Abstract
Background
The positive effects of steroids on lung development are well known, and 1,25-dihydroxy vitamin D3 has been shown to exert positive effects on fetal lung development.
Objective
We aimed to investigate the relationship between 25-hydroxyvitamin D [25(OH)D] levels and respiratory distress syndrome (RDS) in premature infants.
Methods
Infants aged ≤32 gestational weeks who were admitted to the neonatal intensive care unit (NICU) during 1 year were enrolled in this prospective study. 25(OH)D levels were obtained at the time of admission to NICU. Patients were divided into three groups according to their 25(OH)D levels: severe (group 1), moderate (group 2), and mild (group 3) 25(OH)D deficiencies.
Results
The study comprised 72 patients; of them, RDS was observed in 49 and not observed in 23 patients. The mean 25(OH)D levels were significantly lower in RDS patients (p=0.04). Multivariate analysis showed that patients with higher 25(OH)D levels can be preventive for the development of RDS (odds ratio 0.89; 95% confidence interval 0.8–0.99; p=0.04).
Conclusion
Our study revealed that 25(OH)D deficiency is an independent risk factor for RDS in premature infants. However, further studies are necessary to explore the association between 25(OH)D deficiency and RDS.
Keywords: 25-hydroxyvitamin D, prematurity, respiratory distress syndrome
Introduction
25-hydroxyvitamin D [25(OH)D] is an important steroid hormone involved in bone metabolism and neuromuscular functions; in recent years, an increasing number of reports in the literature have revealed incidences of maternal and neonatal 25(OH)D deficiency.1 Management of vitamin D deficiency is crucial during pregnancy because 25(OH)D deficiency affects fetal development besides bone metabolism.2 25(OH)D exhibits antiproliferative, proapoptotic, and immunomodulatoy properties; additionally, it plays a role in cell development and embryogenesis and helps in fetal lung maturation.3,4
Respiratory distress syndrome (RDS) is the most common respiratory problem in premature infants. The critical factors in the pathogenesis of RDS are surfactant deficiency and pulmonary immaturity.5 The positive effects of steroids on lung development and maturation are well known, and 1,25-dihydroxy vitamin D3 [1,25(OH)2D], which is the active form of 25(OH)D with steroid structure, has been shown to exert positive effects on fetal lung development. Animal studies have shown 1,25(OH)2D to increase surfactant synthesis and secretion by increasing the number of type 2 alveolar cells.1,6 In this study, we aimed to identify the relationship between RDS and 25(OH)D levels in premature infants.
Materials and methods
All preterm infants aged ≤32 gestational weeks admitted to the neonatal intensive care unit (NICU) of the Uludag University Medical School between January 2014 and 2015 were enrolled in this prospective study. Exclusion criteria included refusal of parental consent; infants with major congenital abnormalities, chromosomal anomalies, or cyanotic congenital heart disease; major maternal infection; and chorioamnionitis. The Ethics Committee of the Uludag University Medical Faculty approved this study; a written informed consent was obtained from the parents before enrollment.
RDS diagnosis was established by evaluating x-ray and clinical findings. All patients were treated with nasal continuous positive airway pressure (nCPAP) with 7 cm H2O mean airway pressure. To maintain the arterial oxygen pressure above 60 mm Hg, the required fraction of inspired oxygen (FiO2) was 0.3 for infants aged ≤26 weeks and 0.4 for infants aged >26 weeks; the infants who needed FiO2 above 0.4 were treated with “early rescue therapy” and 200 mg/kg poractant alfa (Curosurf®, Chiesi Pharmaceuticals, Italy). Infants who did not show clinical improvement during follow-up and needed FiO2≥0.4 were administered a second dose 6 h after the first dose. During the follow-up, up to three doses of surfactant were administered due to RDS. Duration of hospital stay, duration of mechanical ventilation, bronchopulmonar dysplasia (BPD) development, and survival rates were recorded.
Demographic data such as maternal age, education level, socioeconomic status, and maternal disease status were documented. All infants were recorded for gestational age, birth weight, gender, type of delivery, and birth period. Birth seasons were separated into four groups: fall (September, October, November), winter (December, January, February), spring (March, April, May), and summer (June, July, August).
Maternal 25(OH)D usage was classified into three groups: those who never used it during pregnancy, those who used it intermittently (<3 months in total), and those who were regular users (≥3 months in total). Patients were divided into three groups according to their 25(OH)D levels, <5 ng/mL as severe deficiency group (group 1); 5–15 ng/mL as moderate deficiency group (group 2); and 15–30 ng/mL as mild deficiency group (group 3).1,7,8
Blood samples of patients were obtained in NICU within 6 h of birth; maternal blood samples were collected within the first 4 h of birth. Plasma of the blood samples from the infants and mothers were stored at −80°C. Samples were analyzed using a Shimadzu LC-20AT high-performance liquid chromatography system (Shimadzu Scientific Instruments, SSI Kyoto, Japan) attached to a UV detector at the Biochemistry Laboratory of the Uludag University Faculty of Medicine (Bursa, Turkey).
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 20.0 software (SPSS Inc., Chicago, IL, USA). Besides evaluating the study data for quantitative data comparison using descriptive statistical methods (mean, standard deviation, median, frequency, rate, minimum, and maximum), Student's t-test was used for two-group comparisons of normal distributions, and Mann-Whitney U test was used for two-group comparisons of non-normal distributions. One-way analysis of variance was used to compare three or more groups with a normal distribution. Logistic regression analysis was used to examine the risk factors affecting RDS. Chi-square, Fisher-Freeman-Halton, and Fisher's exact tests were used for comparison of qualitative data. A p value of <0.05 was considered significant.
Results
Of the 72 patients included in the study, RDS was observed in 49 and not observed in 23 patients. The demographic characteristics of the patients with and without RDS are summarized in Table 1. Age in gestational weeks and birth weights of RDS patients were found to be significantly lower than those of the non-RDS patients, whereas the rates of cesarean section (C/S) and spring-summer births were found to be significantly higher in the former than in the latter (Table 1).
Table 1.
Demographic characterics of the study groups
| RDS (+) (n=49) |
RDS (-) (n=23) |
p | |
| GA at birth, wk median (IQR) |
29 (25–32) | 30 (26–32) | 0.001a |
| Birth weight, g Median (IQR) |
1020 (620–2260) | 1400 (755–3140) | 0.001a |
| Sex, n (%) Male Female |
23 (63.9) 26 (72.2) |
13 (36.1) 10 (28.8) |
0.4b |
| Type of delivery, n (%) NVD C/S |
1 (12.5) 48 (75) |
7 (87.5) 16 (25) |
0.001c |
| Antenatal steroid: n(%) Used Not used |
23 (71.9) 26 (65) |
7 (87.5) 16 (25) |
0.6b |
| Season: n(%) Fall Winter Spring Summer |
12 (50) 6 (54.5) 19 (82.6) 12 (85.7) |
12 (50) 5 (45.5) 4 (17.4) 2 (14.3) |
0.03d |
Mann-Whitney U test
Chi Square test
Ficher's Exact test
Ficher Freeman Halton test
GA: gestational age; IQR: interquartile range; NVD: normal vaginal delivery; C/S: caesarean section
When the patients were classified according to their vitamin D levels, there was no significant difference in terms of perinatal characteristics such as birth weight, gestational age, gender, ethnicity, delivery type, and antenatal steroid usage that could be a risk factor for RDS among the groups (Table 2).
Table 2.
Demographic data in terms of 25(OH)D levels
| Group 1 (n=29) |
Group 2 (n=31) |
Group 3 (n=12) |
p | |
| GA at birth: wk median (IQR) |
29 (25–32) | 30 (26–32) | 29 (26–32) | 0.6a |
| Sex: n (%) Male Female |
10 (27.8) 19 (52.8) |
19 (52.8) 12 (33.3) |
7 (19.4) 5 (13.9) |
0.1b |
| Type of delivery: n (%) NVD C/S |
2(25) 27 (42.2) |
4 (50) 27(42.2) |
2 (25) 10 (15.6) |
0.7c |
| Antenatal Steroid: n (%) Used Not used |
12 (37.5) 17 (42.5) |
14 (43.8) 17 (42.5) |
6 (18.8) 6 (15) |
0.9b |
Kruskal Wallis test
Chi-Square test
Fisher Freeman Halton test
Group 1 (Severe deficiency): 25(OH)D ≤ 5ng/ml; Group 2 (Moderete deficiency): 25(OH)D 5–15ng/ml; Group 3 (Mild deficiency): 25(OH)D 15–30 ng/ml
GA: gestational age; IQR: interquartile range; NVD: normal vaginal delivery; C/S: caesarean section
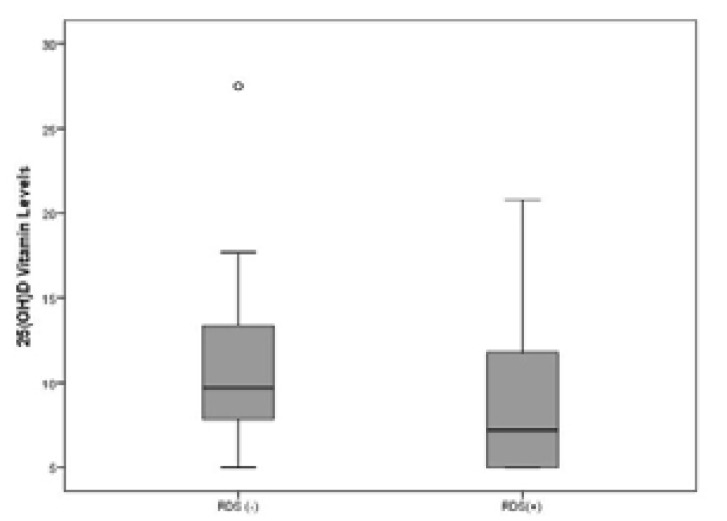
25(OH)D levels were 7.2 ng/mL (median) in the RDS group and 9.7 ng/mL (median) in the no-RDS group, and there was a significant difference in the levels between the groups (p=0.04) (Fig. 1, Table 3). There was no significant association between maternal vitamin D levels and RDS development and it was noteworthy that the rate of RDS in group 1 (patients with severe 25(OH)D deficiency) was higher (82.8%) (Table 3). All mothers included in the study were homogeneous regarding their ethnicity/race. All the mothers were Turkish and of white race. There was no significant difference between the RDS and no-RDS groups regarding maternal age, educational status, maternal vitamin use, and disease status.
Figure 1.
25(OH)D levels of RDS and no-RDS patients
Table 3.
Maternal and infant 25(OH)D levels and RDS ratios of the study groups
| RDS (n=49) |
No-RDS (n=23) |
P | |
| Maternal, 25(OH)D level (ng/ml), median (IQR) |
11.1 (2.6–28.8) | 10.2 (8–21.9) | 0.75a |
| Infant, 25(OH)D level (ng/ml), median (IQR) |
7.2 (5–20.8) | 9.7 (5–27.5) | 0.04a |
| Infant; n(%) Group 1 Group 2 Group 3 |
24 (82.8) 17 (54.8) 8 (66.7) |
5 (17.2) 14 (45.2) 4 (33.3) |
0.06b |
Mann-Whitney U test
Fisher Freeman Halton Test
IQR: interquartile range
Group 1(Severe deficiency): 25(OH)D.5ng/ml; Group 2 (Moderete deficiency): 25(OH)D 5–15 ng/ml; Group 3 (Mild deficiency): 25(OH)D 15–30 ng/ml
When the RDS group was evaluated for the severity of RDS, patients with low vitamin D levels showed a longer duration of mechanical ventilation, which was statistically significant (p=0.002). BPD was not observed in group 3 (mild vitamin D deficiency), whereas BPD rate in group 1 was 45.8% (p=0.3).
When the gestational week, antenatal steroid use, and 25(OH)D levels of the patients were considered as possible independent risk factors in the multiple regression analysis, it was observed that high 25(OH)D levels of the patients (odds ratio [OR] 0.89; 95% confidence interval [CI] 0.80–0.99; p=0.04) and gestational age (OR 0.59; 95% CI 0.42–0.83; p=0.003) can be preventive for the development of RDS.
In multivariate analysis, factors that could affect the duration of hospitalization were assessed, and late neonatal sepsis, BPD, and 25(OH)D levels were included in the model. Of these three factors, BPD was found to have a significant effect on the duration of hospitalization (OR 70.26; 95% CI 53.4–87.0; p<0.001).
Discussion
In this study, we investigated the association between RDS development and vitamin D levels and showed that vitamin D deficiency is an independent risk factor for RDS development. 25(OH)D is thought to prevent surfactant insufficiency by increasing the proliferation of type 2 pneumocytes and, thus, increasing surfactant synthesis, a critical factor in RDS pathophysiology9,10. Our results are compatible with the recent literature reporting a similar relationship between vitamin D deficiency and RDS.1,11,12 Age in gestational weeks is another important risk factor for RDS besides vitamin D deficiency, and patients with RDS have a lower age in gestational weeks and lower birth weight.5 However, even when the cases were controlled according to their age in gestational weeks, vitamin D deficiency was identified as an important risk factor. Moreover, a literature review for RDS risk factors reveals that low birth weight and low 25(OH)D levels can increase the frequency and severity of RDS.12
The active form of 25(OH)D is known to play an essential role in many tissues and epithelial barriers.13 Although there is no clear evidence on the effects of vitamin D on fetal and neonatal lung development, animal studies investigating the effects of vitamin D and vitamin D receptors (VDRs) on the development of pulmonary system have indicated a positive correlation between type 2 pneumocyte and fibroblast proliferation, surfactant synthesis, and VDR upregulation in pulmonary tissue.4,6,14
Surfactant reduces surface tension and helps in controlling lung inflammation. Many factors regulate surfactant synthesis, including cytokine release, hormones, and growth factors.6 Corticosteroids induce fetal lung maturation by aiding type 2 pneumocyte differentiation and stimulating surfactant release.4,15 Nguyen et al. 9 conducted a study on rats and showed an association between VDR expression and lung maturation level. They stated that 1,25(OH)2D may increase surfactant synthesis through gluconeogenesis and that vitamin D plays a crucial role in epithelial cell-mesenchymal cell interactions during pulmonary system development. Vitamin D increases the production as well as secretion of surfactant-related phospholipids in type 2 pneumocytes; furthermore, it has been reported that during the most active period of alveolarization, 1,25(OH)2D positively affects type 2 pneumocytes and fibroblasts by inhibiting apoptosis and stimulating secretion of surfactant-related phospholipids.16 The inhibition of apoptosis results in an increased number of cells postnatally.17,18 In a study by Sakurai et al. 14 C-3 epimer, which is a metabolite of 1,25(OH)2D, was shown to play a crucial role in antenatal lung maturation, lipofibroblast proliferation, and epithelial mesenchymal interaction mechanisms. In our study, vitamin D levels of the RDS patients were significantly lower similar to the findings in the literature. It is noteworthy that the RDS rate was markedly higher in patients with severe vitamin D deficiency.11
In our study, patients' age in gestational weeks and birth weights were low, as expected, and C/S birth rate was high in RDS cases. In a study by Tochie et al.19 C/S was reported to be an independent predictor for RDS. Regular pregnancy follow-up and avoiding unnecessary early elective C/S may reduce the incidence of premature births and RDS.
For patients in group 1, the duration of mechanical ventilation was significantly longer and the BPD rate was 47.1%. Due to the positive effects of high 25(OH)D levels on lung development, it can be speculated that patients in group 3 had a better prognosis for RDS. The reason behind this speculation is that in group 3, a markedly reduced duration of mechanical ventilation was observed and no BPD was observed in any case. A similar observation was recently reported by Cetinkaya et al.20 who detected a severe 25(OH)D deficiency in all premature BPD patients. BPD is one of the most severe morbidities in premature patients, and we found a significant effect of BPD on the duration of hospitalization. In premature infants, positive effects of high 25(OH)D levels on preventing BPD are promising.
The literature reveals no clarity on the adequate 25(OH) D levels, particularly in premature infants. Fettah et al.11 have defined 25(OH)D deficiency for premature infants as levels ≤ 15 ng/mL. Likewise, in this study, we defined vitamin D levels <5 ng/mL as severe deficiency and levels between 5 and 15 ng/mL as moderate deficiency. In the literature, 25(OH)D levels >30 ng/ml have been accepted as normal. Therefore, we defined levels between 15 and 30 ng/mL as mild deficiency.8,21,22
Multivitamin supplements containing 500 IU of vitamin D are recommended routinely for all pregnant women in Turkey. In our study, no difference was found between the RDS and non-RDS groups regarding maternal vitamin use. Consistent with previous studies, maternal and neonatal 25(OH)D vitamin levels were correlated, and both maternal and fetal vitamin D levels were often low.23 Although vitamin D supplementation is recommended in pregnancy, both neonatal and maternal vitamin D levels remain low probably due to the lack of regular use of vitamin D supplements. Also vitamin D deficiency is more likely to be observed in premature infants aged <32 gestational weeks owing to shorter gestation periods and insufficient vitamin D stores.24 In our study, infants with severe 25(OH)D deficiency exhibited higher RDS rates, a longer need for mechanical ventilation, and higher BPD rates. Therefore, vitamin D deficiency at birth may predispose infants to prematurity-related complications.
Our study had several limitations. One of them was the small number of cases in the groups. Furthermore, 25(OH)D levels were low in all patients; thus, it was unclear whether vitamin D deficiency was related to prematurity and all prematurity morbidities or only RDS. Additionally, we used common vitamin D deficiency definitions because of the lack of clarity in the literature regarding normal 25(OH)D vitamin levels corresponding to gestational weeks. Nonetheless, we believe that largescale studies on 25(OH)D vitamin levels according to gestational weeks are needed.
Conclusion
25(OH)D deficiency is an independent risk factor for RDS development in premature infants, and high neonatal 25(OH)D levels at birth may protect premature infants against RDS. However, further studies with more patients are necessary to assess the association between 25(OH)D deficiency and RDS.
Conflict of interest
None.
Funding
None.
References
- 1.Fettah ND, Zenciroğlu A, Dilli D, Beken S, Okumuş N. Is higher 25-hydroxyvitamin D level preventive for respiratory distress syndrome in preterm infants? Am J Perinatol. 2015;32(3):247–250. doi: 10.1055/s-0034-1383849. [DOI] [PubMed] [Google Scholar]
- 2.Thandrayen K, Pettifor JM. Maternal vitamin D status: implications for the development of infantile nutritional rickets. Rheum Dis Clin North Am. 2012;38(1):61–79. doi: 10.1016/j.rdc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lykkedegn S, Sorensen GL, Beck-Nielsen SS, Christesen HT. The impact of vitamin D on fetal and neonatal lung maturation. A systematic review. Am J Physiol Lung Cell Mol Physiol. 2015;308(7):L587–L602. doi: 10.1152/ajplung.00117.2014. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez RJ. Management of respiratory distress syndrome: an update. Respir Care. 2003;48(3):279–286. discussion 286–287. [PubMed] [Google Scholar]
- 6.Phokela SS, Peleg S, Moya FR, Alcorn JL. Regulation of human pulmonary surfactant protein gene expression by 1alpha, 25-dihydroxyvitamin D3. Am J Physiol Lung Cell Mol Physiol. 2005;289(4):L617–L626. doi: 10.1152/ajplung.00129.2004. [DOI] [PubMed] [Google Scholar]
- 7.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M, Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen M, Trubert CL, Rizk-Rabin M, Rehan VK, Besançon F, Cayre YE, et al. 1,25-Dihydroxyvitamin D3 and fetal lung maturation: immunogold detection of VDR expression in pneumocytes type II cells and effect on fructose 1,6 bisphosphatase. J Steroid Biochem Mol Biol. 2004;89–90(1–5):93–97. doi: 10.1016/j.jsbmb.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Ballard PL, Ertsey R, Gonzales LW, Gonzales J. Transcriptional regulation of human pulmonary surfactant proteins SP-B and SP-C by glucocorticoids. Am J Respir Cell Mol Biol. 1996;14(6):599–607. doi: 10.1165/ajrcmb.14.6.8652188. [DOI] [PubMed] [Google Scholar]
- 11.Yu RQ, Chen DZ, Hao XQ, Jiang SH, Fang GD, Zhou Q. Relationship between serum 25(OH)D levels at birth and respiratory distress syndrome in preterm infants. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19(11):1134–1137. doi: 10.7499/j.issn.1008-8830.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazzi SNJ, Karnati S, Puthuraya S, Thomas R. Vitamin D deficiency and respiratory morbidity among African American very low birth weight infants. Early Hum Dev. 2018;119:19–24. doi: 10.1016/j.earlhumdev.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G208–G216. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 14.Sakurai R, Shin E, Fonseca S, Sakurai T, Litonjua AA, Weiss ST, et al. 1alpha,25(OH)2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. Am J Physiol Lung Cell Mol Physiol. 2009;297(3):L496–L505. doi: 10.1152/ajplung.90539.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Renzo GC, Anceschi MM, Cosmi EV. Lung surfactant enhancement in utero. Eur J Obstet Gynecol Reprod Biol. 1989;32(1):1–11. doi: 10.1016/0028-2243(89)90115-9. [DOI] [PubMed] [Google Scholar]
- 16.Lee P, Nair P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med. 2009;360(18):1912–1914. doi: 10.1056/NEJMc0809996. [DOI] [PubMed] [Google Scholar]
- 17.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181(10):7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett N, Zhao Z, Koyama T, Janz DR, Wang CY, May AK, et al. Vitamin D deficiency and risk of acute lung injury in severe sepsis and severe trauma: a case-control study. Ann Intensive Care. 2014;4(1):5. doi: 10.1186/2110-5820-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tochie JN, Choukem SP, Langmia RN, Barla E, Koki-Ndombo P. Neonatal respiratory distress in a reference neonatal unit in Cameroon: an analysis of prevalence, predictors, etiologies and outcomes. Pan Afr Med J. 2016;24:152. doi: 10.11604/pamj.2016.24.152.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Çetinkaya M, Çekmez F, Erener-Ercan T, Buyukkale G, Demirhan A, Aydemir G, et al. Maternal/neonatal vitamin D deficiency: a risk factor for bronchopulmonary dysplasia in preterms? J Perinatol. 2015;35(10):813–817. doi: 10.1038/jp.2015.88. [DOI] [PubMed] [Google Scholar]
- 21.Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202(5):429 e1–429 e9. doi: 10.1016/j.ajog.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cizmeci MN, Kanburoglu MK, Akelma AZ, Ayyildiz A, Kutukoglu I, Malli DD, et al. Cord-blood 25-hydroxyvitamin D levels and risk of early-onset neonatal sepsis: a case-control study from a tertiary care center in Turkey. Eur J Pediatr. 2015;174(6):809–815. doi: 10.1007/s00431-014-2469-1. [DOI] [PubMed] [Google Scholar]
- 23.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79(5):717–726. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 24.Marshall I, Mehta R, Petrova A. Vitamin D in the maternal-fetal-neonatal interface: clinical implications and requirements for supplementation. J Matern Fetal Neonatal Med. 2013;26(7):633–638. doi: 10.3109/14767058.2012.746306. [DOI] [PubMed] [Google Scholar]