Abstract
Opioids and opioid-conditioned stimuli (CS) negatively alter host immunity, impairing the response to pathogens during opioid use and following drug cessation. Using male rats, our laboratory has determined that heroin or heroin-CS exposure preceding a lipopolysaccharide (LPS) challenge markedly suppresses normal induction of peripheral pro-inflammatory biomarkers. Presently, it is unknown if these heroin-induced and -conditioned effects extend to the female immune response. To begin this venture, the current study tested the direct effects of heroin and heroin-CS on LPS-induced peripheral nitric oxide (NO) production in female rats. We focused investigations on peripheral NO as it is a critical pro-inflammatory molecule necessary for pathogen resistance. In Experiment 1, male and female Lewis rats were administered 0 (Saline), 1, or 3 mg/kg heroin subcutaneously (s.c). Sixty minutes later, animals were injected with LPS (1 mg/kg, s.c.). Spleen and plasma samples were collected 6 hours later to examine NO production through inducible NO synthase (iNOS) expression and nitrate/nitrite concentration, respectively. In Experiment 2, female Lewis rats underwent five, 60-minute context conditioning sessions with heroin (1 mg/kg, s.c.) or saline. On test day, CS-exposed and control (home cage) animals were injected with LPS (1 mg/kg, s.c.). Tissue was collected 6 hours later to examine splenic iNOS expression and plasma nitrate/nitrite concentration. Both heroin administration alone and exposure to heroin-CS suppressed LPS-induced indices of NO production in spleen and plasma. Our results are the first to indicate that, similar to males, female rats express heroin-induced and -conditioned immunomodulation to a LPS challenge.
Keywords: heroin, opioid, heroin conditioning, immune, immune conditioning, female, LPS
1. Introduction
The current heroin epidemic is a devastating public health crisis that poses a significant economic burden [e.g. (Mark et al., 2001; Shipton et al., 2018)]. The detrimental consequences of opioid use are exacerbated by conditioned responses that stem from the association between a drug-paired context and drug use. Drug-associated contexts serve an important role in maintaining addiction by increasing the likelihood of relapse [e.g. (Bossert et al., 2016; Childress et al., 1993; Childress et al., 1986; See, 2002)]. Additionally, these heroin-associated stimuli drive other conditioned responses, such as heroin-conditioned peripheral immune alterations (Lysle and Ijames, 2002). Heroin negatively alters host immunity and decreases pathogen resistance by dampening the induction of peripheral pro-inflammatory agents such as cytokines, circulating lymphocytes, and inducible nitric oxide (Govitrapong et al., 1998; McCarthy et al., 2001; Nair et al., 1986). Moreover, heroin-induced immunomodulation may be particularly harmful for users diagnosed with HIV, a disease occurring at a disproportionally high rate in this population (Todd et al., 2007), as it could dampen the already impaired pro-inflammatory response to infection (Meijerink et al., 2015).
Our laboratory was the first to establish that heroin-induced immunomodulation can become conditioned to environmental contexts repeatedly paired with use (Lysle and Ijames, 2002). We reliably demonstrate that exposure to heroin-paired stimuli alone is sufficient to elicit pronounced suppression of peripheral nitric oxide and cytokine production following endotoxin exposure [e.g. (Lysle and Ijames, 2002; Saurer et al., 2008; Szczytkowski and Lysle, 2008)]. Our laboratory has extensively characterized these effects using male rats and determined that it is a Pavlovian conditioned response. Further, both heroin-induced and -conditioned immunomodulation are mediated by the central nervous system (Fecho et al., 1996; Hutson et al., 2014; Lebonville et al., 2016; Lebonville et al., 2020; Lysle et al., 1996; Paniccia et al., 2018; Saurer et al., 2009; Szczytkowski et al., 2011; Szczytkowski et al., 2013; Szczytkowski and Lysle, 2007; Szczytkowski and Lysle, 2008). Our results suggest that conditioning processes extend heroin’s deleterious health effects past drug cessation. Thus, investigations into heroin-induced and -conditioned peripheral immunomodulation could have clinical relevance, particularly for long-term heroin users and those in recovery.
Traditionally, animal models investigating opioid-related health consequences have focused on males. However, females experience distinct behavioral patterns and biological responses associated with opioid use (Iversen et al., 2010; Jamison et al., 2010) that might make them more vulnerable to opioid-related effects, and in particular, heroin-induced and -conditioned immunomodulation. For instance, women are more likely to be prescribed opioid analgesics and are at a greater risk of misusing prescription opioids (Koons et al., 2018), which is often a precursor for heroin use (Lankenau et al., 2012). Consistent with this, heroin use has disproportionally increased among women in recent years, with the incidence of heroin use doubling among women in the United States (Jones et al., 2015). Further, heroin-paired cues appear to elicit stronger physiological and motivated responses in women than in men (Yu et al., 2007). Sex differences in opioid use/taking behavior exist in the pre-clinical literature as well. Female rats have been shown to acquire heroin self-administration faster than males (Lynch and Carroll, 1999), suggesting a distinct sex-specific behavioral pattern in drug-taking behavior.
Although the literature indicates females display increased heroin taking behavior and reactivity to heroin-related cues, there are existing discrepancies regarding sex differences in some of these same opioid-induced responses in both clinical populations and pre-clinical models of heroin use disorders (Lynch et al., 2002). For example, Kennedy and colleagues found no sex differences in amount of heroin used or treatment retention, and that women were less likely to crave drug after seeing others use heroin (Kennedy et al., 2013). Similarly, some pre-clinical results demonstrate no sex-related effects in heroin self-administration, total heroin intake, breakpoint, or heroin craving following abstinence in male and female rats (Lynch et al., 2002; Venniro et al., 2017). It is apparent that there are conflicting reports on whether sex differences exist in heroin-induced responses, such as self-administration and craving caused by heroin-paired cues. Given this, it is important to test potential sex differences continually in heroin-induced behaviors and responses and gain insight into how the response in question is affected based on biological sex.
Importantly for the current study, sex differences exist in the immune response (Klein, 2004; Schwarz and Bilbo, 2012) and in opioid-immune interactions (Doyle and Murphy, 2017). There is evidence to suggest females have a more robust innate immune response than males and tend to show higher levels of cytokine production following exposure to an immune challenge with lipopolysaccharide [LPS; (Engler et al., 2016)]. LPS stimulation of innate immune toll-like receptor 4 (TLR4) signaling may contribute to this exacerbated cytokine response in females (Kovats, 2015). Interestingly, opioids also bind to TLR4, and TLR4 stimulation modulates opioid pharmacodynamics (Shah et al., 2016). Opioids have alternatively been shown to negatively modulate activity downstream of TLR4 stimulation, disrupting pro-inflammatory responses, including production of cytokines and NO (Eisenstein, 2019). Sex differences in innate immunity, combined with differences in direct opioid-immune interaction may, in part, account for sex-based differences in the behavioral effects of opioids (Doyle and Murphy, 2017; Doyle and Murphy, 2018), but testing opioid-related health consequences mostly in males has left a disparity in understanding how opioids affect female health. Presently, it is unknown if heroin’s deleterious impact on immune function extends to females, and if present, whether this heroin-induced immunomodulation can become conditioned to environmental stimuli.
The current set of experiments investigated whether heroin suppresses the peripheral immune response in female rats as compared to males (Experiment 1) and if female rats express context-heroin conditioned immunomodulation (Experiment 2). Our laboratory has a long history of examining the effect of heroin and heroin-paired cues on the immune response in male rats [e.g. (Fecho and Lysle, 2000; Lanier et al., 2002; Lysle and How, 2000; Lysle and Ijames, 2002)], and is well-positioned to investigate heroin-induced and -conditioned alterations in peripheral nitric oxide (NO) production in females. NO is a critical component of host immunity (Breitbach et al., 2006; Hoffmann et al., 2006; Oates and Gilkeson, 2006). For instance, NO is an essential modulator of T-cell activation and proliferation, B-cell maturation and proliferation, immunoglobulin production, and the synthesis of numerous cytokines (Bogdan et al., 2000). To assess the impact of heroin and heroin-conditioned stimuli (CS) on peripheral NO, we analyze the pro-inflammatory indices of NO production: inducible nitric oxide synthase (iNOS; the enzyme that is responsible for NO production) and plasma nitrate/nitrite concentration (the degradation product of NO). These indices of NO production are potently upregulated by an immune challenge like LPS (Bogdan, 2001) and changes in peripheral iNOS expression and nitrate/nitrite concentration are some of the most reliable and sensitive indices of alterations of immune status in vivo.
The current study employed LPS to mimic bacterial infection and trigger pro-inflammatory responses that permit observation of heroin-induced and -conditioned changes in NO production. We have reliably used LPS in male rats to stimulate the immune system and examine how heroin and heroin-CS alter peripheral indices of NO production in vivo [e.g. (Hutson et al., 2014; Lebonville et al., 2016; Lebonville et al., 2020; Paniccia et al., 2018; Szczytkowski et al., 2013)]. LPS is a component of the cell wall of Gram-negative bacteria, and it is the most widely recognized agent that effectively and reliably stimulates immune responses (Liu et al., 1993; Liu et al., 1997; Tracey et al., 1995). Thus, we administer LPS following heroin and heroin-CS exposure to examine alterations in the pro-inflammatory biomarkers of NO production, specifically within the spleen and blood plasma of female rats. Naturally cycling female rats were used in both experiments, and estrous phase was examined at the time of tissue collection for potential indication of cycle effects on heroin-induced or -conditioned NO suppression. Collectively, these studies provide insight into the negative health consequences of heroin use in females and examine if females, like males, are susceptible to heroin-conditioned immunomodulation that would extend these deleterious effects past drug cessation.
2. Materials and Methods
2.1. Animals
Adult male and female Lewis rats (N = 68; 18 males, 50 females) were purchased from Charles River Laboratories (Kingston, NY, USA). Historically, Lewis rats have been the preferred strain for investigating the inflammatory response of nitric oxide (Misiewicz et al., 1996; Salvemini et al., 1995), as well as opioid-induced effects [e.g., (Ambrosio et al., 1995; Guitart et al., 1992; Guitart et al., 1993; Suzuki et al., 1988)], in a rat model. Rats were individually housed on a 12-hour reverse light-dark cycle, received ad libitum home cage access to food and water, and were handled regularly throughout experimentation. All procedures took place during the dark phase of the light cycle and were conducted in compliance with regulations set forth by the National Research Council’s Guide for the Care and Use of Laboratory Animals and the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
2.2. Drug Administration
Heroin (diacetylmorphine, National Institute on Drug Abuse Drug Supply Program, Bethesda, MD, and USA) was dissolved in 0.9% sterile saline vehicle and stored at 4°C until use at room temperature. Heroin was administered subcutaneously (s.c.) at a dose of either 1 or 3 mg/kg in Experiment 1 and at a dose of 1 mg/kg in Experiment 2. Lipopolysaccharide (LPS; derived from E. coli, serotype O55:B5, Millipore-Sigma, Burlington, MA, USA) was dissolved in 0.9% sterile, pyrogen-free saline to a final dose of 1 mg/kg and was administered s.c. in all experiments. This LPS dose reliably produces sickness behavior, induces production of peripheral NO, and can be used to examine heroin-induced and -conditioned effects on NO production (Lysle and How, 2000; Lysle and Ijames, 2002).
2.3. Heroin-induced and -conditioned Immunomodulation and Testing
2.3.1. Experiment 1: Heroin-induced Peripheral Immunomodulation
Male and female rats (N = 18/sex) were randomly assigned to groups in a 2 (sex) × 3 (heroin dose) between-subjects design. Animals received one of three drug doses: 0 (0.9% sterile saline vehicle), 1, or 3 mg/kg, and were left undisturbed in home cage for 1 hour. Subsequently, rats received an LPS injection to stimulate an immune response and remained in home cage until tissue collection 6 hours later. We have reliably observed heroin-related alteration of LPS-induced peripheral NO production through measurement of splenic iNOS expression and plasma degradation products at this time point, which is near the peak of LPS-induced NO production (Lysle and How, 1999, 2000; Lysle and Ijames, 2002).
2.3.2. Experiment 2: Heroin-conditioned Peripheral Immunomodulation
Naturally cycling female rats (N = 32) were randomly assigned to groups in a 2 (drug condition) × 2 (CS exposure) between-subjects design. The heroin conditioning paradigm employed herein has been previously described [see (Paniccia et al., 2018)]. In short, animals were subject to five, 1-hour conditioning sessions in which heroin (or saline vehicle as a control) was paired with a standard conditioning chamber (BRS/LVE, Laurel, MD, USA; H 26.7 cm × D 24.1 cm × W 30.5 cm). The chamber served as the conditioned stimulus (CS) and was located in a room adjacent to the vivarium. Each chamber contained metal bar flooring and cedar bedding. Conditioning chambers were located within sound- and light-attenuating chambers (H 36.8 cm × D 34.3 cm × W 50.8 cm) with a fan used to mask background noise. This setup created an environmental CS with olfactory, tactile, auditory, and visual cues distinct from home cage stimuli. Conditioning sessions were separated by 48 hours. Following the conditioning procedure, animals remained undisturbed in home cage for six days until the conditioning test. On test day, rats were either reexposed to the CS for 1 hour or remained in home cage as controls. Immediately following CS exposure or home cage stay, animals were administered LPS and remained in home cage for 6 hours until tissue collection. Saline-conditioned animals controlled for ancillary effects from exposure to heroin, the conditioning chamber, and the injection experience.
2.4. Tissue Collection
Animals were sacrificed via rapid cervical dislocation without anesthesia. Spleen tissue and blood plasma were collected for later analysis of iNOS expression and nitrate/nitrite concentration, respectively. Spleen tissue for RNA and protein analyses was divided into ~100 mg samples and were stored at −80°C in either RNAlater (Ambion, ThermoFisher Scientific, Waltham, MA, USA) or cOmplete protease inhibitor cocktail (Roche, Millipore-Sigma) in 1X phosphate buffered saline until assay.
2.5. Vaginal Lavage and Histology
Vaginal lavages were taken post mortem to assess estrous stage on test day. Samples were smeared onto SuperFrost Plus slides (ThermoFisher Scientific), allowed to dry, and stained using a Wright Giemsa Stain, modified (Sigma-Aldrich) method. Vaginal cell distribution was examined using a Leica DM 6000B microscope (Leica Microsystems, Buffalo Grove, IL, USA) and estrous phase (diestrus, proestrus, estrus, or metestrus) was determined according to methods described by Cora and colleagues (Cora et al., 2015).
2.6. RT-qPCR for Splenic iNOS mRNA Expression
2.6.1. RNA Extraction and cDNA Synthesis
RNA was extracted from spleen tissue to measure iNOS mRNA expression. Tissue was added to 1 mL of cold TriReagent (Molecular Research Center, Cincinnati, OH, USA) and homogenized using a bead homogenizer (Precellys Instruments, Montigny-le-Bretonneux, France). Spleen homogenates were briefly centrifuged to remove debris and transferred to a second tube. Next, BCP (1-Bromo-3-Chloropropane; Molecular Research Center, Inc, Cincinnati, OH, USA) was added to the homogenate and samples were thoroughly mixed, incubated at room temperature for 7 minutes, and centrifuged at 4° C for phase separation. The aqueous layer was mixed with isopropanol and incubated at room temperature for 7 min. Following this, samples were centrifuged at 4° C to collect the RNA pellet. RNA pellets were washed three times in 75% ethanol and allowed to air dry to remove residual ethanol. Purified RNA was reconstituted in warm, PCR-grade water. RNA concentration and purity were assessed via spectrophotometer (Epoch™, BioTek Instruments Inc., Winooski, VT, USA) using the Take3 Application and Gen5 Software for Nucleic Acid Quantification (BioTek Instruments Inc.).
Sample RNA concentration was equalized to 1 μg in PCR-grade water for the cDNA reaction. cDNA was synthesized using the Advantage for RT-PCR Kit and Oligo(DT) primers (ClonTech, Takara, Mountain View, CA, USA) according to manufacturer’s protocol on a Veriti 96 Well Fast Thermal Cycler (Applied Biosystems, ThermoFisher Scientific). Samples were diluted 1:5 in PCR-grade water for qPCR.
2.6.2. RT-qPCR
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) was performed using the TaqMan™ Fast Advanced Master Mix Kit (Applied Biosystems, ThermoFisher Scientific), following the manufacturer’s instructions, to quantify splenic iNOS mRNA expression. Sample reactions were carried out in triplicate on a 384-well plate, with individual reactions containing 1.5 μL of cDNA. Predesigned fluorescein (FAM) assays were used to quantify expression of iNOS (NOS2, Assay ID: Rn00561646_m1, ThermoFisher Scientific) and reference gene 60S ribosomal protein L13a (Rpl13a, reference gene, Assay ID: Rn01475911_g1; ThermoFisher Scientific). No template control wells were run to ensure reaction purity. RT-qPCR was run on QuantStudio™ 6 Flex RealTime PCR System (Applied Biosystems, ThermoFisher Scientific) using the QuantStudio™ RealTime PCR Software with a PCR Run Method as follows: 2 min at 50°C for PCR product contamination degradation; 20 sec hold at 95°C for polymerase activation; 45 PCR cycles (1 sec at 95° C then 20 sec at 60° C) with data collection at the end of each cycle. The comparative delta delta CT (ΔΔCT) method was used for data analysis (Schmittgen and Livak, 2008). iNOS CT values were first normalized to the reference gene (L13a) and then to the mean ΔCT of the entire data set. Linearly transformed values were used to display the data graphically.
2.7. ELISA for Splenic iNOS Protein
Protein was extracted from spleen tissue using bead homogenization and freeze-thaw lysis. A Bradford Assay was used to quantify total protein concentration for each sample. Briefly, 10 μL of diluted (1:200) sample, in duplicate, was incubated with 200 μL of diluted (1:4) and filtered Bio-Rad Protein Assay Dye Reagent (BioRad Laboratories, Hercules, CA, USA) for 10 min. Absorbance was measured via spectrophotometer (Epoch™, BioTek Instruments Inc.) at 595 nm, averaged across sample duplicates, and compared to a concurrently run BSA standard curve to determine concentration (μg/μL). To measure splenic iNOS protein, samples were run in duplicate in a rat iNOS sandwich ELISA (Cat #: abx256135, Abbexa Ltd., Cambridge, UK) according to the manufacturer’s protocol. Input protein was equalized across all samples to 15 μg, and pg quantity of iNOS protein per total protein was calculated based on a concurrently run standard curve. The sample duplicate mean iNOS protein quantity was used in data analysis.
2.8. Griess Reagent Assay for Plasma Nitrate/Nitrite Concentration
The degradation products of NO, nitrate and nitrite, were measured in blood plasma using the Griess reagent assay as previously described (Szczytkowski and Lysle, 2007). Briefly, samples were assayed in triplicate, and diluted plasma was incubated with nitrate reductase (1.0 U/mL), 0.31 M phosphate buffer (pH = 7.5), 0.86 mM NADPH (Sigma-Aldrich Inc., Milwaukee, WI, USA), and 0.11 mM flavin adenine dinucleotide at room temperature in the dark for 90 minutes. Subsequently, Griess reagent [1:1 (vol:vol) solution 1% sulfanilamide in 5% phosphoric acid and 0.1% N-(1-napthyl)ethylenediamine dihydrochloride in distilled H2O] was added to the samples and incubated for 10 minutes at room temperature. Absorbance was read at 550 nm using a spectrophotometer (Epoch™, BioTek Instruments Inc), and concentration for each sample was determined based on a concurrently run standard curve. Mean concentration of sample triplicate was used in analysis.
2.9. Statistical Analysis
SPSS Statistics (IBM, Armonk, NY) was used to analyze data from each experiment. Results were analyzed using between-subjects analysis of variance (ANOVA). Statistically significant outliers were detected using Grubb’s test and were removed from data analysis. For all tests, alpha was set at p = .05. Tukey’s Honestly Significant Difference (HSD) post-hoc comparison was used to probe significant interactions and ancillary effects.
3. Results
3.1. Experiment 1: Heroin-induced Suppression of Peripheral NO Production Occurs in Both Sexes
Experiment 1 investigated whether female rats exhibit comparable heroin-induced immunomodulation as male counterparts (see Fig. 1A for experimental timeline). Heroin administration significantly suppressed LPS-induced pro-inflammatory indices of NO production (Fig. 1B–D). Final group sizes were n = 5–6 after accounting for statistical outliers: four animals were removed from mRNA and one from protein analyses.
Figure 1.
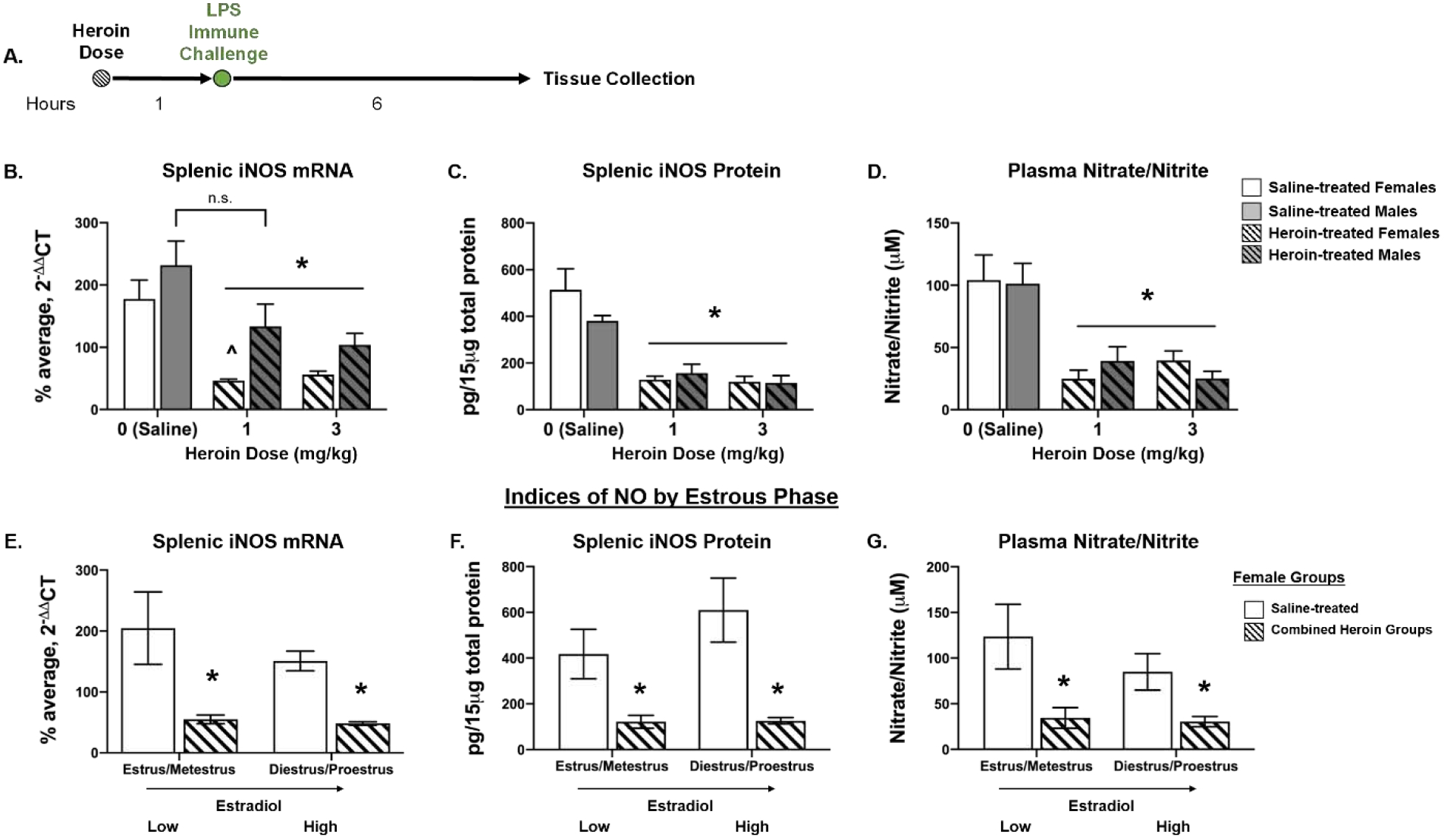
Heroin administration suppresses LPS-induced indices of peripheral NO production. Experimental timeline (A). For females, both heroin doses (1- and 3-mg/kg) significantly suppressed iNOS mRNA levels relative to saline-treated counterparts; while only 3 mg/kg heroin suppressed LPS-induced iNOS mRNA in males as compared to saline counterparts (B). In both sexes, a 1- or 3-mg/kg dose of heroin blunts LPS-induced splenic iNOS protein (C) and plasma nitrate/nitrite concentration (D) relative to saline-treated controls. E–G depicts the data of female rats separated by estrous stages with low or high/increasing estradiol in saline- and heroin-treated groups. Regardless of estrous distinction, heroin significantly suppressed LPS-induced splenic iNOS mRNA (E) and protein (F), as well as plasma nitrate/nitrite concentration (G) relative to saline-treated controls. *, statistically significant main effect of heroin. ^, statistically significant difference from male counterpart. n.s., no significant differences between saline treated and 1-mg/kg heroin males. Error bars indicate SEM.
A 2 × 3 ANOVA of splenic L13a mRNA levels revealed a main effect of sex (F(1, 28) = 4.521, p = .042), but no effect of drug treatment (F(2,28) = 0.197, p = .823) nor interaction of the two (F(2,28) = 0.385, p = .684). Post hoc analysis revealed there were no significant differences between male and females in the saline-treated group (p = .992), 1 mg/kg heroin group (p = .803), or 3 mg/kg group heroin (p = .437). Thus, L13a was used as a reference gene in analysis. For splenic iNOS mRNA, a 2 × 3 ANOVA of ΔΔCT values revealed a main effect of sex (F(1,26) = 21.16, p < .001) and a main effect of drug treatment (F(2,26) = 24.293, p < .001) but no interaction between the two [F(2, 26) = 2.137, p = .138; Fig. 1B]. Tukey’s HSD post hoc comparisons revealed that heroin significantly suppressed LPS-induced splenic iNOS mRNA expression relative to saline-treated counterparts in males at doses of 3 mg/kg (p = .021), but not at 1 mg/kg (p = .101). In females, heroin significantly blunted LPS-induced iNOS mRNA levels at 1 mg/kg (p < .001) and 3 mg/kg (p = .001) as compared to saline-treated controls. There were no differences in iNOS mRNA levels between the 1-mg/kg and 3-mg/kg heroin-treated females (p = .957), indicating both heroin doses produced comparable suppression of iNOS mRNA expression. Additionally, post hoc analysis revealed no significant difference between the saline-treated male and female groups (p = .793), whereas within the 1 mg/kg heroin treatment females had significantly lower iNOS levels than their male counterparts (p = .003). At 3 mg/kg, iNOS mRNA expression did not differ across sex (p = .162).
Heroin exposure significantly blunted LPS-induced splenic iNOS protein expression (Fig. 1C). A 2 × 3 ANOVA of splenic iNOS protein concentration revealed a main effect of drug treatment (F(2, 29) = 31.356, p < .001), but no main effect of sex (F(1, 29) = 0.967, p = .333) nor sex × drug interaction (F(2, 29) = 1.701, p = .20). Tukey’s HSD post hoc comparisons revealed that as compared to saline-treated controls, 1 mg/kg of heroin significantly blunted LPS-induced iNOS protein in both females (p < .001) and males (p = .024). Similarly, 3 mg/kg heroin suppressed iNOS protein expression across sex, in that both males (p = .005) and females (p < .001) had significantly lower levels of iNOS protein than their saline-treated counterparts. There were no significant sex differences within the saline-treated group (p = .364), 1-mg/kg heroin group (p = .998), or 3-mg/kg heroin group (p = .999), demonstrating comparable levels of heroin-induced immunomodulation in both sexes. Additionally, there was no difference in iNOS protein expression between the 1- and 3-mg/kg heroin-treated females (p = .999), indicating comparable heroin-induced iNOS protein suppression for both heroin doses.
Consistent with splenic iNOS measures, heroin administration significantly suppressed LPS-induced plasma nitrate/nitrate concentration (Fig. 1D). A 2 × 3 ANOVA of plasma nitrate/nitrite concentration revealed a main effect of drug treatment (F(2, 30) = 21.258, p < .001), but no main effect of sex (F(1, 30) = 0.01, p = .923) nor interaction between the two (F(2, 30) = 0.515, p = .515). For males, post hoc analysis revealed that when heroin administration preceded LPS challenge, doses of 1- (p = .016) or 3-mg/kg (p = .002) suppressed nitrate/nitrite concentration relative to saline controls. Similarly, females that were administered heroin at 1- (p = .001) or 3-mg/kg (p = .011) had significantly lower plasma nitrate/nitrite concentration compared to saline-treated counterparts. There were no significant sex differences within the saline-treated group (p = .999), 1-mg/kg heroin group (p = .962), or 3-mg/kg heroin group (p = .962), demonstrating comparable levels of heroin-induced immunomodulation across sex. There was no difference in heroin-induced suppression of nitrate/nitrite concentration between females that were administered 1- or 3-mg/kg heroin (p = .958), suggesting that both doses produced suppression of this measure.
Although naturally cycling females exhibited heroin-induced suppression of NO in peripheral tissue and plasma, estrous phase was determined for each sample and categorized into diestrus, proestrus, estrus, or metestrus. Statistical analysis did not reveal significant differences in female heroin-induced immunomodulation between the 1- and 3-mg/kg doses of heroin, and as such, the heroin groups were combined to assess NO measures across estrous cycle. Final analyses of splenic iNOS expression and plasma nitrate/nitrite concentration (n = 6 in the saline-treated group and n = 11–12 in the heroin groups) when split across cycle did not yield a large enough sample size per stage to probe effects of all four estrous phases on measures of peripheral NO. Given that rat samples in diestrus and proestrus are characterized as “increasing/high estradiol” and those in estrus and metestrus are characterized as “low estradiol” (Miller and Takahashi, 2014; Santmyire et al., 2010), a 2 (low vs high estradiol phase) × 2 (saline vs heroin) ANOVA was used to examine estradiol effects on NO production. Final group sizes per estradiol group was n = 3 for saline-treated and n = 4–7 for heroin-treated females. Analysis of iNOS ΔΔCT values revealed a significant main effect of heroin (F(1, 13) = 60.266, p < .001) but no main effect of estradiol phase (F(1, 13) = 1.135, p = .306) or heroin treatment × estradiol interaction (F(1, 13) = .088, p = .772) (Fig. 1E). For iNOS protein, a 2 × 2 ANOVA revealed a main effect of heroin (F(1, 14) = 40.112, p < .001), but no main effect of estradiol phase (F(1, 14) = 2.549, p = .133) or drug × estradiol interaction (F(1, 14) = 2.33, p = .149) (Fig. 1F). Similarly, analysis of nitrate/nitrite concentration revealed a significant main effect of heroin (F(1, 14) = 20.746, p < .001), but no main effect of estradiol phase (F(1, 14) = 1.834, p = .197) or drug × estradiol interaction (F(1, 14) = 1.209, p = .29) (Fig. 1G). Thus, estradiol phase did not significantly alter heroin-induced immunomodulation, since heroin consistently blunted LPS-induced indices of NO production.
3.2. Experiment 2: Female Rats Exhibit Heroin-conditioned Immunomodulation
Experiment 2 examined whether females would express heroin-conditioned peripheral immunomodulation following re-exposure to a heroin-paired context (see Fig. 2A for experimental timeline). Following context-heroin conditioning with a 1 mg/kg dose of heroin, CS exposure significantly suppressed the LPS-induced peripheral pro-inflammatory indices of NO production (Fig. 2B–D). Final group sizes were n = 7–8 after accounting for statistical outliers: two animals were excluded from mRNA and one from nitrate/nitrite analyses.
Figure 2.
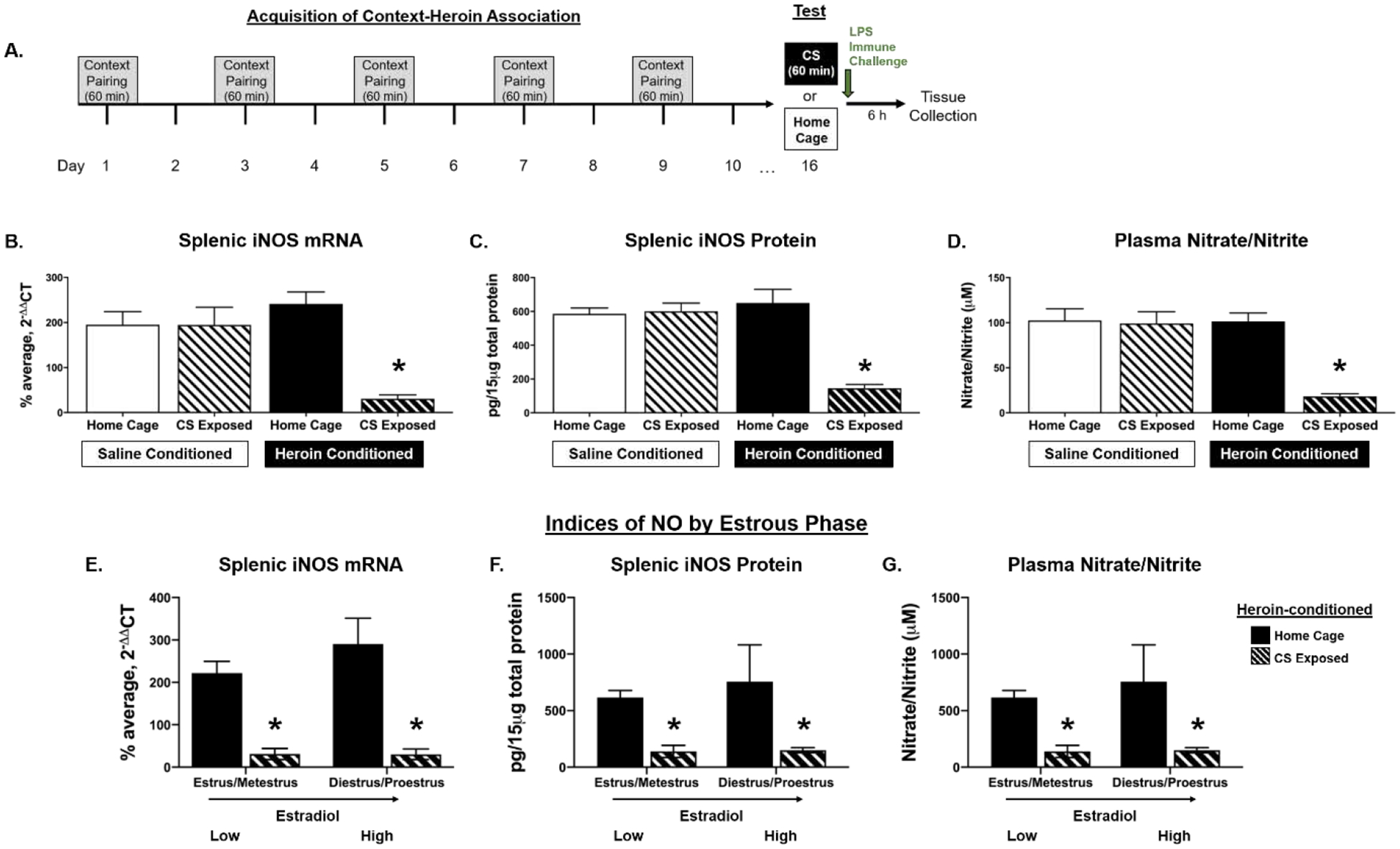
Females express heroin-conditioned suppression of LPS-induced indices of NO production. Experimental timeline (A). Following context-heroin conditioning with 1 mg/kg heroin, exposure to heroin-conditioned stimuli (CS) blunts LPS-induced splenic iNOS mRNA (B) and protein (C), as well as plasma nitrate/nitrite concentration (D) relative to heroin-conditioned home cage controls and both saline-conditioned groups. E–G depicts data from heroin-conditioned female rats separated into low or increasing/high estradiol groups. Regardless of estrous distinction, CS exposure significantly suppressed LPS-induced splenic iNOS mRNA (E) and protein (F) as well as plasma nitrate/nitrite concentration (G) relative to home cage controls. *, statistically significant difference from heroin-conditioned control. Error bars indicate SEM.
A 2 × 2 ANOVA of splenic L13a mRNA levels revealed no main effect of drug treatment (F(1, 28) = .421, p = .522), CS exposure (F(1, 28) = .486, p = .492), or drug treatment × CS exposure interaction (F(1, 28) = 1.24, p = .275), validating L13a’s use as an appropriate reference gene. For splenic iNOS ΔΔCT values, a 2 × 2 ANOVA revealed a significant drug treatment × CS exposure interaction (F(1, 26) = 25.476, p < .001), as well as significant main effects of drug treatment (F(1, 26) = 14.989 , p = .001) and CS exposure (F(1, 26) = 31.441, p < .001) (Fig. 2B). Post hoc analysis revealed that heroin-conditioned, CS-exposed animals had significantly suppressed LPS-induced iNOS mRNA levels relative to heroin-conditioned home cage counterparts (p < .001), as well as to saline-conditioned home cage (p < .001) and CS-exposed (p < .001) controls.
Similar to splenic iNOS mRNA expression, prior CS exposure significantly blunted LPS-induced splenic iNOS protein concentration in heroin conditioned animals (Fig. 2C). A 2 × 2 ANOVA of splenic iNOS protein revealed a significant drug treatment × CS exposure interaction (F(1, 28) = 25.687, p < .001), as well as significant main effects of drug treatment (F(1, 28) = 14.509, p = .001) and CS exposure (F(1, 28) = 22.842, p < .001). Post hoc analysis revealed that heroin-conditioned, CS-exposed animals had significantly suppressed splenic iNOS protein concentrations relative to heroin-conditioned home cage counterparts (p < .001), as well as to saline-conditioned home cage (p < 0.001) and CS-exposed (p < 0.001) controls.
Consistent with splenic iNOS measures, CS exposure significantly suppressed LPS-induced plasma nitrate/nitrite concentration in heroin-conditioned animals (Fig. 2D). A 2 × 2 ANOVA of plasma nitrate/nitrite concentration revealed a significant drug treatment × CS exposure interaction (F(1, 27) = 13.925, p = .001), as well as significant main effects of drug treatment (F(1, 27) = 14.754, p = .001) and CS exposure (F(1, 27) = 16.413, p < .001). Post hoc analysis revealed that when CS exposure preceded the LPS challenge, heroin-conditioned animals had lower plasma nitrate/nitrite concentration relative to heroin-conditioned home cage counterparts (p < .001), saline-conditioned home cage controls (p < .001) and saline-conditioned, CS-exposed controls (p < .001).
Although naturally cycling females expressed heroin-conditioned suppression of NO in peripheral tissue and plasma, estrous phase was determined for each sample and categorized into diestrus, proestrus, estrus, or metestrus. Final analyses of splenic iNOS expression and plasma nitrate/nitrite concentration in the heroin conditioned groups (n = 7–8) when split across stage did not yield a large enough sample size to probe effects of estrous on CS-induced suppression of peripheral NO production. As in Experiment 1, samples in diestrus and proestrus were characterized as “increasing/high estradiol,” while those in estrus and metestrus were characterized as “low estradiol” (Miller and Takahashi, 2014; Santmyire et al., 2010). Indices of NO production in heroin-conditioned animals were analyzed using a 2 (estradiol phase, low vs high) × 2 (CS exposure, home cage vs CS) ANOVA. After combining, final group sizes for heroin-conditioned females per estradiol group was n = 2–6 (home cage controls) and n = 3–5 (CS exposed). Analysis of iNOS ΔΔCT values revealed a significant main effect of CS exposure (F(1, 11) = 51.195, p < .001), but no main effect of estradiol phase (F(1, 11) = .108, p = .748) or CS× estradiol interaction (F(1, 11) = .29, p = .601) (Fig. 2E). For iNOS protein, a 2 × 2 ANOVA revealed a main effect of CS (F(1, 12) = 32.899, p < .001), but no main effect of estradiol phase (F(1, 12) = .657, p = .433) or CS exposure × estradiol interaction (F(1, 12) = .484, p = .50) (Fig. 2F). Similarly, a 2 × 2 ANOVA of nitrate/nitrite concentration revealed a significant main effect of CS (F(1, 11) = 45.242, p < .001), but no main effect of estradiol phase (F(1, 11) = .442, p = .52) or CS × estradiol interaction (F(1, 11) = .323, p = .581) (Fig. 2G). Thus, estradiol phase did not significantly alter heroin-conditioned immunomodulation as CS exposure consistently blunted LPS-induced indices of NO production in heroin-conditioned animals.
4. Discussion
The present experiments establish that heroin directly modulates peripheral NO in female rats and that this effect can become conditioned to environmental stimuli previously paired with heroin administration. At two doses (1 and 3 mg/kg), heroin suppressed LPS-induced indices of peripheral NO production in both sexes, demonstrating for the first time that heroin-induced immunomodulation occurs in female rats to a comparable magnitude as in males. Further, both doses of heroin dampened multiple indices of LPS-induced NO production to the same degree in females and males, indicating that 1 mg/kg is sufficient to elicit heroin-induced immunomodulation in both sexes. Using the same context-heroin conditioning parameters we have reliably and reproducibly employed with males, the current study demonstrates that exposure to heroin-conditioned stimuli (CS) alone elicits heroin-conditioned suppression of LPS-induced peripheral NO production in naturally cycling female rats. Thus, when either heroin or heroin-paired cues precede pathogen exposure, typical induction of peripheral pro-inflammatory NO biomarkers is attenuated in both sexes. Collectively, our studies provide evidence that heroin elicits comparable immunomodulation in both sexes and suggests that the context-heroin association formed over the course of an opioid use disorder can extend health consequences past drug cessation for both men and women.
The current study focused on iNOS expression in the spleen and circulating plasma degradation products, two indicators of the immune response and peripheral NO production. The spleen is key in initiating both the innate and adaptive immune response, and monitors the blood for pathogens and filters out damaged and dead blood cells (Lewis et al., 2019). Although there was an observed main effect of sex in heroin-induced suppression of splenic iNOS mRNA, this appears to an artifact produced by the main effect of sex seen in the reference gene, L13a. This idea is supported by the fact that both splenic iNOS protein and circulating plasma NO degradation products are comparably suppressed in both sexes following heroin administration. Further, we observe a main effect of drug in both sexes, yet, in males, 1 mg/kg heroin did not significantly suppress splenic iNOS mRNA expression relative to saline controls. We have previously shown 1 mg/kg is sufficient to suppress LPS-induced iNOS mRNA expression [see (Lysle and How, 2000)], and in the current study induction of both splenic iNOS protein and plasma nitrate/nitrite concentration were significantly blunted relative to saline controls following 1 mg/kg heroin. Taken together, we are confident in our findings that both 1- and 3-mg/kg of heroin comparably suppress LPS-induced NO production in both sexes. Given that heroin and heroin-CS consistently blunted multiple measures of peripheral LPS-induced NO in spleen and blood plasma, we hypothesize that across sex, immune impairment would also be observed in other tissues involved in the immune response, such as the liver and lung. This possibility is supported by our previous work establishing that in male rats, heroin-induced and conditioned immunomodulation is wide-spread and is found in liver and lung tissue in addition to the spleen [see (Lanier et al., 2002; Lysle and How, 2000; Lysle and Ijames, 2002; Szczytkowski and Lysle, 2008)].
The current investigations focused on NO production in females due to its reliable and meaningful suppression by heroin and heroin-CS in males. Previously, we have shown that in males the immunomodulatory effects of heroin and heroin-paired CS extend beyond suppression of NO to include suppression of peripheral pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and TNFα, as well as natural killer cell activity (Saurer et al., 2009; Szczytkowski et al., 2011; Szczytkowski and Lysle, 2008). Although the present results demonstrate comparable heroin-induced and -conditioned suppression of NO production in males and females, other pro-inflammatory immune agents may be differentially regulated based on sex. Females tend to have a more robust immune response than males that is in part mediated through direct action of female sex hormones on the immune system (Khan and Ansar Ahmed, 2016). For example, estradiol has a complex relationship with the immune response and has been shown to exert biphasic effects on peripheral pro-inflammatory cytokine production, either enhancing or attenuating expression based on estrogen receptor activity. Interestingly, LPS and TLR-signaling has been shown to downregulate anti-inflammatory estrogen receptor action, ultimately increasing expression of peripheral pro-inflammatory agents (Kovats, 2015). While we did not see any evidence of enhanced NO production in females to LPS nor an effect of estrous cycle on the actions of heroin or heroin-CS on NO, other immune measures may be modified by female sex hormones. The current study was optimized to maximally detect heroin-related changes in LPS-induced NO production and is a direct comparison to our previous work using this LPS dose and time course to examine heroin-induced and -conditioned NO suppression in male rats [e.g. (Lebonville et al., 2020; Lysle and How, 2000; Lysle and Ijames, 2002; Paniccia et al., 2018)]. Our results are the first to demonstrate comparable heroin-induced suppression of NO across multiple measures in both sexes and provide the vital first steps to understanding how heroin and heroin-CS alter the immune response in female rats. To examine whether heroin-induced and -conditioned NO effects extend to other pro-inflammatory agents or differ based on LPS amount and/or frequency, future experiments can alter the time course based on the immune marker in question and test larger or repeated doses of LPS to explore the interaction of heroin, LPS, sex hormones, and cytokine production in female rats.
The current studies employed naturally cycling female rats and examined estrous stage at the point of tissue collection on test day. There appeared to be no effect of estrous cycle, as all female samples demonstrate both heroin-induced and -conditioned dampening of the peripheral immune response through suppression of peripheral NO production. When samples were separated across estrous stage, there was insufficient sample size to adequately assess if magnitude of suppression varied across all four phases of the cycle. As such, stages were combined and samples characterized as either increasing/higher estradiol (diestrus and proestrus) or low estradiol (estrus and metestrus) stages. We observed that females in both the high and low estradiol phases exhibit both heroin-induced and -conditioned suppression splenic iNOS expression and plasma nitrate/nitrite concentration. Although there are documented sex differences in the response to endotoxin challenge (Engler et al., 2016), the current set of experiments demonstrate that heroin produces comparable immunomodulation across sex and heroin-CS elicits robust suppression of LPS-induced NO production. We have optimized our methodology to detect heroin-related changes in NO production, yet, there is the possibility that some of our measures (iNOS protein, nitrate/nitrate concentration) are not sensitive enough to capture small differences in the magnitude of NO suppression between the sexes. Additionally, estrous stages were grouped based on characteristic levels of estradiol across cycle and did not account for potential effects of other female sex hormones on NO production, such as progesterone. Given these possibilities, future experiments can examine whether female sex hormones, including estradiol and progesterone, directly modulate the mechanisms regulating the magnitude of heroin-induced and -conditioned suppression of NO in female rats.
Results from the current study indicate that there is comparable heroin-induced suppression of NO production to endotoxin exposure across sex. This raises the possibility that similar neurobiological mechanisms regulate this response in both males and females. Previously, we established that heroin’s impact on the peripheral immune response in males is mediated by central μ-opioid receptor action [μ-OR; (Nelson et al., 2000)]. As both sexes exhibit heroin-induced immunomodulation, it is possible μ-OR signaling is also involved in the suppression of LPS-induced NO production in females. Interestingly, our laboratory has shown μ-OR signaling exacerbates contact hypersensitivity, an antigen-specific type of cutaneous inflammation, in both sexes, with the magnitude of this response being significantly greater in female rats (Elliott et al., 2003). Moreover, the increased potentiation of contact hypersensitivity in females was contingent on the presence of gonadal sex hormones. As we did not observe sex-based differences in heroin-induced immunomodulation, data from our laboratory indicates that opioid-immune responses are not uniform and differ based on the response in question and biological sex, although they may be mediated through similar central mechanisms.
The current study is the first to demonstrate context-heroin conditioned suppression of typical peripheral NO induction following LPS in female rats. We have extensively characterized the neural mechanisms governing heroin-conditioned immunomodulation in male rats and found that it is a learned Pavlovian response mediated through hippocampal processes (Szczytkowski et al., 2013; Szczytkowski and Lysle, 2007). Using males, we found that dorsal hippocampal (DH) signaling is critical for the expression of heroin-conditioned immunomodulation to occur (Szczytkowski et al., 2013), and that DH neural immune mechanisms play a causal role in the conditioned immune effect (Paniccia et al., 2018). Specifically, astrocytes are a critical component during CS exposure for context-heroin conditioned suppression of peripheral NO production to occur (Paniccia et al., 2018). DH astrocytes express estrogen receptors, opening the possibility that female sex hormones directly modulate astrocyte activity. In fact, estradiol has been shown to mediate astrocyte excitability through Ca2+-induced intracellular signaling (Chaban et al., 2004) and influence LPS-induced inflammation in glia (Loram et al., 2012). While no sex differences were found in the current study, this could have implications for how heroin-conditioned immunomodulation is regulated in females. Future experiments can extend this line of work to investigate whether female sex hormones modulate astrocyte activity and therefore encoding or recall of the context-heroin association responsible for conditioned immunomodulation.
In addition to the hippocampus, there is considerable overlap in the neural substrates governing heroin-conditioned immunomodulation with those involved in other heroin-related responses, such as conditioned motivation to take drug (Crombag et al., 2008; Szczytkowski et al., 2011). Specifically, dopamine D1 receptor stimulation within the nucleus accumbens shell mediates both context-heroin conditioned immunomodulation (Saurer et al., 2008; Saurer et al., 2009) and heroin seeking behavior (Bossert et al., 2007). The current results demonstrating that heroin-induced and -conditioned immunomodulation is present in both sexes are in line with literature suggesting males and females respond similarly to heroin and heroin-paired stimuli (Lynch et al., 2002; Venniro et al., 2017). Given this, we hypothesize that across sex common neurobiological mechanisms govern heroin-induced and -conditioned immunomodulatory and motivated responses, albeit there may be crosstalk with steroid sex hormones. For instance, dopamine can interact with the female hormone system, mimicking the effects of progesterone and stimulating estrogen receptors (Olesen and Auger, 2008; Power et al., 1991). This raises questions about whether there is dopamine-hormone interplay regulating heroin-related responses in females, despite the behavioral output to heroin and heroin-CS appearing comparable across sex. Moreover, occasional interaction between female sex hormones and neurobiological factors may account for some of the existing discrepancies in the literature regarding sex differences in heroin-related responses, such as drug taking behavior and reactivity to heroin-paired cues. Although we did not observe sex differences in heroin-induced and conditioned immunomodulation, there is ample evidence that female sex hormones interact with and may influence the established central mechanisms regulating these immune effects (i.e. dopamine activity, astrocyte reactivity, μ-OR signaling).
In conclusion, the present study is the first to establish that exposure to heroin or heroin-CS alter the immune response to a LPS challenge in female rats. When heroin or exposure to a heroin-paired context precedes administration of this endotoxin, peripheral NO production, as indicated by splenic iNOS expression and plasma nitrate/nitrite concentration, is suppressed in females as it is in males. Thus, we have demonstrated that exposure to heroin and heroin-CS interferes with normal immune processes and has the potential to alter resistance to infection both during and long after drug use, regardless of sex. These experiments are a vital first step to fully understanding the negative health consequences associated with heroin use disorders. Future lines of research can extend these findings to identify novel treatment strategies that can be effective in both sexes to ameliorate the negative impact of heroin and heroin-conditioned stimuli on the immune response.
Highlights:
Heroin attenuates typical induction of the peripheral immune response in male rats
Heroin similarly suppresses endotoxin-induced peripheral nitric oxide in females
Females express context-heroin conditioned suppression of nitric oxide production
Funding and Disclosure:
This research was supported by National Institute on Drug Abuse (NIDA) grants R01 DA034721 (DTL), T32 DA007244 (JEP, CLL), and F31 DA047054 (JEP), a National Science Foundation grant DGE-1144081 (CLL), a Baughman Dissertation Award (JEP), a King Research Excellence Award (JEP), Dashiell Dissertation Award (JEP), a Lindquist Undergraduate Research Award (TNW), and a David Bray Peele Memorial Research Award (TNW). The authors declare no potential conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrosio E, Goldberg SR, Elmer GI, 1995. Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol 6, 229–237. [PubMed] [Google Scholar]
- Bogdan C, 2001. Nitric oxide and the immune response. Nat Immunol 2, 907–916. [DOI] [PubMed] [Google Scholar]
- Bogdan C, Röllinghoff M, Diefenbach A, 2000. The role of nitric oxide in innate immunity. Immunological reviews 173, 17–26. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Adhikary S, St Laurent R, Marchant NJ, Wang H-L, Morales M, Shaham Y, 2016. Role of projections from ventral subiculum to nucleus accumbens shell in contextinduced reinstatement of heroin seeking in rats. Psychopharmacology 233, 1991–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y, 2007. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 12655–12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach K, Klocke S, Tschernig T, van Rooijen N, Baumann U, Steinmetz I, 2006. Role of inducible nitric oxide synthase and NADPH oxidase in early control of Burkholderia pseudomallei infection in mice. Infect Immun 74, 6300–6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban VV, Lakhter AJ, Micevych P, 2004. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology 145, 3788–3795. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP, 1993. Cue reactivity and cue reactivity interventions in drug dependence. NIDA research monograph 137, 73–95. [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP, 1986. Role of conditioning factors in the development of drug dependence. The Psychiatric clinics of North America 9, 413–425. [PubMed] [Google Scholar]
- Cora MC, Kooistra L, Travlos G, 2015. Vaginal Cytology of the Laboratory Rat and Mouse:Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicologic Pathology 43, 776–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y, 2008. Context-induced relapse to drug seeking: a review. Philosophical Transactions of the Royal Society B: Biological Sciences 363, 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle HH, Murphy AZ, 2017. Sex differences in innate immunity and its impact on opioid pharmacology. J Neurosci Res 95, 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle HH, Murphy AZ, 2018. Sex-dependent influences of morphine and its metabolites on pain sensitivity in the rat. Physiol Behav 187, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein TK, 2019. The Role of Opioid Receptors in Immune System Function. Frontiers in Immunology 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JC, Picker MJ, Nelson CJ, Carrigan KA, Lysle DT, 2003. Sex differences in opioid-induced enhancement of contact hypersensitivity. The Journal of investigative dermatology 121, 1053–1059. [DOI] [PubMed] [Google Scholar]
- Engler H, Benson S, Wegner A, Spreitzer I, Schedlowski M, Elsenbruch S, 2016. Men and women differ in inflammatory and neuroendocrine responses to endotoxin but not in the severity of sickness symptoms. Brain, behavior, and immunity 52, 18–26. [DOI] [PubMed] [Google Scholar]
- Fecho K, Lysle DT, 2000. Heroin-induced alterations in leukocyte numbers and apoptosis in the rat spleen. Cellular immunology 202, 113–123. [DOI] [PubMed] [Google Scholar]
- Fecho K, Maslonek KA, Dykstra LA, Lysle DT, 1996. Assessment of the involvement of central nervous system and peripheral opioid receptors in the immunomodulatory effects of acute morphine treatment in rats. J Pharmacol Exp Ther 276, 626–636. [PubMed] [Google Scholar]
- Govitrapong P, Suttitum T, Kotchabhakdi N, Uneklabh T, 1998. Alterations of immune functions in heroin addicts and heroin withdrawal subjects. Journal of Pharmacology and Experimental Therapeutics 286, 883–889. [PubMed] [Google Scholar]
- Guitart X, Beitnerjohnson D, Marby DW, Kosten TA, Nestler EJ, 1992. Fischer and Lewis Rat Strains Differ in Basal Levels of Neurofilament Proteins and Their Regulation by Chronic Morphine in the Mesolimbic Dopamine System. Synapse 12, 242–253. [DOI] [PubMed] [Google Scholar]
- Guitart X, Kogan JH, Berhow M, Terwilliger RZ, Aghajanian GK, Nestler EJ, 1993. Lewis and Fischer Rat Strains Display Differences in Biochemical, Electrophysiological and Behavioral Parameters - Studies in the Nucleus-Accumbens and Locus-Ceruleus of Drug Naive and Morphine-Treated Animals. Brain Res 611, 7–17. [DOI] [PubMed] [Google Scholar]
- Hoffmann O, Zweigner J, Smith SH, Freyer D, Mahrhofer C, Dagand E, Tuomanen EI, Weber JR, 2006. Interplay of pneumococcal hydrogen peroxide and host-derived nitric oxide. Infect Immun 74, 5058–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson LW, Szczytkowski JL, Saurer TB, Lebonville C, Fuchs RA, Lysle DT, 2014. Region-specific contribution of the ventral tegmental area to heroin-induced conditioned immunomodulation. Brain Behav Immun 38, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen J, Wand H, Gonnermann A, Maher L, 2010. Gender differences in hepatitis C antibody prevalence and risk behaviours amongst people who inject drugs in Australia 19982008. The International journal on drug policy 21, 471–476. [DOI] [PubMed] [Google Scholar]
- Jamison RN, Butler SF, Budman SH, Edwards RR, Wasan AD, 2010. Gender differences in risk factors for aberrant prescription opioid use. The journal of pain : official journal of the American Pain Society 11, 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Logan J, Gladden RM, Bohm MK, 2015. Vital Signs: Demographic and Substance Use Trends Among Heroin Users - United States, 2002–2013. MMWR Morb Mortal Wkly Rep 64, 719–725. [PMC free article] [PubMed] [Google Scholar]
- Kennedy AP, Epstein DH, Phillips KA, Preston KL, 2013. Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug and alcohol dependence 132, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan D, Ansar Ahmed S, 2016. The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. Frontiers in Immunology 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, 2004. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite immunology 26, 247–264. [DOI] [PubMed] [Google Scholar]
- Koons AL, Rayl Greenberg M, Cannon RD, Beauchamp GA, 2018. Women and the Experience of Pain and Opioid Use Disorder: A Literature-based Commentary. Clinical therapeutics 40, 190–196. [DOI] [PubMed] [Google Scholar]
- Kovats S, 2015. Estrogen receptors regulate innate immune cells and signaling pathways. Cellular Immunology 294, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier RK, Ijames SG, Carrigan KA, Carelli RM, Lysle DT, 2002. Self-administration of heroin produces alterations in the expression of inducible nitric oxide synthase. Drug Alcohol Depen 66, 225–233. [DOI] [PubMed] [Google Scholar]
- Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M, 2012. Initiation into prescription opioid misuse amongst young injection drug users. The International journal on drug policy 23, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebonville CL, Jones ME, Hutson LW, Cooper LB, Fuchs RA, Lysle DT, 2016. Acquisition of heroin conditioned immunosuppression requires IL-1 signaling in the dorsal hippocampus. Brain Behav Immun 56, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebonville CL, Paniccia JE, Parekh SV, Wangler LM, Jones ME, Fuchs RA, Lysle DT, 2020. Expression of a heroin contextually conditioned immune effect in male rats requires CaMKIIα-expressing neurons in dorsal, but not ventral, subiculum and hippocampal CA1. Brain, Behavior, and Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SM, Williams A, Eisenbarth SC, 2019. Structure and function of the immune system in the spleen. Science Immunology 4, eaau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Adcock IM, Old RW, Barnes PJ, Evans TW, 1993. Lipopolysaccharide treatment in vivo induces widespread tissue expression of inducible nitric oxide synthase mRNA. Biochemical and biophysical research communications 196, 1208–1213. [DOI] [PubMed] [Google Scholar]
- Liu SF, Barnes PJ, Evans TW, 1997. Time course and cellular localization of lipopolysaccharide-induced inducible nitric oxide synthase messenger RNA expression in the rat in vivo. Critical care medicine 25, 512–518. [DOI] [PubMed] [Google Scholar]
- Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HE, Maier SF, Watkins LR, 2012. Sex and estradiol influence glial proinflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology 37, 1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME, 1999. Sex differences in the acquisition of intravenously selfadministered cocaine and heroin in rats. Psychopharmacology (Berl) 144, 77–82. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME, 2002. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 164, 121–137. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Hoffman KE, Dykstra LA, 1996. Evidence for the involvement of the caudal region of the periaqueductal gray in a subset of morphine-induced alterations of immune status. J Pharmacol Exp Ther 277, 1533–1540. [PubMed] [Google Scholar]
- Lysle DT, How T, 1999. Endogenous opioids regulate the expression of inducible nitric oxide synthase by splenocytes. The Journal of pharmacology and experimental therapeutics 288, 502–508. [PubMed] [Google Scholar]
- Lysle DT, How T, 2000. Heroin modulates the expression of inducible nitric oxide synthase. Immunopharmacology 46, 181–192. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Ijames SG, 2002. Heroin-associated environmental stimuli modulate the expression of inducible nitric oxide synthase in the rat. Psychopharmacology (Berl) 164, 416–422. [DOI] [PubMed] [Google Scholar]
- Mark TL, Woody GE, Juday T, Kleber HD, 2001. The economic costs of heroin addiction in the United States. Drug Alcohol Depen 61, 195–206. [DOI] [PubMed] [Google Scholar]
- McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ, 2001. Opioids, opioid receptors, and the immune response. Drug Alcohol Depen 62, 111–123. [DOI] [PubMed] [Google Scholar]
- Meijerink H, Indrati A, Utami F, Soedarmo S, Alisjahbana B, Netea MG, van Crevel R, Wisaksana R, van der Ven AJ, 2015. Heroin use is associated with suppressed proinflammatory cytokine response after LPS exposure in HIV-infected individuals. PLoS One 10, e0122822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Takahashi JS, 2014. Central circadian control of female reproductive function. Front Endocrinol (Lausanne) 4, 195–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiewicz B, Zelazowska E, Raybourne RB, Cizza G, Sternberg EM, 1996. Inflammatory responses to carrageenan injection in LEW/N and F344/N rats: LEW/N rats show sex- and age-dependent changes in inflammatory reactions. Neuroimmunomodulation 3, 93–101. [DOI] [PubMed] [Google Scholar]
- Nair MP, Laing TJ, Schwartz SA, 1986. Decreased natural and antibody-dependent cellular cytotoxic activities in intravenous drug abusers. Clinical immunology and immunopathology 38, 68–78. [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Schneider GM, Lysle DT, 2000. Involvement of central mu- but not delta- or kappa-opioid receptors in immunomodulation. Brain Behav Immun 14, 170–184. [DOI] [PubMed] [Google Scholar]
- Oates JC, Gilkeson GS, 2006. The biology of nitric oxide and other reactive intermediates in systemic lupus erythematosus. Clinical Immunology 121, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen KM, Auger AP, 2008. Dopaminergic activation of estrogen receptors induces fos expression within restricted regions of the neonatal female rat brain. PloS one 3, e2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniccia JE, Lebonville CL, Jones ME, Parekh SV, Fuchs RA, Lysle DT, 2018. Dorsal hippocampal neural immune signaling regulates heroin-conditioned immunomodulation but not heroin-conditioned place preference. Brain, Behavior, and Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power R, Mani S, Codina J, Conneely O, O’Malley B, 1991. Dopaminergic and ligandindependent activation of steroid hormone receptors. Science 254, 1636–1639. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Manning PT, Zweifel BS, Seibert K, Connor J, Currie MG, Needleman P, Masferrer JL, 1995. Dual inhibition of nitric oxide and prostaglandin production contributes to the antiinflammatory properties of nitric oxide synthase inhibitors. The Journal of clinical investigation 96, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santmyire BR, Venkat V, Beinder E, Baylis C, 2010. Impact of the estrus cycle and reduction in estrogen levels with aromatase inhibition, on renal function and nitric oxide activity in female rats. Steroids 75, 1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer TB, Ijames SG, Carrigan KA, Lysle DT, 2008. Neuroimmune mechanisms of opioid-mediated conditioned immunomodulation. Brain, behavior, and immunity 22, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer TB, Ijames SG, Lysle DT, 2009. Evidence for the Nucleus Accumbens as a Neural Substrate of Heroin-Induced Immune Alterations. Journal of Pharmacology and Experimental Therapeutics 329, 1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ, 2008. Analyzing real-time PCR data by the comparative CT method. Nature Protocols 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD, 2012. Sex, glia, and development: interactions in health and disease. Hormones and behavior 62, 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, 2002. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacology, biochemistry, and behavior 71, 517–529. [DOI] [PubMed] [Google Scholar]
- Shah M, Anwar MA, Yesudhas D, Krishnan J, Choi S, 2016. A structural insight into the negative effects of opioids in analgesia by modulating the TLR4 signaling: An in silico approach. Scientific Reports 6, 39271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton EA, Shipton EE, Shipton AJ, 2018. A Review of the Opioid Epidemic: What Do We Do About It? Pain Ther 7, 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA, 1988. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. The Journal of pharmacology and experimental therapeutics 245, 164–170. [PubMed] [Google Scholar]
- Szczytkowski JL, Fuchs RA, Lysle DT, 2011. Ventral tegmental area-basolateral amygdala-nucleus accumbens shell neurocircuitry controls the expression of heroin-conditioned immunomodulation. J Neuroimmunol 237, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczytkowski JL, Lebonville C, Hutson L, Fuchs RA, Lysle DT, 2013. Heroin-induced conditioned immunomodulation requires expression of IL-1beta in the dorsal hippocampus. Brain Behav Immun 30, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczytkowski JL, Lysle DT, 2007. Conditioned effects of heroin on the expression of inducible nitric oxide synthase in the rat are susceptible to extinction and latent inhibition. Psychopharmacology 191, 879–889. [DOI] [PubMed] [Google Scholar]
- Szczytkowski JL, Lysle DT, 2008. Conditioned effects of heroin on proinflammatory mediators require the basolateral amygdala. Eur J Neurosci 28, 1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd CS, Abed AMS, Strathdee SA, Scott PT, Botros BA, Safi N, Earhart KC, 2007. HIV, hepatitis C, and hepatitis B infections and associated risk behavior in injection drug users, Kabul, Afghanistan. Emerg Infect Dis 13, 1327–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey WR, Tse J, Carter G, 1995. Lipopolysaccharide-induced changes in plasma nitrite and nitrate concentrations in rats and mice: pharmacological evaluation of nitric oxide synthase inhibitors. J Pharmacol Exp Ther 272, 1011–1015. [PubMed] [Google Scholar]
- Venniro M, Zhang M, Shaham Y, Caprioli D, 2017. Incubation of Methamphetamine but not Heroin Craving After Voluntary Abstinence in Male and Female Rats. Neuropsychopharmacology 42, 1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang S, Epstein DH, Fang Y, Shi J, Qin H, Yao S, Le Foll B, Lu L, 2007. Gender and stimulus difference in cue-induced responses in abstinent heroin users. Pharmacology Biochemistry and Behavior 86, 485–492. [DOI] [PubMed] [Google Scholar]


