Abstract
Background
Infections caused by Extended spectrum beta lactamase (ESBL) producing bacterial are global challenge. There is limited information on the magnitude of bacteriospermia, ESBL producing Gram-negative bacteria (GNB) causing bacteriospermia and factors associated with male infertility. This study determined magnitude of bacteriospermia, ESBL-GNB and other factors association with infertility among presumptive infertile men in Mwanza, Tanzania.
Methods
A cross-sectional hospital-based study was conducted between May 2017 and July 2018 among 137 presumptive infertile men. Semen specimens were self-collected by masturbation into clean, sterile and none-spermicidal containers and processed following laboratory standard operating procedures (SOPs). Data analysis was done using STATA 13.0.
Results
Gram-negative bacteria were predominantly isolated (86.4%), of which 31.6% were ESBL producers. In a total 44 bacteria were isolated from semen culture. The blaCTX-M gene was detected in 75% of phenotypically confirmed ESBL producers. Infertility was independently found to be associated with abnormal spermatozoa morphology (OR (95%CI): 14.48(3.17–66.05)) and abnormal spermatozoa motility (OR (95%CI): 0.05(0.01–0.24)). However, neither bacteriospermia (OR (95%CI): 0.86(0.29–2.59)) nor ESBL bacteriospermia (OR (95%CI): 0.13(0.01–1.22)) was found to be associated with infertility.
Conclusion
One third of bacteriospermia is due to ESBL-producers with history of antibiotic use being protective factor for infertility. Abnormal spermatozoa morphology and poor spermatozoa forward motility independently predicted infertility.
Keywords: bacteriospermia, blaCTX-M, male infertility, extended spectrum beta lactamase, Mwanza, Tanzania
Introduction
Bacteria, fungi, protozoa and viruses are agents implicated in male urogenital tract and accessory sex gland infections and account for about 15% of male infertility1,2. Bacteria like; Staphylococcus aureus, Enterococcus faecalis, Staphylococcus saprophyticus, Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae are the most common pathogens reported to be isolated from semen culture and causing bacteriospermia3,4. Bacteriospermia has been found to cause infertility due to various factors such as: deterioration of spermatogenesis, alteration of acrosome and sperm morphology, auto-immune processes induced by inflammation, increased sperm DNA fragmentation due to formation of reactive oxygen species and obstruction of genital tracts due to fibrosis and inflammation3,5.
Production of Extended spectrum beta lactamase (ESBL) among multi-drug resistant (MDR) Gram-negative bacteria is the common antibiotic resistance mechanism6. E. coli, K. pneumoniae, Citrobacter spp., Enterobacter spp., Acinetobacter spp. and Pseudomonas aeruginosa are common ESBL producing Gram-negative bacteria (GNB)6,7. The blaCTX-M gene is documented to account over 75% of ESBL producing gram negative bacteria clinically isolated however little is known on its prevalence among ESBL producing Gram-negative bacteria causing bacteriospermia8. Here in, we report the magnitude of bacteriospermia, blaCTX-M among ESBL producing Gram-negative bacteria and other factors associated with male infertility in Mwanza, Tanzania. This is the first study to report magnitude of bacteriospermia, ESBL producing GNB and other factors associated with male infertility from Tanzania. These data are important in the management of bacteriospermia in our setting where there is high prevalence of ESBL producing Gram-negative bacteria9–11.
Methods
Study design, duration, population and setting
This cross-sectional hospital-based study was conducted between May 2017 and July 2018 involving 137 presumptive infertile men (whose female couples were medically confirmed fertile) attending reproductive health/infertility clinics in Mwanza, Tanzania. Standardized data collection tools used to collect socio-demographic and clinical characteristics of the study participants. Semen specimens were self-collected by masturbation into wide mouth, clean, sterile and spermatozoa non-toxic specimen containers (Hunter Scientific Limited, UK) after consented voluntarily and sexual abstinence of a minimum of 3 days. Patients were instructed to pass urine and then thoroughly cleaned their hands and penis with clean water and non-antiseptic soap12. Specimens were brought to Central Pathology Laboratory, department of Histopathology at the Bugando Medical Centre (BMC) for semen analysis at room temperature within 30 minutes after collection and Catholic University of Health and Allied Sciences (CUHAS) multipurpose laboratory in cold box (2–8°C) for semen culture within one hour of collection. PCR to detect blaCTX-M gene was done at National Institute of Medical Research (NIMR), Mwanza.
In this study, presumptive male infertility refers to male's inability to make fertile female partner pregnant for a period of ≥ 1 year of active sexual practices without protections while medically confirmed infertility among men refers male infertility13–15. Male infertility can be medically confirmed by examining spermatozoa quality and quantity in an ejaculate13,14. This includes: spermatozoa concentration, morphology and/or forward motility13,14.
Semen analysis, culture and identification of significant isolated bacteria
Semen analysis involved the following parameters; colour (grey to opalescent), volume (2–6 ml), viscosity (< 2 cm dropping threads from pipette), pH (7.2–8.2), motility, morphology and spermatozoa count (20–120 million/milliliter) per SOPs and WHO guidelines12. Semen analysis was performed by skilled and experienced laboratory scientist (> 5 working years) and two other laboratory technicians (> 3 working years) were used to confirm for the validity of the results. Semen specimens were inoculated onto blood agar (BA) and MacConkey agar (MCA) plates followed by aerobic incubation at 37°C for 24–48 hours. A pure significant growth (≥ 103 CFU/ml growth) of bacteria were further identified to species level by inhouse biochemical identification tests; Gram stain, catalase, slide coagulase, novobiocin, bacitracin, bile esculin and optochin for Gram-positive bacteria and Gram stain, triple sugar iron (TSI), sulfur indole motility (SIM), Simmons citrate, urease and oxidase for Gram-negative bacteria16.
Antibiotic susceptibility testing
Antibiotic susceptibility testing (AST) was performed on Muller Hinton agar (MHA) plates by Kirby-Bauer disc diffusion method as per CLSI:2010 guidelines17. Erythromycin 15µg, clindamycin 2µg, vancomycin 30µg, gentamicin 10µg, cefoxitin 30µg (for S. aureus only) and ciprofloxacin 5µg were used for gram positive bacteria while ampicillin 10µg, sulphamethoxazole-trimethoprim 1.25/23.75µg, gentamicin 10µg, ciprofloxacin 5µg, amoxycillin-clavulanic acid 20/10µg, ceftriaxone 30µg, ceftazidime 30µg, piperacillin-tazobactam 100/10µg and meropenem 10µg were used for gram negative bacteria.
Phenotypic detection of ESBL producing gram negative bacteria
Double disc synergy (DDS) technique was used to detect ESBL producing gram negative bacteria as reported previously17. Briefly, ceftazidime-clavulanic acid and ceftazidime plain discs were seeded on MHA plate with test organisms. The plates were incubated for 24 hours at 37°C. The difference of zones of inhibitions of ≥ 5mm between ceftazidime-clavulanic acid and ceftazidime plain was interpreted as ESBL producer18,19.
Molecular characterization of blaCTX-M gene from ESBL producing gram negative bacteria
Heat treatment technique was performed to extract bacterial DNA with minor modification from previous study20. Two colonies of fresh grown bacteria were suspended into DNase/RNase free tubes containing 500 µL of sterile de-ionized water, mixed by vortexing and boiled at 100°C for 10 minutes. Tubes were centrifuged at 12000 rpm for 10 minutes to obtain 5 µL of supernatant of each test bacteria for PCR.
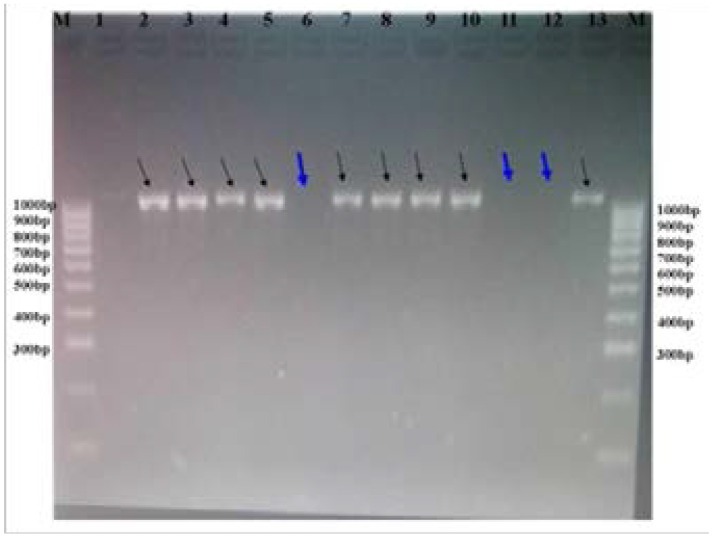
PCR was performed for phenotypically confirmed ESBL producing gram negative bacteria to determine the presence of blaCTX-M gene as previously reported21. CTX-M3G; forward primer: 5′-GTTACAATGTGTGAGAAGCAG-3′ and reverse primer: 5′-CCGTTTCCGCTATTACAAAC-3′ were used. Briefly, PCR amplification was carried on thermocycler machine (GeneAmp® PCR System 9700, ThermoFishers Scientific, Singapore) as previously explained. Briefly, PCR were conditioned at; initial denaturation at 94°C for 5 minutes and cycles: 1; denaturation at 94°C for 60 seconds, 2; annealing at 55°C for 30 seconds and 3; extension at 72°C for 60 seconds and final extension at 72°C for 5 minutes. PCR products were visualized under UV illumination on gel electrophoresis by using 2% agarose gel stained with redsafe (7.5 µL of redafe were added into 150 ml of TBE suspended with 3g of agarose powder). The amplicon with band size of 1000bp was annotated as blaCTX-M gene, (Figure 1). E. coli ATCC 25922 was used as negative control organisms.
Figure 1.
Visualization of PCR products on 2% agarose gel stained with redsafe. Lane M; ladder marker and lanes 1–13; phenotypic confirmed bacterial isolates. Bacteria with positive blaCTX-M genes are shown by black arrows while negative are shown with with thick blue arrows.
Statistical analysis
Data analysis was done by using STATA 13.0 version. Continuous data were presented as mean (± SD) and categorical data as percentages. Logistic regression analysis was used to show association between male infertility and independent variables. A p-value of less than 0.05 at 95% confidence interval was considered as statistically significant.
Ethical considerations
Ethical clearance to conduct this study was obtained from a joint BMC/CUHAS ethics and review committee and given ethical numbers: CREC 329/2017 and updated in 2018 by certificate number 719/2018. Written informed consent forms were obtained from study participants before enrollment in this study. Laboratory results; semen analysis, and culture and sensitivity were submitted to respective clinicians for patient management
Results
Socio-demographic and clinical characteristics of study participants
A total of 137 participants were enrolled during this study period. The mean age (±SD) and mean infertility duration (±SD) was 33±6.9 years and 2.7±2 years, respectively. The majorities of participants were living in urban areas (64.2%), 54.0% had tertiary education and 97.1% enrolled from BMC. The following participants reported history of; 2.2% fever, 16.1% antibiotic use within one month prior to enrollment in this study, 18.3% UTI and 3.7% sexually transmitted diseases (STDs). Of the 22 participants with history of antibiotic use, 13 used for ≤ 5 days while of 25 participants with history of UTI, 7 purchased antibiotic without prescription, (Table 1).
Table 1.
Socio-demographic and clinical characteristics of study participants
| Variables | Frequency (n) | Percentage (%) | |
| Mean (+/− SD) age (years) | 33 (+/− 6.9) | - | |
| Mean (+/−SD) infertility duration (years) | 2.7 (+/− 2) | - | |
| Residence (N=137) | Urban | 88 | 64.2 |
| Rural | 49 | 35.8 | |
|
Level of education (N=137) |
Uneducated | 3 | 2.2 |
| Primary | 10 | 7.3 | |
| Secondary | 50 | 36.5 | |
| Tertiary | 74 | 54.0 | |
|
Recruitment clinic (N=137) |
Bugando Medical Centre | 133 | 97.1 |
| Kamanga Hospital | 2 | 1.4 | |
| Manjis health care centre | 2 | 1.4 | |
|
History of fever (N=137) |
Yes | 3 | 2.2 |
| No | 134 | 97.8 | |
|
Previous antibiotic (N=137) |
Yes | 22 | 16.1 |
| No | 115 | 83.9 | |
|
Duration of antibiotic use (N=22) |
≤ 5 days | 13 | 59.1 |
| 1 week | 2 | 9.1 | |
| 2 weeks | 6 | 27.3 | |
| 1 month | 1 | 4.5 | |
|
Type of antibiotic used (N=22) |
Ceftriaxone | 9 | 40.9 |
| Ciprofloxacin | 8 | 36.4 | |
| Azythromycin | 3 | 13.6 | |
| Cotrimoxazole | 1 | 4.5 | |
| Amoxycillin | 1 | 4.5 | |
| History of UTI (N=137) | Yes | 25 | 18.3 |
| No | 112 | 81.7 | |
|
UTI treatment facility (N=25) |
Healthcare facility | 18 | 72 |
| Pharmacy or drug shops | 7 | 28 | |
| History of STD (N=137) | Yes | 5 | 3.7 |
| No | 132 | 96.3 | |
| STD treatment (N=137) | Complete | 4 | 80 |
| Incomplete | 1 | 20 | |
| Catheterization (N=137) | Yes | 1 | 0.7 |
| No | 136 | 99.3 | |
|
Other co-morbid (N=137) |
Yes (Hypertension) | 1 | 0.7 |
| No | 136 | 99.3 | |
Semen analysis, bacteriospermia and ESBL bacteriospermia
All participants (100%) had normal semen appearance (color) during semen analysis. Of 137 studied participants, majority of participants had abnormal semen volume (63.5%): 92.0% hypospermia and 8.0% hyperspermia, and poor forward motility of spermatozoa 63.5%. Infertility was observed among 35.0% of participants of which 64.6% had oligospermia (Table 2).
Table 2.
Semen analysis, bacteriospermia and ESBL bacteriospermia results
| Variables | Frequency (n) | Percentage (%) | |
| Quality of spermatozoa and semen | |||
| Semen appearance (N=137) | Normal | 137 | 100 |
| Poor | 0 | 0 | |
| Semen PH (N=137) | Normal (7.2 – 8.2) | 131 | 95.6 |
| Increased alkaline (≥ 9) | 6 | 4.4 | |
| Semen viscosity (N=137) | Normal | 55 | 40.1 |
| Abnormal | 82 | 59.9 | |
| Semen volume (N=137) | Normal | 50 | 36.5 |
| Abnormal | 87 | 63.5 | |
| Abnormal semen volume (N=87) | Hypospermia | 80 | 92.0 |
| Hyperspermia | 7 | 8.0 | |
|
Spermatozoa morphology (N=137) |
Normal | 115 | 83.9 |
| Abnormal | 22 | 16.1 | |
| Spermatozoa motility (N=137) | Good forward motility | 50 | 36.5 |
| Poor forward motility | 87 | 63.5 | |
| Quantity of spermatozoa in semen | |||
| Male infertility (N=137) | Normalspermia | 89 | 65.0 |
| Infertility | 48 | 35.0 | |
| Infertility types (N=48) | Oligospermia(<20 mil/ml) | 31 | 64.6 |
| Azoospermia (no sperms) | 16 | 33.3 | |
| Necrospermia (dead) | 1 | 2.1 | |
| Bacteriospermia | |||
| Bacteriospermia (N=137) | Positive | 44 | 32.1 |
| Negative | 93 | 67.9 | |
| Isolated bacteria spp (N=44) | K. pneumoniae | 12 | 27.3 |
| E. coli | 9 | 20.5 | |
| Acinetobacter spp | 7 | 15.9 | |
| Enterobacter aerogenes | 3 | 6.8 | |
| Enterococcus faecalis | 3 | 6.8 | |
| K. oxytoca | 3 | 6.8 | |
| P. aeruginosa | 3 | 6.8 | |
| Others* | 3 | 6.8 | |
| ESBL Bacteriospermia | |||
| ESBL producing GNB (N=38) | Producers | 12 | 31.6 |
| None producers | 26 | 64.8 | |
| ESBL-GNB species | K. pneumoniae | 7 | 58.3 |
| Acinetobacter spp | 3 | 25 | |
| Enterobacter aerogenes | 1 | 8.3 | |
| Pseudomonas aeruginosa | 1 | 8.3 | |
| ESBL blaCTX-M3G gene (N=12) | Positive | 9 | 75 |
| Negative | 3 | 25 | |
Among 137 semen culture, 32.1% had positive bacteriospermia of which Gram-negative bacteria were predominantly isolated (86.4%). K. pneumoniae (27.3%) was the most frequently isolated bacteria followed by E. coli (20.5%) and Acinetobacter spp. (15.9%). Out of 38 Gram-negative bacteria, 31.6% were phenotypically ESBL producers. K. pneumoniae was predominant ESBL producer detected (58.3%). ESBL blaCTX-M gene was found among 75% of phenotypically confirmed ESBL producers (Table 2).
The mean age (±SD) and mean infertility duration from seeking medical intervention (±SD) of the 15 infertile participants with bacteriospermia was 31.5 (±8.1) years and 2.5 (±1.5) years, respectively. The majority of infertile participants with bacteriospermia had semen hyperviscosity (73.3%, n=11), hypospermia (66.7%, n=10) and poor forward spermatozoa motility (66.7%, n=10) while 4 (26.7%) participants had no spermatozoa in their semen (Table 3).
Table 3.
Description of 15 infertile participants with bacteriospermia
| Age (years) |
Infertility duration |
History of UTI |
Semen analysis | Bacteria spp | ESBL | ||||
| Viscosity | Volume | Morphology | Motility | Remarks | |||||
| 25 | 1 | Yes | Abnormal | Reduced | Normal | Normal | Oligospermia | E. faecalis | N/A |
| 20 | 2 | No | Abnormal | Reduced | N/A | N/A | Azoospermia | S. pyogenes | N/A |
| 33 | 2 | No | Abnormal | Increased | Normal | Poor | Oligospermia | E. faecalis | N/A |
| 26 | 1 | No | Abnormal | Normal | Normal | Poor | Oligospermia | E. coli | NEG |
| 32 | 3 | No | Abnormal | Increased | Normal | Poor | Oligospermia |
K. pneumoniae |
NEG |
| 26 | 1 | No | Abnormal | Normal | Normal | Poor | Oligospermia | E. coli | NEG |
| 45 | 5 | No | Normal | Reduced | Normal | Poor | Oligospermia |
K. pneumoniae |
POS |
| 36 | 4 | No | Normal | Reduced | N/A | N/A | Azoospermia |
K. pneumoniae |
POS |
| 35 | 5 | No | Abnormal | Reduced | Abnormal | Poor | Oligospermia | E. coli | NEG |
| 32 | 2 | Yes | Abnormal | Reduced | Normal | Poor | Oligospermia |
K. pneumoniae |
NEG |
| 35 | 3 | No | Normal | Increased | Abnormal | Poor | Oligospermia | Acinetobacter spp | NEG |
| 29 | 1 | No | Abnormal | Reduced | Normal | Poor | Oligospermia | E. coli | NEG |
| 49 | 5 | No | Normal | Reduced | Normal | Poor | Oligospermia | K. oxytoca | NEG |
| 19 | 1 | No | Abnormal | Reduced | N/A | N/A | Azoospermia | P. aeruginosa | NEG |
| 31 | 2 | No | Abnormal | Reduced | N/A | N/A | Azoospermia |
Acinetobacter spp |
NEG |
KEY: ID=Identification number, N/A=Not Applicable, NEG=Negative and POS=Positive
Antibiotics resistance pattern
Percentage resistance of Gram-negative bacteria to antibiotics ampicillin, trimethoprim-sulphamethoxazole and amoxycillin-clavulanic acid was 100%, 100% and 92.1% respectively while ESBL-GNB resistance to ampicillin, trimethoprim-sulphamethoxazole, amoxycillin-clavulanic acid and gentamicin was 100%, 100%, 91.7% and 66.7%. Percentage resistances of Gram-positive bacteria to erythromycin w ere 66.7% (Table 4).
Table 4.
Antibiotics susceptibility patterns of isolated bacteria causing bacteriospermia and ESBL producing GNB
| ISOLATES | INT | ANTIBIOTIC SUSCEPTIBILITY PROFILES |
||||||||||||
| AMP n(%) |
SXT n(%) |
AK n(%) |
CIP n(%) |
GEN n(%) |
AMC n(%) |
CRO n(%) |
CAZ n(%) |
TZP n(%) |
MEM n(%) |
E n(%) |
VA n(%) |
CD n(%) |
||
| GNB (N=38) |
R | 38(100%) | 38 (100%) |
2(5.3%) | 5(13.2%) | 9(23.7%) | 35(92.1%) | 15(39.5%) | 11(28.9%) | 3(7.9%) | - | N/A | N/A | N/A |
| I | - | - | 4(10.5%) | 1(2.6%) | 1(2.6%) | 2(5.3%) | 3(7.9%) | 5(13.2%) | 3(7.9%) | - | ||||
| S | - | - | 32(84.2%) | 32(84.2%) | 28(73.7%) | 1(2.6%) | 20(52.6%) | 22(57.9%) | 32(84.2%) | 38(100%) | ||||
| ESBL-GNB (N=12) |
R | 12(100%) | 12(100%) | 2(16.7%) | 2(16.7%) | 8(66.7) | 11(91.7%) | 10(83.3%) | 10(83.3%) | 2(16.7%) | - | N/A | ||
| I | - | - | 1(8.3%) | - | - | 1(8.3%) | 2(16.7%) | 2(16.7%) | 1(8.3%) | - | ||||
| S | - | - | 9(75%) | 10(83.3%) | 4(33.3%) | - | - | - | 9(75%) | 12(100%) | ||||
| GPB (N=6) | R | N/A | N/A | N/A | 1(16.7%) | 2(33.3%) | N/A | N/A | N/A | N/A | N/A | 4(66.7%) | ||
| I | - | 1(16.7%) | 1(16.7%) | - | - | |||||||||
| S | 5(83.3%) | 3(50%) | 1(16.7%) | 6(100%) | 6(100%) | |||||||||
AMP=ampicillin, SXT=cotrimoxazole, AK=amikacin, CIP=ciprofloxacin, GEN=gentamicin, AMC=amoxycillin-clavulanic acid, CRO=ceftriaxone, CAZ=ceftazidime, TZP=piperacillin-tazobactam, MEM=meropenem, E=erythromycin, VA=vancomycin, CD=clindamycin, INT=interpretation, R=resistance, I=intermediate, S=sensitive and NA=not applicable
Factors associated with male infertility
On Chi square analysis, male infertility was significantly associated with: semen hyper-viscosity (p=0.022), abnormal semen volume (p=0.015), abnormal spermatozoa morphology (p<0.001) and poor spermatozoa motility (p<0.001).On multivariate regression analysis, male infertility was significantly associated with: antibiotic use (OR (95%CI): 0.14(0.02–0.85),p=0.033), abnormal spermatozoa morphology (OR (95%CI): 14.48(3.17–66.05),p=0.001) and abnormal spermatozoa motility (OR (95%CI): 0.05(0.01–0.24),p<0.001). Bacteriospermia and ESBL bacteriospermia did not have significant association with infertility on both; univariate and multivariate regression analysis (Table 5).
Table 5.
Factors associated with male infertility among presumptive infertile men
| Variables | Infertility N=48, % |
Chi square | P value | Multivariate | ||
| OR(95%CI) | P value | |||||
| Antibiotics use | Yes (22) | 2 (9.1) | 7.7514 | 0.005 | 0.14(0.02–0.85) | 0.033 |
| No (115) | 46 (40) | |||||
| History of UTI | Yes (25) | 3 (6.3) | 7.1299 | 0.008 | * | * |
| No (112) | 45 (93.7) | |||||
| History of STDs | Yes (5) | 2 (40) | 0.0562 | 0.811 | 3.39(0.33–34.82) | 0.304 |
| No (132) | 46 (34.8) | |||||
| Semen viscosity | Hyper (82) | 35 (42.6) | 5.2469 | 0.022 | 1.18(0.39–3.53) | 0.762 |
| Normal (55) | 13 (23.6) | |||||
| Semen volume | Abnormal (87) | 37 (42.5) | 5.8790 | 0.015 | 2.85(0.97–8.39) | 0.057 |
| Normal (50) | 11 (22) | |||||
|
Spermatozoa morphology |
Abnormal (22) | 19 (39.6) | 30.3354 | 0.000 | 14.48(3.17–66.05) | 0.001 |
| Normal (115) | 29 (60.4) | |||||
|
Spermatozoa motility |
Poor (87) | 45 (51.7) | 29.1655 | 0.000 | 0.05(0.01–0.24) | 0.000 |
| Normal (50) | 3 (6.0) | |||||
| Bacteriospermia | Positive (44) | 15 (30.1) | 0.0255 | 0.533 | 0.86(0.29–2.59) | 0.797 |
| Negative (93) | 33 (35.5) | |||||
|
ESBL Bacteriospermia |
Positive (12) | 2 (16.7) | 1.9499 | 0.163 | 0.13(0.01–1.22) | 0.073 |
| Negative (125) | 46 (36.8) | |||||
had collinearity with previous history of antibiotic use
Discussion
In the current study, male infertility was found among one third of the participants, of which oligospermia was prevalent encountered type followed with azoospermia while one participant had necrospermia as observed previous22. Significantly, male infertility was associated with poor forward spermatozoa motility and abnormal spermatozoa morphology. Poor forward spermatozoa motility means that spermatozoa cannot swim properly hence unable to reach the egg for fertilization and abnormal morphology of the spermatozoa means that spermatozoa may be unable to penetrate an egg for fertilization23,24. Therefore, the two factors reduce spermatozoa quality and ability of fetilization25. These factors may be used as surrogate markers for diagnosis of male infertility14.
Neither bacteriospermia nor ESBL bacteriospermia was associated with male infertility as observed elsewhere3,4. This might be due to small sample size of this study resulting to wide 95%CI and imprecise estimate of the effect therefore results didn't have statistical significance26. However, about one third of infertile men had bacteriospermia, suggesting that bacteria might have adverse impact on spermatozoa quantity and/or quality. This is further supported by the fact that history of antibiotic use was protective factor of male infertility. Therefore, there is a need of infectology to be part of infertility diagnosis and management among presumptive infertile men.
Gram-negative bacteria, specifically K. pneumoniae, E. coli and Acinetobacter spp. were prevalently isolated in this study as previously reported from other studies22,27. This is contrary to a study3 which reported that, Gram-positive bacteria, specifically Enterococcus faecalis and Staphylococcus aureus were predominantly isolates causing bacteriospermia. This difference may be due to overall increase trend of multi resistant gram negative infections as the commonest cause of bacterial infections, in the current study's setting Gram-negative bacteria are the most leading causative agents of bacterial infections10.
About one third of the Gram-negative bacteria were found to be ESBL producers with three quarters of phenotypic ESBL producers carrying blaCTX-M gene. The other quarter might be carrying other CTX-M groups and/or other ESBL families (SHV and TEM) as previously observed28. It should be noted previously studies21,29,30 have found blaCTX-M-15 which is a member of group 1 to be the commonest allele (>75%) in the majority of ESBL producers in Tanzania. The observation of about 30% of Gram-negative bacteria from semen culture to carry ESBL genes is significantly higher than what has been observed in other studies31,32. This could be due to high ESBL carriage in our setting due to overuse of antibiotics29,33. In this study it was observed that 16.1% of participants used antibiotics mainly cephalosporins (40.9%) without prescriptions. As previously observed, in the current study, resistance to non -beta lactam antibiotics was very high among ESBL- producers33. The observation is worrisome as treatment options for ESBL producing bacteria are expensive and most of the time not available in most health facilities in developing countries.
Conclusion
The magnitude of bacteriospermia and ESBL bacteriospermia is high among presumptive infertile men. We recommend that, infectology should be part of diagnosis and management of male infertility.
Limitations of the study
Due to limited funds, this study neither characterized other ESBL families (SHV and TEM) nor other CTX-M groups. Furthermore, we did not investigate other pathogens such as fungi and viruses which could have adverse impact on the quality and/or quantity of spermatozoa in semen of infertile men. Another limitation is, we lack data schistosomiasis screening which is endemic in this study setting and reported elsewhere to be associated with male infertility.
Acknowledgment
The authors wish to thank laboratory technicians and scientists from Central Pathology Laboratory, department of Histopathology at Bugando Medical Centre for technical assistance and Mr. Bernard Okamo for optimization of PCR for characterizations of CTX-M gene.
Competing interests
None declared.
Authors' contributions
VS, JI, FC, MFM and SEM conceived and designed this study; YM and ALH collected study data; VS, YM and ALH participated in laboratory procedures; VS, JI, FC, MFM and SEM participated in data analysis; VS wrote the first draft of manuscript; all authors critically revised and approved the final draft of manuscript.
References
- 1.Diemer T, Huwe P, Ludwig M, Hauck E, Weidner W. Urogenital infection and sperm motility. Andrologia. 2003;35(5):283–287. [PubMed] [Google Scholar]
- 2.Ochsendorf F. Sexually transmitted infections: impact on male fertility. Andrologia. 2008;40(2):72–75. doi: 10.1111/j.1439-0272.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- 3.Vilvanathan S, Kandasamy B, Jayachandran AL, et al. Bacteriospermia and its impact on basic semen parameters among infertile men. Interdisciplinary Perspectives on Infectious Diseases. 2016;2016 doi: 10.1155/2016/2614692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golshani M, Taheri S, Eslami G, Rahbar AS, Fallah F, Goudarzi H. Genital tract infection in asymptomatic infertile men and its effect on semen quality. Iranian Journal of Public Health. 2006;35(3):81–84. [Google Scholar]
- 5.Bukharin O, Kuz'min M, Ivanov I. The role of the microbial factor in the pathogenesis of male infertility. Zhurnal Mikrobiologii, Epidemiologii, I Immunobiologii. 2000;2:106–110. [PubMed] [Google Scholar]
- 6.Heffernan H, Pope C, Carter P. Identification of extended-spectrum β-lactamase types, plasmid mediated AmpC β-lactamases and strains among urinary Escherichia coli and Klebsiella in New Zealand in 2006 Communicable Disease Group, ESR. 2007. FW07103. [Google Scholar]
- 7.Iredell J, Brown J, Tagg K. Antibiotic resistance in Enterobacteriaceae: mechanisms and clinical implications. BMJ. 2016;352:h6420. doi: 10.1136/bmj.h6420. [DOI] [PubMed] [Google Scholar]
- 8.Mshana SE, Hain T, Domann E, Lyamuya EF, Chakraborty T, Imirzalioglu C. Predominance of Klebsiella pneumoniae ST14 carrying CTX-M-15 causing neonatal sepsis in Tanzania. BMC Infectious Diseases. 2013;13(1):466. doi: 10.1186/1471-2334-13-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marando R, Seni J, Mirambo MM, et al. Predictors of the extended-spectrum-beta lactamases producing Enterobacteriaceae neonatal sepsis at a tertiary Hospital, Tanzania. International Journal of Medical Microbiology. 2018 doi: 10.1016/j.ijmm.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayange N, Kamugisha E, Mwizamholya DL, Jeremiah S, Mshana SE. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza-Tanzania. BMC Pediatrics. 2010;10(1):39. doi: 10.1186/1471-2431-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seni J, Sweya E, Mabewa A, Mshana SE, Gilyoma JM. Comparison of antimicrobial resistance patterns of ESBL and non ESBL bacterial isolates among patients with secondary peritonitis at Bugando Medical Centre, Mwanza-Tanzania. BMC Emergency Medicine. 2016;16(1):41. doi: 10.1186/s12873-016-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization D, author. WHO laboratory manual for the Examination and processing of human sperm. World Health Organiz; 2010. [Google Scholar]
- 13.WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interlaction [press release] Cambridge Cambridge University Press; 1999. [Google Scholar]
- 14.Cooper TG, Noonan E, Von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Human Reproduction Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 15.Plachot M, Belaisch-Allart J, Mayenga J-M, Chouraqui A, Tesquier L, Serkine AM. Outcome of conventional IVF and ICSI on sibling oocytes in mild male factor infertility. Human Reproduction. 2002;17(2):362–369. doi: 10.1093/humrep/17.2.362. [DOI] [PubMed] [Google Scholar]
- 16.Koneman EW, Allen SD, Janda W, Schreckenberger P, Winn W. Diagnostic microbiology. The nonfermentative gram-negative bacilli. Philedelphia: Lippincott-Raven Publishers; 1997. pp. 253–320. [Google Scholar]
- 17.Wayne P. Clinical and Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing: 20th informational supplement. 2010. CLSI document M100-S20. [Google Scholar]
- 18.Drieux L, Brossier F, Sougakoff W, Jarlier V. Phenotypic detection of extended-spectrum β-lactamase production in Enterobacteriaceae: review and bench guide. Clinical Microbiology and Infection. 2008;14:90–103. doi: 10.1111/j.1469-0691.2007.01846.x. [DOI] [PubMed] [Google Scholar]
- 19.Livermore DM. beta-Lactamases in laboratory and clinical resistance. Clinical Microbiology Reviews. 1995;8(4):557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dashti AA, Jadaon MM, Abdulsamad AM, Dashti HM. Heat treatment of bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med J. 2009;41(2):117–122. [Google Scholar]
- 21.Mshana SE, Imirzalioglu C, Hossain H, Hain T, Domann E, Chakraborty T. Conjugative IncFI plasmids carrying CTX-M-15 among Escherichia coli ESBL producing isolates at a University hospital in Germany. BMC Infectious Diseases. 2009;9(1):97. doi: 10.1186/1471-2334-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad S, Wasim S, Tiwari N, Verma V, Gupta N, Mishra N. Evaluation of Bacteriospermia as Etiology for Oligospermia: An Analysis. International Journal of Scientific Study. 2016;4(2):194–197. [Google Scholar]
- 23.Kristiansen S. The Causes of Low Sperm Motility and How It Can Be Treated. 2015. [02 September 2018]. https://www.infertilityivfhouston.com/blog/2015/06/16/the-causes-of-low-sperm-159391.
- 24.Madormo C. How Does Sperm Morphology Affect Fertility? 2017. [02 September 2018]. https://www.healthline.com/health/sperm-morphology.
- 25.Fisch H. Declining worldwide sperm counts: disproving a myth. Urologic Clinics of North America. 2008;35(2):137–146. doi: 10.1016/j.ucl.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Hackshaw A. Small studies: strengths and limitations. Eur Respiratory Soc. 2008 doi: 10.1183/09031936.00136408. [DOI] [PubMed] [Google Scholar]
- 27.Bhatt C, Mishra S, Bhatt A, Lakhey M. Bacterial pathogens in semen culture and their antibiotic susceptibility pattern in vitro. Int J Biomed Res. 2015;6(11):909–914. [Google Scholar]
- 28.Moremi N, Claus H, Vogel U, Mshana SE. Faecal carriage of CTX-M extended-spectrum beta-lactamase-producing Enterobacteriaceae among street children dwelling in Mwanza city, Tanzania. PloS One. 2017;12(9):e0184592. doi: 10.1371/journal.pone.0184592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mshana SE, Hain T, Domann E, Lyamuya EF, Chakraborty T, Imirzalioglu C. Predominance of Klebsiella pneumoniaeST14 carrying CTX-M-15 causing neonatal sepsis in Tanzania. BMC Infectious Diseases. 2013;13(1):466. doi: 10.1186/1471-2334-13-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moremi N, Manda EV, Falgenhauer L, et al. Predominance of CTX-M-15 among ESBL producers from environment and fish gut from the shores of Lake Victoria in Mwanza, Tanzania. Frontiers in Microbiology. 2016;7 doi: 10.3389/fmicb.2016.01862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibrahim ME, Bilal NE, Magzoub MA, Hamid ME. Prevalence of extended-spectrum β-lactamases-producing Escherichia coli from Hospitals in Khartoum State, Sudan. Oman Medical Journal. 2013;28(2):116. doi: 10.5001/omj.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adeyankinnu FA, Motayo BO, Akinduti A, et al. A multicenter study of beta-lactamase resistant Escherichia coli and Klebsiella pneumoniae reveals high level chromosome mediated extended spectrum β lactamase resistance in Ogun State, Nigeria. Interdisciplinary Perspectives on Infectious Diseases. 2014;2014 doi: 10.1155/2014/819896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marwa KJ, Mushi MF, Konje E, Alele PE, Kidola J, Mirambo MM. Resistance to cotrimoxazole and other antimicrobials among isolates from HIV/AIDS and Non-HIV/AIDS patients at bugando medical centre, Mwanza, Tanzania. AIDS Research and Treatment. 2015;2015 doi: 10.1155/2015/103874. [DOI] [PMC free article] [PubMed] [Google Scholar]