Abstract
More than a quarter of veterans of the 1990–1991 Persian Gulf War suffer from Gulf War Illness (GWI), a chronic, multi-symptom illness that commonly includes musculoskeletal pain. Exposure to a range of toxic chemicals, including sarin nerve agent, are a suspected root cause of GWI. Moreover, such chemical exposures induce a neuroinflammatory response in rodents, which has been linked to several GWI symptoms in rodents and veterans with GWI. To date, a neuroinflammatory basis for pain associated with GWI has not been investigated. Here, we evaluated development of nociceptive hypersensitivity in a model of GWI. Male Sprague Dawley rats were treated with corticosterone in the drinking water for 7 days, to mimic high physiological stress, followed by a single injection of the sarin nerve agent surrogate, diisopropyl fluorophosphate. These exposures alone were insufficient to induce allodynia. However, an additional sub-threshold challenge (a single intramuscular injection of pH 4 saline) induced long-lasting, bilateral allodynia. Such allodynia was associated with elevation of markers for activated microglia/macrophages (CD11b) and astrocytes/satellite glia (GFAP) in the lumbar dorsal spinal cord and dorsal root ganglia (DRG). Additionally, Toll-like receptor 4 (TLR4) mRNA was elevated in the lumbar dorsal spinal cord, while IL-1β and IL-6 were elevated in the lumbar dorsal spinal cord, DRG, and gastrocnemius muscle. Demonstrating a casual role for such neuroinflammatory signaling, allodynia was reversed by treatment with either minocycline, the TLR4 inhibitor (+)-naltrexone, or IL-10 plasmid DNA. Together, these results point to a role for neuroinflammation in male rats in the model of musculoskeletal pain related to GWI. Therapies that alleviate persistent immune dysregulation may be a strategy to treat pain and other symptoms of GWI.
Keywords: Cytokines, immune cell, glia, neuroimmune, sphingolipids, pain, gulf war illness
1. Introduction
Over the past three decades, approximately 25–30% of veterans of the 1990–1991 Gulf War have consistently suffered from chronic health symptoms, including mood disturbance, cognitive dysfunction, fatigue, and musculoskeletal pain (Institute of Medicine, 2013, 2014; Fukuda et al., 1998; Iannacchione et al., 2011; Steele, 2000; White et al., 2016). This chronic, multi-symptom illness has been termed Gulf War Illness (GWI) and lacks effective treatment (Cory-Slechta and Wedge, 2016; Fukuda et al., 1998; Golomb, 2008; Heng, 2016; Steele, 2000; White et al., 2016). Returning veterans from many wars, including the recent Iraq, Afghanistan, and Balkan conflicts, have suffered from chronic, multi-symptom illness. However, illness rates among veterans of the Gulf War are significantly higher, which is especially striking given the brevity of this conflict (Institute of Medicine, 2013; Jones et al., 2002; Powell et al., 2012). Chemical exposures are widely suspected to be a cause of GWI, given the low-level release of chemical warfare agents, prevalent use of pesticides, prophylactic medications to protect against nerve agent attacks, and oil well fires that are unique to this conflict (Cory-Slechta and Wedge, 2016; Golomb, 2008; Heng, 2016; Steele, 2000; Sullivan et al., 2018; White et al., 2016).
Several animal models of GWI have been developed, based on the chemical exposures described above (Cory-Slechta and Wedge, 2016; White et al., 2016). Many have implicated neuroinflammation in the central nervous system, including glial activation and subsequent production of pro-inflammatory cytokines and sphingomyelins (Abdullah et al., 2012, 2013; Alhasson et al., 2017; Hernandez et al., 2019; Joshi et al., 2019; Koo et al., 2018; Madhu et al., 2019; Michalovicz et al., 2019; O’Callaghan et al., 2015; Michalovicz et al., 2020; Parihar et al., 2013; White et al., 2016; Zakirova et al., 2015). In support of these rodent studies, inflammatory and neuroinflammatory processes are elevated in veterans with GWI (Alshelh et al., 2020; Broderick et al., 2013; Emmerich et al., 2017; Joshi et al., 2019; Parkitny et al., 2015). Pain has been investigated in several GWI models, implicating increased activity of NaV1.9 and Transient Receptor Potential Ankyrin 1 (TRPA1) and concurrent decrements in KV7 activity in nociceptors (Cooper et al., 2018; Flunker et al., 2017; Nizamutdinov et al., 2018; Nutter et al., 2015). Whether neuroinflammatory mechanisms also underpin pain in GWI—as in other forms of persistent pain (Beggs et al., 2012b; Grace et al., 2014, 2016b; McMahon et al., 2015; Ji et al., 2016; Inoue and Tsuda, 2018; Malcangio, 2019)—is not yet known.
In this study, we investigated the development of pain in a model of GWI in which rodents are exposed to the sarin nerve agent surrogate diisopropyl fluorophosphate (DFP) following treatment with corticosterone (CORT) (Koo et al., 2018; Locker et al., 2017; O’Callaghan et al., 2015). As CORT potentiates neuroinflammatory responses (Frank et al., 2015, 2019), high physiological stress is a possible sensitizing factor that could predispose soldiers to GWI in the presence of chemical exposures (Freeman, 2013; Locker et al., 2017). Other factors, such as fatiguing exercise or muscle injury that cause tissue acidosis, could further increase risk of musculoskeletal pain (Gregory et al., 2016; Issberner et al., 1996; Woo et al., 2004). With modifications to induce robust musculoskeletal pain, we examined markers of neuroinflammation and the therapeutic effects of immunomodulatory agents.
2. Methods
2.1. Animals
Pathogen-free adult male Sprague Dawley rats (10 weeks old on arrival, Envigo, USA) were used. Rats were housed 2–3 per cage in a light- and temperature-controlled room (12:12-h light-dark cycle, lights on at 7:00 am) with food and water available ad libitum. Group sizes were n = 6–8 for all experiments, except for sphingolipid analyses, where group sizes were n = 4. All procedures were approved by the MD Anderson Cancer Center Animal Care and Use Committee.
2.2. GWI pain model
The GWI pain model was performed with slight modifications from previous descriptions in mice and rats (Koo et al., 2018; O’Callaghan et al., 2015), and is summarized in Figure 1. Corticosterone (CORT) was administered via the drinking water (200 mg/L in 0.6% ethanol) for 7 days to mimic high physiological stress. Vehicle (0.6% ethanol in drinking water) was used as the negative control. On the final day of CORT treatment (day 7), a single dose of the sarin nerve agent surrogate diisopropyl fluorophosphate (DFP) was subcutaneously (s.c.) administered (1.5 mg/kg). Vehicle (saline) was used as the negative control. Preliminary studies indicated that these insults did not induce nociceptive hypersensitivity. Therefore, 7 days after DFP, tissue acidosis was induced with a single 100 μL injection of acidic saline (pH = 4.0) into the left gastrocnemius muscle, under brief isoflurane anesthesia. Saline was adjusted to pH 4.0 using 0.1 M HCl or NaOH. Negative control was normal saline (pH = 7.2). A single intramuscular injection of acidic saline is a sub-threshold nociceptive stimulus; two injections are usually required to induce robust and persistent nociceptive hypersensitivity (Sluka et al., 2001). We reasoned that the CORT/DFP would substitute for the first, priming injection of acidic saline. Intramuscular acidic saline was selected as a well-characterized model of musculoskeletal pain (Sluka et al., 2001, 2002, 2003; Sharma et al., 2009; Sutton and Opp, 2015), reminiscent of the pain experienced by veterans with GWI.
Figure 1. Timeline of experimental procedures.
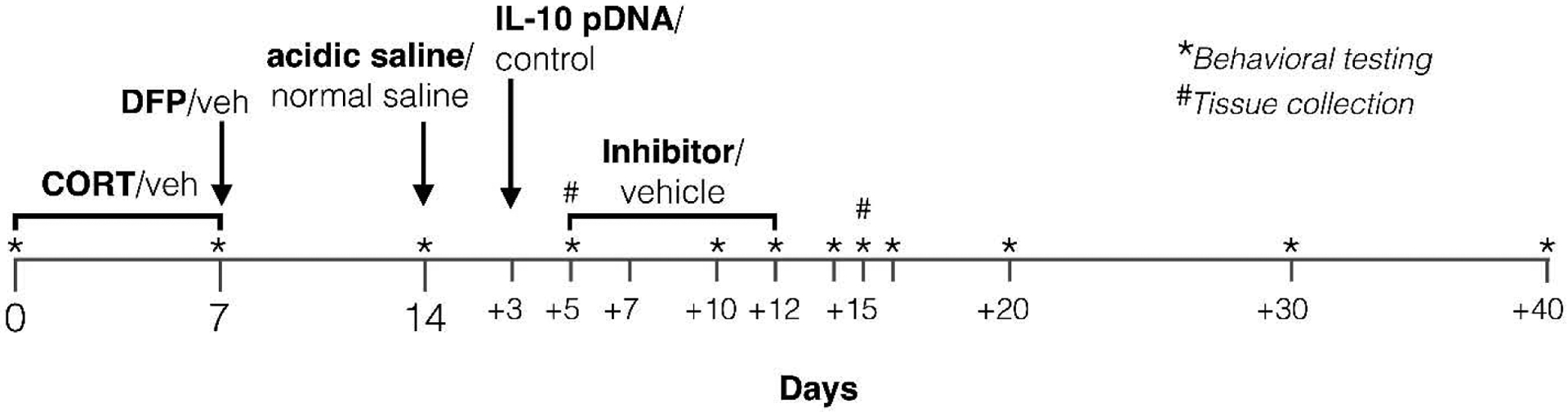
CORT: corticosterone; DFP: diisopropyl fluorophosphate; Veh: vehicle.
2.3. Drug administration
The macrophage/microglial inhibitor minocycline (Sigma Aldrich, St. Louis, MO) was administered by oral gavage at 50 mg/kg/5 mL once per day in water for 7 consecutive days, beginning 5 days after the acidic saline injection (Figure 1) (Hutchinson et al., 2008a). The TLR4 inhibitor, and opioid receptor-inactive isomer (+)-naltrexone (gifted by Dr. Kenner Rice) was administered s.c. at 6 mg/kg three times per day in saline for 7 consecutive days, beginning 5 days after the acidic saline injection (Figure 1) (Ellis et al., 2014; Hutchinson et al., 2008b; Wang et al., 2016; Zhang et al., 2018). Respective equivolume vehicles were used as negative controls for minocycline and (+)-naltrexone. IL-10 gene therapy was acutely administered on day 3 post acidic saline injection (Figure 1). The timing of administration differed from above so as to allow more time for the plasmid to be expressed and reverse allodynia. The plasmid DNA encoding rat IL-10 (pDNA-IL-10) and empty pDNA control vectors have been described previously (Milligan et al., 2006), and were a generous gift from Dr. Linda Watkins (University of Colorado Boulder). The pDNA vectors were prepared and intrathecally administered according to our previously described methods (Grace et al., 2016c, 2017; Lacagnina et al., 2017): the lines were loaded with 3 μg of plasmid DNA (in 7.5 μL of 0.9% sterile saline), followed by 25 μg D-mannose (Sigma Aldrich; dissolved in 3 μL of 0.9% sterile saline) (Dengler et al., 2014).
2.4. Mechanical allodynia
Testing was conducted blind with respect to group assignment. Rats received at least three 60 min habituations to the test environment on separate days prior to behavioral testing. Rats were placed in a small plexiglass enclosure on a mesh stand, and the von Frey test (Chaplan et al., 1994) was performed as described previously (Grace et al., 2016a, 2016c; Li et al., 2020). Assessments were made prior to CORT/vehicle (baseline), prior to DFP/vehicle, prior to acidic/normal saline, and at regular intervals thereafter (summarized in Figure 1). A logarithmic series of 10 calibrated Semmes–Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL) were applied randomly to the left vs right hind paws to define the threshold stimulus intensity required to elicit a paw withdrawal response. Log stiffness of the hairs ranged from manufacturer-designated 3.61 (0.40 g) to 5.18 (15.14 g) filaments. The behavioral responses were used to calculate absolute threshold (the 50% probability of response) by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method (Harvey, 1986; Treutwein and Strasburger, 1999) as previously described (Milligan et al., 2001, 2000). The steepness of the psychometric function was assumed to be 3.32 (corresponding to a standard deviation of 0.301 of the underlying Gaussian probability distribution) and to range from 0.0 response probability to a maximum of 1.0. Estimated thresholds derived from a Gaussian integral function yield a mathematical continuum and, thus, are appropriate for parametric statistical analyses. The computer program, PsychoFit, may be downloaded from L.O. Harvey’s website (http://psych.colorado.edu/~lharvey).
2.5. Thermal hyperalgesia
Testing was conducted blind with respect to group assignment. Rats received at least three 60 min habituations to the test environment on separate days prior to behavioral testing. Latencies for behavioral response to radiant heat stimuli applied to the plantar surface of each hind paw and tail were assessed using a modified Hargreaves test (Hargreaves et al., 1988). Briefly, baseline withdrawal values were calculated from an average of two consecutive withdrawal latencies of the left and the right hind paws, measured at 15 min intervals. Latencies at baseline ranged from 8 to 10 s, and a cut-off time of 20 s was imposed to avoid tissue damage. Assessments were made prior to CORT/vehicle (baseline), prior to DFP/vehicle, prior to acidic/normal saline, and at regular intervals thereafter (summarized in Figure 1).
2.6. Tissue collection
On days 5 or 15 post saline administration, rats were overdosed with sodium pentobarbital (100 mg/kg; i.p.) and transcardially perfused with ice-cold saline (Figure 1). The left gastrocnemius muscles, left L4/5 dorsal root ganglia (DRG), and left L4/5 dorsal segments of the spinal cord were dissected and rapidly frozen for subsequent analysis.
2.7. RT-PCR
Total RNAs were extracted using TRIzol (ThermoFisher Scientific, Waltham, MA) from spinal cord and DRG samples. One μg RNA was used for reverse transcription with iScript Reverse Transcription Supermix (Bio-Rad, Hercules, CA). Real-time polymerase chain reaction was carried out in a final volume of 20 μL with iTaq Universal SYBR Green Supermix (Bio-Rad) containing 2 μL of five times diluted cDNA and monitored by CFX Connect Real-Time PCR Detection System (Bio-Rad) as we have previously described (Li et al., 2020). Primer sequences were as follows: Itgam (CD11b): F: CTGGTACATCGAGACTTCTC, R: TTGGTCTCTGTCTGAGCCTT; Gfap (glial fibrillary acidic protein): F: AGATCCGAGAAACCAGCCTG, R: CCTTAATGACCTCGCCATCC; Tlr4 (TLR4): F: TCCCTGCATAGAGGTACTTC, R: CACACCTGGATAAATCCAGC; S1pr1 (Sphingosine-1-Phosphate Receptor 1): F: TTCAGCCTCCTTGCTATCGC, R: AGGATGAGGGAGATGACCCAG; Gapdh (glyceraldehyde 3-phosphate dehydrogenase): F: AGGGACAATCTCACACAGG R: GACTCAACCTTCCTCTCCA. The level of the target mRNA was quantified relative to the housekeeping gene (Gapdh) using the ΔΔCT method. Gapdh was not significantly different between treatments.
2.8. ELISAs
Tissue samples were dissociated with a gentleMACS Octo Dissociator (Miltenyi Biotec, Auburn, CA) or a mortar and pestle in tissue extraction reagent (50 mM Tris buffer containing 100 mM 6-amino-n-caproic acid, 1 mM EDTA, 5 mM benzamidine, 0.2 mM phenylmethyl sulfonyl fluoride (in 100% ethanol)) supplied with protease and phosphatase inhibitors as previously described (Grace et al., 2016a, 2016c; Li et al., 2020). Assay kits for IL-1β (RLB00, R&D Systems, Minneapolis, MN) (detection range: 5 – 2,000 pg/mL), IL-6 (R6000B, R&D Systems) (detection range: 62.5 – 4,000 pg/mL), and TNF (RTA00, R&D Systems) (detection range: 5 – 800 pg/mL) were used according to manufacturer instructions. Results were normalized to total protein levels (Bradford protein assay).
2.9. Liquid chromatography-mass spectrometry
Lipids were extracted from DRG and spinal cord samples with water/MeOH/CHCl3 (1:1:3), under acidic conditions. Targeted quantification of sphingolipids (D-sphingosine, sphingosine-1-phosphate, C12-ceramide, C16-ceramide, C18-ceramide, C24-ceramide) was performed using LC-ESI-MS/MS (Thermo TSQ Quantiva) as previously described (Stockstill et al., 2018). The concentration range of the standards was 0.01–6.25 μM. Sphingolipid concentrations were normalized to tissue weight.
2.10. Statistics
Mechanical allodynia was analyzed as the interpolated 50% thresholds (absolute threshold) and averaged between left and right hindpaws. One-way ANOVAs were used to confirm that there were no baseline differences in absolute thresholds between treatment groups. Differences between treatment groups were determined using repeated measures three-way ANOVA, followed by Tukey post hoc tests, where appropriate. Gene expression and protein levels were analyzed by two-way ANOVA followed by Tukey post hoc tests, as appropriate. Sphingolipid levels were analyzed by T-tests. Results are expressed as mean ± SD. P < 0.05 was considered statistically significant.
3. Results
3.1. Behavioral assessment of nociceptive hypersensitivity in a putative model of GWI pain
We first tested whether nociceptive hypersensitivity was induced in CORT+DFP model of GWI. Confirming our preliminary studies, there was no change in mechanical thresholds in the von Frey test following treatment with CORT, DFP, or their combination (Figure 2A; time × CORT × DFP: F5, 110 = 2.07, P = 0.074; CORT × DFP: F1, 22 = 0.12, P = 0.734; time × DFP: F5, 110 = 0.17, P = 0.974; time × CORT: F5, 110 = 0.99, P = 0.427; DFP: F1, 22 = 0.01, P = 0.962; CORT: F1, 22 = 0.03, P = 0.861; time: F1, 22 = 0.03, P = 0.861). Furthermore, treatment with CORT, DFP, or their combination did not alter withdrawal latencies in the Hargreaves assay (data not shown; time × CORT × DFP: F2, 44 = 0.70, P = 0.502; CORT × DFP: F1, 22 = 4.31, P = 0.055; time × DFP: F2, 44 = 0.28, P = 0.755; time × CORT: F2, 44 = 0.75, P = 0.480; DFP: F1, 22 = 0.12, P = 0.737; CORT: F1, 22 = 2.25, P = 0.148; time: F1.98, 43.47 = 0.91, P = 0.909).
Figure 2. Mechanical allodynia in a putative model of GWI pain.
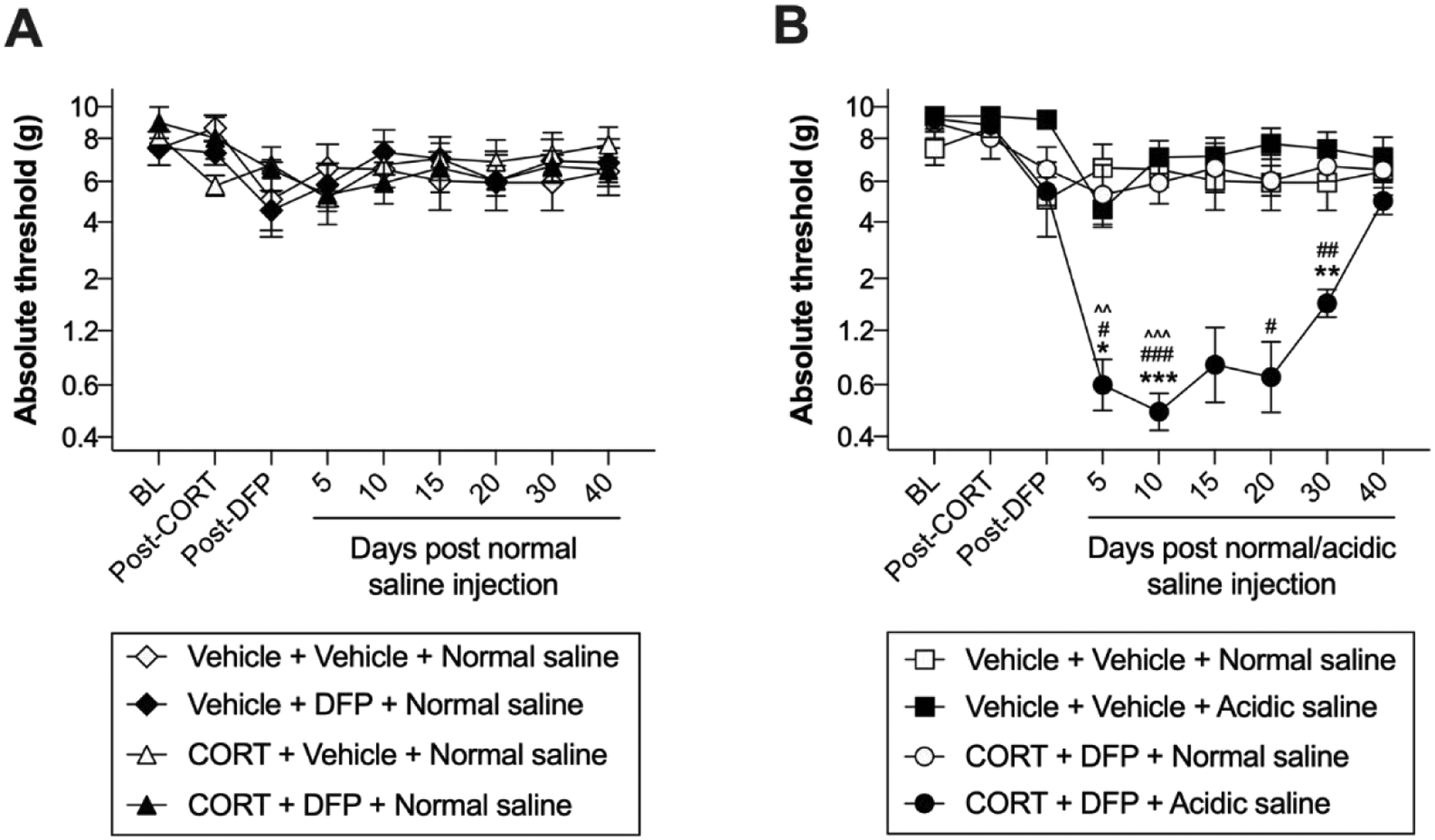
Corticosterone (CORT) or vehicle was administered via the drinking water for 7 days to mimic high physiological stress. On the final day of CORT treatment, a single dose of diisopropyl fluorophosphate (DFP) or vehicle was administered. Seven days later, rats received a single injection of normal (pH = 7.2) or acidic saline (pH = 4.0) into the left gastrocnemius muscle. (A) Treatment with CORT alone, DFP alone, or in combination (all following treatment with intramuscular normal saline) did not alter mechanical thresholds. (B) Acidic saline in combination with CORT and DFP significantly reduced mechanical thresholds. Relative to CORT+DFP+Normal saline: *P < 0.05, **P < 0.01, ***P < 0.001; Relative to Vehicle+Vehicle+Acidic saline: #P < 0.05, ##P < 0.01, ###P < 0.001; Relative to Vehicle+Vehicle+Normal saline: ^^P < 0.01, ^^^P < 0.001. N = 6–8/group. Data are mean ± SD.
Next, we tested whether CORT+DFP would serve as a nociceptive priming stimulus for a single intramuscular injection of acidic saline. A single intramuscular injection of acidic saline is a sub-threshold nociceptive stimulus; two injections, 5 days apart, are usually required to elicit long-lasting mechanical allodynia (Sluka et al., 2001). The combination of CORT+DFP with acidic saline induced bilateral allodynia (Figure 2B; time × CORT+DFP × acidic saline: F5, 120 = 8.49, P < 0.001; CORT+DFP × acidic saline: F1, 24 = 20.34, P = 0.001; time × acidic saline: F5, 120 = 9.50, P < 0.001; time × CORT+DFP: F5, 120 = 9.809, P < 0.001; acidic saline: F1, 24 = 14.61, P < 0.001; CORT+DFP: F1, 24 = 20.89, P < 0.001; time: F2.96, 70.99 = 12.90, P < 0.001). Following exposure to CORT+DFP, acidic saline induced allodynia at days 5 (P = 0.045), 10 (P < 0.001), and 30 (P = 0.004), compared to normal saline (Figure 2B). Neither CORT nor DFP alone with acidic saline was sufficient to alter mechanical thresholds (data not shown; CORT × acidic saline: F1, 14 = 0.19, P = 0.666; DFP × acidic saline: F1, 11 = 0.63, P = 0.445), indicating that the combination of insults is necessary to induce allodynia.
The combination of CORT+DFP with acidic saline did not induce thermal hyperalgesia (data not shown; time × CORT+DFP × acidic saline: F2, 42 = 0.83, P = 0.826; CORT+DFP × acidic saline: F1, 21 = 0.01, P = 0.947; time × acidic saline: F2, 42 = 2.94, P = 0.064; time × CORT+DFP: F2, 42 = 0.69, P = 0.504; acidic saline: F1, 21 = 1.64, P = 0.504; CORT+DFP: F1, 21 = 0.61, P = 0.445; time: F1.91, 40.13 = 0.91, P = 0.404).
3.2. Inflammatory signaling: dorsal spinal cord
Pro-inflammatory glial activation has been implicated in the CORT+DFP model of GWI (Koo et al., 2018; Locker et al., 2017; O’Callaghan et al., 2015). Here, we examined the influence of these challenges, combined with acidic saline, on several representative markers implicated previously in pain: microglia (CD11b) astrocytes (GFAP), the pattern recognition receptor Toll-like receptor 4 (TLR4), and proinflammatory cytokines (TNF, IL-1β and IL-6) (Beggs et al., 2012b; Grace et al., 2014, 2016b; McMahon et al., 2015; Ji et al., 2016; Inoue and Tsuda, 2018; Malcangio, 2019; Lacagnina et al., 2018). We observed increases in expression of Itgam (CD11b) at day 5 (Figure 3A; acidic saline × CORT+DFP: F1, 26 = 15.21, P < 0.001; acidic saline: F1, 26 = 30.81, P < 0.001; CORT+DFP: F1, 26 = 15.80, P < 0.001) and day 15 (Figure 3B; acidic saline × CORT+DFP: F1, 28 = 85.94, P < 0.001; acidic saline: F1, 28 = 56.59, P < 0.001; CORT+DFP: F1, 28 = 70.50, P < 0.001). Gfap was modestly elevated by the combination of CORT+DFP+Acidic saline at day 5 (Figure 3C; acidic saline × CORT+DFP: F1, 26 = 8.04, P = 0.008; acidic saline: F1, 26 = 3.60, P = 0.069; CORT+DFP: F1, 26 = 0.95, P = 0.338) and robustly at day 15 (Figure 3D; acidic saline × CORT+DFP: F1, 25 = 111.10, P < 0.001; acidic saline: F1, 25 = 86.10, P < 0.001; CORT+DFP: F1, 25 = 89.59, P < 0.001). Expression of Tlr4 was increased by the combined challenges at day 5 (Figure 3E; acidic saline × CORT+DFP: F1, 28 = 65.57, P < 0.001; acidic saline: F1, 28 = 50.10, P < 0.001; CORT+DFP: F1, 28 = 60.46, P < 0.001) and day 15 (Figure 3F; acidic saline × CORT+DFP: F1, 27 = 56.50, P < 0.001; acidic saline: F1, 27 = 65.63, P < 0.001; CORT+DFP: F1, 27 = 31.82, P < 0.001). We also observed increases in the proinflammatory cytokine IL-1β at day 5 (Figure 3G; acidic saline × CORT+DFP: F1, 28 = 24.46, P < 0.001; acidic saline: F1, 28 = 25.21, P < 0.001; CORT+DFP: F1, 28 = 38.69, P < 0.001) but not at day 15 (Figure 3H; acidic saline × CORT+DFP: F1, 21 = 0.66, P = 0.427; acidic saline: F1, 21 = 5.43, P = 0.030; CORT+DFP: F1, 21 = 0.01, P = 0.98). IL-6 levels were increased by CORT+DFP+Acidic saline at day 5 (Figure 3I; acidic saline × CORT+DFP: F1, 27 = 126.50, P < 0.001; acidic saline: F1, 27 = 119.60, P < 0.001; CORT+DFP: F1, 27 = 128.40, P < 0.001) and at day 15 (Figure 3J; acidic saline × CORT+DFP: F1, 28 = 391.50, P < 0.001; acidic saline: F1, 28 = 515.60, P < 0.001; CORT+DFP: F1, 28 = 361.80.59, P < 0.001). TNF was not detectable at either timepoint.
Figure 3. Neuroinflammatory markers in the lumbar dorsal spinal cord.
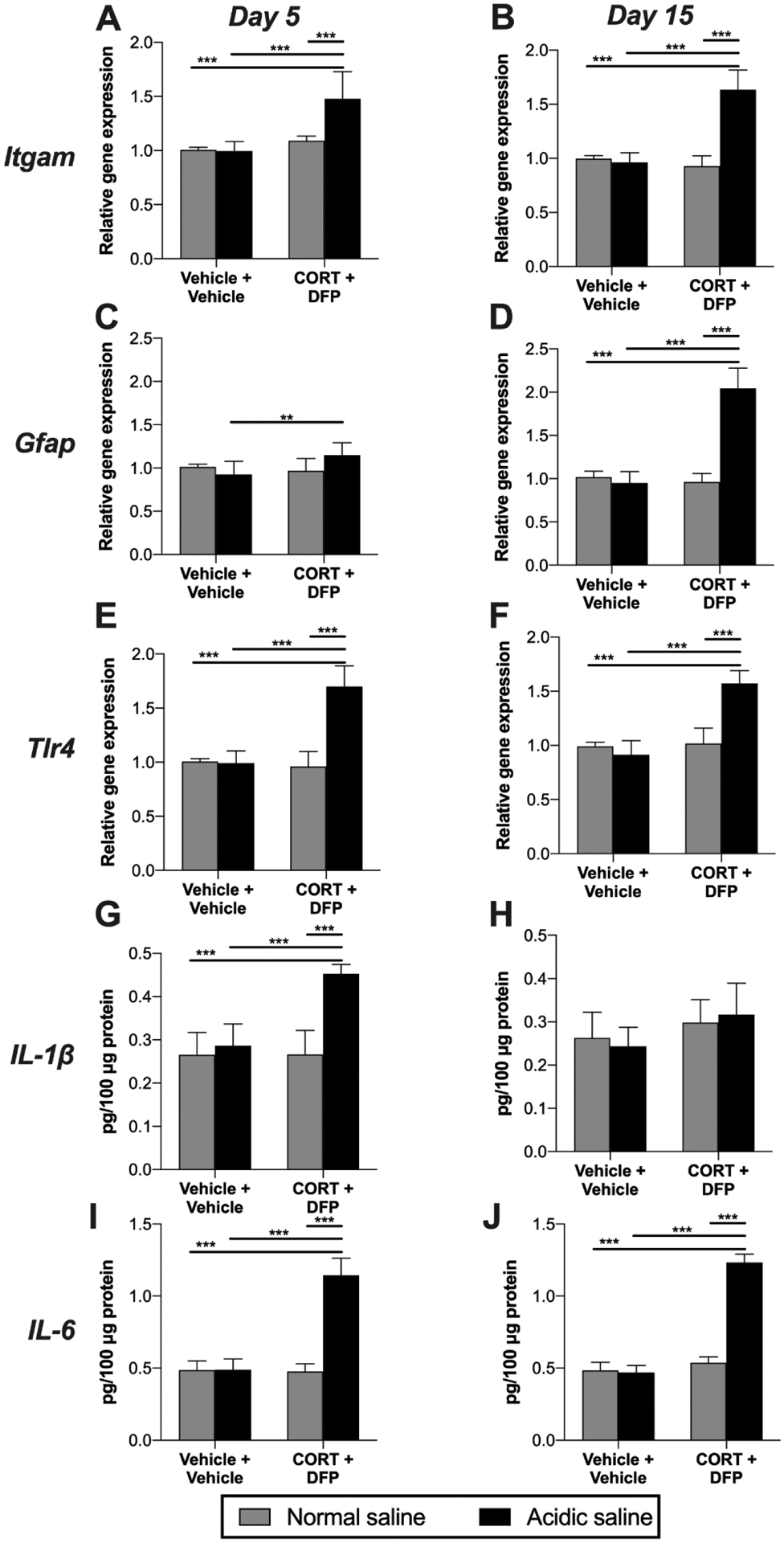
Corticosterone (CORT) or vehicle was administered via the drinking water for 7 days to mimic high physiological stress. On the final day of CORT treatment, a single dose of diisopropyl fluorophosphate (DFP) or vehicle was administered. Seven days later, rats received a single injection of normal (pH = 7.2) or acidic saline (pH = 4.0) into the left gastrocnemius muscle. Left L4/5 dorsal spinal cord quadrants were collected 5 or 15 days later. Expression levels of (A, B) Itgam (CD11b), (C, D) Gfap, and (E, F) Tlr4 were assessed. Protein levels of (G, H) IL-1β, and (I, J) IL-6 were also assayed. *P < 0.05, **P < 0.01, ***P < 0.001. N = 6–8/group. Data are mean ± SD.
3.3. Inflammatory signaling: dorsal root ganglia (DRG)
Neuroinflammatory mechanisms in the DRG also contribute to nociceptive hypersensitivity (Grace et al., 2016b; McMahon et al., 2015; Ji et al., 2016; Malcangio, 2019). We therefore assayed expression levels of representative markers of macrophages (CD11b) and satellite glia (GFAP), as well as protein levels of proinflammatory cytokines TNF, IL-1β and IL-6. The combination of CORT+DFP+Acidic saline increased expression of Itgam (CD11b) at day 5 (Figure 4A; acidic saline × CORT+DFP: F1, 27 = 22.37, P < 0.001; acidic saline: F1, 27 = 31.12, P < 0.001; CORT+DFP: F1, 27 = 44.05, P < 0.001) and day 15 (Figure 4B; acidic saline × CORT+DFP: F1, 28 = 21.69, P < 0.001; acidic saline: F1, 28 = 30.75, P < 0.001; CORT+DFP: F1, 28 = 17.12, P < 0.001). Gfap mRNA was also elevated by the combination of CORT+DFP+Acidic saline at day 5 (Figure 4C; acidic saline × CORT+DFP: F1, 24 = 14.53, P < 0.001; acidic saline: F1, 24 = 2.12, P = 0.158; CORT+DFP: F1, 24 = 24.31, P < 0.001) and day 15 (Figure 4D; acidic saline × CORT+DFP: F1, 24 = 12.35, P = 0.002; acidic saline: F1, 24 = 11.86, P = 0.002; CORT+DFP: F1, 24 = 11.39, P = 0.003). We also observed increases in proinflammatory cytokines. IL-1β was elevated by CORT+DFP+Acidic saline at day 5 (Figure 4E; acidic saline × CORT+DFP: F1, 23 = 15.69, P < 0.001; acidic saline: F1, 23 = 23.79, P < 0.001; CORT+DFP: F1, 23 = 36.09, P < 0.001) and to a lesser extent at day 15 (Figure 4F; acidic saline × CORT+DFP: F1, 25 = 5.38, P = 0.029; acidic saline: F1, 25 = 8.39, P = 0.008; CORT+DFP: F1, 25 = 7.59, P = 0.011). IL-6 levels were increased by CORT+DFP+Acidic saline at day 5 (Figure 4G; acidic saline × CORT+DFP: F1, 22 = 10.35, P = 0.004; acidic saline: F1, 22 = 8.20, P = 0.009; CORT+DFP: F1, 22 = 9.89, P = 0.005) and further elevated at day 15 (Figure 4H; acidic saline × CORT+DFP: F1, 24 = 36.16, P < 0.001; acidic saline: F1, 24 = 37.52, P < 0.001; CORT+DFP: F1, 24 = 26.94, P < 0.001). TNF was not detectable at either timepoint.
Figure 4. Levels of neuroinflammatory markers in DRG.
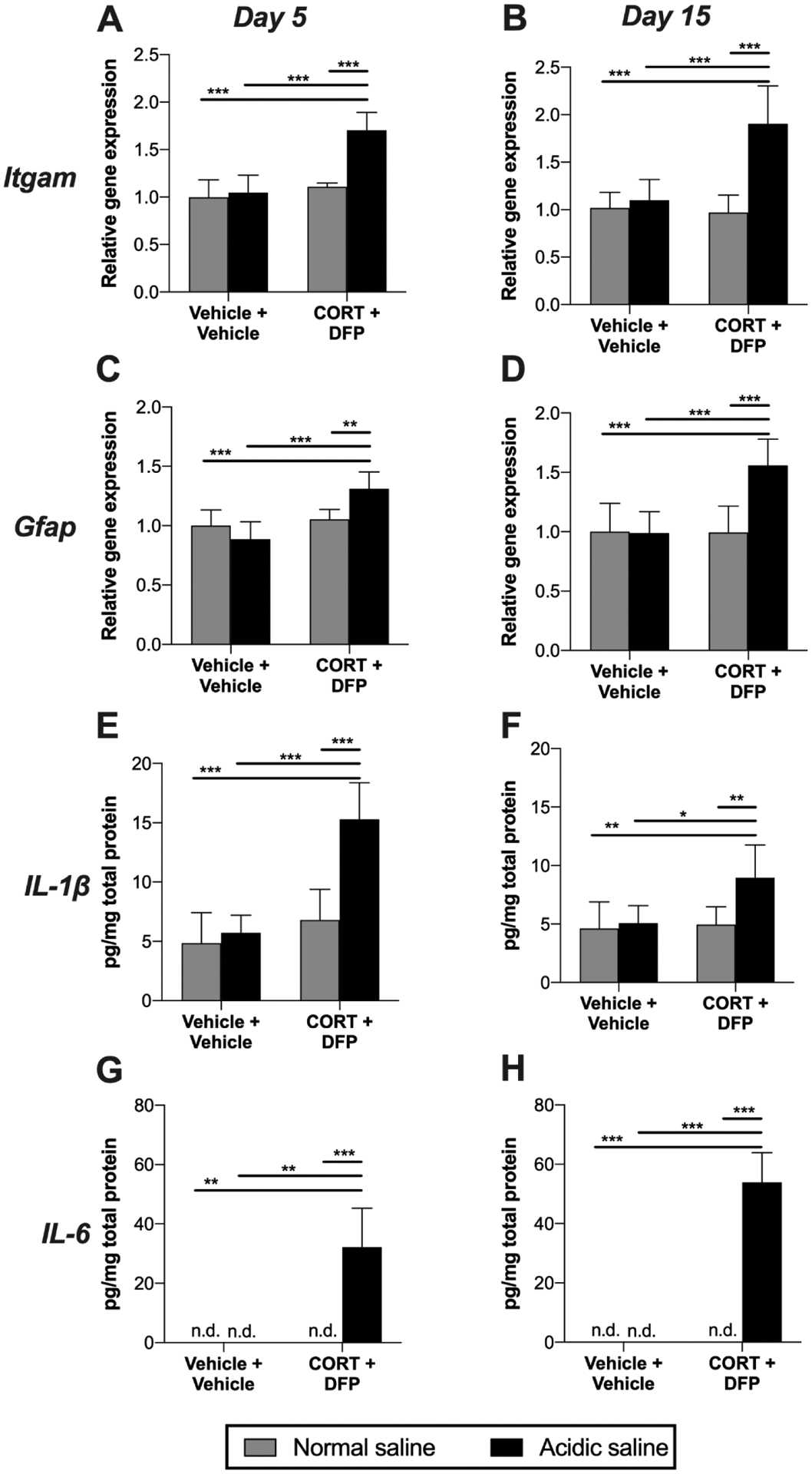
Corticosterone (CORT) or vehicle was administered via the drinking water for 7 days to mimic high physiological stress. On the final day of CORT treatment, a single dose of diisopropyl fluorophosphate (DFP) or vehicle was administered. Seven days later, rats received a single injection of normal (pH = 7.2) or acidic saline (pH = 4.0) into the left gastrocnemius muscle. Left L4/5 DRG were collected 5 or 15 days later. Expression levels of Itgam (CD11b) were determined at (A) day 5, (B) and day 15, as well as Gfap at (C) day 5, (D) and day 15. Protein levels of IL-1β were assayed at (E) day 5 (F) and day 15. IL-6 levels were also assayed at (G) day 5 (H) and day 15, using the lower limit of detection for statistical analysis of undetectable values. *P < 0.05, **P < 0.01, ***P < 0.001. N = 6–8/group. Data are mean ± SD.
3.4. Inflammatory signaling: gastrocnemius muscle
As pro-inflammatory cytokines are causally implicated in the acidic saline model of musculoskeletal pain (Sutton and Opp, 2015), we assayed TNF, IL-1β and IL-6 in the gastrocnemius muscle at days 5 and 15 after injection of acidic or normal saline. The combination of CORT+DFP+Acidic saline elevated IL-1β at day 5 (Figure 5A; acidic saline × CORT+DFP: F1, 24 = 25.25, P < 0.001; acidic saline: F1, 24 = 100.90, P < 0.001; CORT+DFP: F1, 24 = 97.07, P < 0.001) and day 15 (Figure 5B; acidic saline × CORT+DFP: F1, 22 = 12.58, P = 0.002; acidic saline: F1, 22 = 2.95, P = 0.100; CORT+DFP: F1, 22 = 2.32, P = 0.142). IL-6 was also elevated by the combination of CORT+DFP+Acidic saline at day 5 (Figure 5C; acidic saline × CORT+DFP: F1, 22 = 18.12, P < 0.001; acidic saline: F1, 25 = 14.15, P < 0.001; CORT+DFP: F1, 25 = 28.82, P < 0.001) and day 15 (Figure 5D; acidic saline × CORT+DFP: F1, 27 = 35.29, P < 0.001; acidic saline: F1, 27 = 43.23, P < 0.001; CORT+DFP: F1, 27 = 45.45, P < 0.001). TNF was again not detectable at either timepoint.
Figure 5. Pro-inflammatory cytokine levels in gastrocnemius muscle.
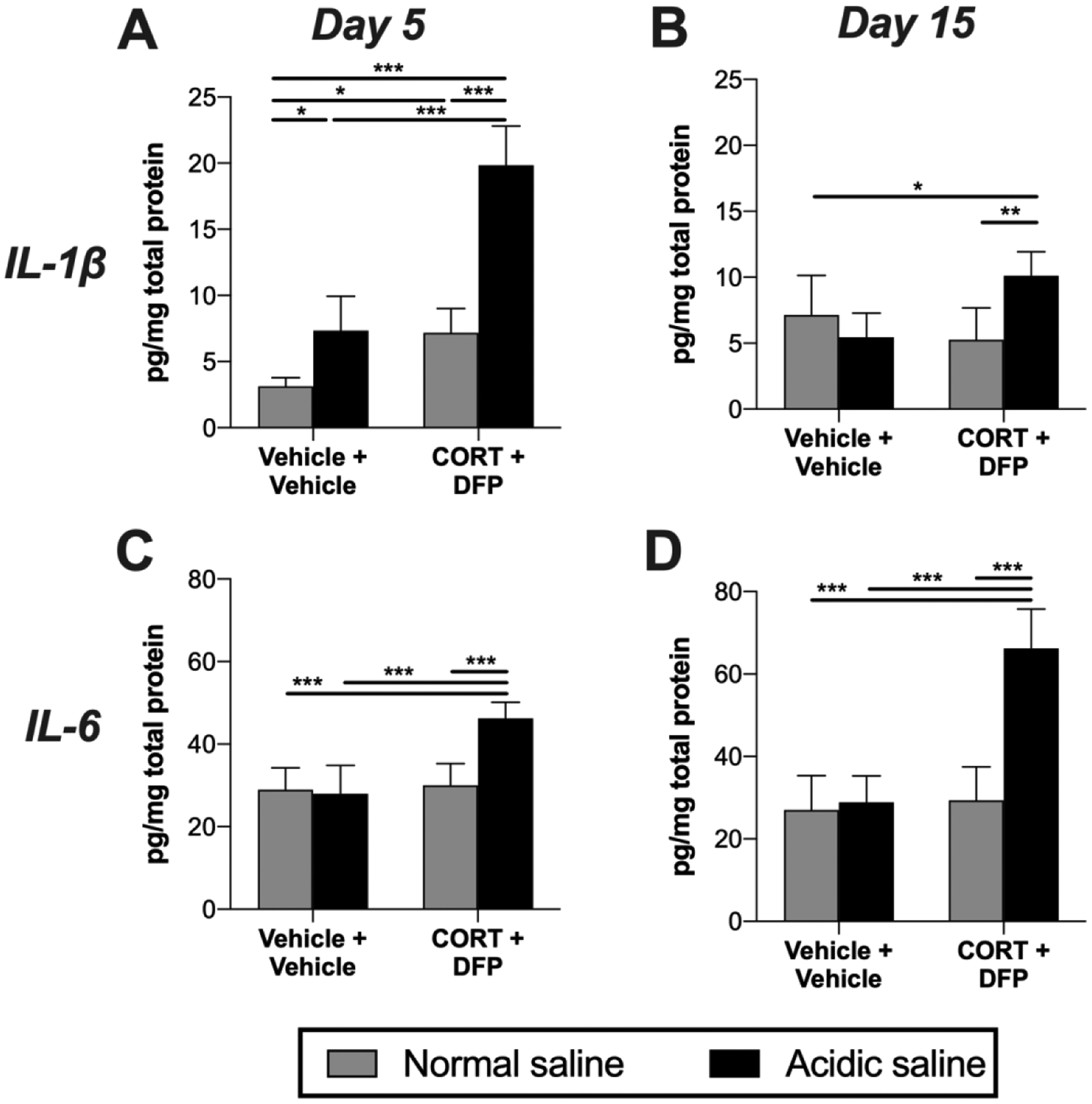
Corticosterone (CORT) or vehicle was administered via the drinking water for 7 days to mimic high physiological stress. On the final day of CORT treatment, a single dose of diisopropyl fluorophosphate (DFP) or vehicle was administered. Seven days later, rats received a single injection of normal (pH = 7.2) or acidic saline (pH = 4.0) into the left gastrocnemius muscle. Gastrocnemius muscle was collected 5 or 15 days later. (A) IL-1β levels were assayed at day 5, (B) and day 15. (C) IL-6 levels were assayed at day 5, (D) and day 15. *P < 0.05, **P < 0.01, ***P < 0.001. N = 6–8/group. Data are mean ± SD.
3.5. Sphingolipid signaling in the lumbar spinal cord and DRG
Given a role for sphingolipid signaling in pain (Patti et al., 2012; Stockstill et al., 2018) and GWI (Abdullah et al., 2013, 2012), we tested whether key sphingolipids were upregulated in the GWI model of pain. Analysis was performed on two key experimental groups (CORT+DFP+Acidic saline vs. Vehicle+Vehicle+Normal saline) at Day 5 only as a go/no-go for full analysis. Sphingolipid levels in the DRG and spinal cord did not significantly differ between treatment groups. Summary results are presented in Table 1. Transcript levels of sphingosine-1-phosphate receptor 1, previously implicated in pain (Stockstill et al., 2018), were not altered in DRG or lumbar spinal cord by any treatment group at any timepoint (data not shown).
Table 1. Sphingolipid levels in GWI model of pain.
Comparisons were made between CORT+DFP+Acidic saline vs. Vehicle+Vehicle+Normal saline at Day 5 after acidic saline/normal saline injection. Ipsilateral L4/5 DRG and ipsilateral L4/5 dorsal quadrants of the spinal cord were analyzed. Mean (SD). N=4/group.
| DRG | Spinal cord | |||
|---|---|---|---|---|
| Vehicle+Vehicle+Normal saline (nM/mg) | CORT+DFP+Acidic saline (nM/mg) | Vehicle+Vehicle+Normal saline (nM/mg) | CORT+DFP+Acidic saline (nM/mg) | |
| D-Sphingosine | 66.58 (19.17) | 36.22 (5.24) | 33.56 (1.76) | 30.19 (7.83) |
| Sphingosine-1-phosphate | 995.13 (455.73) | 946.78 (299.96) | 514.10 (178.05) | 535.47 (113.43) |
| C12-Ceramide | 44.90 (24.48) | 24.73 (3.42) | 10.95 (1.16) | 8.08 (1.43) |
| C16-Ceramide | 607.20 (36.98) | 336.51 (139.88) | 314.98 (44.49) | 283.45 (50.72) |
| C18-Ceramide | 7906.77 (5084.76) | 3879.24 (1040.64) | 715.75 (186.70) | 465.88 (120.96) |
| C24-Ceramide | 97.75 (48.82) | 49.04 (15.07) | 33.5075 (7.08) | 31.00 (18.00) |
3.6. Effects of immunomodulators on behavior in the putative GWI model of pain
The biochemical analyses indicated a role for pro-inflammatory signaling, including microglia/macrophages and TLR4. We therefore tested the anti-allodynic action of several immunomodulators that can resolve such inflammatory signaling (Grace et al., 2014; Hutchinson et al., 2008a, 2008b; Kwilasz et al., 2015; Milligan et al., 2006). The microglia/macrophage inhibitor minocycline attenuated allodynia induced by the combination of CORT, DFP, and acidic saline (Figure 6A; time × CORT+DFP+Acidic saline × treatment: F6, 100 = 12.67, P < 0.001; CORT+DFP+Acidic saline × treatment: F1, 20 = 50.09, P < 0.001; time × treatment: F5, 100 = 16.62, P < 0.001; time × CORT+DFP+Acidic saline: F5, 100 = 13.46, P < 0.001; treatment: F1, 20 = 44.32, P < 0.001; CORT+DFP+Acidic saline: F1, 20 = 413.50, P < 0.001; time: F3.58, 71.53 = 23.99, P < 0.001). With the 7-day systemic treatment beginning on day 5 after acidic saline, allodynia was reversed on days 10 (P = 0.049) and 12 (P < 0.001), persisting after treatment had concluded on 14 (P < 0.001), but returning to pre-treatment levels by day 16.
Figure 6. Effects of immunomodulators on allodynia in a putative model of GWI pain.
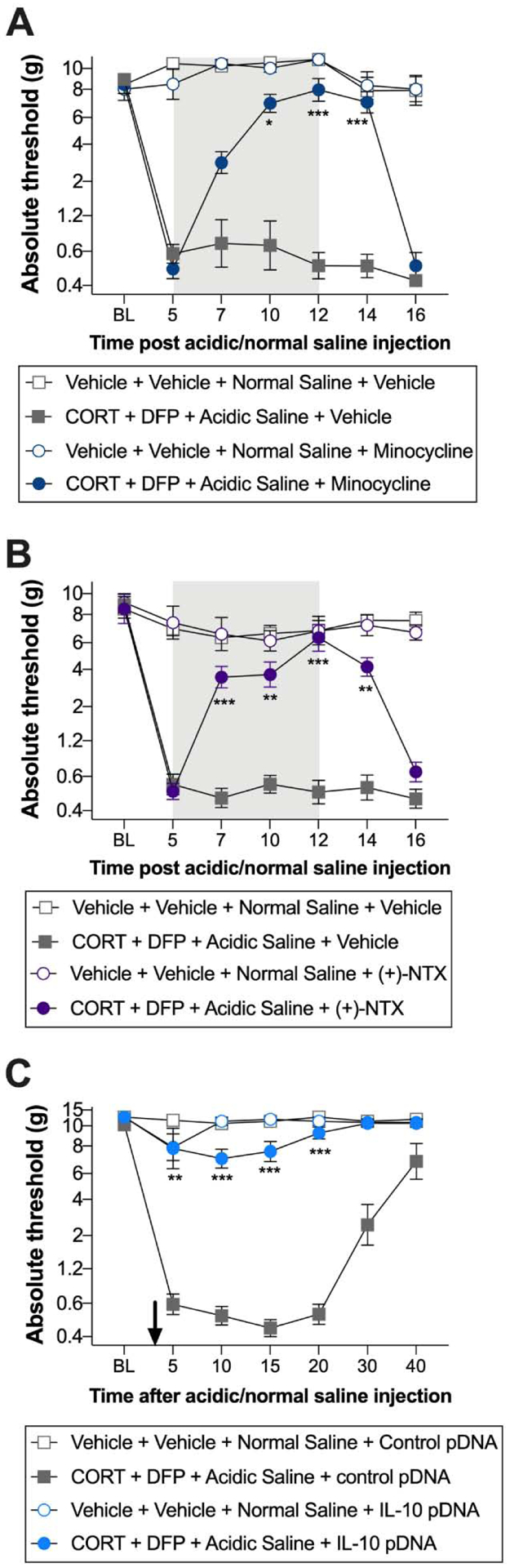
Corticosterone (CORT) or vehicle was administered via the drinking water for 7 days to mimic high physiological stress. On the final day of CORT treatment, a single dose of diisopropyl fluorophosphate (DFP) or vehicle was administered. Seven days later, rats received a single injection of normal (pH = 7.2) or acidic saline (pH = 4.0) into the left gastrocnemius muscle. Allodynia was attenuated by (A) treatment with the macrophage/microglia inhibitor minocycline (50 mg/kg once per day), beginning on day 5 and concluding on day 12 (indicated by the gray rectangle), (B) the TLR4 inhibitor (+)-naltrexone (6 mg/kg three times per day), beginning on day 5 and concluding on day 12 (indicated by the gray rectangle), and (C) IL-10 gene therapy (3 μg of plasmid DNA with 25 μg D-mannose), intrathecally administered on day 3 (indicated by the arrow). *P < 0.05, **P < 0.01, ***P < 0.001. N = 6–8/group. Data are mean ± SD.
Systemic treatment with the TLR4 inhibitor (+)-naltrexone also reversed allodynia induced by CORT, DFP and acidic saline (Figure 6B; time × CORT+DFP+Acidic saline × treatment: F5, 100 = 13.00, P < 0.001; CORT+DFP+Acidic saline × treatment: F1, 20 = 41.36, P < 0.001; time × treatment: F5, 100 = 12.72, P < 0.001; time × CORT+DFP+Acidic saline: F5, 100 = 15.08, P < 0.001; treatment: F1, 20 = 36.92, P < 0.001; CORT+DFP+Acidic saline: F1, 20 = 280.60, P < 0.001; time: F3.91, 78.11 = 11.31, P < 0.001). The 7-day treatment began on day 5 after acidic saline, reversing allodynia on days 7 (P < 0.001), 10 (P = 0.004) and 12 (P < 0.001), and after treatment had concluded on day 14 (P = 0.002). However, mechanical thresholds returned to pre-treatment levels by day 16.
IL-10 gene therapy was administered by acute intrathecal injection on day 3 after induction of allodynia by the combination of CORT, DFP and acidic saline (Figure 6C; time × CORT+DFP+Acidic saline × treatment: F5, 100 = 10.43, P < 0.001; CORT+DFP+Acidic saline × treatment: F1, 20 = 243.00, P < 0.001; time × treatment: F5, 100 = 9.72, P < 0.001; time × CORT+DFP+Acidic saline: F5, 100 = 19.31, P < 0.001; treatment: F1, 20 = 208.20, P < 0.001; CORT+DFP+Acidic saline: F1, 20 = 350.40, P < 0.001; time: F1.95, 38.89 = 21.44, P < 0.001). Rats treated with IL-10 gene therapy did not develop allodynia, with significant differences between the control pDNA group at days 5 (P = 0.010), 10 (P < 0.001), 15 (P < 0.001), and 20 (P < 0.001).
4. Discussion
In this study, we observed no changes in hindpaw nociceptive hypersensitivity after rats were treated with CORT, followed by exposure to the sarin surrogate DFP. However, robust and persistent hindpaw allodynia developed when these challenges were followed by a single, sub-threshold intramuscular injection of acidic saline. The allodynia was associated with neuroinflammation in the L4/5 dorsal spinal cord and DRG, as well as induction of cytokines in the gastrocnemius muscle. Finally, we showed that treatment with either minocycline, the TLR4 antagonist (+)-naltrexone (the opioid receptor-inactive isomer), or IL-10 pDNA all attenuated allodynia associated with the model of GWI.
Musculoskeletal pain is a principal symptom of GWI, yet effects in prior rodent models are subtle in the absence of an appropriate pain-inducing challenge. For example, prolonged exposure to chemical agents like chlorpyrifos, permethrin, or pyridostigmine bromide alters home-cage activity and is associated with pro-nociceptive neurophysiological changes, but nociceptive hypersensitivity is absent or modest (Cooper et al., 2018; Flunker et al., 2017; Nizamutdinov et al., 2018; Nutter et al., 2015). Our results largely agree with these reports. Robust mechanical allodynia was only induced after a single, normally sub-threshold, intramuscular injection of acidic saline. These findings suggest that additional factors besides exposure to chemicals and high stress may be necessary for the development of pain related to GWI. This idea is concordant with the clinical manifestation, as some, but not all veterans with GWI suffer from chronic, widespread pain (Cory-Slechta and Wedge, 2016; Stimpson et al., 2006). There is support in the literature for the premise that repeated challenges can interact to cause persistent pain (Beggs et al., 2012a; Grace et al., 2016c; Melemedjian et al., 2010; Reichling and Levine, 2009; Sutton and Opp, 2014; Wang et al., 2013). Thus, exposures in the Gulf War theater may have increased the vulnerability of veterans to develop persistent pain in response to subsequent sub-threshold nociceptive stimuli. Such stimuli could be related to the rigors of physical training and deployment (Gregory et al., 2016; Issberner et al., 1996; Woo et al., 2004). In contrast to the clinical manifestation of GWI pain, the nociceptive hypersensitivity resolved after a month in our model. This suggests that additional maintenance factors may be required, for example, continued exposure to stressors (Kelly et al., 2018).
The expression of nociceptive hypersensitivity in our model of CORT + DFP + Acidic saline is similar in some aspects to the original repeated acidic saline model (Sluka et al., 2001). That is, allodynia is bilateral, and heat hyperalgesia does not develop (Sluka et al., 2001, 2002, 2003; Sharma et al., 2009; Sutton and Opp, 2015). Some common underlying mechanisms may also be engaged, as we report increases in muscle IL-1β and IL-6, but no changes in TNF levels, as observed previously in the repeated acidic saline model (Sutton and Opp, 2015). However, there are also important differences. In contrast to our results, a previous study found little evidence of glial activation in the lumbar spinal cord after repeated acidic saline (Ledeboer et al., 2006). Where we found increased IL-1β mRNA in the lumbar spinal cord and a therapeutic effect of IL-10 pDNA in our model, intrathecal administration of several immunomodulators, including IL-1ra and IL-10 pDNA, had no effect on mechanical thresholds after repeated acidic saline (Ledeboer et al., 2006). While neuroinflammatory mechanisms evidently do not contribute to the central sensitization responsible for maintaining the long-lasting allodynia after repeated acidic saline (Sluka et al., 2001, 2003; Ledeboer et al., 2006), the prior exposure to CORT and DFP may alter the spinal cord microenvironment differentially to facilitate neuroimmune activation in the spinal cord.
Previous studies have shown that markers of neuroinflammation are elevated throughout the brain at acute timepoints (6 h) after CORT and DFP exposure (Ashbrook et al., 2018; Craddock et al., 2018; Koo et al., 2018; Locker et al., 2017; O’Callaghan et al., 2015). When we assessed the spinal cord and DRG 12 days after DFP, we saw little evidence of neuroinflammation. However, markers for glial activation, TLR4 signaling, and cytokine induction—which have causal roles in other types of pain (Beggs et al., 2012b; Grace et al., 2014, 2016b; McMahon et al., 2015; Ji et al., 2016; Inoue and Tsuda, 2018; Malcangio, 2019; Lacagnina et al., 2018)—were persistently induced following intramuscular acidic saline. Some of these markers may be temporally regulated, with an early preference towards IL-1β and later induction of IL-6 and astrocyte/satellite glia activation. While sphingolipid signaling, initiated by glia, is induced in other models of GWI and contributes to other forms of pain (Abdullah et al., 2013, 2012; Patti et al., 2012; Stockstill et al., 2018), we did not detect changes in these pathways at the timepoints or tissues assessed. However, the neuroinflammatory signaling induced through microglia/macrophages and TLR4 is causally implicated in our model of GWI pain, as allodynia was alleviated by treatment with minocycline, (+)-naltrexone, and IL-10 pDNA. Together, these results point to dysregulated immunity following CORT and DFP exposure that is unmasked following additional challenges.
A limitation of our study is the inclusion of only male rats, especially considering that females are at greater risk of developing moderate to severe GWI (Heboyan et al., 2019). There are numerous sex differences with respect to immune responses after injury, including those relevant to pain (e.g., (Mapplebeck et al., 2018; Sorge et al., 2015; Taves et al., 2016; Tawfik et al., 2020)). As it cannot be assumed that neuroinflammatory mechanisms are sexually monomorphic, future studies should include female rodents.
In conclusion, widespread musculoskeletal pain is a cardinal symptom of Gulf War Illness. We have reproduced such pain by modelling some of the chemical and stress exposures that occurred in theater, lending further support to the “multiple hit” hypothesis of GWI (Janulewicz et al., 2018). In addition to pain, neuroinflammation underlies other symptoms of GWI in animal models, such impaired memory, depression, anxiety, lethargy, with support from clinical studies in veterans with GWI (Abdullah et al., 2012; Alhasson et al., 2017; Hernandez et al., 2019; Michalovicz et al., 2020; Parihar et al., 2013; White et al., 2016; Zakirova et al., 2015; Alshelh et al., 2020; Broderick et al., 2013; Emmerich et al., 2017; Joshi et al., 2019; Parkitny et al., 2015). Strategies to alleviate neuroinflammation may therefore have efficacy in alleviating several symptoms of GWI, including pain.
Highlights.
A neuroinflammatory basis for pain associated with GWI has not been investigated.
A combination of corticosterone, diisopropyl fluorophosphate, and intramuscular acidic saline induces long-lasting, bilateral, musculoskeletal pain.
The pain is associated with elevated neuroinflammatory markers in the spinal cord, dorsal root ganglia, and gastrocnemius muscle.
Minocycline, the TLR4 inhibitor (+)-naltrexone, and IL-10 pDNA each reversed the musculoskeletal pain.
Acknowledgements
This work was supported by U.S. Army Medical Research and Development Command grant W81XWH-16-1-0717 / GW150187 (P.M.G.), Congressionally Directed Medical Research Program Consortium award W81XWH-13-2-0072 / GW120037 (K.S.), University of Texas Rising STARS Award (P.M.G), National Institutes of Health grants CA016672, S10OD012304-01, U01CA235510, and P30CA016672 (MD Anderson Cancer Center), Cancer Prevention Research Institute of Texas grant RP130397 (MD Anderson Cancer Center), and by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse Intramural Research Programs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullah L, Evans JE, Bishop A, Reed JM, Crynen G, Phillips J, Pelot R, Mullan MA, Ferro A, Mullan CM, Mullan MJ, Ait-Ghezala G, Crawford FC, 2012. Lipidomic Profiling of Phosphocholine Containing Brain Lipids in Mice with Sensorimotor Deficits and Anxiety-Like Features After Exposure to Gulf War Agents. Neuromol Med 14, 349–361. 10.1007/s12017-012-8192-z [DOI] [PubMed] [Google Scholar]
- Abdullah L, Evans JE, Montague H, Reed JM, Moser A, Crynen G, Gonzalez A, Zakirova Z, Ross I, Mullan C, Mullan M, Ait-Ghezala G, Crawford F, 2013. Chronic elevation of phosphocholine containing lipids in mice exposed to Gulf War agents pyridostigmine bromide and permethrin. Neurotoxicol Teratol 40, 74–84. 10.1016/j.ntt.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Alhasson F, Das S, Seth R, Dattaroy D, Chandrashekaran V, Ryan CN, Chan LS, Testerman T, Burch J, Hofseth LJ, Horner R, Nagarkatti M, Nagarkatti P, Lasley SM, Chatterjee S, 2017. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS ONE 12, e0172914 10.1371/journal.pone.0172914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshelh Z, Albrecht DS, Bergan C, Akeju O, Clauw DJ, Conboy L, Edwards RR, Kim M, Lee YC, Protsenko E, Napadow V, Sullivan K, Loggia ML, 2020. In-vivo imaging of neuroinflammation in veterans with Gulf War illness. Brain Behav. Immun 87, 498–507. 10.1016/j.bbi.2020.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbrook DG, Hing B, Michalovicz LT, Kelly KA, Miller JV, de Vega WC, Miller DB, Broderick G, O’Callaghan JP, McGowan PO, 2018. Epigenetic impacts of stress priming of the neuroinflammatory response to sarin surrogate in mice: a model of Gulf War illness. J Neuroinflammation 15, 86 10.1186/s12974-018-1113-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Currie G, Salter MW, Fitzgerald M, Walker SM, 2012a. Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain 135, 404–417. 10.1093/brain/awr288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Trang T, Salter MW, 2012b. P2X4R+ microglia drive neuropathic pain. Nat. Neurosci 15, 1068–1073. 10.1038/nn.3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick G, Ben-Hamo R, Vashishtha S, Efroni S, Nathanson L, Barnes Z, Fletcher MA, Klimas N, 2013. Altered immune pathway activity under exercise challenge in Gulf War Illness: An exploratory analysis. Brain, Behavior, and Immunity 28, 159–169. 10.1016/j.bbi.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, 1994. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63. [DOI] [PubMed] [Google Scholar]
- Cooper BY, Flunker LD, Johnson RD, Nutter TJ, 2018. Behavioral, cellular and molecular maladaptations covary with exposure to pyridostigmine bromide in a rat model of gulf war illness pain. Toxicol. Appl. Pharmacol 352, 119–131. 10.1016/j.taap.2018.05.023 [DOI] [PubMed] [Google Scholar]
- Cory-Slechta D, Wedge R (Eds.), 2016. Gulf War and Health: Volume 10: Update of Health Effects of Serving in the Gulf War. National Academies Press (US). [PubMed] [Google Scholar]
- Craddock TJA, Michalovicz LT, Kelly KA, Rice MA, Miller DB, Klimas NG, Morris M, O’Callaghan JP, Broderick G, 2018. A Logic Model of Neuronal-Glial Interaction Suggests Altered Homeostatic Regulation in the Perpetuation of Neuroinflammation. Front Cell Neurosci 12, 336 10.3389/fncel.2018.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler EC, Alberti LA, Bowman BN, Kerwin AA, Wilkerson JL, Moezzi DR, Limanovich E, Wallace JA, Milligan ED, 2014. Improvement of spinal non-viral IL-10 gene delivery by D-mannose as a transgene adjuvant to control chronic neuropathic pain. J Neuroinflammation 11, 92 10.1186/1742-2094-11-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis A, Wieseler J, Favret J, Johnson KW, Rice KC, Maier SF, Falci S, Watkins LR, 2014. Systemic administration of propentofylline, ibudilast, and (+)-naltrexone each reverses mechanical allodynia in a novel rat model of central neuropathic pain. J Pain 15, 407–421. 10.1016/j.jpain.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerich T, Zakirova Z, Klimas N, Sullivan K, Shetty AK, Evans JE, Ait-Ghezala G, Laco GS, Hattiangady B, Shetty GA, Mullan M, Crynen G, Abdullah L, Crawford F, 2017. Phospholipid profiling of plasma from GW veterans and rodent models to identify potential biomarkers of Gulf War Illness. PLoS ONE 12, e0176634 10.1371/journal.pone.0176634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flunker LK, Nutter TJ, Johnson RD, Cooper BY, 2017. DEET potentiates the development and persistence of anticholinesterase dependent chronic pain signs in a rat model of Gulf War Illness pain. Toxicol. Appl. Pharmacol 316, 48–62. 10.1016/j.taap.2016.12.014 [DOI] [PubMed] [Google Scholar]
- Frank MG, Annis JL, Watkins LR, Maier SF, 2019. Glucocorticoids mediate stress induction of the alarmin HMGB1 and reduction of the microglia checkpoint receptor CD200R1 in limbic brain structures. Brain Behav. Immun 80, 678–687. 10.1016/j.bbi.2019.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF, 2015. The permissive role of glucocorticoids in neuroinflammatory priming: mechanisms and insights. Curr Opin Endocrinol Diabetes Obes 22, 300–305. 10.1097/MED.0000000000000168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R, 2013. Objective evidence of autonomic dysfunction and the role of stress in the Gulf War syndrome. JAMA Neurol 70, 158–159. 10.1001/jamaneurol.2013.1494 [DOI] [PubMed] [Google Scholar]
- Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, Noah DL, Barrett DH, Randall B, Herwaldt BL, Mawle AC, Reeves WC, 1998. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA 280, 981–988. 10.1001/jama.280.11.981 [DOI] [PubMed] [Google Scholar]
- Golomb BA, 2008. Acetylcholinesterase inhibitors and Gulf War illnesses. PNAS 105, 4295–4300. 10.1073/pnas.0711986105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Fabisiak TJ, Green-Fulgham SM, Anderson ND, Strand KA, Kwilasz AJ, Galer EL, Walker FR, Greenwood BN, Maier SF, Fleshner M, Watkins LR, 2016a. Prior voluntary wheel running attenuates neuropathic pain. Pain 157, 2012–2023. 10.1097/j.pain.0000000000000607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Gaudet AD, Staikopoulos V, Maier SF, Hutchinson MR, Salvemini D, Watkins LR, 2016b. Nitroxidative Signaling Mechanisms in Pathological Pain. Trends Neurosci. 39, 862–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR, 2014. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol 14, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Loram LC, Christianson JP, Strand KA, Flyer-Adams JG, Penzkover KR, Forsayeth JR, van Dam A-M, Mahoney MJ, Maier SF, Chavez RA, Watkins LR, 2017. Behavioral assessment of neuropathic pain, fatigue, and anxiety in experimental autoimmune encephalomyelitis (EAE) and attenuation by interleukin-10 gene therapy. Brain Behav. Immun 59, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR, 2016c. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. U.S.A 113, E3441–3450. 10.1073/pnas.1602070113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory NS, Brito RG, Fusaro MCGO, Sluka KA, 2016. ASIC3 Is Required for Development of Fatigue-Induced Hyperalgesia. Molecular Neurobiology 53, 1020–1030. 10.1007/s12035-014-9055-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J, 1988. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88. [DOI] [PubMed] [Google Scholar]
- Harvey LO, 1986. Efficient estimation of sensory thresholds. Behavior Research Methods, Instruments, & Computers 18, 623–632. 10.3758/BF03201438 [DOI] [Google Scholar]
- Heboyan V, Krengel MH, Sullivan K, Iobst S, Klimas N, Wilson C, Coughlin SS, 2019. Sex Differences in Gulf War Illness: A Reanalysis of Data From the CDC Air Force Study Using CDC and Modified Kansas Case Definitions. J. Occup. Environ. Med 61, 610–616. 10.1097/JOM.0000000000001620 [DOI] [PubMed] [Google Scholar]
- Heng HHQ, 2016. Challenges and new strategies for Gulf War illness research. Environmental disease 1, 118–125. [Google Scholar]
- Hernandez S, Fried DE, Grubišić V, McClain JL, Gulbransen BD, 2019. Gastrointestinal neuroimmune disruption in a mouse model of Gulf War illness. FASEB J. 33, 6168–6184. 10.1096/fj.201802572R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Chao LW, Kearney JJ, Zhang Y, Berkelhammer DL, Loram LC, Rozeske RR, Bland ST, Maier SF, Gleeson TT, Watkins LR, 2008a. Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav. Immun 22, 1248–1256. 10.1016/j.bbi.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR, 2008b. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur. J. Neurosci 28, 20–29. 10.1111/j.1460-9568.2008.06321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannacchione VG, Dever JA, Bann CM, Considine KA, Creel D, Carson CP, Best H, Haley RW, 2011. Validation of a research case definition of Gulf War illness in the 1991 US military population. Neuroepidemiology 37, 129–140. 10.1159/000331478 [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M, 2018. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci 19, 138–152. 10.1038/nrn.2018.2 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, 2014. Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined, Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined. National Academies Press (US). [PubMed] [Google Scholar]
- Institute of Medicine, 2013. Gulf War and Health: Treatment for Chronic Multisymptom Illness. National Academies Press (US), Washington (DC). [PubMed] [Google Scholar]
- Issberner U, Reeh PW, Steen KH, 1996. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neuroscience Letters 208, 191–194. 10.1016/0304-3940(96)12576-3 [DOI] [PubMed] [Google Scholar]
- Janulewicz P, Krengel M, Quinn E, Heeren T, Toomey R, Killiany R, Zundel C, Ajama J, O’Callaghan J, Steele L, Klimas N, Sullivan K, 2018. The Multiple Hit Hypothesis for Gulf War Illness: Self-Reported Chemical/Biological Weapons Exposure and Mild Traumatic Brain Injury. Brain Sci 8 10.3390/brainsci8110198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R-R, Chamessian A, Zhang Y-Q, 2016. Pain regulation by non-neuronal cells and inflammation. Science 354, 572–577. 10.1126/science.aaf8924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Hodgins-Vermaas R, McCartney H, Everitt B, Beech C, Poynter D, Palmer I, Hyams K, Wessely S, 2002. Post-combat syndromes from the Boer war to the Gulf war: a cluster analysis of their nature and attribution. BMJ 324, 321–324. 10.1136/bmj.324.7333.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi U, Pearson A, Evans JE, Langlois H, Saltiel N, Ojo J, Klimas N, Sullivan K, Keegan AP, Oberlin S, Darcey T, Cseresznye A, Raya B, Paris D, Hammock B, Vasylieva N, Hongsibsong S, Stern LJ, Crawford F, Mullan M, Abdullah L, 2019. A permethrin metabolite is associated with adaptive immune responses in Gulf War Illness. Brain Behav. Immun 81, 545–559. 10.1016/j.bbi.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KA, Michalovicz LT, Miller JV, Castranova V, Miller DB, O’Callaghan JP, 2018. Prior exposure to corticosterone markedly enhances and prolongs the neuroinflammatory response to systemic challenge with LPS. PloS One 13, e0190546 10.1371/journal.pone.0190546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo B-B, Michalovicz LT, Calderazzo S, Kelly KA, Sullivan K, Killiany RJ, O’Callaghan JP, 2018. Corticosterone potentiates DFP-induced neuroinflammation and affects high-order diffusion imaging in a rat model of Gulf War Illness. Brain Behav. Immun 67, 42–46. 10.1016/j.bbi.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR, 2015. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology 96, 55–69. 10.1016/j.neuropharm.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina MJ, Kopec AM, Cox SS, Hanamsagar R, Wells C, Slade S, Grace PM, Watkins LR, Levin ED, Bilbo SD, 2017. Opioid Self-Administration is Attenuated by Early-Life Experience and Gene Therapy for Anti-Inflammatory IL-10 in the Nucleus Accumbens of Male Rats. Neuropsychopharmacology 42, 2128–2140. 10.1038/npp.2017.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina MJ, Watkins LR, Grace PM, 2018. Toll-like receptors and their role in persistent pain. Pharmacol. Ther 184, 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A, Mahoney JH, Milligan ED, Martin D, Maier SF, Watkins LR, 2006. Spinal cord glia and interleukin-1 do not appear to mediate persistent allodynia induced by intramuscular acidic saline in rats. J Pain 7, 757–767. 10.1016/j.jpain.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Li J, Ma J, Lacagnina MJ, Lorca S, Odem MA, Walters ET, Kavelaars A, Grace PM, 2020. Oral Dimethyl Fumarate Reduces Peripheral Neuropathic Pain in Rodents via NFE2L2 Antioxidant Signaling. Anesthesiology 132, 343–356. 10.1097/ALN.0000000000003077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker AR, Michalovicz LT, Kelly KA, Miller JV, Miller DB, O’Callaghan JP, 2017. Corticosterone primes the neuroinflammatory response to Gulf War Illness-relevant organophosphates independently of acetylcholinesterase inhibition. J. Neurochem 142, 444–455. 10.1111/jnc.14071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhu LN, Attaluri S, Kodali M, Shuai B, Upadhya R, Gitai D, Shetty AK, 2019. Neuroinflammation in Gulf War Illness is linked with HMGB1 and complement activation, which can be discerned from brain-derived extracellular vesicles in the blood. Brain Behav. Immun 81, 430–443. 10.1016/j.bbi.2019.06.040 [DOI] [PubMed] [Google Scholar]
- Malcangio M, 2019. Role of the immune system in neuropathic pain. Scand J Pain 20, 33–37. 10.1515/sjpain-2019-0138 [DOI] [PubMed] [Google Scholar]
- Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, Halievski K, Assi S, Mogil JS, Trang T, Salter MW, 2018. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain. 10.1097/j.pain.0000000000001265 [DOI] [PubMed] [Google Scholar]
- McMahon SB, La Russa F, Bennett DLH, 2015. Crosstalk between the nociceptive and immune systems in host defence and disease. Nat. Rev. Neurosci 16, 389–402. 10.1038/nrn3946 [DOI] [PubMed] [Google Scholar]
- Melemedjian OK, Asiedu MN, Tillu DV, Peebles KA, Yan J, Ertz N, Dussor GO, Price TJ, 2010. IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J. Neurosci 30, 15113–15123. 10.1523/JNEUROSCI.3947-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovicz LT, Kelly KA, Sullivan K, O’Callaghan JP, 2020. Acetylcholinesterase inhibitor exposures as an initiating factor in the development of Gulf War Illness, a chronic neuroimmune disorder in deployed veterans. Neuropharmacology 171, 108073 10.1016/j.neuropharm.2020.108073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovicz LT, Locker AR, Kelly KA, Miller JV, Barnes Z, Fletcher MA, Miller DB, Klimas NG, Morris M, Lasley SM, O’Callaghan JP, 2019. Corticosterone and pyridostigmine/DEET exposure attenuate peripheral cytokine expression: Supporting a dominant role for neuroinflammation in a mouse model of Gulf War Illness. Neurotoxicology 70, 26–32. 10.1016/j.neuro.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR, 2000. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 861, 105–116. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR, 2001. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J. Neurosci 21, 2808–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, Mahoney JH, Levkoff LH, Maier SF, Cruz PE, Flotte TR, Johnson KW, Mahoney MM, Chavez RA, Leinwand LA, Watkins LR, 2006. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain 126, 294–308. 10.1016/j.pain.2006.07.009 [DOI] [PubMed] [Google Scholar]
- Nizamutdinov D, Mukherjee S, Deng C, Stauss HM, Shapiro LA, 2018. Gulf War agents pyridostigmine bromide and permethrin cause hypersensitive nociception that is restored after vagus nerve stimulation. Neurotoxicology 69, 93–96. 10.1016/j.neuro.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Nutter TJ, Johnson RD, Cooper BY, 2015. A delayed chronic pain like condition with decreased Kv channel activity in a rat model of Gulf War Illness pain syndrome. Neurotoxicology 51, 67–79. 10.1016/j.neuro.2015.09.010 [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Kelly KA, Locker AR, Miller DB, Lasley SM, 2015. Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War Illness. J. Neurochem 133, 708–721. 10.1111/jnc.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Shuai B, Shetty AK, 2013. Mood and Memory Deficits in a Model of Gulf War Illness Are Linked with Reduced Neurogenesis, Partial Neuron Loss, and Mild Inflammation in the Hippocampus. Neuropsychopharmacology 38, 2348–2362. 10.1038/npp.2013.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkitny L, Middleton S, Baker K, Younger J, 2015. Evidence for abnormal cytokine expression in Gulf War Illness: A preliminary analysis of daily immune monitoring data. BMC Immunol. 16, 57 10.1186/s12865-015-0122-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti GJ, Yanes O, Shriver L, Courade J-P, Tautenhahn R, Manchester M, Siuzdak G, 2012. Metabolomics Implicates Altered Sphingolipids in Chronic Pain of Neuropathic Origin. Nat Chem Biol 8, 232–234. 10.1038/nchembio.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell TM, Smith TC, Jacobson IG, Boyko EJ, Hooper TI, Gackstetter GD, Phillips CJ, Smith B, Millennium Cohort Study Team, 2012. Prospective assessment of chronic multisymptom illness reporting possibly associated with open-air burn pit smoke exposure in Iraq. J. Occup. Environ. Med 54, 682–688. 10.1097/JOM.0b013e318255ba39 [DOI] [PubMed] [Google Scholar]
- Reichling DB, Levine JD, 2009. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 32, 611–618. 10.1016/j.tins.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma NK, Ryals JM, Liu H, Liu W, Wright DE, 2009. Acidic Saline-Induced Primary and Secondary Mechanical Hyperalgesia in Mice. The Journal of Pain 10, 1231–1241. 10.1016/j.jpain.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA, 2001. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 24, 37–46. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ, 2003. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106, 229–239. 10.1016/S0304-3959(03)00269-0 [DOI] [PubMed] [Google Scholar]
- Sluka KA, Rohlwing JJ, Bussey RA, Eikenberry SA, Wilken JM, 2002. Chronic muscle pain induced by repeated acid injection is reversed by spinally administered μ- and δ-, but not κ-, opioid receptor agonists. Journal of Pharmacology and Experimental Therapeutics 302, 1146–1150. 10.1124/jpet.102.033167 [DOI] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin J-S, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji R-R, Zhang J, Salter MW, Mogil JS, 2015. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci 18, 1081–1083. 10.1038/nn.4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele L, 2000. Prevalence and Patterns of Gulf War Illness in Kansas Veterans: Association of Symptoms with Characteristics of Person, Place, and Time of Military Service. Am J Epidemiol 152, 992–1002. 10.1093/aje/152.10.992 [DOI] [PubMed] [Google Scholar]
- Stimpson NJ, Unwin C, Hull L, David T, Wessely S, Lewis G, 2006. Prevalence of reported pain, widespread pain, and pain symmetry in veterans of the Persian Gulf War (1990–1991): the use of pain manikins in Persian Gulf War health research. Mil Med 171, 1181–1186. 10.7205/milmed.171.12.1181 [DOI] [PubMed] [Google Scholar]
- Stockstill K, Doyle TM, Yan X, Chen Z, Janes K, Little JW, Braden K, Lauro F, Giancotti LA, Harada CM, Yadav R, Xiao WH, Lionberger JM, Neumann WL, Bennett GJ, Weng H-R, Spiegel S, Salvemini D, 2018. Dysregulation of sphingolipid metabolism contributes to bortezomib-induced neuropathic pain. J. Exp. Med 215, 1301–1313. 10.1084/jem.20170584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K, Krengel M, Bradford W, Stone C, Thompson TA, Heeren T, White RF, 2018. Neuropsychological functioning in military pesticide applicators from the Gulf War: Effects on information processing speed, attention and visual memory. Neurotoxicol Teratol 65, 1–13. 10.1016/j.ntt.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Sutton BC, Opp MR, 2015. Acute increases in intramuscular inflammatory cytokines are necessary for the development of mechanical hypersensitivity in a mouse model of musculoskeletal sensitization. Brain Behav. Immun 44, 213–220. 10.1016/j.bbi.2014.10.009 [DOI] [PubMed] [Google Scholar]
- Sutton BC, Opp MR, 2014. Sleep fragmentation exacerbates mechanical hypersensitivity and alters subsequent sleep-wake behavior in a mouse model of musculoskeletal sensitization. Sleep 37, 515–524. 10.5665/sleep.3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves S, Berta T, Liu D-L, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S, Ji R-R, 2016. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav. Immun 55, 70–81. 10.1016/j.bbi.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik VL, Huck NA, Baca QJ, Ganio E, Haight ES, Culos A, Ghaemi S, Phongpreecha T, Angst MS, Clark JD, Aghaeepour N, Gaudilliere B, 2020. Systematic Immunophenotyping Reveals Sex-Specific Responses After Painful Injury in Mice. Frontiers in Immunology In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutwein B, Strasburger H, 1999. Fitting the psychometric function. Percept Psychophys 61, 87–106. [DOI] [PubMed] [Google Scholar]
- Wang H, Heijnen CJ, Velthoven C.T.J. van, Willemen HLDM, Ishikawa Y, Zhang X, Sood AK, Vroon A, Eijkelkamp N, Kavelaars A, 2013. Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Invest 123, 5023–5034. 10.1172/JCI66241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Peng Y, Hutchinson MR, Rice KC, Yin H, Watkins LR, 2016. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br. J. Pharmacol 173, 856–869. 10.1111/bph.13394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, Hardie A, Klimas N, Knox M, Meggs WJ, Melling J, Philbert MA, Grashow R, 2016. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex, What’s your poison? Neurobehavioural consequences of exposure to industrial, agricultural and environmental chemicals 74, 449–475. 10.1016/j.cortex.2015.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YC, Park SS, Subieta AR, Brennan TJ, 2004. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology 101, 468–475. 10.1097/00000542-200408000-00029 [DOI] [PubMed] [Google Scholar]
- Zakirova Z, Tweed M, Crynen G, Reed J, Abdullah L, Nissanka N, Mullan M, Mullan MJ, Mathura V, Crawford F, Ait-Ghezala G, 2015. Gulf War Agent Exposure Causes Impairment of Long-Term Memory Formation and Neuropathological Changes in a Mouse Model of Gulf War Illness. PLoS One 10 10.1371/journal.pone.0119579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cui F, Chen H, Zhang T, Yang K, Wang Y, Jiang Z, Rice KC, Watkins LR, Hutchinson MR, Li Y, Peng Y, Wang X, 2018. Dissecting the Innate Immune Recognition of Opioid Inactive Isomer (+)-Naltrexone Derived Toll-like Receptor 4 (TLR4) Antagonists. J Chem Inf Model 58, 816–825. 10.1021/acs.jcim.7b00717 [DOI] [PMC free article] [PubMed] [Google Scholar]


