Abstract
Background:
There are many theories about the cause of trigeminal neuralgia (TN). None of them satisfactorily explains how demyelination alone through the ephaptic mechanism can contribute to the development of the TN crisis. The main characteristic of TN pain is its dynamic nature, which is difficult to explain based only on anatomical findings. With these antecedents, the exact mechanism by which radiosurgery produces pain relief in TN is unknown.
Methods:
It is based on the trigeminal ganglion (TG) cytoarchitecture and the pathophysiological findings observed after an injury to a trigeminal branch. TG seems to have a predominant role given its cellular structure. The neuronal component in sensory ganglia is generally surrounded by a single layer of satellite glial cells (SGC), which forms a sheath around each body cell. There is increasing evidence that SGCs play a key role in nociception. This depends on their ability to influence the neuronal excitability that occurs in conditions of neuropathic and inflammatory pain; contributing to both the generation and maintenance of pain.
Results:
We have already published the beneficial effects of radiosurgery on the TG for the treatment of idiopathic TN and secondary to vertebrobasilar ectasia. Now, we are investigating the functioning of the TG and how radiosurgery could act on the SGC, deactivating them, and contributing to the decrease or disappearance of the painful condition.
Conclusion:
We are postulating a theory on how radiosurgery in TG produces changes in the SGC, with implications in the pathological mechanisms initiated by the alteration caused in the neuron after a nerve injury.
Keywords: Gamma knife, Radiosurgery, Satellite glial cells, Theory, Trigeminal ganglion, Trigeminal neuralgia

INTRODUCTION
At present, the pathogenesis of trigeminal neuralgia (TN) and the exact mechanism by which radiosurgery produces pain relief is unknown.[18,30] It has been postulated that there is an ephaptic transmission caused by demyelination in the trigeminal nerve (TN). This is assumed to be caused by vascular compression at the level of the fifth nerve entry zone in the protuberance. Under this premise, radiosurgery would act decreasing pain intensity through the interruption of the ephaptic transmission, although the radiosurgical treatment itself produces, in turn, demyelination of the nerve fibers.[1,10,14,17,18,30]
In the past decades, many investigations show the role that glial cells of the trigeminal ganglion (TG) play in the origin and maintenance of the painful status in TN, which gives a new perspective.[7,10-12,14,16-18] Radiosurgery could have a determining role in the deactivation of these cells causing changes in perineural homeostasis to eliminate the painful condition. In two previous publications on patients treated with gamma knife radiosurgery for TN secondary to vertebrobasilar ectasia[29] and idiopathic TN, we discussed the results from a clinical point of view.[30] The treatment has shown high effectiveness with very few side effects, maintaining pain relief for a longer period, when compared to the results obtained when the target is the TN.
We investigate the anatomical, cellular, and functional characteristics of the TG. The results of our research allow us, on the one hand, to postulate the implication of cellular architecture and physiological aspects of TG, which provides a new, more coherent approach to the causes of TN and, second, to present a theory on how radiosurgery works in TG to relieve pain in TN.
MATERIALS AND METHODS
We analyzed the findings obtained in our patients, which were previously published.[29,30] To find an explanation for what was observed, we proceeded to review the anatomical and histological characteristics of TG. We also reviewed its physiology and reaction after a pathological condition such as the ones present in TN. The anatomical and functional organization is of great importance to explain the results obtained from the clinical point of view.
RESULTS
Clinical background
In 2014, we reported a case of a patient with NT secondary to vertebrobasilar ectasia. We treated TG for the painful condition because the TN could not be visualized adequately in the neuroimaging studies. Three days after the procedure, the pain intensity had decreased and 15 days after the procedure, the patient was pain-free. During the 48 months of follow-up, the patient remained without pain and any alteration in facial sensation.[29]
Based on the good results obtained, we decided to prospectively evaluate the technique in 30 patients with idiopathic TN that was reported in 2019.[30] In our series, the radiosurgical target was conformed through two isocenters, 8 and 4 mm in the Meckel’s cave. The maximum dose was 86 Gy (43 Gy at 50% isodose prescribed), as shown in [Figure 1]. The average latency period was 7 days (range, 1–40 days); all patients were pain-free. In 18 patients (60%), pain relief had a latency period of fewer than 7 days.
Figure 1:
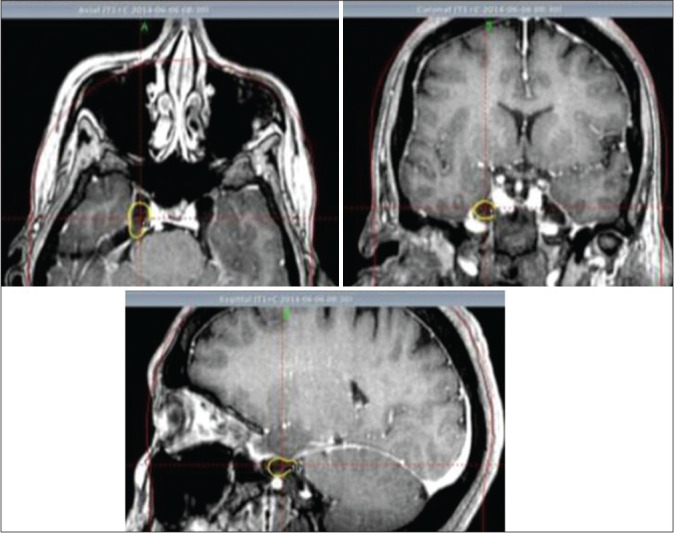
Axial, coronal, and sagittal T1-weighted images after gadolinium. Right trigeminal ganglion and exit zone of trigeminal nerve from the ganglion are visualized.
The primary outcome after radiosurgery was based on pain intensity, which was defined and assessed using the pain intensity scoring criteria of the Barrow Neurological Institute (BNI). Before undergoing GKS, all patients classified their pain as BNI IV or V. At the last follow-up, significant pain relief was observed in 86.6% of patients. The adverse effects of radiosurgery were presented in 13.3%. Patients with a long history of TN had good responses as those with a short history; the history of the previous surgery did not influence the results.[30]
These findings led us to investigate two important aspects: the current theories about the cause of the TN and the implication of the TG with its anatomic and functional characteristics.
Anatomical features
The TG, also known as the Gasserian or lunate ganglion, is a large, flattened ganglion found in the middle cranial fossa in the Meckel cave, which is a rigid structure. It is an easy target and there are many fewer possibilities of movement during the radiosurgical treatment.[7,29]
TG had been considered as a simple transition site for sensory information from the periphery to the central nervous system (CNS). Now, it is very well known that it can act as an integrating structure located in the peripheral nervous system regulating the intracellular modulating mechanisms as well as intercellular and autocrine signaling. It is a key component in the nociception of craniofacial pain that contributes to the peripheral modulation of pain pathways in TN.[22,32]
In the TG, the regions V1, V2, and V3 are interconnected and stimulation of V3 neurons could cause an increase in the levels of active signaling proteins in neuronal and satellite glial cells (SGC) in other regions of the ganglion, which contributes to signal propagation and chronic pain.[3,12,22,30]
The cytoarchitecture of the TG
As in the CNS, the sensory ganglia contains neurons, glia, and fibroblasts that form collagen fibers, small capillary-type blood vessels, and several types of immune cells, such as microglia-like resident macrophages and peripheral support cells.[19,22]
The two most numerous and best-defined glial cells in the ganglia are equivalent to the CNS macroglia and they are the Schwann cells and the SGC.[23,32]
The neuronal component and the proximal portion of its axon in the sensory ganglia are usually surrounded by a single layer of SGC, forming a sheath around each cell body. They are organized in discrete bands or groups within each region of the TG and are connected by gap junctions, a space of approximately 20 nm, forming a path between the connective tissue and the neuronal surface.[2,6]
Physiology and the physiopathological responses of the TG. Role of SGC
Under physiological conditions, each cell body surrounded by an SGC sheath forms a morphological and functional differentiation that allows a close bidirectional interaction through paracrine signaling between the neuronal body and the SGC that facilitates the maintenance of neuronal homeostasis.[3,7,13,24,32] Several ion channels, receptors, and adhesion molecules have been identified in these cells. In this way, the glial cells control the perineural environment modulating its activity and facilitating non-synaptic communication between these two types of cells. SGCs are considered equivalent cells in the peripheral nervous system to the CNS astrocytes, with analogous characteristics. In sensory ganglia, particularly in dorsal spinal ganglia (DSG) and TG, SGC establish a privileged relationship with the surrounding neuronal bodies.[3,24] The communication with the intercellular gap helps to maintain a homeostatic environment by buffering important cellular ions, such as K+, Na+, and Ca2+ with the regulation of the concentration in the extracellular space and the recycling of neurotransmitters.[3,7] Through the unions, there is a direct transfer not only of ions but also of metabolic precursors and second messengers that regulate the excitability of the cells. As a result of this activity, glial cells exhibit a highly negative membrane potential at rest.[3]
In DSG, the excitation of neurons leads to the development of an action potential in neighboring neurons, a property called cross-excitation. In vitro studies have shown that repeated stimulation of these neurons induced a transient depolarization of neighboring neurons in the ganglion, probably mediated by chemical messengers. ATP seems to be the main mediator in the interaction between neurons and SGC in the sensory ganglia.[3,28]
In pathological conditions, those molecules initiate and maintain neurogenic inflammation, whose results are peripheral sensitization of trigeminal nociceptors. This will cause the excitation of second-order neurons within the brainstem and spinal cord involved in the transmission of nociceptive information that leads to pain, central sensitization, hyperalgesia, and allodynia.[7] The process starts once the SGC are activated.
These cells undergo profound changes in response to nerve injury. There is an increase in intracellular Ca2 + in both cell types and, consequently, the release of ATP from neurons and the SGCs. In this way, there is a growth of bidirectional communication between the neurons and the SGC which will activate P2 receptors around SGCs and in the neuron itself. The increase in the ATP, together with the multiplication of the number of gaps between the SGCs of neighboring perineural sheaths, will allow the propagation of Ca2 + waves to these SGCs and neighboring neurons, which influences the excitability of the neurons that are not directly affected by the injury, as shown in [Figure 2]. The changes observed after the activation of the SGCs have been present in different chronic pain models with an increase in the expression of glial fibrillary acidic protein (GFAP), a lower expression and sensitivity of potassium channels, a greater coupling of SGCs by unions, an increase in the number of GAP junctions, the formation of bridges that connect previously separated perineuronal sheaths, a greater sensitivity to ATP, altered expression of purinergic receptors, and the release of ATP and cytokines.[3,12,23,27,31,33]
Figure 2:
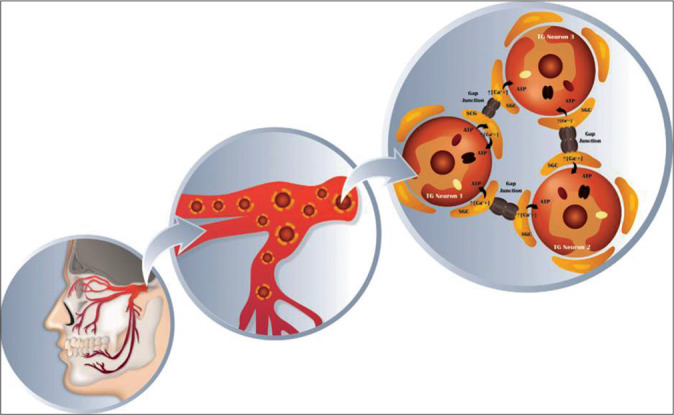
Schematic of trigeminal ganglion neurons and surrounding satellite glial cells. Glial cells play a primary role in the processes of nervous system dysfunction, such as the generation and/or maintenance pain. After a nerve injury, there is a release of some neurotransmitters as CGPR, substance P and ATP, which activate satellite glial cells (SGC), increasing the intracellular calcium concentration in those cells. Through the gap junctions, the SGC communicate with others SGC using the wave propagation of Ca2+, affecting those neurons in the same manner as the first neuron was affected. Thus, the excitability process of the last neurons was not directly affected by the injury. Concomitantly, there is an increasing number of gap junctions and alterations with a rapid redistribution which increases K+ and Ca2+ in neurons as well as in SGC.
Those ultrastructural and biochemical changes in the axon and myelin are seen not only in the TG but also in the root or both structures.[3,13,18] Luo et al.[20] investigated the glial plasticity changes in oligodendrocytes, Schwann cells, astrocytes, and microglia/macrophages in the entry zone of the TN in rats with TN induced by compression injury. The results showed that mechanical compression injury in the TN induced glial plasticity in the entry zone. The GFAPimmunoreactive astrocyte processes significantly proliferated and extended distally from the central region to the peripheral side of the trigeminal root entry zone after nerve compression injury in the TN group. The results reported in the TN are similar to those obtained in the plasticity of the SGC in the TG and could explain the results observed in the treatment of TN with radiosurgery in both the TN and the TG.
Demyelination and other factors may delay the restoration of membrane potentials and excitability after an episode of TN. The appearance of a refractory period from seconds to minutes after a TN attack is well known, during which no further attacks can be triggered. Devor et al.[5] described the mechanism by which after the intense pain that occurs in TN, it stops.[10,14,19,28] The maintenance of low concentrations of extracellular K+, mediated by SGC, can be crucial to control the resting membrane potential and neuronal excitability. During a trigeminal pain crisis, calcium ions enter the neuron and activate K channels. Potassium ions flow from the neuron through these channels, which causes the neuron to hyperpolarize and the trigger to stop. As a result, the attacks are brief. The hyperpolarization after the burst may last a minute or more. This is probably the basis of the refractory period that often follows a pain attack of TN, during which a second attack cannot be triggered. Consequently, knowledge about the SGCs and its mechanisms of interaction with the neuronal body assumes increasing importance in the search for new targets for the treatment of TN.[3,13,31] The SGCs are responsible for the perineural K+ homeostasis.[3,23,31,33]
DISCUSSION
Theories about the cause of TN
Dandy proposed compression of the TN in the entry zone into the pons by an arterial vessel as a possible cause of TN.[4] The pathological substrate of this condition is mainly associated with the demyelination in the entry zone of the trigeminal root.[10,26] This is assumed to be a critical region where central myelin (oligodendrocytes) is converted to peripheral myelin (Schwann cells). The oligodendrocytes are more sensitive to irradiation than Schwann cells, and consequently, a stronger radiobiological effect would occur on the root entry zone.[16] However, Régis et al.,[25] treated the TN at the pontine cistern where the myelin is peripheral (Schwan cells) obtaining the same results and invalidating such an assumption.
For many years, the most popular theory of the peripheral mechanism of the disease was the “short connection” theory proposed by Dott in 1951.[6] According to this theory, the TN attack starts from the interconnection of demyelinated axons, spontaneous activity, and the generation of ectopic impulses.[26] The cross-excitation between the sensory fibers and those delegated to transport painful stimuli was proposed as a possible explanation of the discharge observed in TN after the tactile stimulation of the activation points.[10] However, even though ephapsis can amplify the sensation caused by the applied stimuli, generating hyperesthesia and even pain, it does not explain why the paroxysms of TN pain last longer than the trigger stimulus, and why its intensity is not related to the intensity of the stimulus.[5] Nor does it explain why pain occurs spontaneously. Hence, a simple compression is not a generator of ectopic pain at the level of the injury and by itself does not explain the clinical findings. Similarly, demyelination alone does not provide clear evidence of the characteristic symptoms of the disease.[19]
The knowledge that clinical improvement occurs after microvascular decompression (MVD) supports the concept that the development of TN involves two different and concurrent processes in the same pathological condition. The rapid clinical and electrophysiological recovery that often follows MVD has led to questioning the central role of demyelination in the development of NT. The process of myelination does not occur immediately after the MVD, so it cannot explain the rapid relief of neuralgia. In the long-term, however, remyelination can help ensure sustained symptomatic relief.[10] There is some evidence contrary to the hypothesis of neurovascular compression. In two studies on cadavers without TN, neurovascular contact was observed in 13–32% of cadavers with neurovascular compression ranging between 8 and 10%.[10] Majoie et al.[21] presented a magnetic resonance imaging (MRI) study in which they examined 170 trigeminal nerves in 85 non-TN patients. Seventy-nine nerves (46%) had some point of contact with some vascular structure, 24 (14%) had contact with cisterns, 52 (30%) had a contact in the root entry zone, and the remaining 3 (2%) had a real deformity of the root entry zone.
Thus, the main feature of the pain of TN is its dynamic nature, which is difficult to explain based only on the anatomical findings. The TG seems to have a predominant role given its cellular architecture.[3,19]
How does radiosurgery work in trigeminal pain?
There are interesting animal research studies evaluating the effect of radiosurgery. Kondziolka et al.[17] showed the effects of radiosurgery on TN fibers in a baboon model. They administered to the nerve 80 or 100 Gy by a single isocenter of 4 mm. The trigeminal nerves that received radiosurgery of 80 Gy had no inflammation. Focal myelin pallidness and vacuolation without fibrosis was observed in the preganglionic nerve segments. Immunoreactivity for the neurofilament revealed substantial axonal loss, fragmentation, and some inflammation. The nuclei of Schwann cells were necrotic. The surrounding region contained only rare degenerative axons in which the TG appeared normal, as did the distal nerve beyond the radiosurgery target. The TN that received 100 Gy exhibited necrosis with axonal degeneration, vacuolation of myelin, and expansion of the endoneurial intercellular matrix compatible with edema. The ganglion remained normal. The histological effect was related to the dose.
The authors concluded that through partial axonal (focal) degeneration, radiosurgery probably relieves the pain of TN by affecting a population of axons large enough to relieve pain. They assumed that the low incidence of loss of facial sensation indicated that the remaining intact axonal population was sufficient to maintain neurological function in the majority of patients. This balance between pain relief and preservation of sensation, as well as the histological effect, was related to the dose.
Unlike what happens in the TN where there are studies of pathological anatomy after radiosurgical treatment, there are no such studies at the level of the TG that allow us to expand our knowledge on the effects of radiosurgery. Nevertheless, recently Goldschmidt et al.[8] evaluated those effects in the DRG. They developed an animal model to evaluate the response of the DRG that contains the body of sensory neurons responsible for pain sensitivity, considering that it could be useful to determine the clinical response of radiosurgery in chronic patients with spinal radicular pain susceptible to neuromodulation. They based on the experience obtained with radiosurgery as an effective technique to create TN injuries to treat TN.
The animals were assessed to detect motor and sensory deficiencies every 2 weeks and were sacrificed at 3 and 6 months after the SRS. No detectable deficit was observed in any of the animals at any time. They verified the hypothesis that 80 Gy administered in a single fraction would induce changes similar to those described for the TN without compromising the sensory or motor function of the nerve root.
These findings mimic those observed after SRS in the TN in experimental animals. Using the same dose as for TN, similar histological changes were obtained without clinical toxicity. These results suggest that radiosurgery may be a possible option in the treatment of chronic spinal radicular pain. It can be inferred, based on these findings, that similar doses to the TG could have a beneficial clinical effect with low clinical toxicity.
The similarity found between astrocytes and SGCs, both anatomically and functionally, is also reflected in the response obtained at high doses of radiation such as those commonly used in radiosurgery for TN. This, together with the results obtained in radiosurgery in the DRG for chronic radicular pain; coincide in many aspects with the clinical results obtained in the treatment of the TG in patients with idiopathic TN.
Kamiryo et al.[15] studied the effect on astrocytes by high doses of radiation. They observed changes induced by radiation in the parietal cortex of Wistar rats at several time points after GKS. The maximum doses of 50, 75, and 120 Gy were administered with an isocenter of radiation using a 4 mm collimator. Conventional histochemical and immunocytochemical analyses were used to examine the brain tissue fixed by perfusion. The higher the dose of radiosurgery the faster the observed changes. Irradiation at a dose of 50 Gy caused morphological changes of the astrocytes in the parietal cortex at 3 months. Anti-GFA revealed hypertrophied astrocytes in the irradiated area at 3 months. Irradiation at a dose of 75 Gy resulted in morphological changes of the astrocytes in 1 month. Anti-GFAP staining showed hypertrophied astrocytes 1 month after irradiation and this effect was observed at 4 months. Irradiation at a dose of 120 Gy caused changes in astrocytic morphology within 3 days. Necrosis was observed at 4 weeks; however, no significant inflammation was observed. These findings demonstrated dose-dependent and time-dependent changes in normal brain tissue after irradiation with GKS, and reinforce our hypothesis that similar effects could be obtained on the SGCs with the use of radiosurgery in the TG, using the doses usually prescribed for TN.
The results obtained with GKS on TG are also based on precision, which is a critical aspect when it comes to TN where a shot with a 4 mm isocenter is placed. In addition to the neurosurgeon’s experience in determining the correct area to place the shot, there are other variables related to precision such as the quality of the images, the mechanical errors of the stereotactic frame, and the mechanical errors of the radiation equipment used. The sum of errors can be superior to 2 mm. Moreover, there is a minimal submillimetric respiratory movement of the cranial nerves while crossing cerebrospinal fluid space within the skull. Minimal variations of the nerve position during prolonged radiation delivery time may negatively impact the amount of clinically relevant fibers of the TN receiving the minimal radiation dose necessary to produce pain relief in conjunction with the previously mentioned aspects and help explain why some patients fail radiosurgery and some patients respond sooner than others.[9] On the other hand, The TG lies in Meckel’s cave, which is a rigid structure easily identified on CT or MRI.[2] With these characteristics, TG is an easy target and there are fewer possibilities of movement during treatment, which could also explain the results obtained in our series. The few adverse effects observed in our cases could be explained by the less compact conformation of the nerve fibers in the ganglion.
In summary, once a nerve injury occurs, it induces changes in the SGC, with an increase in GAP junctions and the formation of bridges that interconnect the perineuronal sheaths, all of which increase the sensitivity of nociception receptors to a variety of chemical mediators and ionic changes. These alterations can occur not only in the TG but also in the TN.
The results suggest that radiosurgery on the TG produces elimination of pain or the reduction of its intensity in a short period. Concomitantly, there is a disruption of ephaptic transmission, secondary to demyelination. It remains to be determined whether the dose of radiosurgical treatment used is adequate or whether it is possible to decrease it. The same consideration could be made concerning the use of a single collimator located at the level of the Meckel’s cave.
Our follow-up is short to assess long-term pain relief. However, the results are very promising regarding the short term to obtain pain relief, and the long duration of this status over time. This maintained condition could further improve the results when compared with those where the target is the TN. To answer this assumption, we must have a longer follow-up.
CONCLUSSION
We postulate that radiosurgery in the TG produces an injury to the SGC that leads to the cessation of the pathological mechanisms initiated by the alteration in the neuron after a nerve injury. Reducing the gaps and functionality of the SGCs would produce a prolonged extracellular hyperpolarization. Clinically, there is a decrease in pain discharges and the maintenance of this state for a long time. In addition, there is a short latency period, minor side effects, and a high percentage of pain control.
Conflicts of interest
There are no conflicts of interest.
Footnotes
How to cite this article: Somaza S, Montilla EM. Novel theory about radiosurgery’s action mechanisms on trigeminal ganglion for idiopathic trigeminal neuralgia: Role of the satellite glial cells. Surg Neurol Int 2020;11:412.
Contributor Information
Salvador Somaza, Email: ssomaza@gmail.com.
Eglee M. Montilla, Email: egleesomaza@gmail.com.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
REFERENCES
- 1.Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996;16:4733–41. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen M, Shao Z, Zhang W, Wang Z, Zhang W, Hu H. X-knife stereotactic radiosurgery on the trigeminal ganglion to treat trigeminal neuralgia: A preliminary study. Minim Invasive Neurosurg. 2010;53:223–8. doi: 10.1055/s-0030-1269926. [DOI] [PubMed] [Google Scholar]
- 3.Costa FA, Neto FL. Satellite glial cells in sensory ganglia: Its role in pain. Rev Bras Anestesiol. 2015;65:73–81. doi: 10.1016/j.bjan.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Dandy WE. The treatment of trigeminal neuralgia by the cerebellar route. Ann Surg. 1932;96:787–95. doi: 10.1097/00000658-193210000-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: The ignition hypothesis. Clin J Pain. 2002;18:4–13. doi: 10.1097/00002508-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Dott NM. Vol. 44. London: Proceedings of the Royal Society of Medicine; 1951. Facial Pain; p. 1034. [PMC free article] [PubMed] [Google Scholar]
- 7.Durham PL, Garrett FG. Emerging importance of neuron-satellite glia interactions within trigeminal ganglia in craniofacial pain. Open Pain J. 2010;3:3–13. [Google Scholar]
- 8.Goldschmidt E, Fellows-Mayle W, Paschel EE, Niranjan A, Flickinger JC, Lunsford LD, et al. Evaluation of clinical and histologic effects of high-dose radiosurgery on rat dorsal root ganglion. World Neurosurg. 2019;124:e276–80. doi: 10.1016/j.wneu.2018.12.082. [DOI] [PubMed] [Google Scholar]
- 9.Gorgulho A. Radiation mechanisms of pain control in classical trigeminal neuralgia. Surg Neurol Int. 2012;3(Suppl 1):S17–25. doi: 10.4103/2152-7806.91606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasso G, Landi A, Alafaci C. A novel pathophysiological mechanism contributing to trigeminal neuralgia. Mol Med. 2016;22:452–4. doi: 10.2119/molmed.2016.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haines SJ, Jannetta PJ, Zorub DS. Microvascular relations of the trigeminal nerve, An anatomical study with clinical correlation. J Neurosurg. 1980;52:381–6. doi: 10.3171/jns.1980.52.3.0381. [DOI] [PubMed] [Google Scholar]
- 12.Hanani M. Intercellular communication in sensory ganglia by purinergic receptors and gap junctions: Implications for chronic pain. Brain Res. 2012;1487:183–91. doi: 10.1016/j.brainres.2012.03.070. [DOI] [PubMed] [Google Scholar]
- 13.Hanani M. Satellite glial cells in sensory ganglia: From form to function. Brain Res Brain Res Rev. 2005;48:457–76. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Jannetta PJ. Microsurgical management of trigeminal neuralgia. Arch Neurol. 1985;42:800. doi: 10.1001/archneur.1985.04210090068018. [DOI] [PubMed] [Google Scholar]
- 15.Kamiryo T, Kassell NF, Thai Q, Lopes MB, Lee KS, Steiner L. Histological changes in the normal rat brain after gamma irradiation. Acta Neurochir (Wien) 1996;138:451–9. doi: 10.1007/BF01420308. [DOI] [PubMed] [Google Scholar]
- 16.Kondziolka D, Lunsford L, Flickinger J, Young R, Vermeulen S, Duma C, et al. Stereotactic radiosurgery for trigeminal neuralgia: A multiinstitutional study using the gamma unit. J Neurosurg. 1996;84:940–5. doi: 10.3171/jns.1996.84.6.0940. [DOI] [PubMed] [Google Scholar]
- 17.Kondziolka D, Lunsford LD, Flickinger JC. Trigeminal neuralgia radiosurgery. Semin Neurosurg. 2004;15:135–41. [Google Scholar]
- 18.Kondziolka D, Perez B, Flickinger JC, Habeck M, Lunsford LD. Gamma knife radiosurgery for trigeminal neuralgia: Results and expectations. Arch Neurol. 1998;55:1524–9. doi: 10.1001/archneur.55.12.1524. [DOI] [PubMed] [Google Scholar]
- 19.Love S, Coakham H. Trigeminal neuralgia: Pathology and pathogenesis. Brain. 2001;124:2347–60. doi: 10.1093/brain/124.12.2347. [DOI] [PubMed] [Google Scholar]
- 20.Luo D, Lin R, Luo L, Li Q, Chen T, Qiu R, et al. Glial plasticity in the trigeminal root entry zone of a rat trigeminal neuralgia animal model. Neurochem Res. 2019;44:1893–902. doi: 10.1007/s11064-019-02824-2. [DOI] [PubMed] [Google Scholar]
- 21.Majoie CB, Hulsmans FJ, Verbeeten B, Jr, Castelijns JA, van Beek EJ, Valk J, et al. Trigeminal neuralgia: Comparison of two MR imaging techniques in the demonstration of neurovascular contact. Radiology. 1997;204:455–60. doi: 10.1148/radiology.204.2.9240535. [DOI] [PubMed] [Google Scholar]
- 22.Messlinger K, Russo AF. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia. 2018;39:1661–74. doi: 10.1177/0333102418786261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohara PT, Vit JP, Bhargava A, Romero M, Sundberg C, Charles AC, et al. Gliopathic pain: When satellite glial cells go bad. Neuroscientist. 2009;15:450–63. doi: 10.1177/1073858409336094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pannese E. The structure of the perineuronal sheath of satellite glial cells (SGCs) in sensory ganglia. Neuron Glia Biol. 2010;6:3–10. doi: 10.1017/S1740925X10000037. [DOI] [PubMed] [Google Scholar]
- 25.Régis J, Tuleasca C, Resseguier N, Carron R, Donnet A, Gaudart J, et al. Long-term safety and efficacy of gamma knife surgery in classical trigeminal neuralgia: A 497-patient historical cohort study. J Neurosurg. 2016;124:1079–87. doi: 10.3171/2015.2.JNS142144. [DOI] [PubMed] [Google Scholar]
- 26.Sabalys G, Juodzbalys G, Wang HL. Aetiology and pathogenesis of trigeminal neuralgia: A comprehensive review. J Oral Maxillofac Res. 2013;3:e2. doi: 10.5037/jomr.2012.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scemes E, Giaume C. Astrocyte calcium waves: What they are and what they do. Glia. 2006;54:716–25. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinder V, Devor M. Structural basis of neuron-to-neuron cross-excitation in dorsal root ganglia. J Neurocytol. 1994;23:515–31. doi: 10.1007/BF01262054. [DOI] [PubMed] [Google Scholar]
- 29.Somaza S, Hurtado W, Montilla E, Ghaleb J. Gamma knife radiosurgery to the trigeminal ganglion for treatment of trigeminal neuralgia secondary to vertebrobasilar ectasia. Surg Neurol Int. 2014;5(Suppl 16):S580–5. doi: 10.4103/2152-7806.148056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somaza S, Montilla E, Mora M. Gamma knife radiosurgery on the trigeminal ganglion for idiopathic trigeminal neuralgia: Results and review of the literature. Surg Neurol Int. 2012;3(Suppl 1):S17–25. doi: 10.25259/SNI-134-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev. 2009;33:784–92. doi: 10.1016/j.neubiorev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Vinterhøj HS, Stensballe A, Duroux M, Gazerani P. Characterization of rat primary trigeminal satellite glial cells and associated extracellular vesicles under normal and inflammatory conditions. J Proteomics. 2019;190:27–34. doi: 10.1016/j.jprot.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Vit JP, Jasmin L, Bhargava A, Ohara PT. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biol. 2006;2:247–57. doi: 10.1017/s1740925x07000427. [DOI] [PMC free article] [PubMed] [Google Scholar]


