) | 41.6% | 9.70 | .000 | 2.29 | |
| Temporal* | 5.28 (1.16) | 7.46 (1.60) | 42.1% | 8.66 | .000 | 2.04 | |
| Thalamus* | 5.91 (1.63) | 7.66 (1.70) | 31.1% | 13.37 | .000 | 3.15 | |
| Cognitive Tasks | Psychomotor Speed | 2.49 (0.11) | 2.49 (0.10) | 0.1% | 0.14 | .89 | 0.03 |
| Visual Attention | 2.69 (0.09) | 2.71 (0.09) | 0.8% | 1.42 | .17 | 0.33 | |
| Executive Function | 48.44 (15.94) | 46.94 (14.76) | −3.1% | 0.47 | .65 | 0.11 | |
| Visual Working Memory | 1.33 (0.22) | 1.26 (0.25) | −5.4% | 1.73 | .10 | 0.43 | |
| Visual Learning | 1.00 (0.14) | 0.93 (0.19) | −7.2% | 2.40 | .03 | 0.58 | |
| Verbal Learning* | 27.06 (4.28) | 24.06 (5.17) | −10.7% | 4.21 | .001 | 0.99 | |
| Verbal Recall* | *9.47 (1.94) | 7.35 (3.37) | −22.3% | 4.24 | .001 | 1.03 | |
Note. LPS = lipopolysaccharide. Cognitive tasks are from the computerized Cogstate battery (www.cogstate.com).
Asterisks denote a statistically significant change following LPS administration after Bonferroni-correction for multiple comparisons: p<.005 (.05/10 brain regions) for [11C]PBR28 VT values and p<.007 (.05/7 cognitive tasks) for cognitive measures. Cohen’s d effect size interpretation: ‘small’ ≤0.2; ‘moderate’ =0.5; and ‘large’ ≥0.8.
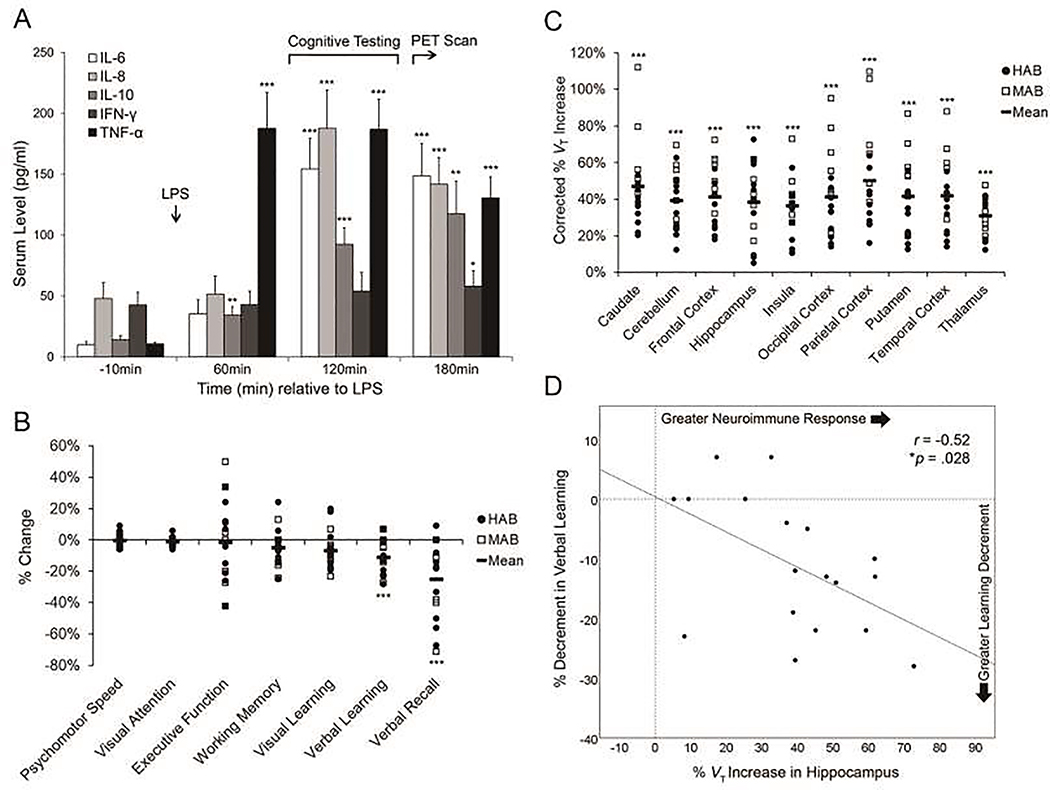
Figure 1. LPS-induced neuroimmune response and cognitive performance decrements.
A) Serum cytokine concentrations (pg/ml) are depicted. Error bars: ± 1 standard error of the mean. B) Individual subject values of the LPS-induced percentage change in cognitive task performance are depicted (HABs = filled circles; MABs = unfilled squares; black bars = task mean). C) Individual subject values for the LPS-induced percentage increase in [11C]PBR28 VT for each ROI, corrected for rs6971 genotype, are depicted (HABs = filled circles; MABs = unfilled squares; black bars = ROI mean). D) The LPS-induced percentage increase in hippocampal TSPO levels was correlated with the LPS-induced percentage decrement in verbal learning (r=−0.52, *p=.028). Note: *p<.05; **p<.01; ***p<.001.
Results
There were no differences between baseline and post-LPS scans for [11C]PBR28 injected activity (14.2±5.1 mCi vs. 15.6±4.4 mCi) or mass (1.6±2.2 μg vs. 1.8±2.7 μg) or radiotracer plasma-free fraction (2.7±0.8% vs. 2.8±1.3%; ps>.40). LPS significantly increased [11C]PBR28 VT in the new subject cohort by 36% (n=10; p<.001; range: 3140%), and the full sample by 41% (N=18; p<.001; range: 31–50%; Figure 1C; Table 1), indicating robust neuroimmune stimulation. Post-hoc analyses showed that LPS evoked a whole-brain neuroimmune response (all ROIs: p<.001; Bonferroni-corrected threshold: p<.005; Cohen’s ds>1.60). LPS impaired verbal learning and verbal recall proficiency by 10.7% and 22.3%, respectively (ps≤.001; Bonferroni-corrected threshold: p<.007), indicating large decrements in performance (Cohen’s ds>0.90; Figure 1B; Table 1). A Pearson correlation revealed that greater LPS-induced TSPO increases in the hippocampus were associated with greater decrements in verbal learning (r=−0.52, p=.028; Figure 1D). Visual learning was also impaired by LPS (p=.03) but did not survive Bonferroni-correction (p<.007). No other cognitive domains approached statistical significance (uncorrected ps≥.10). Additionally, greater LPS-induced TSPO increases in putamen and thalamus were associated with greater decrements in verbal learning (r=−0.50 and −0.55, respectively; ps<.05). No other regions exhibited significant correlations with verbal learning or recall (ps>.10). A subset of participants (n=10) repeated the cognitive battery an average of 5 months after LPS administration (range: 0.7–9.8 months). Follow-up verbal learning and recall proficiency did not differ from baseline levels (ps>.60), suggesting that LPS-induced cognitive decrements were transient in nature.
Discussion
Results of this study confirmed that LPS increased brain TSPO availability (Sandiego et al., 2015), indicative of microglial activation/proliferation (Hannestad et al., 2012; Tournier et al., 2019), and leads to a substantial and specific disruption in memory processes. Furthermore, the magnitude of neuroimmune response in the hippocampus was associated with the decrement in verbal learning (and explained 27% of the variance). Together, these data suggest that hippocampally-mediated memory processes may be sensitive to neuroimmune stimulation and/or microglial activation. Two neurobiological phenomena may explain these findings. First, the hippocampus expresses a relatively high density of cytokine receptors (Ban, 1994). Second, in rodents, LPS has been shown to suppress long term potentiation in the hippocampus (Jo et al., 2001) suggesting a link between neuroinflammation and memory dysfunction.
LPS did not alter attention, psychomotor, or executive processes. The specificity of the LPS effects on memory suggests that general experimental factors, such as LPS-induced ‘sickness symptoms’ (fatigue and malaise), cannot explain our findings (Cunningham and Sanderson, 2008). Exploratory correlations further indicated that neuroimmune responses in the putamen and thalamus were also related to verbal learning decrements, suggesting possible network-level LPS effects, consistent with an emerging literature implicating the basal ganglia and thalamus in memory dysfunction (de Jong et al., 2008; Foerde and Shohamy, 2011). Whereas animal models have shown that neuroimmune stimulation disrupts episodic memory processes, this is the first experimental evidence, to our knowledge, that specifically links neuroimmune responses to memory dysfunction in humans.
Consistent with our previous study (Sandiego et al., 2015), LPS evoked a robust whole-brain neuroimmune response, evidenced by increased TSPO availability across ROIs. Prior research indicates that elevated brain TSPO availability after LPS is associated with microglial activation and proliferation (Hannestad et al., 2012; Tournier et al., 2019). LPS combined with PET TSPO imaging and brief, reliable cognitive testing (Lim et al., 2013) provides a powerful experimental paradigm model that can be used to investigate the mechanistic nature of neuroimmune-related cognitive dysfunction, and may inform etiologic models of neurologic and psychiatric disorders. This paradigm may also be useful for evaluating pharmacotherapies designed to rescue cognitive deficits in neuroinflammatory diseases (McAfoose and Baune, 2009); often the primary endpoint in clinical trials. The magnitude of the acute memory impairment observed herein was consistent with that observed among individuals with mild cognitive impairment due to Alzheimer’s disease (Maruff et al., 2013) and major depression (Davis et al., 2017), indicating that the nature and magnitude of the memory dysfunction induced herein was clinically relevant and that this experimental paradigm may have utility for investigating neuroimmune-related cognitive dysfunction in such disorders. Limitations of this study were its ‘open label’ design and lack of control group; though, it is unlikely a placebo response can robustly increase TSPO availability or selectively impair verbal memory function. Additionally, we were unable to investigate sex differences in this sample. Finally, we were limited to a single post-LPS PET TSPO measurement. Future studies are needed to determine the time course of TSPO normalization to baseline levels, which may occur by 24 hours after immune stimulation (Hannestad et al., 2012; Nettis et al., 2020).
In summary, results from this paradigm development study illustrate the sensitivity of verbal memory processes to acute neuroimmune stimulation and provide initial validation of an experimental paradigm that can be used to investigate relationships between neuroinflammatory processes and cognitive dysfunction. Future research is needed to investigate these relationships in clinical populations.
Highlights.
LPS increased whole-brain TSPO availability, indicative of a neuroimmune response
LPS impaired verbal memory but not psychomotor, attention, or executive processes
The TSPO increase in the hippocampus correlated with the decrement in verbal memory
Hippocampal memory processes may be sensitive to neuroimmune stimulation
Acknowledgments
Funding/Support: This study was supported by grants from the National Institute on Drug Abuse [K02 DA031750 (KPC), K01 AA024788 (ATH), T32 DA022975 (postdoctoral fellow: EAW), and K99 DA048125 (EAW)], the National Institute of Mental Health [R01 MH110674 (KPC, RHP)] and the Clinical Neurosciences Division of the U.S. Department of Veterans Affairs National Center for Posttraumatic Stress Disorder.
Footnotes
This study is registered as a clinical trial on clinicaltrials.gov: NCT04233593
Disclosure: Dr. Maruff is a full-time employee of Cogstate.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ban EM-H, 1994. Interleukin-1 receptors in the brain: characterization by quantitative in situ autoradiography. Immunomethods 5, 31–40. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Sanderson DJ, 2008. Malaise in the water maze: Untangling the effects of LPS and IL-1β on learning and memory. Brain, behavior, and immunity 22, 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MT, DellaGioia N, Matuskey D, Harel B, Maruff P, Pietrzak RH, Esterlis I, 2017. Preliminary evidence concerning the pattern and magnitude of cognitive dysfunction in major depressive disorder using cogstate measures. Journal of affective disorders 218, 82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong LW, van der Hiele K, Veer IM, Houwing J, Westendorp R, Bollen E, de Bruin PW, Middelkoop H, van Buchem MA, van der Grond J, 2008. Strongly reduced volumes of putamen and thalamus in Alzheimer’s disease: an MRI study. Brain 131, 3277–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Shohamy D, 2011. The role of the basal ganglia in learning and memory: insight from Parkinson’s disease. Neurobiology of learning and memory 96, 624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Gallezot J-D, Schafbauer T, Lim K, Kloczynski T, Morris ED, Carson RE, Ding Y-S, Cosgrove KP, 2012. Endotoxin-induced systemic inflammation activates microglia:[11 C] PBR28 positron emission tomography in nonhuman primates. Neuroimage 63, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer AT, Holden D, Fowles K, Nabulsi N, West BL, Carson RE, Cosgrove KP, 2017. Microglial depletion and activation: A [11 C] PBR28 PET study in nonhuman primates. EJNMMI research 7, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J-H, Park E-J, Lee J-K, Jung M-W, Lee C-J, 2001. Lipopolysaccharide inhibits induction of long-term potentiation and depression in the rat hippocampal CA1 area. European journal of pharmacology 422, 69–76. [DOI] [PubMed] [Google Scholar]
- Lim YY, Jaeger J, Harrington K, Ashwood T, Ellis KA, Stöffler A, Szoeke C, Lachovitzki R, Martins RN, Villemagne VL, 2013. Three-month stability of the CogState brief battery in healthy older adults, mild cognitive impairment, and Alzheimer’s disease: results from the Australian Imaging, Biomarkers, and Lifestyle-rate of change substudy (AIBL-ROCS). Archives of clinical neuropsychology 28, 320–330. [DOI] [PubMed] [Google Scholar]
- Maruff P, Lim YY, Darby D, Ellis KA, Pietrzak RH, Snyder PJ, Bush AI, Szoeke C, Schembri A, Ames D, 2013. Clinical utility of the cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer’s disease. BMC psychology 1, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfoose J, Baune B, 2009. Evidence for a cytokine model of cognitive function . Neuroscience & Biobehavioral Reviews 33, 355–366. [DOI] [PubMed] [Google Scholar]
- Nettis M, Veronese M, Nikkheslat N, Mariani N, Lombardo G, Sforzini L, Enache D, Harrison N, Turkheimer F, Mondelli V, 2020. PET imaging shows no changes in TSPO brain density after IFN-α immune challenge in healthy human volunteers. Translational psychiatry 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Gallezot J-D, Delgadillo A, Liu S, Planeta B, Lin S-F, O’Connor KC, Lim K, Lee J-Y, Chastre A, 2015. 11 C-PBR28 imaging in multiple sclerosis patients and healthy controls: test-retest reproducibility and focal visualization of active white matter areas. European journal of nuclear medicine and molecular imaging 42, 10811092. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T, 2001. Cytokine-associated emotional and cognitive disturbances in humans. Archives of general psychiatry 58, 445–452. [DOI] [PubMed] [Google Scholar]
- Sandiego CM, Gallezot J-D, Pittman B, Nabulsi N, Lim K, Lin S-F, Matuskey D, Lee J-Y, O’Connor KC, Huang Y, 2015. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proceedings of the National Academy of Sciences 112, 12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier BB, Tsartsalis S, Ceyzériat K, Medina Z, Fraser BH, Grégoire M-C, Kövari E, Millet P, 2019. Fluorescence-activated cell sorting to reveal the cell origin of radioligand binding. Journal of Cerebral Blood Flow & Metabolism, 0271678X19860408. [DOI] [PMC free article] [PubMed] [Google Scholar]