Abstract
Objective:
Elevated inflammation is associated with worse late-life cognitive functioning and brain health. Our goal was to examine the relationship between inflammation trajectories and white matter integrity in midlife.
Methods:
Participants were 508 adults from the Coronary Artery Risk Development in Young Adults Study (CARDIA; 51% female). Latent class analysis was used to identify inflammation trajectories based on repeated measures of the inflammatory marker C-reactive protein (CRP) over the 18 years before brain magnetic resonance imaging (MRI). Outcomes were brain MRI measures of total and region-specific white matter volume and integrity at a mean age of 50.6±3.4 years. Linear regression was used to examine if inflammation trajectories were associated with brain MRI outcomes, adjusting for potential confounds in all models and for disease and health behaviors in follow-up models.
Results:
Lower-stable (38%), moderate-increasing (7%), and consistently-higher (54%), trajectories emerged. Compared to the lower-stable group, the moderate-increasing group showed lower white matter volume (β = −0.18, 95% CI −0.29, −0.06) and worse white matter integrity as indexed by lower fractional anisotropy (FA; β = −0.37, 95% CI −0.70, −0.04) and higher mean diffusivity (β = 0.44, 95% CI 0.11, 0.78) in the whole brain. The consistently-higher group showed lower whole-brain FA (β = −0.20, −0.38, −0.03). In exploratory analyses, the moderate-increasing group showed lower white matter volume, lower FA and higher MD in the frontal, temporal, and parietal lobes compared to the lower-stable group. The consistently-higher group showed lower white matter volume in the parietal lobe and lower FA in the frontal, temporal, and parietal lobes, but similar MD, compared to the lower-stable group. Findings for the moderate-increasing, but not the consistently-higher, group were robust to adjustment for disease and lifestyle factors.
Conclusion:
Increasing or high inflammation trajectories from early to mid adulthood are associated with worse brain health, as indexed by lower white matter volume and/or worse white matter integrity.
Keywords: Inflammation, White Matter, Midlife, Brain Structure, DTI
Introduction
As the global population ages, there is a pressing need to identify biomarkers and mechanisms of age-related cognitive decline.1 Elevated inflammation has emerged as a predictor and potential mechanism of both cognitive impairment and dementia in older individuals.2–4 Although the neural underpinnings of the relationship between inflammation and cognitive decline remain unclear, it appears that elevated peripheral inflammation negatively impacts brain structure, and particularly white matter integrity, in late life.5–8 In turn, impairments in white matter integrity appear to play a role in age-related cognitive decline.9–11 Given that cognitive impairment in late life typically emerges due to changes that occur over decades, it is critically important that we better understand the relationship of inflammation and brain health across the lifespan.
Research in non-human model systems has shown that neuroinflammation can lead to neurodegeneration, the death of neurons, and impair neurogenesis, the growth of new neurons.12–16 In vitro evidence also indicates that neuroinflammation can damage neurons and oligodendrocytes.13,16–18 A key issue is whether peripheral inflammation, which can be easily measured in humans, is related to the neuroinflammation that impacts brain structure. Although the brain was long considered an immune-privileged site, there is increasing recognition of great potential for crosstalk between peripheral and central immune systems.19,20 In line with this, experimental studies in rodents have shown that peripheral inflammation can elicit neuroinflammation.21–23 Moreover, circulating blood levels of the systemic inflammatory marker C-reactive protein (CRP) appear strongly associated with cerebrospinal fluid levels of multiple inflammatory markers including CRP in humans.24 Thus, peripheral inflammation may be a valuable index of neuroinflammation and possibly even a clinically useful biomarker.
A large body of literature has documented cross-sectional associations of elevated peripheral inflammatory markers with reduced white matter volume and worse white matter integrity in late life.5,7,8,25 Accumulating evidence indicates that elevated inflammation may also prospectively predict late-life white matter volume and integrity.26 A better understanding of the relationship between inflammation and white matter degradation will require that we clarify the effects of chronic patterns of inflammation on white matter. In this vein, an emerging literature has begun to define the trajectories of inflammatory activity in mid to late life that predict late-life white matter volume and integrity.6,8,27 Overall, this literature indicates that increasing or consistently high levels of inflammation have particularly deleterious effects on late-life brain structure.6,27 Trajectories of inflammation may also be important for white matter integrity in midlife, a time when intervention may be more effective.
Using prospective data from the Coronary Artery Risk Development in Young Adults (CARDIA) study, we examined whether early to midlife inflammation trajectories influence brain structure in midlife. We hypothesized that “problematic” trajectories of inflammation (i.e., increasing or consistently high) would be associated with lower white matter volumes and reduced white matter integrity, as assessed by lower fractional anisotropy (FA) and higher mean diffusivity (MD), at midlife.
Methods
Study Design and Sample
Participants are from CARDIA, a multi-site longitudinal study of 5,115 adults, initially healthy and aged between 18 and 30 years (55% female, 52% Black) at enrollment in 1985–1986.28 CARDIA participants were recruited from four U.S. cities (Birmingham, AL; Minneapolis, MN; Chicago, IL; and Oakland, CA) and completed a baseline visit and eight follow-up visits at Years 2, 5, 7, 10, 15, 20, 25, and 30.28,29 Among all CARDIA participants, 3,929 had at least three CRP measures from Years 7, 15, 20, or 25, which were in the 18 years prior to and the same year as the Brain MRI visit. Of these, 2,194 were eligible for inclusion in our trajectory analysis.
At the Year 25 visit, a subset of CARDIA participants completed neuroimaging as part of the CARDIA Brain Magnetic Resonance Imaging (MRI) Ancillary Study,30 which is a sub-study designed to identify predictors of brain structure. This sub-study was conducted at three sites (Birmingham, AL; Minneapolis, MN; and Oakland, CA). The CARDIA Brain MRI sub-study enrolled participants at the time of scheduling of the Year 25 appointments. The sub-study aimed for balance within four strata of ethnicity/race (black, white) and sex (male/female), and across three CARDIA field centers (Birmingham, AL, Minneapolis, MN, and Oakland, CA). Each field center had a Brain MRI sub-study target sample size. When they reached their target enrollment, recruitment ceased. Exclusion criteria for the MRI substudy included any contra-indication to MRI or a body size that was too large for the MRI tube bore. Further details regarding the design of CARDIA and the Brain MRI ancillary study have been previously reported.33
Out of the 695 participants who had data available from the neuroimaging sub-study at Year 25, 508 (72%) were eligible for inclusion in our trajectory analysis. These 508 participants (mean age at brain scan = 50.6±3.4, 51% female, 34% Black) were included in the analytic models to investigate the association between the inflammatory trajectories and midlife brain structure. Characteristics of individuals included versus excluded based on eligibility for the trajectory analysis are included in Supplementary Table 1. Participants with MRI data who were excluded from our models had a lower educational level, were more likely to be younger, male, White, and smokers, and to have elevated body mass index, lower levels of physical activity, and liver disease (BMI) (p < 0.05).
Participants provided written informed consent at each study visit and separately for participation in the CARDIA Brain MRI Ancillary Study. Study protocols were reviewed and approved by the institutional review boards at each site and by the CARDIA Coordinating Center.
Measures
Inflammation.
The systemic inflammatory marker CRP was measured in Years 7, 15, 20, and 25. Participants were asked to fast for at least eight hours and to avoid smoking and heavy physical activity for at least two hours before each blood collection. Plasma for CRP assays was separated from whole blood and stored at −70°C before being shipped on dry ice to a central laboratory for storage. A particle-enhanced immunonepholometric assay was conducted on a Siemens Dade Behring BN II Nephelometer to assess CRP levels in Years 7 to 20 (range: 0.175–1,000 mg/L). Intra-assay coefficients of variation (CVs) were 2.3–4.4% and inter-assay CVs were 2.1–5.7%. A Roche latex-particle enhanced immunoturbidimetric assay kit and the Roche Modular P Chemistry analyzer was used to assess CRP levels in Year 25 (range: 0.175–1,000 mg/L) with an intra-assay CV of 3.7%. Participants who had three detectable (≥0.175mg/L) CRP levels ≤ 10 mg/L were included in our analyses because at least three measures are required to define a trajectory over time.31 CRP data was missing for 4.1%, 1.6%, 1.5% and 0.7% of participants at Year 7, 15, 20, and 25 respectively. Values >10 mg/L were excluded because of the possibility that they were due to acute inflammation from recent infection or injury, and our inability to distinguish between high inflammation due to acute illness or chronic factors.32 Values > 10 mg/L were excluded for 5.7%, 7.2%, 4.8%, and 7% of cases in Years 7, 15, 20, and 25 respectively.
Brain Structure.
Structural MRI and diffusion tensor imaging (DTI) scans were completed in 3 Tesla (3T) magnetic resonance scanners (Philips 3T Achieva/2.6.3.6 platform in Birmingham, AL; Siemens 3T Tim Trio/VB 15 platform in Minneapolis, MN; and Siemens 3T Tim Trio/VB 15 platform in Oakland, CA) using standardized protocols in Year 25. More detailed neuroimaging methods are provided in Launer et al. (2015).33 In brief, structural MRI images were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using an automated multispectral computer algorithm that classifies all supratentorial brain tissue into gray matter, white matter, and CSF.33 White matter was further characterized as normal and abnormal and then parcellated into regions of interest based on the Jakob Atlas.34–36 We examined total, frontal, temporal, parietal, and occipital white matter volumes corrected for intracranial volume (ICV; sum of total brain volume plus cerebrospinal fluid) to account for variability in head size. Normal volumes in each lobe were combined across left and right hemispheres, then divided by ICV to calculate the volume to ICV ratio. DTI was used to compute voxel-wise maps of white matter integrity, as indexed by FA and MD: Plane Axial Coil 12-channel Psd File name ep2d diff MDDW: TR 7300 TE 84 Fov 245 Thickness 2.2mm distance factor 0% Diff Directions- 33 Concatenations = 1 number sl = 64 Flip 90 Matrix 128×128 NSA 1 Center Freq. water Phase FOV = 100 Pixel BW = 1860 diff mode = Free Phase part fourier = 7/8 Echo spacing = .59 diff weighting = 1 Accel factor = 3 EPI factor = 112 Base Res 112 Phase res 100%; 2 times.37 The FA measure estimates the degree or uniformity to which water diffuses along the direction of myelinated tracks in the white matter. FA scores range from 0 to 1 with lower scores indicating worse white matter integrity. The MD measure reflects the diffusivity of water such that reduced tissue density is associated with higher MD, indicating worse white matter integrity. We examined white matter integrity (FA, MD) overall and by lobe, such that mean values overall and within specific lobes were averaged across the left and right hemispheres.
Other Covariates.
We used covariates as assessed at Year 25, which was the same annual assessment as the Brain MRI. Demographics, current smoking (coded as smoker vs. non-smoker), and alcohol use (drinks per week) were based on self-report. BMI was defined as the average weight in kilograms divided by height in meters squared. Physical activity total scores were measured using the CARDIA Physical Activity questionnaire.38 Presence of chronic disease (asthma, kidney problems, liver disease, heart problems, cancer/tumor, HIV, sleep apnea, stroke/TIA and multiple sclerosis) was assessed via a combination of self-report, physician diagnoses, and medication use. Cardiovascular risk factors including diabetes, hypertension, and high cholesterol were defined via a combination of self-report, medication use, and clinic assessments. The Center for Epidemiologic Studies (CESD) was used to assess depressive symptoms.39
Data Analysis
We generated inflammation trajectories using latent class growth analysis, a semi-parametric approach that differentiates groups of individuals based on their probability of following a similar trajectory on an outcome over time.40,41 We used SAS PROC TRAJ to estimate trajectories of inflammation as indexed by CRP across visits at Years 7, 15, 20, and 25. We used natural log transformations for CRP to account for skewed distributions and modeled repeated CRP measurements as censored normal.42 Model selection procedures were guided according to recommended procedures and we required that each trajectory include at least 5% of participants.42 We fit 2-, 3-, and 4-class models40,41 and for each model in which a given number of trajectories was selected, we evaluated cubic, quadradic, and linear terms to identify the trajectory shapes that best fit the data. We then determined the number of trajectories to keep by calculating the Log Bayes factor, which compares the Bayesian Information criterion (BIC) values between models and indicates that the more complex model with lower BIC better fits the data.40
Next, we selected the subset of original CARDIA participants who completed the neuroimaging sub-study and used descriptive statistics to compare characteristics of those with different inflammation trajectories in Year 25, the same year as the Brain MRI sub-study. To examine the association between inflammation trajectories and brain structure, we estimated a series of linear regression models. Our primary outcomes were whole-brain measures of white matter volume, FA and MD. Exploratory outcomes included lobe-specific measures of white matter volume, FA and MD for the frontal, temporal, parietal, and occipital lobes. Model 1 was unadjusted. Model 2 adjusted for demographics (age, sex, race, and education) and study site in order to examine associations of inflammatory trajectories with brain health adjusted only for potential confounds. Models 3 and 4 additionally adjusted for potential contributors to the relationship between inflammation trajectory and brain health. First, Model 3 adjusted for Model 2 covariates plus medical illnesses (sleep apnea, diabetes, and hypertension) that differed between trajectory groups (p < .10) because these diseases could be a source of inflammation that contributes to the effects of inflammation on the brain.43 Model 4 adjusted for Model 2 covariates plus lifestyle factors (BMI, physical activity, alcohol, smoking, and depression) that could cause inflammation that then impacts brain health.44 Analyses were conducted in SAS version 9.4 and R version 3.4.3.
Data Availability Statement
Anonymized data are available from the CARDIA Coordinating Center (cardia.dopm.uab.edu/contact-cardia). A description of the National Heart, Lung, and Blood Institute policies governing the data and describing access to the data can be found online (cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data).
Results
Inflammation Trajectories
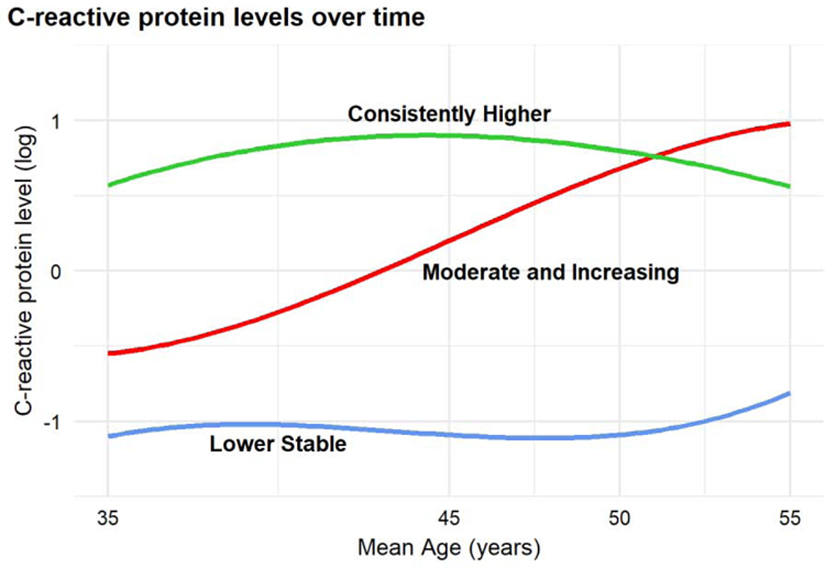
Our trajectory analysis in the 2,194 participants who had at least three measures of CRP during the study period indicated that a 4-trajectory model was associated with improvements in model fit over a 3-trajectory model (For details, see Supplementary Table 2). However, this model had fewer than 5% of participants in one group. Therefore, we selected the 3-trajectory model for our follow-up models. The three identified inflammation trajectories reflected overall patterns of low, moderate, and high CRP during the 18-year period during young to middle age, with high inflammation defined as having CRP level ≥ the sample median across all years. We labeled the trajectories as lower-stable, moderate-increasing, and consistently-higher (Figure 1). The trajectories of the 2,194 participants were characterized as follows: lower-stable (n = 786 [36%]), moderate increasing (n = 169 [8%]), and consistently higher (n = 1,239 [56%]). Of those in the MRI sub-study (n = 508), 38% (n = 195) were classified as lower-stable, 7% (n = 38) as moderate increasing, and 54% (n = 275) as consistently higher.
Figure 1.
C-reactive protein (CRP) latent class trajectories for the 2194 CARDIA participants who had at least 3 eligible CRP measures from Year 7 to Year 25.
Inflammation trajectory group membership was significantly associated with other participant characteristics including demographics, BMI, physical activity, alcohol consumption, current smoking, sleep apnea, diabetes, hypertension, and depression. Overall, the moderate-increasing and consistently-higher groups tended to have worse health behaviors and more health problems than those in the lower-stable group (Table 1). A similar, but not entirely overlapping, pattern of characteristics was observed in the larger cohort of 2,194 participants (Supplementary Table 3).
Table 1.
Characteristics of sample with brain MRI by inflammatory trajectory
| Characteristic mean (sd), n (%), or median (IQR) | Inflammatory Trajectory Group, No. (%) |
|||
|---|---|---|---|---|
| Lower stable | Moderate-increasing | Consistently-higher | pa | |
| (n = 195) | (n = 38) | (n = 275) | ||
| Age, years | 51.0 (3.3) | 48.8 (3.6) | 50.6 (3.4) | <0.001 |
| Black | 146 (74.9) | 19 (50.0) | 170 (61.8) | 0.001 |
| Female | 99 (50.8) | 16 (42.1) | 141 (51.3) | 0.56 |
| Education, years | 15.5 (2.4) | 15.2 (2.1) | 14.7 (2.5) | 0.003 |
| Smoking: current | 19 (9.7) | 7 (18.4) | 45 (16.4) | 0.08 |
| Alcoholb: drinks per week | 10 (4 – 20) | 3 (0–5) | 8(4–13) | 0.05 |
| Body mass index: kg/m2 | 25.37 (3.7) | 30.3 (4.5) | 29.8 (5.6) | <0.001 |
| Physical activity | 416.3 (272.9) | 361.1 (233.4) | 356.0 (293.5) | 0.06 |
| Asthma | 10 (5.1) | 3 (7.9) | 24 (8.7) | 0.33 |
| Kidney problems | 14 (7.2) | 3 (7.9) | 17 (6.2) | 0.87 |
| Heart problems | 19 (9.7) | 4 (10.5) | 35 (12.7) | 0.59 |
| Liver disease | 5 (2.6) | 1 (2.6) | 14 (5.1) | 0.34 |
| Cancer/tumor | 16 (8.2) | 1 (2.6) | 24 (8.7) | 0.43 |
| Diabetes | 8 (4.1) | 3 (7.9) | 33 (12.0) | 0.01 |
| HIV | 3 (1.5) | 0 (0.0) | 2 (0.7) | 0.55 |
| Sleep apnea | 7 (3.6) | 2 (5.3) | 29 (10.5) | 0.01 |
| Multiple sclerosis | 19 (9.7) | 4 (10.5) | 35 (12.7) | 0.59 |
| Stroke/TIA | 0 (0.0) | 0 (0.0) | 3 (1.1) | 0.27 |
| High cholesterol | 14 (7.2) | 6 (15.8) | 23 (8.4) | 0.21 |
| Hypertension | 36 (18.5) | 12 (31.6) | 103 (37.5) | <0.001 |
| Depression: CESD > 16 | 20 (10.3) | 5 (13.2) | 49 (17.8) | 0.07 |
| CRP: ≥ medianc | 18 (9.2) | 32 (84.2) | 203 (73.8) | <0.001 |
CARDIA, coronary artery risk development in young adults; sd, standard deviation; IQR, interquartile range; CESD, Center for Epidemiological Studies Depression scale.
P values are from 1-way analysis of variance for age, education, physical activity, and BMI; χ2 tests for black race, female sex, smoking, self-reported medical conditions, cardiovascular risk factors, and depression; Kruskal-Wallis tests for alcohol.
Number of drinks per week among current drinkers.
CRP ≥ median refers to CRP values across all years.
Inflammation Trajectories and White Matter Volume and Integrity
We first examined if inflammation trajectories were associated with white matter volume in the whole brain at midlife. In demographics-adjusted models, those in the moderate-increasing group showed lower (β = −0.18, 95% CI −0.29, −0.06), and the consistently-higher group showed similar, whole-brain white matter volume compared to the lower-stable group. Further adjustment for disease and lifestyle factors did not alter this pattern of results and the effect sizes remained similar to those in the demographics-adjusted models (Table 2).
Table 2.
White Matter Volume by Inflammation Trajectory Group
| Standardized Difference in Each Brain Measure (95% CI) |
||||
|---|---|---|---|---|
| Inflammation trajectory group | Unadjusted Model β (95% CI) |
Demographics Adjusteda β (95% CI) |
Disease Adjustedb β (95% CI) |
Lifestyle Adjustedd β (95% CI) |
| White matter (WM) brain volume | ||||
| Total WM | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | −0.47 (−0.80, −0.13) | −0.18 (−0.29, −0.06) | −0.17 (−0.29, −0.06) | −0.18 (−0.30, −0.06) |
| Consistently-higher | −0.27 (−0.45, −0.09) | −0.04 (−0.10, 0.01) | −0.04 (−0.10, 0.01) | −0.04 (−0.10, 0.02) |
| P-value | 0.001 | 0.01 | 0.01 | 0.01 |
| Frontal WM | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | −0.47 (−0.84, −0.15) | −0.21 (−0.36, −0.07) | −0.21 (−0.36, −0.07) | −0.25 (−0.37, −0.07) |
| Consistently-higher | −0.27 (−0.38, −0.02) | −0.01 (−0.07, 0.08) | 0.01 (−0.06, 0.08) | 0.01 (−0.07, 0.08) |
| P-value | 0.001 | 0.01 | 0.01 | 0.01 |
| Temporal WM | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | −0.38 (−0.73, −0.04) | −0.09 (−0.23, 0.03) | −0.10 (−0.24, 0.03) | −0.09 (−0.25, 0.02) |
| Consistently-higher | −0.25 (−0.43, −0.07) | −0.08 (−0.05, 0.05) | −0.02 (−0.09, 0.04) | −0.02 (−0.10, 0.05) |
| P-value | 0.01 | 0.37 | 0.34 | 0.32 |
| Parietal WM | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | −0.47 (−0.81, −0.12) | −0.17 (−0.31, −0.01) | −0.16 (−0.32, −0.01) | −0.17 (−0.33, −0.01) |
| Consistently-higher | −0.34 (−0.53, −0.16) | −0.11 (−0.20, −0.03) | −0.10 (−0.18, −0.01) | −0.10 (−0.19, −0.01) |
| P-value | <0.001 | 0.01 | 0.02 | 0.03 |
| Occipital WM | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | −0.43 (−0.78, −0.09) | −0.16 (−0.34, 0.01) | −0.13 (−0.31, 0.05) | −0.08 (−0.27, 0.10) |
| Consistently-higher | −0.10 (−0.42, −0.10) | −0.06 (−0.16, 0.24) | −0.06 (−0.16, 0.02) | −0.03 (−0.13, 0.07) |
| P-value | 0.01 | 0.14 | 0.22 | 0.64 |
CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval
Adjusted for age, sex, race/ethnicity, education, intercranial volume (WM models only), and site.
Additionally adjusted for sleep apnea, diabetes, and hypertension.
Additionally adjusted for BMI, alcohol, smoking, physical activity, and depression.
We next examined if inflammation trajectories over time were associated with white matter integrity at midlife as indexed by whole-brain FA and MD. Compared to the lower-stable group, those displaying a moderate-increasing trajectory had worse white matter integrity as indexed by lower whole-brain FA (β = −0.37, 95% CI −0.70, −0.04) and higher whole-brain MD (β = 0.44, 95% CI 0.11, 0.78) after demographics adjustment. The consistently-higher trajectory group also displayed worse white matter integrity as indexed by significantly lower whole-brain FA (β = −0.20, 95% CI −0.38, −0.03) compared to the lower-stable group, but their levels of MD were similar. When we additionally adjusted for lifestyle factors and disease, the moderate-increasing group still showed significantly lower FA and higher MD in the whole brain, but the finding of lower FA in the consistently-higher group was not significant (Table 3). Thus, the moderate-increasing group showed lower FA and higher MD that remained significant when adjusting for disease and lifestyle factors, whereas associations of the consistently-higher trajectory with lower FA were no longer significant when adjusting for disease and lifestyle factors.
Table 3.
White Matter Integrity by Inflammation Trajectory Group
| Standardized Difference in Each Brain Measure (95% CI) | ||||
|---|---|---|---|---|
| Inflammation trajectory group | Unadjusted Model β (95% CI) |
Demographics Adjusteda β (95% CI) |
Disease Adjustedb β (95% CI) |
Lifestyle Adjustedd β (95% CI) |
| Fractional Anisotropy (FA) | ||||
| Total FA | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Lower-stable | −0.29 (−0.62, −0.04) | −0.37 (−0.70, −0.04) | −0.34 (−0.67, −0.02) | −0.32 (−0.66, −0.01) |
| Moderate-increasing | −0.23 (−0.41, −0.05) | −0.20 (−0.38, −0.03) | −0.14 (−0.31, 0.03) | −0.13 (−0.31, 0.05) |
| Consistently-higher | 0.02 | 0.01 | 0.06 | 0.12 |
| P-value | ||||
| Frontal FA | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | −0.21 (−0.56, −0.13) | −0.33 (−0.66, −0.01) | −0.32 (−0.66, 0.01) | −0.28 (−0.62, 0.05) |
| Consistently-higher | −0.19 (−0.37, −0.01) | −0.18 (−0.36, −0.01) | −0.11 (−0.29, 0.06) | −0.08 (−0.27, 0.09) |
| P-value | 0.04 | 0.04 | 0.13 | 0.25 |
| Temporal FA | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | −0.37 (−0.72, −0.02) | −0.43 (−0.84, −0.15) | −0.46 (−0.78, −0.08) | −0.44 (−0.79, −0.08) |
| Consistently-higher | −0.24 (−0.42, −0.05) | −0.20 (−0.70, −0.02) | −0.39 (−0.39, −0.02) | −0.16(−0.35, 0.02) |
| P-value | 0.01 | 0.01 | 0.01 | 0.03 |
| Parietal FA | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | −0.32 (−0.67, −0.01) | −0.38 (−0.69, −0.07) | −0.39 (−0.70, −0.08) | −0.35 (−0.68, −0.03) |
| Consistently-higher | −0.26 (−0.44, −0.07) | −0.24 (−0.41, −0.08) | −0.18 (−0.35, −0.02) | −0.17 (−0.34, 0.02) |
| P-value | 0.01 | 0.01 | 0.01 | 0.04 |
| Occipital FA | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | −0.23 (−0.58, 0.11) | −0.23 (−0.57, 0.11) | −0.22 (−0.58, 0.11) | −0.27 (−0.63, 0.08) |
| Consistently-higher | −0.13 (−0.37, −0.10) | −0.14 (−0.32, 0.03) | −0.09 (−0.28, 0.08) | −0.14 (−0.33, 0.05) |
| P-value | 0.08 | 0.18 | 0.37 | 0.20 |
| Mean diffusivity (MD) | ||||
| Total MD | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Lower-stable | 0.39 (0.05, 0.72) | 0.44 (0.11, 0.78) | 0.41 (0.08, 0.74) | 0.42 (0.08, 0.76) |
| Moderate-increasing | 0.10 (−0.06, 0.28) | 0.13 (−0.05, 0.29) | 0.05 (−0.10, 0.27) | 0.05 (−0.13, 0.24) |
| Consistently-higher | 0.04 | 0.02 | 0.03 | 0.04 |
| P-value | ||||
| Frontal MD | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | 0.41 (0.06, 0.75) | 0.50 (0.16, 0.85) | 0.45 (0.10, 0.80) | 0.44 (0.08, 0.79) |
| Consistently-higher | 0.12 (−0.05, 0.30) | 0.14 (−0.03, 0.33) | 0.07 (−0.10, 0.26) | 0.06 (−0.12, 0.25) |
| P-value | 0.04 | 0.02 | 0.03 | 0.05 |
| Temporal MD | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | 0.45 (0.10, 0.80) | 0.49 (0.15, 0.84) | 0.47 (−0.02, 0.66) | 0.31 (−0.04, 0.77) |
| Consistently-higher | 0.04 (−0.06, 0.29) | 0.13 (−0.48, 0.31) | 0.07 (−0.16, 0.19) | 0.01 (−0.20, 0.14) |
| P-value | 0.03 | 0.01 | 0.16 | 0.18 |
| Parietal MD | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | 0.32 (0.01, 0.67) | 0.38 (0.07, 0.69) | 0.36 (0.12, 0.82) | 0.13 (0.02, 0.85) |
| Consistently-higher | 0.06 (−0.27, 0.40) | 0.13 (−0.16, 0.44) | 0.17 (−0.12, 0.47) | −0.10 (−0.13, 0.28) |
| P-value | 0.01 | 0.01 | 0.03 | 0.02 |
| Occipital FA | ||||
| Lower-stable | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Moderate-increasing | 0.27 (−0.06, 0.62) | 0.25 (−0.09, 0.60) | 0.26 (−0.09, 0.61) | 0.26 (−0.08, 0.61) |
| Consistently-higher | 0.04 (−0.13, 0.23) | 0.03 (−0.14, 0.22) | −0.01 (−0.19, 0.18) | 0.12 (−0.20, 0.45) |
| P-value | 0.28 | 0.35 | 0.30 | 0.21 |
CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval
Adjusted for age, sex, race/ethnicity, education, intercranial volume (WM models only), and site.
Additionally adjusted for sleep apnea, diabetes, and hypertension.
Additionally adjusted for BMI, alcohol, smoking, physical activity, and depression.
Inflammation Trajectories and Region-Specific White Matter Volume and Integrity
In exploratory analyses, we examined if inflammation trajectories were associated with white matter volume and integrity in specific brain regions. Compared to the lower-stable group, those in the moderate-increasing group showed lower white matter volume in the frontal (β = −0.21, 95% CI −0.36, −0.07) and parietal (β = −0.17, 95% CI −0.31, −0.01) lobes, and those in the consistently-higher group showed lower white matter volume in the parietal lobe (β = −0.11, 95% CI −0.20, −0.03), in demographics-adjusted models (Table 2). All of these findings remained significant in models additionally adjusted for disease and lifestyle factors.
With regard to region-specific white matter integrity, the moderate-increasing trajectory group showed lower FA in the frontal (β = −0.33, 95% CI −0.66, −0.01), temporal (β = −0.43, 95% CI −0.84, −0.15) and parietal lobes (β = −0.38, 95% CI −0.69, −0.07), but not in the occipital lobe, after demographics adjustment. Similarly, the moderate-increasing group showed significantly higher frontal (β = 0.50, 95% CI 0.16, 0.85), temporal (β = 0.49, 95% CI 0.15, 0.84), and parietal (β = 0.38, 95% CI 0.07, 0.69), but not occipital, MD compared to the lower-stable group in demographics-adjusted models. The consistently-higher trajectory group had significantly lower FA in the frontal (β = −0.18, 95% CI −0.36, −0.01), temporal (β = −0.20, 95% CI −0.70, −0.02), and parietal (β = −0.24, 95% CI −0.41, −0.08) lobes, but not the occipital lobe, compared to the lower-stable group, in demographics-adjusted analyses. However, the consistently-higher group did not show significantly different MD than the lower-stable group in any regions.
Findings of lower FA for the moderate-increasing group in the temporal and parietal, but not the frontal, lobe remained significant when models were adjusted for disease and lifestyle factors (Table 3). With regard to MD, the moderate-increasing group showed higher MD in the frontal and parietal lobes, but not in the temporal lobe, when adjusting for disease and lifestyle factors. When we adjusted for lifestyle factors, the consistently-higher group only showed lower FA in the temporal and parietal lobes, and adjusting for disease rendered these associations non significant with marked reductions in effect sizes (Table 3).
Discussion
Results indicate that inflammation trajectories across almost two decades during early to middle adulthood are associated with worse white matter volume and integrity at midlife. Individuals showing a moderate-increasing trajectory of inflammation over time had lower white matter volume and worse white matter integrity, as indexed by lower FA and higher MD, in the whole brain, compared to a lower-stable trajectory group. Individuals in the consistently-higher group had lower whole-brain FA, but similar whole-brain white matter volume and MD, compared to the lower-stable group. Overall, these data indicate that moderate-increasing and consistently-higher trajectories of inflammation in early to mid adulthood might be risk factors for lower white matter quantity and quality in midlife. In turn, an accumulating literature has found that white matter volume and integrity are associated with cognitive impairment.9,10 Thus, trajectories of inflammation may impact brain health even at midlife.
Our findings are in line with previous studies linking trajectories of inflammatory activity with white matter volume and integrity, although prior studies have examined brain outcomes in late life. In the Atherosclerosis Risk in Communities study, increasing levels of CRP levels during midlife predicted worse late-life white matter integrity.6 Similarly, in a sample with an average age of 83 taken from the Health Aging and Body Composition Study, larger decreases in CRP values over a period of six years were associated with higher FA in several brain regions.8 Thus, it appears that increasing levels of inflammation are most closely linked with degraded white matter. Our findings are also in line with prior work linking elevated peripheral inflammation with lower gray and white matter volume cross-sectionally.45 Taken together, this literature raises the possibility that regulating inflammation, particularly to avoid increasing levels over time, may be beneficial for brain health throughout adulthood.
Identifying factors that contribute to deleterious inflammation trajectories, and particularly to increasing levels of inflammation, may point us in the direction of interventions that improve brain health across the lifespan. One possibility is that factors related to health behaviors, such as smoking, obesity, and physical inactivity, contribute to increasing levels of inflammation over time.46–48 Stress-related psychiatric symptoms, including depressive symptoms, may also contribute to increases in inflammation.49,50 Another possibility is that pathophysiological inflammatory processes associated with chronic diseases such as sleep apnea, diabetes, and hypertension may contribute to rises in inflammatory activity.51–53 All of these factors and conditions were less common in the lower-stable group in our analyses. However, adjusting for them reduced some, but not all, of the associations of inflammation trajectories with white matter volume and integrity. Notably, the characteristics of our different trajectory groups differed somewhat between those with (Table 1) and without (Supplementary Table 1) MRI data available. A comparison of the trajectory-group characteristics for the full sample of 2,194 and the MRI subsample of 508 indicates that women and Black people may be underrepresented in the moderate-increasing group in our MRI analyses. Although our models adjusted for both gender and race, data from key demographic groups may be missing. Findings for the moderate-increasing trajectory group were generally robust to adjustment for disease and lifestyle factors, whereas most results for the consistently-higher trajectory were not independent of these factors. Thus, interventions targeting health behaviors and disease may be particularly beneficial for white matter in those with consistently-higher levels of inflammation in early to mid-adulthood. Other mechanisms may be at play in the moderate-increasing trajectory group.
In exploratory analyses focused on region-specific white matter, we found that the moderate-increasing group showed lower white matter volume and worse white matter integrity in the frontal, temporal, and parietal lobes compared to the lower-stable group. The consistently-higher group showed reduced white matter volume in the parietal lobe and lower FA in the frontal, temporal, and parietal lobes, but similar MD, compared to the lower-stable group. There were no associations of inflammation trajectories with white matter volume and integrity in the occipital lobe. As these findings are based on exploratory analyses, they must be interpreted with caution. However, they suggest that inflammation trajectories may be associated with a general decrease in white matter volume and integrity across many brain areas, but perhaps less so in the occipital lobe. Previous research has indicated that the highest age-related changes in inflammation may occur in the temporal and frontal lobes,54 and it will be important to understand how inflammation may impact brain-region specific changes in white matter that underlie different forms of dementia.55
Limitations
Several limitations should be considered when interpreting our findings. First, our moderate sample size may have limited our power to detect some effects. Our moderate-increasing trajectory group was particularly small and many of our findings are related to that group. Second, we rely on peripheral CRP as a marker of inflammation in this study. Although peripheral levels of CRP have been found to correlate highly with cerebrospinal fluid levels of inflammatory markers,24 the inflammatory system is highly complex and future research should consider multiple markers as well as direct assessments of neuroinflammation. Moreover, although we attempted to exclude people with levels of CRP > 10 mg/L, which could be reflective of acute inflammation, it remains possible that some of the CRP measures are partly reflective of acute inflammation. In addition, our efforts to remove values reflective of acute inflammation may have led to the omission of extremely high CRP values reflective of high levels of chronic inflammation.56,57 Future studies with larger samples will be needed to clarify the effects of extremely high inflammation on brain health. Third, although we examined a role for lifestyle and disease factors by including them as covariates in our models, further research will be necessary to clarify the source of high and increasing levels of inflammation that may influence brain structure. Importantly, change in these factors over time may be crucial to understanding their impact on an individual’s inflammation trajectory. Future studies should examine how change in lifestyle and disease factors influence trajectories of inflammation over time in population-based samples. In addition, our classification of covariates into the categories of “health behaviors” and “diseases” is imperfect. In particular, while BMI may index health behaviors, high BMI may also be a clinical condition. Moreover, apart from our adjustment for depressive symptoms, we did not assess the contribution of psychiatric disorders to our findings. Fourth, this paper is focused on brain health and did not examine cognitive functioning directly. Fifth, we did not adjust for multiple comparisons in this paper and our brain-region specific analyses were exploratory and should be interpreted cautiously. Finally, our study included only a subset of eligible participants from the CARDIA cohort who tended to have higher levels of education and were more likely to be older, female, White, and non-smoking, and to have less liver disease and lower BMI than those who were excluded (Supplementary Table 1). Population-based MRI studies will be needed to examine the generalizability of our findings.
Conclusions
Increasing and consistently high levels of inflammation in early to mid-adulthood are associated with reduced white matter volume and lower white matter integrity at midlife. Individuals who display such trajectories of inflammation in adulthood may be at risk for adverse neurological outcomes over time, which may index risk for accelerated cognitive decline. When considering when to intervene to enhance brain health in late life, we need to begin at least as early as midlife.
Supplementary Material
Highlights.
From early adulthood to midlife, inflammation trajectories fell into three groups.
Moderate-increasing trajectory associated with lower midlife white matter volume.
Moderate-increasing trajectory associated with worse midlife white matter integrity.
Some evidence for worse midlife white matter integrity in consistently-higher group.
Acknowledgements
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). CARDIA was also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). Dr. O’Donovan was funded by a NIMH K01 Career Development Award (K01MH109871) and a University of California Hellman Fellowship. Dr. Yaffe was supported by a NIA K24 Award (K24AG031155). This manuscript has been reviewed by CARDIA for scientific content.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Aoife O’Donovan, PhD, University of California, San Francisco, CA, None
Amber Bahorik, PhD, University of California, San Francisco, CA, None
Stephen Sidney, MD, MPH, Kaiser Permanente, Oakland, CA, None
Lenore Launer, PhD, National Institute on Aging, Bethesda, MD, None
Kristine Yaffe, MD, University of California, San Francisco, CA, None
References
- 1.Robine J-M, Michel J-P. Looking Forward to a General Theory on Population Aging. J Gerontol Ser A. 2004;59:M590–M597. [DOI] [PubMed] [Google Scholar]
- 2.Yaffe K, Kanaya A, Lindquist K, et al. The Metabolic Syndrome, Inflammation, and Risk of Cognitive Decline. JAMA. 2004;292:2237–2242. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham C, Hennessy E. Co-morbidity and systemic inflammation as drivers of cognitive decline: new experimental models adopting a broader paradigm in dementia research. Alzheimers Res Ther. 2015;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory Proteins in Plasma and the Risk of Dementia: The Rotterdam Study. Arch Neurol. 2004;61:668–672. [DOI] [PubMed] [Google Scholar]
- 5.Jefferson AL, Massaro JM, Wolf PA, et al. Inflammatory biomarkers are associated with total brain volume. Neurology. 2007;68:1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker KA, Windham BG, Power MC, et al. The association of mid-to late-life systemic inflammation with white matter structure in older adults: The Atherosclerosis Risk in Communities Study. Neurobiol Aging. 2018;68:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly The 3C-Dijon Study. Neurology. 2012;78:720–727. [DOI] [PubMed] [Google Scholar]
- 8.Bettcher BM, Yaffe K, Boudreau RM, et al. Declines in inflammation predict greater white matter microstructure in older adults. Neurobiol Aging. 2015;36:948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vernooij MW, Ikram MA, Vrooman HA, et al. White Matter Microstructural Integrity and Cognitive Function in a General Elderly Population. Arch Gen Psychiatry. 2009;66:545–553. [DOI] [PubMed] [Google Scholar]
- 10.Madden DJ, Spaniol J, Costello MC, et al. Cerebral White Matter Integrity Mediates Adult Age Differences in Cognitive Performance. J Cogn Neurosci. 2008;21:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Power MC, Su D, Wu A, et al. Association of white matter microstructural integrity with cognition and dementia. Neurobiol Aging;83:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry VH, Teeling J. Microglia and macrophages of the central nervous system: The contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol. 2013;35:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monje ML, Toda H, Palmer TD. Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science. 2003;302:1760–1765. [DOI] [PubMed] [Google Scholar]
- 14.Ekdahl CT, Claasen J-H, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci. 2003;100:13632–13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreisel T, Frank MG, Licht T, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19:699–709. [DOI] [PubMed] [Google Scholar]
- 16.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. [DOI] [PubMed] [Google Scholar]
- 17.Penta A di, Moreno B, Reix S, et al. Oxidative Stress and Proinflammatory Cytokines Contribute to Demyelination and Axonal Damage in a Cerebellar Culture Model of Neuroinflammation. PLOS ONE. 2013;8:e54722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Dev Brain Res. 2003;140:205–214. [DOI] [PubMed] [Google Scholar]
- 19.Louveau A, Harris TH, Kipnis J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015;36:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesquita SD, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murta V, Farías MI, Pitossi FJ, Ferrari CC. Chronic systemic IL-1β exacerbates central neuroinflammation independently of the blood–brain barrier integrity. J Neuroimmunol. 2015;278:30–43. [DOI] [PubMed] [Google Scholar]
- 22.Silverman HA, Dancho M, Regnier-Golanov A, et al. Brain Region-Specific Alterations in the Gene Expression of Cytokines, Immune Cell Markers and Cholinergic System Components during Peripheral Endotoxin-Induced Inflammation. Mol Med. 2014;20:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho Y-H, Lin Y-T, Wu C-WJ, Chao Y-M, Chang AYW, Chan JYH. Peripheral inflammation increases seizure susceptibility via the induction of neuroinflammation and oxidative stress in the hippocampus. J Biomed Sci. 2015;22:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felger JC, Haroon E, Patel TA, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. Epub 2018. June 12:1. [DOI] [PMC free article] [PubMed]
- 25.Bettcher BM, Wilheim R, Rigby T, et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun. 2012;26:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker KA, Hoogeveen RC, Folsom AR, et al. Midlife systemic inflammatory markers are associated with late-life brain volume: The ARIC study. Neurology. 2017;89:2262–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadkarni NK, Boudreau RM, Studenski SA, et al. Slow gait, white matter characteristics, and prior 10-year interleukin-6 levels in older adults. Neurology. 2016;87:1993–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 29.Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials. 1987;8:68S–73S. [DOI] [PubMed] [Google Scholar]
- 30.Launer LJ, Lewis CE, Schreiner PJ, et al. Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PloS One. 2015;10:e0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curran PJ, Obeidat K, Losardo D. Twelve Frequently Asked Questions About Growth Curve Modeling. J Cogn Dev Of J Cogn Dev Soc. 2010;11:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers Gary L, Rifai Nader, Tracy Russell P, et al. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease. Circulation. 2004;110:e545–e549. [DOI] [PubMed] [Google Scholar]
- 33.Launer LJ, Lewis CE, Schreiner PJ, et al. Vascular Factors and Multiple Measures of Early Brain Health: CARDIA Brain MRI Study. PLOS ONE. 2015;10:e0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabani NJ, MacDonald DJ, Holmes CJ, Evans AC. 3D Anatomical Atlas of the Human Brain. NeuroImage. 1998;7:S717. [Google Scholar]
- 35.Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging. 2002;21:1421–1439. [DOI] [PubMed] [Google Scholar]
- 36.Goldszal A, Davatzikos C, Pham D, Yan M, Bryan RN, Resnick S. An Image-Processing System for Qualitative and Quantitative Volumetric Analysis of Brain Images. J Comput Assist Tomogr. 1998;22:827–837. [DOI] [PubMed] [Google Scholar]
- 37.Le Bihan D, Mangin J, Poupon C, et al. Diffusion tensor imaging: Concepts and applications. J Magn Reson Imaging. 2001;13:534–546. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs DR, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and Reliability of Short Physical Activity History: Cardia and the Minnesota Heart Health Program. J Cardpulm Rehabil. 1989;9:448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 40.Jones BL, Nagin DS, Roeder K. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociol Methods Res. 2001;29:374–393. [Google Scholar]
- 41.Jones BL, Nagin DS. Advances in Group-Based Trajectory Modeling and an SAS Procedure for Estimating Them. Sociol Methods Res. 2007;35:542–571. [Google Scholar]
- 42.Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent Class Growth Modelling: a tutorial. Tutor Quant Methods Psychol. 2009;5:11–24. [Google Scholar]
- 43.Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connor M-F, Irwin MR. Links Between Behavioral Factors and Inflammation. Clin Pharmacol Ther. 2010;87:479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marsland AL, Gianaros PJ, Kuan DC-H, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. 2015;48:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McEvoy John W, Nasir Khurram, DeFilippis Andrew P., et al. Relationship of Cigarette Smoking With Inflammation and Subclinical Vascular Disease. Arterioscler Thromb Vasc Biol. 2015;35:1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkins J, Ghosh P, Vivar J, Chakraborty B, Ghosh S. Exploring the associations between systemic inflammation, obesity and healthy days: a health related quality of life (HRQOL) analysis of NHANES 2005–2008. BMC Obes. 2018;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abramson JL, Vaccarino V. Relationship Between Physical Activity and Inflammation Among Apparently Healthy Middle-aged and Older US Adults. Arch Intern Med. 2002;162:1286–1292. [DOI] [PubMed] [Google Scholar]
- 49.Niles AN, Smirnova M, Lin J, O’Donovan A. Gender differences in longitudinal relationships between depression and anxiety symptoms and inflammation in the health and retirement study. Psychoneuroendocrinology. 2018;95:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: Depression Fans the Flames and Feasts on the Heat. Am J Psychiatry. 2015;172:1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H-L, Lu C-H, Lin H-C, et al. White Matter Damage and Systemic Inflammation in Obstructive Sleep Apnea. Sleep. 2015;38:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. [DOI] [PubMed] [Google Scholar]
- 53.Miguel CD, Rudemiller NP, Abais JM, Mattson DL. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep. 2015;17:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.López-González I, Tebé Cordomí C, Ferrer I. Regional Gene Expression of Inflammation and Oxidative Stress Responses Does Not Predict Neurodegeneration in Aging. J Neuropathol Exp Neurol. 2017;76:135–150. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Schuff N, Du A-T, et al. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain. 2009;132:2579–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mac Giollabhui N, Ellman LM, Coe CL, Byrne ML, Abramson LY, Alloy LB. To exclude or not to exclude: Considerations and recommendations for C-reactive protein values higher than 10 mg/L. L. Brain Behav. Immun. 2020. [DOI] [PMC free article] [PubMed]
- 57.Ishii S, Karlamangla AS, Bote M, Irwin MR, Jacobs DR Jr, Cho HJ, Seeman TE. Gender, obesity and repeated elevation of C-reactive protein: data from the CARDIA cohort. PloS ONE. 2012;7:e36062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data are available from the CARDIA Coordinating Center (cardia.dopm.uab.edu/contact-cardia). A description of the National Heart, Lung, and Blood Institute policies governing the data and describing access to the data can be found online (cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data).