Abstract
Background:
Spontaneous intracranial hypotension (SIH) is a rare condition that can be very debilitating. SIH is well understood to be due to a CSF leak, however, identifying the source of the leak is still a challenge. We are presenting a case of Type 4 CSF leak and reviewing the related literature.
Case Description:
A 46-year-old female presenting with intractable orthostatic headaches was diagnosed with SIH. She was unable to mobilize due to the severity of her symptoms. MRI scans of the brain and spine did not identify a source of the leak. After failing conservative therapy and multiple epidural blood patches, the patient underwent surgery which resulted in significant improvement in symptoms.
Conclusion:
This study has shown that surgical intervention improves symptoms in patients who do not have an identifiable source of CSF leak. Further studies need to be done to fully understand the role of surgery in Type 4 CSF leaks.
Keywords: Spontaneous intracranial hypotension, Surgery, Type 4 CSF leak

INTRODUCTION
Spontaneous intracranial hypotension (SIH) is a rare condition that occurs in about 5 out of 100,000 individuals. It commonly affects middle-aged individuals and tends to occur more frequently in women.[2] The most common presentation of this condition is a headache in the upright position with onset occurring within 15 min after sitting up or standing.[14] The headaches can range from mild to severely debilitating. Other associated symptoms are the improvement in headaches when lying down, nausea and vomiting which occur in about 50% of patients, stiff neck, and blurry vision.[14] The current understanding of the pathophysiology of SIH is that a weakening in the dura causes a leak in the epidural space.[14] Connective tissue abnormalities and/or disorders such as Marfan syndrome or autosomal polycystic kidney disease are seen in a large proportion of patients with SIH.[4,14] Mechanical injury has also been considered a possible cause of the CSF leak. CSF leaks in SIH have been classified into four categories based on the etiology of the leak. Type 1 leaks occur when there is a tear in the dura, Type 2 leaks occur due to meningeal diverticula, Type 3 leaks are a result of CSF venous fistulas, and leaks that do not have an identifiable source are categorized as Type 4 leaks.[10] SIH is often treated conservatively with bed rest, oral hydration, copious amounts of caffeine, and an abdominal binder.[14] When conservative management fails or if the patient wants more immediate relief from symptoms, blood patches are used to seal the CSF leak. Surgical intervention is considered when blood patches fail to resolve a patient’s symptoms.[14] To the best of our knowledge, no randomized controlled trials have studied treatment options for SIH and best practices for the treatment of SIH are not yet fully established.[5] Surgery has commonly been performed on CSF leaks where the source of the leak is identified, however, surgical intervention is rarely performed on Type 4 leaks where the cause of the CSF leak is unknown. According to case report (CARE) guidelines,[9] we present a case of a Type 4 leak that failed to respond to nonsurgical treatments with a subsequent surgical intervention that resulted in remarkable improvement in symptoms and functionality. Furthermore, we reviewed the literature on type 4 leaks. The patient provided informed consent for the writing of this case report.
CASE REPORT
A 46-year-old female presented with severe bifrontal orthostatic headaches with vomiting. She could not continue working due to her symptoms. MRI scan of the brain showed pachymeningeal enhancement and a progressive acquired Chiari malformation in keeping with findings of intracranial hypotension [Figure 1a and b]. The intracranial hypotension was idiopathic as the patient had not had any trauma, fractures, surgery, or lumbar puncture before the onset of her orthostatic headaches. No abnormalities were noted on her physical examination including the fundus examination. Multiple MRI scans of the brain and spinal cord were done to identify the source of the leak, but the source could not be identified. The patient was given dexamethasone 1.5 mg daily to help with the headaches and an epidural blood patch was done. After the failure of the first blood patch, the patient underwent two more epidural blood patches and dihydroergotamine mesylate 0.3 mg IV every 8 h for 72 h was started for symptomatic relief. Dihydroergotamine mesylate resulted in symptomatic relief for the first 5 hours but the headache reoccurred in the 3 hours before the next dose. The blood patches resulted in a mild reduction in the severity of her headaches but did not completely resolve the symptoms. The patient also developed a headache above the left eye with blurry vision after the third blood patch that resolved after 1.5 weeks. The patient was reluctant to have any further blood patches after this experience. A CT myelogram of the cervical, thoracic, and lumbar spine was done to identify the source of the leak but was found to be negative. Dexamethasone 1.5 mg and oxycodone/ acetaminophen 2.5 mg every 4 hours were the only two agents that provided any persistent improvements in her symptoms. The patient was admitted to the hospital due to intractable vomiting secondary to her headaches on several occasions. Ondansetron 8 mg PO before meal 3 times a day improved her nausea and vomiting. Pharmacological treatments for the headaches proved to be ineffective and an MRI of the brain 3 years after the original MRI scans showed an acquired Chiari malformation [Figure 1c]. An MRI of the thoracic spine was done at this point which did not identify the source of the CSF leak. The patient was referred to neurosurgery for a posterior fossa decompression with a C1 laminectomy which relieved her symptoms mildly for two months, however, it got worse so a T11-T12 laminotomy and open thoracic and lumbar blood patching were done. An epidural catheter was inserted in the thoracic region and 10 cc of blood was injected. 8 cc of blood was injected in the lumbar spine through another epidural catheter. Two months after surgery, the patient had a reduction in her headaches and was able to walk, and there was a reversal of the cerebellar ectopia on MRI [Figure 1d-f]. Three months after the surgery, the patient continued to improve with on and off headaches. An 8-year follow-up phone call was done, and the patient is still having mild on and off headaches.
Figure 1:
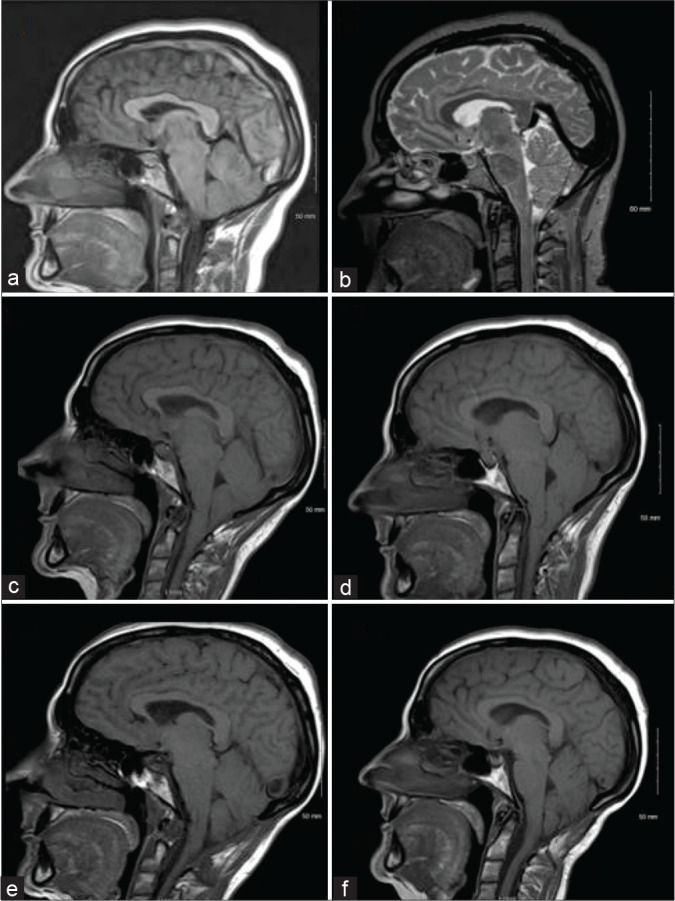
Progression of the cerebellar ectopia. The patient’s symptoms began in September 2007 and posterior fossa decompression was done in June 2012. (a) April 2008, (b) October 2008, (c) December 2011 (showing acquired Chiari malformation, flattening of the pons, and sagging of the cerebellar tonsils slightly below the level of the C1 arch), (d) February 2012, (e) September 5, 2012, (f) December 2014.
Surgical procedures
Posterior fossa decompression
The patient was intubated, her head was held in Sugita pins and she was turned prone on the table. The patient was given preoperative antibiotics. A midline incision was made between the level of C2 and the occiput. The lower part of the occipital bone and the foramen magnum was removed. As the tonsils were going below the C1 level on the MRI, a C1 laminectomy was also conducted. The dura was opened with the arachnoid left intact with no evidence of CSF leak. The lower part of the cerebellar tonsils was seen at the upper edge of the C2 lamina. A duraplasty was done using a piece of fascia lata graft with no attempt to dissect the arachnoid or the tonsils and the incision was closed in layers.
Open thoracic and lumbar blood patching
A midline incision was made over the T11-T12 spinous process. The T11 and T12 spinous process and lamina were exposed in subperiosteal fascial dissection. A laminotomy at the T11-T12 level was done on the patient’s left side. The ligamentum flavum was removed and a 6 French (Fr) pediatric catheter was passed in the epidural space cranially for 20 cm. A laminotomy at the T11-T12 level was also done on the right side with an epidural catheter passed caudally into the lumbar spine for 15 cm. Venous blood was collected from an intravenous line in the patient’s forearm and 10 CCs were injected into the thoracic spine and 8 CCs in the lumbar spine while withdrawing the catheter. The catheters were removed, and the incision was closed in layers.
DISCUSSION
We presented a case where a patient with an unidentifiable CSF leak, debilitating headaches, and intracranial hypotension had significant improvements in symptoms and functional outcome after surgery. We reviewed the PubMed using the search terms “spontaneous intracranial hypotension” and “type 4 CSF leak.” In about 10–30% of patients with SIH, symptoms persist even after repeated epidural blood patches.[1] In such cases, surgery is recommended.[14] Several case studies have reported benefit for patients suffering from SIH after surgery.[1,7,11,12] A case series of 10 patients with SIH underwent either ligation of diverticula or packing of the epidural space.[11] All 10 cases resulted in the resolution of headaches.[11] Schievink et al. (1994) presented a case of SIH with a subarachnoid diverticulum that was successfully treated with ligation.[12] A study conducted by Inenaga et al. (2001) reported a complete resolution of symptoms after the C2-C3 spinal nerve root pouch was sealed off from the subarachnoid space.[7] About 90% of patients report symptomatic relief after surgery in cases where the source of the CSF leak is known and relapses are rare.[1] However, surgical intervention is rarely performed on Type 4 leaks where the cause of the CSF leak is unknown. One study briefly mentioned that saline infusion in the epidural space and lumbar dural reduction surgery have shown some symptomatic relief for Type 4 leaks.[2] Lumbar dural reduction surgery has also proven to be useful in a case where a CSF leak was no longer detectable on imaging.[13]
The headaches seen in patients with spontaneous intracranial hypotension are postulated to occur due to low intracranial CSF volume.[1] The buoyancy created by the CSF fluid reduces the effective weight of the brain from 1500 g to 50 g.[1] The remaining weight is distributed across pain-sensitive structures in the cranium including meninges, cranial nerves, and veins.[1] Reduced buoyancy due to low intracranial CSF volume causes traction on these structures resulting in headaches.[1] Cerebellar ectopia and brain sagging may decrease the flow of CSF into the brain from the spinal cord.[1] Doing a posterior fossa decompression in this case may have increased the flow of CSF from the spinal cord into the cranium, thus, increasing intracranial CSF volume and reducing the pressure on pain-sensitive structures in the cranium. Epidural blood patches work by redistributing CSF from the spinal canal into the intracranial region, thereby, increasing intracranial CSF volume.[13] In our case, after the failure of several blind blood patches, a blood patch was injected at the thoracic and lumbar region. The efficacy of blind blood patches compared to targeted blood patches is still unclear. One retrospective case series reported better long-term symptomatic relief with targeted thoracic blood patches compared to lumbar blood patches.[6] A study by Cho et al. (2011) showed symptomatic relief in 87% of patients with a targeted blood patch compared to 52% in patients with a blind blood patch.[3] One study reported lower rates of receiving a second blood patch with the first blood patch being targeted instead of being blind.[8]
CONCLUSION
The current evidence, including the case presented in this report, shows that surgical intervention can be an effective way to manage SIH in patients who fail to respond to conservative management. Surgery is rarely considered a treatment option for Type 4 CSF leaks. As there is a paucity of case–control studies, cohort studies, and randomized controlled trials on this topic, the current level of evidence is weak and is only based on case series and case reports. Thus, further studies need to be conducted to fully understand the efficacy of targeted blood patches for clinical improvement in SIH with an unknown etiology of CSF leak, and to date, the management of these types of CSF leaks should be individualized to try to obtain the best patients’ outcome.
Footnotes
How to cite this article: Shahab S, Soliman MA, Alkhamees AF, Eaton S, Quint E, Im J, et al. Surgical intervention for spontaneous intracranial hypotension Type 4 CSF leak: A case report. Surg Neurol Int 2020;11:421.
Contributor Information
Saba Shahab, Email: sshahab2021@meds.uwo.ca.
Mohamed A. R. Soliman, Email: moh.ar.sol@kasralainy.edu.eg.
Abdullah F. Alkhamees, Email: alkhamees@qumed.edu.sa.
Sydney Eaton, Email: seaton2021@meds.uwo.ca.
Elise Quint, Email: equint2021@meds.uwo.ca.
Jacob Im, Email: jacob.im.edu@gmail.com.
Avalon O’Connor, Email: aoconnor2021@meds.uwo.ca.
Erika Haberfellner, Email: ehaberfellner2021@meds.uwo.ca.
Abdalla Shamisa, Email: ashamisa@yahoo.ca.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Beck J, Häni L, Ulrich CT, Fung C, Jesse CM, Piechowiak E, et al. Diagnostic challenges and therapeutic possibilities in spontaneous intracranial hypotension. Clin Transl Neurosci. 2018;2:1–11. [Google Scholar]
- 2.Chan SM, Chodakiewitz YG, Maya MM, Schievink WI, Moser FG. Intracranial hypotension and cerebrospinal fluid leak. Neuroimaging Clin N Am. 2019;29:213–26. doi: 10.1016/j.nic.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Cho KI, Moon HS, Jeon HJ, Park K, Kong DS. Spontaneous intracranial hypotension: Efficacy of radiologic targeting vs blind blood patch. Neurology. 2011;76:1139–44. doi: 10.1212/WNL.0b013e318212ab43. [DOI] [PubMed] [Google Scholar]
- 4.Davenport RJ, Chataway SJ, Warlow CP. Spontaneous intracranial hypotension from a CSF leak in a patient with Marfan’s syndrome. J Neurol Neurosurg Psychiatry. 1995;59:516–9. doi: 10.1136/jnnp.59.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson B, Nassiri F, Mansouri A, Badhiwala JH, Witiw CD, Shamji MF, et al. Spontaneous intracranial hypotension: A review and introduction of an algorithm for management. World Neurosurg. 2017;101:343–9. doi: 10.1016/j.wneu.2017.01.123. [DOI] [PubMed] [Google Scholar]
- 6.Feltracco P, Galligioni H, Barbieri S, Ori C. Thoracic epidural blood patches in the treatment of spontaneous intracranial hypotension: A retrospective case series. Pain Physician. 2015;18:343–8. [PubMed] [Google Scholar]
- 7.Inenaga C, Tanaka T, Sakai N, Nishizawa S. Diagnostic and surgical strategies for intractable spontaneous intracranial hypotension, Case report. J Neurosurg. 2001;94:642–5. doi: 10.3171/jns.2001.94.4.0642. [DOI] [PubMed] [Google Scholar]
- 8.Rettenmaier LA, Park BJ, Holland MT, Hamade YJ, Garg S, Rastogi R, et al. Value of targeted epidural blood patch and management of subdural hematoma in spontaneous intracranial hypotension: Case report and review of the literature. World Neurosurg. 2017;97:27–38. doi: 10.1016/j.wneu.2016.09.076. [DOI] [PubMed] [Google Scholar]
- 9.Riley DS, Barber MS, Kienle GS, Aronson JK, von SchoenAngerer T, Tugwell P, et al. CARE guidelines for case reports: Explanation and elaboration document. J Clin Epidemiol. 2017;89:218–35. doi: 10.1016/j.jclinepi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Schievink WI, Maya MM, Jean-Pierre S, Nuño M, Prasad RS, Moser FG. A classification system of spontaneous spinal CSF leaks. Neurology. 2016;87:673–9. doi: 10.1212/WNL.0000000000002986. [DOI] [PubMed] [Google Scholar]
- 11.Schievink WI, Morreale VM, Atkinson JL, Meyer FB, Piepgras DG, Ebersold MJ. Surgical treatment of spontaneous spinal cerebrospinal fluid leaks. J Neurosurg. 1998;88:243–6. doi: 10.3171/jns.1998.88.2.0243. [DOI] [PubMed] [Google Scholar]
- 12.Schievink WI, Reimer R, Folger WN. Surgical treatment of spontaneous intracranial hypotension associated with a spinal arachnoid diverticulum, Case report. J Neurosurg. 1994;80:736–9. doi: 10.3171/jns.1994.80.4.0736. [DOI] [PubMed] [Google Scholar]
- 13.Schievink WI. A novel technique for treatment of intractable spontaneous intracranial hypotension: Lumbar dural reduction surgery. Headache. 2009;49:1047–51. doi: 10.1111/j.1526-4610.2009.01450.x. [DOI] [PubMed] [Google Scholar]
- 14.Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286–96. doi: 10.1001/jama.295.19.2286. [DOI] [PubMed] [Google Scholar]


