Abstract
Over 4000 preventable injuries due to medication errors occur each year in any given hospital. Smart pumps have been widely introduced as one means to prevent these errors. Although smart pumps have been implemented to prevent errors, they fail to prevent specific types of errors in the medication administration process and may introduce new errors themselves. As a result, unique prevention strategies have been implemented by providers. No catalog of smart pump error types and prevention strategies currently exist. The aim of this study is to review and catalog the types of human-based errors related to smart pump use identified in the literature and to summarize the associated error prevention strategies. Literature pertaining to human-based errors associated with smart pumps was searched in MEDLINE, PubMed, PubMed Central, and CINAHL. Studies related to smart pump implementation, other types of pumps, and mechanical failures were excluded. Final selections were mapped for error types and associated prevention strategies. A total of 1,177 articles were initially identified, and 105 articles were included in the final review. Extraction of error types and prevention strategies resulted in the identification of 18 error types and 10 prevention strategies. Through a comprehensive literature review, we compiled a catalog of smart pump-related errors and associated prevention strategies. Strategies were mapped to error types to provide an initial framework for others to use as a resource in their error reviews and improvement work. Future research should assess the application of the resources provided by this review.
1.0. Introduction
Medication administration errors in hospitals are common and dangerous, with 3–7% of doses administered by nurses having an error and approximately 4000 preventable injuries due to medication administration errors occurring per hospital annually [1,2]. When considering the medication use cycle, administration is one of the final steps in which healthcare providers can intervene before a drug exposure occurs [3]. As such, errors in medication administration are less likely to be intercepted and are more likely to reach the patient than other types of errors [4]. Drug infusions are particularly high risk due to the complexity of calculations (rate, timing) and the need to hang multiple infusions at once [5]. Several high-risk medications are run as infusions, including insulin drips, narcotic drips, vasopressors, and electrolytes such as potassium in parenteral nutrition. In a study of critically ill adults, 20% of patients had an injury caused by healthcare - half of which were preventable. Sixty-one percent of these injuries were caused by medications [6].
In the past decade, tremendous resources have gone into the development and implementation of technology to prevent errors in medication use among hospitalized patients [7]. Early single site research showed that computerized physician order entry, which primarily targets physician ordering errors, can reduce impatient medication errors [8]. Bar coding is used to prevent a variety of errors, including medication administration errors. By scanning the bar code on a medication, the computer can compare the medication label with the medication order. Bar coding has been shown to reduce adverse drug events, in some studies by as much as 23% [4,9].
Smart infusion pumps are medication infusion pumps that contain medication libraries, with preset dosing limits, which can provide point of care feedback on under- or overdose infusion errors to the nurse programming the pump. The few adult studies evaluating smart pump impact on errors have had mixed results. A pre-post study in a pediatric hospital found that infusion errors reported in hospital incident reports declined by 73% when smart pumps were introduced at the same time as standard drug concentrations and new medication labels [10]. In a rigorous time-series study no change in errors was found with implementation of smart pumps in a general hospital [6]. A systematic review found that smart pumps do not eliminate errors for multiple reasons and that there are many opportunities to improve the safety of medication infusion via these devices [11].
In the ideal state, these technologies work together and seamlessly integrate to prevent or intercept medication errors before they reach the patient. These systems are, of course, imperfect and may fail to prevent some errors. In addition, any major change to a system can create new types of errors caused by the technology itself. For example, introduction of computerized order entry systems was associated with introduction of new errors due to failure in the human-technology interface, such as keypad entry errors or drop-down menu selection errors [8]. For smart pumps in particular, available studies describe errors caused by smart pump technology, including programming errors such as entry of wrong drug, wrong rate, wrong concentration or wrong weight, and varying levels of trust in smart pumps by nurses who use them [12].
The objectives of this study are to: (1) review and catalog the types of human-based errors related to smart infusion pump use identified in the literature and (2) to summarize the prevention strategies for these errors.
2.0. Methods
2.1. Search Strategy
A literature search was initiated by the investigative team, with the intention of finding articles that contained information about human-based errors that may occur when working with smart pumps, and prevention strategies for those errors. The MEDLINE, PubMed, PubMed Central and CINAHL (The Cumulative Index to Nursing and Allied Health Literature) databases are the databases where literature about infusion pumps and patient safety aspects are most frequently found and were the databases searched for the study. The first literature search was conducted in 2015 through PubMed for the period of January 2000 through December 2015. A subsequent search was done on January 8, 2020 for CINAHL and PubMed for the period of January 2000 through December 2019 to refresh the search. Study authors were not contacted for additional data.
2.2. Inclusion Criteria
We searched for the following terms and compiled an initial list of candidate articles:
infusion pump AND error, smart AND pump AND error, infusion AND pump AND error, smart AND pump AND safety
medication AND safety AND (“smartpump” OR “infusion pump” OR “smart pump”)
In addition, we reviewed the “similar articles” section on PubMed.gov for related articles for inclusion. The research team reviewed the titles and abstracts to determine whether each article should be investigated further. Duplicate articles were identified and manually removed. We applied exclusion criteria to filter out inapplicable literature.
2.3. Exclusion Criteria
Articles were removed from the final corpus if they were (a) not pertaining to use in humans, (b) not in the English language, (c) were editorials or presentations, or (d) did not include original research data. Additionally, articles were excluded if they (e) did not cover any infusion pump-related errors or prevention interventions, (f) only covered pump implementation issues or errors, (g) focused on other forms of pumps that were not smart pumps (i.e.: implantable, internal, bulb-based, non-programmable pumps, etc.), (h) focused on errors related to non-human or mechanical failures, or (i) did not focus on intravenous delivery of medications.
2.4. Extraction of Relevant Data
Qualifying articles were reviewed and analyzed by a review team of all investigators (EK, KM, HH, KT, KW), with notes recorded on the article’s design, results, conclusions, error types, prevention interventions, and any additional information or notes deemed pertinent for all literature types. These results were then grouped and sorted to create hierarchical error categories as well as prevention strategy categories with specific action types by all members of the investigative team. The prevention strategies and action types were then mapped to the associated error types. Mapping was synchronously completed by three members of the investigative team (EK, KM, KT) and reviewed by the rest of the team. Disagreements on inclusion, exclusion, categorization, and mapping were discussed as a full investigative team and decided upon unanimously.
3.0. Results
3.1. Characteristics of Included Literature
Initial search results yielded articles from the year 2000 to present. This reflects the appearance of the terms related to “smart pumps” as the technology was just beginning to be implemented into clinical environments. The PRISMA diagram in Figure 1 details the process of inclusion from an original return of 1,177 articles to a final inclusion of 105 articles [13]. After the exclusion of duplicates, 546 articles were screened for abstract and title relevancy. Upon review, 257 articles were excluded for not meeting the inclusion criteria. A total of 289 articles were reviewed in-depth by the investigative team, and 184 were excluded for not meeting the inclusion criteria. Articles reviewed in-depth were most frequently excluded for only covering implementation issues or errors, focusing on other forms of pumps or techniques, focusing on errors related to non-human or mechanical failures, or not focusing on intravenous delivery of medications. One hundred and five articles met the inclusion criteria and were included in the study for final review.
Figure 1: Literature Search Inclusion PRISMA Flow Diagram.
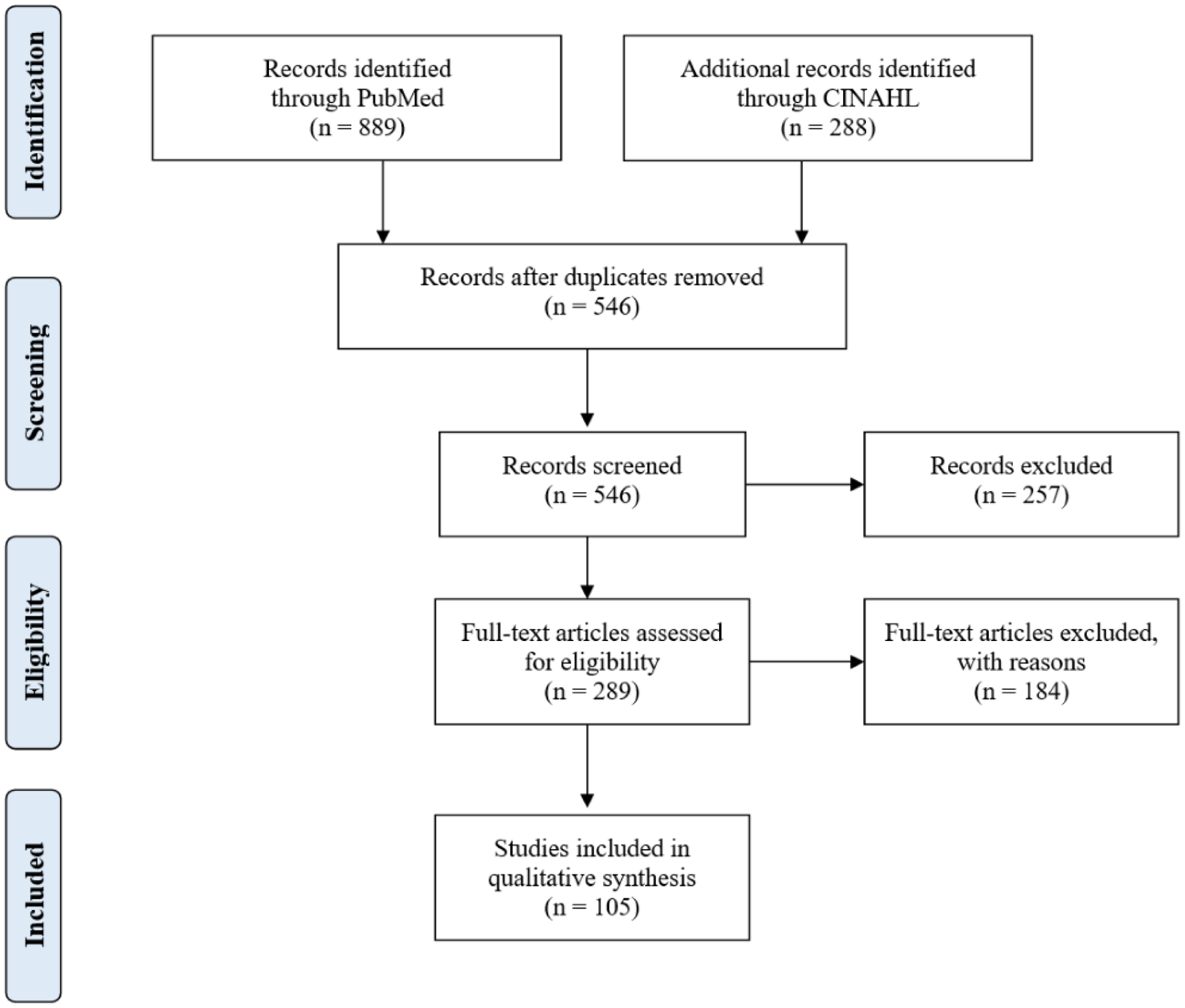
Flow diagram of the search strategy results and filtering of literature according to the inclusion and exclusion criteria.
Literature included in the review was published between 2000–2019 with 63.8% (n=67) of the studies investigating only a single healthcare site. Articles included can be classified as case studies, literature reviews, and original research studies. The literature reflected different populations with 17.1% (n=18) studying an adult population, 12.4% (n=13) specifically studying pediatric populations, 4.8% (n=5) studying a neonatal population, 12.4% (n=13) studying a population of all ages, and 53.3% (n=56) with an undescribed or unidentified population. A complete review of each article and its characteristics can be found in Appendix 1.
3.2. Error Types
A diverse set of infusion pump-related errors were identified during the literature review. The resulting types of errors were collated, then sorted and grouped by similarity. This process resulted in five main categories: undocumented orders, drug library errors, programming errors, administration errors, and ancillary equipment errors. Within these five main categories of errors, the investigative team identified specific types and even more granular subtypes within the literature for error categorization. The hierarchal categorization schema was derived by consensus of the investigative team. A detailed list of the types (n=18) and subtypes (n=21) of errors within each of the above categories can be referenced in Table 1. Following development of the list of smart pump error types identified in the literature, we cross-referenced the list to the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) taxonomy, which has been developed for the standardization of medication error classification. We found that many of the smart pump error types that we identified aligned exactly with the taxonomy, while other categories, like drug library errors, were unique to smart pumps and expanded on the current taxonomy. Aligned and new categories are designated in Table 1. Using the NCC MERP taxonomy, we also identified the categories of potential contributing causes. Although most of the contributing causes that we identified in the literature were found in the taxonomy, we found several unique causes that were not part of the existing taxonomy, including misunderstanding or misinterpreting pump alerts, forgetting to close tube clamps, and bag misalignment with multiple infusions. Medication errors are also often graded in severity according to the NCC MERP taxonomy. Smart pump errors identified in the literature span in severity from Category A, events occurred that had the capacity to cause an error, to Category I, an error occurred that may have resulted in the patient’s death [14].
Table 1.
Identified Error Types and Subtypes Associated with Smart Infusion Pumps
| Error Category | Error Type (NCC MERP Taxonomy Number) | Error Subtype | Potential Causes (NCC MERP Taxonomy Number) |
|---|---|---|---|
| Undocumented Errors (E1.0) | Undocumented verbal orders for medications administered (E1.1) |
Communication (81) Human Factors (87) System-related (90) |
|
| Unauthorized fluid/med - no order for it in the system (E1.2) |
Communication (81) Human Factors (87) System-related (90) |
||
| Drug Library Errors (E2.0) | Wrong drug library selected (E2.1) |
Communication (81) Name Confusion (83) Human Factors (87) Packaging/Design (89) System-related (90) |
|
| Drug library insufficient (E2.2) | Medication not in drug library (E2.2.1) |
Packaging/Design (89) System-related (90) |
|
| Medication concentration not in the drug library (E2.2.2) | |||
| Drug library does not match hospital policies (E2.2.3) | |||
| Units in drug library don’t match orders (e.g., orders used mg, limits in gm) (E2.2.4) | |||
| Bypassing an available drug library e.g., Basic infusion selected instead of a drug-specific library (E2.3) |
Human Factors (87) Packaging/Design (89) System-related (90) |
||
| Programming Errors (E3.0) | Wrong concentration programmed (E3.1) 70.3 Wrong Strength/Concentration |
Communication (81) Name Confusion (83) Human Factors (87) Packaging/Design (89) System-related (90) |
|
| Wrong volume programmed (E3.2) 70.2 Improper Dose | VTBI (Volume to Be Infused) not programmed (E3.2.1) |
Communication (81) Name Confusion (83) Human Factors (87) Packaging/Design (89) System-related (90) |
|
| Programmed extra volume for extra fluid in bag/priming (E3.2.2) | |||
| Wrong VTBI programmed (doesn’t match order) (E3.2.3) | |||
| Wrong dose programmed (E3.3) 70.2 Improper Dose |
Dose infused doesn’t match the order (E3.3.1) |
Communication (81) Name Confusion (83) Human Factors (87) Packaging/Design (89) System-related (90) |
|
| Non-titratable medication order - medication dose was titrated (E3.3.2) | |||
| Titratable med order - dose was not titrated as ordered (E3.3.3) | |||
| Extra doses administered (intermittent medication administration) (E3.3.4) | |||
| Using drug calculation to provide dose outside of library - drug calculator workaround (E3.3.5) | |||
| Programming with incorrect units (e.g. mg instead of gm) (E3.3.6) | |||
| Wrong 4-hour dose limit *applicable to PCAs only (E3.3.7) | |||
| Accidentally adding or subtracting an extra digit (e.g., “factor of 10” error) (E3.3.8) | |||
| Drugs dosed at decimal level (e.g. 0.01 mg/mL) (E3.3.9) | |||
| Wrong rate programmed (E3.4) 70.8 Wrong Rate |
Rate of infusion doesn’t match the order (E3.4.1) |
Communication (81) Name Confusion (83) Human Factors (87) Packaging/Design (89) System-related (90) |
|
| Providing bolus by increasing rate (E3.4.2) | |||
| Infusion as bolus infusion ends and the pump defaults to historical infusion parameters (E3.4.3) | |||
| Wrong patient weight programmed (E3.5) | Wrong patient’s weight entered (E3.5.1) |
Communication (81) Human Factors (87) Packaging/Design (89) System-related (90) |
|
| 2.2x weight error (patient’s weight in lbs. entered instead of kg) (E3.5.2) | |||
| Programming errors- other (E3.6) |
Communication (81) Name Confusion (83) Human Factors (87) Packaging/Design (89) System-related (90) |
||
| Administration Errors (E4.0) | Administered to the wrong patient (E4.1) 70.11 Wrong Patient |
Communication (81) Human Factors (87) Packaging/Design (89) System-related (90) |
|
| Administered via the wrong route (e.g., intrathecal vs intravenous) (E4.2) 70.7 Wrong Route |
Communication (81) Human Factors (87) Packaging/Design (89) System-related (90) |
||
| Wrong administration technique (E4.3) 70.6 Wrong Technique |
Communication (81) Human Factors (87) Packaging/Design (89) System-related (90) |
||
| Not administered - omitted or missed medication dose (e.g., no medication was given an ordered time) (E4.4) 70.1 Dose Omission |
Communication (81) Human Factors (87) System-related (90) |
||
| Administered the wrong drug (E4.5) 70.4 Wrong Drug |
Communication (81) Name Confusion (83) Human Factors (87) Packaging/Design (89) System-related (90) |
||
| Ancillary Equipment Errors (E5.0) | Switched lines; wrong line running through pump (E5.1) |
Human Factors (87) Packaging/Design (89) |
|
| Wrong syringe size (E5.2) | Packaging/Design (89; 89.3.2) |
Abbreviations: VTBI, volume to be infused; PCA, patient-controlled analgesia; EHR, electronic health record.
3.3. Prevention Strategies
Many articles discussed unique prevention strategies developed in response to medication error events. A collection of the prevention types (n=10) and subtypes (n=33) can be found in Table 2. Identified strategies ranged from improved communication to policy change with many articles including reformed training as a key error prevention strategy.
Table 2.
Identified Prevention Strategy Types and Subtypes
| Prevention Strategy | Subtype | Example References |
|---|---|---|
| Training (P1.0) | Training refreshers (P1.1) | A51, A64, A82 |
| Train on how and why (P1.2) | A5, A64, A88 | |
| Nurse Involvement in Design and Evaluation (P2.0) | Resource super users (P2.1) | A5, A64 |
| Nurse input for design (P2.2) | A64, A81 | |
| Uniform Pump Brand/Function (P3.0) | Standardize pump fleet (P3.1) | A43, A94 |
| Consistency Between Systems (P3.2) | A64 | |
| Improve Communication & Reliability (P4.0) | Redundant, independent medication checks by 2 people (P4.1) | A45, A49 |
| Medication review on transfer (P4.2) | A43 | |
| Automation/Logic (P5.0) | Automate medication titration (P5.1) | A69 |
| Logic for titrated drugs (P5.2) | A8 | |
| Integrate pumps with lab or vital sign data (P5.3) | A69 | |
| Automated error alert systems (P5.4) | A24, A92 | |
| Technological Integration of Systems (P6.0) | Barcode Scanner (BCMA) integrated with EHR (P6.1) | A73, A95 |
| Infusion Pumps Interfaced with EHR and BCMA (P6.2) | A90, A92, A95 | |
| Standardize and Update Drug Libraries (P7.0) | Limit and standardize medication concentrations (P7.1) | A77, A86, A87 |
| Include bolus doses in library (P7.2) | A8 | |
| Scheduled library updates (P7.3) | A62, A71 | |
| Increase Compliance (P8.0) | Compliance with library, default to DERS, monitor compliance (P8.1) | A35, A65, A84 |
| Compliance with pump protocol (P8.2) | A83 | |
| Simplify programming process to improve compliance (P8.3) | A3 | |
| Decrease unnecessary warnings (P8.4) | A8 | |
| Minimize workarounds (P8.5) | A8, A88, A96 | |
| Interpump constraints – checking of infusion parameters between and across pumps (P8.6) | A49 | |
| Hard limits on new smart pumps (P8.7) | A43 | |
| Organizational Factors (P9.0) | Eliminate Resource Constraints (P9.1) | A64 |
| No ‘One Size Fits All’ Policies (P9.2)– policies are specific and tailored to the needs of the patient population and clinical context | A64 | |
| Policies and procedures to standardize processes and best practice (P9.3) | A9, A19, A105 | |
| Limitation of Interruptions (P9.4) | A10, A74 | |
| Human Factors/Quality Improvement (P10.0) | Continuous QI with scheduled data review to improve pump use and compliance with DERS (P10.1) | A6, A11, A53, A71 |
| Use of Failure Mode & Effects Analyses (FMEA) (P10.2) | A17, A31, A73, A98 | |
| Standardized medication concentrations (P10.3) | A77, A86, A87 | |
| Human factors informed labels, e.g., redesigning med labels to have higher readability, formatting consistent with pump programming info needs, color-coding, etc. (P10.4) |
A87, A94 | |
| Checklists (P10.5) | A49 |
Numbers in column 3 refer to the IDs for each article in the Appendix. Abbreviations: BCMA, barcode scanner; EHR, electronic health record; QI, quality improvement; FMEA, failure mode & effects analyses
The identified prevention strategies were mapped to specific error types and subtypes in Table 3. Most notably, programming errors were prevented by implementing and standardizing the pump brands and functions, development and implementation of pump automation and logic, technological integration of systems including barcode scanners and pump integration with EHRs, and the use of human factors engineering and quality improvement techniques. Administrative-based errors were prevented by improving communication and implementing a barcode scanner. Frequently proposed prevention strategies included compliance with the drug library and improved communication through independent checks by two healthcare providers and medication reviews upon transfer.
Table 3.
Medication Errors Mapped to Associated Prevention Strategies
| ERRORS | PREVENTION STRATEGIES | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Error Category | Error Type | Error Subtype | Training | Nurse Involvement In Design and Evaluation | Uniform Pump Brand/Function | Improve Communication | Automation/Logic | Tech. Integration of System | Standardize & Update Drug Libraries | Increase Compliance | Organizational Factors | Human Factors / QI |
| Undocumented Orders (E1.0) | X | X | X | X | ||||||||
| Unauthorized fluid/med - no order for it (E1.2) | X | X | X | X | ||||||||
| Drug Library Errors (E2.0) | X | X | X | X | X | X | ||||||
| Medication not in library (E2.2.1) | X | X | X | X | X | |||||||
| Medication concentration not in the library (E2.2.2) | X | X | X | X | X | |||||||
| Library policies don’t match hospital policies (E2.2.3) | X | X | X | X | X | |||||||
| Units in the library don’t match orders (E2.2.4) | X | X | X | X | X | |||||||
| Bypassing an available drug library (E2.3) | X | X | X | X | X | X | ||||||
| Programming Errors (E3.0) | X | X | X | |||||||||
| Wrong volume (E3.2) | VTBI not programmed (E3.2.1) | X | X | X | X | X | X | |||||
| Programmed extra volume for extra fluid in bag/priming (E3.2.2) | X | X | X | X | X | |||||||
| Wrong VTBI programmed (doesn’t match order) (E3.2.3) | X | X | X | X | ||||||||
| Wrong dose (E3.3) | Dose infused doesn’t match the order (E3.3.1) | X | X | X | ||||||||
| Non -titratable med order (E3.3.2) | X | X | X | X | ||||||||
| Titratable med order (E3.3.3) | X | X | X | X | ||||||||
| Extra doses administered *N/A to continuous meds (E3.3.4) | X | X | X | |||||||||
| Using drug calc to provide dose outside of library - drug calculator workaround (E3.3.5) | X | X | X | X | X | X | X | X | ||||
| Programming with incorrect units, e.g., mg instead of gm (E3.3.6) | X | X | X | X | ||||||||
| Wrong 4-hour dose limits *applicable to PCAs only (E3.3.7) | X | X | X | |||||||||
| Rate of infusion doesn’t match the order (E3.4.1) | X | X | X | X | ||||||||
| Providing bolus by increasing rate (E3.4.2) | X | X | X | X | X | X | ||||||
| Infusion as bolus ends and the pump defaults to historical infusion parameters (E3.4.3) | X | X | X | X | ||||||||
| Wrong patient’s weight entered (E3.5.1) | X | X | X | X | ||||||||
| 2.2x weight error (patient’s weight in ibs. entered in kgs) (E3.5.2) | X | X | X | X | X | X | X | |||||
| X | X | X | ||||||||||
| Drugs dosed at decimal level (e.g., 0.01 mg/ml) (E3.7) | X | X | X | |||||||||
| Programming errors - other (E3.8) | X | X | X | X | X | X | ||||||
| Administration Errors (E4.0) | X | X | X | X | ||||||||
| Wrong administration route (E4.2) | X | X | X | |||||||||
| Wrong administration technique (E4.3) | X | X | X | X | X | X | ||||||
| Not administered - omitted or missed medication (E4.4) | X | X | X | X | ||||||||
| Administered wrong drug (E4.5) | X | X | X | X | ||||||||
| Ancillary Equipment Errors (E5.0) | Switched lines; wrong line running through pump (E5.1) | X | X | X | ||||||||
| Wrong syringe size (E5.2) | X | X | X | |||||||||
Abbreviations: VTBI, volume to be infused; PCA, patient-controlled analgesia; EHR, electronic health record
3.4. Quality of Evidence
Overall, the quality of the evidence was low, as the majority of the studies that we identified were observational (N=80/105) and single site studies (N=67/105). Of the 105 studies that we evaluated, only two were randomized studies evaluating the benefit of smart pump clinical decision support in an on-off design, with one showing benefit and the other demonstrating no benefit [15,16]. Two studies were comparative analyses, comparing error rates using smart pumps versus infusion pumps without DERS, with again one simulation study demonstrating a decrease in specific error types and the other retrospective study demonstrating similar rates of error [17,18]. Many of the observational studies suggesting benefit were pre/post implementation studies that identified cancelled or reprogrammed infusions as intercepted errors. Whether these are true errors is difficult to define. No studies evaluated the effect of mitigation strategies in a randomized fashion, although several studies employed strong quality improvement methodology for assessing prevention strategies. With the large number of single center studies and lack of randomized trials, reporting bias is suspected to be high. Given the imprecision of the results, the magnitude of effect of smart pumps, or the mitigation strategies, is difficult to judge.
4.0. Discussion
Smart pump technology was created and implemented into healthcare systems to address pertinent clinical concerns surrounding high rates of medication errors. The effectiveness of this technology is, however, inconclusive. Many of the studies we identified demonstrate the effectiveness of smart pumps in intercepting specific types of errors, such as wrong rate and wrong dose errors, including eliminating wrong concentration errors when integrated pumps were used [11,16,18–24]. Other studies have failed to show a significant benefit of smart pumps on medication errors [5,6,25,26]. Low rates of compliance, which are as low as 70–75% within some healthcare settings, may prevent the benefits of the implemented smart pump technology from being realized [15,20]. One study from the United Kingdom identified that only 32% of infusions were delivered using a smart pump despite availability in the majority of hospitals [25]. Many publications relay descriptions of different error types and some offer prevention strategies, but no source to date has compiled all described error types into a unified list. In response to this need, we cataloged the errors associated with smart pump technology and the error prevention strategies.
In Table 1 we present the catalog of the different error types attributed in the literature to the use of smart pumps for medication infusions. Some identified error types - undocumented orders, administration errors, and human errors - are not new to the implementation of smart pump technology and persist in the clinical setting regardless of the technological implementation. Other error types - programming errors, drug library errors, and ancillary equipment errors - however, permeate the clinical setting as new error types introduced by the implementation of smart pump technology; some errors may be the result of workarounds performed by clinicians to intentionally bypass safety features. New error types such as these often require new error prevention strategies, as seen in Table 2.
To combine this data into a useful tool to use in efforts to decrease the likelihood of smart pump-related errors, the strategies were mapped to each error type and subtype in Table 3. This mapping is in response to previous research which argues that the evaluation of smart pump data alone is insufficient for understanding medication errors and that we must understand the environmental context to identify errors and implement effective prevention strategies [27–29]. Dunford et al. even demonstrates that 46% of workarounds and prevention strategies are related to issues with the technology itself, including inflexibility of smart pumps for specific patient populations [30]. Smart pumps are not likely to be able to prevent all medication errors on their own, and it is clear from prior research and our own that causes of medication errors are multi-factorial and must be addressed using multi-dimensional strategies [31]. One suggested strategy is an interoperable system that combines computerized provider order entry (CPOE) and bar-coding smart pumps to create a closed-loop system. While some, like Trbovich and Tran, have shown these systems to be capable of eliminating specific error types, such as wrong concentration errors and wrong patient errors, other error types continue to be permitted and issues arise with drug libraries and titratable medications, amongst others [18,19,32].
It is intended for the mapped prevention strategies to serve as a tool for healthcare teams to engage with in the event of a medication error. Medication errors are often complex and may require multiple prevention strategies to overcome. Importantly, just as smart infusion pumps have been able to be utilized in all populations, strategies to prevent infusion pump errors do not seem to be restricted to specific patient populations but rather can be broadly applied to all populations, making our toolkit generalizable to many situations. To provide a suggestion for meaningful use of the toolkit, we have developed a hypothetical example of a medication error event and corresponding toolkit use.
A pediatric ICU nurse receives an alert that Patient X has been prescribed 50mcg of fentanyl intravenously over a period of 45 minutes before their scheduled surgery. The nurse finds fentanyl in the drug library, clicks it, and manually inputs the rate of infusion. Upon preparing for transport to the operating room, the patient complains of pain and the nurse notices the IV infusion has not been completed. It was then realized that the medication was programmed for 50mcg over 450 minutes. At this point, the error was reported through the organization’s incident reporting system. An investigative team convenes, reviews the EHR of the patient, the incident report, and other associated data. As part of their efforts to decrease the likelihood of another event like this occurring, a member of their team finds this paper and reviews Table 1, identifying the error type(s) that occurred in their specific example. The team then references Table 3 and finds that “improved communication” and “technological intervention” are listed as effective prevention strategies for medication errors involving programming the wrong rate. Now knowing which types of prevention strategies are recommended for that error type, they can use Table 2 to determine more specific actions they can take to prevent that error from happening again and briefly review the suggested articles for more information. Upon referencing article A49, they ultimately agree that they will begin doing redundant checks by two people, and after referencing article A95, they submit a proposal to their department to investigate integrating their smart pumps with their already integrated barcode scanner and EHR.
The previous example highlights one approach to using the tools produced by this review. It should be noted that another recurring theme from our review, and one to consider in the above example, is that systems in which smart pumps are optimally utilized should be constantly assessed. This assessment should be viewed as a continuous quality improvement initiative, to promote increased compliance with existing libraries, regular review of smart pump data to make necessary library adjustments and address gaps, and to standardize pump use to reduce risk, among other strategies [33,34]. With a heightened understanding of the types of errors encountered by healthcare providers in conjunction with smart pumps, future studies should investigate the effectiveness of the listed prevention strategies on reducing medication errors, as well as how best to apply the tools and modify them optimally. Understanding the context and environment in which errors occur will also be important as the context of errors currently remains understudied. We know that human errors will exist as they do for any complex sociotechnical system. Often the solution to address these errors is to identify solutions that can remove the driving human factors. We present the findings of this study as an initial important step towards an evidence-based smart infusion pump-related error prevention toolkit.
5.0. Limitations
The list of error types and strategies to address them is not exhaustive; it is a starting point and the list may be modified. It also could be made more granular, but for sake of brevity and applicability, we chose to keep the list at a fairly high level and not cumbersome to implement. However, the list reflects the items we found expressly called out in the literature. Additionally, as we refreshed our search, we did not uncover many new error types or strategies, indicating that we had reached a reasonable point of saturation in our review. However, underreporting is a limitation to any literature review pertaining to errors in the clinical setting. It is important to note that other sources of information outside of literature exist to inform the implementation and use of smart infusion pumps, including resources provided by the Institute for Safe Medication Practice (ISMP), the ECRI Institute, and the Association for the Advancement of Medical Instrumentation (AAMI). Although identification of all resources was beyond the scope of this review, such might be leveraged by institutions in addition to the peer-reviewed literature to best inform smart infusion pump use. In addition, researchers or clinicians who apply the lists should keep in mind that the lists do not contain mutually exclusive items. For instance, an error in the Human Error category, error type of “misinterpretation of order” could lead to a Programming Error, “wrong dose” error, in which case both types of errors could be attributed to a single error. In this case, we recommend documenting the error types in a hierarchal format, e.g., a misinterpretation error led to a programming error that led to a wrong dose. Finally, the quality of the evidence supporting any one mitigation strategy type is low, but randomized assessments are unlikely and the potential to prevent rare but harmful events is important.
6.0. Conclusions
Existing research demonstrates that while smart pumps are effective in reducing many types of medication errors, some error types prevail. Additionally, smart pumps introduce their own class of medication errors. Through a comprehensive literature review and analysis, we compiled a catalog of smart infusion pump-related errors and prevention strategies for those errors. Interventions included interfacing the pumps with EHRs, maximizing use of barcoding technology, promoting compliance with pump drug libraries, drug library standardization and updating, optimal training and education, and pump standardization. It is intended for this compilation and mapping of prevention strategies to serve as a toolkit for clinical use. Future research should investigate best practices associated with smart pumps and quantitatively assess the effectiveness of mitigation strategies developed to prevent medication errors, as well as the application of the resources provided by this review.
Supplementary Material
Key Points.
Smart infusion pumps have been implemented to prevent errors but fail to prevent specific error types and introduce new errors themselves.
Strategies to prevent smart infusion pump-related errors include interfacing smart pumps with EHRs, maximizing use of barcoding technology, promoting compliance with pump drug libraries, drug library standardization and updating, optimal training and education, and pump standardization.
We compiled a catalog of smart infusion pump-related errors and mapped prevention strategies to serve as a toolkit for clinical use.
Acknowledgments
The authors would like to acknowledge the assistance of Yizhao Ni, Eric Hall, and Teresa Couch. Drs. Yizhao and Hall provided guidance regarding infusion pump data availability and utility. Ms. Couch assisted the team with guidance around clinical practice and use of smart infusion pumps at Cincinnati Children’s Hospital Medical Center.
Funding
This research was supported by the National Institute of Health under award number R01LM012230 (sponsored by the National Library of Medicine).
Footnotes
Conflicts of Interest
None of the authors have any conflicts of interest to declare.
Consent for Publication
All authors consent to the publication of this paper.
Availability of Data and Material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Barker KN, Flynn EA, Pepper GA, Bates DW, Mikeal RL. Medication errors observed in 36 health care facilities. Arch Intern Med. 2002;162(16):1897–1903. [DOI] [PubMed] [Google Scholar]
- 2.Kale A, Keohane CA, Maviglia S, Gandhi TK, Poon EG. Adverse drug events caused by serious medication administration errors. BMJ Qual Saf. 2012;21(11):933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billstein-Leber M, Carrillo CJD, Cassano AT, Moline K, Robertson JJ. ASHP Guidelines on Preventing Medication Errors in Hospitals. Am J Health Syst Pharm. 2018;75(19):1493–1517. [DOI] [PubMed] [Google Scholar]
- 4.Truitt E, Thompson R, Blazey-Martin D, NiSai D, Salem D. Effect of the Implementation of Barcode Technology and an Electronic Medication Administration Record on Adverse Drug Events. Hosp Pharm. 2016;51(6):474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husch M, Sullivan C, Rooney D, et al. Insights from the sharp end of intravenous medication errors: implications for infusion pump technology. Qual Saf Health Care. 2005;14(2):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothschild JM, Landrigan CP, Cronin JW, et al. The Critical Care Safety Study: The incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med. 2005;33(8):1694–1700. [DOI] [PubMed] [Google Scholar]
- 7.Shah PK, Irizarry J, O’Neill S. Strategies for Managing Smart Pump Alarm and Alert Fatigue: A Narrative Review. Pharmacotherapy. 2018;38(8):842–850. [DOI] [PubMed] [Google Scholar]
- 8.Walsh KE, Adams WG, Bauchner H, et al. Medication errors related to computerized order entry for children. Pediatrics. 2006;118(5):1872–1879. [DOI] [PubMed] [Google Scholar]
- 9.Hutton K, Ding Q, Wellman G. The Effects of Bar-coding Technology on Medication Errors: A Systematic Literature Review. J Patient Saf. 2017. [DOI] [PubMed] [Google Scholar]
- 10.Larsen GY, Parker HB, Cash J, O’Connell M, Grant MC. Standard drug concentrations and smart-pump technology reduce continuous-medication-infusion errors in pediatric patients. Pediatrics. 2005;116(1):e21–25. [DOI] [PubMed] [Google Scholar]
- 11.Ohashi K, Dalleur O, Dykes PC, Bates DW. Benefits and risks of using smart pumps to reduce medication error rates: a systematic review. Drug Saf. 2014;37(12):1011–1020. [DOI] [PubMed] [Google Scholar]
- 12.Montague E, Asan O, Chiou E. Organizational and technological correlates of nurses’ trust in a smart intravenous pump. Comput Inform Nurs. 2013;31(3):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartwig SC, Denger SD, & Schneider PJ Severity-indexed, incident report-based medication error-reporting program. American Journal of Health-System Pharmacy. 1991;48:2611–2616. [PubMed] [Google Scholar]
- 15.Rothschild JM, Keohane CA, Cook EF, Orav EJ, Burdick E, Thompson S, Hayes J, Bates DW. A controlled trial of smart infusion pumps to improve medication safety in critically ill patients. Crit Care Med. 2005;33:533–540. [DOI] [PubMed] [Google Scholar]
- 16.Schilling MB, Sandoval S. Impact of intelligent intravenous infusion pumps on directing care toward evidence-based standards: a retrospective data analysis. Hosp Pract (1995). 2011;39(3):113–121. [DOI] [PubMed] [Google Scholar]
- 17.Blandford A, Dykes PC, Franklin BD, et al. Intravenous Infusion Administration: A Comparative Study of Practices and Errors Between the United States and England and Their Implications for Patient Safety. Drug Saf. 2019;42(10):1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trbovich PL, Pinkney S, Cafazzo JA, Easty AC. The impact of traditional and smart pump infusion technology on nurse medication administration performance in a simulated inpatient unit. Qual Saf Health Care. 2010;19(5):430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran M, Ciarkowski S, Wagner D, Stevenson JG. A case study on the safety impact of implementing smart patient-controlled analgesic pumps at a tertiary care academic medical center. Jt Comm J Qual Patient Saf. 2012;38(3):112–119. [DOI] [PubMed] [Google Scholar]
- 20.Ibarra-Pérez R, Puértolas-Balint F, Lozano-Cruz E, Zamora-Gómez SE, Castro-Pastrana LI. Intravenous Administration Errors Intercepted by Smart Infusion Technology in an Adult Intensive Care Unit. J Patient Saf. 2017. [DOI] [PubMed] [Google Scholar]
- 21.Manrique-Rodríguez S, Sánchez-Galindo AC, de Lorenzo-Pinto A, et al. Implementation of smart pump technology in a paediatric intensive care unit. Health Informatics J. 2015;21(3):209–222. [DOI] [PubMed] [Google Scholar]
- 22.Manrique-Rodríguez S, Sánchez-Galindo AC, Fernández-Llamazares CM, Calvo-Calvo MM, Carrillo-Álvarez Á, Sanjurjo-Sáez M. Safe intravenous administration in pediatrics: A 5-year Pediatric Intensive Care Unit experience with smart pumps. Med Intensiva. 2016;40(7):411–421. [DOI] [PubMed] [Google Scholar]
- 23.Manrique-Rodríguez S, Sánchez-Galindo AC, López-Herce J, et al. Impact of implementing smart infusion pumps in a pediatric intensive care unit. Am J Health Syst Pharm. 2013;70(21):1897–1906. [DOI] [PubMed] [Google Scholar]
- 24.Melton KR, Timmons K, Walsh KE, Meinzen-Derr JK, Kirkendall E. Smart pumps improve medication safety but increase alert burden in neonatal care. BMC Med Inform Decis Mak. 2019;19(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons I, Furniss D, Blandford A, et al. Errors and discrepancies in the administration of intravenous infusions: a mixed methods multihospital observational study. BMJ Qual Saf. 2018;27(11):892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuckols TK, Bower AG, Paddock SM, et al. Programmable infusion pumps in ICUs: an analysis of corresponding adverse drug events. J Gen Intern Med. 2008;23 Suppl 1(Suppl 1):41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuo J, Lambert G, Hicks RW. Intralipid medication errors in the neonatal intensive care unit. Jt Comm J Qual Patient Saf. 2007;33(2):104–111. [DOI] [PubMed] [Google Scholar]
- 28.Kirkbride G, Vermace B. Smart pumps: implications for nurse leaders. Nurs Adm Q. 2011;35(2):110–118. [DOI] [PubMed] [Google Scholar]
- 29.Nemeth CP, Brown J, Crandall B, Fallon C. The mixed blessings of smart infusion devices and health care IT. Mil Med. 2014;179(8 Suppl):4–10. [DOI] [PubMed] [Google Scholar]
- 30.Dunford BB, Perrigino M, Tucker SJ, et al. Organizational, Cultural, and Psychological Determinants of Smart Infusion Pump Work Arounds: A Study of 3 U.S. Health Systems. J Patient Saf. 2017;13(3):162–168. [DOI] [PubMed] [Google Scholar]
- 31.Kunac DL, Reith DM. Identification of priorities for medication safety in neonatal intensive care. Drug Saf. 2005;28(3):251–261. [DOI] [PubMed] [Google Scholar]
- 32.Furniss D, Dean Franklin B, Blandford A. The devil is in the detail: How a closed-loop documentation system for IV infusion administration contributes to and compromises patient safety. Health Informatics J. 2019:1460458219839574. [DOI] [PubMed] [Google Scholar]
- 33.Apkon M, Leonard J, Probst L, DeLizio L, Vitale R. Design of a safer approach to intravenous drug infusions: failure mode effects analysis. Qual Saf Health Care. 2004;13(4):265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orto V, Hendrix CC, Griffith B, Shaikewitz ST. Implementation of a smart pump champions program to decrease potential patient harm. J Nurs Care Qual. 2015;30(2):138–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


