Abstract
Background
Thumb carpometacarpal (CMC) osteoarthritis (OA), a degenerative condition affecting hand use, is typically evaluated through radiographs and clinical examination. Although this can determine treatment, it is difficult to evaluate functional limitations. Shear wave elastography (SWE) is a quantitative ultrasound technique that characterizes tissue stiffness.
Questions/Purposes
This pilot study aimed to establish data of the SWE findings in the thenar eminence muscles in patients with first CMC OA and correlate these findings with the clinical tests of hand function.
Methods
This cross-sectional study correlated the SWE stiffness of thenar eminence muscles to clinical tests of hand function in patients with first CMC OA and in asymptomatic control subjects, using Spearman’s correlation coefficient. Mean SWE values of the thenar eminence muscles in patients were compared with those in control subjects. The study was performed in a non-profit tertiary care hospital setting. Patients and control subjects were recruited on a volunteer basis.
Results
SWE values in the abductor pollicis brevis and flexor pollicis brevis muscles showed moderate to very strong correlation with multiple measures of hand function. Mean SWE values of the thenar eminence muscles in first CMC OA patients were lower than those in asymptomatic control subjects.
Conclusions
Correlations between mean SWE values in the thenar eminence muscles and clinical measures of hand function suggest decreased function in subjects with less stiff thenar eminence muscles.
Electronic supplementary material
The online version of this article (10.1007/s11420-020-09795-z) contains supplementary material, which is available to authorized users.
KEYWORDS: ultrasound, elastography, osteoarthritis, thumb, hand
Introduction
Osteoarthritis (OA) of the thumb carpometacarpal (CMC) joint affects greater than 30% of the general population, with a higher prevalence in women [5, 9]. Thumb CMC OA, typically idiopathic in etiology, leads to pain and functional impairment that if non-surgical measures fail to reverse may require surgery. The current modality of choice for imaging and evaluating CMC OA is radiographs and clinical examinations. However, radiographs are unable to assess the role that surrounding structures including muscles and ligaments play in the degradation of the articulating cartilage surfaces, although it has been hypothesized that increased ligamentous laxity may be associated with a higher risk of first CMC OA [19]. The interplay between the thenar eminence muscles and the ligaments is thought to contribute to stability in the CMC joint [19]. Thumb CMC OA leads to reduced grip strength, decreased range of motion (ROM), and joint stiffness [19], and thenar muscle atrophy is expected in association. There is a need to further explore how these changes in thenar eminence muscle quality associate with function in thumb CMC OA.
Few studies have found weak relationships between radiographic staging of first CMC OA with functional hand performance [2, 6, 7]. Exploration of the relationship between the radiographic staging of first CMC OA and functional hand use is important, especially as radiographs are frequently used to guide intervention. If radiographic severity of the first CMC OA does not reflect the patient’s functional ability, the determination of treatment can be limited. Studies have yet to quantify muscle changes in first CMC arthrosis or assess how they may relate to hand function.
Recent studies have revealed promising results on the use of shear wave elastography (SWE), a quantitative ultrasound technique, in the evaluation of various musculoskeletal soft tissues, including nerves, muscles, tendons, and ligaments [1, 3, 8, 10, 14–16, 20]. SWE allows for the assessment of intrinsic tissue properties, which can help with early diagnosis of disease when no abnormality can be visualized in conventional ultrasound [17]. Currently, SWE has been applied to the evaluation of muscles, tendons, and peripheral nerve conditions [3, 10, 14, 17]. SWE characterizes tissue stiffness and may be useful in the quantification of muscle stiffness around the first CMC joint.
This pilot study therefore aimed to establish data of the SWE findings in the thenar eminence muscles in patients with first CMC OA, as compared with asymptomatic control subjects, and correlate these findings with clinical tests of hand function.
Methods
This cross-sectional, observational pilot study received institutional review board approval. Patients with varying stages of basilar joint OA were enrolled in a non-profit tertiary care hospital setting from March 2016 to July 2018, based on modified Eaton-Littler (E-L) grades of thumb CMC OA on standard radiographs [9]. Inclusion criteria were patients 18 years old or older with first CMC OA (as determined by clinical and/or radiological measures). Participants were recruited on a volunteer basis, and non-patient volunteers were enrolled as controls. Those with prior surgery to the affected hand were excluded. Patient participants had undergone radiography as a standard of care for evaluation and treatment of first CMC OA; control subjects did not undergo radiography. The modified Eaton-Littler classification system stages thumb CMC arthrosis as 4 grades. Grade 1 indicates normal radiographs with subtle CMC joint space widening [9]. Grade 2 describes a slight narrowing of the CMC joint space with sclerosis, and cystic changes with osteophytes or loose bodies < 2mm [9]. Advanced CMC joint space narrowing, sclerosis, and cystic changes with osteophytes or loose bodies > 2 mm characterize grade 3 [9]. Grade 4 indicates arthritic changes in the CMC joint as in stage III with scaphotrapezial arthritis [9].
All patients and control subjects underwent SWE evaluation of the thenar eminence muscles (abductor pollicis brevis (APB), flexor pollicis brevis (FPB), and opponens pollicis (OP)) and the flexor pollicis longs (FPL) tendon, performed by a single musculoskeletal radiologist with 8 years of experience with musculoskeletal ultrasound and elastography. Muscle stiffness was measured in kilopascal (kPa) using a 9-MHz transducer on a LOGIQ E9 ultrasound system (GE Healthcare, Waukesha, WI). An average of 3 SWE measurements was obtained per muscle in each subject. The appropriate muscles were identified and measured in a short axis, using the first metacarpal and the flexor pollicis longus tendon as landmarks for thenar eminence musculature. The ultrasound evaluation was performed with the subjects recumbent and prone, with the forearm pronated and the palm up—thus stabilizing the hand and minimizing active strain in the thenar eminence. Minimal transducer pressure was applied while obtaining SWE measurements.
Patients and volunteers subsequently underwent a clinical evaluation of hand function by an occupational therapist certified in hand therapy with 10 years of experience. This clinical evaluation consisted of physical, performance, and self-report outcome measures (Patient-Specific Functional Scale [PSFS]; Patient Rated Wrist/Hand Evaluation [PRWHE]; Disabilities of the Arm, Shoulder, and Hand [DASH]; Modified Score for the Assessment and Quantification of Chronic Rheumatic Affections of the Hands [M-SACRAH]; Arthritis Hand Function Test [AHFT]) [18]. Higher PSFS scores indicate greater functional hand use, whereas lower DASH, PRWHE, and M-SACRAH scores indicate greater functional hand use. In the AHFT, higher numbers in the strength sections (grip, pinch, lifting cans, pouring water) indicate greater functional use, and lower numbers in the dexterity sections (timed tasks) indicate greater functional hand use.
Spearman’s ρ with 95% subject clustered bootstrapped confidence intervals (CI) were calculated to assess for associations between SWE values and measures of hand function. Differences in mean SWE between patients and controls were estimated from a mixed-effects linear model. Analyses were performed in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
There were 5 patients/9 OA thumbs and 9 volunteers/9 control thumbs enrolled. Patient mean age was 62.8 (range 51–71) years. Non-patient control mean age was 38 years (range 26–57). Based on the x-rays in the 9 symptomatic thumbs, there were 2 thumbs each in the grades 1, 2, and 3 first CMC OA groups, and 3 thumbs with grade 4 OA. Mean SWE values in patients were negatively correlated with the OA grade (ρ ranging − 0.1 to − 0.7), although correlation was weak.
Average ABP SWE showed a very strong positive correlation with the AHFT two-point pinch (right, ρ = 0.8 [95% CI: 0.4, 1.0]) (Fig. 1), strong negative correlation with the AHFT button board test (ρ = − 0.7 [− 1.0, − 0.1]), strong negative correlation with the DASH work (ρ = − 0.7 [− 0.9, − 0.1]), PRWHE usual (ρ = − 0.6 [− 0.9, − 0.2]) and PRWHE specific (ρ = − 0.6 [− 0.9, − 0.1]), and moderate negative correlation with MSACRAH pain (ρ = − 0.5 [− 0.9, 0.0]). Average FPB scores showed a strong negative correlation with the PRWHE usual (ρ = − 0.6 [− 0.9, − 0.1]) and moderate positive correlation with the AHFT two-point pinch (right, ρ = 0.6 [0.0, 0.9]), lifting tin can (ρ = 0.4 [0.2, 0.8]), and pouring water scores (ρ = 0.4 [0.2, 0.8]). There was a weak correlation between OP SWE scores and the measures of hand function.
Fig. 1.
SWE of the thenar eminence in a first CMC OA patient and control subject. SWE map of the thenar eminence in a 51-year-old first CMC OA patient with AFHT two-point pinch (right) of 4.7 lbs. showed APB stiffness of 5.2 kPa (a). Compare that to the SWE map in a 38-year-old asymptomatic control subject with AFHT of 14.7 lbs. and APB stiffness of 9.6 kPa (b).
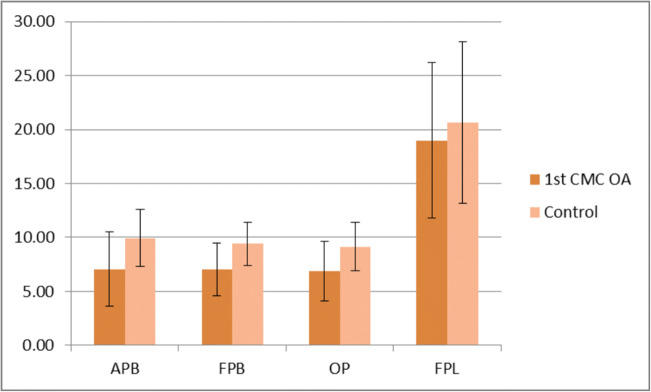
The mean SWE values of the thenar eminence muscles and the FPL in first CMC OA patients were lower than those in volunteers (Fig. 2), although the differences were marginal.
Fig. 2.
Column chart demonstrating mean SWE values (kPa) in first CMC OA patients vs. control subjects. Error bars depict differences in mean thenar eminence muscle SWE values between first CMC OA patients and controls.
Discussion
This pilot study aimed to establish data of the SWE findings in the thenar eminence muscles in patients with first CMC OA and correlate these findings with the clinical tests of hand function. This study revealed a negative but weak correlation between SWE values in the thenar eminence muscles and radiographic grades of the first CMC OA. However, moderate to very strong correlations were seen between mean SWE values in the ABP and FPB muscles and clinical measures of hand function, with poorer function seen in subjects with less stiff thenar eminence muscle. Mean SWE values of the thenar eminence muscles and the FPL tendon were lower in first CMC OA patients when compared with control subjects.
The study has several limitations. The small sample size limited more robust analyses, such as the effect of hand dominance. Also, the small sample size limited adjusting for any confounders such as age, sex, and comparison of dominant and non-dominant hands. Our use of a convenience sample limits the generalizability of our findings. Additionally, our patient and non-patient volunteers were not age matched; the control group had a younger mean age. This was largely due to the difficulty of finding age-matched asymptomatic volunteers, given the prevalence of first CMC OA. The tester evaluating hand function also could not be blinded during the study as the participants were interviewed to assess for thumb pain and dysfunction, making those with clinically diagnosed arthritis apparent. This study examined SWE in muscles comprising the thenar eminence, thus excluding the adductor pollicis, an important contributor to the first CMC function. Stabilizing ligaments of the first CMC joint were similarly not considered in this study but have also been indicated in the first CMC OA [11].
The limitations of radiographic scoring systems of first CMC OA such as the E-L system have been well reviewed [9, 12] and include poor correlation with clinical symptoms and intraoperative findings, and limited ability to guide surgical treatment. In a recent publication [18], our group demonstrated a poor correlation between basilar joint x-ray findings and measures of hand function. It follows that SWE measures of thenar eminence muscle stiffness in this study are poorly correlated with x-ray grades of first CMC OA.
Conversely, associations between thenar eminence muscle stiffness on SWE and hand function were found in this study. The thenar eminence muscles, especially the APB and FPB, are directly involved in pinch and grip functions [4, 13], and the correlations in this study mirrored this. More explicitly, subjects in this study with stiffer APB and FPB muscles showed better functional hand use, strength, and dexterity. When specifically comparing groups, our aforementioned prior investigation found significant differences in hand function between first CMC OA patients and control subjects, with control subjects performing better [18]. SWE metrics of muscle stiffness in the current study also revealed differences between groups, where the less stiff muscle was seen in first CMC patients. A direct relationship between thenar eminence muscle function or stiffness and the degree of first CMC joint cartilage loss is not clearly shown by these results. However, one can assume that muscles are less well used across an arthritic joint. Future expansive investigations will include assessment of the adductor pollicis, the stabilizing ligaments of the thumb CMC joint, and the degree of cartilage loss.
In summary, the findings in this pilot study suggest that SWE metrics in the thenar eminence muscles may better correlate to hand function than radiographic grades of first CMC OA. Less stiff thenar eminence muscle appears to correlate with poorer function in first CMC OA and strengthening of these muscles may be a mechanism for improvement of hand function. These preliminary results will serve as a foundation for a more robust quantitative imaging study of first CMC OA, with the goal to inform on techniques for preventative care and effective treatment.
Electronic Supplementary Material
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
Compliance with Ethical Standards
Conflict of Interest
O. Kenechi Nwawka, MD; Gwen Weinstock-Zlotnick, PhD, OTR/L, CHT; Bin Lin, ScM; and Lydia M. Ko, MPH, declare that they have no conflicts of interest.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent
Informed consent was waived from all patients for being included in this study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Level IV: Cross-sectional Study
References
- 1.Arda K, Ciledag N, Aktas E, Aribas BK, Köse K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol. 2011;197(3):532–536. doi: 10.2214/AJR.10.5449. [DOI] [PubMed] [Google Scholar]
- 2.Dahaghin S, Bierma-Zeinstra SMA, Ginai AZ, Pols HAP, Hazes JMW, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study) Ann Rheum Dis. 2005;64(5):682–687. doi: 10.1136/ard.2004.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeWall RJ, Slane LC, Lee KS, Thelen DG. Spatial variations in Achilles tendon shear wave speed. J Biomech. 2014;47(11):2685–2692. doi: 10.1016/j.jbiomech.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Michelsen-Jost H. Anatomy and function of the thenar muscles. Hand Clin. 2012;28(1):1–7. doi: 10.1016/j.hcl.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Haugen IK, Englund M, Aliabadi P, Niu J, Clancy M, Kvien TK, Felson DT. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2011;70:1581–1586. doi: 10.1136/ard.2011.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haugen I, Slatkowsky-Christensen B, Bøyesen P, van der Heijde D, Kvien TK. Cross-sectional and longitudinal associations between radiographic features and measures of pain and physical function in hand osteoarthritis. Osteoarth Cart. 2013;21(9):1191–1198. doi: 10.1016/j.joca.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Hoffler CE, 2nd, Matzon JL, Lutsky KF, Kim N, Beredjiklian PK. Radiographic stage does not correlate with symptom severity in thumb basilar joint osteoarthritis. J Am Acad Ortho Surg. 2015;23(12):778–782. doi: 10.5435/JAAOS-D-15-00329. [DOI] [PubMed] [Google Scholar]
- 8.Kantarci F, Ustabasioglu FE, Delil S, et al. Median nerve stiffness measurement by shear wave elastography: a potential sonographic method in the diagnosis of carpal tunnel syndrome. Eur Radiol. 2014;24(2):434–440. doi: 10.1007/s00330-013-3023-7. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy CD, Manske MC, Huang JI. Classifications in brief: The Eaton-Littler classification of thumb carpometacarpal joint arthrosis. Clin Ortho Rel Res. 2016;474:2729–2733. doi: 10.1007/s11999-016-4864-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacourpaille L, Hug F, Guével A, et al. Non-invasive assessment of muscle stiffness in patients with Duchenne muscular dystrophy. Muscle Nerve. 2015;51(2):284–286. doi: 10.1002/mus.24445. [DOI] [PubMed] [Google Scholar]
- 11.Ladd AL, Weiss AC, Crisco JJ, Hagert E, Wolf JM, Glickel SZ, Yao J. The thumb carpometacarpal joint: anatomy, hormones, and biomechanics. AAOS Instr Course Lect. 2013;62:165–179. [PMC free article] [PubMed] [Google Scholar]
- 12.Ladd AL, Messana J, Berger AJ, Weiss APC. Correlation of clinical disease severity to radiographic thumb osteoarthritis index. J Hand Surg Am. 2015;40(3):474–482. doi: 10.1016/j.jhsa.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SK, Wisser JR. Restoration of pinch in intrinsic muscles of the hand. Hand Clin. 2012;28(1):45–51. doi: 10.1016/j.hcl.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Palmeri ML, Dahl JJ, MacLeod DB, Grant SA, Nightingale KR. On the feasibility of imaging peripheral nerves using acoustic radiation force impulse imaging. Ultrason Imaging. 2009;31(3):172–182. doi: 10.1177/016173460903100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen ZL, Vince DG, Li ZM. In vivo study of transverse carpal ligament stiffness using acoustic radiation force impulse (ARFI) imaging. PLoS One. 2013;8(7):e68569. doi: 10.1371/journal.pone.0068569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinohara M, Sabra K, Gennisson JL, Fink M, Tanter M. Real-time visualization of muscle stiffness distribution with ultrasound shear wave imaging during muscle contraction. Muscle Nerve. 2010;42(3):438–441. doi: 10.1002/mus.21723. [DOI] [PubMed] [Google Scholar]
- 17.Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, Gao L, Witte RS. Shear-Wave elastography: basic physics and musculoskeletal applications. Radiographics. 2017;37(3):855–870. doi: 10.1148/rg.2017160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstock-Zlotnick G, Lin B, Nwawka OK. Clinical assessments of hand function in first carpometacarpal osteoarthritis do not appear to correlate with radiographic findings. HSS J. 2019;15(3):269–275. doi: 10.1007/s11420-019-09705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss AC, Goodman AD. Thumb basal joint arthritis. J Am Acad Orthop Surg. 2018;26(16):562–571. doi: 10.5435/JAAOS-D-17-00374. [DOI] [PubMed] [Google Scholar]
- 20.Wu CH, Chen WS, Wang TG. Elasticity of the coracohumeral ligament in patients with adhesive capsulitis of the shoulder. Radiology. 2016;278(2):458–464. doi: 10.1148/radiol.2015150888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)