Abstract
Background
Although long-term exposure to particulate matter<2.5μm (PM2.5) has been linked to chronic debilitating brain disorders (CDBD), the role of short-term exposure in health care demand, and increased susceptibility for PM2.5-related health conditions, among Medicare enrollees with CDBD has received little attention. We used a causal modeling approach to assess the effect of short-term high PM2.5 exposure on all-cause admissions, and prevalent cause-specific admissions among Medicare enrollees with CDBD (Parkinson’s disease-PD, Alzheimer’s disease-AD and other dementia).
Methods
We constructed daily zipcode counts of hospital admissions of Medicare beneficiaries older than 65 across the United-States (2000-2014). We obtained daily PM2.5 estimates from a satellite-based model. A propensity score matching approach was applied to match high-pollution (PM2.5 >17.4 μg/m3) to low-pollution zip code-days with similar background characteristics. Then, we estimated the percent change in admissions attributable to high pollution. We repeated the models restricting the analysis to zipcode-days with PM2.5 below of 35 μg/m3.
Results
We observed significant increases in all-cause hospital admissions (2.53% in PD and 2.49% in AD/dementia) attributable to high PM2.5 exposure. The largest observed effect for common causes was for pneumonia and urinary tract infection. All the effects were larger in CDBD compared to the general Medicare population, and similarly strong at levels of exposure considered safe by the EPA.
Conclusion
We found Medicare beneficiaries with CDBD to be at higher risk of being admitted to the hospital following acute exposure to PM2.5 levels well below the National Ambient Air Quality Standard defined as safe by the EPA.
Keywords: particulate air pollution, dementia, PM2.5, Parkinson’s disease
Graphical abstract
1. INTRODUCTION
In recent years, long-term exposure to PM2.5 has been associated with chronic debilitating brain disorders (CDBD) (Parkinson’s disease (PD), Alzheimer’s disease (AD), and other dementia) (Fu et al., 2019; Kioumourtzoglou et al., 2016; Paul et al., 2019), possibly due to neuroinflammation and accumulation of peptides in the brain, induced by particulate matter<2.5μm (PM2.5) exposure (Calderon-Garciduenas et al., 2008). PM2.5 may infiltrate the brain directly through the olfactory bulb neurons or through the blood-brain barrier (Paul et al., 2019), or indirectly through vascular neuropathology (Paul et al., 2019).
Evidence of chronic air pollution effects on CDBD is beginning to accumulate (Paul et al., 2019). However, evidence of the role of acute PM2.5 exposure in health care demand, and of increased susceptibility for various PM2.5-related health conditions among elderly people with CDBD, is yet minimal.
Unlike commonly used regression methods, causal inference analyses formalize the concepts and assumptions (Rubin, 1997) that allow the investigation of causal effects in observational studies. The idea motivating our causal inference approach is that each zipcode in our study area, on a given day, could have been exposed either to low or high PM2.5 pollution. However, for each zipcode-day, we only observe the outcome under the pollution scenario that occurred in reality. Causal inference methods estimate and compare the outcomes that would have occurred if all zipcode-days had experienced high pollution versus if all zipcode-days had experienced low pollution. In doing so, they flexibly eliminate confounding, mimicking a randomized study.
We used a propensity score (PS) matching approach to assess the causal effect of short-term high PM2.5 exposure on all-cause admissions, and most prevalent cause-specific admissions, among a susceptible subgroup of Medicare enrollees with CDBD. We then compared these effects to the effects observed among all Medicare enrollees across the United States (U.S.). Unlike most current studies that assess the health effect of PM2.5 increases continuously (Di et al., 2017b), we simulated a scenario in which an intervention shifts the entire PM2.5 distribution below the 90th percentile of exposure, to answer the question: what is the risk of hospital admissions associated with high versus low PM2.5 exposure?
In sensitivity analyses, we limited our focus to zipcode-days with PM2.5 exposure below the Environmental Protection Agency (EPA) National Ambient Air Quality (NAAQS) 24-hours standard of 35 μg/m3 to investigate whether the current guidelines are sufficient to eliminate the harmful PM2.5 effects.
2. MATERIALS AND METHODS
2.1. Data and study population
This study was approved by the Harvard School of Public Health Institutional Review Board. We included all Medicare beneficiaries across the contiguous U.S., 65 years or older, enrolled in the fee-for-service program between the years 2000 and 2014. Medicare is a federally funded insurance that covers over 95% of the population≥65 years of age in the United States. We obtained all Medicare inpatient hospital claims from the Medicare Provider and Analysis Review database, these data provides information on principal and secondary hospital admission codes, age, race, eligibility to Medicaid, sex, and zipcode of residence (Medicare., 2019).
2.2. Outcomes
From this data, we constructed zipcode-daily counts of primary emergency hospital all-cause admissions, both among all Medicare enrollees and among Medicare enrollees with CDBD specifically. The International Classification and Disease, Ninth Revision (ICD 9 discharge code) was used to characterize admissions types. All-cause admissions among the CDBD cohort, were defined as any admissions having a primary or secondary admission code of Parkinson’s disease (ICD 9: 332), or Alzheimer’s disease (ICD 9: 3310) and other dementia (ICD 9: 290).
We additionally identified the most prevalent admission causes among the CDBD cohort, i.e., the most common co-occurring diagnosis codes in CDBD admissions, and then constructed zipcode-daily counts of these admission causes among all Medicare enrollees, and among the CDBD cohort. The four most prevalent causes of admission among the CDBD cohort were: cerebral degenerations (ICD 9: 331), pneumonia, organism unspecified (ICD 9: 486); urinary tract infection–UTI (ICD 9: 559); and hip fractures (ICD 9: 820).
2.3. Exposure
We obtained PM2.5 exposure predictions from a well-validated ensemble-based model, which predicts daily PM2.5 levels at a spatial resolution of 1 km × 1 km across the contiguous U.S. In brief, the model integrates three machine learning algorithms (Di et al., 2016), and incorporate more than 100 predictor variables from satellite data (e.g. aerosol optic depth, absorbing aerosol index), land-use information (e.g. elevation, road density), weather monitoring sites (e.g. temperature, precipitation), and chemical transport model simulations. These variables were incorporated into a neural network-based hybrid model. The model was calibrated using daily PM2.5 concentrations measured at EPA monitoring sites and demonstrated excellent model performance (average cross-validated R2=0.86). In addition, the mean slope between predictions and PM2.5 measured at held out monitors was 1.0, indicating the error was random about the true value. Also, the trends in correlations among all pairs of PM2.5 levels as a function of distance, were identical when plotting monitored and modeled PM2.5. For more details please refer to Di et al. (Di et al., 2016). In a previous study by our group (Yitshak-Sade et al., 2018), the exposure of interest was defined as the two days moving average exposure to PM2.5. For consistency, in this study we also utilized the two days moving average exposure to PM2.5 concentration. To align with the Medicare data, we aggregated gridded exposures to zipcodes by averaging the daily exposures of the grid cells within each zipcode.
2.4. Statistical analysis
We conducted the statistical analysis in two phases. First, in the design phase, we utilized a PS matching approach to create a sample of matched high and low pollution zipcode-days in which confounding is eliminated. Second, in the analysis phase, we used the matched sample to estimate the percent change in hospital admission rates attributable to high PM2.5 exposure.
2.4.1. The design phase
We used a PS matching approach (Austin, 2011; Baccini et al., 2017) to eliminate confounding by spatial and temporal confounders and produce a matched sample in which the distributions of confounders are balanced across the high-pollution and low-pollution zipcode-days. We defined high pollution zipcode-days as zipcode-days with PM2.5 exposure over the 90th percentile of the distribution across the U.S. (≥17.4 μg/m3), and low-pollution zipcode-days as zipcode-days with PM2.5 exposure<17.4 μg/m3. Because year and state are strong spatial and temporal confounders, we implemented exact matching on those features, i.e., we stratified the sample by year and state prior to PS estimation and matching.
Within each subsample, we used a logistic regression model to construct the PS. The PS was defined as the probability of observing PM2.5 exposure ≥17.4 μg/m3 in a zipcode-day, conditional on the confounders included in the model. The following confounders were included in the PS model: season, day of the week, daily mean temperature, and zipcode level socioeconomic characteristics (median house value, percent poverty, percent with less than high school diploma, median household income, population density, and percent of black and Hispanic residents). We obtained the daily mean temperatures for each zipcode-day from the North American Regional Reanalysis data (Kalnay et al., 1996). We obtained the zipcode level socioeconomic variables from the U.S Census Bureau 2000 and 2010, and the American Community Survey 5-year estimates of 2009–2013. Since values are not available for all study years, we updated these variables each year by linearly extrapolating between the measured years.
We employed the PS to match low- and high-pollution zipcode-days in a 1:1 ratio. We used the ‘MatchIt’ package (2018) in R software (R Core Team; http://cran.r-project.org/) and created the matches using the ‘nearest neighbor’ method without replacement. We assessed the confounder balance by comparing the standardized mean differences (SMD) of each confounder before and after the matching procedure. An SMD lower than 0.1 was considered an indication of proper balance (Austin, 2011), and we chose the model that yielded the lowest SMD values for all confounders in the matched sample.
2.4.2. The analysis phase
After creating a well-balanced sample, we estimated the percent change in hospital admissions rates in high pollution days, compared to low pollution days. We applied conditional Poisson regression models to the matched sample, with adjustment for season and temperature. We chose this doubly robust approach to assure that there is no residual confounding by season and temperature, which were harder to balance. Among the CDBD cohort, we estimated the percent change in the following primary admission causes attributable to high pollution exposure: all-cause admissions, and the four most prevalent causes (cerebral degeneration, pneumonia of an unspecified organism, UTI, and hip fractures). We then repeated the models to estimate the same quantities for all Medicare enrollees, for each admission type with the exception of cerebral degeneration, which was irrelevant for Medicare enrollees without CDBD. We report the results as percent changes in admissions rates with 95% confidence intervals (CI). These changes can be interpreted as causal effects under the assumptions provided in Baccini et al (Baccini et al., 2017).
To test whether the effects remain when the PM2.5 exposure levels do not exceed the 24hr NAAQS defined by the EPA(EPA), we estimated the PM2.5 effects in a subsample restricted to zipcode-day pairs in which the PM2.5 levels on high pollution zipcode-days did not exceed 35 μg/m3 (i.e. between 17.4 and 35 μg/m3). We ensured that the covariates’ balance was not violated and then repeated our models within the restricted sample.
To make sure the effects found in our study are not confounded by NO2, we repeated the models with adjustment for NO2.
2.5. Data availability statement
Restricted by our Data Use Agreement with the U.S. Centers for Medicare & Medicaid Services, the Medicare data that support the findings of this study are neither sharable nor publicly available. Academic and non-profit researchers who are interested in using Medicare data shall contact with the U.S. Centers for Medicare & Medicaid Services directly.
3. THEORY
There are studies that assessed the effect of acute air pollution on CDBD primary admissions (Lee et al., 2017; Linares et al., 2017; Zanobetti et al., 2014), however, to our knowledge, no study to date has addressed the overall susceptibility of Medicare enrollees with CDBD to being admitted to the hospital due to high PM2.5 exposure, especially not within a causal modeling framework. Routine chronic disease management is more difficult among people with CDBD, which may lead to higher hospital admissions rates due to disease exacerbations or acute exacerbations of comorbidities (Phelan et al., 2012). It is, therefore, essential to assess the overall hospital admission burden rather than investigating dementia, AD, or PD related admissions alone.
4. RESULTS
Of the zipcode-days in the study period (2000-2014), we observed 21,026,131 high-pollution zipcode-days, i.e., zipcode-days with two-day average PM2.5 exposure above 17.4 μg/m3. All the measured confounders were well balanced in the matched sample (Supplementary Figure 1).
Our matched study sample included 30,079,287 primary emergency hospital admissions, 8.8% of these were among Medicare enrollees with CDBD. The mean daily count of admissions per zipcode-day was 0.71 among the general Medicare population, 0.016 among Medicare enrollees with PD, and 0.05 among Medicare enrollees with AD or other dementia. The most prevalent admissions-causes among Medicare enrollees with CDBD were cerebral degeneration (n=195,669), pneumonia due to an unspecified organism (n=147,253), UTI (n=138,153), and hip fractures (n=112,471). Climate and demographic features for the matched sample are summarized in Table 1. Features for the original unmatched population are summarized in Supplementary Table 1.
Table1.
Zipcode characteristics of low and high air pollution days.
| High pollution day | No (n=21,026,131) | Yes (n=21,026,131) |
|---|---|---|
| Temporal characteristics, n (%) | ||
| Season | ||
| Winter | 5,121,137 (24.4) | 4,865,190 (23.1) |
| Spring | 3,291,420 (15.7) | 3,202,507 (15.2) |
| Summer | 8,067,889 (38.4) | 8,537,690 (40.6) |
| Fall | 4,545,685 (21.6) | 4,420,744 (21.0) |
| SES characteristics, Mean (SD) | ||
| Median house value | 144,016 (130249) | 143,322 (124871) |
| Poverty | 0.11 (0.10) | 0.11 (0.10) |
| Median household income | 46,468 (21477) | 46,172 (20691) |
| Population density | 1,990 (6384) | 2,090 (6222) |
| Percent black | 0.11 (0.19) | 0.11 (0.19) |
| Percent Hispanic | 0.08 (0.15) | 0.08 (0.15) |
| Percent no high school diploma | 0.34 (0.19) | 0.34 (0.19) |
| ZIP code Medicare population | 1,068 (1573) | 1,075 (1579) |
| Environmental exposures, Mean (SD) | ||
| Temperature C° | 16.32 (10.39) | 16.94 (11.12) |
| PM2.5 | 11.10 (3.78) | 23.08 (6.51) |
SES=socioeconomic status; PM2.5= particulate matter<2.5μm
We observed significant increases in all-cause admissions attributable to high pollution exposure. The effects were larger among Medicare enrollees with PD (2.53% increase, 95% CI 2.40%; 2.63%), and among Medicare enrollees with AD/other dementia (2.49% increase, 95% CI 2.38%; 2.60%), compared to the general Medicare population (0.76%, 95% CI 0.69%; 0.83%). All the estimated risks remained similar after restricting the analysis to zipcode-days matched pairs with a high pollution day exposure below 35 μg/m3 (Table 2). All the observed effects remained similar when adjusting the models to NO2 (Table S2–S3).
Table 2.
The percent change in all-cause admissions among all Medicare beneficiaries, Medicare beneficiaries with AD/other dementia or PD, associated with a high pollution day.
| All-cause admissions | Percent increase in admissions rate (95% confidence interval) | |
|---|---|---|
| All zipcode-days in the matched sample | Restricted matched sample a | |
| Among all | 0.76% (0.69%: 0.83%) * | 0.65% (0.58%; 0.73%) * |
| Among AD/Other dementia | 2.49% (2.38%: 2.60%) * | 2.31% (2.20%; 2.42%) * |
| Among PD | 2.53% (2.40%: 2.63%) * | 2.45% (2.33%; 2.57%) * |
Table 2 shows the percent change in all-cause admissions rates in high PM2.5 pollution days, compared to low pollution days. We first analyzed all zipcode-days that were included in the matched sample. We defined high pollution zipcode-days as zipcode-days with PM2.5 exposure over the 90th percentile of the distribution across the U.S. (≥17.4 μg/m3), and low-pollution zipcode-days as zipcode-days with PM2.5 exposure<17.4 μg/m3.
We then restricted the sample to matched pairs with a high pollution zipcode-day that did not exceed 35 μg/m3 (i.e. between 17.4 and 35 μg/m3). Pairs of zipcode-days with high-pollution day PM2.5 exposure (≥17.4 μg/m3 were excluded from this analysis.
Each model was adjusted for temperature and season.
p<0.05
PD= Parkinson’s disease; AD= Alzheimer’s disease.
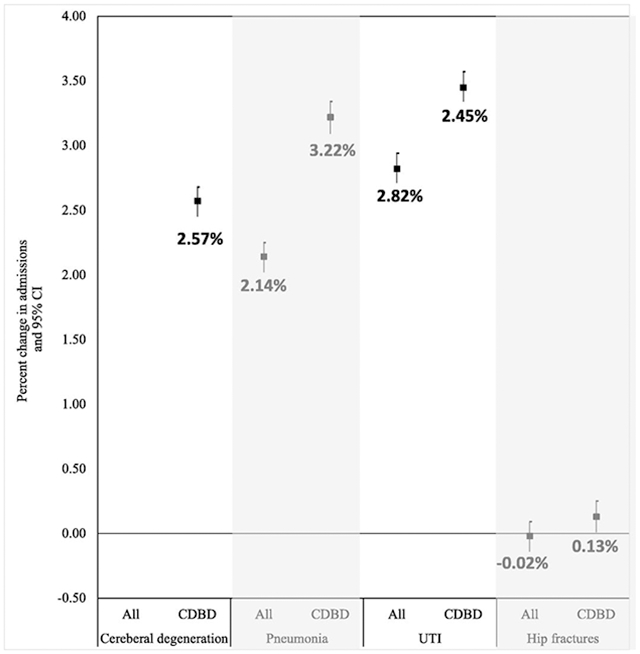
Among Medicare enrollees with CDBD, we found significant increases in all four most prevalent admission causes, attributable to high PM2.5 exposure. All effects were larger than the general Medicare population, with the largest effect observed for UTI (3.45% increase, 95%CI 3.34%; 3.57%) and pneumonia (3.22% increase, 95% CI 3.09%; 3.34%) (Table 3).
Table 3.
The percent change in cause-specific admissions among all Medicare beneficiaries, and among Medicare beneficiaries with CDBD, associated with a high pollution day.
| Admission cause | Percent increase in admissions rate (95% confidence interval) | |
|---|---|---|
| Medicare beneficiaries with CDBD | All Medicare beneficiaries | |
| Cerebral degenerations (ICD 9: 331) | 2.57% (2.45%; 2.68%) * | |
| Pneumonia NOS (ICD 9: 486) | 3.22% (3.09%; 3.34%) * | 2.14% (2.02%: 2.25%) * |
| UTI (ICD 9: 559) | 3.45% (3.34%; 3.57%) * | 2.82% (2.71%: 2.94%) * |
| Hip fractures (ICD 9: 820) | 0.13% (0.01%; 0.25%) * | −0.02% (−0.14%: 0.09%) |
Table 3 shows the percent change in cause-specific admissions rates in high PM2.5 pollution days, compared to low pollution days, among all Medicare enrollees and specifically among people with CDBD. Each model was fit to the matched sample and the model was adjusted for temperature and season. We identified the four most common causes of admission among people with CDBD, and then analyzed the association between high pollution day and these cause specific admissions among all people, and in a subgroup of people with CDBD.
p<0.05
ICD 9: International Classification and Disease, Ninth Revision; CDBD= chronic debilitating brain disease; NOS= no organism specified; UTI= urinary tract infection.
5. DISCUSSION
We found elderly with CDBD to be more susceptible to the effects of high PM2.5 exposure on all-cause admissions, and common cause-specific admissions. Although we observed significant PM2.5 effects in the general Medicare population as well, the effects were smaller. The effects found in our study remained significant and of similar magnitude when we restricted the analysis to days with PM2.5 exposure below the EPA NAAQS 24-hours standard of 35 μg/m3.
In recent years, some studies found links between long-term PM2.5 exposure and the development of CDBD (Babadjouni et al., 2017; Fu et al., 2019). Our study suggests that, in addition to inducing the disease, high air pollution exposure increases the risk of being admitted to the hospital due to other causes in elderly with CDBD. CDBD is known to be associated with higher rates of hospital admissions for causes such as pneumonia, congestive heart failure, dehydration, or UTI (Phelan et al., 2012). A study among Medicare enrollees found that elderly people with AD had a higher overall illness burden compared to the controls. UTI and injuries were among the top reasons for the emergency room visits (Zhao et al., 2008). This is supported by our study which showed that the most prevalent causes of admission among Medicare enrollees with CDBD were cerebral degeneration, pneumonia, UTI, and hip fractures. And more importantly, it emphasizes the importance of finding preventable exposures that are associated with risk for emergency admission due to various causes, rather than restricting the analysis to CDBD-related admission codes.
A recent national study that examined the short-term effect of PM2.5 exposure on cause-specific hospital admissions among the Medicare population has found increased risks for multiple prevalent and rarely studied admissions associated with higher exposure (Wei et al., 2019). Like our study, they found significant increases in the risk for Pneumonia and UTI.
The main finding of our study is the higher susceptibility of elderly people with CDBD to the PM2.5 effect. In most CDBD, as cognition worsens, patients are more prone to pneumonia, UTI, and age-related diseases such as stroke or cancer. The most common causes of death among CDBD patients in the terminal stages of the disease, other than age-related conditions, are complications of pneumonia, UTI, or decubitus ulcerations (Cummings and Pillai., 2016). People with CDBD may also be more susceptible to the PM2.5 harmful effect due to an inflammatory response in nervous tissue (Campbell et al., 2005; Zanobetti et al., 2014). Campbell et al has found increases levels of immune-related components and proinflammatory cytokines in the brain following exposure to concentrated particulate matter. Oxidative stress and increased inflammatory response have been associated with progression of neurodegenerative processes. Therefore, it is possible that exposure to PM may have an adverse effect on the progression CDBD (Campbell et al., 2005). The combination of these factors, alongside reduced mobility, can explain the higher susceptibility to air pollution-related hospital admissions in this population.
Another important finding of our study is the significant harmful effects observed in exposure to PM2.5 levels below the current EPA NAAQS threshold. Although these guidelines were set to protect the public from the harmful effects of air pollution, we show that health consequences occur even below the threshold defined as safe by the EPA. The same pattern was observed in other studies that investigated the effects of air pollution on health in the U.S (Di et al., 2017a; Makar et al., 2017).
A major strength of our study is the use of a causal modeling approach to minimize the possibility of confounding bias. Other studies have used PS methods to assess potential PM2.5 effects on health (Baccini et al., 2017; Makar et al., 2017). However, to the best of our knowledge, no previous studies have used this approach to assess the effect of short-term exposure to PM2.5 on various admission-causes among elderly people with CDBD in a national population-based study. This causal evidence is crucial for clinicians who can use this knowledge to tailor interventions and health education to reduce their patients’ risk. This can be done, for example, by providing information on ways of limiting exposure (i.e., maintaining proper ventilation, avoiding dust accumulation indoors, or using air filters), prioritizing preventive treatments, and considering dietary supplements to strengths immunity and reduce oxidative stress (Hadley et al., 2018), and taking special care in reducing air pollution exposure in nursing houses and similar facilities, where dementia and PD are highly prevalent.
This study has several limitations. First, this study investigates a binary exposure rather than a continuous exposure-response curve, therefore the observed effects may depend on the selected threshold. Second, there is always a possibility of exposure measurement error. However, since we use a highly spatially and temporally resolved prediction model to estimate the exposure, we expect the error to be minor and non-differential. In addition, although the PS matching approach eliminated confounding by measured confounders and omitted confounders that are highly correlated with those included in the model, there is still a possibility of confounding by omitted confounders that are not accounted for in the PS model. For example, we expect noise and gaseous air pollutants to be fairly balanced between low and high pollution days because these exposures are correlated with SES and temporal confounders that were included in the model. Also, the exposure estimates we used were at the neighborhood rather than street level, therefore the correlation with NO2 and noise is lower. This was confirmed by our sensitivity analysis that showed effects of similar magnitude with additional adjustment for NO2. and Lastly, since we only identified CDBD based on hospital diagnoses, we might have misclassified the disease status among subjects for whom the disease was not registered as a diagnosis.
In conclusion, we found Medicare beneficiaries with CDBD to be at higher risk of being admitted to the hospital following acute exposure to PM2.5 levels well below the NAAQS defined as safe by the EPA.
Supplementary Material
HIGHLIGHTS.
We tested the effect of exposure to high PM2.5 (i.e. PM2.5≥17.4 μg/m3)
PM2.5 exposure was associated with increases in all-cause hospital admissions
People with Parkinson’s disease and dementia were more susceptible to PM2.5
The effects remained when restricting the sample to PM2.5 levels under the EPA 24h standard.
6. ACKNOWLEDGMENTS
7. FUNDING
This study was supported by the Harvard Data Science Initiative; the National Institute of Health (NIH) (grant number ES024332-01 and AG066793-01); the National Institute of Environmental Health Sciences (NIEHS) (grant number P30 ES000002); the united states Environmental protection Agency (USEPA) (grant numbers RD83615601 and RD83587201); and the Health Effects Institute (HEI) (grant number 4953-RFA14-3/16-4). The contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA.
ABBREVIATIONS
- AD
Alzheimer’s disease
- CDBD
chronic debilitating brain disorders
- EPA
Environmental protection agency
- ICD9
International Classification and Disease, Ninth Revision
- NAAQS
national ambient air quality standards
- PD
Parkinson’s disease
- PM2.5
particulate matter<2.5μm
- PS
propensity score
- SMD
standardized mean differences
- UTI
urinary tract infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
8. REFERENCES
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behavioral Research 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babadjouni RM, Hodis DM, Radwanski R, Durazo R, Patel A, Liu QH, et al. Clinical effects of air pollution on the central nervous system; a review. Journal of Clinical Neuroscience 2017; 43: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccini M, Mattel A, Mealli F, Bertazzi PA, Carugno M. Assessing the short term impact of air pollution on mortality: a matching approach. Environmental Health 2017; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, et al. Long-term Air Pollution Exposure Is Associated with Neuroinflammation, an Altered Innate Immune Response, Disruption of the Blood-Brain Barrier, Ultrafine Particulate Deposition, and Accumulation of Amyloid beta-42 and alpha-Synuclein in Children and Young Adults. Toxicologic Pathology 2008; 36: 289–310. [DOI] [PubMed] [Google Scholar]
- Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology 2005; 26: 133–140. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Pillai JA. Neurodegenerative diseases: unifying principles. Oxford: Oxford University Press, 2016. [Google Scholar]
- Di Q, Dai LZ, Wang Y, Zanobetti A, Choirat C, Schwartz JD, et al. Association of Short-term Exposure to Air Pollution With Mortality in Older Adults. Jama-Journal of the American Medical Association 2017a; 318: 2446–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Kloog I, Koutrakis P, Lyapustin A, Wang YJ, Schwartz J. Assessing PM2.5 Exposures with High Spatiotemporal Resolution across the Continental United States. Environmental Science & Technology 2016; 50: 4712–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Koutrakis P, Choirat C, Dominici F, et al. Air Pollution and Mortality in the Medicare Population. New England Journal of Medicine 2017b; 376: 2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental protection agency (EPA). National ambient air quality standards (NAAQS) table. Avialble at: https://www.epa.gov/criteria-air-pollutants/naaqs-table.
- Fu PF, Guo XB, Cheung FMH, Yung KKL. The association between PM2.5 exposure and neurological disorders: A systematic review and meta-analysis. Science of the Total Environment 2019; 655: 1240–1248. [DOI] [PubMed] [Google Scholar]
- Hadley MB, Baumgartner J, Vedanthan R. Developing a Clinical Approach to Air Pollution and Cardiovascular Health. Circulation 2018; 137: 725–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D, Imai K, King G, Stuart E, Whitworth A. Package ‘MatchIt’. 2019, 2018. Avilable at: https://cran.r-project.org/web/packages/MatchIt/MatchIt.pdf. [Google Scholar]
- Kalnay E, Kanamitsu M, Kistler R, Collins W, Deaven D, Gandin L, et al. The NCEP/NCAR 40-year reanalysis project. Bulletin of the American Meteorological Society 1996; 77: 437–471. [Google Scholar]
- Kioumourtzoglou M-A, Schwartz JD, Weisskopf MG, Melly SJ, Wang Y, Dominici F, et al. Long-term PM2.5 Exposure and Neurological Hospital Admissions in the Northeastern United States. Environmental Health Perspectives 2016; 124: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Myung W, Kim DK, Kim SE, Kim CT, Kim H. Short-term air pollution exposure aggravates Parkinson’s disease in a population-based cohort. Scientific Reports 2017; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares C, Culqui D, Carmona R, Ortiz C, Diaz J. Short-term association between environmental factors and hospital admissions due to dementia in Madrid. Environmental Research 2017; 152: 214–220. [DOI] [PubMed] [Google Scholar]
- Makar M, Antonelli J, Di Q, Cutler D, Schwartz J, Dominici F. Estimating the Causal Effect of Low Levels of Fine Particulate Matter on Hospitalization. Epidemiology 2017; 28: 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicare. Medicare Provider Analysis and Review (MEDPAR), 2019. Avilable at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareFeeforSvcPartsAB/MEDPAR.
- Paul KC, Haan M, Mayeda ER, Ritz BR. Ambient Air Pollution, Noise, and Late-Life Cognitive Decline and Dementia Risk. Annual Review of Public Health, Vol 40 2019; 40: 203–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of Incident Dementia With Hospitalizations. Jama-Journal of the American Medical Association 2012; 307: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. Estimating causal effects from large data sets using propensity scores. Annals of Internal Medicine 1997; 127: 757–763. [DOI] [PubMed] [Google Scholar]
- Wei Y, Wang y, Di Q, Choirat C, Wang Y, Koutrakis P, et al. Short term exposure to fine particulate matter and hospital admission risks and costs in the Medicare population: time stratified, case crossover study. BMJ 2019; 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yitshak-Sade M, Bobb JF, Schwartz JD, Kloog I, Zanobetti A. The association between short and long-term exposure to PM2.5 and temperature and hospital admissions in New England and the synergistic effect of the short-term exposures. Science of the Total Environment 2018; 639: 868–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Dominici F, Wang Y, Schwartz JD. A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environmental Health 2014; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Kuo TC, Weir S, Kramer MS, Ash AS. Healthcare costs and utilization for Medicare beneficiaries with Alzheimer’s. Bmc Health Services Research 2008; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Restricted by our Data Use Agreement with the U.S. Centers for Medicare & Medicaid Services, the Medicare data that support the findings of this study are neither sharable nor publicly available. Academic and non-profit researchers who are interested in using Medicare data shall contact with the U.S. Centers for Medicare & Medicaid Services directly.


