Abstract
Background and Aims
Altered microbiota can affect gut-liver-brain axis in cirrhosis and hepatic encephalopathy (HE), but the impact of sex on these changes is unclear. We aimed to determine differences in fecal microbiota composition/functionality in men compared to women with cirrhosis and HE on differing treatments.
Methods
Men and women with cirrhosis and controls underwent stool microbiome composition (16srRNA sequencing) and microbial functional analysis between groups cross-sectionally. HE on rifaximin+lactulose(HE-Rif), HE on lactulose only(HE-Lac) and cirrhosis without HE(No-HE) were compared to controls within men and then within women using random forest classifier. Men and women were also compared.
Results
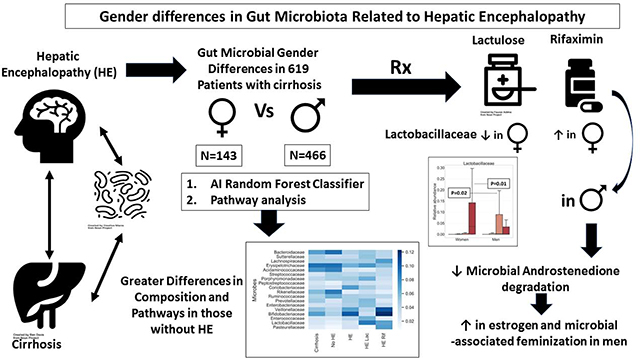
761 subjects, 619 cirrhosis (466 men,153 women) and 142 controls (92 men, 50 women) were included. Men were older and used more PPI but MELD score, No-HE (n=319), HE-lac (n=130) and HE-Rif (n=170) proportion were similar. PPI/age-adjusted AUC of differentiation between controls versus cirrhosis, and controls versus No-HE were higher within women than men but was reversed No-HE versus HE-Lac. Control versus HE-Rif differentiation was similar across sexs. Men versus women were different in all cirrhosis, No-HE and HE-Lac but not HE-Rif on PERMANOVA and AUC analyses. Autochthonous taxa decreased and pathobionts increased with disease progression regardless of sex. In men, Lactobacillaceae were higher in HE-Lac but decreased in HE-Rif, with Veillonellaceae. Pathways related to glutamate and aromatic compound degradation were higher in men at all stages. Degradation of androstenedione, an estrogenic precursor, was lower in men versus women in HE-Rif, likely enhancing feminization.
Conclusions
In this cross-sectional study, there is a sex difference with HE therapies focused on Lactobacillaceae and Veillonellaceae and androstenedione degradation. Further studies linking these to sex-specific outcomes are needed.
Keywords: Cirrhosis, Artificial Intelligence, Rifaximin, Lactulose, Lactobacillus, Androgen, Random forest classifier
Lay Summary
Patients with liver cirrhosis develop changes in their brain function, and men often develop feminization with disease progression. However, the interaction between sex, microbiota and disease severity is unclear. We found that as disease progressed, microbiota in men that were different from women approached their composition with changes in specific microbes that are associated with male hormone metabolism.
Graphical Abstract
INTRODUCTION
Cirrhosis is associated with changes in the gut-brain axis that leads to hepatic encephalopathy (HE)[1]. HE affects clinical, psycho-social and functional outcomes and is a major burden on the patient, their family and the healthcare system[2]. Altered gut microbiota plays a role in the diagnosis and prognosis of cirrhosis and HE and most therapeutic regimens are focused on modulating these microbial changes[3, 4]. The diagnostic cut-offs for cognitive dysfunction, a hallmark of HE, needed to be adjusted by sex to create norms for testing in multi-center US studies [5, 6]. However, potential differences in the gut-brain axis and responses to HE therapies between sexs has not been investigated in cirrhosis. This is relevant because differences in microbiota between sexs have been found across the lifespan, which can also impact the gut-brain axis and influence immune responses[7, 8]. In addition, several liver disease etiologies and processes related to bile acid and cholesterol metabolism have sex-specific differences[9–11]. However, their impact on cirrhosis and subsequent impact of HE and related therapies are unclear. Therefore, determining changes in microbial composition between sexs may be important in potentially individualizing therapy.
Our aim was to determine differences in fecal microbiota composition and functionality in men compared to women with cirrhosis and HE on differing therapeutic regimens in a cross-sectional analysis. Our hypothesis was that sex is associated with gut microbiota composition and function changes with HE.
PATIENTS AND METHODS
In this cross-sectional study, we included outpatients with cirrhosis who were recruited prospectively from Virginia Commonwealth University and McGuire VA Medical Centers between 2014–2019 after written informed consent and IRB approval. Cirrhosis was diagnosed based on either liver biopsy, evidence of varices or portal hypertension in chronic liver disease patients or those with frank decompensation. Diagnosis of HE was made based on history of confusion and hospitalizations attributable to HE which required therapy with lactulose and/or rifaximin. Only patients with mini-mental status>25 could participate to ensure that there was no active HE. We excluded patients with recent (<6 weeks) infection or antibiotic use apart from rifaximin, use of probiotics within the last 6 weeks, active alcohol use disorder (AUDIT-10>8), or use of illicit drugs, or those who could not provide informed consent or provide samples. We also excluded patients with primary biliary cholangitis, autoimmune hepatitis and primary sclerosing cholangitis. Healthy controls that were age-balanced were included by word of mouth or by community screening and were required to be free of chronic diseases and not be on any prescription medications. All controls also provided samples after written informed consent. None of the subjects included were on hormone replacement therapy, antipsychotic medications, or were undergoing or had undergone sex reassignment procedures or medications. All subjects also underwent a 3-day dietary recall at the time of sample collection and average caloric intake and type of dietary intake was analyzed.
Statistical analyses
Demographics were compared between men and women between and within control and patient groups. Patients with cirrhosis were divided a priori into those (i) without HE (no HE) (ii) with HE only on lactulose (HE-Lac) and (iii) with HE on lactulose + rifaximin (HE-Rif). This categorization of HE patients was performed because HE-Rif are likely to have a worse disease severity that HE-Lac in the US[1, 12]. Comparison of cirrhosis severity and other medications was performed between sexs with cirrhosis and their subgroups.
Microbiota analysis
Microbial DNA was isolated from stool samples as previously described [13] after home collection using ParaPak kits in RNALater to bring within 8 hours of collection to ensure stability. Samples were kept at −80°C until the time of analysis. Bacterial ribosomal RNA Gene Sequencing: The V1 and V2 variable regions of the bacterial 16S ribosomal RNA (rRNA) gene were sequenced using Multitag fusion primers and sequenced on an Ion Torrent PGM next-generation sequencer[14]. We utilized the Quantitative Insights into Microbial Ecology (QIIME2) package for UNIFRAC analysis and diversity analysis. The relative abundance table was then used to compare subgroups (Supplementary data).
Functional prediction analysis
For functional prediction and pathway inferences we used Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2)[15] (details in Supplement). PICRUSt generates a pathway enrichment table by estimating the contribution of gene families for each microbiome sample and annotating those genes with several internal pre-calculated databases such as MetaCyc[16]. To evaluate the differential enrichment pattern of each pathway between two groups we used Metastats[17]. Metastats performs Fisher’s exact test and calculates p-value. Comparisons were performed using Metastats to rank these pathways and log10p-values and log2 fold changes of the comparisons and expression of pathways were studied between sexs. These comparisons were performed for cirrhosis, No-HE, HE-Lac and HE-Rif after adjustment for age and PPI use[18]. PERMANOVA comparisons for PICRUSt were performed for Jaccard and Bray-Curtis distances between men and women.
AI Methods to compare disease-associated microbes between sexs
AI methods allow for identifying disease-associated microbes by considering the effect of a given microbe in the presence of all other microbes, providing an advantage over univariate statistical analysis. We used a random forest classifier (RFC) AI method to discriminate between disease groups based on their microbiota composition and evaluated the importance of each microbe in the classifier’s decision. Microbes with a higher feature importance in the classifier’s decision are more important for differentiating between the groups than the microbes with lower feature importance, resulting in a small set of microbes for which post-hoc statistical analysis can be done. We classified the overall groups (control vs cirrhosis, control vs No-HE, control vs HE-Lac and control vs HE-Rif) first and then divided these classifications into those with men alone and women alone. To reduce dimensionality, we removed the same features (microbes) for all the sub-populations and removed the microbes if they were present in less than 10% of men and 10% of women, i.e., only the microbes present in at least 10% of men or 10% of women were considered for further analysis. Reducing dimensionality in such a fashion provides the following advantages: (i) it allows for the analysis of microbes that are sufficiently well represented in at least one sex, and (ii) it provides a better statistical power to the study by a priori elimination of microbes that are unlikely to be different between the sexs. RFC with 21 trees and a maximum depth of 4 was created. Monte Carlo cross-validation for 30 iterations was performed, where each iteration included an 80–20 split of the data. Area under the curve (AUC) of receiver operating characteristics for classifications were recorded to evaluate the classifier’s performance. Comparisons of the classification performance between disease groups by sex were performed using Welch’s t-test of the AUC and standard deviation (SD). AUC comparisons were FDR corrected. The most important microbes were identified by averaging the feature importance of a microbe across iterations of cross-validation and selecting the top microbes which had a cumulative importance of 0.5 (out of 1). The first analyses were performed within sexs classifying subgroups (i.e. controls vs cirrhosis in men and same comparisons in women) and comparing the AUCs of these classifications.
The second analysis directly classified men vs women within individual subgroups based on their microbiota composition using the RFC and the classifier performance was evaluated with AUC. To evaluate if the composition between sexs within a disease group were distinguishable better than chance level (AUC=0.5), the AUCs were compared using 1 sample t-test with mean 0.5. The p-values were FDR corrected. Average feature importance of each microbe was also computed for the men vs women classifiers within individual subgroups. Post-hoc Wilcoxon rank-sum tests were used to compare median relative abundances between men and women for microbial families that appeared different in the AI based classification-and-feature-importance analysis. Analysis of potential confounders on specific microbiota driving these differences were also performed using classification performance of models after adding or removing a potential confounder. AUC changes and independence of the prior variables in the model along with confounder were analyzed.
RESULTS
Demographics
We enrolled 619 outpatients with cirrhosis (466 men and 153 women) and 142 healthy controls (92 men and 50 women) between October 2014 to October 2019. There were no significant differences in ages between men or women with and without cirrhosis. Within healthy controls, the mean±SD age for women was statistically similar to men (59.0±12.8 women vs 60.9±11.6 men, p=0.37). Similar number of healthy control men and women were on proton pump inhibitors (PPI, 8 each, p=0.62). Within patients with cirrhosis, men were older than women, but remaining cirrhosis-related variables were statistically similar (Table 1). A higher proportion of patients with cirrhosis were on PPI compared to controls (p<0.0001) and more men than women were on PPI (Table 1). There were no significant differences in daily caloric intake (Table 1) and all patients were following a Western non-vegetarian diet.
Table 1:
Demographic and Cirrhosis-related comparisons between men and women
| All Patients with Cirrhosis (n=619) | |||
| Men (n=466) | Women (n=153) | P value | |
| Age (years) | 58.2±7.5 | 56.4±6.7 | 0.006 |
| Diabetes | 153 (33%) | 54 (35%) | 0.57 |
| MELD score | 13.6±6.6 | 13.1 ±9.4 | 0.54 |
| Hepatitis C/Alcohol/Others | 161/116/189 | 4¾3/66 | 0.21 |
| Hepatic encephalopathy | 227 (49%) | 73 (48%) | 0.84 |
| Lactulose use only | 94 (20%) | 36 (24%) | 0.37 |
| Rifaximin use also | 133 (29%) | 37 (24%) | 0.32 |
| Proton Pump Inhibitor use | 247 (53%) | 65 (42%) | 0.02 |
| Daily caloric intake | 2310±367 | 2291±215 | 0.44 |
| Only No-HE (n=319) | |||
| Men (n=239) | Women (n=80) | P value | |
| Age (years) | 60.1±7.3 | 57.0±6.8 | 0.001 |
| Diabetes | 72 (30%) | 28 (35%) | 0.08 |
| MELD score | 10.7±5.3 | 11.6±7.1 | 0.28 |
| Hepatitis C/Alcohol/Others | 99/59/81 | 23/25/32 | 0.13 |
| Proton Pump Inhibitor use | 94 (39%) | 31 (39%) | 0.93 |
| Daily caloric intake | 2434±393 | 2381±572 | 0.32 |
| HE-Lac (n=130) | |||
| Men (n=94) | Women (n=36) | P value | |
| Age (years) | 55.2±6.9 | 56.4±7.1 | 0.36 |
| Diabetes | 54 (57%) | 14 (39%) | 0.06 |
| MELD score | 16.4±5.8 | 14.5±5.2 | 0.08 |
| Hepatitis C/Alcohol/Others | 28/24/42 | 9/10/16 | 0.89 |
| Proton Pump Inhibitor use | 55 (59%) | 14 (39%) | 0.04 |
| Daily caloric intake | 2201±529 | 2094±739 | 0.43 |
| HE-Rif (n=170) | |||
| Men (n=133) | Women (n=37) | ||
| Age (years) | 58.9±5.6 | 59.4±7.9 | 0.69 |
| Diabetes | 65 (49%) | 12 (32%) | 0.08 |
| MELD score | 17.1 ±7.1 | 15.4±7.8 | 0.21 |
| Hepatitis C/Alcohol/Others | 34/33/66 | 11/8/18 | 0.85 |
| Proton Pump Inhibitor use | 98 (74%) | 20 (54%) | 0.02 |
| Daily caloric intake | 2238±782 | 2104±629 | 0.31 |
Ability of microbiota to differentiate between subject groups differed between men and women
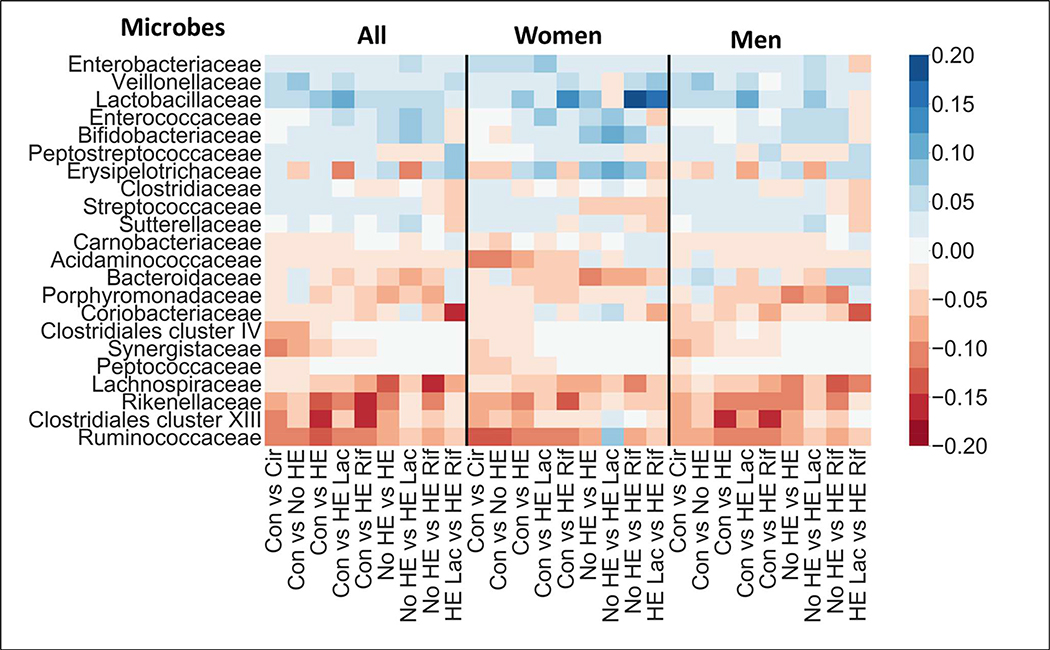
Microbe data is high-dimensional which can pose challenges for classification algorithms by affecting classification performance and in estimation of feature importance[19–21]. To reduce the dimensionality of the data (reduce the number of microbes used in the analysis), stool microbes present in less than 10% of the men and 10% of the women were removed. This criterion also allowed in focusing the analysis on microbes that were well-represented in at least one sex and are likely to play a large role to play due to the compositional nature of the data. Of the 149 families, this resulted in 29 microbes being used for classification (Figure 1). Difference in representation between sex, i.e., the difference in percentages of men and women for whom the microbe relative abundance was non-zero, was <5% for all 120 excluded families. This resulted in similar representation in both sexs for these excluded families and it is unlikely that their representation influences sex differences in HE between men and women (Supplement and Figure S1).
Figure 1: Composition Changes Between Healthy and Diseased Groups in Cirrhosis within sex.
These comparisons are within sex and a heatmap of bacterial families that were high in importance in comparison between all groups, within men and within women. Warmer colors indicate negative relationship while cooler colors indicate positive linkages in the more advanced category of comparison. Blank or white areas indicate that there is no linkage. HE: Hepatic encephalopathy, Rif: rifaximin, Lac: Lactulose, Cir: Cirrhosis, Con: healthy control.
The AUC for classifying controls vs cirrhosis and controls vs No-HE patients using microbiota was significantly higher within women than within men. AUC was similar regardless of sex for classifying controls vs HE (total, HE-Lac or HE-Rif) and for HE patients on lactulose vs HE patients on lactulose+rifaximin (Table 2). AUC was higher for differentiating HE vs No-HE within men than within women. This difference was primarily driven by the higher AUC for differentiating HE-Rif vs No-HE within men than within women, since No-HE vs HE-Lac classification performance was similar within both sexs.
Table 2:
Area under the curve comparison between groups with differing stages of disease for all patients, within men, and within women
| Data presented as AUC (SD) | All samples | Within Women | Within Men | FDR corrected P-value between AUCs |
|---|---|---|---|---|
| Control vs Cirrhosis | 0.81 (0.04) | 0.85 (0.06) | 0.78 (0.05) | 2.3 e-5 |
| Control vs Cirrhosis without HE | 0.75 (0.04) | 0.89 (0.06) | 0.73 (0.06) | 1.8 e-13 |
| Control vs Cirrhosis with HE | 0.89 (0.03) | 0.87 (0.08) | 0.88 (0.03) | 0.96 |
| Control vs HE-Lac | 0.87 (0.4) | 0.84 (0.09) | 0.86 (0.07) | 0.51 |
| Control vs HE-Rif | 0.90 (0.03) | 0.92 (0.08) | 0.89 (0.02) | 0.22 |
| Cirrhosis without HE vs Cirrhosis with HE | 0.78 (0.05) | 0.72 (0.12) | 0.80 (0.04) | 0.003 |
| Cirrhosis without HE vs HE-Lac | 0.72 (0.06) | 0.71 (0.10) | 0.74 (0.06) | 0.24 |
| Cirrhosis without HE vs HE-Rif | 0.83 (0.03) | 0.74 (0.10) | 0.84 (0.05) | 1.4 e-4 |
| HE on lactulose vs HE also on rifaximin | 0.69 (0.07) | 0.68 (0.12) | 0.67 (0.10) | 0.87 |
Comparisons are within women and within men to determine how well the microbial taxa differentiate the two compared groups within the sexs to the other group in the row. Rif: patients on lactulose and rifaximin, HE-Lac: patients only on lactulose.
As shown in Table S1, PPI and age were separately added to these models and only age significantly added to the differences between diseased and healthy groups when classification performance within men was compared to within women. Additionally, the microbes important in the analysis without confounders continued to have a high feature importance when confounders were included. Therefore, the classification analyses presented are adjusted for age and PPI.
Microbiota composition comparisons using the AI models between men and women
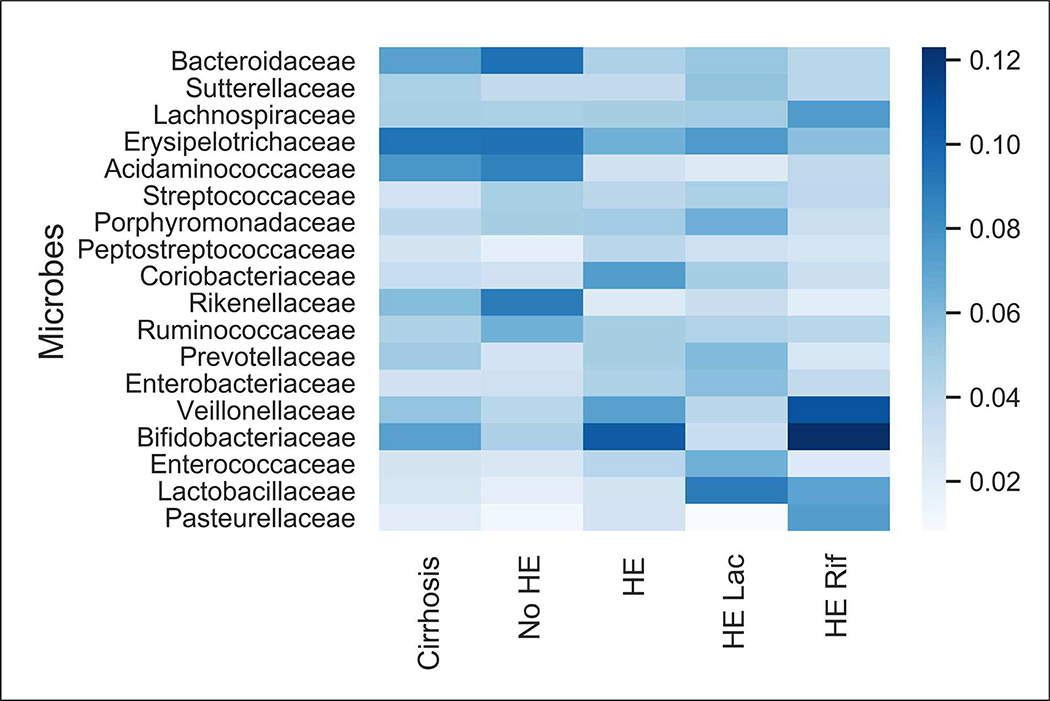
In addition to distinguishing within sexs, we also classified men vs women within the same disease/control group based on composition (Figure 2). As shown in Table 3, classification performance was significantly better than chance in all groups except in those with HE-Rif. There was also a similar pattern of difference using PERMANOVA of pathways that were activated between sexs in the PiCRUSt comparisons.
Figure 2: Microbial Composition Changes between Men and Women.
These comparisons are between sex and a heatmap of bacterial families that were high in importance in comparison between men and women are shown. Darker colors indicate greater importance of that family in differentiating between men and women. Blank or white areas indicate that there is no linkage. HE: Hepatic encephalopathy, Rif: rifaximin, Lac: Lactulose
Table 3:
Comparison between men and women for specific subgroups
| Group | Composition | PiCRUSt | ||||
|---|---|---|---|---|---|---|
| Random Forest Classifier Model | PERMANOVA Bray-Curtis | PERMANOVA Jaccard | ||||
| AUC (SD) | P value | R value | P value | R value | P value | |
| Cirrhosis | 0.67 (0.05) | 3.7 e-16 | 3.67 | 0.001 | 3.67 | 0.003 |
| No-HE | 0.74 (0.06) | 2.5 e-18 | 5.57 | 0.001 | 4.9 | 0.001 |
| HE on Lactulose only | 0.61 (0.14) | 1.5 e-4 | 2.2 | 0.05 | 2.0 | 0.05 |
| HE also on Rifaximin | 0.55 (0.15) | 0.08 | 0.52 | 0.81 | 0.60 | 0.83 |
AUC (Area under the curve) and top microbes for various classification tasks. P<0.05= statistical significance from 1 sample t-test (that the performance is better than chance level AUC=0.5). Top microbe importance score adds up to 0.5. Bray-Curtis distance evaluates the relative importance of a specific pathway, while Jaccard studies the presence or absence of variables. PiCRUSt: Phylogenetic Investigation of Communities by Reconstruction of Unobserved States, SD: Standard deviation
Analysis of Individual families
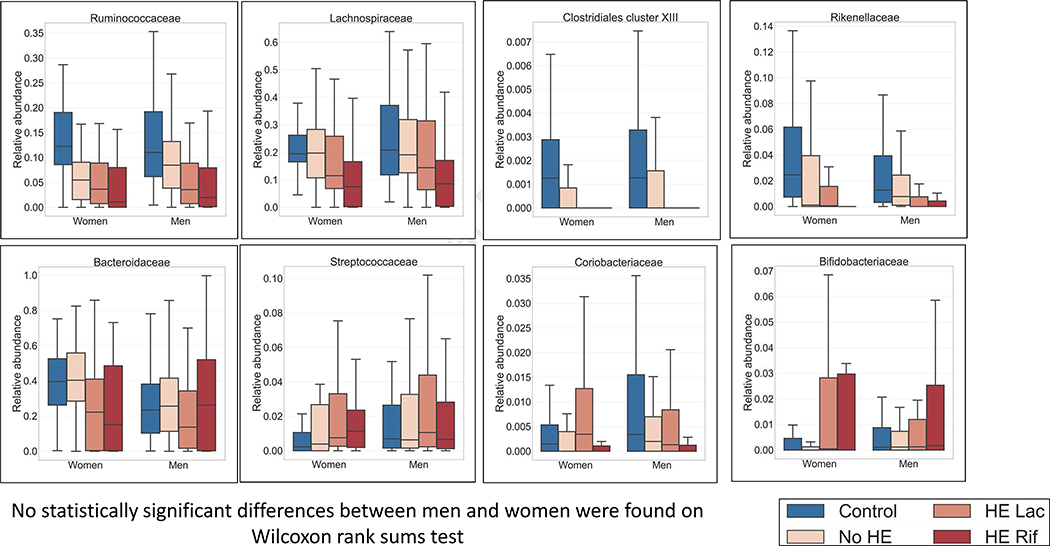
Sex-agnostic changes
As the disease progressed, there was relatively lower Ruminococcaceae, Lachnospiraceae, Rikenellaceae, Clostridium cluster XIII and Bacteroidaceae while there was higher Streptococcaceae and Bifidobacteriaceae in both groups. (Figure 3). Coriobacteriaceae reduced after rifaximin in both groups similarly. None of these differences at any stages were different between men and women.
Figure 3: Microbial taxa composition whose pattern of change over disease stages remains relatively similar between sex.
Median and IQR of microbial taxa in men and women across disease states whose relative abundance changed similarly regardless of sex are presented. HE: Hepatic encephalopathy, Rif: rifaximin, Lac: Lactulose
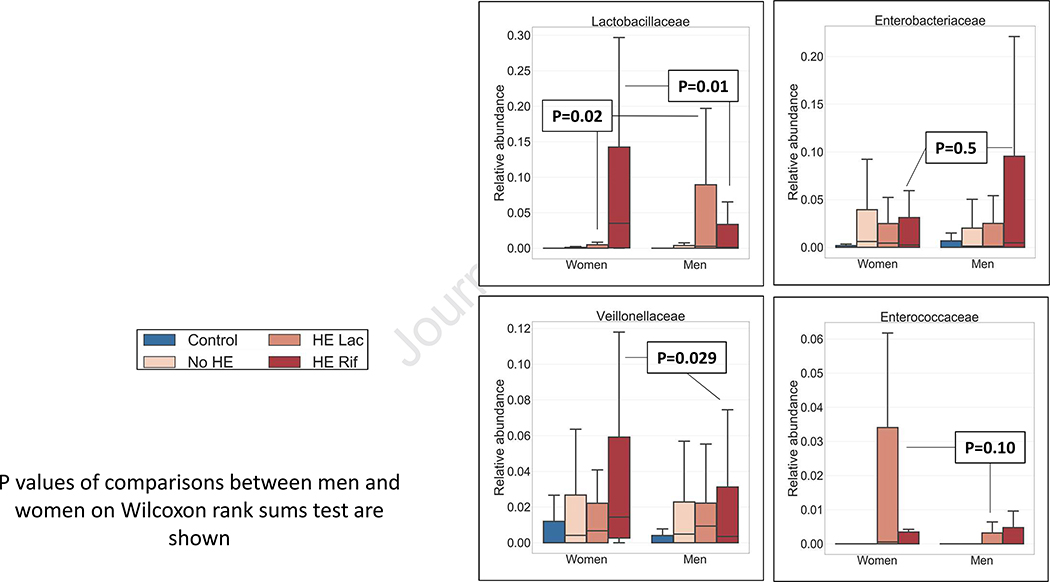
Sex-associated changes
Lactobacillaceae relative abundance was significantly higher in men after lactulose and low after rifaximin and vice-versa for women. A non-significant increase in Enterococcaceae was seen in women after lactulose compared to men but reduced after rifaximin in both sexs similarly. Veillonellaceae relative abundance also significantly increased in women after rifaximin but not in men (Figure 4).
Figure 4: Microbial taxa composition whose pattern of change after HE development is different between sex.
Median and IQR of microbial taxa in men and women across disease states whose relative abundance changed in different patterns with disease progression are presented. HE: Hepatic encephalopathy, Rif: rifaximin, Lac: Lactulose
Functional analyses between men and women
Entire cirrhosis group
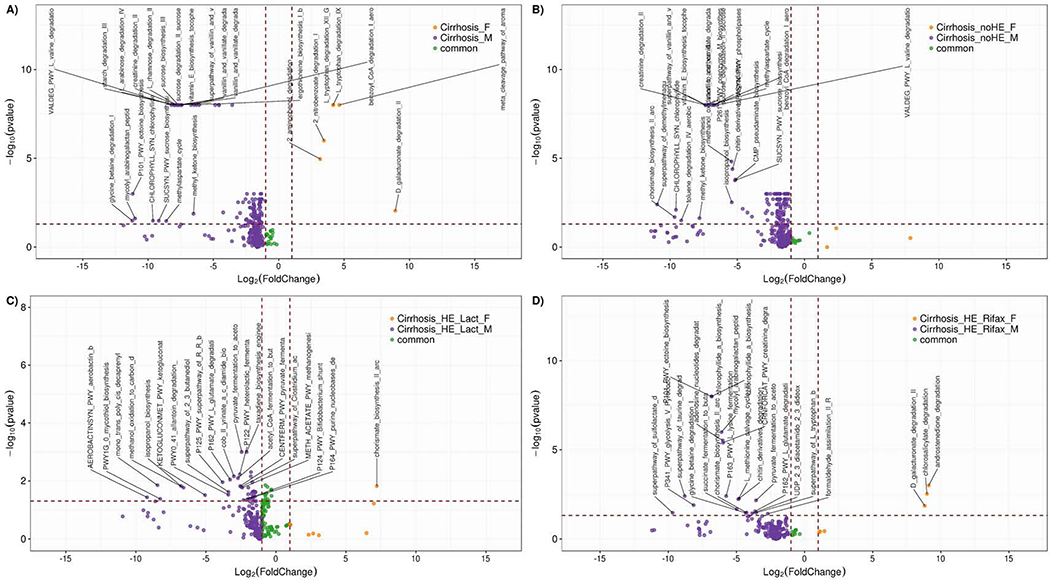
Several pathways were higher in men that focused on bacterial cell wall synthesis, carbohydrate and creatinine metabolism, glutamate degradation and methylaspartate cycle and lignin degradation, while D-galacturonate degradation was higher in women with cirrhosis. Interestingly, plant degradation with gallate and methylgallate, aromatic amino acids and androstenedione and vitamin B12 synthesis pathways were similarly expressed regardless of sex in all patients with cirrhosis (Figure 5A, Table S2).
Figure 5: Differential enriched pathways volcano plot.
Volcano plot showing Pathway fold changes on X-axis and the negative logarithm (base 10) of the Bonferroni-adjusted p-value on Y-axis. Dashed vertical and horizontal lines reflect the filtering criteria (fold change= ±1.0 and Bonferroni-adjusted p-value > −log (0.05)). Blue or Orange dots represent pathways that their log2 foldchange is greater or less than 1.0. The green dots represent common pathways which their absolute value of their foldchange is lower than 1. To be considered as a significant feature; the foldchange value should be greater than 1 or less than −1, and the negative logarithm (base 10) of the Metastats p-value should be above 1.13(-log (0.05)). We labeled top 20 of significant pathways for each group. Fold-change directions of men versus women are presented with log10 p value and log 2-fold change. Orange: pathways expressed more in women, Purple: pathways expressed more in men, Green: pathways expressed similarly in both. Pathways that are found above and to the right of the crossing of the dashed lines are significant. All pathway comparison details are in supplementary tables 1-4.
5A: Comparison between men and women with cirrhosis
5B: Comparison between men and women without hepatic encephalopathy (HE)
5C: Comparison between men and women with HE only on lactulose
5D: Comparison between men and women with HE on rifaximin also
In No-HE patients
Common features were similar to those found in the entire group. None of the pathways were increased in women. In men, there were higher changes pertaining to cell wall synthesis, carbohydrate metabolism, valine and creatinine degradation and the glutamate/methyl-aspartate cycle compared to women (Figure 5B, Table S3).
In HE-Lac patients
Common changes between sexs in those with HE on lactulose were related to cell wall, urea cycle and vitamin synthesis in addition to the ones that were found between cirrhosis patients as a whole. Chorismate, which is an intermediate in the synthesis of aromatic amino acids and vitamin synthesis in prokaryotes, was higher in women. In men there was higher component of cell wall synthesis, cobalamin and glutamate degradation. Men also had a higher heterolactic fermentation pathway and fermentation of pyruvate and lysine towards butanoate and acetate, which are short-chain fatty acids (SCFA) compared to women (Figure 5C, Table S4).
In HE-Rif patients
In women androstenedione, D-galacturonate, and chlorosalicylate degradation pathways showed higher expression while in men, there was more tryptophan and cell wall component synthesis along with glutamate, lysine, and glycine degradation. HE-Rif women demonstrated greater androstenedione and galacturonate degradation compared to men, while in the remaining comparisons in other cirrhosis sub-groups, this pathway remained common between sexs (Figure 5D, Table S5).
DISCUSSION
Our results demonstrate differences in microbiota composition and function between men and women with cirrhosis, which are most apparent with lactulose and rifaximin therapy.
In cirrhosis, the interplay of impaired bile flow and local immune response predisposes to higher pathobiont and lower autochthonous taxa relative abundance[22–24]. The microbiota and their products in turn lead to changes in brain function that spans the gamut from cognitive alterations to frank coma[22]. Therapies focused on the microbiota related to prebiotic/laxatives (lactulose), non-absorbable antibiotics (rifaximin) and fecal microbiota transplant have been used in HE[25]. The response and consequences of therapy with these medications is not uniform and one feature that is often overlooked could be the sex of the patient[26, 27]. Prior studies have found a better cognitive performance in women compared to men, with implications for HE diagnosis [5]. Differentiating between sexs based on microbiota composition using the random forest classifier showed that the strongest differences between men and women within HE was when those only on lactulose were compared. However, once the disease progressed further enough to require rifaximin, which is usually used in more advanced cirrhosis, these differences reduced.
Lactulose, being a prebiotic, is hypothesized to increase potentially beneficial microbiota[28, 29]. The ability to tolerate lactulose and prevent further HE could partly depend on improving fermentation and increase in constituents of Lactobacillaceae[29]. We found that men only on lactulose had relatively higher Lactobacillaceae and greater expression of SCFA-generating pathways focused on acetate and butanoate[30]. However, with rifaximin, Lactobacillaceae and Veillonellaceae increased in women. The Veillonellaceae family includes several SCFA forming lactate-fermenting taxa, which may be relevant in HE[31]. This extends another translational study in which SCFA generation varied between individuals with cirrhosis into a sex-based analysis[32].Also, pathway expression of ammonia-generating glutamate degradation, tryptophan biosynthesis accompanied by increase in cell wall synthesis, and creatinine degradation were greater in men, likely leading to a more ammoniagenic inflammatory milieu[33]. This differential response when on lactulose and rifaximin could provide insight into potential sex-related differences in cognition between men and women with cirrhosis.
It is also interesting that, in all comparisons but HE-Rif, androstenedione and galacturonate degradation expressions were common between sexs. In HE-Rif, these pathways were lower in men versus women. D-galacturonate is the main monomeric constituent of plant cell wall pectin, which can be degraded by several Lactobacillus spp [34]. Therefore, the relatively higher Lactobacillaceae in HE-Rif women could be linked to this.
Androstenedione is high in men with cirrhosis due to suppression of the hypothalamic-pituitary axis with higher circulating steroids[35]. We observed a lower microbially-driven androstenedione degradation in men on rifaximin versus women. This would lead to greater feminization since androstenedione is also a more efficient precursor for estrogen than testosterone. Also, because some Lactobacillaceae taxa can produce androgens, their relative reduction in HE-Rif men but not women could be associated with this overall microbially-associated feminization in men with advanced cirrhosis[36, 37].
Ultimately, we found overall reductions in sex-related differences in composition as the cirrhosis progressed from the PiCRUSt and RFC analysis. This means that along with the increasing overall feminization found in men with cirrhosis, the microbiota also becomes similar overall to that of women in advanced disease. However, specific pathways and microbiota focused on Lactobacillaceae, androstenedione degradation and cell wall synthesis remain different between sexs, which could be implicated in feminization of men.
Since this was a cross-sectional study and patients on rifaximin likely experienced a worse HE course than only on lactulose, it is also important to consider whether these changes are related to disease severity itself. Since men and women on rifaximin were similar with respect to their clinical liver disease characteristics when HE-Lac and HE-Rif were compared between sexs, it is unlikely that this is entirely related to the disease stage. Moreover, functional rather than compositional changes after rifaximin and lactulose replicate prior pre/post therapeutic trials[28, 38].
Our data extend prior observations in human and animal trials that sex plays an important role in gut microbiota composition and function in cirrhosis and HE[39–41]. The trial is limited by the relatively lower number of women compared to men. However, the number included is still higher than most HE-related studies and at each stage of disease women were equivalently positioned from a cirrhosis standpoint. While some etiologies are over-represented in some sexs, we excluded cholangiopathies and autoimmune diseases and had an equitable distribution of other etiologies. On review of our data 10 patients (6 men and 4 women) were excluded, which would not have substantially altered the results. Further larger studies focused on these autoimmune etiologies may give additional insight. The results at this stage may be relevant from a physiological rather than clinical perspective but could set the stage for sex-specific management strategies in the future. While these were not significant on random forest or other analyses, there was a tendency of greater diabetes and MELD score in men compared to women on lactulose. We analytically controlled for age and PPI, and none of the other non-antibiotic drugs, such as estrogen derivatives and anti-psychotics were used in our subjects[42]. However, there may be other data that are related to microbiota that could need further study. The random forest classifier excluded microbiota that were present in less than 10% men and 10% women, which could decrease the relative ability of the model to discriminate between groups. However, reducing data dimensionality can improve the performance of the classifier (by making it more robust to noise) and improve the estimation of feature importance values assigned to the microbes which aid in identification of important microbes driving the differences (and discrimination) between groups[19–21]. We also found that the included bacteria were indeed the ones that drove the sex-based differences. We also performed PiCRUSt and 16S rather than the metagenomics or metatranscriptomics; the latter could improve the understanding of these differences at a deeper level.
We conclude that our cross-sectional study provides cues for microbial compositional and functional differences in the earlier stages of cirrhosis, which converge as disease progresses to HE. There are differences in gut microbial function and composition focused on Lactobacillaceae and Veillonellaceae between men and women with cirrhosis, which could implicate these in differential responses to lactulose and rifaximin. Changes in microbial function related to bacterial cell wall, aromatic amino acids, and sex steroids could be relevant towards the increasing feminization of men with advanced cirrhosis and play a role in defining sex as a determinant of hepatic encephalopathy therapy. After further verification in other geographical and cultural contexts, prospective studies could prove if sex differences may play a role in HE response to treatment with non-absorbable disaccharides, with or without rifaximin.
Supplementary Material
Highlights.
Patients with cirrhosis have variable hepatic encephalopathy (HE) therapy responses
Men with cirrhosis demonstrate feminization but the role of microbiota are unclear
Microbial changes between genders are highest in compensated cirrhosis
Changes in Lactobacillaceae and androgen metabolism differentiate between genders
Gender-microbiota interactions can influence feminization and HE therapy response
Acknowledgments
Financial Support Statement: Partly supported by VA Merit Review 2I0CX001076, RO1HS024512 and R21TR002024 to JSB, NSF CNS-1337732, NSF CNS-1624790 and Mayo Clinic and Illinois Alliance Fellowships for Technology based Healthcare Research for the UIC team. None of the sponsors had any role in the study design, conduct and decision to publish.
Footnotes
Conflict of Interest: None for any author
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- [1].Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–735. [DOI] [PubMed] [Google Scholar]
- [2].Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol 2012;10:1034–1041 e1031. [DOI] [PubMed] [Google Scholar]
- [3].Acharya C, Bajaj JS. Altered Microbiome in Patients With Cirrhosis and Complications. Clin Gastroenterol Hepatol 2019;17:307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Iebba V, Guerrieri F, Di Gregorio V, Levrero M, Gagliardi A, Santangelo F, et al. Combining amplicon sequencing and metabolomics in cirrhotic patients highlights distinctive microbiota features involved in bacterial translocation, systemic inflammation and hepatic encephalopathy. Sci Rep 2018;8:8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Allampati S, Duarte-Rojo A, Thacker LR, Patidar KR, White MB, Klair JS, et al. Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. Am J Gastroenterol 2016;111:78–86. [DOI] [PubMed] [Google Scholar]
- [6].Bajaj JS, Duarte-Rojo A, Xie JJ, Acharya C, Wade J, Robles C, et al. Minimal Hepatic Encephalopathy and Mild Cognitive Impairment Worsen Quality of Life in Elderly Patients with Cirrhosis. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jasarevic E, Morrison KE, Bale TL. Sex differences in the gut microbiome-brain axis across the lifespan. Philosophical transactions of the Royal Society of London Series B, Biological sciences 2016;371:20150122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. Sex bias in autoimmunity is influenced by microbiota. Immunity 2013;39:400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Durazzo M, Belci P, Collo A, Prandi V, Pistone E, Martorana M, et al. Sex specific medicine in liver diseases: a point of view. World J Gastroenterol 2014;20:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019;70:1457–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Phelps T, Snyder E, Rodriguez E, Child H, Harvey P. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biol Sex Differ 2019;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010;362:1071–1081. [DOI] [PubMed] [Google Scholar]
- [13].Liu TC, Gurram B, Megan T. Baldridge RH, 3 Vy Lam,2 Chengwei Luo,4 Yumei Cao,5 Pippa Simpson,5 Michael Hayward,2 Mary L. Holtz,2 Pavlos Bousounis,2 Joshua Noe,2 Diana Lerner,2 Jose Cabrera,2 Vincent Biank,2 Michael Stephens,2 Curtis Huttenhower,4,6 Dermot P.B. McGovern,7 Ramnik J. Xavier,4,8 Thaddeus S. Stappenbeck,1 and Nita H. Salzman2 Paneth cell defects in Crohn’s disease patients promote dysbiosis. JCI Insight 2016;1:e86907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bajaj JS, Fagan A, White MB, Wade JB, Hylemon PB, Heuman DM, et al. Specific Gut and Salivary Microbiota Patterns Are Linked With Different Cognitive Testing Strategies in Minimal Hepatic Encephalopathy. Am J Gastroenterol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Douglas GM, Maffei VJ, Zaneveld J, Yurgel SN, Brown JR, Taylor CM, et al. PICRUSt2: An improved and customizable approach for metagenome inference. bioRxiv 2020:672295. [Google Scholar]
- [16].Caspi R, Altman T, Billington R, Dreher K, Foerster H, Fulcher CA, et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res 2014;42:D459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].White JR, Nagarajan N, Pop M. Statistical Methods for Detecting Differentially Abundant Features in Clinical Metagenomic Samples. PLoS Computational Biology 2009;5:e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol 2009;5:e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, et al. Best practices for analysing microbiomes. Nat Rev Microbiol 2018;16:410–422. [DOI] [PubMed] [Google Scholar]
- [20].Winham SJ, Colby CL, Freimuth RR, Wang X, de Andrade M, Huebner M, et al. SNP interaction detection with Random Forests in high-dimensional genetic data. BMC Bioinformatics 2012;13:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bishop CM. Pattern recognition and machine learning: Springer; 2006. [Google Scholar]
- [22].Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol 2017;67:1084–1103. [DOI] [PubMed] [Google Scholar]
- [23].Hatton G, Shawcross DL. Is treating the gut microbiome the key to achieving better outcomes in cirrhosis? Expert Rev Gastroenterol Hepatol 2019;13:1–2. [DOI] [PubMed] [Google Scholar]
- [24].Monteiro S, Grandt J, Uschner FE, Kimer N, Madsen JL, Schierwagen R, et al. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bajaj JS, Khoruts A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J Hepatol 2020;72:1003–1027. [DOI] [PubMed] [Google Scholar]
- [26].Rathi S, Fagan A, Wade JB, Chopra M, White MB, Ganapathy D, et al. Patient Acceptance of Lactulose Varies Between Indian and American Cohorts: Implications for Comparing and Designing Global Hepatic Encephalopathy Trials. J Clin Exp Hepatol 2018;8:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bajaj JS, Sanyal AJ, Bell D, Gilles H, Heuman DM. Predictors of the recurrence of hepatic encephalopathy in lactulose-treated patients. Aliment Pharmacol Ther 2010;31:1012–1017. [DOI] [PubMed] [Google Scholar]
- [28].Wang JY, Bajaj JS, Wang JB, Shang J, Zhou XM, Guo XL, et al. Lactulose improves cognition, quality of life, and gut microbiota in minimal hepatic encephalopathy: A multicenter, randomized controlled trial. J Dig Dis 2019;20:547–556. [DOI] [PubMed] [Google Scholar]
- [29].Riggio O, Varriale M, Testore GP, Di Rosa R, Di Rosa E, Merli M, et al. Effect of lactitol and lactulose administration on the fecal flora in cirrhotic patients. J Clin Gastroenterol 1990;12:433–436. [DOI] [PubMed] [Google Scholar]
- [30].Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilan CG, Salazar N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front Microbiol 2016;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med 2019;25:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jin M, Kalainy S, Baskota N, Chiang D, Deehan EC, McDougall C, et al. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int 2019;39:1437–1447. [DOI] [PubMed] [Google Scholar]
- [33].al Mardini H, Harrison EJ, Ince PG, Bartlett K, Record CO. Brain indoles in human hepatic encephalopathy. Hepatology 1993;17:1033–1040. [PubMed] [Google Scholar]
- [34].Valk LC, Luttik MAH, de Ram C, Pabst M, van den Broek M, van Loosdrecht MCM, et al. A Novel D-Galacturonate Fermentation Pathway in Lactobacillus suebicus Links Initial Reactions of the Galacturonate-Isomerase Route With the Phosphoketolase Pathway. Front Microbiol 2019;10:3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Southren AL, Gordon GG, Olivo J, Rafii F, Rosenthal WS. Androgen metabolism in cirrhosis of the liver. Metabolism 1973;22:695–701. [DOI] [PubMed] [Google Scholar]
- [36].Poutahidis T, Springer A, Levkovich T, Qi P, Varian BJ, Lakritz JR, et al. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS One 2014;9:e84877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fransen F, van Beek AA, Borghuis T, Meijer B, Hugenholtz F, van der Gaast-de Jongh C, et al. The Impact of Gut Microbiota on Sex-Specific Differences in Immunity. Front Immunol 2017;8:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One 2013;8:e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Markle JG, Frank DN, Adeli K, von Bergen M, Danska JS. Microbiome manipulation modifies sex-specific risk for autoimmunity. Gut Microbes 2014;5:485–493. [DOI] [PubMed] [Google Scholar]
- [40].Cross TL, Kasahara K, Rey FE. Sexual dimorphism of cardiometabolic dysfunction: Gut microbiome in the play? Mol Metab 2018;15:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Collden H, Landin A, Wallenius V, Elebring E, Fandriks L, Nilsson ME, et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am J Physiol Endocrinol Metab 2019;317:E1182–E1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018;555:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.