Abstract
Background:
Peripheral schwannomas can be misdiagnosed or mistreated as they can mimic other subcutaneous lesions, leading to wrong diagnosis and, therefore, to improper treatment.
Case Description:
A 23-years-old male presented a painful growing nodule at the left popliteal fossa, with distally irradiated pain. A first magnetic resonance imaging depicted a heterogeneous lesion between common peroneal and sural nerves but, surprisingly, the patient was submitted to perilesional injection of ozone-oxygen mixture, causing the onset of intense neuropathic pain. A second MRI showed a morphological change of tumor characteristics. He finally underwent surgery but, intraoperatively, inter-fascicular fibrous adherences were noticed, making the tumor removal more difficult and riskier. The histopathological diagnosis was of schwannoma with areas of foreign body reaction.
Conclusion:
The injection of ozone or other substances within a subcutaneous swelling should be avoided, before a complete imaging assessment; because of such swelling could be a peripheral nerve schwannoma. The correct assessment of a lesion of the limbs determining radiating pain should be carefully demanded to a thorough history, clinical examination, and appropriate imaging technique. To avoid incorrect management, the treatment of such tumors should be performed in the first place by dedicated equips with proven expertise in this field.
Keywords: Common peroneal nerve, Foreign body reaction, Nerve tumors, Oxygen-ozone therapy, Schwannoma

INTRODUCTION
Schwannomas, the most common peripheral nerve sheath tumors (PNST), are well-circumscribed lesions within peripheral nerves.[17] They typically appear as progressive enlarging subcutaneous swelling, with local and radiating pain. To prevent neurological impairment and to reduce pain, surgical removal is usually indicated.[4,17,20] Safe excision relies on a sufficiently large tumor exposure, followed by pseudocapsule or true capsule intraoperative electrical stimulation, to identify functional fascicles running outside the lesion. Then, after the incision of the silent entry zone, intraneural dissection leads to progressive enucleation.[7,13,16] A complete preoperative evaluation consists of ultrasonography, electromyography (EMG), and contrast-enhanced magnetic resonance imaging (MRI). PNST can be misdiagnosed or mistreated because they can mimic other subcutaneous lesions (epidermoid cysts, lipomas, and lymphomas); despite fine-needle aspiration and needle core biopsy are sometimes proposed,[10] these procedures carry the risk of transient or persistent fascicle damages, especially when performed by a physician other than a neurosurgeon.
During the past years, oxygen-ozone therapy (OT) has been employed in several pathologies, mainly in painful syndromes,[2,12] through intraforaminal or paravertebral injection.[8] OT is based on the chemical properties of ozone, an unstable allotropic form of oxygen.[1] OT is generally a safe procedure, widely spread among orthopedists, radiologists, anesthesiologists, pain specialists, and neurosurgeons too. However, its effect has not been proven;[15] also, its indiscriminate use should be carefully evaluated, due to the induced adhesions in the target zone and the surrounding tissues.[18]
We report a case of a young male harboring a lower limb schwannoma, previously treated with percutaneous intralesional OT, causing morphological, and architectural tumor’s alteration. The subsequent the formation of strong adhesions between the neoplasm and the originating nerve finally led to a more dangerous surgical procedure, resulting in transient slight neurological worsening.
CASE PRESENTATION
In May 2019, a 23-year-old male was evaluated at our institution for a 1-year history of a painful subcutaneous nodule at the left popliteal fossa. The pain, described as an “electric shock.” was also irradiated to the lateral surface of the leg. He then underwent ultrasonography, with a first diagnosis of subcutaneous cyst; subsequently, he was submitted in another institution, under local anesthesia, to an attempt for surgical removal by a dermatology specialist; however, the procedure was aborted due to the onset of acute pain. A first MRI [Figure 1a and b] was, therefore, performed in November 2018, depicting a homogeneous lesion of about 12 × 13 mm at the branching point between the left common peroneal and sural nerves. Surprisingly, the patient was not evaluated by a neurosurgeon at that moment. He was instead evaluated by another physician in an outpatient clinic and submitted to 5 mL intra- and perilesional injection of ozone-oxygen mixture. The procedure caused a dramatic, intense, and durable local pain in addition to the previously experienced radiating pain to the lateral leg surface. He was finally referred to our institution, 6 months after the first MRI, for definitive treatment. On admission, the neurological examination showed a slight left foot dorsiflexion deficit, without sensory impairment. An EMG confirmed a mild impairment of motor conduction at the level of the fibula’s head. The subcutaneous swelling was suspected for schwannoma of the common peroneal nerve. A second MRI [Figure 1c and d] with contrast medium showed an increase of tumor’s dimensions (14 × 16 mm) and, noticeably, also a change in tumor morphology: It had become heterogeneous, with a rim of contrast enhancement and a hypointense central core.
Figure 1:
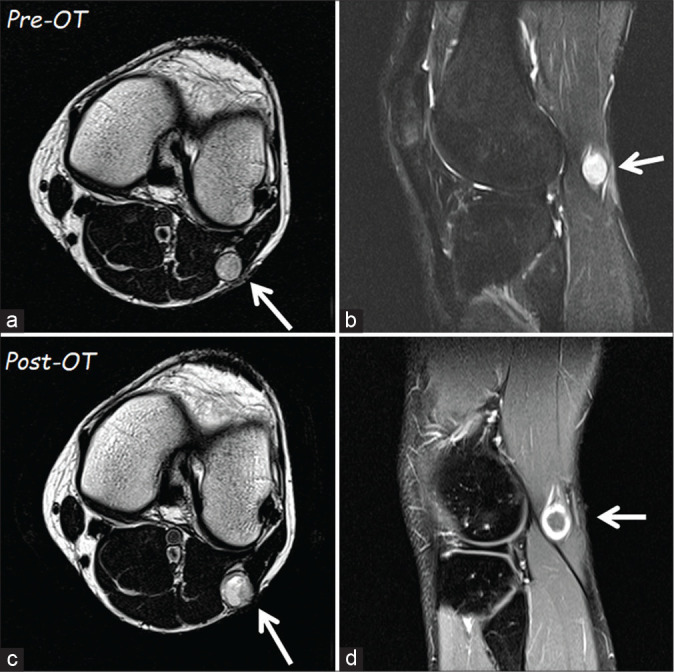
The first magnetic resonance imaging (MRI) of November 2018 (a – T1 axial and b – sagittal scans) showed a homogeneous lesion (arrow) of 12 × 13 mm at the fibula’s head level. The second MRI in axial (c) scan, performed after oxygen-ozone therapy, documented a slight increase of the tumor size. It is evident a change in tumor intensity, which had become inhomogeneous, and with a rim of contrast enhancement (d).
Surgical treatment
The patient finally underwent surgery under microscopic view, with intraoperative neurophysiological monitoring. Continuous free-running and stimulus-triggered EMG was used to identify functioning nerves and to localize the safest entry point inside the tumor capsule. Sodium fluorescein 1 mg/kg was intravenously injected by the anesthesiologist, on completion of the induction, as previously described.[19,21]
A slight curvilinear incision at the level of the lateral and inferior part of the popliteal fossa was performed and, after subcutaneous dissection, we identified an encapsulated lesion between the common peroneal and sural nerves. Since the dissection of the pseudocapsule, a fibrous reaction was noticed, which made it slightly more challenging to identify the tumor capsule and a silent, safe zone without positive electrophysiological responses. Several strong adherences were present, and the consistency of the neoplasm was harder than usual. These factors determined the impossibility to remove the tumor en block; it was instead removed through a gentle piecemeal resection [Figure 2]. Postremoval IOM showed slight impairment in motor nerve response, while the sensory response (sural nerve) was unchanged.
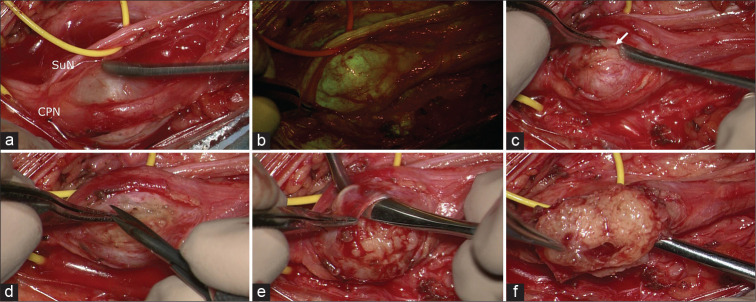
Figure 2:
Intraoperative photographs under microscopic view: in (a), the schwannoma is visible between common peroneal nerve and sural nerve. After the YELLOW560 filter activation, the tumor showed a fluorescein uptake more intense than the surrounding nerves (b). The capsule (arrow in c) appeared extremely thick and adherent to the tumor and the originating nerve, requiring multiple manipulations for intraneural dissection and schwannoma removal (d-f).
Histopathological analysis
Histological examination [Figure 3] revealed a nerve sheath tumor constituted by well-differentiated Schwann cells, with a thin capsule, loose Antoni B, and compact Antoni A areas, characterized by nuclear palisades consistent with Verocay bodies. Ectatic vessels, cystic-hemorrhagic areas, and foci of acute and chronic inflammatory infiltrates were also identified. The immunohistochemistry showed S100 positivity. The microscopic study highlighted areas of foreign body reaction with hematoxylin and eosin stain (H and E), along with gray-yellowish, uniformly sized microspheres phagocytized by multinucleated giant cells (CD68 immunostained). The final diagnosis was of schwannoma with foreign body reaction.
Figure 3:
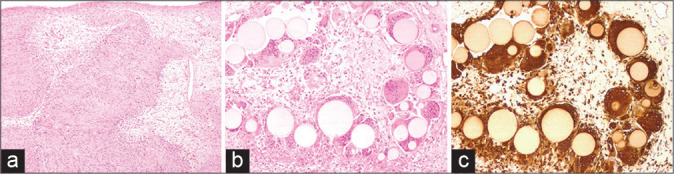
The histological examination revealed (a) a nerve sheath tumor constituted by well-differentiated Schwann cells, characterized by nuclear palisades consistent with Verocay bodies (H and E, ×40). The microscopic study at ×100 highlighted foci of foreign body reaction (H and E in b) with uniformly sized, grey-yellowish microspheres phagocytized by multinucleated giant cells (immunostained with CD68 in c).
Postoperative outcome
After surgery, the patient presented worsening of the left foot extension, while local and irradiated pain disappeared. He was then referred to physical therapy: 3-months after surgery, the motor deficit was almost completely regressed. At 12-months follow-up examination, the motor function had completely recovered. The last nerve ultrasonography depicted a discreetly preserved structure, without focal section enlargement.
DISCUSSION
Surgery is the gold-standard treatment in peripheral nerve schwannomas: after capsule and pseudocapsule incision, it is possible to remove the tumor safely, following the right surgical steps.[13,17] In our case, this procedure was more complex, due to the anatomical changes secondary to OT and the subsequent inflammatory reaction to foreign bodies, as demonstrated by histopathology. The manipulation of peritumoral fascicles determined, in our case, a slight postoperative worsening of the pre-existent foot extension deficit. It is noticeable that the patient was evaluated 1 year after the onset of symptoms, and 6-month after the neuroradiological imaging.
Despite the lack of specific literature about the delay in the diagnosis of the lower limb schwannomas, PNST can sometimes be confused with other diseases, as lipomas.[3,14] For example, misdiagnoses and consequent mistreatments have been reported for pelvic schwannoma originating from the sacral nerves, confused with ovarian teratoma,[23] or in retroperitoneal schwannoma determining left hip pain radiating into the posterolateral portion of the thigh, leading to hip replacement before the correct therapeutic strategy.[9] Therefore, a prompt preoperative diagnosis plays a relevant role in guiding the treatment. This consideration is also correct for the lower limb schwannomas: Nerve imaging provides information about lesion morphology, anatomic location, relationship of lesions to surrounding soft tissue, also identifying peripheral nerve lesions that are not clearly identifiable on neurophysiological testing.[22] All these findings are used to establish the correct treatment, which is in the majority of cases, surgical removal.
OT has been employed for the treatment of several conditions, in particular loco-regional pain syndromes, low back pain, arteriosclerosis, and arthritis.[11] The ozone for medical use, mainly in degenerative spinal diseases, has a direct mechanical effect on proteoglycans composing the nucleus pulposus, whereas the specific effects on neural structures are not well known. In an experimental setting regarding acute spinal injuries, ozone combined with steroid therapy seems to hasten clinical and to increase histological recovery compared with steroids alone.[5] Intralesional OT has been described for the treatment of subcutaneous swellings as lipomas: Kara recently reported a case of a 31-year-old woman, harboring a painful mass on the forearm which determined local pain and paresthesia, treated with local ozone injection.[6] This treatment determined the sudden disappearance of local pain and the reduction of tumor dimensions. The author assessed that the effectiveness of OT in lipodystrophies is secondary not only to fatty acids splitting but also to local increase of oxygen. As a matter of fact, OT determines morphological changes inside the injected tissues through several mechanisms. In 2015 Vanni et al. presented a series of 186 patients who underwent microsurgery for lumbar disc herniation or lumbar stenosis. Among them, 23 patients were previously treated with OT through intraforaminal approach, while 28 received intraforaminal steroid injections before surgery.[18] Intraoperatively, the authors noted strong adhesions between the roots and the dural sac or the disc, sometimes with a difficult-to-resect envelope, and a complex subversion of normal local anatomy. These findings were observed only in the patients who received OT, but not in patients submitted to steroid injections.
In our case, the schwannoma was harder and with several adherences within the fascicles, leading to maneuvers that are more complex and, potentially, more dangerous, to separate the tumor from the intact fascicles. The need for such maneuvers, despite the intraoperative neurophysiological monitoring, finally led to the transient neurological worsening.
CONCLUSION
The injection of ozone or other substances within a subcutaneous swelling should be avoided, before a complete imaging evaluation; because of such swelling could be a peripheral nerve schwannoma. The management of tumors should be demanded to neurosurgeons with expertise in this field to avoid a wrong treatment.
Footnotes
How to cite this article: Vetrano IG, Acerbi F, Marucci G, Nazzi V. The effect of ozone injection within a common peroneal nerve schwannoma: A mistreatment due to a misdiagnosis. Surg Neurol Int 2020;11:413.
Contributor Information
Ignazio Gaspare Vetrano, Email: ignazio.vetrano@istituto-besta.it.
Francesco Acerbi, Email: francesco.acerbi@istituto-besta.it.
Gianluca Marucci, Email: gianluca.marucci@istituto-besta.it.
Vittoria Nazzi, Email: vittoria.nazzi@istituto-besta.it.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated during the current study.
Declaration of patient consent
The study protocol was in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration of 1970 as amended in 2000.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Apuzzo D, Giotti C, Pasqualetti P, Ferrazza P, Soldati P, Zucco GM. An observational retrospective/horizontal study to compare oxygen-ozone therapy and/or global postural reeducation in complicated chronic low back pain. Funct Neurol. 2014;29:31–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Elvis AM, Ekta JS. Ozone therapy: A clinical review. J Nat Sci Biol Med. 2011;2:66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fass G, Hossey D, Nyst M, Smets D, Saligheh EN, Duttmann R, et al. Benign retroperitoneal schwannoma presenting as colitis: A case report. World J Gastroenterol. 2007;13:5521–4. doi: 10.3748/wjg.v13.i41.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guha D, Davidson B, Nadi M, Alotaibi NM, Fehlings MG, Gentili F, et al. Management of peripheral nerve sheath tumors: 17 years of experience at Toronto Western Hospital. J Neurosurg. 2018;128:1226–34. doi: 10.3171/2017.1.JNS162292. [DOI] [PubMed] [Google Scholar]
- 5.Gürkan G, Sayin M, Kizmazoglu C, Erdogan MA, Yigitturk G, Yilmaz HE, et al. Evaluation of the neuroprotective effects of ozone in an experimental spine injury model. J Neurosurg Spine. 2020:1–9. doi: 10.3171/2020.2.SPINE191439. [DOI] [PubMed] [Google Scholar]
- 6.Kara Ö Kara M. Lipolysis of a painful lipoma with ozone: The role of ultrasound in the diagnosis and quantification of the treatment. Med Gas Res. 2019;9:168. doi: 10.4103/2045-9912.267000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University health sciences center. J Neurosurg. 2005;102:246–55. doi: 10.3171/jns.2005.102.2.0246. [DOI] [PubMed] [Google Scholar]
- 8.Muto M, Giurazza F, Silva RP, Guarnieri G. Rational approach, technique and selection criteria treating lumbar disk herniations by oxygen-ozone therapy. Interv Neuroradiol. 2016;22:736–40. doi: 10.1177/1591019916659266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ongley D, Shipton E. Pain images: Hip pain-wrong diagnosis, wrong operation. Pain Med. 2010;11:942–5. doi: 10.1111/j.1526-4637.2010.00834.x. [DOI] [PubMed] [Google Scholar]
- 10.Resnick JM, Fanning CV, Caraway NP, Varma DG, Johnson M. Percutaneous needle biopsy diagnosis of benign neurogenic neoplasms. Diagn Cytopathol. 1997;16:17–25. doi: 10.1002/(sici)1097-0339(199701)16:1<17::aid-dc5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 11.Rilling S, Viebahn R. Heidelberg: KF Haug Publishers; 1987. The Use of Ozone in Medicine. [Google Scholar]
- 12.Rowen RJ, Robins H. Ozone therapy for complex regional pain syndrome: Review and case report. Curr Pain Headache Rep. 2019;23:41. doi: 10.1007/s11916-019-0776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell SM. Preserve the nerve: Microsurgical resection of peripheral nerve sheath tumors. Neurosurgery. 2007;61:113–7. doi: 10.1227/01.neu.0000289724.89588.bc. discussion 117-8. [DOI] [PubMed] [Google Scholar]
- 14.Singh V, Kapoor R. Atypical presentations of benign retroperitoneal schwannoma: Report of three cases with review of literature. Int Urol Nephrol. 2005;37:547–9. doi: 10.1007/s11255-004-4705-5. [DOI] [PubMed] [Google Scholar]
- 15.Smith N, Wilson A, Gandhi J, Vatsia S, Khan S. Ozone therapy: An overview of pharmacodynamics, current research, and clinical utility. Med Gas Res. 2017;7:212–9. doi: 10.4103/2045-9912.215752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone JJ, Spinner RJ. Go for the gold: A plane and simple technique for resecting Benign peripheral nerve sheath tumors. Oper Neurosurg (Hagerstown) 2020;18:60–8. doi: 10.1093/ons/opz034. [DOI] [PubMed] [Google Scholar]
- 17.Tiel R, Kline D. Peripheral nerve tumors: Surgical principles, approaches, and techniques. Neurosurg Clin N Am. 2004;15:167–75. doi: 10.1016/j.nec.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Vanni D, Galzio R, Kazakova A, Pantalone A, Sparvieri A, Salini V, et al. Intraforaminal ozone therapy and particular side effects: Preliminary results and early warning. Acta Neurochir (Wien) 2016;158:491–6. doi: 10.1007/s00701-015-2545-y. [DOI] [PubMed] [Google Scholar]
- 19.Vetrano IG, Acerbi F, Falco J, Devigili G, Rinaldo S, Messina G, et al. Fluorescein-guided removal of peripheral nerve sheath tumors: A preliminary analysis of 20 cases. J Neurosurg. 2019:1–10. doi: 10.3171/2019.9.JNS19970. [DOI] [PubMed] [Google Scholar]
- 20.Vetrano IG, Lucarella F, Dalolio M, di Cristofori A, Nataloni IF, Tiberio F, et al. The importance of predicting factors in the surgical outcome of peripheral nerve sheath tumors. J Neurol Surg A Cent Eur Neurosurg. 2014;75:104–9. doi: 10.1055/s-0033-1348351. [DOI] [PubMed] [Google Scholar]
- 21.Vetrano IG, Saletti V, Nazzi V. Fluorescein-guided resection of plexiform neurofibromas: How i do it. Acta Neurochir (Wien) 2019;161:2141–5. doi: 10.1007/s00701-019-04038-5. [DOI] [PubMed] [Google Scholar]
- 22.Zaidman CM, Seelig MJ, Baker JC, Mackinnon SE, Pestronk A. Detection of peripheral nerve pathology: Comparison of ultrasound and MRI. Neurology. 2013;80:1634–40. doi: 10.1212/WNL.0b013e3182904f3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou F, Dai M, Zhang B, Nie T. Misdiagnosis of a giant intrapelvic schwannoma: A case report. Oncol Lett. 2013;6:1646–8. doi: 10.3892/ol.2013.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated during the current study.